Abstract
Background and Aims
The mechanism of auxin action on ion transport in growing cells has not been determined in detail. In particular, little is known about the role of chloride in the auxin-induced growth of coleoptile cells. Moreover, the data that do exist in the literature are controversial. This study describes experiments that were carried out with maize (Zea mays) coleoptile segments, this being a classical model system for studies of plant cell elongation growth.
Methods
Growth kinetics or growth and pH changes were recorded in maize coleoptiles using two independent measuring systems. The growth rate of the segments was measured simultaneously with medium pH changes. Membrane potential changes in parenchymal cells of the segments were also determined for chosen variants. The question of whether anion transport is involved in auxin-induced growth of maize coleoptile segments was primarily studied using anion channel blockers [anthracene-9-carboxylic acid (A-9-C) and 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS)]. In addition, experiments in which KCl was replaced by KNO3 were also performed.
Key Results
Both anion channel blockers, added at 0·1 mm, diminished indole-3-acetic acid (IAA)-induced elongation growth by ∼30 %. Medium pH changes measured simultaneously with growth indicated that while DIDS stopped IAA-induced proton extrusion, A-9-C diminished it by only 50 %. Addition of A-9-C to medium containing 1 mm KCl did not affect the characteristic kinetics of IAA-induced membrane potential changes, while in the presence of 10 mm KCl the channel blocker stopped IAA-induced membrane hyperpolarization. Replacement of KCl with KNO3 significantly decreased IAA-induced growth and inhibited proton extrusion. In contrast to the KCl concentration, the concentration of KNO3 did not affect the growth-stimulatory effect of IAA. For comparison, the effects of the cation channel blocker tetraethylammonium chloride (TEA-Cl) on IAA-induced growth and proton extrusion were also determined. TEA-Cl, added 1 h before IAA, caused reduction of growth by 49·9 % and inhibition of proton extrusion.
Conclusions
These results suggest that Cl– plays a role in the IAA-induced growth of maize coleoptile segments. A possible mechanism for Cl– uptake during IAA-induced growth is proposed in which uptake of K+ and Cl– ions in concert with IAA-induced plasma membrane H+-ATPase activity changes the membrane potential to a value needed for turgor adjustment during the growth of maize coleoptile cells.
Keywords: Anion channel blockers, auxin, cell growth, chloride uptake, coleoptile segments, elongation growth, membrane potential, Zea mays maize
INTRODUCTION
Although research on the effects of auxin on plant growth has been carried out for a long time, the mechanism of auxin action on ion transport in growing cells has still not been precisely investigated. The ‘acid growth’ theory of auxin action, independently proposed by Cleland (1971) and Hager et al. (1971), still evokes discussion and undergoes multiple modifications (for a critical evaluation see Kutschera, 1994, 2006; Niklas and Kutschera 2012). For example, Kutschera (1994, 2006) suggests that it is the fungal phytotoxin fusicoccin, not the auxin indole-3-acetic acid (IAA), that fulfils the predictions of the acid growth hypothesis of coleoptile elongation. Upon addition of fusicoccin, the equilibrium pH in the incubation medium of coleoptile segments is 3·5–4·0 (Rubinstein and Cleland, 1981; Kutschera, 1994, 2006; Karcz and Burdach, 2002; Karcz et al., 2008; Rudnicka et al., 2014), whereas in the presence of IAA a pH of only 4·8–5·0 is observed (Kutschera, 1994, 2006; Karcz and Burdach, 2002; Hager, 2003; Rudnicka et al., 2014). Although much is known about the action of IAA in grass coleoptiles, the question of the extent to which endogenous and IAA-induced growth depends on cell wall acidification remains open. As new techniques for the analysis of membrane channels have been developed, particularly patch-clamp techniques, the acid growth theory has been supported by new data on the role of various ions in auxin action. Patch-clamp techniques applied to maize coleoptile protoplasts showed that K+ uptake channels (ZMK1) are involved in auxin-induced elongation growth (Philippar et al., 1999; Becker and Hedrich, 2002). ZMK1 channels exhibit typical properties of voltage-dependent, proton-stimulated K+ channels, which are activated by a hyperpolarizing membrane potential and by extracellular apoplastic protons. These channels mediate K+ uptake into cortex cells, increasing their turgor and cell expansion. It has been shown that, apart from posttranslational, auxin-dependent upregulation of K+ uptake channels, auxin also regulates the expression of the maize K+ uptake channel gene ZMK1 (Philippar et al., 1999). In turn, this leads to incorporation of newly synthesized K+ channels into the plasma membrane and to an increase in the number of active K+ channels in the plasma membrane (Philippar et al., 1999). Interestingly, a scenario similar to that of the auxin-induced activation of K+ channels has been reported for the stimulation of H+-pumping ATPase in the plasma membrane of maize coleoptile cells (Hager et al., 1991). Experiments performed with the patch-clamp technique have confirmed earlier studies showing that auxin-induced growth depends strictly on external K+ supply (Claussen et al., 1997).
Significantly less is known about the role of Cl– ions in the auxin-induced growth of coleoptile cells. In addition, the existing data are often controversial. For example, in their early experiments Rubinstein and Light (1973) and Rubinstein (1974) showed that IAA enhanced Cl– uptake into Avena coleoptile cells, whereas Haschke and Lüttge (1975a, b) did not find such dependence in studies performed on the same material. Somewhat later, the stimulating effect of auxin on Cl– uptake into oat coleoptile cells was also reported by Stevenson and Cleland (1981).
Starting in the late 1990s, there was renewed emphasis on studying the role of Cl– ions in auxin-induced growth of coleoptile cells. Keller and Van Volkenburgh (1996a) showed that depolarization of membrane potential preceding the auxin-induced membrane hyperpolarization in Avena sativa coleoptiles was sensitive to external Cl– and was blocked in the presence of an anion channel blocker [anthracene-9-carboxylic acid (A-9-C)]. Interestingly, they also showed that at high concentrations A-9-C inhibited endogenous and auxin-induced growth. The same authors (Keller and Van Volkenburgh, 1996b), on the basis of experiments performed on oat coleoptile protoplasts, reported that auxin-induced protoplast swelling was independent of the presence of Cl– in the medium. Results obtained with oat coleoptiles by Babourina et al. (1998) are in sharp contrast with data recorded by Keller and Van Volkenburgh (1996a) and suggest that the Cl– transport system of the plasma membrane, causing increased Cl– uptake, is one of the targets of auxin action in cells.
Experiments carried out by Marten et al. (1991) and Thomine et al. (1997) on Vicia faba guard cells and Arabidopsis thaliana hypocotyls, respectively, should also be mentioned. The former group of authors found that voltage-dependent anion channels in guard cells were modulated by auxin. However, the latter showed that anion channel blockers [A-9-C and 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS)], which produced no or little effect by themselves, were able to counteract the auxin-induced growth inhibition in hypocotyl cells.
The main question we addressed was whether Cl– ions participate in IAA-induced growth of maize coleoptile segments. To answer this question, we undertook experiments in which we studied (1) the effects of KCl and KNO3 on both IAA-induced growth and the pH of the medium, measured simultaneously with growth; (2) the impact of anion and cation channel blockers on both IAA-induced growth and medium pH; and (3) the effects of KCl and an anion channel blocker (A-9-C) on the membrane potential in parenchymal cells of maize coleoptile segments incubated in the presence and absence of IAA.
MATERIALS AND METHODS
Plant material
Experiments were carried out with 10-mm-long segments obtained from 4-day-old maize (Zea mays ‘Cosmo’) coleoptiles grown in the dark at 27 ± 1 °C. Coleoptile segments, with the first leaves removed, were excised 3 mm below the tip and collected in a control medium of the following composition: 1 or 10 mm KCl, 0·1 mm NaCl, 0·1 mm CaCl2; initial pH 5·8–6·0. Conditions for growing the maize seedlings have been described previously (Karcz and Burdach, 2002; Kurtyka et al., 2011).
Chemicals
An aqueous stock solution (1 mm) of IAA (Serva, Heidelberg, Germany) was prepared using the potassium salt of IAA. IAA was used at a final concentration of 10 µm. The potassium channel blockers tetraethylammonium chloride (TEA-Cl; Sigma, USA) and BaCl2 (POCh, Poland) were dissolved in deionized water and used at a final concentration of 30 and 10 mm, respectively. The anion channel blockers A-9-C (Sigma, USA) and DIDS (Aldrich, USA) were used at a final concentration of 0·1 mm. The A-9-C solution was prepared by dissolving the substance in 1 m KOH, afterwards supplemented with deionized water. DIDS was dissolved in 0·1 m KHCO3. In selected experiments, KCl was replaced with KNO3 (POCh, Poland) at a final concentration of 1 or 10 mm. It is worth pointing out that the replacement of KCl with K-gluconate significantly buffered medium pH (Supplementary Data Fig. S1).
Growth and pH measurements
Growth experiments were carried out in two independent elongation measurement systems. In the first system, high-resolution measurements of growth rate were performed with an angular position transducer (TWK Electronik, Düsseldorf, Germany), which resulted in a precise record of the growth kinetics. In this system, as previously described (Karcz et al., 1999; Karcz and Burdach, 2002; Polak et al., 2011), five unabraded coleoptile segments, 10 mm in length, were strung on a stainless steel needle and inserted vertically in an intensively aerated control solution (5 ml per segment). The length of coleoptile segments was sampled every 3 min with a CX 721 converter (Elmetron, Poland). Data were stored on a diskette and analysed with the Statistica program (version 10.0, Statsoft, USA, http://www.statsoft.com). The growth rate was expressed in µm s–1 cm–1.
In the second system, an apparatus for simultaneous measurements of elongation growth and pH of the incubation medium was used, as recently described by Polak et al. (2012). Briefly, measurements of growth rate were performed, similarly to the first system, using an angular position transducer (TWK, Düsseldorf, Germany). In this apparatus, 60 coleoptile segments were arranged vertically in three narrow glass pipettes (20 segments in each) connected by means of a silicone hose. Coleoptile segments were incubated in an intensively aerated medium. The volume of the incubation medium in the elongation and pH-measuring apparatus was 18 ml (0·3 ml segment–1). The incubation medium also flowed through the lumen of the coleoptile cylinders. This feature permitted the experimental solutions to be in direct contact with the interior of segments, which significantly enhanced both elongation growth of coleoptile segments and proton extrusion (Karcz et al., 1995). Medium circulation was driven by a peristaltic pump (Type Peri-Star PRO, World Precision Instruments Inc., USA). Extension growth of a stack of 20 segments and pH of the incubation medium were sampled every 3 min using a multifunctional computer (CX-771, Elmetron, Poland). A pH electrode (OSH 10-10, Metron, Poland) measured the pH of incubation solutions. The data were collected using a data logger (CX-771, Elmetron, Poland).
Electrophysiology
Electrophysiological experiments were performed on intact, 10-mm-long coleoptile segments, prepared in the same manner as for growth experiments. A standard electrophysiological technique was used for membrane potential measurements, as previously described (Karcz and Burdach, 2002; Kurtyka et al., 2011). Briefly, membrane potential (Em) was measured by recording the voltage between a glass micropipette filled with 3 m KCl inserted into the parenchymal cells and a reference electrode in the bathing medium of the same composition as that used in the growth experiments. Before electrophysiological experiments, coleoptile segments were preincubated in an intensively aerated control medium (1 or 10 mm KCl, 0·1 mm NaCl, 0·1 mm CaCl2; initial pH 5·8–6·0). A-9-C and IAA were added to the medium in accordance with the time protocol used for the growth experiments (details of electrophysiological experiments are included in Table 1). The flow of medium was driven with a peristaltic pump (Type Peri-Star PRO, World Precision Instruments Inc., USA), which allowed a change of the bathing medium in the electrophysiological chamber (usually 4-fold within <2 min). Microelectrodes were inserted into parenchymal cells under a microscope by means of a micromanipulator (Hugo Sachs Elektronik, Germany). After stabilization of the membrane potential, IAA was added. Micropipettes were prepared as previously described by Karcz and Burdach (2002) and Kurtyka et al. (2011).
Table 1.
Membrane potential (Em, mV) in parenchymal coleoptile cells. Data (mean ± s.e.) are means of at least seven independent experiments
| Treatment | A | B | C | D | E | F | Em at depolarization | F–A |
|---|---|---|---|---|---|---|---|---|
| 0 min | 3 min | 6 min | 10 min | 20 min | 30 min | |||
| 1 mm KCla | –120·1 ± 4·3 | –119·8 ± 3·9 | –119·2 ± 4·6 | –120·5 ± 5·8 | –121·0 ± 5·4 | –122·4 ± 6·1 | – | –2·3ns |
| 1 mm KCl + IAAb | –120·6 ± 7·4 | –121·0 ± 6·6 | –116·6 ± 4·9 | –117·3 ± 5·2 | –126·1 ± 6·7 | –131·3 ± 7·5 | –115·5 ± 5·9 | –10·7* |
| 1 mm KCl + A-9-Cc + IAAb | –108·4 ± 4·7 | –107·9 ± 5·4 | –105·4 ± 6·2 | –108·1 ± 5·2 | –115·8 ± 6·1 | –118·5 ± 6·8 | –105·3 ± 5·8 | –10·1* |
| 10 mm KCl | –70·6 ± 4·1 | –68·8 ± 3·9 | –67·9 ± 4·3 | –68·2 ± 4·5 | –68·4 ± 3·8 | –67·9 ± 4·2 | – | 2·7ns |
| 10 mm KCl + IAAb | –68·7 ± 4·5 | –69·2 ± 4·7 | –67·8 ± 3·8 | –70·0 ± 3·4 | –72·8 ± 4·1 | –74·1 ± 4·3 | –64·7 ± 4·2 | –5·4* |
| 10 mm KCl + A-9-Cc + IAAb | –64·8 ± 3·9 | –60·8 ± 4·2 | –62·4 ± 3·6 | –63·6 ± 4·5 | –63·1 ± 3·3 | –62·2 ± 3·5 | –60·8 ± 4·2 | 2·6ns |
aColeoptile segments were incubated in the indicated medium (the same as in growth experiments) for 110 min, after which a single segment was transferred into an electrophysiological chamber containing the same medium. Measurements of membrane potential (30 min) were carried out after insertion of a microelectrode into the cell and stabilization of the Em ( < 10 min) at 2 h (0 min).
bIAA was added after 2 h of segment preincubation in medium with 1 or 10 mm KCl (for the last 10 min the coleoptile segments were incubated in the electrophysiological chamber).
cA-9-C was added after 1 h of segment preincubation in medium with 1 or 10 mm KCl (for the last 10 min the coleoptile segments were incubated in the electrophysiological chamber).
nsNot statistically significant.
*Statistically significant at P < 0·05.
Statistical analysis
Data were analysed with Statistica software for Windows (version 8.0). Differences between individual treatments and the control were analysed using one-way ANOVA and the least significant difference (LSD) test.
RESULTS
Effect of IAA on the growth of coleoptile segments incubated in the presence of KCl or KNO3
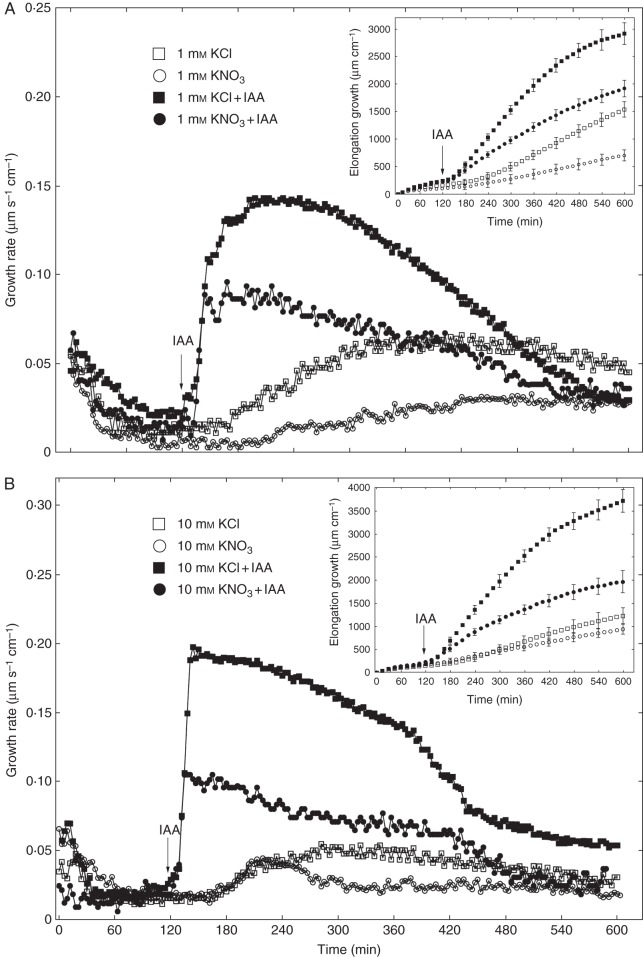
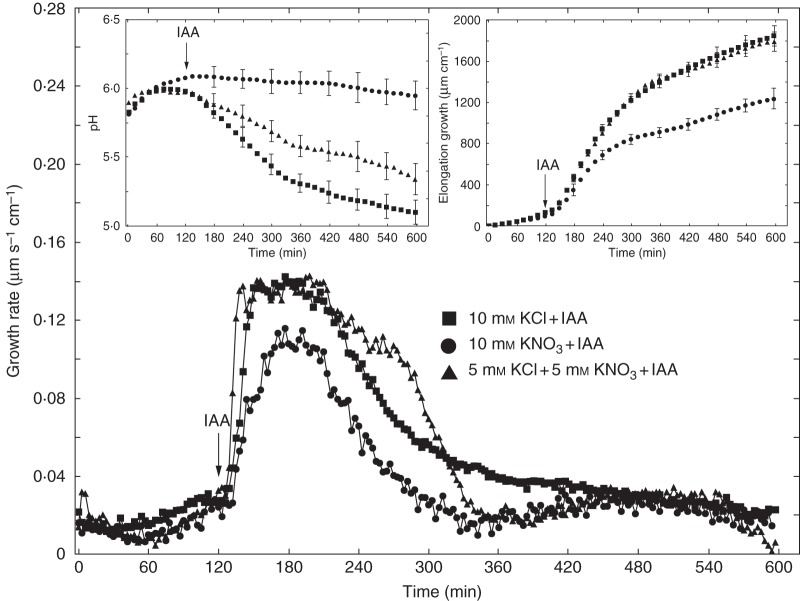
Experiments described in this section were performed using the first elongation measurement system. Figure 1 shows the growth-promoting activity of 10 µm IAA in maize coleoptile segments incubated in the presence of KCl or KNO3. The segments were first preincubated for 2 h in auxin-free medium until a low, constant growth rate (∼0·02 µm s–1 cm–1) was achieved, whereupon IAA was added at a final concentration of 10 µm. As can be seen in Fig. 1A, auxin added to incubation medium containing 1 mm KCl induced rapid growth with a maximal growth rate of ∼0·14 µm s–1 cm–1. When IAA was added to medium with 1 mm KNO3, a significant decrease was observed in growth rate, which did not exceed 0·1 µm s–1 cm–1. The total IAA-induced elongation growth (calculated between 120 and 600 min as the sum of extensions from measurements at 3-min intervals) of maize coleoptile segments incubated in the presence of 1 mm KNO3 was 36·4 % lower than that measured in the presence of 1 mm KCl (Fig. 1A). When the KCl concentration in the incubation medium was increased from 1 to 10 mm (Fig. 1B), auxin-induced elongation of coleoptile segments was 24·3 % greater. However, the growth-stimulatory effect of IAA did not depend on KNO3 concentration (Fig. 1A, B). Interestingly, the difference between IAA-induced elongation growth of maize coleoptile segments incubated in the presence of 10 mm KCl and 10 mm KNO3 was ∼46 %. Replacement of KCl by KNO3 also diminished endogenous growth (growth in a medium without growth effectors, here an auxin-free medium) of the coleoptile segments. In the presence of 1 and 10 mm KNO3, endogenous growth of coleoptile segments was 43·3 % and 24 % lower than that measured for 1 and 10 mm KCl, respectively (Fig. 1A, B). It should also be mentioned that, upon replacement of KCl by KNO3, the first, very rapid, phase of auxin-induced growth was reduced by 50 %. These data suggest that elongation growth of maize coleoptile segments measured in the presence of IAA depends specifically on Cl– . To confirm this suggestion, we performed growth and electrophysiological experiments using anion channel blockers. For comparison, the effect of K+ channel blockers on growth of maize coleoptile segments was also studied.
Fig. 1.
Effects of 1 mm (A) and 10 mm (B) KCl or KNO3 on the growth rate of maize coleoptile segments incubated in the presence or absence of 10 μm IAA. The growth rate of a stack of five segments was measured as described in Materials and methods (first measuring system). IAA was added to the incubation medium at 2 h. Inset shows total elongation growth calculated as the sum of extensions measured at 3-min intervals for 10 h. All curves represent means of at least nine independent experiments. Bars indicate ± s.e. The LSD test is included in Supplementary Data Fig. S2.
Effect of anion and cation channel blockers on IAA-induced elongation growth of coleoptile segments
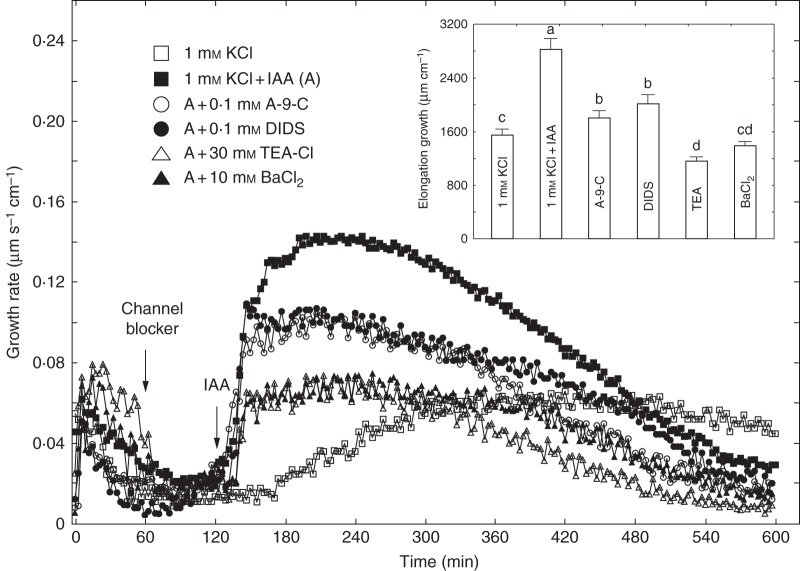
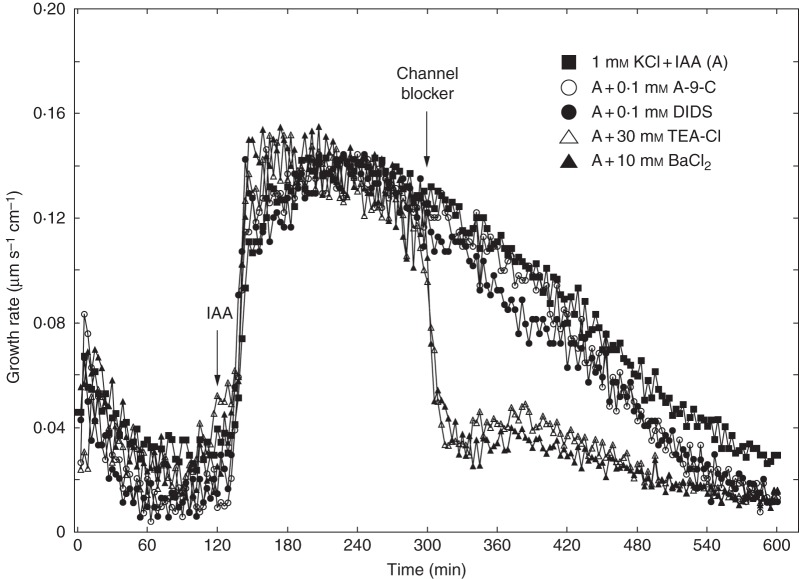
The anion channel blockers (A-9-C and DIDS belong to distinct chemical families: A-9-C is a polycyclic molecule derived from anthracene while DIDS is a stilbene derivative. Figure 2 shows the effects of A-9-C and DIDS on IAA-induced growth of maize coleoptile segments incubated in the first system. Coleoptile segments were first preincubated (1 h) in control medium, and A-9-C or DIDS was then added at a final concentration of 0·1 mm. At 2 h, IAA was added to the incubation medium. Data in Fig. 2 indicate that A-9-C and DIDS diminished the growth of maize coleoptile segments incubated in the presence of IAA by 32·3 and 24·6 %, respectively. Both anion channel blockers reduced the first (rapid) phase of IAA-induced growth practically to the same level (∼0·1 µm s–1 cm–1). If coleoptile segments were first treated with IAA (at 2 h) and subsequently with A-9-C or DIDS (at 5 h), a small reduction was observed in elongation growth (Figs 2 and 3). Application of A-9-C and DIDS 3 h after addition of IAA inhibited growth by 14 % and 22 %, respectively, during the next 5 h (Fig. 3). Addition of potassium channel blockers (TEA-Cl and BaCl2) reduced elongation growth of maize coleoptile segments to a similar levels. Elongation growth of coleoptile segments incubated with IAA was reduced by 57 % in the presence of TEA-Cl and 52 % in the presence of BaCl2. However, addition of potassium channel blockers at 5 h caused a rapid reduction in growth rate (Fig. 3).
Fig. 2.
Effects of anion (A-9-C or DIDS) and cation (TEA-Cl or BaCl2) channel blockers on the growth rate of maize coleoptile segments incubated in the presence of IAA (10 μm). Coleoptile segments were preincubated (1 h) in a control medium and the channel blockers were then added; IAA was added to the incubation medium at 2 h. Curves represent data obtained from the first measuring system. Inset shows total elongation growth as a bar graph, calculated as the sum of extensions between 120 and 600 min of the experiment. Values are means of at least nine independent experiments. Bars indicate s.e. Means followed by the same letter are not significantly different from each other according to the LSD test (P < 0·05).
Fig. 3.
Effects of anion (A-9-C or DIDS) and cation (TEA-Cl or BaCl2) channel blockers on the growth rate of maize coleoptile segments incubated in the presence of IAA (10 μm). Auxin was added to the incubation medium at 2 h and channel blockers 3 h later. Curves represent data obtained from the first measuring system. All curves are means of at least eight independent experiments.
Effects of IAA on simultaneously measured growth and medium pH of coleoptile segments incubated in the presence of KCl or KNO3
The experiments described below were performed using the apparatus for simultaneous measurements of both the elongation growth and pH of the incubation medium (the second system). IAA, at a final concentration of 10 µm, was added to the incubation medium using the same time protocol as in the first system.
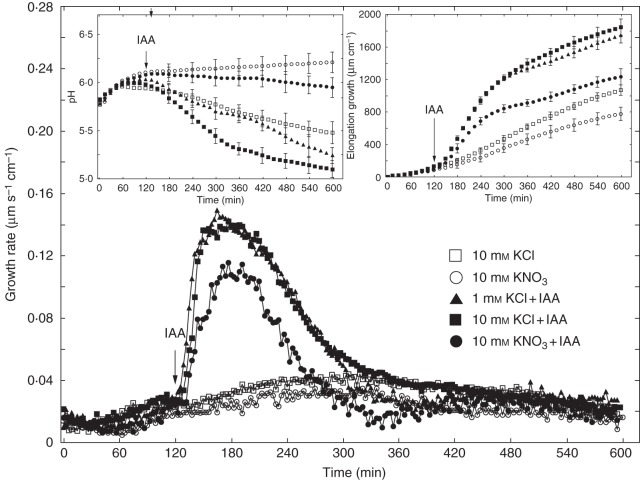
As IAA induced practically the same growth when added to medium containing 1 or 10 mm KCl (maximal growth rate ∼0·14 µm s–1 cm–1; Fig. 4), we decided to use 10 mm KCl in further experiments. Such a choice was dictated by (1) increased accessibility of K+ and Cl– ions to coleoptile segments, considering their density (60 segments per 18 ml), and (2) a significantly higher total IAA-induced efflux of H+ ions observed in the presence of 10 mm KCl compared with 1 mm KCl (Fig. 4, left inset). When IAA was added to medium containing 10 mm KCl, the total IAA-induced elongation of coleoptile segments (calculated between 120 and 600 min) was 33·2 % greater than for 10 mm KNO3 (Fig. 4, right inset). Medium pH changes, measured simultaneously with growth, indicated that KNO3 inhibited both IAA-induced proton extrusion and proton extrusion in auxin-free medium (Fig. 4, left inset). To prove that IAA-induced growth of maize coleoptile segments depends specifically on Cl– ions, experiments were performed in which 5 mm of KCl plus 5 mm KNO3 was used. Data presented in Fig. 5 revealed no differences between the growth of coleoptile segments incubated in medium containing 10 mm KCl or 5 mm KCl plus 5 mm KNO3, suggesting that NO3– ions did not inhibit IAA-induced growth in the presence of KCl. Interestingly, in medium with 5 mm KCl plus 5 mm KNO3, IAA-induced medium acidification was lower than in medium with 10 mm KCl.
Fig. 4.
Effect of 10 mm KCl or 10 mm KNO3 on the growth rate of maize coleoptile segments incubated in the presence or absence of 10 μm IAA, added to the incubation medium at 2 h. The growth rate of a stack of 20 segments was measured as described in Materials and methods (second measuring system). Inset on the right shows total elongation growth, calculated as the sum of extensions measured at 3-min intervals for 10 h. Inset on the left presents medium pH changes when maize coleoptile segments were incubated in the presence of IAA. All curves represent means of at least nine independent experiments. Bars indicate ± s.e. The LSD test for growth is included in Supplementary Data Fig. S2.
Fig. 5.
Effects of 10 mm KCl, 10 mm KNO3 and 5 mm KCl plus 5 mm KNO3 on the growth rate of maize coleoptile segments incubated in the presence of 10 μm IAA added to the incubation medium at 2 h. Inset on the left presents medium pH changes for maize coleoptile segments measured simultaneously with growth. All curves represent means of at least nine independent experiments. Bars indicate ± s.e. The LSD test for growth is included in Supplementary Data Fig. S2.
Effect of IAA on simultaneously measured growth and medium pH of coleoptile segments incubated in the presence of anion and cation channel blockers
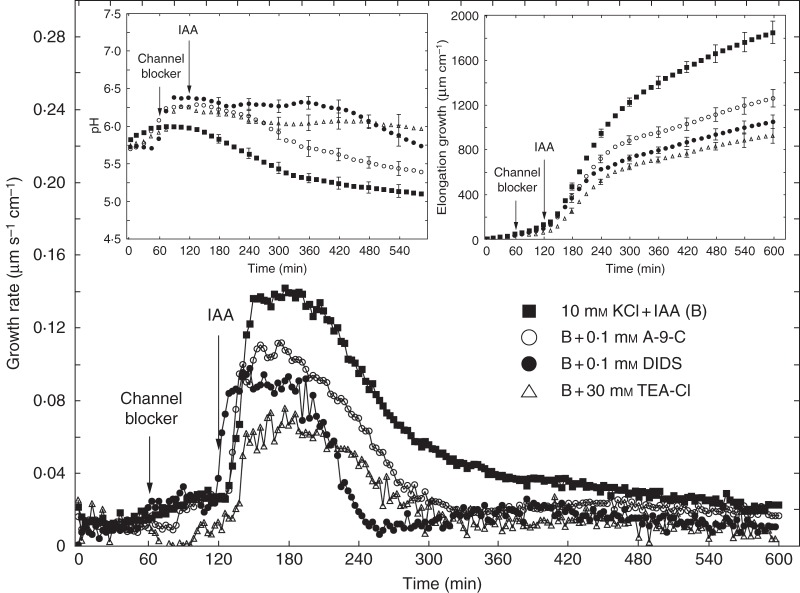
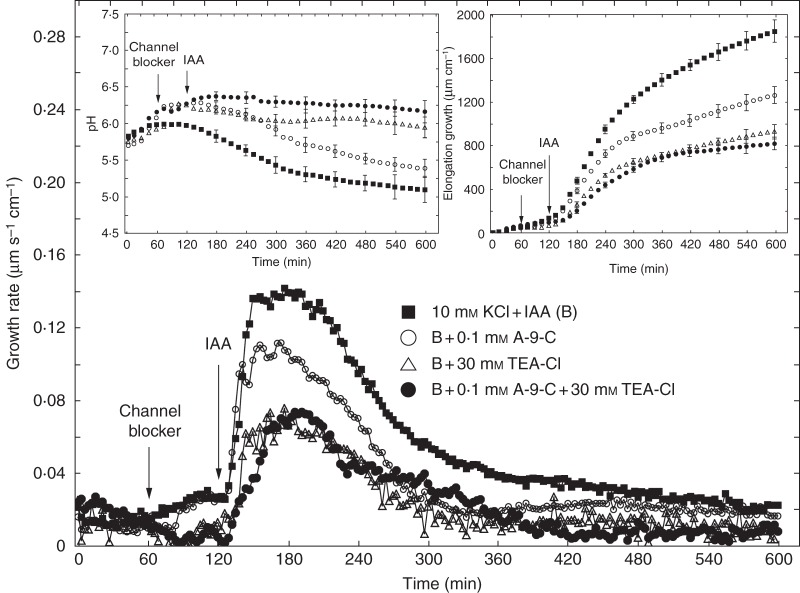
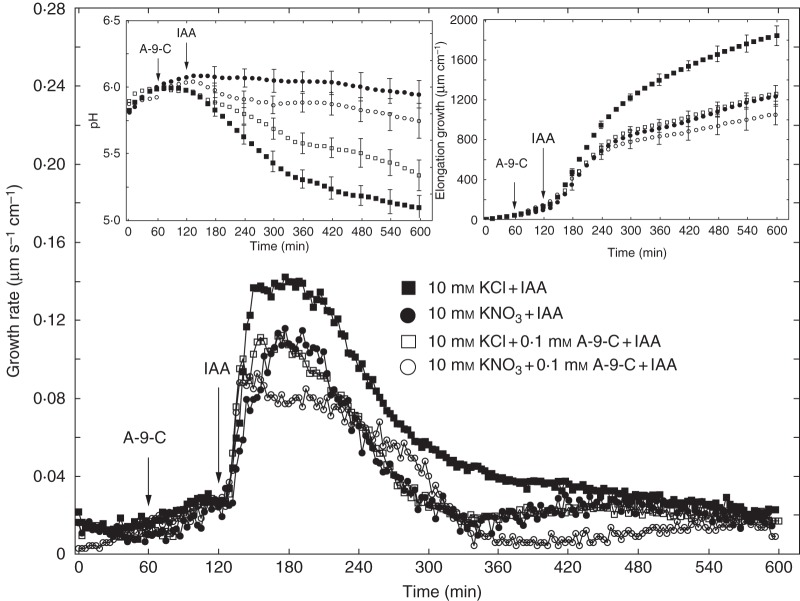
Figure 6 shows the effects of anion and cation channel blockers (A-9-C, DIDS and TEA-Cl) on IAA-induced growth and medium pH measured in the second system. As in the first system, coleoptile segments were first preincubated for 1 h before the blockers were added. IAA was added to the incubation medium at 2 h. Data in Fig. 6 indicate that A-9-C and DIDS decreased IAA-induced growth of coleoptile segments by 31·7 and 43·0 %, respectively. TEA-Cl added to the incubation medium using the same time protocol as for anion channel blockers reduced IAA-induced growth by 49·9 %. Medium pH changes measured simultaneously with growth (Fig. 6, left inset) showed that, whereas DIDS and TEA-Cl abolished IAA-induced proton extrusion, A-9-C diminished it by 50 % (expressed as H+ concentration in the medium at 10 h). When A-9-C and TEA-Cl were added together, after 1 h of segment preincubation, IAA-induced growth was inhibited by 56 % (Fig. 7, right inset). This result indicates that, in the presence of both blockers, inhibition of IAA-induced growth was similar to that observed for TEA-Cl only. Changes in the pH of the medium, measured simultaneously with growth, indicated that addition of both blockers together caused strong inhibition of IAA-induced proton extrusion, characteristic of the action of TEA-Cl (Fig. 7, left inset). Experiments in which TEA-Cl and A-9-C were added together suggest coupling between the transport of K+ and Cl– ions. Figure 8 shows a comparison of the effects of the anion channel blocker A-9-C on IAA-induced growth and medium pH of coleoptile segments incubated in the presence of 10 mm KCl or 10 mm KNO3. Addition of A-9-C to medium containing 10 mm KNO3 diminished IAA-induced growth of maize coleoptile segments only slightly (∼10 %) compared with medium containing 10 mm KCl. Interestingly, in the presence of A-9-C the inhibitory effect of KNO3 on IAA-induced proton extrusion was somewhat lower.
Fig. 6.
Effects of anion (A-9-C or DIDS) and cation (TEA-Cl) channel blockers on the growth rate of maize coleoptile segments incubated in the presence of IAA (10 μm). Coleoptile segments were preincubated (1 h) in control medium, the channel blockers were then added to the incubation medium, and IAA was added at 2 h. Curves represent data obtained using the second measuring system. Inset on the right shows total elongation growth, calculated as the sum of extensions measured at 3-min intervals for 10 h. Inset on the left presents medium pH changes for maize coleoptile segments incubated in the presence of IAA. All curves represent means of at least seven independent experiments. Bars indicate ± s.e. The LSD test for growth is included in Supplementary Data Fig. S2.
Fig. 7.
Effects of A-9-C and TEA-Cl added together or separately (1 h after starting the experiment) on the growth rate of maize coleoptile segments incubated in the presence of 10 μm IAA. IAA was added to the incubation medium at 2 h. Curves represent data obtained using the second measuring system. Inset on the right shows total elongation growth, calculated as the sum of extensions measured at 3-min intervals for 10 h. Inset on the left presents medium pH changes for maize coleoptile segments incubated in the presence of IAA. All curves represent means of at least nine independent experiments. Bars indicate ± s.e. The LSD test for growth is included in Supplementary Data Fig. S2.
Fig. 8.
Comparison of the effect of an anion channel blocker (A-9-C) on the growth rate of maize coleoptile segments incubated in the presence of 10 mm KCl or 10 mm KNO3 and 10 μm IAA, added to the medium at 2 h. Inset on the right shows total elongation growth, calculated as the sum of extensions measured at 3-min intervals for 10 h. Inset on the left presents medium pH changes measured simultaneously with growth. All curves represent means of at least seven independent experiments. Bars indicate ± s.e. The LSD test for growth is included in Supplementary Data Fig. S2.
Effect of A-9-C on membrane potential of parenchymal cells of coleoptile segments incubated in the presence or absence of IAA
Results shown in Table 1 indicate that the membrane potential of parenchymal cells of maize coleoptile segments depended on the K+ concentration in the medium. A 10-fold increase in K+ concentration caused depolarization by ∼50 mV, which is near the value predicted from the Nernst equation (the difference probably resulted from the presence of Ca2+ ions, which inhibit the ZMK1 inwardly rectifying K+ channels, in the bathing medium). Addition of IAA to incubation medium containing 1 mm KCl produced characteristic changes in the membrane potential of parenchymal cells: the initial, transient depolarization by 5·1 mV was followed by a delayed hyperpolarization, during which the potential was 10·7 mV more negative than the original value (–120·6 ± 7·4 mV, mean ± s.e., n = 14). At 10 mm KCl (Table 1), IAA-induced membrane hyperpolarization was 50 % less than that seen in medium with 1 mm KCl. However, transient membrane depolarization was similar in both cases. Adding the anion channel blocker A-9-C at 1 h to bathing medium with 1 mm KCl resulted, after 1 h, in depolarization by 11·7 mV (from –120·1 ± 4·3 to –108·4 ± 4·7 mV, Table 1, column A). In turn, IAA added to bathing medium containing both 1 mm KCl and A-9-C resulted, after 30 min, in hyperpolarization by –10·1 mV, similar to the change observed in the presence of 1 mm KCl only. In the case of medium with 10 mm KCl, addition of A-9-C led to depolarization by 5·8 mV (Table 1, column A). Interestingly, in the presence of 10 mm KCl and A-9-C, auxin did not cause membrane potential hyperpolarization.
DISCUSSION
The main question we addressed was whether Cl– ions participate in IAA-induced elongation growth of maize coleoptile segments. We tested this hypothesis by replacing KCl with KNO3 in the incubation medium and using two anion channel blockers (A-9-C and DIDS). These anion channel blockers have previously been used in animal (Greger, 1990) and plant (Marten et al., 1992, 1993; Schwartz et al., 1995; Keller and Van Volkenburgh, 1996a; Thomine et al., 1997) cells to study the mechanisms of anion channel regulation (for example, DIDS acts by covalent modification of ε-amino groups of lysine residues) and cellular anion efflux, respectively. For comparison, the effect of K+ channel blockers (TEA-Cl and BaCl2) was also studied. It is well established that anion channels play a role in cell volume regulation (e.g. stomatal movement), plant nutrition and membrane potential regulation (for reviews see White and Broadley, 2001; Tavares et al., 2011). The electrochemical gradient for chloride is in the direction of passive efflux. However, the uptake of chloride (and potassium) is mediated by two systems: transporters and ion channels. Both transport systems are energized by the plasma membrane H+-ATPase (Sanders, 1990). It should also be mentioned that most Cl– channel blockers are also able to interact with anion transporters (Greger, 1990). Independently of the K+ concentration and measuring system, replacement of Cl– by NO3– led to a decrease in growth in the presence of IAA (Figs 1 and 4), suggesting that part of this growth depends (positively) on Cl–.
Similar dependence has also been found for the endogenous growth of maize coleoptile segments. It is well established that in isolated coleoptile segments the rate of endogenous growth is not stable with time (Evans and Schmitt, 1975; Vesper and Evans, 1978; Tamimi et al., 1996). In excised maize coleoptile segments, the endogenous growth rate increases strongly 3–4 h after excision (Evans et al., 1977; Tamimi et al., 1996), which is in good agreement with the data obtained using our first system (Figs 1A, B and 2). In the second system, an intensified endogenous growth rate was recorded 1 h earlier (for an explanation of the nature of accelerated growth, commonly referred to as the spontaneous growth response, see Hager, 2003). Medium pH measured simultaneously with growth (Fig. 4, left inset) indicated that, in the presence of 10 mm KNO3, both IAA-induced proton extrusion and proton extrusion observed in auxin-free medium were inhibited. As can also be seen in Fig. 4 (left inset), proton extrusion was stimulated by auxin much more effectively at 10 mm KCl than at 1 mm KCl, supporting the hypothesis that auxin enhances H+/K+ antiport at the plasma membrane (for review see Hager, 2003). Data in Fig. 4 (right inset) also indicate that the enhanced proton extrusion observed in the presence of IAA and 10 mm KCl does not necessarily result in elongation growth of coleoptile segments being significantly greater than in medium with IAA and 1 mm KCl. The idea that Cl– ions participate in IAA-induced growth of maize coleoptile segments is also supported by data obtained in experiments with anion channel blockers (Figs 2, 3 and 6). Both anion channel blockers, A-9-C and DIDS, independently of experimental conditions (first or second measuring system), diminished IAA-induced elongation growth, by 32 and 33·8 % respectively (values are means calculated for each blocker and each measuring system). Inhibition of auxin (α-naphthalene acetic acid)-induced growth by 0·1 mm A-9-C has also been shown by Keller and Volkenburgh (1996a) in experiments performed with oat coleoptile segments. According to these authors, growth inhibition by A-9-C suggests that the opening of rapidly activating Cl– channels is an essential component of auxin-induced growth response. Interestingly, in their preliminary experiments Keller and Volkenburgh (1996a) also found that 0·1 mm A-9-C depolarized the membrane by 30–35 mV after 15 min, which was not a result expected by the authors after blocking Cl– channel activity. Our data also showed that in medium containing 1 mm KCl an anion channel blocker (A-9-C) caused depolarization by 11·7 mV 60 min after its application (Table 1, column A). In medium with 10 mm KCl this effect was 50 % lower. This result may be evidence that A-9-C blocks the uptake of Cl– ions. In the presence of 1 mm KCl and A-9-C, IAA-induced membrane potential changes in parenchymal cells were similar to those observed in the presence of IAA alone (Table 1). In contrast to medium with 1 mm KCl and A-9-C, IAA added to medium containing 10 mm KCl and A-9-C did not cause membrane hyperpolarization. At present, there is no doubt that plasma membrane hyperpolarization in the presence of IAA (Cleland et al., 1977; Felle et al., 1991; Peters et al., 1992; Keller and Volkenburgh, 1996a; Karcz and Burdach 2002, 2007) is a consequence of stimulated proton extrusion through H+-ATPase (Rücke et al., 1993; Hedrich et al., 1995). These findings suggest that IAA-stimulated plasma membrane H+-ATPase activity may be inhibited in the presence of 10 mm KCl and A-9-C.
With arabidopsis hypocotyls, Thomine et al. (1997) reported that auxin treatment reduced hypocotyl length to ∼20–30 % of the control value, while in the presence of 0·1 mm A-9-C or DIDS hypocotyl length recovered practically to the control value. Although Thomine et al. (1997) did not propose any precise mechanism of interaction between auxin-signalling pathways and anion transport blockers, they suggested a contribution of anion channels to the regulation of arabidopsis hypocotyl growth by auxin. Interestingly, Thomine et al. (1997) rejected the hypothesis that anion channel blockers (A-9-C, DIDS) interacted with auxin efflux carriers, although they did not exclude such an interaction with auxin influx carriers. It should also be mentioned that we performed additional experiments examining the effect of A-9-C on the content of indolic compounds in maize coleoptile segments incubated in the presence of IAA (Supplementary Data Table S1). The results clearly showed that A-9-C did not change the content of indolic compounds in coleoptile segments incubated with IAA. In some early experiments it was also shown that DIDS at 0·1 mm inhibited Cl– uptake into protoplasts and segments from maize roots (Lin, 1981; Kochian et al., 1985). Medium pH, here measured simultaneously with the growth of maize coleoptile segments, indicated that while DIDS at 0·1 mm abolished IAA-induced proton extrusion, A-9-C at the same concentration diminished it by only 50 %. This suggests that plasma membrane H+-ATPase might be involved in Cl– uptake. We have also shown that both anion channel blockers, similarly to KNO3, diminished the first, very rapid phase of IAA-induced growth, which, in accordance with the model proposed by Becker and Hedrich (2002) for maize coleoptile segments, is associated with stimulation of the plasma membrane H+-ATPase (acid growth) and activation of the voltage-dependent K+ uptake channel ZMK1. A-9-C and DIDS, added to the incubation medium 3 h after addition of IAA, decreased growth only slightly compared with that recorded in the presence of IAA, suggesting that anion channel blockers are predominantly active in the first phase of IAA-induced growth. This finding is in good agreement with the hypothesis proposed by Thomine et al. (1997), indicating that A-9-C and DIDS act on anion channels involved in early auxin signal transduction. When TEA-Cl was applied 1 h before addition of IAA, 57 % growth reduction was observed. Administration of BaCl2 using the same time protocol as that for TEA-Cl caused a similar decrease in auxin growth response. It should be added that the kinetics of IAA-induced growth rate responses were similar in the presence of TEA-Cl and BaCl2 (Fig. 2). In contrast to anion channel blockers, application of both cation channel blockers 3 h after addition of IAA brought about rapid inhibition of growth, suggesting that K+ ions are also involved in the long-lasting phase of IAA-induced growth (for the model of IAA-induced growth see Becker and Hedrich, 2002). Inhibition of IAA-induced growth of maize coleoptile segments by TEA-Cl and BaCl2 (used at the same concentrations as in our experiments) was shown previously by Claussen et al. (1997). These authors also found that TEA-Cl inhibited IAA-induced proton extrusion, which supports our data (Figs 6 and 7). Results provided by Pesci (1988) and recently by Visnovitz et al. (2013) should also be mentioned. Pesci (1988) showed that DIDS and TEA-Cl caused a reduction in K+ and Cl– influx into cells of barley leaf segments, suggesting coupling between the transport of these ions. With barley leaves, Visnovitz et al. (2013) reported that when K+ uptake into cells of elongating leaf tissue (exhibiting acid-growth-type mechanisms) was blocked in the presence of cation channel blockers (TEA, Cs+), leaf growth was reduced by ∼50 %. The authors observed a similar growth reduction in response to vanadate (500 µm), which also increased apoplast pH. Experiments with maize coleoptile segments, performed by Polak (2010) using the second measuring system, as described here, also showed that vanadate, added at a final concentration of 1000 µm to medium with 1 mm KCl, diminished IAA-induced growth (acid growth) by ∼50 % and abolished the medium acidification measured simultaneously with growth. Thus, the investigations carried out by Polak (2010) and data obtained in the present study support the hypothesis proposed by Visnovitz et al. (2013) for barley leaves, assuming that ∼50 % of leaf growth does not depend on apoplast acidification.
On the basis of our data, we propose the hypothesis that Cl– ion uptake is involved in the IAA-induced growth of maize coleoptile segments. This hypothesis is supported by two facts: (1) the diminished IAA-induced growth found when KCl was replaced by KNO3 and (2) the decrease in IAA-induced growth, to a level similar to that seen with KNO3, by anion channel blockers (A-9-C and DIDS). The changes in pH measured simultaneously with growth indicate that KNO3 and anion channel blockers inhibited IAA-induced proton extrusion, suggesting involvement of plasma membrane proton pumps in Cl– uptake.
Considering both our own results and literature data, a possible scenario for Cl– uptake in the presence of IAA can be proposed. In this scenario, uptake of K+ and Cl– ions in concert with IAA-induced plasma membrane H+-ATPase activity should change the plasma membrane potential to a value needed for turgor adjustment during the growth of maize coleoptile cells. This hypothesis is similar to that proposed for turgor recovery in osmotically stressed arabidopsis epidermal root cells (Shabala et al., 2000; Shabala and Lew, 2002). These authors found that turgor recovery after hyperosmotic stress was accompanied by a significant increase in the uptake of K+, Cl– and Na+ into root cells. Interestingly, hyperosmotic stress, like IAA (for review see Hager, 2003), results in plasma membrane hyperpolarization, proton extrusion and activation of inwardly rectifying K+ channels (Li and Delrot, 1987; Curti et al., 1993; Shabala et al., 2000; Shabala and Lew, 2002).
SUPPLEMENTARY DATA
LITERATURE CITED
- Babourina O, Shabala S, Newman I. Auxin stimulates Cl- uptake by oat coleoptiles. Annals of Botany. 1998;82:331–336. [Google Scholar]
- Becker D, Hedrich R. Channelling auxin action: modulation of ion transport by indole-3-acetic acid. Plant Molecular Biology. 2002;49:349–356. [PubMed] [Google Scholar]
- Claussen M, Lüthen H, Blatt M, Böttger M. Auxin-induced growth and its linkage to potassium channels. Planta. 1997;201:227–234. [Google Scholar]
- Cleland RE. Cell wall extension. Annual Review of Plant Physiology. 1971;22:197–222. [Google Scholar]
- Cleland RE, Prins HBA, Harper JR, Higinbotham N. Rapid hormone-induced hyperpolarization of the oat coleoptile transmembrane potential. Plant Physiology. 1977;59:395–397. doi: 10.1104/pp.59.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti G, Massardi F, Lado P. Synergistic activation of plasma membrane H+-ATPase in Arabidopsis thaliana cells by turgor decrease and by fusicoccin. Physiologia Plantarum. 1993;87:592–600. [Google Scholar]
- Evans ML, Schmitt MR. The nature of spontaneous changes in growth rate in isolated coleoptile segments. Plant Physiology. 1975;55:757–762. doi: 10.1104/pp.55.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ML, Simon M, Vesper MJ. Further characterization of the spontaneous growth response in Zea coleoptile segments. Plant and Cell Physiology. 1977;18:441–452. [Google Scholar]
- Felle HH, Peters WS, Palme K. The electrical response of maize to auxins. Biochimica et Biophysica Acta. 1991;1064:199–204. doi: 10.1016/0005-2736(91)90302-o. [DOI] [PubMed] [Google Scholar]
- Greger R. Chloride channel blockers. Methods in Enzymology. 1990;191:793–809. doi: 10.1016/0076-6879(90)91048-b. [DOI] [PubMed] [Google Scholar]
- Hager A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. Journal of Plant Research. 2003;116:483–505. doi: 10.1007/s10265-003-0110-x. [DOI] [PubMed] [Google Scholar]
- Hager A, Menzel H, Krauss A. Versuche und Hypothese zur Primarwirkung des Auxins beim Streckungwachstum. Planta. 1971;100:47–75. doi: 10.1007/BF00386886. [DOI] [PubMed] [Google Scholar]
- Hager A, Debus G, Edel HG, Stransky H, Serrano R. Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta. 1991;185:527–537. doi: 10.1007/BF00202963. [DOI] [PubMed] [Google Scholar]
- Haschke HP, Lüttge U. Interactions between IAA, potassium, and malate accumulation, and growth in Avena coleoptile segments. Zeitschrift fur Pflanzenphysiologie. 1975a;76:450–455. [Google Scholar]
- Haschke HP, Lüttge U. Stoichiometric correlation of malate accumulation with auxin-dependent K+-H+ exchange and growth in Avena coleoptile segments. Plant Physiology. 1975b;56:696–698. doi: 10.1104/pp.56.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Bregante M, Dreyer I, Gambale F. The voltage-dependent potassium-uptake channel of corn coleoptiles has permeation properties different from other K+ channels. Planta. 1995;197:193–199. [Google Scholar]
- Karcz W, Burdach Z. A comparison of the effects of IAA and 4-Cl-IAA on growth, proton secretion and membrane potential in maize coleoptile segments. Journal of Experimental Botany. 2002;53:1089–1098. doi: 10.1093/jexbot/53.371.1089. [DOI] [PubMed] [Google Scholar]
- Karcz W, Burdach Z. Effect of temperature on growth, proton extrusion and membrane potential in maize (Zea mays L.) coleoptile segments. Plant Growth Regulation. 2007;52:141–150. [Google Scholar]
- Karcz W, Stolarek J, Lekacz H, Kurtyka R, Burdach Z. Comparative investigation of auxin and fusicoccin-induced growth and H+-extrusion in coleoptile segments of Zea mays L. Acta Physiologiae Plantarum. 1995;17:3–8. [Google Scholar]
- Karcz W, Lüthen H, Böttger M. Effect of IAA and 4-Cl-IAA on growth rate in maize coleoptile segments. Acta Physiologiae Plantarum. 1999;21:133–139. [Google Scholar]
- Karcz W, Burdach Z, Lekacz H, Polak M. Fusicoccin counteracts inhibitory effects of high temperature on auxin-induced growth and proton extrusion in maize coleoptile segments. Plant Signaling & Behavior. 2008;3:821–822. doi: 10.4161/psb.3.10.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CP, Van Volkenburgh E. The electrical response of Avena coleoptile cortex to auxins. Evidence in vivo for activation of a Cl- conductance. Planta. 1996a;198:404–412. [Google Scholar]
- Keller CP, Van Volkenburgh E. Osmoregulation by oat coleoptile protoplasts (effect of auxin) Plant Physiology. 1996b;110:1007–1016. doi: 10.1104/pp.110.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Xin-Zhi J, Lucas WJ. Potassium transport in corn roots: IV. Characterization of the linear component. Plant Physiology. 1985;79:771–776. doi: 10.1104/pp.79.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtyka R, Kita A, Karcz W. Fusicoccin counteracts the toxic effect of cadmium on the growth of maize coleoptile segments. Archives of Environmental Contamination and Toxicology. 2011;61:568–577. doi: 10.1007/s00244-011-9662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U. The current status of the acid-growth hypothesis. New Phytologist. 1994;126:549–569. [Google Scholar]
- Kutschera U. Acid growth and plant development. Science. 2006;311:952–954. doi: 10.1126/science.311.5763.952b. [DOI] [PubMed] [Google Scholar]
- Li ZS, Delrot S. Osmotic dependence of the transmembrane potential difference of broad bean mesocarp cells. Plant Physiology. 1987;84:895–899. doi: 10.1104/pp.84.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplasts. Plant Physiology. 1981;68:435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten I, Loshe G, Hedrich R. Plant growth hormones control voltage-dependent activity of anion channels in plasma membrane of guard cells. Nature. 1991;353:758–762. [Google Scholar]
- Marten I, Zeilinger C, Redhead C, Landry DW, al-Awqati Q, Hedrich R. Identification and modulation of a voltage-dependent anion channel in the plasma membrane of guard cells by high-affinity ligands. EMBO Journal. 1992;11:3569–3575. doi: 10.1002/j.1460-2075.1992.tb05440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten I, Busch H, Raschke K, Hedrich R. Modulation and block of the plasma membrane anion channel of guard cells by stilbene derivatives. European Biophysics Journal. 1993;21:403–408. [Google Scholar]
- Niklas KJ, Kutschera U. Plant development, auxin, and the subsystem incompleteness theorem. Frontiers in Plant Science. 2012;3:37. doi: 10.3389/fpls.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci P. Ion fluxes and abscisic acid-induced proline accumulation in barley leaf segments. Plant Physiology. 1988;86:927–930. doi: 10.1104/pp.86.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters WS, Richter U, Felle HH. Auxin-induced H+-pump stimulation does not depend on the presence of epidermal cells in corn coleoptiles. Planta. 1992;186:313–316. doi: 10.1007/BF00196261. [DOI] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Lüthen H, et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. Proceedings of the National Academy of Sciences of the USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak M. Katowice, Poland: University of Silesia; 2010. The interdependences between growth, medium pH and membrane potential in maize coleoptile segments incubated in the presence of auxin (IAA), fusicoccin (FC) and allicin. Doctoral thesis. [Google Scholar]
- Polak M, Tukaj Z, Karcz W. Effect of temperature on the dose-response curves for auxin-induced elongation growth in maize coleoptile segments. Acta Physiologiae Plantarum. 2011;33:437–442. [Google Scholar]
- Polak M, Zaborska W, Tukaj Z, Karcz W. Effect of thiosulphinates contained in garlic extract on growth, proton fluxes and membrane potential in maize (Zea mays L.) coleoptile segments. Acta Physiologiae Plantarum. 2012;34:41–52. [Google Scholar]
- Rubinstein B. Effect of pH and auxin on chloride uptake into Avena coleoptile cells. Plant Physiology. 1974;54:835–839. doi: 10.1104/pp.54.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B, Cleland RE. Responses of Avena coleoptiles to suboptimal fusicoccin: kinetics and comparisons with indoleacetic acid. Plant Physiology. 1981;68:543–547. doi: 10.1104/pp.68.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B, Light EN. Indoleacetic-acid-enhanced chloride uptake into coleoptile cells. Planta. 1973;110:43–56. doi: 10.1007/BF00386921. [DOI] [PubMed] [Google Scholar]
- Rudnicka M, Polak M, Karcz W. Cellular responses to naphthoquinones: juglone as a case study. Plant Growth Regulation. 2014;72:239–248. [Google Scholar]
- Rücke A, Palme K, Venis MA, Napier RM, Felle HH. Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant Journal. 1993;4:41–46. [Google Scholar]
- Sanders D. Kinetic modeling of plant and fungal membrane transport systems. Annual Review of Plant Physiology and Molecular Biology. 1990;41:77–108. [Google Scholar]
- Shabala SN, Lew RR. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiology. 2002;129:290–299. doi: 10.1104/pp.020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Ilan N, Schwarz M, Scheaffer J, Assmann SM, Schroeder JI. Anion-channel blockers inhibit S-type anion channels and abscisic acid responses in guard cells. Plant Physiology. 1995;109:651–658. doi: 10.1104/pp.109.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Babourina O, Newman IA. Ion-specific mechanisms of osmoregulation in bean mesophyll cells. Journal of Experimental Botany. 2000;51:1243–1253. [PubMed] [Google Scholar]
- Stevenson TT, Cleland RE. Osmoregulation in the Avena coleoptile in relation to auxin and growth. Plant Physiology. 1981;67:749–753. doi: 10.1104/pp.67.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamimi S, Trebacz K, Sievers A. The spontaneous growth response of maize coleoptile segments and the change in tissue sensitivity to endogenous auxin. Journal of Experimental Botany. 1996;47:993–998. [Google Scholar]
- Tavares B, Domingos P, Dias PN, Feijó JA, Bicho A. The essential role of anionic transport in plant cells: the pollen tube as a case study. Journal of Experimental Botany. 2011;62:2273–2298. doi: 10.1093/jxb/err036. [DOI] [PubMed] [Google Scholar]
- Thomine S, Lelievre F, Boufflet M, Guern J, Barbier-Brygoo H. Anion-channel blockers interfere with auxin responses in dark-grown Arabidopsis hypocotyls. Plant Physiology. 1997;115:533–542. doi: 10.1104/pp.115.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper MJ, Evans ML. Time-dependent changes in the auxin sensitivity of coleoptile segments. Plant Physiology. 1978;61:204–208. doi: 10.1104/pp.61.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnovitz T, Touati M, Miller AJ, Fricke W. Apoplast acidification in growing barley (Hordeum vulgare L.) leaves. Journal of Plant Growth Regulation. 2013;32:131–139. [Google Scholar]
- White PJ, Broadley MR. Chloride in soils and its uptake and movement within the plant: a review. Annals of Botany. 2001;88:967–988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.