Abstract
Trauma is the leading cause of death in individuals less than 45 years old worldwide, and up to 50% of trauma fatalities are because of brain injury. Prediction of outcome is one of the major problems associated with severe traumatic brain injury (TBI), and research efforts have focused on the investigation of biomarkers with prognostic value after TBI. Therefore, our aim was to investigate whether cell-free DNA concentrations correlated to short-term primary outcome (survival or death) and Glasgow Coma Scale (GCS) scores after severe TBI. A total of 188 patients with severe TBI were enrolled in this prospective study; outcome variables comprised survival and neurological assessment using the GCS at intensive care unit (ICU) discharge. Control blood samples were obtained from 25 healthy volunteers. Peripheral venous blood was collected at admission to the ICU. Plasma DNA was measured using a real-time quantitative polymerase chain reaction (PCR) assay for the β-globin gene. There was correlation between higher DNA levels and both fatal outcome and lower hospital admission GCS scores. Plasma DNA concentrations at the chosen cutoff point (≥171,381 kilogenomes-equivalents/L) predicted mortality with a specificity of 90% and a sensitivity of 43%. Logistic regression analysis showed that elevated plasma DNA levels were independently associated with death (p<0.001). In conclusion, high cell-free DNA concentration was a predictor of short-term mortality after severe TBI.
Key words: : biomarkers, outcome, plasma DNA, real-time PCR, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the leading cause of injury, death, and disability in young persons worldwide. In the period of 2002 through 2006, approximately 1.7 million U.S. civilians sustained a TBI annually; of these, approximately 1.4 million were treated and discharged from emergency departments (EDs), 275,000 were hospitalized and discharged alive, and 52,000 had a fatal outcome.1,2 Severe TBI is associated with a 30–70% mortality rate and, generally, the survivors are, to a greater or lesser extent, permanently disabled.3
Prediction of outcome is one of the major problems associated with severe TBI.4 Early assessment of patients' brain damage may be quite difficult during the stay in the intensive care unit (ICU). ICU scores are used to predict hospital outcome in critically ill patients, but have shortcomings.5,6 Therefore, the wide range of conditions associated and the relatively variable predictive value of clinical assessments in severe TBI complicate the identification of patients at higher risk for development of secondary brain injury and fatal outcome.7 Clearly, a practical and sensitive biomarker is needed to identify these patients, as early as possible, to initiate intervention and to indicate those persons to be targeted with higher risk therapeutic strategies.8,9
In the last few decades, a rapidly growing number of molecules have been tested as potential biomarkers of TBI. Several potential biomarkers have been proposed, among which are: Protein S100B, neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), myelin basic protein, creatine-kinase-BB, Hsp 70, sFas, tumor necrosis factor alpha (TNFα), brain-derived neirotrophic factor (BDNF), plasma von Willebrand factor, cleaved tau protein, spectrin breakdown products (SBDPs), ubiquitin C-terminal hydrolase-L1 (UCH-L1), plasma DNA, and serum interleukin-10.8–28 Nevertheless, at present, no single molecule presented adequate specificity and sensitivity as a comprehensive clinical diagnostic tool to predict the extent of neural tissue damage, or to aid in monitoring care and forecasting outcome.
The presence of extracellular nucleic acids in the bloodstream was first described by Mandel and Metals (1948).29 Only in the last 15 years, however, has the potential use of circulating cell-free DNA in the plasma or serum been investigated for the establishment of diagnosis, prognosis, and monitoring of a variety of conditions. Tumor, fetus, and donor-derived sequences have been detected in the plasma and serum of cancer patients, pregnant women, and transplant recipients, respectively.30–34 In addition, plasma DNA has also demonstrated potential as a clinical biomarker in several acute pathologic conditions with high mortality risk.35–39
Significant increases of circulating DNA in the plasma of trauma patients have been reported as a promising marker for risk stratification of patients with minor, moderate, and severe injury and presented correlation with injury severity and development of posttraumatic complications.40,41 A previous study of our group showed that severe TBI is associated with elevated DNA plasma levels and suggested that persistent DNA elevations correlated with mortality.22 Recently, Macher and associates27 investigated plasma DNA in 65 patients after severe TBI in the first 96 h after ICU admission. After an initial peak, a higher decrease was detected within the first 24 h among survivors compared with non-survivors.27
Therefore, our aim was to investigate in a large sample of patients with severe TBI whether plasma DNA concentrations correlated to short-term primary outcome (survival or death) and Glasgow Coma Scale (GCS) scores within the first 24 h after injury.
Methods
Patients and control subjects
Ethical approval for the study protocol was granted by the Research Ethics Committee of the Universidade Luterana do Brasil (CEP ULBRA 2008-239H). From September 2008 to September 2011, in three regional trauma centers, 188 persons with severe TBI (GCS 3–8 at emergency department admission) were enrolled in this prospective study. Only patients ≥16 years old and without a history of neurological or psychiatric disease were included in this cohort. On admission to the trauma emergency department, patients were initially evaluated, resuscitated (with crystalloids), and underwent emergency operation when necessary. Only the patients transferred to the trauma ICU within 12 h of the head injury were included in the study.
Clinical outcome variables of severe TBI comprised short-term survival, time for ICU discharge, and neurological assessment using the GCS at the ICU discharge. The circulatory function and GCS scores were monitored at hospital admission, ICU admission, and during ICU stay. All patients were sedated and mechanically ventilated. Corticosteroids were not administered. To establish normal values of plasma DNA, a negative control group was included consisting of 14 healthy male and nine female volunteers without a history of brain damage (median age 30 years; range 19–45 and 44 years; range 34–65 years, respectively).
Blood sampling
Peripheral venous blood was collected into ethylenediaminetetraacetic acid-containing tubes at ICU admission. Blood samples were centifuged at 1000 g for 10 min; then the plasma was removed (with great care taken not to disturb the pellet) and stored at −20°C until batch evaluation. Blood samples from the control group were collected and processed in the same way.
DNA extraction from plasma samples
DNA from plasma samples was extracted according to the protocol developed by Boom and associates.42 Briefly, 100 μL of plasma samples were lysed in 900 μL of a guanidine thiocyanate (GuSCN) buffer. After lysis, nucleic acids were bound to silica particles and subsequently washed with several solvents (a GuSCN-containing wash buffer, 70% ethanol and acetone) in consecutive steps. After being dried, the nucleic acids were released from the silica particles in 50 μL of elution buffer.
Real-time quantitative polymerase chain reaction (PCR)
Theoretical and practical aspects of real-time quantitative PCR were described by Heid and colleagues.43 Real-time quantitative PCR analysis was performed using StepOnePlus™ Real-Time PCR System (Life Technologies, Carlsbad, CA). The amplification and product reporting system was based on the 5′ nuclease assay (Taqman assay).44 Plasma DNA was measured using a real-time quantitative PCR assay for the β-globin gene.31 Our study used primers and probe sequences from human beta globin gene, based in several previous studies related with circulating cell-free DNA. This target is the most studied for this purpose.31,32,35,40,41,45 The β-globin Taqman system consisted of the amplification primers beta-globin-354F (5′-GTG CAC CTG ACT CCT GAG GAG A-3′), beta-globin-455R (5′-CCT TGA TAC CAA CCT GCC CAG-3′), and a dual-labeled fluorescent probe beta-globin-402T [5′-(FAM) AAG GTG AAC GTG GAT GAA GTT GGT GG (TAMRA)-3′].31 The expression of quantitative results as kilogenome-equivalents/L was described previously.31 One genome-equivalent was defined as the amount of a target sequence contained in a single diploid human cell.
Statistical analysis
Continuous variables were analyzed by the Kolmogorov-Smirnov test to determine the distribution type. Those with a normal distribution were analyzed by the Student t test, while those with a non-parametric distribution were analyzed by the Mann-Whitney U test or Kruskal-Wallis analysis followed by the Dunn post-test. Correlations were analyzed using the Spearman nonparametric correlation method or linear regression method. The extent to which the DNA concentrations differed between persons surviving or dying in the ICU after severe TBI was assessed using receiver operator characteristics (ROC) plots. The ROC plot is obtained by calculating the sensitivity and specificity for every distinct observed data value, and plotting sensitivity against 1-(specificity). The ROC curve was used to evaluate the optimal cutoff values measured at study entry for prediction of unfavorable outcome. A cutoff point on the curves was chosen to attain the best compromise between sensitivity and specificity for death in the ICU. Logistic regression analysis was performed to eliminate confounding factors, and the dependent variable was the primary outcome (dead/alive). The independent variables tested were age, associated injury, craniotomy, GCS score at hospital admission, and plasma DNA levels. All p values presented are two-tailed and the values of p<0.05 were considered statistically significant.
Results
Plasma DNA concentrations were determined in all 188 patients with severe TBI at ICU admission (mean time for blood sampling after hospital admission was 6.0±4.9 h). Characteristics of the severe TBI population stratified for the primary outcome measure (survivors/non-survivors) are depicted in Table 1. The mean age was 34.8±13.9 years and 88.0% of the persons were males. Forty-one percent of the patients presented isolated severe TBI. There were no significant differences concerning age, incidence of pre-hospital care, mechanism of injury, and proportion of associated extracranial injuries between survivors and non-survivors. Craniotomy was performed in 55.3% of the patients, and there were no significant differences in the rate of craniotomy between survivors and non-survivors. Severe TBI was associated with a 35.1% mortality rate, mostly occurring within 72 h after ICU admission. The mean time between the traumatic event and death was 5.0±3.9 days. In contrast, in the survivors group, the mean time between trauma and ICU discharge was 15.5±11.5 days, and mean GCS score at ICU discharge was 11.1±3.4 (Table 1). GCS scores at hospital admission differed significantly between the survivor and non-survivor groups, with the non-survivors presenting lower scores than the survivors (5.2±2.0 and 6.3±2.6, respectively, p=0.002) (Table 1).
Table 1.
Characteristics of the Severe Traumatic Brain Injury Study Population Stratified for the Primary Outcome Measure (Survivors/Non-Survivors)
| Characteristic* | All patients (n=188) | Survivors (n=122) | Non-survivors (n=66) | p value |
|---|---|---|---|---|
| Age, years | 34.8 (13.9) | 33.4 (12.6) | 37.2 (15.8) | 0.081 |
| Pre-hospital care, n (%) | 124 (66) | 84 (69) | 40 (61) | 0.863 |
| Sex, male, n (%) | 165 (88) | 107 (88) | 58 (88) | 0.462 |
| GCS at hospital admission | 5.7 (2.2) | 6.3 (2.6) | 5.2 (2.0) | <0.001 |
| Systolic blood pressure | 128 (29) | 129 (28) | 126 (33) | 0.552 |
| Diastolic blood pressure | 77 (22) | 77 (23) | 76 (19) | 0.892 |
| Mechanism of injury, n (%) | 0.168 | |||
| Motor vehicle accident | 80 (43) | 55 (45) | 25 (38) | |
| Assault | 41 (22) | 24 (20) | 17 (26) | |
| Auto pedestrian | 40 (21) | 28 (23) | 12 (18) | |
| Fall | 27 (14) | 15 (12) | 12 (18) | |
| Craniotomy, n (%) | 104 (55) | 63 (52) | 41 (62) | 0.236 |
| Associated injuries, n (%) | 110 (59) | 77 (63) | 33 (50) | 0.090 |
| Mortality, n (%) | 66 (35) | - | 66 (100) | |
| Time between trauma and outcome, days | 11.9 (10.8) | 15.5 (11.5) | 5.0 (3.9) | <0.001 |
| GCS at discharge from ICU | 11.1 (3.4) | 11.1 (3.4) | - |
Data are shown as mean (standard deviation) or, when indicated, number (%) of patients.
GCS, Glasgow coma scale; ICU, intensive care unit.
Characteristics of the TBI population stratified for the type of severe TBI (isolated TBI or TBI associated with multitrauma) are shown in Table 2. There were no significant differences in age, incidence of pre-hospital care, GCS scores at either hospital or ICU admission, and diastolic blood pressure at hospital admission. There were significant differences in systolic blood pressure, craniotomy rate and time between event and outcome between isolated TBI and TBI associated with multitrauma (Table 2).
Table 2.
Characteristics of the Traumatic Brain Injury Study Population Stratified for the Type of Trauma (Isolated or Associated with Multitrauma)
| Characteristic* | Isolated TBI (n=78) | TBI+multitrauma (n=110) | P value |
|---|---|---|---|
| Age, years | 37.1 (14.8) | 33.2 (12.8) | 0.063 |
| Sex, male, n (%) | 69 (88) | 96 (87) | 0.876 |
| GCS at hospital admission | 5.9 (2.6) | 5.8 (2.2) | 0.434 |
| Systolic blood pressure | 135 (31) | 124 (27) | 0.023 |
| Diastolic blood pressure | 80 (23) | 75 (20) | 0.251 |
| Time for blood sampling (h after ICU admission) | 5.4 (3.5) | 6.3 (5.4) | 0.219 |
| Mechanism of injury, n (%) | 0.710 | ||
| Motor vehicle accident | 33 (42) | 47 (43) | |
| Assault | 20 (26) | 21 (19) | |
| Auto pedestrian | 15 (19) | 25 (23) | |
| Fall | 10 (13) | 17 (15) | |
| Craniotomy, n (%) | 63 (81) | 41 (37) | 0.004 |
| Mortality, n (%) | 33 (42) | 33 (29) | 0.090 |
| Time between trauma and outcome, days | 9.9 (9.7) | 13.3 (11.4) | 0.040 |
| GCS at discharge from ICU | 10.3 (3.4) | 11.1 (3.3) | 0.215 |
Data are shown as mean (standard deviation) or, when indicated, number (%) of patients.
GCS, Glasgow coma scale; ICU, intensive care unit.
The plasma concentration of cell–free DNA was estimated for all persons enrolled in the study. The control group (n=25) presented a mean plasma DNA concentration of 2156±591 kilogenomes-equivalents/L (mean±standard error of the mean [SEM]). There were no significant differences in cell-free DNA levels among men and women (p=0.109, Mann Whitney U test). In addition, cell–free DNA was estimated in patients with severe TBI at ICU admission (mean time 6.0±4.9 h after hospital admission). Mean plasma DNA concentrations were significantly higher in the severe TBI group (429856±162311 kilogenomes-equivalents/L, mean±SEM) when compared with the control group (p<0.001, Mann-Whitney U test) (Fig. 1). Noteworthy, mean plasma DNA concentrations were significantly higher in the non-survivor group (986750±260548 kilogenomes-equivalents/L, mean±SEM) when compared with the survivor group (130699±427496 kilogenomes-equivalents/L, mean±SEM) (p<0.001, Mann-Whitney U test) (Fig. 1). In fact, there was a significant correlation between higher plasma DNA concentrations and fatal outcome (Spearman's rho=0.320, p<0.001). There were no significant correlations between plasma cell-free DNA and either age (linear regression, p=0.718) or craniotomy (Spearman rank, p=0.724) (data not shown).
FIG. 1.
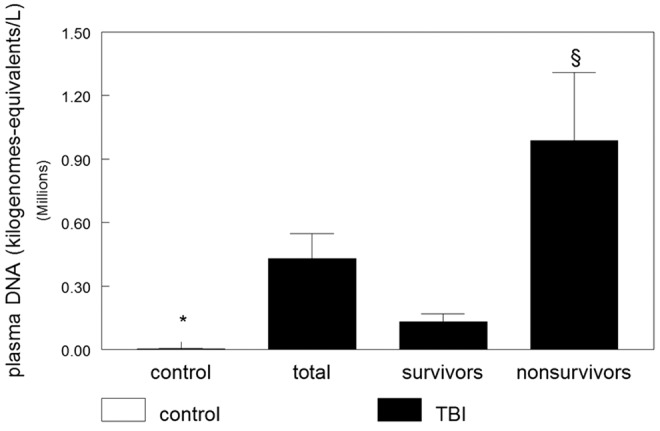
Plasma DNA concentrations in control and severe traumatic brain injury (TBI) persons stratified by the primary outcome (survival or death). Data are shown as mean±standard error of the mean stratified by primary outcome. There was a significant correlation between higher plasma DNA concentrations and fatal outcome (Spearman rho=0.320, p=0.001). *Significantly different from other groups (p<0.001, Kruskal-Wallis, followed by Dunn test). §Significantly different from control and TBI survivor groups (p<0.001, Kruskal-Wallis, followed by Dunn test).
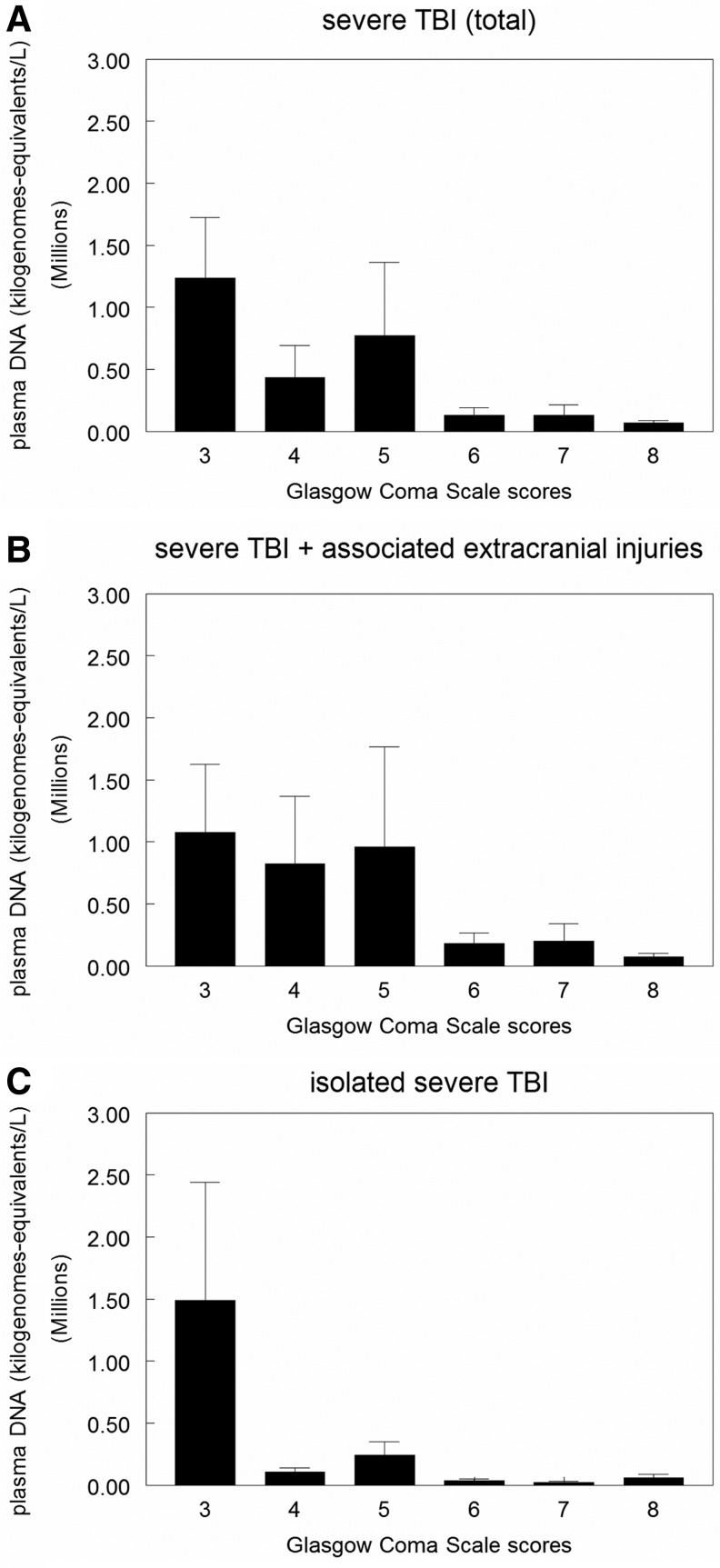
Furthermore, when plasma cell-free DNA levels and GCS scores were analyzed, a significant correlation between higher DNA levels and lower GCS scores at hospital admission was observed (1234043±491479, 436573±257432, 770442±592283, 130611±58329, 130269±84581, 66347±21288, mean plasma cell-free DNA levels for 3, 4, 5, 6, 7, and 8 GCS scores, respectively; Spearman rho = −0.327, p=0.001) (Fig. 2). Indeed, this correlation between higher plasma cell-free DNA levels and lower GCS scores was detected despite the type of severe TBI the patient had (either isolated TBI [Spearman rho = −0.335, p=0.003] or TBI associated with extracerebral lesions [Spearman rho = −0.275, p=0.004]) (Fig. 2B, C).
FIG. 2.
Plasma DNA concentrations and hospital admission Glasgow Coma Scale scores in severe traumatic brain injury (TBI) persons stratified by the type of TBI lesion. In (A), data represent correlation between plasma DNA concentrations and hospital admission GCS scores in all persons with severe TBI (n=188). There was a significant correlation between higher DNA levels and lower GCS scores (p=0.001). In (B), data represent correlation between plasma DNA concentrations and hospital admission GCS scores in the group with severe TBI associated with extracranial lesions (n=110). There was a significant correlation between higher DNA levels and lower GCS scores (p=0.003). In (C), data represent correlation between plasma DNA concentrations and hospital admission GCS scores in the isolated severe TBI group (n=78). There was a significant correlation between higher DNA levels and lower GCS scores (p=0.004). Data are expressed as mean±S.E.M.
ROC curve was plotted (Fig. 3) and a cutoff point that would ensure the detection of the highest proportion of persons with fatal outcome with the least compromise of specificity was chosen. Therefore, a cutoff point of 171381 kilogenomes-equivalents/L plasma DNA concentrations within 12 h after hospital admission was chosen. The diagnostic characteristics of this cutoff point was a specificity of plasma DNA concentration for predicting mortality of 90% and a sensitivity of 43%. The area under the curve for cell-free DNA plasma concentration was 0.694 (p<0.001) (Fig. 3). Interestingly, considering the effect of the type of severe TBI (isolated or associated with extracerebral lesions) on the diagnostic characteristics of the chosen cutoff point of plasma cell-free DNA, we observed that isolated severe TBI ensured higher specificity than TBI associated with multitrauma for predicting mortality within the first 12 h after trauma (specificity of 92% and 89% and sensitivity of 33% and 55% for isolated TBI or TBI associated with extracerebral lesions, respectively) (Fig. 4).
FIG. 3.
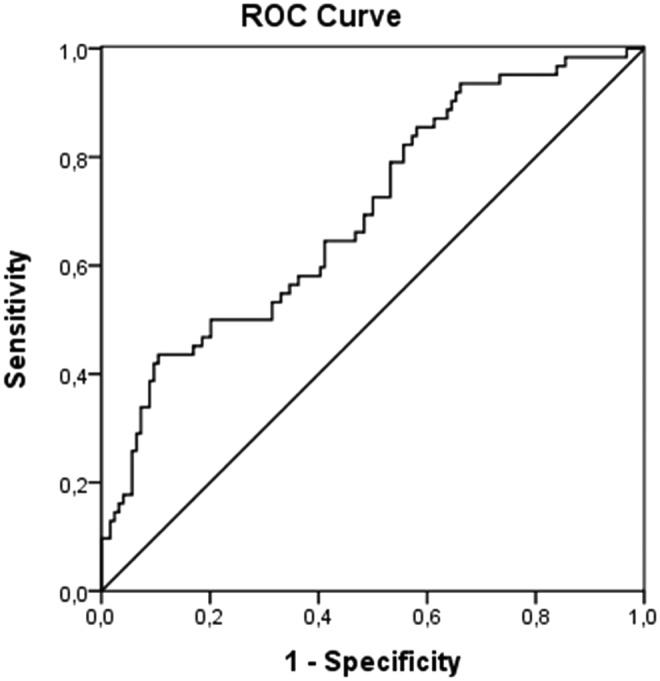
Receiver operator characteristics (ROC) curves plasma DNA concentrations for predicting fatal outcome after severe TBI. ROC curve analysis showed area under the curve of 0.694±0.040 (standard error of the mean) (p<0.001). A cutoff point of 171381 kilogenomes-equivalents/L plasma DNA concentrations was chosen and presented a specificity and sensitivity for predicting mortality of 90% and 43%, respectively.
FIG. 4.
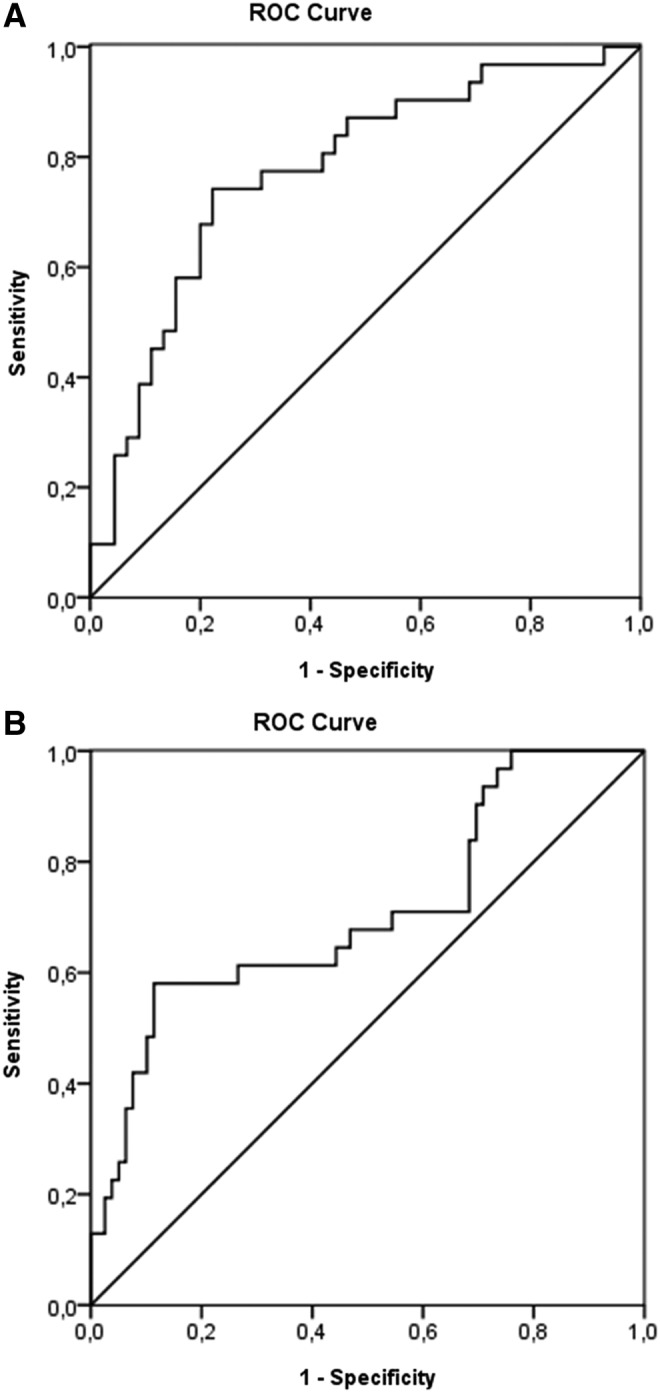
Receiver operator characteristics (ROC) curves plasma DNA concentrations stratified by type of lesion for predicting fatal outcome after severe TBI. In (A), plasma DNA concentrations in the isolated severe TBI group, ROC curve analysis showed area under the curve of 0.777±0.056 (standard error of the mean [SEM]) (p<0.001). In (B), plasma DNA concentrations in the severe TBI associated with the extracerebral lesions group, ROC curve analysis showed area under the curve of 0.679±0.060 (S.E.M.) (p=0.003). A cutoff point of 171381 kilogenomes-equivalents/L plasma DNA concentrations was chosen. The specificity of plasma DNA concentration predicting mortality according to the chosen cutoff point was 92% and 89% for isolated severe TBI or severe TBI associated with extracerebral injuries, respectively, and sensitivity was 33% and 55%, respectively.
Logistic regression analysis was performed to assess the independent influence of cell-free DNA plasma levels on the TBI primary outcome (dead/alive). After adjusting for confounding variables we found that lower GCS at hospital admission (p=0.007), absence of associated injuries (p=0.018), and higher plasma cell-free DNA levels (>171381 kilogenomes-equivalents/L; p<0.001) were variables independently associated with poor outcome (death).
Discussion
In this study, we evaluated circulating plasma DNA as a predictor of fatal outcome in patients with severe TBI. The study showed a positive correlation between high plasma cell-free DNA levels and both lower GCS scores and fatal outcome within the initial 12 h after hospital admission despite the presence of associated extracerebral injuries. To our knowledge, cell-free DNA has not hitherto been investigated as an early predictor of mortality in a large series of exclusively patients with severe TBI. In accordance with the literature, victims of severe TBI enrolled in our study were mostly young men involved in motor vehicle accidents and interpersonal violence. Lower GCS scores at hospital admission were associated with worst prognosis, and the short-term mortality rate was of 35%.1
Previous studies have reported elevated plasma cell-free DNA in trauma; correspondingly, in the present study, we observed that patients with severe TBI presented higher cell-free DNA levels than healthy persons.22,37,40,41,45 The precise mechanism by which DNA is released into the bloodstream remains uncertain, because both necrosis and apoptosis have been observed in this scenario.46 Further, decreased efficiency of DNA clearance mechanisms after injury may play a role in the increase of cell-free DNA in trauma.40 In fact, it is possible that direct damage or hemodynamic compromise of the organ systems responsible for circulating DNA clearance could also lead to increased plasma cell-free DNA.40 Recently, it has been suggested that circulating plasma DNA may play a role in cell communication. Indeed, evidence supporting the active release of free circulating DNA by living cells has been reported.47,48
Previous studies investigating plasma cell-free DNA and trauma showed association of increased plasma cell-free DNA levels with both injury severity and the development of post-traumatic complications.27,40,41 In a previous study, our group has demonstrated that high concentrations of plasmatic cell-free DNA correlated to fatal outcome after severe TBI in males.22 In the present study, we enrolled a larger sample of patients with severe TBI (either isolated severe TBI or TBI associated with extracerebral lesions) from both sexes and determined plasma cell-free DNA concentrations at ICU admission, within 12 h after trauma. In addition, we analyzed the association between plasma cell-free DNA levels and hospital admission GCS scores.
To our knowledge, this is the largest series investigating plasma cell-free DNA for short-term outcome prediction in patients with severe TBI. We observed a positive correlation between higher plasma cell-free DNA levels and fatal outcome within 12 h after trauma. In contrast with previous studies, in the present study, a single determination early after the trauma (mean 6 h) predicted fatal outcome of the patients with severe TBI admitted to the ICU.22,27 Temporal profile post-injury may influence assessment of the predictive value of plasma cell-free DNA after severe TBI. Accordingly, Lo and coworkers49 reported in a study investigating healthy pregnant women that DNA has a rapid clearance from plasma, with a mean estimated half-life of 16.3 min (range 4–30 min). Therefore, because of the short half-life of plasma cell-free DNA, an early determination to establish prognosis in patients with severe TBI may be more suitable and cost-effective in clinical practice.
Since Teasdale and Jennett50 introduced the Glasgow Coma Scale in 1974, GCS scores have been widely used as a quantative measure of the level of consciousness in patients with TBI.50 Nevertheless, few studies investigated the GCS as the sole variable in predicting outcome in patients with head injury.51–53 Interestingly, in the present study, a positive correlation between higher plasma cell-free DNA levels and lower hospital admission GCS scores was demonstrated. This correlation was also demonstrated when patients were stratified for type of severe TBI (isolated or associated with extracerebral lesions). Therefore, we established a correlation between plasma cell-free DNA levels and the severity of injury at hospital admission despite the presence of extracerebral lesions. In fact, major extracranial injury has been reported as a prognostic factor for mortality in patients with TBI, but the strength of the effect is smaller in patients with more severe brain injury as is the most likely to occur in our series of patients who presented a high rate of isolated severe TBI from assault.54,55
Besides the brain, the release of cell-free DNA from injured cells may occur peripherally, because DNA is present in the nucleus/mitochondria of the majority of cell types and can be released into the circulation. Thus, extracerebral sources may contribute to the increase plasma DNA levels after multitrauma. Because many patients with severe head injury have extracranial injuries, they were included in the present study to investigate the value of cell-free measurements after severe TBI in the scenario of clinical practice. Associated extracranial injuries was observed in both the survivor (63%) and non-survivor (50%) TBI groups and did not correlate with outcome. Consequently, the impact of associated injuries on mortality seems to be limited. We recognize, however, that for a blood biomarker to be valuable in therapeutic trials in TBI, the ability to contribute to the successful prediction of the level of the neurological outcome of the survivors rather than solely mortality is essential. Indeed, in our study, the remarkably high levels of cell-free DNA observed in non-survivors early after TBI could be reflecting extensive tissue necrosis eliciting greater DNA release into the circulation. We could not establish a direct correlation, however, between cerebral tissue destruction and plasma DNA concentrations. In this sense, it would also have been important to cross-validate the findings for cell-free DNA with other paraclinical measures. A shortcoming of the present study is that image analysis was not defined as a primary outcome measure and therefore was not standardized.
The specificity of plasma cell-free DNA concentrations for prediction of poor outcome (death) at the chosen cutoff point (≥171381 kilogenomes-equivalents/L) was high (specificity of 90%, 92%, and 89%, for all patients with severe TBI, isolated severe TBI, and severe TBI associated with extracerebral lesions, respectively). These rates for prediction of mortality were higher than those of clinically established predictors such as GCS scores and age.
Conclusion
The overall mortality rate in the severe TBI group was 35%, with most deaths occurring in the first 72 h after injury, and high plasma cell-free DNA concentrations within the initial 12 h after hospital admission was associated with lower GCS scores and increased death risk despite the presence of associated extracerebral injuries.
Acknowledgments
The present study received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; research Grant 568691/2008-3) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS; research Grant PPSUS 09/0041-5).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths, 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.Coronado V.G., Xu L., Basavaraju S.V., Mcguire L.C., Wald M.M., Faul M.D., Guzman B.R., and Hemphill J.D; Centers for Disease Control and Prevention. (2011). Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveil Summ 60, 1–32 [PubMed] [Google Scholar]
- 3.Zaloshnja E., Miller T., Langlois JA., and Selassie AW. (2008). Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehab. 23, 394–400 [DOI] [PubMed] [Google Scholar]
- 4.Woertgen C., Rothoerl RD., Metz C., and Brawanski A. (1999). Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. J. Trauma 47, 1126–1130 [DOI] [PubMed] [Google Scholar]
- 5.Pelinka LE., Toegel E., Mauritz W., and Redl H. (2003). Serum S 100B: a marker of brain damage in traumatic brain injury with and without multiple trauma. Shock 19, 195–200 [DOI] [PubMed] [Google Scholar]
- 6.Toschlog E.A., MacElligot J., Sagraves S.G., Schenarts P.J., Bard M.R., Goettler C.E., Rotondo M.F., and Swanson M.S. (2003). The relationship of Injury Severity Score and Glasgow Coma Score to rehabilitative potential in patients suffering traumatic brain injury. Am. Surg. 69, 491–498 [PubMed] [Google Scholar]
- 7.Ghajar J. (2000). Traumatic brain injury. Lancet 356, 923–929 [DOI] [PubMed] [Google Scholar]
- 8.Townend W.J., Guy M.J., Pani M.A., Martin B., and Yates D.W. (2002). Head injury outcome prediction in the emergency department: a role for protein S-100B? J. Neurol. Neurosurg. Psychiatry 73, 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coplin W.M. (2001). Intracranial pressure and surgical decompression for traumatic brain injury: biological rationale and protocol for a randomized clinical trial. Neurol. Res. 23, 277–290 [DOI] [PubMed] [Google Scholar]
- 10.Raabe A., Grolms C., Sorge O., Zimmermann M., and Seifert V. (1999). Serum S-100B protein in severe head injury. Neurosurgery 45, 477–483 [DOI] [PubMed] [Google Scholar]
- 11.Raabe A., Kopetsch O., Woszczyk A., Lang J., Gerlach R., Zimmermann M., and Seifert V. (2003). Serum S-100B protein as a molecular marker in severe traumatic brain injury. Restor. Neurol. Neurosci. 21, 159–169 [PubMed] [Google Scholar]
- 12.Herrmann M., Curio N., Jost S., Grubich C., Ebert A.D., Fork M.L., and Synowitz H. (2001). Release of biochemical markers of damage to neuronal and glial brain tissue is associated with short and long term neuropsychological outcome after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry, 70, 95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regner A., Kaufman M., Friedman G., and Chemale I. (2001). Increased serum S100beta protein concentrations following severe head injury in humans: a biochemical marker of brain death? Neuroreport 12, 691–694 [DOI] [PubMed] [Google Scholar]
- 14.Petzold A., Green A.J., Keir G., Fairley S., Kitchen N., Smith M., and Thompson E.J. (2002). Role of serum S100B as an early predictor of high intracranial pressure and mortality in brain injury: a pilot study. Crit. Care Med. 30, 2705–2710 [DOI] [PubMed] [Google Scholar]
- 15.Mussack T., Biberthaler P., Kanz K.G., Wiedmann E., Gippner-Steppert C., Mutschler W., and Jochum M. (2002). Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit. Care Med. 30, 2669–2674 [DOI] [PubMed] [Google Scholar]
- 16.Pelinka L.E., Kroepfl A., Schmidhammer R., Krenn M., Buchinger W., Redl H., and Raabe A. (2004). Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J. Trauma 57, 1006–1012 [DOI] [PubMed] [Google Scholar]
- 17.Savola O., Pythinen J., Leino T.K., Siitonen S., Niemela O., and Hillbom M. (2004). Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J. Trauma 56, 1229–1234 [DOI] [PubMed] [Google Scholar]
- 18.da Rocha A.B., Zanoni C., de Freitas G.R., André C., Himelfarb S., Schneider R.F., Grivicich I., Borges L., Schwartsmann G., Kaufmann M., and Regner A. (2005). Serum Hsp70 as an early predictor of fatal outcome after severe traumatic brain injury in males. J. Neurotrauma 22, 966–977 [DOI] [PubMed] [Google Scholar]
- 19.da Rocha A.B., Schneider R.F., de Freitas G.R., André C., Grivicich I., Zanoni C., Fossá A., Gehrke J.T, Pereira Jotz G., Kaufmann M., Simon D., and Regner A. (2006). Role of serum S100B as a predictive marker of fatal outcome following isolated severe head injury or multitrauma in males. Clin. Chem. Lab. Med. 44, 1234–1242 [DOI] [PubMed] [Google Scholar]
- 20.Crespo A.R., Rocha A.B., Jotz G.P., Schneider R.F., Grivicich I., Pinheiro K., Zanoni C., and Regner A. (2007). Increased serum sFas and TNFα following isolated severe head injury in males. Brain Inj. 21, 441–447 [DOI] [PubMed] [Google Scholar]
- 21.de Oliveira C.O., Reimer A.G., da Rocha A.B., Grivicich I., Schneider R.F., Roisenberg I., Regner A., and Simon D. (2007). Plasma von Willebrand factor levels correlate with clinical outcome of severe traumatic brain injury. J. Neurotrauma 24, 1331–1338 [DOI] [PubMed] [Google Scholar]
- 22.Campello Yurgel V., Ikuta N., Brondani da Rocha A., Lunge V.R., Fett Schneider R., Kazantzi Fonseca A.S., Grivicich I., Zanoni C., and Regner A. (2007). Role of plasma DNA as a predictive marker of fatal outcome following severe head injury in males. J. Neurotrauma 24, 1172–1181 [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues E., Oliveira C., Cambrussi A., Gomes G., Godoy D., da Rocha A.B., Grivicich I., and Regner A. (2008). Increased serum brain derived neurotrophic factor (BDNF) following isolated severe traumatic brain injury in humans. Brain Inj. 22, Suppl 1, 165 [Google Scholar]
- 24.Hergenroeder G.W., Redell J.B., Moore A.N., and Dash P.K. (2008). Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol. Diagn. Ther. 12, 345–358 [DOI] [PubMed] [Google Scholar]
- 25.Papa L., Akinyi L., Liu M.C., Pineda J.A., Tepas J.J., Oli M.W., Zheng W., Robinson G., Robicsek S.A., Gabrielli A., Heaton S.C., Hannay H.J., Demery J.A., Brophy G.M., Layon J., Robertson C.S., Hayes R.L., and Wang K.K. (2010). Ubiquitin c-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 38, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svetlov S.I., Larner S.F., Kirk D.R., Atkinson J., Hayes R.L., and Wang K.K. (2009). Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury. J. Neurotrauma 26, 913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macher H., Egea-Guerrero J.J., Revuelto-Rey L., Gordillo-Escobar E., Enamorado-Enamorado J., Boza A., Rodriguez A., Molinero P., Guerrero J.M., Dominguez-Roldán J.M., Murillo-Cabezas F., and Rubio A. (2012). Role of early cell-free DNA levels decreased as a predictive marker of fatal outcome after severe traumatic brain injury. Clin. Chim. Acta, 414, 12–17 [DOI] [PubMed] [Google Scholar]
- 28.Schneider Soares F.M., Menezes d Souza N., Liborio Schwarzbold M., Paim Diaz A., Costa Nunes J., Hohl A., Nunes Abreu da Silva P., Vieira J., Lisboa de Souza R., Moré Bertotti M., Schoder Prediger R.D., Neves Linhares M., Bafica A., and Walz R. (2012). Interleukin-10 is an independent biomarker of severe traumatic brain injury prognosis. Neuroimmunomodulation 19, 377–385 [DOI] [PubMed] [Google Scholar]
- 29.Mandel P., and Metais P. (1948). Les acides nucleiques du plasma sanguin chez l'homme. C. R. Acad. Sci. Paris, 142, 241–243C [PubMed] [Google Scholar]
- 30.Lo Y.M., Corbetta N., Chamberlain P.F., Rai V., Sargent I.L., Redman C.W., and Wainscoat JS. (1997) Presence of fetal DNA in maternal plasma and serum. Lancet 350, 485–487 [DOI] [PubMed] [Google Scholar]
- 31.Lo Y.M., Tein M.S., Pang C.C., Yeung C.K., Tong K.L., and Hjelm NM. (1998). Presence of donor-specific DNA in plasma of kidney and liver-transplant recipients. Lancet 351, 1329–1330 [DOI] [PubMed] [Google Scholar]
- 32.Lo Y.M., Tein M.S., Lau T.K., Haines C.J., Leung T.N., Poon P.M., Wainscoat J.S., Johnson P.J., Chang A.M., and Hjelm N.M. (1998). Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am. J. Hum. Genet. 62, 768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson P.J., and Lo Y.M. (2002). Plasma nucleic acids in the diagnosis and management of malignant disease. Clin. Chem. 48, 1186–1193 [PubMed] [Google Scholar]
- 34.Wang B.G., Huang H., Chen Y., Bristow R.E., Kassauei K., Cheng C., Roden R., Skoll L.J., Chan D.W., and Shih IeM. (2003). Increased plasma DNA integrity in cancer patients. Cancer Res. 63, 3966–3968 [PubMed] [Google Scholar]
- 35.Rainer T.H., Wong L.K., Lam W., Yuen E., Lam N.Y., Metreweli C., and Lo Y.M. (2003). Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin. Chem. 49, 562–569 [DOI] [PubMed] [Google Scholar]
- 36.Rhodes A., Wort S.J., Thomas H., Collinson P., and Bennett E.D. (2006). Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit. Care 10, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rainer T.H., Lam N.Y., Man C.Y., Chiu R.W., Woo K.S., and Lo Y.M. (2006). Plasma beta-globin DNA as a prognostic marker in chest pain patients. Clin. Chim. Acta. 368, 110–113 [DOI] [PubMed] [Google Scholar]
- 38.Saukkonen K., Lakkisto P., Pettilä V., Varpula M., Karlsson S., Ruokonen E., and Pulkki K.; Finnsepsis Study Group. (2008). Cell-free plasma DNA as a predictor of outcome in severe sepsis and septic shock. Clin. Chem. 54, 1000–1007 [DOI] [PubMed] [Google Scholar]
- 39.Arnalich F., Menéndez M., Lagos V., Ciria E., Quesada A., Codoceo R., Vazquez J.J., López-Collazo E., and Montiel C. (2010). Prognostic value of cell-free plasma DNA in patients with cardiac arrest outside the hospital: an observational cohort study. Crit. Care 14, R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo Y.M., Rainer T.H., Chan L.Y., Hjelm N.M., and Cocks R.A. (2000). Plasma DNA as a prognostic marker in trauma patients. Clin. Chem. 46, 319–323 [PubMed] [Google Scholar]
- 41.Lam N.Y., Rainer T.H., Chan l.Y.S., Joynt G.M., and Lo Y.M. (2003). Time course of early and late changes in plasma DNA in trauma patients. Clin. Chem. 49, 1286–1291 [DOI] [PubMed] [Google Scholar]
- 42.Boom R., Sol C.J., Salimans M.M., Jansen C.L., Wertheim-van Dillen P.M, and Van Der Noordaa J. (1990). Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28, 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heid C.A., Stevens J., Livak K.J., and Williams P.M. (1996). Real time quantitative PCR. Genome Res. 6, 986–994 [DOI] [PubMed] [Google Scholar]
- 44.Holland P.M., Abramson R.D., Watson R., and Gelfand D.H. (1991). Detection of specific polymerase chain reaction product by utilizing the 5′-3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 88, 7276–7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam N.Y., Rainer T.H., Chiu R.W., Joynt G.M., and Lo Y.M. (2004). Plasma mitochondrial DNA concentrations after trauma. Clin. Chem. 50, 213–216 [DOI] [PubMed] [Google Scholar]
- 46.Jahr S., Hentze H., Englisch S., Hardt D., Fackelmayer F.O., Hesch R.D., and Knippers R. (2001). DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 61, 1659–1665 [PubMed] [Google Scholar]
- 47.Gahan P.B., Anker P., and Stroun M. (2008). Metabolic DNA as the origin of spontaneously released DNA? Ann. N.Y. Acad. Sci. 1137, 7–17 [DOI] [PubMed] [Google Scholar]
- 48.Van der Vaart M., and Pretorius P.J. (2008). Circulating DNA. Its origin and fluctuation. Ann. N.Y. Acad. Sci. 1137, 18–26 [DOI] [PubMed] [Google Scholar]
- 49.Lo Y.M., Zhang J., Leung T.N., Lau T.K., Chang A.M., and Hjelm N.M. (1999). Rapid clearance of fetal DNA from maternal plasma. Am. J. Hum. Genet. 64, 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness: a practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 51.Zafonte R.D., Hammond F.M., Mann N.R., Wood D.L., Black K.L., and Millis S.R. (1996). Relationship between Glasgow coma scale and functional outcome. Am. J. Phys. Med. Rehabil. 75, 364–369 [DOI] [PubMed] [Google Scholar]
- 52.Diringer M.N., and Edwards D.F. (1997). Does modification of the Innsbruck and the Glasgow Coma Scales improve their ability to predict functional outcome? Arch. Neurol. 54, 606–611 [DOI] [PubMed] [Google Scholar]
- 53.Balestreri M., Czosnyka M., Chatfield D.A., Steiner L.A., Schmidt E.A., Smielewski P., Matta B., and Pickard J.D. (2004). Predictive value of Glasgow Coma Scale after brain trauma: change in trend over the past ten years. J. Neurol. Neurosurg. Psychiatry 75, 161–162 [PMC free article] [PubMed] [Google Scholar]
- 54.van Leeuwen N., Lingsma H.F., Perel P., Lecky P., Roozenbeek B., Lu J., Shakur H., Weir J., Steyerberg E.W., and Maas A.I. (2012). Prognostic value of major extracranial injury in traumatic brain injury: an individual patient data meta-analysis in 39,274 patients. Neurosurgery 70, 811–818 [DOI] [PubMed] [Google Scholar]
- 55.Andriessen T.M., Horn J., Franchsman G., van der Naalt J., Haitsma I., Jacobs B., Steyerberg E.W., and Vos P.E. (2011). Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: a prospective multicenter study. J. Neurotrauma 28, 2019–2031 [DOI] [PubMed] [Google Scholar]