Abstract
A multisite, randomized, controlled clinical effectiveness trial was conducted for osteoarthritis patients with chronic pain of the knee or hip. Adult health nurse practitioners provided a 10-session intervention, pain coping skills training (PCST), in patients’ doctors’ offices (N = 129 patients); the control group received usual care (N = 127 patients). Primary outcomes assessed at baseline, posttreatment, 6-month follow-up, and 12-month follow-up were: pain intensity, physical functioning, psychological distress, self-efficacy, catastrophizing, use of coping strategies, and quality of life. Secondary measures included fatigue, social functioning, health satisfaction, and use of pain medication. Methods favoring external validity, consistent with pragmatic, effectiveness research, were utilized. Primary ITT and secondary per-protocol analyses were conducted. Attrition was within the expected range: 11% at posttreatment and 29% at 12-month follow-up; rates did not differ between groups. Omnibus ITT analyses across all assessment points indicated significant improvement for the PCST group compared with the control group for pain intensity, physical functioning, psychological distress, use of pain coping strategies, and self-efficacy, as well as fatigue, satisfaction with health, and reduced use of pain medication. Treatment effects were robust to covariates (demographics and clinical sites). Trends in the outcomes across the assessments were examined. All outcomes, except for self-efficacy, were maintained through the 12-month follow-up; effects for self-efficacy degraded over time. Per-protocol analyses did not yield greater effect sizes. Comparisons of PCST patients who were more vs less treatment adherent suggested greater effectiveness for patients with high adherence. Results support the effectiveness of nurse practitioner delivery of PCST for chronic osteoarthritis pain.
Keywords: Chronic pain, Clinical nursing research, Coping skills, Osteoarthritis, Treatment effectiveness
1. Introduction
Arthritis pain is a major international health challenge. In the United States alone, arthritis affects over 22% of the population (50 million), making it one of the most common chronic medical conditions [14], and women are afflicted at higher rates than men [59]. It impacts substantial portions of the population by middle age, with more than half of the adult population >65 years of age diagnosed (60%), and the percent continues to increase with age [13, 58]. It is not an inconsequential disease [63]. The 2010 Global Burden of Disease study reported that musculoskeletal disorders are among the major causes of disability-adjusted life years (DALYs) [47]. Health-related quality of life is consistently worse for persons with arthritis primarily due to chronic pain, difficulty standing, and walking [14]. Interventions include analgesics, joint injections, exercise, and surgical joint replacement [24]. Despite utilization of these treatments, many patients still report significant chronic pain and activity limitation.
Pain coping skills training (PCST), a treatment for patients with persistent pain, has demonstrated efficacy for osteoarthritis (OA) [30, 31]. It teaches patients cognitive strategies and behavioral skills to reduce the effects of chronic pain on functioning and quality of life. A recent meta-analysis of psychological therapies for chronic pain documents its efficacy [60].
However, patient access to PCST currently is very limited, depriving patients of this opportunity to actively participate in improving their pain, functioning, and quality of life. In most instances, health care providers delivering PCST have been clinical psychologists in academic medical/mental health settings, where PCST has been developed and studied. Few practitioners in the community are trained in PCST. Whereas the physician focus is typically not on training patients in pain coping skills, the nursing profession, with its emphasis on implementing self-management and patient education counseling for improved care of chronic illness, is well suited to deliver PCST. Because PCST is designed to treat pain caused by a medical condition and is not a form of psychotherapy, we believe that nurses can be readily trained to deliver PCST and that training in managing patients’ mental health is not a prerequisite for successful delivery of the intervention. Nevertheless, attention to the interaction of emotional state and the pain experience is a fundamental component of PCST work with the patient.
This randomized, multisite clinical trial was the first to evaluate the effectiveness of a health care delivery model that trains nurse practitioners (NP) to deliver PCST to OA patients in community practices. It used effectiveness trial methods to maximize external validity, with sufficient attention to internal validity to yield results that can be confidently interpreted. The goal of this research was to determine whether NPs can treat OA patients with chronic pain of the knee and/or hip and achieve significant reductions in pain and in physical and psychological disability, and increases in self-efficacy, use of coping strategies, and quality of life compared with a usual care control group through a 12-month posttreatment assessment.
2. Methods
2.1. Study design
This study was a multisite, randomized, controlled trial examining the effectiveness of PCST delivered by trained NP in community primary care and rheumatology offices. Patients with OA were randomized in equal numbers to 1 of 2 conditions: PCST (treatment condition) or usual care (control condition). Patients in the PCST treatment condition received 10 sessions of individual PCST, which was designed to promote the use of cognitive-behavioral pain management coping skills. Patients in the control condition continued with their usual care for OA. Consistent with usual care, patients in both conditions were provided with an OA informational brochure from the Arthritis Foundation and information on programs (support groups, arthritis education, and aquatic exercise classes) offered in the community. Assessments were completed at baseline, posttreatment, 6-month follow-up, and 12-month follow-up.
2.2. Participants
Patients with chronic pain caused by OA of the knee or hip were recruited from community primary care and rheumatology practices in New York, Virginia, and North Carolina. Advertisements with information about the study were posted in the waiting rooms, and participating doctors informed eligible patients of the opportunity to participate in the clinical trial at the time of a regular office visit. Patients were told that the purpose of the study was to evaluate the effectiveness of a 10-session program for coping with pain, and was delivered by nurses in their doctor’s office. Patients randomly assigned to the control group would continue with their usual care and participate in the periodic assessments. Interested patients were invited to contact the research office for further details and to be telephone-screened for eligibility. Eligibility criteria were (1) physician-confirmed diagnosis of hip or knee OA, (2) 21 years of age or older, (3) usual pain ≥4 on a 10-point scale for a duration of at least 6 months, (4) ability to read, write, and understand English, (5) ability to attend 10 treatment sessions at the doctor’s office if randomized to treatment, (6) no cognitive/ mental impairment that would interfere with participation, (7) no expected joint replacement surgery in the next 2 years.
2.3. Measures
2.3.1. Baseline OA disease severity ratings
The widely used Kellgren-Lawrence system for OA joint damage based on radiographs was used to grade disease severity at baseline [34]. Severity was graded from 0 (no radiographic findings of OA) to 4 (definite osteophytes with severe joint space narrowing and subchondral sclerosis). Scoring based on radiographs has been shown to correlate moderately with articular surface grading during knee arthroscopy [35]. All radiographs were graded using K-L criteria by 2 independent raters, and a third rating was obtained in cases in which the ratings disagreed by 2 grades or more (n = 24). Inter-rater reliability was acceptable among the first 2 raters, with linear weighted kappa = .74 (95% confidence interval [CI] = .68 to .79) and Krippendorff [23] ordinal alpha = .76 (95% CI = .71 to .80). Reliability was slightly improved by the third rating (ordinal alpha = .78, 95% CI = .75 to .81).
2.3.2. Outcome measures
2.3.2.1. Arthritis Impact Measurement Scales (AIMS2)
This 78-item questionnaire measures the health status of patients with arthritis and has been used extensively in survey and treatment outcomes research [22, 43]. The AIMS2 subscales address pain, mobility, walking and bending, extremity functioning, self-care, household tasks, social activities and support, work, tension, and mood. The recall period for this instrument was changed from “in the past month” to a 2-week period. Internal consistency subscale estimates ranged from .72 to .90 in the present study.
2.3.2.2. Brief Pain Inventory (BPI)
Four items from the BPI were used to measure current pain and average, worst, and least pain in the past 2 weeks. The inventory is reliable, valid, and has achieved widespread use among medical conditions with chronic pain [15, 16]. The internal consistency of the 4-item scale was .89 in the present study.
2.3.2.3. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
The WOMAC is the most widely used outcome measure in hip and knee arthritis pharmaceutical and surgical studies. Several studies support the reliability and validity of the WOMAC [5, 39]. The instrument has 24 items covering the following domains: pain, stiffness, and physical function experienced during the past 48 hours. Internal consistency estimates ranged from .70 to .95 for the 3 subscales in the present study.
2.3.2.4. Coping Strategies Questionnaire (CSQ)
The 42-item Coping Attempts Scale of the CSQ [31, 53] was used to assess how often a patient engages in 7 different coping strategies when they feel pain: coping self-statements, praying or hoping, ignoring pain sensations, reinterpreting pain sensations, increasing behavioral activities, catastrophizing, and diverting attention. This instrument has shown sensitivity to treatment change in various chronic pain samples [20, 41] as well as good internal consistency and construct validity [31]. Internal consistency estimates for the 7 subscales ranged from .77 to .86 in the present study.
2.3.2.5. Beck Depression Inventory (BDI)
The 21-item self-report questionnaire measures cognitive, affective, and somatic aspects of depressed mood [3, 4]. It is widely used as a treatment outcome measure and is sensitive to the range of depressed mood in chronic pain patients [19, 25, 62]. The BDI has demonstrated validity and sensitivity to treatment change [2]. The internal consistency of the BDI total score was .89 in the present study.
2.3.2.6. Arthritis Self-Efficacy Scale
The 8-item instrument measures patients’ perceived ability to perform specific behaviors aimed at controlling arthritis pain and disability (ranging from 1 = very uncertain to 10 = very certain) [21]. The scale was adapted from the 20-item questionnaire developed by Lorig et al. [40] that has shown sensitivity to increases in a sense of mastery over arthritis pain in many outcome trials [38, 57]. The 8-item version has shown adequate reliability and validity [21]. The internal consistency of the total score was .92 in the present study.
2.3.2.7. Quality of Life Scale
The 16-item instrument measures quality of life across different life domains in patients with chronic illness. The measure is reliable and content-valid; among medical patients, internal consistency coefficients are above .85, and 6-week test-retest reliability is .76 [12]. The internal consistency of the total score was .91 in the present study.
2.3.2.8. Brief Fatigue Inventory (BFI)
Like the BPI after which it was modeled, the BFI was designed to measure fatigue in cancer patients, but its use has expanded to many medical conditions [1, 44]. Four items from the BFI were used to measure current fatigue and average, worst, and least fatigue; the recall period was changed from the past 24 hours to the past 2 weeks. A factor analysis determined that the BFI assesses a single fatigue dimension with good internal consistency and adequate correlations with other fatigue scales [44, 64]. The internal consistency of the four items was .86 in the present study.
2.3.2.9. End-of-day symptom diaries recorded on interactive voice recording (IVR)
Several key constructs that are central to the arthritis pain experience were measured via IVR (a telephone computer interface) for 7 consecutive days at each assessment period (baseline, posttreatment, and 6- and 12-month follow-up). These constructs included ratings of pain intensity; interference with physical, work, and social activities due to pain; ratings of fatigue and life satisfaction; and pain medication usage. IVR data capture is reliable and valid when compared with paper-and-pencil assessment, and compliance is typically good [11, 36, 46].
2.3.3. Creation of composite measures
Several key constructs were a priori specified as primary outcomes: pain intensity, physical functioning, psychological distress, coping strategies, catastrophizing, self-efficacy, and quality of life. Given the large number of scales administered for several domains, and to reduce type 1 error, composite measures were created for 4 of the primary outcomes (pain intensity, physical functioning, psychological distress, coping). The other outcomes were measured with single scales. The pain intensity composite was comprised of the BPI pain, AIMS2 pain, and WOMAC pain subscales (average intercorrelation across scales at baseline = .70). Physical functioning was composed of the AIMS2 physical and WOMAC difficulties performing activities subscales (r = .58). Psychological distress was comprised of the BDI and AIMS2 affect (tension and mood) scales (r = .70). A coping strategies composite was created by averaging the CSQ subscales (average r = .47), excluding the catastrophizing subscale. In each case, scale scores were first standardized based on the baseline means and SDs across all patients, and they were then averaged into composites. Thus, the composite z scores at each assessment time point indicate where a patient scored in relation to all patients at pretreatment.
2.4. Procedures
All study procedures were approved by the Stony Brook and Duke University Medical Center Institutional Review Boards. Eligible patients were scheduled for their baseline visit at the patient’s participating community clinic site. Before initiating study procedures, patients provided written informed consent. During the visit, patients completed a battery of outcome questionnaires, were instructed on how to use the IVR telephone system for the 7 daily ratings after the baseline visit, and had their weight and height measured. Patients also were sent for a radiograph of their most painful OA-diagnosed joint at no cost to them to determine their baseline disease severity. If a recent radiograph (within the past 9 months) was already available, the research staff obtained a copy and no new radiograph was obtained. Patients were informed that they needed to complete their daily ratings and provide a radiograph within 4 weeks of the baseline assessment.
Upon completion of all baseline assessment components, patients were randomized to 1 of the 2 study conditions. Randomization to experimental condition (PCST or usual care) was done using a permuted blocking procedure using 2 block sizes (6 and 8). The study statistician created a randomization program accessed by site coordinators at the time of each patient’s randomization. The outcome of randomization was only known when the patient was entered into the randomization program. The study coordinator then called patients and informed them of their assignment to study treatment group. Patients assigned to PCST were then scheduled for their first appointment with an NP who provided 10 individual weekly sessions at the patient’s doctor’s office (window for treatment completion 10 to 20 weeks from randomization). Patients assigned to usual care were instructed to continue with their regular treatment for their OA. Both study groups were asked to complete a posttreatment assessment, a 6-month follow-up, and a 12-month follow-up assessment. As in the baseline assessment, research assistants met with patients for each assessment when patients completed outcome measures, had height and weight measures, and completed the 7 daily IVR ratings. The research team maintained assessor blinding, but patients sometimes revealed their experimental condition. Data collection was conducted from 2008 to 2013.
2.5. PCST
PCST interventions teach patients cognitive and behavioral skills to manage their pain and enhance their perception of pain control. Four broad coping skills were taught across the ten 30- to 45-minute sessions: relaxation response, attention diversion techniques, altering activity and rest patterns as a way of increasing activity level, and reducing negative pain-related thoughts and emotions. The sessions were outlined in detail in a treatment manual and followed a format of review of home practice assigned at the last session, instruction in a new coping skill, guided practice in that skill, and a home practice assignment. Homework assignments are an integral component of PCST, followed by review and problem-solving in the subsequent session.
Consistent with the goal of testing the effectiveness of NPs delivering PCST in the patients’ doctors’ offices, all treatment sessions were conducted in the clinics or by telephone (phone sessions). Up to 4 sessions could be conducted via telephone with some discretion on the part of the NP and patient. The first 3 sessions and the last session had to be conducted in person. Patients were provided with a treatment binder divided into sections for each session. These sections included handouts and logs to record home practice of the skill, and they were reviewed by the NP at each session. Treatment sessions with a patient were stopped if they were not completed within 20 weeks of randomization.
2.6. NPs delivering the treatment
Treatment sessions were conducted by several NPs hired by the research grant . Eligibility for these positions required a registered nurse license and working and/or engaged in study as an adult health or family practice NP.
NPs received an initial 2-day training workshop in PCST conducted by clinical members of the research team (F.K., J.B., D.M., P.B.). Although not specializing in mental health, the nurses’ training systematically addressed the emotional underpinnings of pain. They were encouraged to be alert to the psychological needs of patients and the role that anxiety and depression play in altering behaviors in ways that could be addressed by PCST. After the initial training, NPs continued training with the instructors at their site to reach competency with delivery of the treatment. Next, each NP delivered PCST treatment to 2 to 3 OA patients who were not included in protocol data collection (pilot patients). NPs did not begin to see patients in the clinical trial until adherence and therapist competence were confirmed. Competence was defined as the NP consistently achieving a score of 3 (satisfactory) to 5 (excellent) on a rating of therapist performance (1 = poor, 5 = excellent). Using audiotapes of the sessions, ratings were made on specific items including: (1) establishing and maintaining rapport, (2) showing professionalism and clinical judgment, (3) covering the protocol in a way that meets patient needs, (4) encouraging patient active engagement in the session, (5) applying the protocol to the patient’s unique challenges and needs, (6) using time efficiently and appropriately pacing the session, and (7) overall performance. These ratings were based on a nurse’s interactions with the patient during a session. For example, if the nurse’s interactions with the patient were flat (lacking enthusiastic voice cues), a rating of poor was assigned for encouraging engagement. In contrast, if the nurse actively engaged the patient and was receptive and enthusiastic, a rating of excellent was assigned.
2.7. Patient sample size
The sample size was chosen to ensure adequate power to detect an intervention effect (group difference in change from baseline) at posttreatment on pain intensity. In a meta-analysis of randomized controlled trials including cognitive behavioral therapy, Morley calculated a mean effect size of 0.33 for pain experience [45], and power analyses were conducted based on this effect size. To achieve 80% power at a significance level of .05 (2-tailed), we estimated that 230 participants with complete data (115 per group) would be necessary, assuming a correlation between baseline and the posttrial assessments of approximately 0.6. To allow for a 10% dropout rate, 256 participants were enrolled.
2.8. Analysis plan
Analyses were conducted in accordance with the intention-to-treat (ITT) principle. Repeated-measures analyses of covariance (RM-ANCOVA) models were used in all primary analyses examining treatment effects. Separate models were estimated for each outcome variable. Scores for all 3 posttreatment assessments (immediate posttreatment, 6-month follow-up, 12-month follow-up) served as correlated response variables and were simultaneously regressed on baseline scores and experimental group. Clinic site was included as an additional covariate. Thus, the main effect of group for a given posttreatment period represents the estimated intervention effect as the group difference in change from baseline to that assessment period, controlling for potential site differences in outcomes. An omnibus test for all posttreatment assessment periods (df = 3) was first carried out to evaluate an overall effect of the intervention, followed by separate significance tests for each posttreatment period. An unstructured covariance matrix was used to account for the correlation of outcomes between posttreatment periods. Multiple imputation procedures were used to account for missing assessments, and to derive intervention effect estimates consistent with the ITT principle. The multiple imputation approach is preferable to single imputation methods such as last observation carried forward because it adequately accounts for the uncertainty associated with filling in missing responses [49]. A set of 10 multiple imputed datasets was generated using Markov chain Monte Carlo estimation, and the results carried out for each dataset were combined using Rubin’s rules to adjust the standard errors for the uncertainty about imputed values [54, 56]. Intervention effect sizes were calculated in 2 ways: (1) Cohen’s dthe group difference in change relative to the SD of pretreatment scores on the variable, and (2) the standardized response mean as the group difference in change relative to the SD of change scores. Whereas statistical power of a study depends on the standardized response mean, Cohen’s d provides a more readily interpretable metric for evaluating the magnitude of treatment effects. All analyses were conduced using SAS [55] and Mplus [48]
3. Results
3.1. Participant flow
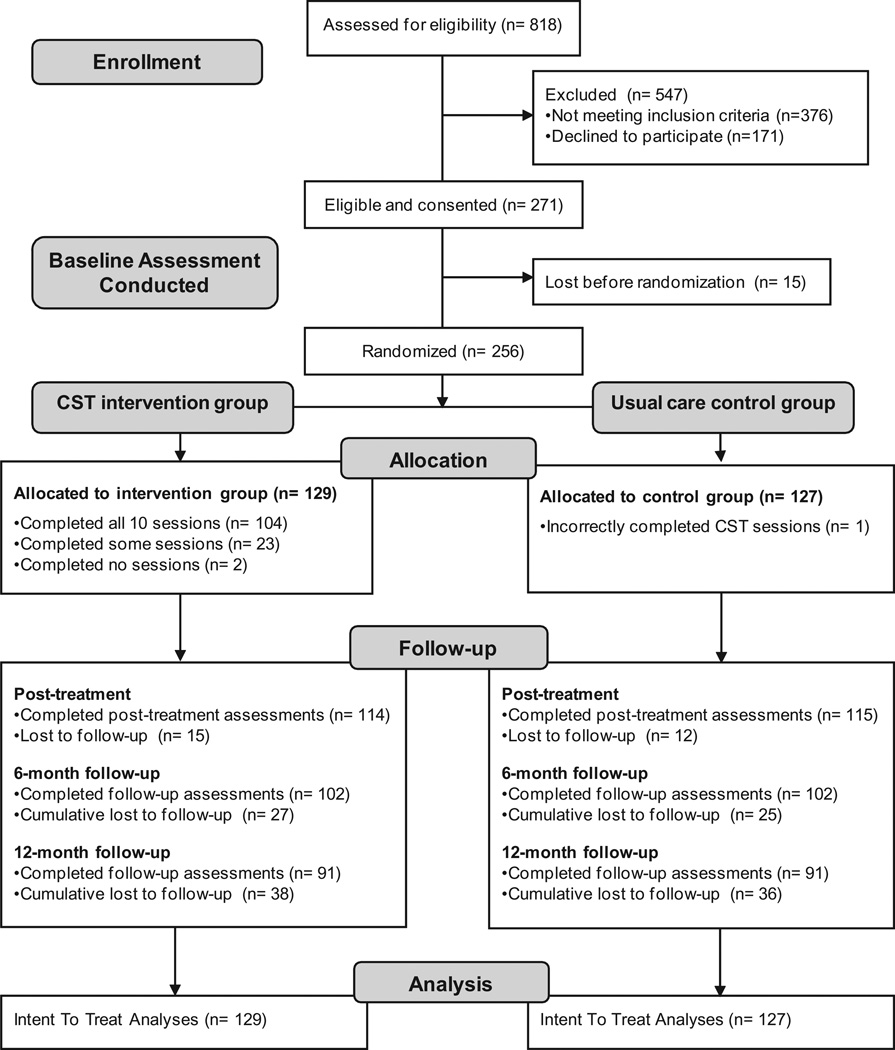
A Consolidated Standards of Reporting Trials (CONSORT) diagram for participant recruitment through 12-month follow-up is shown in Fig. 1. Of the 818 patients in rheumatology and primary care clinics who were screened for eligibility, 46% did not meet 1 or more eligibility criteria, including not having OA of the knee/hip, not having at least moderate chronic pain for at least 6 months, or expecting a joint replacement procedure. Among the 442 eligible patients, 271 (62%) agreed to participate in the study, and 171 (39%) declined. Commonly stated reasons for declining were lack of transportation or time, health problems of self or a family member, or other personal problems. Of the 271 eligible and consenting patients, 15 (6%) dropped out of the study either before or after baseline assessment and before randomization. Thus, a total of 256 were randomized into treatment (N = 129) and control (N = 127).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. CST = coping skills training.
The full 10-session PCST treatment was received by 81% of the patients in the treatment group; 2 did not schedule any treatment, and the other 23 attended fewer than 10 sessions. One patient randomized to the control group inadvertently received treatment, but was analyzed per ITT methods as a control patient.
Based on similar clinical trials, attrition for assessment completion was expected to be approximately 10% to 15% at posttreatment. We observed 11% at posttreatment, 20% at the 6-month follow-up, and 29% 12 months after the end of treatment. Retention rates did not differ between treatment and control groups. The research team and the Data Safety Monitoring Program did not detect any serious adverse effects attributed to the treatment or to participating in the study. A statistical comparison of demographic, medical background, and baseline clinical outcome measures between those who dropped out early and did not complete posttreatment assessment with those who were retained found no significant differences. The only variable that trended (P = .07) was current smoking; more smokers than nonsmokers dropped out.
Compliance with 7 days of daily IVR ratings was tabulated. Of the 256 participants, IVR assessments were completed by 255 (99.6%) at baseline, by 219 (85.5%) at posttreatment, by 198 (77.3%) at 6-month follow-up, and by 163 (63.7%) at 12-month follow-up. For those patients who completed IVR measures, compliance with the 7-day assessment protocol was high at each time point. On average, participants provided IVR ratings on 6.6 (94%) of the 7 days at baseline, on 6.4 (91%) days at posttreatment, on 6.4 (91%) days at 6-month follow-up, and on 6.4 (92%) days at 12-month follow-up.
3.2. Experimental group characteristics and differences
Randomization of patients into treatment and usual care control groups was successful in yielding highly similar groups (Table 1). No significant group differences were found on demographic variables, except for employment (P = .001), with control group participants more likely to be employed. Likewise, no significant baseline group differences were found on clinical outcome variables (Table 2).
Table 1.
Demographic and medical characteristics by experimental group.
| Control group (N = 128) |
Treatment group (N = 129) |
P for difference between groups | |||
|---|---|---|---|---|---|
| n* | Mean (SD) or % | n* | Mean (SD) or % | ||
| Age | 128 | 66.37 (10.26) | 129 | 68.00 (8.67) | .17 |
| Years with osteoarthritis | 121 | 13.59 (9.09) | 128 | 13.95 (10.63) | .77 |
| Body mass index | 123 | 32.87 (8.00) | 124 | 33.77 (8.24) | .38 |
| Disease severity (K–L grading) | 122 | 125 | .22 | ||
| 0 to 1 | 27.0% | 16.8% | |||
| >1 to 2 | 20.5% | 27.2% | |||
| >2 to 3 | 23.0% | 26.4% | |||
| >3 to 4 | 29.5% | 29.6% | |||
| Female | 128 | 78.9% | 129 | 74.4% | .40 |
| White race | 128 | 85.9% | 129 | 87.6% | .69 |
| Married/living with partner | 123 | 62.6% | 126 | 64.3% | .78 |
| Education | 126 | 127 | .18 | ||
| High school graduate | 27.0% | 28.4% | |||
| College graduate | 56.4% | 46.5% | |||
| Master’s degree | 16.7% | 25.2% | |||
| Currently employed | 121 | 39.7% | 128 | 21.1% | .001 |
| Currently on disability | 125 | 15.2% | 128 | 13.3% | .66 |
| Current smoker | 126 | 5.6% | 127 | 7.1% | .62 |
| Past smoker | 123 | 52.9% | 127 | 54.3% | .81 |
| Regular exercise | 121 | 49.6% | 124 | 45.2% | .49 |
| Treatment for psychiatric disorder | 126 | 15.1% | 128 | 16.4% | .77 |
| Treatment for drugs | 125 | 1.6% | 128 | 0.0% | .15 |
| Memory/thinking problems | 125 | 9.6% | 126 | 10.3% | .85 |
| Surgery for osteoarthritis | 121 | 33.9% | 124 | 29.0% | .41 |
| Affected joint is knee (vs hip) | 128 | 73.4% | 129 | 81.4% | .13 |
Numbers indicate available sample size for each variable.
Table 2.
Mean (SD) of outcome variables at pretreatment by experimental condition.
| Range of scale | Control group |
Treatment group |
P for difference | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Primary outcomes | |||||||
| Pain intensity (composite) | [−] | Z score | 0.03 | 0.99 | −0.03 | 1.01 | .61 |
| Physical functioning (composite) | [−] | Z score | 0.02 | 1.04 | −0.02 | 0.96 | .73 |
| Psychological distress (composite) | [−] | Z score | −0.04 | 0.93 | 0.04 | 1.06 | .54 |
| Coping strategies (composite) | [+] | Z score | 0.08 | 1.01 | −0.08 | 0.99 | .20 |
| Catastrophizing | [−] | 0 to 36 | 7.17 | 6.46 | 6.84 | 6.39 | .68 |
| Self-efficacy | [+] | 1 to 10 | 5.97 | 1.82 | 6.12 | 1.91 | .52 |
| Quality of life | [+] | 16 to 112 | 86.57 | 14.64 | 86.29 | 13.00 | .87 |
| Secondary outcomes | |||||||
| Fatigue | [−] | 0 to 10 | 5.06 | 1.90 | 5.14 | 2.01 | .75 |
| AIMS2 social activities | [−] | 0 to 10 | 5.28 | 1.72 | 5.34 | 1.72 | .77 |
| AIMS2 family/friend support | [−] | 0 to 10 | 2.32 | 2.00 | 2.76 | 2.05 | .08 |
| AIMS2 satisfaction with health | [−] | 0 to 10 | 3.69 | 2.10 | 3.83 | 1.98 | .59 |
| IVR outcomes | |||||||
| Daily pain intensity | [−] | 0 to 10 | 4.81 | 1.80 | 4.63 | 1.78 | .42 |
| Daily pain interference | [−] | 0 to 10 | 3.47 | 1.70 | 3.48 | 1.55 | .96 |
| Daily life satisfaction | [−] | 0 to 10 | 6.46 | 1.52 | 6.34 | 1.44 | .34 |
| Daily canceling activities | [−] | 0 to 1 | 0.33 | 0.34 | 0.32 | 0.31 | .83 |
| Daily fatigue | [−] | 0 to 10 | 4.78 | 1.83 | 4.53 | 1.88 | .27 |
| Daily additional pain medication | [−] | 0 to 1 | 0.32 | 0.33 | 0.35 | 0.34 | .58 |
The symbols in brackets indicate the directionality of the measure: [+] = higher scores are more favorable, [−] = lower scores are more favorable.
AIMS2 = Arthritis Impact Measurement Scales; IVR = interactive voice response.
Patient characteristics from the New York and Virginia/North Carolina sites were compared. As anticipated, the southern locations had significantly (P < .0001) more African American patients (26%) compared with suburban New York (2%). Only 14% of the New York patients were male, whereas 35% of the southern patients were (P < .0001). There were overall education differences, with more high school educated patients in the southern locations, and more patients with higher education in New York (P < .006). Finally, more patients in New York had already had some surgery for OA (37%), compared with 25% in the south (P < .04); 84% of patients in New York identified a knee as the most affected joint, whereas 70% of the southern patients did (P < .01). Age, years with OA, body mass index (BMI), and other variables were equivalent between sites. Baseline clinical outcome measures also were compared between sites. Across 17 comparisons of primary and secondary measures, 4 were significant (P ≤ .05). All were in the direction of the New York site patients reporting more psychological distress, less social support, less satisfaction with health, and more frequent canceling of daily activities. We also compared the baseline scores on our patients’ outcome measures with those in the OA literature to try to place this sample in a broader context. In most cases, the mean scores of our groups were somewhat more severe or equivalent to other clinical OA samples (eg, [7, 21, 31, 43, 61]).
3.3. Assessment of treatment effectiveness: ITT
ITT analyses were performed on primary and secondary outcomes across the 4 assessments: baseline, posttreatment, 6-month follow-up, and 12-month follow-up. Table 3 shows the mean change scores for each group as well as treatment effects with effect sizes at each follow-up assessment. Omnibus tests across all follow-up assessments yielded significant group differences, indicating improvement with treatment for 5 of the 7 primary outcomes: pain intensity (F(3, 233) = 2.75, P = .044), physical functioning (F(3, 233) = 3.11, P = .027), psychological distress (F(3, 233) = 2.83, P = .039), use of pain coping strategies (F(3, 233) = 4.97, P = .002), and self-efficacy (F(3, 232) = 10.59, P < .001); catastrophizing showed a trend (F(3, 233) = 2.47, P = .063) and the effect on quality of life was not significant (F(3, 233) = 1.97, P = .119). Two of the 4 secondary outcomes yielded significant treatment effects: fatigue (F(3, 233) = 5.14, P = .002) and AIMS satisfaction with health (F(3, 232) = 3.12, P = .027); AIMS2 family/friend support showed a trend (F(3, 232) = 2.54, P = .057) and the effect for AIMS2 social activities was not significant (F(3, 232) = 0.58, P = .631).
Table 3.
Estimated mean change (standard error) from baseline and treatment effects, adjusted for site.
| Control group |
Treatment group |
Group difference |
Effect size |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | P value | d | SRM | |
| Primary outcomes | |||||||||
| Pain intensity composite | |||||||||
| Posttreatment | −0.17 | 0.07* | −0.38 | 0.07‡ | −0.22 | 0.09* | .017 | 0.22 | 0.28 |
| 6-month follow-up | −0.20 | 0.09* | −0.31 | 0.10† | −0.12 | 0.13 | .390 | 0.12 | 0.13 |
| 12-month follow-up | −0.17 | 0.07* | −0.37 | 0.08‡ | −0.21 | 0.11* | .047 | 0.21 | 0.26 |
| Physical functioning composite | |||||||||
| Posttreatment | −0.10 | 0.06 | −0.28 | 0.06‡ | −0.18 | 0.08* | .024 | 0.18 | 0.27 |
| 6-month follow-up | −0.20 | 0.06† | −0.23 | 0.07† | −0.04 | 0.10 | .719 | 0.04 | 0.05 |
| 12-month follow-up | −0.16 | 0.05† | −0.32 | 0.06‡ | −0.17 | 0.08* | .036 | 0.17 | 0.28 |
| Psychological distress composite | |||||||||
| Posttreatment | −0.03 | 0.06 | −0.20 | 0.06† | −0.16 | 0.08* | .033 | 0.17 | 0.25 |
| 6-month follow-up | 0.00 | 0.06 | −0.11 | 0.07 | −0.11 | 0.09 | .222 | 0.11 | 0.18 |
| 12-month follow-up | −0.05 | 0.05 | −0.25 | 0.06‡ | −0.20 | 0.08* | .012 | 0.20 | 0.33 |
| Coping strategies | |||||||||
| Posttreatment | 0.34 | 0.07‡ | 0.65 | 0.07‡ | 0.31 | 0.10† | .001 | 0.32 | 0.38 |
| 6-month follow-up | 0.30 | 0.09† | 0.53 | 0.09‡ | 0.23 | 0.12* | .043 | 0.23 | 0.24 |
| 12-month follow-up | 0.29 | 0.08‡ | 0.52 | 0.08‡ | 0.23 | 0.10* | .026 | 0.23 | 0.27 |
| Catastrophizing | |||||||||
| Posttreatment | 0.81 | 0.48 | −0.14 | 0.45 | −0.95 | 0.63 | .132 | 0.15 | 0.18 |
| 6-month follow-up | 0.24 | 0.48 | −1.07 | 0.52* | −1.32 | 0.68 | .054 | 0.21 | 0.25 |
| 12-month follow-up | 0.07 | 0.51 | −1.36 | 0.54* | −1.43 | 0.65* | .028 | 0.22 | 0.26 |
| Self-efficacy | |||||||||
| Posttreatment | 0.18 | 0.18 | 1.31 | 0.18‡ | 1.13 | 0.23‡ | <.001 | 0.61 | 0.51 |
| 6-month follow-up | 0.62 | 0.20† | 0.90 | 0.21‡ | 0.28 | 0.27 | .296 | 0.15 | 0.12 |
| 12-month follow-up | 0.50 | 0.19† | 0.84 | 0.20‡ | 0.35 | 0.24 | .158 | 0.19 | 0.16 |
| Quality of life | |||||||||
| Posttreatment | −1.84 | 1.1 | −0.40 | 1.0 | 1.45 | 1.4 | .285 | 0.11 | 0.12 |
| 6-month follow-up | 0.32 | 1.2 | −1.11 | 1.3 | −1.43 | 1.8 | .426 | −0.10 | −0.12 |
| 12-month follow-up | −1.55 | 1.0 | 0.64 | 1.2 | 2.19 | 1.6 | .159 | 0.16 | 0.18 |
| Secondary outcomes | |||||||||
| Fatigue | |||||||||
| Posttreatment | −0.07 | 0.17 | −0.82 | 0.16‡ | −0.76 | 0.22† | .001 | 0.39 | 0.39 |
| 6-month follow-up | 0.16 | 0.18 | −0.24 | 0.21 | −0.40 | 0.28 | .144 | 0.21 | 0.21 |
| 12-month follow-up | 0.29 | 0.18 | −0.47 | 0.21* | −0.75 | 0.27† | .005 | 0.39 | 0.35 |
| AIMS2 social activities | |||||||||
| Posttreatment | 0.11 | 0.14 | −0.10 | 0.13 | −0.21 | 0.18 | .234 | 0.12 | 0.14 |
| 6-month follow-up | −0.24 | 0.14 | −0.24 | 0.14 | −0.00 | 0.19 | .996 | 0.00 | 0.00 |
| 12-month follow-up | −0.31 | 0.16 | −0.18 | 0.15 | 0.12 | 0.21 | .557 | −0.07 | −0.08 |
| AIMS2 family/friend support | |||||||||
| Posttreatment | 0.26 | 0.17 | −0.20 | 0.16 | −0.45 | 0.22* | .040 | 0.23 | 0.25 |
| 6-month follow-up | 0.06 | 0.18 | −0.24 | 0.17 | −0.30 | 0.24 | .205 | 0.15 | 0.15 |
| 12-month follow-up | −0.08 | 0.19 | −0.26 | 0.18 | −0.18 | 0.24 | .455 | 0.09 | 0.09 |
| AIMS2 satisfaction with health | |||||||||
| Posttreatment | −0.36 | 0.13† | −0.65 | 0.13‡ | −0.45 | 0.17† | .009 | 0.23 | 0.30 |
| 6-month follow-up | −0.35 | 0.13† | −0.64 | 0.15‡ | −0.30 | 0.18 | .098 | 0.15 | 0.22 |
| 12-month follow-up | −0.37 | 0.13† | −0.45 | 0.14† | −0.27 | 0.18 | .135 | 0.14 | 0.19 |
| IVR outcomes | |||||||||
| Daily pain intensity | |||||||||
| Posttreatment | −0.08 | 0.13 | −0.78 | 0.13‡ | −0.71 | 0.18‡ | <.001 | 0.40 | 0.48 |
| 6-month follow-up | 0.04 | 0.15 | −0.83 | 0.15‡ | −0.87 | 0.21‡ | <.001 | 0.49 | 0.53 |
| 12-month follow-up | −0.10 | 0.15 | −0.52 | 0.16† | −0.42 | 0.20* | .037 | 0.24 | 0.28 |
| Daily pain interference | |||||||||
| Posttreatment | −0.01 | 0.13 | −0.53 | 0.13‡ | −0.52 | 0.18† | .003 | 0.32 | 0.39 |
| 6-month follow-up | −0.02 | 0.15 | −0.57 | 0.14‡ | −0.55 | 0.21† | .008 | 0.34 | 0.36 |
| 12-month follow-up | 0.03 | 0.17 | −0.39 | 0.15* | −0.42 | 0.23 | .065 | 0.26 | 0.27 |
| Daily life satisfaction | |||||||||
| Posttreatment | 0.24 | 0.12 | 0.42 | 0.13† | 0.18 | 0.17 | .268 | 0.13 | 0.14 |
| 6-month follow-up | 0.11 | 0.14 | 0.33 | 0.14* | 0.22 | 0.19 | .257 | 0.15 | 0.15 |
| 12-month follow-up | 0.30 | 0.13* | 0.34 | 0.11† | 0.05 | 0.16 | .776 | 0.03 | 0.04 |
| Daily canceling activities | |||||||||
| Posttreatment | −0.07 | 0.03† | −0.09 | 0.03† | −0.02 | 0.03 | .492 | 0.07 | 0.08 |
| 6-month follow-up | −0.07 | 0.03† | −0.12 | 0.02‡ | −0.06 | 0.03 | .094 | 0.17 | 0.21 |
| 12-month follow-up | −0.05 | 0.03 | −0.08 | 0.03* | −0.03 | 0.04 | .539 | 0.08 | 0.09 |
| Daily fatigue | |||||||||
| Posttreatment | −0.21 | 0.13 | −0.67 | 0.13‡ | −0.45 | 0.18* | .013 | 0.24 | 0.31 |
| 6-month follow-up | −0.07 | 0.15 | −0.53 | 0.15† | −0.46 | 0.21* | .029 | 0.25 | 0.30 |
| 12-month follow-up | 0.06 | 0.18 | −0.45 | 0.19* | −0.51 | 0.24* | .031 | 0.28 | 0.30 |
| Daily additional pain medication | |||||||||
| Posttreatment | 0.00 | 0.03 | −0.08 | 0.03† | −0.08 | 0.04* | .025 | 0.24 | 0.28 |
| 6-month follow-up | 0.00 | 0.03 | −0.08 | 0.03* | −0.08 | 0.04 | .050 | 0.23 | 0.25 |
| 12-month follow-up | 0.02 | 0.03 | −0.08 | 0.04* | −0.10 | 0.05* | .035 | 0.30 | 0.32 |
SRM = standardized response mean (group difference in change relative to the SD of change scores); AIMS2 = Arthritis Impact Measurement Scales; IVR = interactive voice response. d = Cohen’s d (group difference in change relative to the standard deviation of pretreatment scores).
P < .05.
P < .01
P < .001
These results were buttressed by the aggregated 7 daily IVR ratings of several outcomes (Table 3). Patients in the PCST group reported less pain (F(3, 231) = 9.22, P < .001), less activity interference due to pain (F(3, 231) = 5.68, P = .001), less fatigue (F(3, 231) = 2.95, P = .033), and reduced use of pain medication (F(3, 231) = 3.09, P = .028) across the 3 follow-up assessments compared with the usual care control group. Changes in daily ratings of life satisfaction (F(3, 231) = 0.43, P = .728) and need to cancel activities (F(3, 230) = 1.19, P = .315) did not differ between the groups.
3.4. Secondary analyses: treatment effects controlling for demographic characteristics
Secondary analyses examined the significance of treatment effects when statistically controlling for patient demographic characteristics: (1) in one set of models, employment was added as a covariate because the treatment and control groups differed significantly in rates of employment at baseline; (2) in a second set of models, age, sex, race, education, BMI, and treatment severity (K-L grades) were added as covariates. Adjustment for covariates generally had very little impact on the treatment effects. When employment was added as a covariate, the effect for psychological distress became marginally significant (P = .080) at immediately posttreatment, and at 12-month follow-up the effects for catastrophizing, IVR pain intensity, and IVR fatigue became marginally significant (P = .056, .090, and .052, respectively). Notably, the effect for satisfaction with health became significant (P = .018) at 6-month follow-up and trended (P = .099) at 12 months.
When age, sex, race, education, BMI, and treatment severity were added as covariates, the effect for psychological distress trended (P = .095) at posttreatment. The effect for satisfaction with health also trended (P = .083) at 6-month follow-up and was significant (P = .047) at 12-month follow-up.
3.5. Analysis of changes in magnitude of treatment effects over time
To evaluate how well treatment gains were maintained across time, we compared the magnitude of treatment effects between the assessment points using pairwise contrasts of group differences in change among the 3 follow-up time points. Across 51 separate contrasts for primary and secondary outcomes, we found a high level of maintenance (94% of contrasts were nonsignificant) of the effects through the 12-month posttreatment assessment. Among the primary outcomes, only the effect for self-efficacy, which was strong at posttreatment (effect size d = .61), degraded across the subsequent follow-ups (change in treatment effect from posttreatment to 6 months: P = .001; posttreatment to 12 months: P = .003). The primary pain intensity composite measure showed maintenance of gains, but the aggregated daily IVR ratings of pain showed a decrease in gains from the 6-month to the 12-month follow-up (P = .013), although the treatment effect was still significant at 12 months (P = .037).
3.6. Assessment of clinical site effects
Evidence for significant site differences in treatment effects was evaluated by examining study site by treatment group interaction effects. The interaction effect was not significant for any outcome variable at any assessment period. That is, there was no evidence for site differences in treatment effects.
3.7. Assessment of effectiveness: per protocol analysis
In addition to the conservative ITT results, analyses also were conducted to determine treatment effectiveness for those patients who received an adequate dose of PCST compared with the control group. Two definitions of adequate dose were used. First, we looked at patients who completed at least 7 of the 10 PCST sessions (82% of the randomized PCST patients), and second, we looked at patients who completed at least 50% of weekly homework as assessed each session by the NP (74% of PCST patients). These 2 subgroups were compared with the usual care control group. On average across all outcomes and time points, the per-protocol effects were minimally higher compared with the ITT effects; the median increase in outcome effect size (when comparing per protocol and ITT effects) was d = .01 for session compliance, and d = .02 for homework compliance.
We probed further by comparing the outcomes between patients who received an adequate dose (using the criteria discussed earlier) with those PCST patients who did not meet these levels of protocol compliance. Across all outcome measures and assessment time points, the median difference in the effect size between the groups was d = .08 (session compliance) and d = .06 (homework compliance), favoring compliers. The effect was somewhat greater at the immediate posttreatment assessment (median d = .14 for sessions, d = .11 for homework) compared with 6-month (median d = .11 for sessions, d = .01 for homework) and 12-month follow-up (median d = .02 for sessions, d = .04 for homework).
3.8. Assessment of treatment effect by NP proficiency
Across the 5 years of the clinical trial and the clinical sites, 6 NPs delivered PCST to the study patients. Two clinicians (P.B., D.M.) reviewed 18% of the audiotaped treatment sessions and rated proficiency on a 5-point scale (1 = poor, 3 = satisfactory, 5 = excellent) in delivering the PCST intervention for that session per the PCST manual. Inter rater agreement for audiotaped sessions was 85%. The ratings of NPs across sessions were averaged. None of the nurses received ratings less than satisfactory. Among the 129 PCST patients, 45% received treatment from a nurse with a rating of 3 (satisfactory), 21% a rating of 4, and 34% a rating of 5 (excellent).
We examined whether NP level of proficiency in delivering PCST was associated with the level of improvement that patients achieved. Consistent with the main analyses, an ANCOVA model was used, and site was used as a covariate. Across 7 primary, 4 secondary, and 6 daily outcome measures, there were no significant differences in outcome by nurse proficiency at the posttreatment assessment.
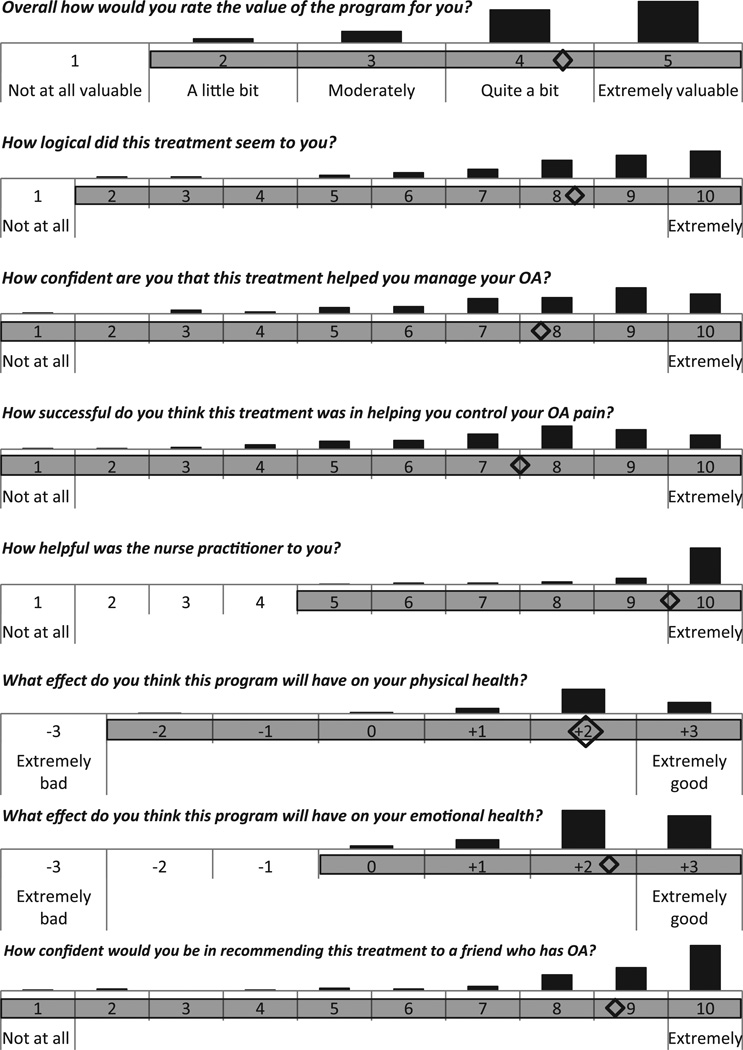
3.9. Patient global evaluations of treatment
At the posttreatment assessment, PCST patients (N = 109) responded to a series of questions about their experience with the treatment (Fig. 2). The mean response for each question, displayed as a diamond, shows an overall very positive view of the treatment. The full range of responses is also displayed, showing that there was variability in their views.
Fig. 2.
Patient ratings of pain coping skills training treatment. Diamonds mark the mean responses. Shading of response options shows the range. Bars show distribution of responses. OA = osteoarthritis.
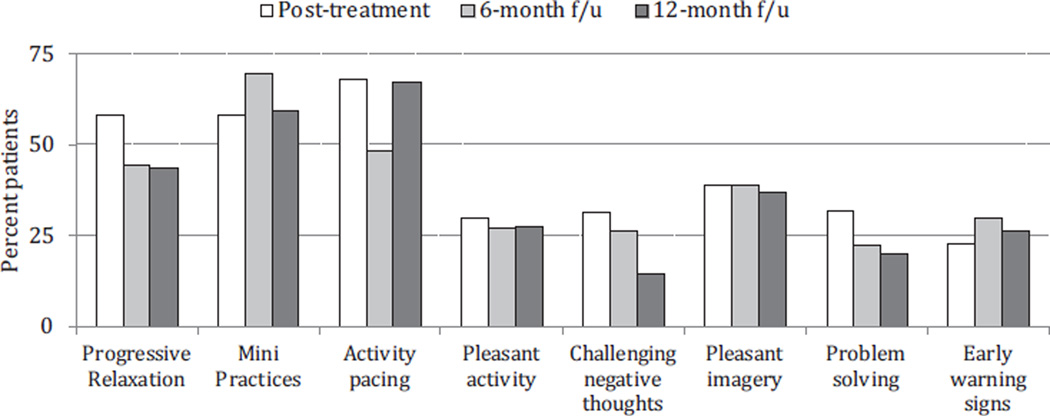
At the end of each of the assessments after treatment ended, patients were asked to report on the 3 PCST skills that they found most helpful. Fig. 3 displays these results with the skills listed from left to right in the order in which they were introduced across the 10 sessions. Progressive muscular relaxation, mini practices of relaxation, and activity pacing were by far the skills that the patients found most helpful. As a result of being introduced early in the treatment, they also were the ones that patients practiced the most across the time in treatment. Pleasant imagery and distraction also were skills that approximately 40% of the patients found most valuable.
Fig. 3.
Patient ratings across posttreatment assessments of the 3 most useful coping strategies.
4. Discussion
This NP-delivered PCST protocol produced significant improvements in a range of pain-related variables including pain intensity, coping with pain, self-efficacy for controlling pain, activity interference due to pain, and the use of pain medication when compared with usual care. Patients showed good attendance at treatment sessions, and a level of attrition over treatment that was within the expected range. Importantly, patients reported finding a number of the pain coping skills to be quite valuable over time. Taken together, these findings support the concept that NPs can effectively deliver PCST to patients with persistent pain.
Given the current global focus on implementing innovative strategies to improve health care systems, these findings are particularly important. Recommendations of 2 recent Institute of Medicine (IOM) reports emphasize the NP role in the delivery of innovative health care strategies such as PCST. Two of 4 key messages from “The Future of Nursing: Leading Change, Advancing Health” support NP-led PCST [26]. These are: (1) nurses should practice to the full extent of their education and training, and (2) nurses should be full partners with physicians and other health professionals in redesigning health care. For many years, the nursing profession has taken a leadership role in designing and implementing self-management and educational counseling approaches for improved care of patients with chronic illness. NPs in this clinical trial showed that they can play an active role in improving outcomes for patients with persistent pain in OA while practicing within their scope of practice in a team-based, patient-centered fashion. A second IOM report, “Relieving Pain in America: A Blue-print for Transforming Prevention, Care, Education, and Research,” recommends promoting and enabling self-management of pain [27]. Emerging literature supports nurse-led self-management interventions as being central to patients’ ability to manage chronic pain. Patients identify nurse care managers as strong sources of support through their approach to individualizing self-management strategies, holding patients accountable for their pain management, and motivating patients [8, 42]. Philosophical tenets of nursing education surrounding holistic, patient-centered care likely contribute to this view and are concordant with our approach to this study. The effectiveness of nurses is inspiring similar efforts by other health professionals, including physical therapists, to incorporate psychosocial and behavioral interventions into their primary treatment approaches [18, 37].
Earlier trials have demonstrated the efficacy of PCST [30, 31, 60], an important first step. However, they do not afford sufficient confidence in the treatment effects that will be observed when implemented in the community under less rigorous conditions. Designed as an effectiveness trial, one of the first PCSTs for OA pain, several features of this study increase confidence for translating the findings into practice. First, eligibility criteria had few restrictions, ensuring that patients enrolled in the trial were more typical of those seen in community settings. Second, PCST was delivered in the patient’s physicians’ offices by adult health NPs, who increasingly are members of the medical team, rather than by highly trained pain psychologists practicing in other settings. In fact, this is part of a growing trend in psychosocial pain intervention toward relying on therapists/trainers who are more likely to be involved in the direct care of patients with painful medical conditions [6, 51]. Third, although working with a treatment manual, the NPs had the flexibility to deliver some of the sessions over the phone to accommodate patients’ illness, physical functioning, and scheduling challenges. Fourth, the nurses were able to adhere to the treatment protocol despite working with patients who varied with respect to disease severity and age. Fifth, this trial was conducted at multiple sites with diversity in the patient populations and NPs. Sixth, to our knowledge this is the largest outcomes study testing the effects of PCST in a sample of patients with OA.
Nurses in this study were enthusiastic about PCST as a valuable tool in their limited armament of interventions for chronic pain in OA. The cognitive and behavioral strategies for coping with pain expanded the nurses’ understanding of factors contributing to the chronic pain experience. However, these advantages of having NPs deliver PCST need to be balanced against potential drawbacks. The level of training and supervision of NPs that we used in this study to enhance the quality of treatment was rigorous. In particular, ongoing supervision of nurses delivering PCST was extensive during the early training of each nurse. Of interest is that within the group of NPs delivering treatment in this study, ratings of therapist competence were not related to treatment outcome, suggesting that a high level of competency is not important. The NPs in this study all reached a moderate to high level of competence, so this finding may not extend to the full pool of NPs. An important direction for future research is exploring the effects of less rigorous, less labor-intensive training, and ongoing supervision protocols to support NPs delivering PCST.
One of the most interesting findings of this study was the breadth and maintenance of treatment effects. NP-delivered PCST not only produced improvements in pain-related outcomes (pain intensity, pain coping, self-efficacy for controlling pain, activity interference due to pain, and the use of pain medication) but also in a broad range of other outcomes important to patients, ie, physical functioning, fatigue, psychological distress, and satisfaction with health. To our knowledge, this study is one of the first in OA patients to demonstrate that a psychosocial pain coping skills intervention can produce such a broad range of significant treatment effects. Importantly, unlike many other studies of cognitive-behavioral treatments, this study also found strong evidence of maintenance of many treatment effects 12 months after treatment ended. Given that OA is a chronic disease, the ability to maintain treatment gains is particularly important.
Also interesting was that the analysis of daily assessments showed larger PCST treatment effects than the traditional questionnaire methods. Based on their daily ratings, patients receiving PCST were much more likely to report reductions in pain intensity, pain interference, fatigue, and use of pain medication than those in the usual care control condition. These findings fit with a growing body of research evidence suggesting that daily ratings are subject to less recall bias than traditional paper and pencil measures [9, 10]. They also suggest the utility of incorporating daily assessment in treatment outcome studies of pain management.
This study had several strengths. First, it was a multisite study conducted in 2 different regions of the country. Although the participants in these 2 regions differed in terms of a number of factors (eg, ethnic background, education, levels of distress and disability, etc), we found no differences in the effects of PCST based on study site. Second, radiographs were used to confirm OA disease severity. Based on the range of disease severity evident on these radiographs, it seems fair to say that the study population was similar to those often encountered in primary care and rheumatology practices and other clinical trials [17, 50]. Third, it was one of a few studies to show that a psychosocial intervention can reduce the reported use of pain medication [28] and have beneficial effects lasting at least 12 months beyond the intervention.
There are several important directions for future research on this topic. First, the results of this study underscore the potential for dissemination/implementation research on PCST. An exciting direction would be to evaluate the effects of nurse training institutes that could teach skills for delivering PCST, provide monitoring of intervention competence, and offer certification of competency. A large cadre of trained nurses who are competent in delivering PCST could have a substantial impact on the management of chronically painful diseases, such as OA. Second, important questions remain about the optimal dose of PCST (ie, how many sessions) needed to achieve treatment effects. To optimize dissemination, treatment needs to be as brief and streamlined as possible. Future studies need to explore the short- and long-term effects achieved with shorter (eg, 4 to 6 sessions) vs more intense (eg, ≥10 sessions) PCST protocols. Third, future studies could conduct head-to-head comparisons of PCST delivered by different providers, eg, psychologist vs NP, physical therapist, or other health provider, as well as by peer leaders. Such studies would provide important information about whether provider discipline, background, or training influences the outcomes of PCST. Fourth, more research is needed on the comparative effectiveness of PCST vs other treatments that may be offered in the primary care setting for managing pain (eg, physical therapy, medications). Fifth, future research should explore other treatment delivery formats. Previously, we showed that PCST can be delivered successfully to groups of OA patients [29, 30, 32, 33], which is an economical format for training a number of patients. Web-based formats, in which the treatment is delivered without the involvement of a therapist, are being explored [52]. If effective, they could offer a very cost-effective strategy for disseminating PCST and other psychosocial interventions for pain. Finally, there is a need to identify the characteristics of patients most likely to benefit from PCST.
In conclusion, the results of this study support the concept that NP-delivered PCST embedded within a practice setting can have significant effects on OA pain and a variety of other important out-comes. Future studies are needed to expand these findings and to investigate other approaches for disseminating psychosocial pain management interventions.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number AR054626. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank our nurse practitioners who provided our patients with the Coping Skills Training intervention at the Stony Brook site (Laureen Diot, Christine Stamatos, Stacey Viteritti) and the Duke site (Verena Knowles, Mary Davidson, Marjorie Thompson). Our trial could not have been completed without the cooperation of several primary care and rheumatology practices (Rheumatology Associates of Long Island, Mark Bernstein, MD, Stony Brook Primary Care, Piedmont Internal Medicine Clinic, Duke University Medical Center Rheumatology Clinic, and Hillsborough Medical Group). We are also grateful to our research assistants at Stony Brook (Amy Stein, GimYen Toh, Laura Wolff, Lauren Cody, and Jessica Latack) and at Duke (Anna Caldwell, Sarah Rowe, Yelena Riordan, Hannah Fisher, and Maggie Presley) and for assistance from Dr. Tamara Somers at Duke. We would also like to thank Dianne Pagani and Lisa McDowell for their administrative assistance throughout the project. Most importantly, we would like to thank the arthritis patients who participated in our trial, and especially those who continued to provide follow-up assessments although assigned to the usual care control group.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Anderson KO, Getto CJ, Mendoza TR, Palmer SN, Wang XS, Reyes-Gibby CC, Cleeland CS. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25:307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 2.Beck A, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 3.Beck AT, Steer RA, Brown GK. Beck Depression Inventory—II manual. San Antonio, Texas: Harcourt Assessment; 1996. [Google Scholar]
- 4.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 5.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 6.Bennell KL, Ahamed Y, Bryant C, Jull G, Hunt MA, Kenardy J, Forbes A, Harris A, Nicholas M, Metcalf B, Egerton T, Keefe FJ. A physiotherapist-delivered integrated exercise and pain coping skills training intervention for individuals with knee osteoarthritis: a randomised controlled trial protocol. BMC Musculoskelet Disord. 2012;13:129. doi: 10.1186/1471-2474-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Vondechend M, Bellamy N, Theiler R. Validation and patient acceptance of a computer touch screen version of the WOMAC 3.1 osteoarthritis index. Ann Rheum Dis. 2005;64:80–84. doi: 10.1136/ard.2003.019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair M, Matthias M, Nyland K. Barriers and facilitators to chronic pain self management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med. 2009;10:1280–1290. doi: 10.1111/j.1526-4637.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. PAIN®. 2008;139:146–157. doi: 10.1016/j.pain.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broderick JE, Stone AA, Calvanese P, Schwartz JE, Turk DC. Recalled pain ratings: a complex and poorly defined task. J Pain. 2006;7:142–149. doi: 10.1016/j.jpain.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Brodey BB, Rosen C, Brodey I, Sheetz B, Unutzer J. Reliability and acceptability of automated telephone surveys among Spanish- and English-speaking mental health services recipients. Ment Health Serv Res. 2005;7:181–184. doi: 10.1007/s11020-005-5786-1. [DOI] [PubMed] [Google Scholar]
- 12.Burckhardt C, Woods S, Schultz A, Ziebarth D. Quality of life of adults with chronic illness: a psychometric study. Res Nurs Health. 1989;12:347–354. doi: 10.1002/nur.4770120604. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Projected prevalence of self reported arthritis or chronic joint symptoms among persons >65 years— United States, 2005–2030. MMWR Morb Mortal Wkly Rep. 2003;52:489–4891. [PubMed] [Google Scholar]
- 14.Cheng Y, Hootman JM, Murphy L, Langmaid G, Helmick C. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation— United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2010;59:1261–1265. [PubMed] [Google Scholar]
- 15.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 16.Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer. 1982;50:1913–1918. doi: 10.1002/1097-0142(19821101)50:9<1913::aid-cncr2820500944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 18.Foster NE, Delitto A. Embedding psychosocial perspectives within clinical management of low back pain: integration of psychosocially informed management principles into physical therapist practice—challenges and opportunities. Phys Ther. 2011;91:790–803. doi: 10.2522/ptj.20100326. [DOI] [PubMed] [Google Scholar]
- 19.Frank R, Beck N, Parker J, Kashani J, Elliott T, Haut A, Smith E, Atwood C, Brownlee-Duffeck M, Kay D. Depression in rheumatoid arthritis. J Rheumatol. 1988;15:920–925. [PubMed] [Google Scholar]
- 20.Gil KM, Abrams MR, Phillips G, Keefe FJ. Sickle cell disease pain: relation of coping strategies to adjustment. J Consult Clin Psychol. 1989;57:725–731. doi: 10.1037//0022-006x.57.6.725. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez VM, Stewart A, Ritter PL, Lorig K. Translation and validation of arthritis outcome measures into Spanish. Arthritis Rheum. 1995;38:1429–1446. doi: 10.1002/art.1780381010. [DOI] [PubMed] [Google Scholar]
- 22.Haavardsholm EA, Kvien TK, Uhlig T, Smedstad LM, Guillemin F. A comparison of agreement and sensitivity to change between AIMS2 and a short form of AIMS2 (AIMS2-SF) in more than 1,000 rheumatoid arthritis patients. J Rheumatol. 2000;27:2810–2816. [PubMed] [Google Scholar]
- 23.Hayes A, Krippendorff K. Answering the call for a standard reliability measure for coding data. Comm Methods Measures. 2007;1:77–89. [Google Scholar]
- 24.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 25.Holzberg AD, Robinson ME, Geisser ME, Gremillion HA. The effects of depression and chronic pain on psychosocial and physical functioning. Clin J Pain. 1996;12:118–125. doi: 10.1097/00002508-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. The future of nursing: leading change, advancing health. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 27.Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 28.Jones AC, Coulson L, Muir K, Tolley K, Lophatananon A, Everitt L, Pringle M, Doherty M. A nurse-delivered advice intervention can reduce chronic non- steroidal anti-inflammatory drug use in general practice: a randomized controlled trial. Rheumatology. 2002;41:14–21. doi: 10.1093/rheumatology/41.1.14. [DOI] [PubMed] [Google Scholar]
- 29.Keefe F, Blumenthal J, Baucom D, Affleck G, Waugh R, Caldwell D, Beaupre P, Kashikar-Zuck S, Wright K, Egert J, Lefebvre J. Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: a randomized controlled study. PAIN®. 2004;110:539–549. doi: 10.1016/j.pain.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Keefe F, Caldwell D, Baucom D, Salley A, Robinson E, Timmons K, Beaupre P, Weisberg J, Helms M. Spouse-assisted coping skills training in the management of osteoarthritis knee pain. Arthritis Care Res. 1996;9:279–291. doi: 10.1002/1529-0131(199608)9:4<279::aid-anr1790090413>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Keefe F, Caldwell D, Queen K, Gil K, Martinez S, Crisson J, Ogden W, Nunley J. Pain coping strategies in osteoarthritis patients. J Consult Clin Psychol. 1987;55:208–212. doi: 10.1037//0022-006x.55.2.208. [DOI] [PubMed] [Google Scholar]
- 32.Keefe F, Caldwell D, Williams D, Gil K, Mitchell D, Robertson C, Martinez S. Pain coping skills training in the management of osteoarthritic knee pain—II: follow-up results. Behav Ther. 1990;21:435–447. [Google Scholar]
- 33.Keefe FJ, Caldwell DS, Williams DA, Gil KM, Mitchell D, Robertson C, Martinez S, Nunley J, Beckham JC, Crisson JE, Helms M. Pain coping skills training in the management of osteoarthritic knee pain: a comparative study. Behav Ther. 1990;21:49–62. [Google Scholar]
- 34.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Anna Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kijowski R, Blankenbaker D, Stanton P, Fine J, De Smet A. Arthroscopic validation of radiographic grading scales of osteoarthritis of the tibiofemoral joint. AJR Am J Roentgenol. 2006;187:794–799. doi: 10.2214/AJR.05.1123. [DOI] [PubMed] [Google Scholar]
- 36.Kranzler HR, Abu-Hasaballah K, Tennen H, Feinn R, Young K. Using daily interactive voice response technology to measure drinking and related behaviors in a pharmacotherapy study. Alcohol Clin Exp Res. 2004;28:1060–1064. doi: 10.1097/01.alc.0000130806.12066.9c. [DOI] [PubMed] [Google Scholar]
- 37.Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, Nichols V, Potter R, Underwood MR. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. Lancet. 2010;375:916–923. doi: 10.1016/S0140-6736(09)62164-4. [DOI] [PubMed] [Google Scholar]
- 38.Lefebvre JC, Keefe FJ, Affleck G, Raezer LB, Starr K, Caldwell DS, Tennen H. The relationship of arthritis self-efficacy to daily pain, daily mood, and daily pain coping in rheumatoid arthritis patients. PAIN®. 1999;80:425–435. doi: 10.1016/s0304-3959(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 39.Lingard EA, Katz JN, Wright RJ, Wright EA, Sledge CB. Validity and responsiveness of the Knee Society Clinical Rating System in comparison with the SF-36 and WOMAC. J Bone Joint Surg Am. 2001;83:1856–1864. doi: 10.2106/00004623-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Lorig K, Chastain R, Ung E, Shoor S, Holman H. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 41.Main CJ, Waddell G. A comparison of cognitive measures in low back pain: statistical structure and clinical validity at initial assessment. PAIN®. 1991;46:287–298. doi: 10.1016/0304-3959(91)90112-B. [DOI] [PubMed] [Google Scholar]
- 42.Matthais M, Miech E, Myers L. An expanded view of health management: patients’ perceptions of education and support in an intervention for chronic musculoskeletal pain. Pain Med. 2012;13:1018–1028. doi: 10.1111/j.1526-4637.2012.01433.x. [DOI] [PubMed] [Google Scholar]
- 43.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 44.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 45.Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. PAIN®. 1999;80:1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 46.Mundt JC, Bohn MJ, King M, Hartley MT. Automating standard alcohol use assessment instruments via interactive voice response technology. Alcohol Clin Exp Res. 2002;26:207–211. [PubMed] [Google Scholar]
- 47.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz- Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 48.Muthén LK, Muthén BO. Mplus user’s guide. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- 49.National Research Council. The prevention and treatment of missing data in clinical trials. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 50.Riddle DL, Jiranek WA, Neff RS, Whitaker D, Hull JR. Extent of tibiofemoral osteoarthritis before knee arthroplasty: multicenter data from the osteoarthritis initiative. Clin Orthop Relat Res. 2012;470:2836–2842. doi: 10.1007/s11999-012-2328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riddle DL, Keefe FJ, Ang D, Khaled J, Dumenci L, Jensen MP, Bair MJ, Reed SD, Kroenke K. A phase III randomized three-arm trial of physical therapist delivered pain coping skills training for patients with total knee arthroplasty: the KASTPain protocol. BMC Musculoskelet Disord. 2012;13:149. doi: 10.1186/1471-2474-13-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rini C, Williams DA, Broderick JE, Keefe FJ. Meeting them where they are: using the internet to deliver behavioral medicine interventions for pain. Transl Behav Med. 2012;2:82–92. doi: 10.1007/s13142-011-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. PAIN®. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 54.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 55.SAS Institute Inc. SAS/STAT 9.2 user’s guide. Cary, NC: SAS Institute; 2008. [Google Scholar]
- 56.Schafer JL. Analysis of incomplete multivariate data. London: Chapman & Hall; 1997. [Google Scholar]
- 57.Schiaffino KM, Revenson TA, Gibofsky A. Assessing the impact of self-efficacy beliefs on adaptation to rheumatoid arthritis. Arthritis Care Res. 1991;4:150–157. doi: 10.1002/art.1790040404. [DOI] [PubMed] [Google Scholar]
- 58.Sharma L. Epidemiology of arthritis. In: Moskowitz R, Howell OS, Altman RD, Buckwalter JA, Goldberg VM, editors. Osteoarthritis: diagnosis and medical/surgical management. Philadelphia: Saunders; 2001. pp. 3–17. [Google Scholar]
- 59.Theis KA, Helmick CG, Hootman JM. Arthritis burden and impact are greater among U.S. women than men: intervention opportunities. J Womens Health (Larchmt) 2007;16:441–453. doi: 10.1089/jwh.2007.371. [DOI] [PubMed] [Google Scholar]
- 60.Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams DA, Farrell MJ, Cunningham J, Gracely RH, Ambrose K, Cupps Mohan N, Clauw DJ. Knee pain and radiographic osteoarthritis interact in the prediction of levels of self-reported disability. Arthritis Rheum. 2004;51:558–561. doi: 10.1002/art.20537. [DOI] [PubMed] [Google Scholar]
- 62.Wilson K, Eriksson M, D’Eon J, Mikail S, Emery P. Major depression and insomnia in chronic pain. Clin J Pain. 2002;18:77–83. doi: 10.1097/00002508-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization. Chronic rheumatic conditions. Geneva, Switzerland: [Google Scholar]
- 64.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]