Abstract
Background
Fluvoxamine, one of the oldest selective serotonin reuptake inhibitors (SSRIs), is prescribed to patients with major depression in many countries. Several studies have previously reviewed the efficacy and tolerability of fluvoxamine for the treatment of major depression. However, these reviews are now outdated.
Objectives
Our objective is to evaluate the effectiveness, tolerability and side effect profile of fluvoxamine for major depression in comparison with other anti‐depressive agents, including tricyclics (TCAs), heterocyclics, other SSRIs, SNRIs, other newer agents and other conventional psychotropic drugs.
Search methods
We searched the Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register. Trial databases and ongoing trial registers in North America, Europe, Japan and Australia, were handsearched for randomised controlled trials. We checked reference lists of the articles included in the review, previous systematic reviews and major textbooks of affective disorder for published reports and citations of unpublished research. The date of last search was 31 August 2008.
Selection criteria
We included all randomised controlled trials, published in any language, that compared fluvoxamine with any other active antidepressants in the acute phase treatment of major depression.
Data collection and analysis
Two independent review authors inspected citations and abstracts, obtained papers, extracted data and assessed the risk of bias of included studies. We analysed dichotomous data using odds ratios (ORs) and continuous data using the standardised mean difference (SMD). A random effects model was used to combine studies.
Main results
A total of 54 randomised controlled trials (n = 5122) were included. No strong evidence was found to indicate that fluvoxamine was either superior or inferior to other antidepressants regarding response, remission and tolerability. However, differing side effect profiles were evident, especially with regard to gastrointestinal side effects of fluvoxamine when compared to other antidepressants. For example, fluvoxamine was generally associated with a higher incidence of vomiting/nausea (versus imipramine, OR 2.23, CI 1.59 to 3.14; versus clomipramine, OR 2.13, CI 1.06 to 4.27; versus amitriptyline, OR 2.86, CI 1.31 to 2.63).
Authors' conclusions
We found no strong evidence that fluvoxamine was either superior or inferior to any other antidepressants in terms of efficacy and tolerability in the acute phase treatment of depression. However, differing side effect profiles were evident. Based on these findings, we conclude that clinicians should focus on practical or clinically relevant considerations, including these differences in side effect profiles.
Keywords: Humans; Antidepressive Agents, Second‐Generation; Antidepressive Agents, Second‐Generation/therapeutic use; Antidepressive Agents, Tricyclic; Antidepressive Agents, Tricyclic/therapeutic use; Depression; Depression/drug therapy; Fluvoxamine; Fluvoxamine/therapeutic use; Randomized Controlled Trials as Topic; Selective Serotonin Reuptake Inhibitors; Selective Serotonin Reuptake Inhibitors/therapeutic use
Plain language summary
Fluvoxamine versus other anti‐depressive agents for depression
Major depression is a severe mental illness characterised by a persistent and unreactive low mood and loss of all interest and pleasure, usually accompanied by a range of symptoms including appetite change, sleep disturbance, fatigue, loss of energy, poor concentration, psychomotor symptoms, inappropriate guilt and morbid thoughts of death. Although pharmacological and psychological interventions are both effective for major depression, antidepressant (AD) drugs remain the mainstay for treatment of moderate or severe depression. Fluvoxamine is one of the oldest selective serotonin reuptake inhibitors (SSRIs) and is prescribed to patients with major depression in many countries. This review reports trials comparing fluvoxamine with other antidepressants for treatment of major depression. We found no strong evidence that fluvoxamine was either superior or inferior to any other antidepressants in terms of efficacy and tolerability in the acute phase treatment of depression. However, there is evidence of differing side‐effect profiles, especially when comparing gastrointestinal side effects between fluvoxamine and tricyclic antidepressants (TCAs). Based on these findings, we conclude that clinicians should focus on practical or clinically relevant considerations including these differences in side effect profiles.
Background
Description of the condition
Major depression is generally diagnosed when a persistent and unreactive low mood and loss of all interest and pleasure are usually accompanied by a range of symptoms including appetite change, sleep disturbance, fatigue, loss of energy, poor concentration, psychomotor symptoms, inappropriate guilt, and morbid thoughts of death (APA 1994). In 2002, major depression was the third leading health burden in the world, following only lower respiratory infections and HIV/AIDS, and accounting for 4.5% of total human suffering related to health concerns. Moreover, the incidence of depression is expected to rise during the next 20 years (WHO 2006). The depressed condition is associated with a marked personal, social and economic morbidity, coupled with a loss of functioning and productivity, which creates significant demands on health service provider workloads (NICE 2004). In the USA, Greenberg 2003 estimated the economic burden of depression to be just over $83 billion in 2000. Of this total, $26 billion came from direct treatment costs, $5 billion came from suicide‐related costs, and $52 billion came from workplace costs. These figures were also suspected to underestimate the true economic burden of the disease, as they did not take into account factors such as the burden on family members and caregivers, the cost of lost productivity while at work, and costs associated with those who remain untreated (Greenberg 2005).
Description of the intervention
Fluvoxamine ((E)‐5‐methoxy‐1‐[4‐(trifluoromethyl)phenyl]pentan‐1‐one O‐2‐aminoethyl oxime) is a selective serotonin reuptake inhibitor (SSRI) that has been available as an antidepressant since 1983 in many countries – 87 countries and regions as of 2006, including some European countries and Japan. It is also available in many countries for anxiety disorders, including obsessive‐compulsive disorder and social anxiety disorder. Fluvoxamine is structurally different from the tricyclic antidepressants (TCAs), heterocyclics, and other classes of antidepressants, and also differs chemically from various other SSRIs. For example, fluvoxamine is the only monocyclic SSRI and it belongs to the 2‐aminoethyloximethers of aralkylketones (Claassen 1977; Fuller 1987). Therefore, some differential clinical potency may be expected, not only between the drugs classes but also among the SSRIs.
How the intervention might work
Fluvoxamine is well absorbed after oral administration and is widely distributed throughout the body. Plasma protein binding of fluvoxamine is low (77%), compared with other SSRIs. Fluvoxamine displays nonlinear, steady‐state pharmacokinetics throughout the therapeutic range, with disproportionately higher plasma concentrations at higher doses (Perucca 1994). However, plasma fluvoxamine concentrations show no clear relationship with patient responses to the antidepressant or to the severity of adverse effects. Fluvoxamine pharmacokinetics remains unaltered by increasing age or by renal impairment. Fluvoxamine is metabolized in the liver by the cytochrome P450 (CYP) enzyme system. It has a prominent affinity for the CYP1A2 isozyme, a lesser affinity for the CYP3A4 and CYP2C isozymes, and a minimal affinity for CYP2D6. Fluvoxamine impairs metabolic elimination of a number of drugs, including TCAs (tertiary, but not secondary, amines), alprazolam, bromazepam, diazepam, theophylline, propranolol and, possibly, carbamazepine. It generates no active metabolites. Smoking is known to increase CYP1A2 activity, and smokers appear to have lower serum concentration of fluvoxamine compared with non‐smokers (Spigset 1995). The drug is eliminated with a mean half‐life of 15 hours, with a range from nine to 28 hours. Excretion is primarily in the urine, predominantly as metabolites (van Harten 1995).
Why it is important to do this review
Although pharmacological and psychological interventions are both effective for major depression, antidepressant (AD) drugs remain the mainstay for treatment of moderate or severe depression (APA 2000; Ellis 2004; NICE 2004). Many different AD agents are available, including TCAs, monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin‐noradrenaline reuptake inhibitors (SNRIs: venlafaxine, duloxetine, milnacipran), and other newer agents (mirtazapine, reboxetine, bupropion). In many Western countries, AD consumption has risen dramatically over the last 20 years, mainly because of the increasing consumption of SSRIs and newer ADs, which have progressively become the most commonly prescribed ADs (Ciuna 2004; Guaiana 2005). SSRIs are generally better tolerated than TCAs (Barbui 2000), and there is evidence of similar efficacy (Anderson 2000; Geddes 2000; Williams 2000). However, head‐to‐head comparisons have provided contrasting findings. Amitriptyline, for example, may have an edge over SSRIs in terms of efficacy (Guaiana 2007), while individual SSRIs and SNRIs may differ in terms of efficacy and tolerability (Cipriani 2005; Cipriani 2009; Puech 1997; Smith 2002; ).
Two systematic reviews on fluvoxamine exist in current literature. Burton (Burton 1991) reviewed 17 double‐blind comparative studies between fluvoxamine and other ADs in depressed patients. Ware (Ware 1997) reviewed 31 controlled trials of fluvoxamine in the pharmacotherapy of depression. These reviews are now quite outdated and neither has provided meta‐analytic summaries.
A group of researchers therefore agreed to join forces under the rubric of the Meta‐Analyses of New Generation Antidepressants Study Group (MANGA Study Group), in order to systematically review all available evidence for each specific newer antidepressant. As of February 2010, we have completed an individual review for fluoxetine (Cipriani 2006), mirtazapine (Watanabe 2008), milnacipran (Nakagawa 2007), escitalopram (Cipriani 2009a) and sertraline (Cipriani 2009b) and have published the protocols for citalopram (Imperadore 2007), duloxetine (Nose 2007), paroxetine (Cipriani 2007b) , venlafaxine (Cipriani 2007c) and reboxetine (Churchill 2009). The multiple‐treatment meta‐analysis of 12 new‐generation antidepressants has also been published (Cipriani 2009).
In the present review, we report head‐to‐head comparisons not only between fluvoxamine and the other 11 new‐generation antidepressants, but also between fluvoxamine and older antidepressants, providing detailed accounts of their comparative side effect profiles.
Objectives
1. To determine the efficacy of fluvoxamine compared to other anti‐depressive agents, including older antidepressants such as TCAs and newer ones such as SSRIs, in alleviating the acute symptoms of depression. 2. To review acceptability of treatment with fluvoxamine compared with that of other antidepressive agents. 3. To investigate the adverse effects of fluvoxamine compared to other antidepressive agents.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were included. Quasi‐randomised trials, such as those allocating by using alternate days of the week, were excluded. For trials that have a crossover design, only results from the first randomisation period were considered.
Types of participants
The review included patients 18 or older, of both sexes, with a primary diagnosis of depression and studies adopting standardised criteria (DSM‐III / DSM‐ III‐R, DSM‐IV (APA 2000), ICD‐10 (WHO 1992), Feighner criteria (Feighner 1972) or Research Diagnostic Criteria (Spitzer 1972) to define patients suffering from unipolar major depression. Studies using ICD‐9 were excluded, as it has only disease names and no diagnostic criteria.
We included the following subtypes of depression: chronic, with catatonic features, with melancholic features, with atypical features, with postpartum onset, and with seasonal pattern. We also included studies in which up to 20% patients presented depressive episodes in bipolar affective disorder. When depressive patients in the trial had psychotic features, we included those studies in which up to 20% patients presented psychotic features. A concurrent secondary diagnosis of another psychiatric disorder was not considered an exclusion criterion. A concurrent primary diagnosis of Axis I or II disorders was an exclusion criterion. AD trials in depressive patients with a serious concomitant medical illness were excluded.
Types of interventions
We have examined fluvoxamine intervention in comparison with conventional treatment of acute depression. We also examined fluvoxamine intervention in comparison with non‐conventional (e.g., herbal products, such as Hypericum) anti‐depressive agents (Linde 2008). Trials in which fluvoxamine was compared to another type of psychopharmacological agent (i.e., anxiolytics, anticonvulsants, antipsychotics or mood‐stabilizers) were excluded. We also excluded trials in which fluvoxamine was used as an augmentation strategy.
Eligible intervention:
1. Fluvoxamine: any dose and mode or pattern of administration.
Eligible comparators:
2. Conventional anti‐depressive agents: any dose and mode or pattern of administration. 2.1 Tricyclics (TCAs) 2.2 Heterocyclics 2.3 SSRIs 2.4 SNRIs 2.5 MAOIs or newer antidepressants (ADs) 2.6 Other conventional psychotropic drugs
3. Non‐conventional anti‐depressive agents 3.1 Herbal products 3.2 Other non‐conventional anti‐depressive agents
Types of outcome measures
Primary outcomes
1. Response ‐ acute phase
We examined cases regarding the number of patients (1) who responded to treatment by showing a reduction of at least 50% on the Hamilton Rating Scale for depression (HRSD) (Hamilton 1960), Montgomery Åsberg Depression Rating Scale (MADRS) (Montgomery 1979), or any other depression scale, depending on the study authors' definition or (2) who were "much or very much improved" (score 1 or 2) on the CGI‐Improvement scale (Guy 1976) out of the total number of randomised patients. Where both are provided, we prefer the former criteria for judging response. The original authors' definitions of response and remission were not used in this review, to avoid possible outcome reporting bias (Furukawa 2007).
When studies report response rates at various time points throughout the trial, we have determined a priori to subdivide the treatment indices ‐ since one systematic review suggested that SSRIs begin to have observable beneficial effects in depression during the first week of treatment ‐ as follows (Taylor 2006):
(i) Response ‐ early phase: between 1 and 4 weeks, with the time point closest to 2 weeks given preference. (ii) Response ‐ acute phase: between 6 and 12 weeks, with preference given to the time point given in the original study as the study endpoint. (iii) Response ‐ follow‐up phase: between 4 and 6 months, with the time point closest to 24 weeks given preference.
The acute phase treatment response rates were our primary outcome of interest.
Secondary outcomes
1. Response ‐ early phase, and follow‐up phase
2. Remission ‐ early phase, acute phase, and follow‐up phase
We are interested in the number of patients who achieved remission, (1) showing =<7 on HRSD‐17, =<8 on for all the other longer versions of HRSD, and =<11 on MADRS or (2) who were "not ill or borderline mentally ill" (score 1 or 2) on the CGI‐Severity score out of the total number of randomised patients. Where both were provided, we preferred the former criterion for judging remission.
3. Group mean scores at the end of the trial and change score on depression scale
4. Social adjustment, social functioning, including the Global Assessment of Function (GAF) scores
5. Health‐related quality of life (QOL)
We limited ourselves to SF‐12 (Ware 1998); SF‐36 (Ware 1992), HoNOS (Wing 1998) and the WHO 2009‐QOL (WHOQOL Group 1998).
6. Costs to health care services.
7. Tolerability
7.1 Total dropout
Number of patients who dropped out during the trial as a proportion of the total number of randomised patients.
7.2 Dropout due to inefficacy
Number of patients who dropped out during the trial because the fluvoxamine was ineffective as a proportion of the total number of randomised patients.
7.3 Dropout due to side effects
Number of patients who dropped out during the trial due to side effects, as a proportion of the total number of randomised patients.
7.4 Number of patients experiencing at least one side effect
7.5 Number of patients experiencing the following specific side effects was sought:
‐ sleepiness/drowsiness
‐ insomnia
‐ dry mouth
‐ constipation
‐ problems urinating
‐ hypotension
‐ agitation/anxiety
‐ suicide wishes/gestures/attempts
‐ completed suicide
‐ vomiting/nausea
‐ diarrhoea
To avoid missing any relatively rare or unexpected side effects in the data extraction phase, we collected all side effect data reported in the literature and discussed ways to summarize them post hoc. Descriptive data regarding side effect profiles were extracted from all available studies. Only studies reporting the number of patients experiencing individual side effects were retained. Due to a lack of consistent reporting of side effects, which came primarily from the study authors' descriptions, we combined terms describing similar side effects; for example, we combined "dry mouth", "reduced salivation" and "thirst" into "dry mouth". All side effect categories were then grouped by organ system, such as neuropsychiatric, gastrointestinal, respiratory, sensory, genitourinary, dermatological and cardiovascular, in accordance with the advice of a previous study (Mottram 2006).
Search methods for identification of studies
Electronic searches
We initially identified RCTs on June 2, 2006 by following the Cochrane Collaboration Depression, Anxiety and Neurosis (CCDAN) criteria for search strategy using the register of CCDAN Review Group Controlled Trials Registers (CCDANCTR‐Studies and CCDANCTR‐References). The registers are compiled from systematic and regularly updated searches of the Cochrane Central Register of Controlled Trials (CENTRAL) ‐ the most comprehensive source of reports of RCTs ‐ , MEDLINE, EMBASE, CINAHL, PsycINFO, PSYINDEX, and LILACS and handsearched of major psychiatric and medical journals as well as conference proceedings. Trial databases (e.g., the Medicines and Healthcare products Regulatory Agency in the UK) and ongoing trial registers (e.g., clinicaltrials.gov in the USA) in North America, Europe, Japan and Australia, were handsearched for published, unpublished and ongoing RCTs.
CCDANCTR‐Studies was searched using the following search strategy: Diagnosis = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Intervention = Fluvoxamine
CCDANCTR‐References was searched using the following search strategy: Keyword = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Free‐Text = Fluvoxamine
The researchers conducted additional searches on the CCDAN Review Group Controlled Trials Registers, MEDLINE and checked various meta‐analysis and review articles on the 26th October 2009 (CCDAN Registers up‐to‐date as of 31 August 2008).
Searching other resources
1. Handsearches
We searched trial databases of the following drug‐approving agencies for published, unpublished and ongoing controlled trials: the Food and Drug Administration (FDA) in the USA, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, the European Medicines Agency (EMEA) in the EU, the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan and the Therapeutic Goods Administration (TGA) in Australia. We also searched ongoing trial registers such as clinicaltrials.gov in the USA, International Standard Randomised Controlled Trial Number Register (ISRCTN) and the National Research Register in the UK, Nederland's Trial Register in the Netherlands, European Union Drug Regulating Authorities Clinical Trials (EudraCT) in the EU, UMIN‐CTR in Japan and the Australian Clinical Trials Registry in Australia. These searches were undertaken in November 2007.
Appropriate journals and conference proceedings relating to fluvoxamine treatment for depression have already been handsearched and incorporated into the CCDANCTR databases.
2. Personal communication
Pharmaceutical companies and experts in this field were asked if they knew of any study that met the inclusion criteria of this review.
3. Reference checking
Reference lists of the included studies, previous systematic reviews and major textbooks of affective disorder written in English were checked for published reports and citations of unpublished research.
Data collection and analysis
Selection of studies
HMG and another independent review author checked to ensure that studies relating to fluvoxamine generated by the search strategies of the CCDANCTR‐References and the other complementary searches met the rough inclusion criteria, firstly based on the title and abstracts. All of the studies that were rated as possible candidates by either of the two review authors were added to the preliminary list, and their full texts were retrieved. TAF and IMO then assessed all of the full text articles in this preliminary list to see if they met the strict inclusion criteria. If the raters disagreed, the final rating was made by consensus with the involvement ‐ if necessary ‐ of another member of the review group. Non‐congruence in selection of trials was reported as akappa statistic. Considerable care was taken to exclude duplicate publications.
Data extraction and management
IMO and NW extracted data from the included studies. Again, any disagreement was discussed, and decisions were documented. If necessary, we contacted authors of studies for clarification. We extracted the following data:
(i) participant characteristics (age, sex, depression diagnosis, comorbidity, depression severity, antidepressant treatment history for the index episode, study setting); (ii) intervention details (intended dosage range, mean daily dosage actually prescribed, co‐intervention if any, fluvoxamine as investigational drug or as comparator drug, sponsorship); (iii) outcome measures of interest from the included studies.
The results were compared with those in the completed reviews of individual antidepressants in the Cochrane Library. If the trial was a three (or more)‐armed trial involving a placebo arm, the data were extracted from the placebo arm as well.
Assessment of risk of bias in included studies
Risk of bias was assessed independently by two review authors (IMO and NW) using criteria described in the Cochrane Collaboration Handbook (Higgins 2008). This set of criteria is based on evidence of associations between effect overestimation and a high risk of bias in an article, such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
The categories are defined below: YES ‐ low risk of bias NO ‐ high risk of bias UNCLEAR ‐ uncertain risk of bias
Measures of treatment effect
All comparisons were performed between fluvoxamine and comparator ADs as a class and as individual ADs.
1. Dichotomous data
For dichotomous, or event‐like, data, odds ratios (ORs) were calculated with its 95% confidence interval (CI). For statistically significant results, we calculated the number needed to treat to provide benefit (NNTB) and the number needed to treat to induce harm (NNTH) as the inverse of the risk difference.
2. Continuous data
For continuous data, mean differences (MD) or standardized mean differences (SMD) ‐ where different measurement scales, were calculated with its 95% CI.
Unit of analysis issues
1. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g., pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, the participants can differ systematically from their initial state, even despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in major depression, we only used data from the first phase of the cross‐over studies.
2. Cluster‐randomised trials
No cluster randomised trials were identified for this version of the review. Should they be identified in a future update, we plan to use the generic inverse variance technique, if such trials have been appropriately analysed taking into account intraclass correlation coefficients to adjust for cluster effects.
Dealing with missing data
1. Dichotomous data
Responders and remitters to treatment were calculated on the strict intention‐to‐treat (ITT) basis: dropouts were included in this analysis. Where participants have been excluded from the trial before the endpoint, we have assumed that they experienced a negative outcome by the end of the trial (e.g., failure to respond to treatment). We examined the validity of this decision in the sensitivity analyses by applying worst‐ and best‐case scenarios. If a statistically significant difference was found, the number needed to treat (NNT) was calculated from an odds ratio obtained by a meta‐analysis (Higgins 2008). We applied the loose ITT analyses for continuous variables, whereby all the patients with at least one post‐baseline measurement were represented by their last observations carried forward (LOCF), with due consideration of the potential bias and uncertainty introduced.
When dichotomous outcomes were not reported but baseline mean, endpoint mean and those standard deviation (SD) of the HRSD (or other depression scale) were provided, we converted continuous outcome data expressed as mean and SD into the number of responding and remitted patients, according to the validated imputation method (Furukawa 2005). We examined the validity of this imputation in the sensitivity analyses. Where SDs were not reported, authors were asked to supply the data. When only the standard error (SE) or t‐statistics or P values are reported, SDs were calculated according to Altman (Altman 1996). In the absence of data from the authors, we substituted SDs by those reported in other studies in the review (Furukawa 2006).
2. Continuous data
When there were missing data and the method of "last observation carried forward" (LOCF) had been used to do an ITT analysis, then the LOCF data were used. When SDs were missing, we presented data descriptively.
Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of the results in the forest plots. Statistic (the I2statistic and the Q statistic) were interpreted with caution, since non‐significant results of statistical tests for heterogeneity cannot be regarded as evidence of heterogeneity (Higgins 2008). If the CIs for the results of individual comparisons had poor overlap, I2 was equal to or more than 50% and P values were smaller than 0.1 (Higgins 2003), potential sources of heterogeneity were investigated. We performed subgroup analyses to investigate heterogeneity (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Data from included studies were entered into a funnel plot (trial effect against trial variance) to investigate small‐study effects (Sterne 2000). We used the tests for funnel plot asymmetry only when there were at least 10 studies included in the meta‐analysis, and results were interpreted cautiously, with visual inspection of the funnel plots (Higgins 2008). When evidence of small‐study effects was identified, possible reasons for funnel plot asymmetry, including publication bias, were investigated.
Data synthesis
The primary analysis used a random effects model (odds ratio [OR]), which had the highest generalisability in our empirical examination of summary effect measures for meta‐analyses (Furukawa 2002a). The robustness of this summary measure was routinely examined by checking the fixed‐effect model OR and the random effects model risk ratios (RRs). Material differences between the models were reported. A p value of less than 0.05 and a 95% confidence interval (CI) were considered statistically significant.
Fixed‐effect analyses were performed routinely for the continuous outcomes as well, to investigate the effect of the choice of method on the estimates. Material differences between the models were reported. Skewed data and non‐quantitative data were presented descriptively. An outcome was considered skewed when the mean was smaller than twice the SD. In terms of change score, data were difficult to depict as skewed or not as the possibility existed for negative values; therefore, we entered all of the results of this outcome into meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses for primary outcome where possible, for the following a priori reasons. Results were interpreted with caution, since multiple comparisons could lead to false positive conclusions (Oxman 1992).
1. Fluvoxamine dosing (fixed low dosage, fixed standard dosage, fixed high dosage; flexible low dosage, flexible standard dosage, flexible high dosage)
Existing evidence implies that low dosage antidepressants may be associated with better outcomes ‐ both in terms of effectiveness and side effects ‐ than standard or high dosage antidepressants (Bollini 1999; Furukawa 2002b). In addition, a fixed versus flexible dosing schedule may affect estimates of treatment effectiveness (Khan 2003). In the case of fluvoxamine, based on the Defined Daily Dosage (DDD) by WHO (WHO 2009), low dosage is referred to as <100, standard dosage to >=100 but <200, and high dosage to >=200 mg/day. We categorized studies by intended maximum dosage of fluvoxamine.
2. Comparator dosing (low dosage, standard dosage, and high dosage)
It is easy to imagine that people taking a comparator drug are less likely to complete a study if they are taking a high dosage of the comparator drug. We categorized studies by the intended maximum dose of fluvoxamine based on the DDD. Since WHO 2009 does not report DDD of milnacipran, we categorized these studies based on previous reports (Lecrubier 1996; Lopez‐Ibor 1996; Okamura 2006), where low dosage refers to <100, standard dosage to >=100 but <150, and high dosage to >=150 mg/day.
3. Depression severity (severe major depression, moderate/mild major depression)
"Severe major depression" was defined by a threshold baseline severity score for entry of 25 or more for HRSD and 31 or more for MADRS (Dozois 2004; Muller 2003).
4. Treatment settings (psychiatric inpatients, psychiatric outpatients, primary care)
Because depressive disorder in primary care has a different profile than that of psychiatric inpatients or outpatients (Suh 1997); it is possible that results obtained from either of these settings may not be applicable to the other settings (Depression Guideline Panel 1993).
5. Elderly patients (>=65 years of age), separately from other adult patients
Older people may be more vulnerable to side effects associated with antidepressants and decreased dosage is often recommended for them (Depression Guideline Panel 1993).
Because the number of a priori planned subgroup analyses now appears excessive in comparison with the identified studies, we will consider reducing the number of subgroup analyses or adjusting the level of significance to account for making multiple comparisons in the next update.
Sensitivity analysis
The following sensitivity analyses for primary outcome were planned a priori. By limiting the included studies to those with higher quality (analysis 1 to 5) or to those free from some "bias" (analysis 6 to 9), we examined whether the results changed and we intended to check for the robustness of the observed findings.
1. We excluded trials with unclear concealment of random allocation and/or unclear double blinding.
2. We excluded trials with a dropout rate greater than 20%.
3. We performed the worst‐case scenario ITT: that all patients in the experimental group experience the negative outcome and all those in the comparison group experience the positive outcome.
4. We performed the best‐case scenario ITT: that all patients in the experimental group experienced the positive outcome and all those in the comparison group experienced the negative outcome.
5. We excluded trials for which the response rates had to be calculated based on the imputation method (Furukawa 2005) and for which the SD had to be borrowed from other trials (Furukawa 2006).
6. We examined a "wish bias" by comparing the trials where fluvoxamine was used as an investigational drug, the drug that was used as a new compound, to the trials where fluvoxamine was used as a comparator, since some evidence suggests that a new antidepressant might perform worse when used as a comparator than when used as an investigational agent (Barbui 2004).
7. We excluded trials funded by, or with at least one author affiliated with, a pharmaceutical company marketing fluvoxamine. This sensitivity analysis is particularly important in light of the recent repeated findings that funding strongly affects outcomes of research studies (Als‐Nielsen 2003; Bhandari 2004; Lexchin 2003; Montgomery 2004; Perlis 2005; Procyshyn 2004) and because industry sponsorship and authorship of clinical trials have increased over the past 20 years (Buchkowsky 2004).
8. We excluded studies that included patients with bipolar depression.
9. We excluded trials studies that included patients with psychotic features.
Our routine application of random effects and fixed‐effect models, as well as our secondary outcomes of remission rates and continuous severity measures, may be considered additional forms of sensitivity analyses.
If the CIs of ORs in the groups did not overlap, potential sources of heterogeneity were investigated.
Results
Description of studies
Results of the search
Initially, we identified 152 references considered relevant for our review. Of these, five trials were unpublished (Coleman 1981a; Coleman 1981b; Coleman 1983; Doogan 1981; van Beek 1981), and one trial was written in Hungarian and was not retrieved. These trials has been placed on the list of studies awaiting assessment (Faludi 1989). The remaining 146 references were retrieved for more detailed evaluation (Figure 1).
1.
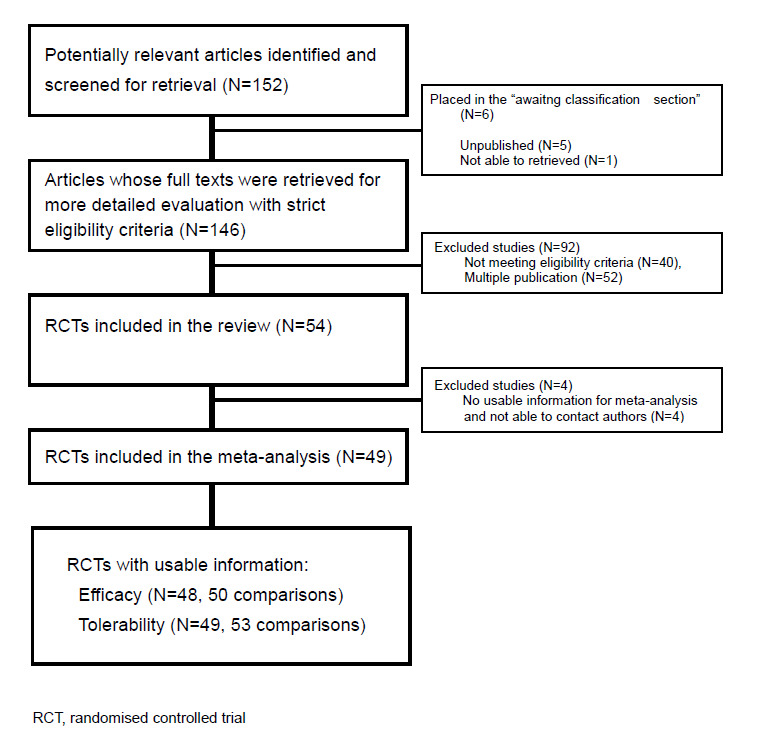
Flow diagram for the trials
Additional searches conducted on the 26th October 2009 (CCDAN Registers up‐to‐date as of 31 August 2008) found 6 trials (Berlin 1998, Donovan 1993, Entsuah 2002b, Mallick 2003, Naito 2007, Ushiroyama 2004) which might be included in the an update of this review. We have placed these articles on the list of studies awaiting assessment.
Included studies
See: Included studies, Figure 1. We were able to include 54 studies (56 comparisons). All studies included were randomised trials. The inter‐rater reliability in selection of trials was satisfactory, with weighted kappa of 0.77.
We mailed or e‐mailed or phoned the authors with known contact details to obtain extra information we sought. We had replies from the authors of 23 trials. Among them, we were able to obtain unpublished data from 15 trials including 17 comparisons (Ansseau 1991a; Ansseau 1991b; Barge‐Schaapveld 1995; Cassano 1986; Dalery 2003; Hackett 1998a; Hackett 1998b; Kasper 1990; Kato 2006; Nathan 1990; Nemeroff 1995; Otsubo 2005; Rechlin 1994; Remick 1994; Rossini 2005; Schoemaker 2002; Ueda 2002).
1. Length of studies
Duration of treatment was relatively brief, with a mean of 5.5 weeks (range 2 to 10 weeks). There was one 2‐week study, 20 4‐week studies, 24 6‐week studies, five 7‐week studies, three 8‐week studies and one 10‐week study.
2. Setting
For 18 studies, treatment occurred in a psychiatric inpatient setting; for 21 studies, treatment occurred in a psychiatric outpatient setting; and in seven studies, treatment occurred in a combined inpatient/outpatient setting. Two studies were based on primary care settings (Barge‐Schaapveld 1995; Moon 1991), and six did not specify their treatment settings.
3. Participants
All trials reported that participants suffered from major depression defined by operationalised diagnostic criteria; however, some studies included less than 20% of patients with bipolar depression (Ansseau 1991a; Ansseau 1991b; Asakura 2005; de Wilde 1983; Guy 1984; Haffmans 1996; Itil 1983; Kasper 1990; March 1990; Murasaki 1998a; Ottevanger 1995; Rossini 2005). In addition, 18 studies used diagnostic criteria such as "major depressive episode" (DSM‐III or IV) , "major affective disorder" (DSM‐III), "depression" (Feighner criteria), or "unipolar or bipolar disorder" (Feighner criteria), and did not exclude patients with bipolar depression. Consequently, some studies might include patients with bipolar depression who were not specifically taken into account (Ansseau 1994; Barrelet 1991; Bocksberger 1993; Bougerol 1992; Brunner 1994; Cassano 1986; Coleman 1982; Dalery 2003; Dick 1983; Gonul 1999; Harris 1991a; Moon 1991; Mullin 1988; Perez 1990; Rahman 1991; Rapaport 1996; Rota 2005; Zohar 2003). Some studies included less than 20% of depressive patients with psychotic features (Ansseau 1991a; Ansseau 1991b; Ansseau 1994; Asakura 2005; Bramanti 1988; Clerc 2001; Haffmans 1996; Kasper 1990). In 25 studies, some elderly subjects (over 65 years old) were included, but the actual number of elderly people was not reported in most trials. One trial was for elderly patients only (Bocksberger 1993), while seven studies did not include any elderly patients. One trial (Claghorn 1996) only included patients with severe depression, defined by a score higher than 25 on the HRSD‐17 at baseline.
4. Study size
Two studies did not report the number of patients included (Kavoussi 1999; Rota 2005). The mean sample size for remaining studies was 93, ranging from 23 (Barge‐Schaapveld 1995) to 481 (Cassano 1986). The majority of the studies (38 RCTs) recruited fewer than 100 participants.
5. Interventions
There were 30 studies comparing fluvoxamine with TCAs, five studies including heterocyclics, 10 including SSRIs, three including SNRIs, four including newer antidepressants, and one comparing fluvoxamine with sulpiride and one four‐arm study comparing fluvoxamine with amitriptyline, doxepine and paroxetine (Rechlin 1994). We could not find studies comparing fluvoxamine with non‐conventional anti‐depressive agents such as herbal products. Regarding fluvoxamine dosing, the trials included five fixed and 43 flexible schedules, and one study did not state dosing schedule. Standard doses were used in 22 studies, and high doses in 28 studies. Four studies did not state fluvoxamine dosing.
6. Outcomes
Of the included 54 studies, five studies (Brown 1986; Gonul 1999; Kavoussi 1999; Rechlin 1994; Rota 2005) did not report efficacy data, and four studies (Kavoussi 1999; Miller 2001;Rechlin 1994; Rota 2005) did not report tolerability data that could be entered into a meta‐analysis. We were unable to obtain further data because we could not contact the authors by any means, nor could we obtain extra information from these authors. The majority of the identified studies (44 studies) used the HRSD as a primary or secondary outcome measure, while a minority of studies used the MADRS and Clinical Global Impression scale (CGI).
Among the 50 studies reporting dropouts due to any reason, 42 reported dropouts due to side effects. Forty‐one studies reported the number of patients experiencing individual side effects. It was unclear how these adverse effects were measured in terms of either severity or duration.
Excluded studies
See: Excluded studies.
By assessing the 146 retrieved full texts, we found 52 articles that were duplicate publications. We assessed the remaining 94 studies for their eligibility and we excluded 40 articles that did not meet our inclusion criteria. Among those excluded, 19 were not randomised trials; three did not use an operationalised criteria to diagnose major depression; three included more than 20 % of participants who suffered from bipolar disorder; four included patients with major depression with psychotic features; five included an Axis I disorder other than major depression or bipolar disorder; two did not compare fluvoxamine with other antidepressants; two had a crossover design and clinical data for the first randomisation period were not reported; one reported clinical data for the maintenance phase only, with no data for the acute phase; one study compared fluvoxamine against imipramine and a placebo, but clinical data were shown for comparison between antidepressants and the placebo, not between fluvoxamine and imipramine.
Risk of bias in included studies
See: Included studies, Figure 2, Figure 3.
2.
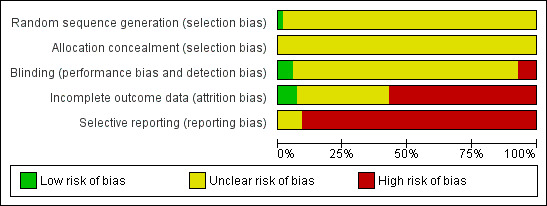
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
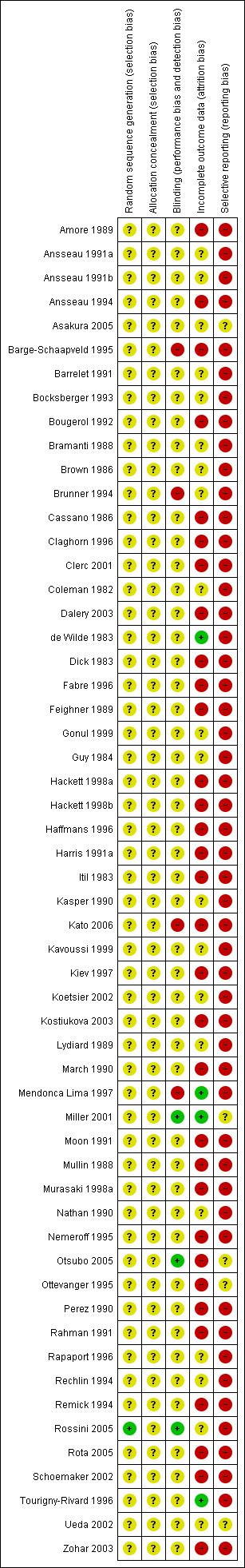
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Our judgment about the overall risk of bias in the individual studies is illustrated in Figure 2 and Figure 3. The methodological quality of these included studies was judged as poor, although judging articles from some time ago by today's standard (Begg 1996; CONSORT) is problematic. Nevertheless, the reporting in these studies was not good. This type of reporting has been associated with an overestimate of the estimate of effect (Schulz 1995) and this should be considered when interpreting the results.
Allocation
Only one study reported the methods of generating random sequence, in which "a computer originated schedule" was used (Rossini 2005). No studies reported the method of allocation concealment. We were not assured that bias was minimised during the allocation procedure, yet 28 studies reported that the participants allocated to each treatment group were "similar", "the same", "not significantly different", "comparable" or "matched" (27/53 studies, 51%).
Blinding
Forty‐three of the trials (80%) described their studies as "double blind"; however, no tests were conducted to ensure successful blinding. We rated only two studies among those 43 trials as having a "low risk of bias" (Miller 2001; Rossini 2005), as blinded raters conducted outcome assessment of those trials. In addition, one "single‐blind" trial (Otsubo 2005) was rated as having a "low risk of bias" because its outcome assessment was blinded to the medication. Four trials were open trials that did not seek blinding (Barge‐Schaapveld 1995; Brunner 1994; Kato 2006; Mendonca Lima 1997).
Incomplete outcome data
Total dropout rate was relatively high, ranging from 0% (de Wilde 1983; Mendonca Lima 1997; Miller 2001; Tourigny‐Rivard 1996) to 59 % (Claghorn 1996). There were twenty‐seven studies (27/54, 50%) where the total dropout rates were more than 20%.
Selective reporting
The study protocol was not available for all studies. Four studies reported only "pituitary‐adrenocortical status" (Brown 1986), "prolactin response to d‐fenfluramine challenge" (Kavoussi 1999), "heart rate" (Rechlin 1994), or "hypothalamic‐pituitary‐adrenocortical axis activity" (Rota 2005) instead of reporting the clinical outcome for each intervention group. One study reported the clinical efficacy outcome only as "we could not find any significant difference" (Gonul 1999). Only ten studies reported SDs of change scores (Asakura 2005; Harris 1991a; Kato 2006; Kiev 1997; Mendonca Lima 1997; Miller 2001; Nemeroff 1995; Otsubo 2005; Schoemaker 2002; Ueda 2002); 26 studies reported SDs of endpoint score of continuous efficacy variables.
Other potential sources of bias
Funding and wish bias
Most of the included studies (38 studies) were funded by industry. Among the 30 trials comparing fluvoxamine to TCAs, a great majority (21 trials) were sponsored by, or had at least one author affiliated with, a pharmaceutical company marketing fluvoxamine, and almost all of the trials (25 trials) set fluvoxamine as an investigational drug. Among the 24 trials comparing fluvoxamine with ADs other than TCAs, pharmaceutical companies marketing fluvoxamine sponsored 8 trials, and a company marketing the comparator drug funded 9 trials; only three trials set fluvoxamine as an investigational drug.
Effects of interventions
Of the 54 included studies (56 comparisons), 48 RCTs (50 comparisons) contributed usable data for the efficacy analyses and 49 RCTs (53 comparisons) did so for the tolerability analyses. No studies reported social adjustment/ functioning, health‐related quality of life, and costs to health care services. ORs for the efficacy data larger than one (falling to the right of the midline) and those for the tolerability data smaller than one indicate a difference in favour of fluvoxamine. Negative SMDs (falling to the left of the midline) indicate a difference in favour of fluvoxamine.
To obtain response rate and remission, we used validated imputation methods, and if SDs were missing, we borrowed from other trials, if possible (See; Table 1, Table 2)
1. Imputation methods and borrowed SD use to obtain response rate.
| Early phase | Acute phase | |||||
| Comparator | Study | Imputation methods | borrowed SD | Imputation methods | borrowed SD | |
| TCAs | Imipramine | Cassano 1986 | Yes | Yes | ‐ | ‐ |
| Amore 1989 | Yes | Yes | ‐ | ‐ | ||
| Lydiard 1989 | Yes | Yes | Yes | Yes | ||
| Bramanti 1988 | Yes | No | ‐ | ‐ | ||
| Claghorn 1996 | Yes | Yes | Yes | Yes | ||
| Fabre 1992 | Yes | Yes | No | Yes | ||
| Feighner 1989 | Yes | Yes | Yes | Yes | ||
| Guy 1984 | Yes | Yes | Yes | Yes | ||
| Itil 1983 | Yes | No | ‐ | ‐ | ||
| Koetsier 2002 | Yes | No | ‐ | ‐ | ||
| March 1990 | Yes | Yes | Yes | Yes | ||
| Miller 2001 | ‐ | ‐ | ‐ | ‐ | ||
| Clomipramine | Coleman 1982 | Yes | Yes | ‐ | ‐ | |
| de Wilde 1983 | Yes | Yes | Yes | Yes | ||
| Dick 1983 | No | No | ‐ | ‐ | ||
| Ottevanger 1995 | Yes | No | ‐ | ‐ | ||
| Zohar 2003 | Yes | Yes | No | Yes | ||
| Amitriptyline | B‐Schaapveld 1995 | No | No | No | No | |
| Harris 1991 | Yes | Yes | Yes | Yes | ||
| Remick 1994 | Yes | No | Yes | No | ||
| Kostiukova 2003 | No | Yes | No | Yes | ||
| Murasaki 1998 | No | No | ‐ | ‐ | ||
| Nortriptyline | Otsubo 2005 | Yes | No | No | No | |
| Dothiepin | Mullin 1988 | Yes | Yes | Yes | Yes | |
| Rahman 1991 | Yes | Yes | Yes | Yes | ||
| Desipramine | Nathan 1990 | Yes | No | ‐ | ‐ | |
| Tourigny Rivard 1996 | ‐ | ‐ | Yes | Yes | ||
| Heterocyclics | Amineptine | Brunner 1994 | Yes | No | ‐ | ‐ |
| Mianserin | Moon 1991 | No | No | No | No | |
| Perez 1990 | Yes | Yes | Yes | Yes | ||
| Maprotoline | Kasper 1989 | Yes | No | ‐ | ‐ | |
| Mendonca Lima 1997 | Yes | Yes | ‐ | ‐ | ||
| SSRIs | Paroxetine | Annseaau 1993 | No | No | No | No |
| Kato 2006 | No | Yes | No | Yes | ||
| Kiev 1997 | Yes | Yes | Yes | No | ||
| Sertraline | Nemeroff 1995 | Yes | Yes | Yes | No | |
| Rossini 2002 | Yes | No | Yes | No | ||
| Fluoxetine | Dalery 1998 | No | Yes | No | Yes | |
| Rapaport 1995 | Yes | Yes | Yes | Yes | ||
| Citalopram | Haffmans 1996 | Yes | Yes | No | Yes | |
| SNRI | Milnacipran | Clerc 2001 | No | No | No | No |
| Ansseau 1991b | No | No | ‐ | ‐ | ||
| Ansseau 1991a | No | No | ‐ | ‐ | ||
| Venlafaxine | Hackett 1998a | Yes | No | Yes | No | |
| Hackett 1998b | Yes | No | Yes | No | ||
| Newer ADs | Mirtazapine | Schoemaker 2002 | No | Yes | No | Yes |
| Moclobemide | Barrelet 1991 | Yes | No | ‐ | ‐ | |
| Bocksberger 1992 | Yes | No | ‐ | ‐ | ||
| Bougerol 1992 | Yes | No | ‐ | ‐ | ||
| Other conventional | Sulpiride | Ueda 2002 | Yes | No | ‐ | ‐ |
2. Imputation methods and borrowed SD use to obtain remission rate.
| Early phase | Acute phase | |||||
| Comparator | Study | Imputation methods | borrowed SD | Imputation methods | borrowed SD | |
| TCAs | Imipramine | Cassano 1986 | Yes | Yes | ‐ | ‐ |
| Amore 1989 | Yes | Yes | ‐ | ‐ | ||
| Lydiard 1989 | Yes | Yes | Yes | Yes | ||
| Bramanti 1988 | Yes | No | ‐ | ‐ | ||
| Claghorn 1996 | Yes | Yes | Yes | Yes | ||
| Fabre 1992 | Yes | Yes | Yes | Yes | ||
| Feighner 1989 | Yes | Yes | Yes | Yes | ||
| Guy 1984 | Yes | Yes | Yes | Yes | ||
| Itil 1983 | No | No | ‐ | ‐ | ||
| Koetsier 2002 | Yes | No | ‐ | ‐ | ||
| March 1990 | Yes | Yes | Yes | Yes | ||
| Miller 2001 | ‐ | ‐ | ‐ | ‐ | ||
| Clomipramine | Coleman 1982 | Yes | Yes | ‐ | ‐ | |
| de Wilde 1983 | Yes | Yes | Yes | Yes | ||
| Dick 1983 | No | No | ‐ | ‐ | ||
| Ottevanger 1995 | Yes | No | ‐ | ‐ | ||
| Zohar 2003 | Yes | Yes | Yes | Yes | ||
| Amitriptyline | B‐Schaapveld 1995 | No | No | No | No | |
| Harris 1991 | Yes | Yes | Yes | Yes | ||
| Remick 1994 | Yes | No | Yes | No | ||
| Kostiukova 2003 | Yes | Yes | Yes | Yes | ||
| Murasaki 1998 | Yes | No | ‐ | ‐ | ||
| Nortriptyline | Otsubo 2005 | Yes | No | No | No | |
| Dothiepin | Mullin 1988 | Yes | Yes | Yes | Yes | |
| Rahman 1991 | Yes | Yes | Yes | Yes | ||
| Desipramine | Nathan 1990 | Yes | No | ‐ | ‐ | |
| Tourigny Rivard 1996 | ‐ | ‐ | Yes | Yes | ||
| Heterocyclics | Amineptine | Brunner 1994 | Yes | No | ‐ | ‐ |
| Mianserin | Moon 1991 | No | No | No | No | |
| Perez 1990 | Yes | Yes | Yes | Yes | ||
| Maprotoline | Kasper 1989 | Yes | No | ‐ | ‐ | |
| Mendonca Lima 1997 | Yes | Yes | ‐ | ‐ | ||
| SSRIs | Paroxetine | Annseaau 1993 | Yes | No | Yes | No |
| Kato 2006 | Yes | Yes | Yes | Yes | ||
| Kiev 1997 | Yes | Yes | Yes | No | ||
| Sertraline | Nemeroff 1995 | Yes | Yes | Yes | No | |
| Rossini 2002 | No | No | No | No | ||
| Fluoxetine | Dalery 1998 | Yes | Yes | Yes | Yes | |
| Rapaport 1995 | Yes | Yes | Yes | Yes | ||
| Citalopram | Haffmans 1996 | Yes | Yes | No | Yes | |
| SNRI | Milnacipran | Clerc 2001 | Yes | No | Yes | No |
| Ansseau 1991b | Yes | No | ‐ | ‐ | ||
| Ansseau 1991a | Yes | No | ‐ | ‐ | ||
| Venlafaxine | Hackett 1998a | No | No | No | No | |
| Hackett 1998b | No | No | No | No | ||
| Newer ADs | Mirtazapine | Schoemaker 2002 | Yes | Yes | Yes | Yes |
| Moclobemide | Barrelet 1991 | Yes | No | ‐ | ‐ | |
| Bocksberger 1992 | Yes | No | ‐ | ‐ | ||
| Bougerol 1992 | Yes | No | ‐ | ‐ | ||
| Other conventional | Sulpiride | Ueda 2002 | Yes | No | ‐ | ‐ |
1. FLUVOXAMINE versus TCAs
Twenty‐eight RCTs contributed usable data for the efficacy analyses and 28 RCTs for the tolerability analyses. Twenty‐one trials reported dichotomous data for a number of patients who experienced each side effect.
1.1 Response ‐ acute phase (between 6 and 12 weeks); Primary outcome
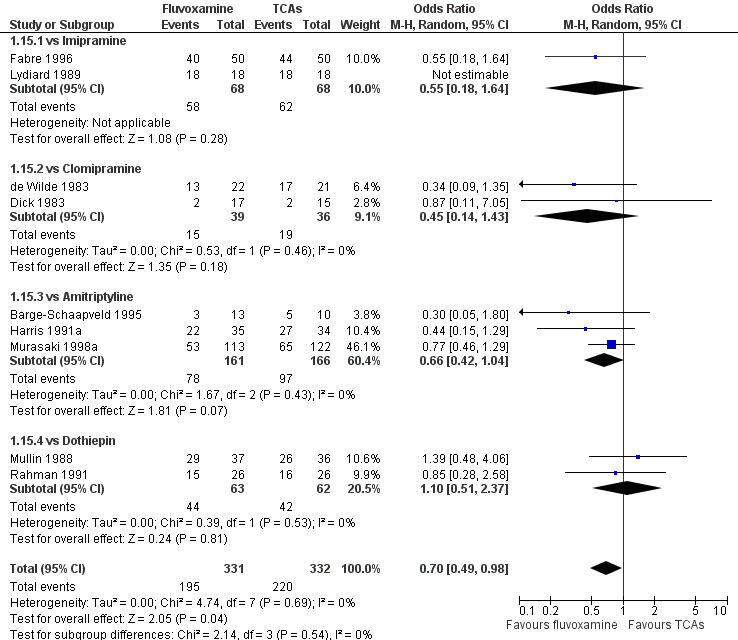
Sixteen studies reported this outcome. Among them, imputation methods were used for 11 studies (Claghorn 1996; de Wilde 1983; Feighner 1989; Guy 1984; Harris 1991a; Lydiard 1989; March 1990; Mullin 1988; Rahman 1991; Remick 1994; Tourigny‐Rivard 1996). There was no strong evidence that fluvoxamine was either superior or inferior to TCAs except desipramine in terms of this dichotomous outcome in head‐to‐head comparisons. However, desipramine were less effective than fluvoxamine based on one small trial (OR: 4.22, 95% CI 0.98 to 18.13, P=0.05; 1 trial, 47 participants) (Analysis 1.1, Figure 4).
1.1. Analysis.
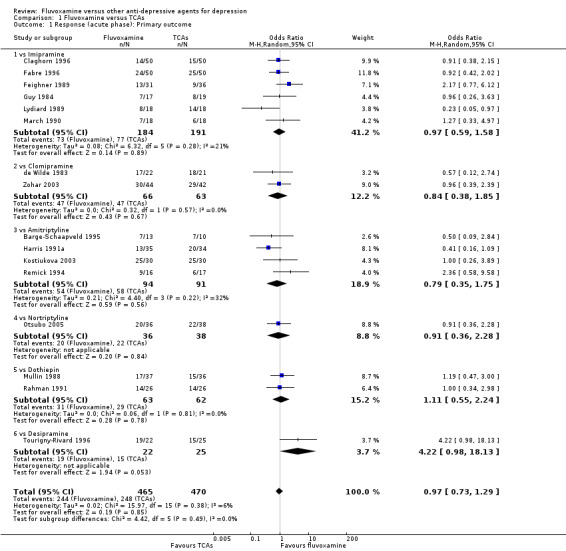
Comparison 1 Fluvoxamine versus TCAs, Outcome 1 Response (acute phase): Primary outcome.
4.
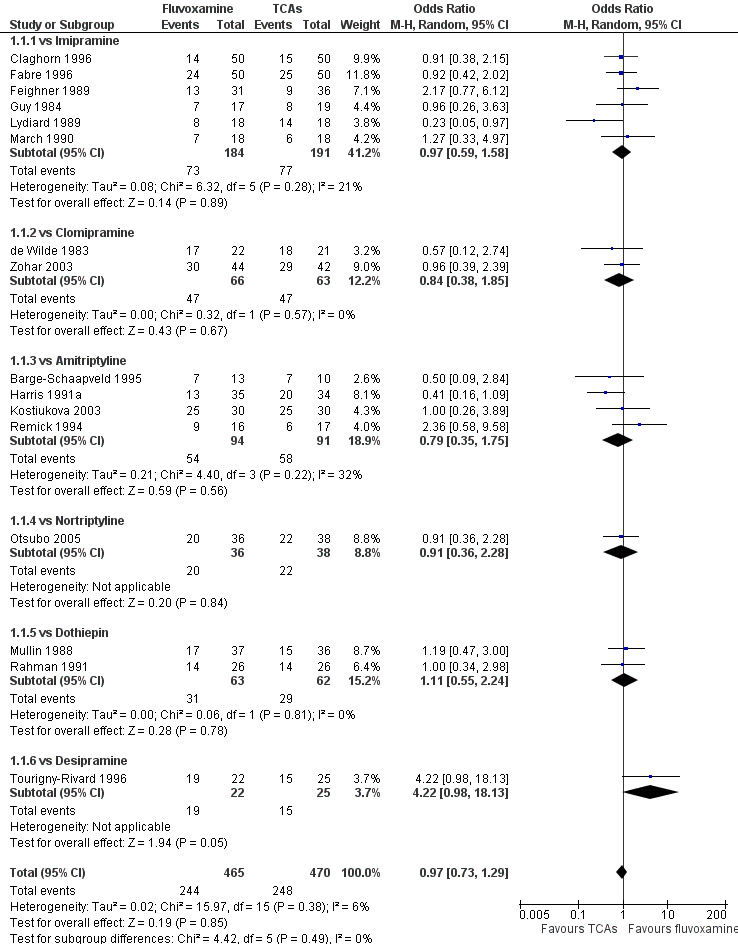
Forest plot of comparison: 1 Fluvoxamine vs TCAs, outcome: 1.1 Response (acute phase).
1.2 Response ‐ early phase and follow‐up phase
1.2.1 Early phase (between 1 and 4 weeks)
No strong evidence indicated that fluvoxamine was either superior or inferior to TCAs in terms of the dichotomous outcome between fluvoxamine and TCAs in head‐to‐head comparisons (Figure 5).
5.
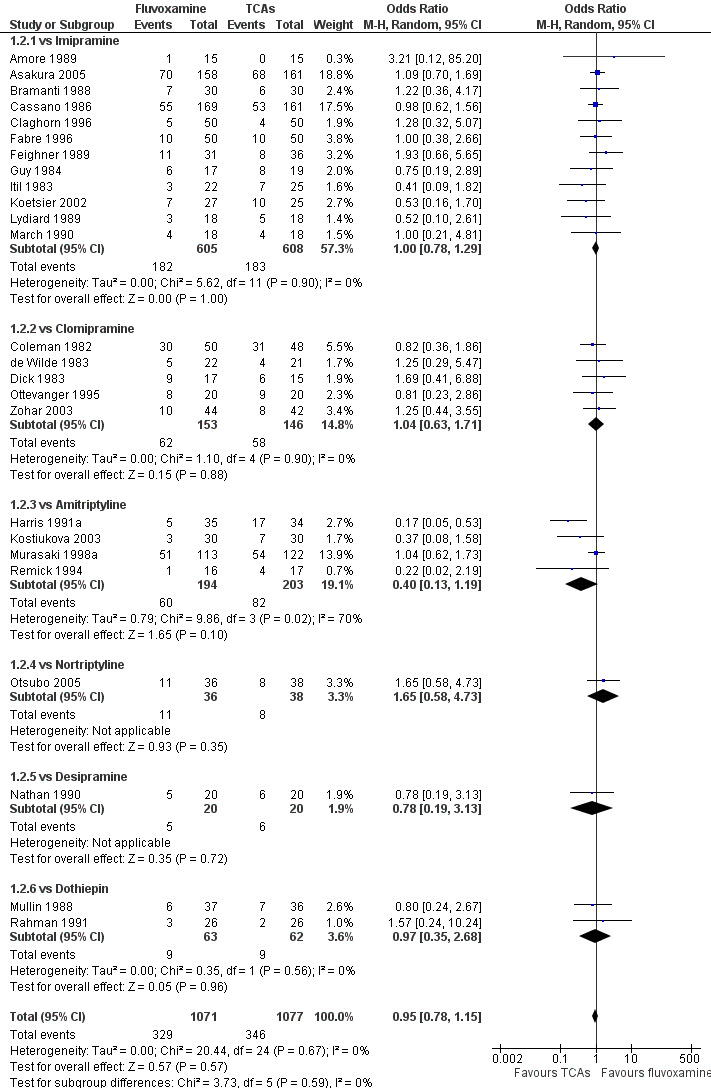
Forest plot of comparison: 1 Fluvoxamine vs TCAs, outcome: 1.2 Response (early phase).
Substantial heterogeneity existed between trials comparing fluvoxamine to amitriptyline, based on four trials (I2= 70 %, P = 0.02, Analysis 1.2). Visual inspection revealed that, among these studies, three smaller ones using the imputation methods for response (Harris 1991a; Kostiukova 2003; Remick 1994) reported results favourable to amitriptyline. However, because of the small number of trials, sources of the heterogeneity cannot be further explained.
1.2. Analysis.
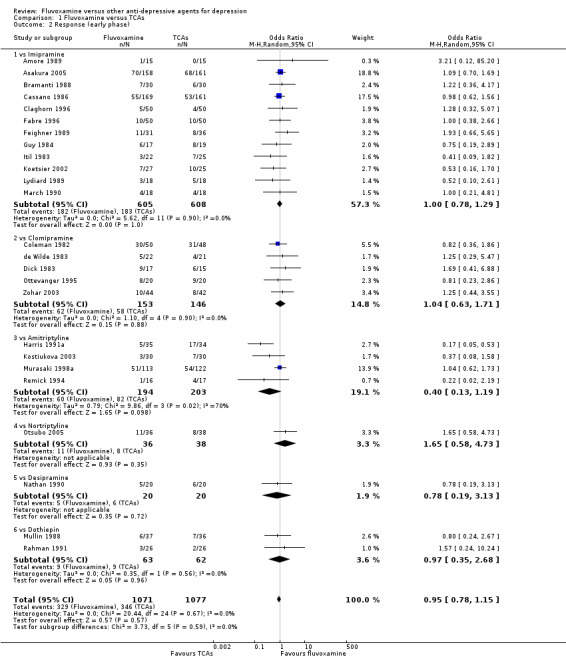
Comparison 1 Fluvoxamine versus TCAs, Outcome 2 Response (early phase).
1.2.2 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
1.3 Remission
1.3.1 Early phase (between 1 and 4 weeks)
In terms of remission at the end of the early phase, the analysis found no strong evidence that fluvoxamine was either superior or inferior to TCA in head‐to‐head comparisons (Analysis 1.3).
1.3. Analysis.
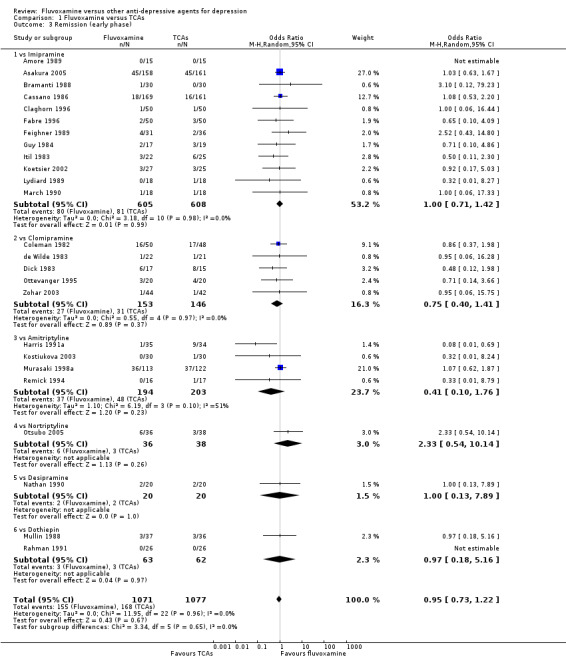
Comparison 1 Fluvoxamine versus TCAs, Outcome 3 Remission (early phase).
1.3.2 Acute phase (between 6 and 12 weeks)
Fluvoxamine was found to be more effective than desipramine based on one trial (OR: 4.50, 95% CI 1.31 to 15.42, P=0.02; 1 trial, 47 participants) (Tourigny‐Rivard 1996); this small study did not report the actual number of patients who experienced remission, so we converted HRSD data expressed as mean and SD into the number of remitted patients, according to the validated imputation method (Furukawa 2005) (Figure 6).
6.
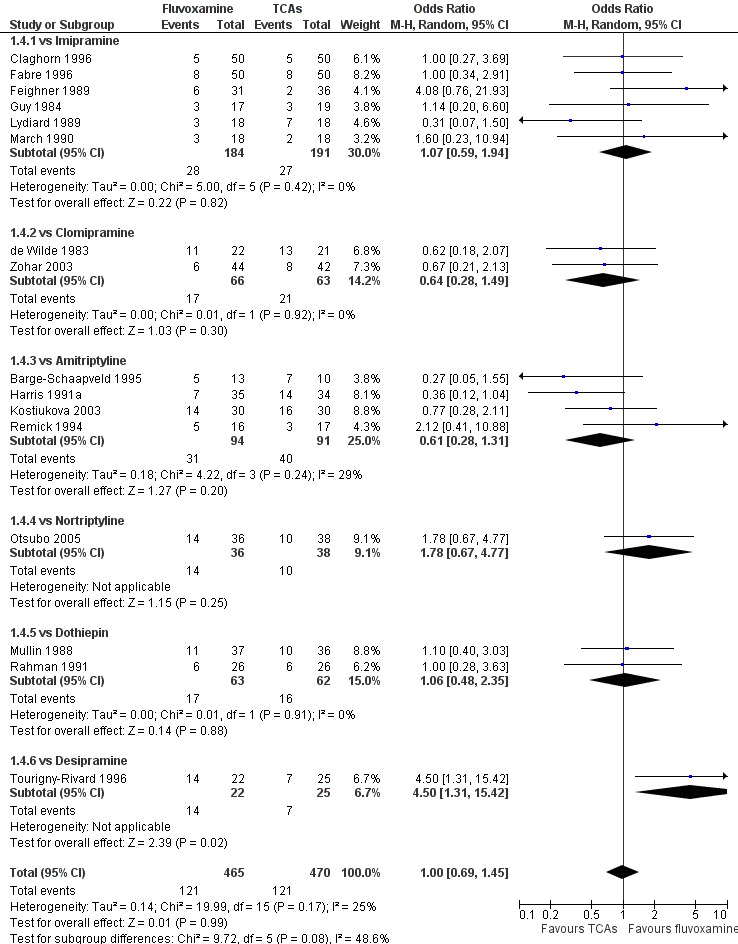
Forest plot of comparison: 1 Fluvoxamine vs TCAs, outcome: 1.4 Remission (acute phase).
1.3.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
1.4 Endpoint score on depression scale
1.4.1 Early phase (between 1 and 4 weeks)
We meta‐analysed non‐skewed data only from 5 trials, and no strong evidence emerged that fluvoxamine was either superior or inferior to TCA as a class or in head‐to‐head comparisons (Analysis 1.5). However, data were skewed in six trials, and SDs were missing in 10 trials. We did not meta‐analyse these data, and presented them descriptively (Analysis 1.6).
1.5. Analysis.
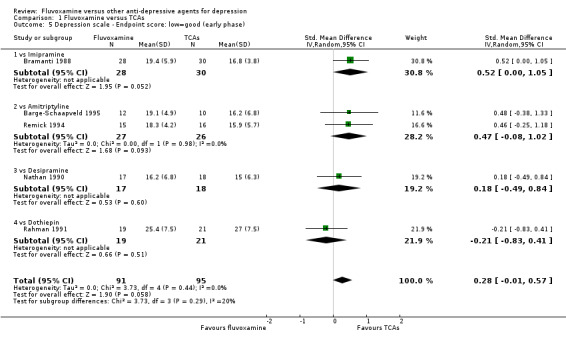
Comparison 1 Fluvoxamine versus TCAs, Outcome 5 Depression scale ‐ Endpoint score: low=good (early phase).
1.6. Analysis.
Comparison 1 Fluvoxamine versus TCAs, Outcome 6 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Imipramine | ||||||||
| Amore 1989 | HRSD‐21 | 22.6 | missing | 15 | 25.4 | missing | 11 | |
| Asakura 2005 | HRSD‐17 | 10.7 | 7.0 | 149 | 10.6 | 7.1 | 149 | skewed. |
| Cassano 1986 | HRSD‐17 | 13.4 | missing | 120 | 13.7 | missing | 119 | |
| Claghorn 1996 | HRSD‐21 | 21.1 | missing | 44 | 21.3 | missing | 44 | |
| Fabre 1996 | HRSD‐21 | 19 | missing | 46 | 18.3 | missing | 48 | |
| Feighner 1989 | HRSD‐17 | 12.5 | missing | 21 | 16.6 | missing | 27 | |
| Guy 1984 | HRSD‐17 | 14.2 | missing | 17 | 13 | missing | 16 | |
| Itil 1983 | HRSD‐16 | 12.7 | 8.2 | 9 | 10.4 | 6.8 | 14 | skewed. |
| Koetsier 2002 | HRSD‐17 | 19.4 | 9.7 | 27 | 15.5 | 8.0 | 25 | skewed. |
| Lydiard 1989 | HRSD‐17 | 18.6 | missing | 17 | 17.2 | missing | 18 | |
| March 1990 | HRSD‐17 | 15.9 | missing | 13 | 17.1 | missing | 15 | |
| vs Clomipramine | ||||||||
| Coleman 1982 | HRSD‐17 | 8.8 | missing | 41 | 8.6 | missing | 43 | |
| Ottevanger 1995 | HRSD‐17 | 15.1 | 8.1 | 20 | 13.9 | 7.4 | 20 | skewed. |
| vs Amitriptyline | ||||||||
| Harris 1991a | HRSD‐17 | 17.1 | missing | 29 | 10.0 | missing | 29 | |
| Murasaki 1998a | HRSD‐17 | 14.5 | 7.8 | 80 | 16.1 | 7.6 | 83 | skewed. |
| vs Nortriptyline | ||||||||
| Otsubo 2005 | HRSD‐17 | 13.5 | 7.7 | 32 | 15.7 | 6.5 | 29 | skewed. |
1.4.2 Acute phase (between 6 and 12 weeks)
Sixteen trials reported this outcome. However, we did not meta‐analyse these data (data were skewed in three trials, and SDs were missing in 13 trials). We presented them descriptively (Analysis 1.7).
1.7. Analysis.
Comparison 1 Fluvoxamine versus TCAs, Outcome 7 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Imipramine | ||||||||
| Claghorn 1996 | HRSD‐21 | 16 | missing | 44 | 15.5 | missing | 44 | |
| Fabre 1996 | HRSD‐21 | 14.2 | missing | 46 | 14.3 | missing | 48 | |
| Feighner 1989 | HRSD‐17 | 10.5 | missing | 21 | 16.4 | missing | 27 | |
| Guy 1984 | HRSD‐17 | 13.0 | missing | 16 | 12.1 | missing | 16 | |
| Lydiard 1989 | HRSD‐17 | 12.8 | missing | 17 | 9.0 | missing | 18 | |
| March 1990 | HRSD‐17 | 12.5 | missing | 13 | 13.8 | missing | 15 | |
| vs Clomipramine | ||||||||
| de Wilde 1983 | HRSD‐17 | 6.4 | missing | 21 | 5.1 | missing | 21 | |
| Zohar 2003 | HRSD‐17 | 13.4 | missing | 42 | 12.3 | missing | 42 | |
| vs Amitriptyline | ||||||||
| Barge‐Schaapveld 1995 | HRSD‐17 | 10.0 | 5.2 | 11 | 8.6 | 8.0 | 10 | skewed. |
| Harris 1991a | HRSD‐17 | 10.4 | missing | 6.6 | missing | 26 | ||
| Kostiukova 2003 | HRSD‐17 | 7.4 | missing | 30 | 6.7 | missing | 30 | |
| Remick 1994 | HRSD‐17 | 8.7 | 7.2 | 13 | 10.3 | 6.0 | 10 | skewed. |
| vs Nortriptyline | ||||||||
| Otsubo 2005 | HRSD‐17 | 11.9 | 8.9 | 36 | 12.1 | 5.9 | 38 | skewed. |
| vs Dothiepin | ||||||||
| Mullin 1988 | HRSD‐17 | 8.3 | missing | 26 | 8.5 | missing | 24 | |
| Rahman 1991 | MADRS | 13.5 | missing | 19 | 14.4 | missing | 21 | |
| vs Desipramine | ||||||||
| Tourigny‐Rivard 1996 | HRSD‐17 | 4.9 | missing | 22 | 10.8 | missing | 25 | |
1.4.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
1.5. Change score on depression scale
1.5.1 Early phase (between 1 and 4 weeks)
We meta‐analysed this outcome and found evidence that fluvoxamine was inferior to amitriptyline (SMD: 1.17, 95% CI 0.61 to 1.73, P<0.0001; 1 trial, 58 participants) (Analysis 1.8). However, four other trials that compared fluvoxamine with amitriptyline did not report SDs for this outcome and we did not meta‐analyse these data (Analysis 1.9).
1.8. Analysis.
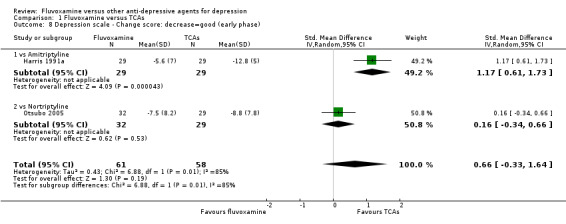
Comparison 1 Fluvoxamine versus TCAs, Outcome 8 Depression scale ‐ Change score: decrease=good (early phase).
1.9. Analysis.
Comparison 1 Fluvoxamine versus TCAs, Outcome 9 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Imipramine | ||||||||
| Amore 1989 | HRSD‐21 | ‐3.1 | missing | 15 | ‐3.4 | missing | 11 | |
| Bramanti 1988 | HRSD‐21 | ‐11.1 | missing | 28 | ‐10.4 | missing | 30 | |
| Cassano 1986 | HRSD‐17 | ‐12.2 | missing | 120 | ‐12.2 | missing | 119 | |
| Claghorn 1996 | HRSD‐21 | ‐5 | missing | 44 | ‐4.6 | missing | 44 | |
| Fabre 1996 | HRSD‐21 | ‐8.7 | missing | 46 | ‐8.2 | missing | 48 | |
| Feighner 1989 | HRSD‐17 | ‐12.5 | missing | 21 | ‐10.6 | missing | 27 | |
| Guy 1984 | HRSD‐17 | ‐10.4 | missing | 17 | ‐12.1 | missing | 16 | |
| Itil 1983 | HRSD‐17 | ‐7.6 | missing | 9 | ‐11.5 | missing | 14 | |
| Koetsier 2002 | HRSD‐17 | ‐7.6 | missing | 27 | ‐12.2 | missing | 25 | |
| Lydiard 1989 | HRSD‐17 | ‐5.9 | missing | 17 | ‐8.2 | missing | 18 | |
| March 1990 | HRSD‐17 | ‐9.1 | missing | 13 | ‐8.4 | missing | 15 | |
| vs Clomipramine | ||||||||
| Coleman 1982 | HRSD‐17 | ‐16.6 | missing | 41 | ‐16 | missing | 43 | |
| de Wilde 1983 | HRSD‐17 | ‐6.9 | missing | 22 | ‐6.5 | missing | 21 | |
| Dick 1983 | HRSD‐17 | ‐11.7 | missing | 15 | ‐8.6 | missing | 13 | |
| Ottevanger 1995 | HRSD‐17 | ‐11.3 | missing | 20 | ‐11.8 | missing | 20 | |
| Zohar 2003 | HRSD‐17 | ‐10.8 | missing | 42 | ‐9.8 | missing | 42 | |
| vs Amitriptyline | ||||||||
| Barge‐Schaapveld 1995 | HRSD‐17 | ‐6.6 | missing | 12 | ‐8.2 | missing | 10 | |
| Kostiukova 2003 | HRSD‐17 | ‐5.3 | missing | 30 | ‐8.6 | missing | 30 | |
| Murasaki 1998a | HRSD‐17 | ‐8.4 | missing | 80 | ‐7.5 | missing | 83 | |
| Remick 1994 | HRSD‐17 | ‐6.6 | missing | 13 | ‐6.9 | missing | 9 | |
| vs Desipramine | ||||||||
| Nathan 1990 | HRSD‐17 | ‐8.4 | missing | 17 | ‐9.3 | missing | 18 | |
| vs Dothiepine | ||||||||
| Mullin 1988 | HRSD‐17 | ‐6.5 | missing | 26 | ‐7.2 | missing | 24 | |
| Rahman 1991 | MADRS | ‐10.2 | missing | 19 | ‐7.9 | missing | 21 | |
1.5.2 Acute phase (between 6 and 12 weeks).
We meta‐analysed non‐skewed data only from 3 trials, and there was no strong evidence that fluvoxamine was superior or inferior to TCA as a class or in head‐to‐head comparisons (Analysis 1.10). However, SDs were missing in 14 trials and we did not meta‐analyse these data, and presented them descriptively instead (Analysis 1.11).
1.10. Analysis.
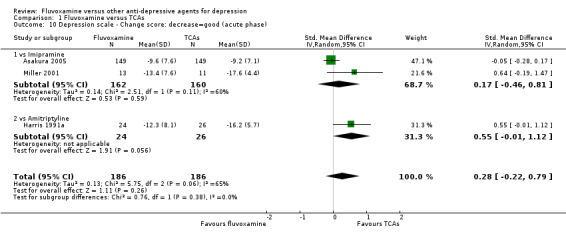
Comparison 1 Fluvoxamine versus TCAs, Outcome 10 Depression scale ‐ Change score: decrease=good (acute phase).
1.11. Analysis.
Comparison 1 Fluvoxamine versus TCAs, Outcome 11 Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Imipramine | ||||||||
| Claghorn 1996 | HRSD‐21 | ‐10.1 | missing | 44 | ‐10.4 | missing | 44 | |
| Fabre 1996 | HRSD‐21 | ‐13.5 | missing | 46 | ‐12.2 | missing | 48 | |
| Feighner 1989 | HRSD‐17 | ‐14.5 | missing | 21 | ‐10.8 | missing | 27 | |
| Guy 1984 | HRSD‐17 | ‐11.6 | missing | 16 | ‐13 | missing | 16 | |
| Lydiard 1989 | HRSD‐17 | ‐11.7 | missing | 17 | ‐17.4 | missing | 18 | |
| March 1990 | HRSD‐17 | ‐12.5 | missing | 13 | ‐11.7 | missing | 15 | |
| vs Clomipramine | ||||||||
| de Wilde 1983 | HRSD‐17 | ‐17 | missing | 21 | ‐19.1 | missing | 21 | |
| Zohar 2003 | HRSD‐17 | ‐17.2 | missing | 42 | ‐18.2 | missing | 42 | |
| vs Amitriptyline | ||||||||
| Barge‐Schaapveld 1995 | HRSD‐17 | ‐15.7 | missing | 11 | ‐15.8 | missing | 10 | |
| Kostiukova 2003 | HRSD‐17 | ‐18.9 | missing | 30 | ‐19 | missing | 30 | |
| Remick 1994 | HRSD‐17 | ‐16.3 | missing | 13 | ‐14.3 | missing | 9 | |
| vs Desipramine | ||||||||
| Nathan 1990 | HRSD‐17 | ‐19.2 | missing | 22 | ‐13.5 | missing | 25 | |
| vs Dothiepine | ||||||||
| Mullin 1988 | HRSD‐17 | ‐13 | missing | 26 | ‐12.7 | missing | 24 | |
| Rahman 1991 | MADRS | ‐22.1 | missing | 19 | ‐20.5 | missing | 21 | |
1.5.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
1.6 Tolerability
1.6.1 Dropout
No strong evidence emerged that fluvoxamine was more or less acceptable in terms of the total numbers of dropouts for any reason, a proxy measure of tolerability, between fluvoxamine and TCAs in head‐to‐head comparisons (Figure 7). Similarly, regarding patients who dropped out because of inefficacy, no strong evidence emerged that fluvoxamine was superior or inferior to TCAs in head‐to‐head comparisons (Analysis 1.13). The analysis of dropouts due to side effects revealed that amitriptyline (OR: 0.59, 95% CI 0.35 to 1.00, P=0.05; 5 trials, 420 participants) and TCA as a class (OR: 0.79, 95% CI 0.60 to 1.04, P=0.09; 21 trials, 1772 participants) were less tolerated than fluvoxamine (Analysis 1.14, Figure 8).
7.
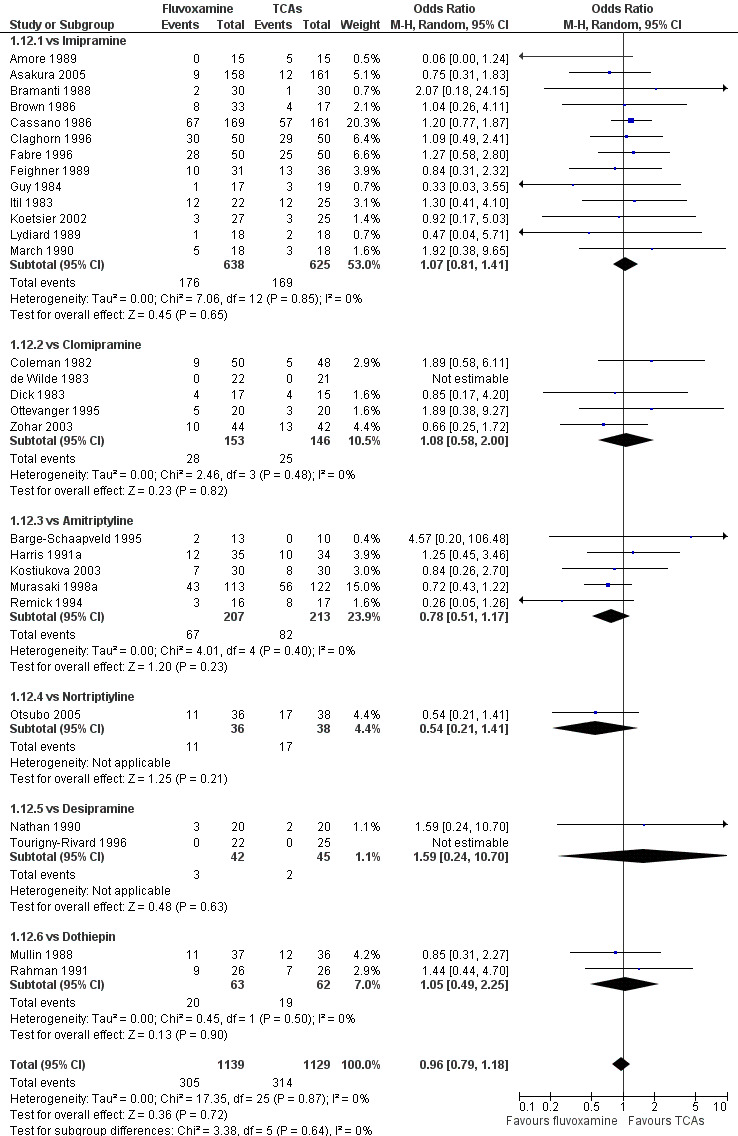
Forest plot of comparison: 1 Fluvoxamine vs TCAs, outcome: 1.11 Total Dropout.
1.13. Analysis.
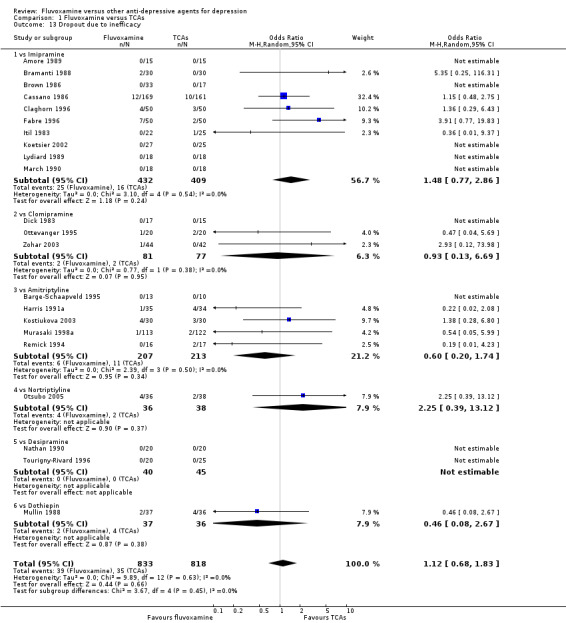
Comparison 1 Fluvoxamine versus TCAs, Outcome 13 Dropout due to inefficacy.
1.14. Analysis.
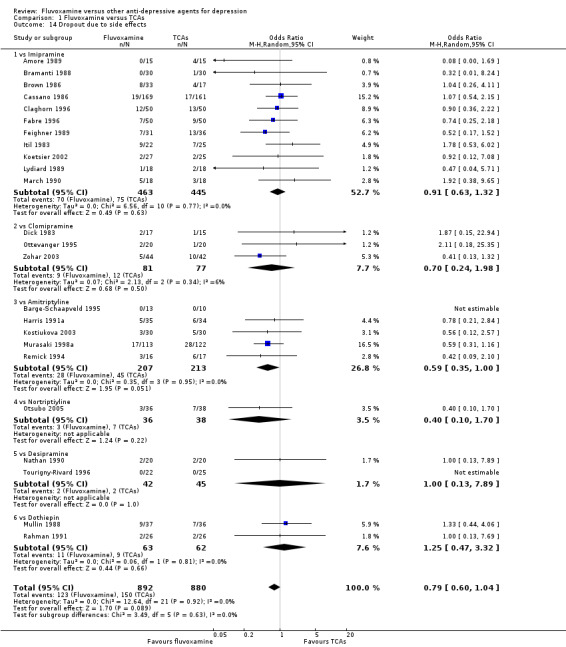
Comparison 1 Fluvoxamine versus TCAs, Outcome 14 Dropout due to side effects.
8.
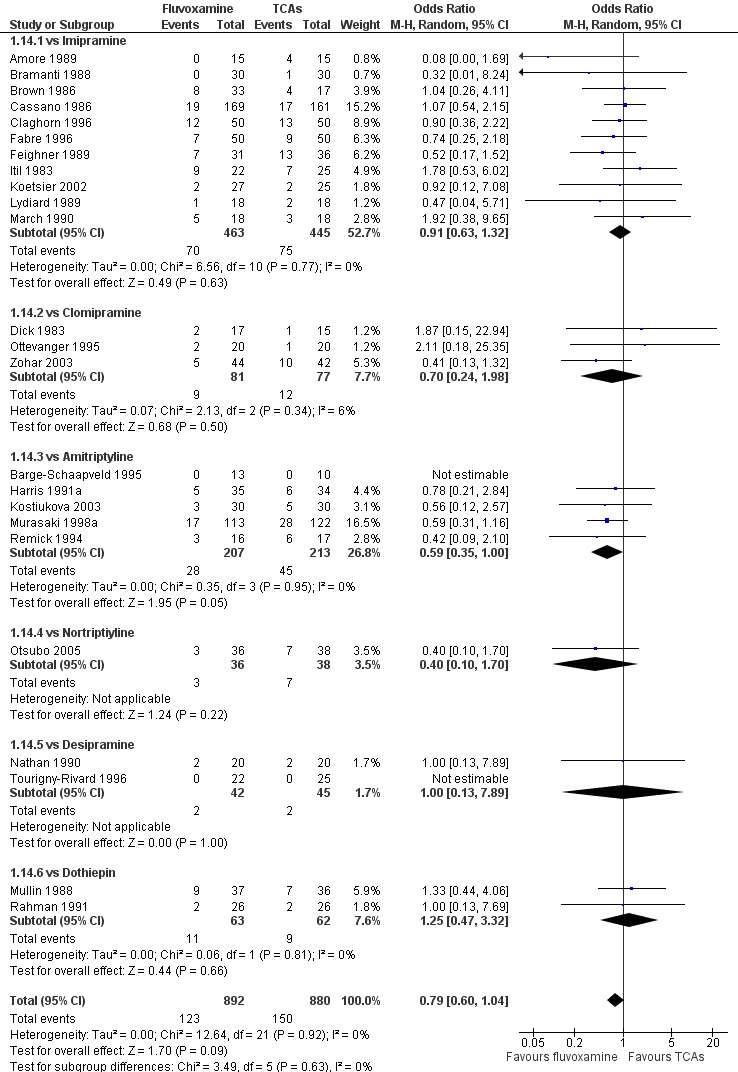
Forest plot of comparison: 1 Fluvoxamine vs TCAs, outcome: 1.13 Dropout due to side effects.
1.6.2 Number of patients experiencing at least one side effect
People allocated to amitriptyline were more likely to have at least one side effect during the trial, even though it was not statistically significant (OR 0.66, 95%CI 0.42 to 1.04, P=0.07; 3 trials, 327 participants) (Analysis 1.15, Figure 9).
9.
Forest plot of comparison: 1 Fluvoxamine vs TCAs, outcome: 1.14 Number of patients experiencing at least one side effect.
1.7 Side effects profile by body system
See: Table 3.
3. Side effect profiles by body system.
| Control drug | Body system | Side effect | N of comparisons | N of participants | OR | 95% CI | NNT | 95% CI |
| versus TCAs | ||||||||
| Imipramine | Cardiovascular | Hypotension / bradycardia | 4 | 560 | 0.24 | 0.10, 0.62 | 16 | 13, 33 |
| Dermatological | Sweating | 7 | 972 | 0.32 | 0.16, 0.66 | 14 | 11, 28 | |
| Gastrointestinal | Dry mouth | 9 | 1055 | 0.24 | 0.16, 0.34 | 4 | 3, 5 | |
| Vomiting / nausea | 9 | 1055 | 2.23 | 1.59, 3.14 | ‐9 | ‐6, ‐17 | ||
| Constipation | 8 | 1008 | 0.50 | 0.27, 0.93 | 11 | 8, 86 | ||
| Diarrhoea | 2 | 136 | 6.38 | 1.27, 32.04 | ‐8 | ‐3, ‐133 | ||
| Neuropsychiatric | Dizziness / vertigo / faintness | 9 | 1055 | 0.24 | 0.15, 0.38 | 7 | 6, 8 | |
| Anxiety / agitation | 5 | 644 | 2.24 | 1.01, 4.97 | ‐17 | ‐6, ‐1893 | ||
| Genitourinary | Problems urinating | 2 | 409 | 0.18 | 0.04, 0.71 | 18 | 15, 51 | |
| Clomipramine | Gastrointestinal | Dry mouth | 3 | 216 | 0.43 | 0.22, 0.81 | 5 | 4, 20 |
| Nausea / vomiting | 3 | 216 | 2.13 | 1.06, 4.27 | ‐9 | ‐4, ‐138 | ||
| Neuropsychiatric | Dizziness / vertigo /faintness | 1 | 86 | 0.21 | 0.05, 0.80 | 6 | 5, 25 | |
| Amitriptyline | Gastrointestinal | Vomiting / nausea | 4 | 387 | 2.86 | 1.31, 6.23 | ‐13 | ‐6, ‐68 |
| Neuropsychiatric | Dizziness / vertigo / faintness | 2 | 304 | 0.31 | 0.11, 0.83 | 7 | 5, 30 | |
| Nortriptyline | Neuropsychiatric | Dizziness / vertigo / faintness | 1 | 74 | 0.22 | 0.07, 0.70 | 4 | 3, 12 |
| Dothiepine | Gastrointestinal | Dry mouth | 1 | 73 | 0.08 | 0.01, 0.70 | 5 | 5, 17 |
| versus Heterocyclics | ||||||||
| Maprtiline | Neuropsychiatric | Dizziness / vertigo / faintness | 1 | 42 | 0.13 | 0.03, 0.56 | 3 | 2, 6 |
| Mianserin | Gastrointestinal | Nausea/vomiting | 2 | 125 | 9.62 | 1.96, 47.30 | ‐5 | ‐2, ‐36 |
| versus SSRIs | ||||||||
| Paroxetine | Dermatological | Sweating | 1 | 60 | 0.22 | 0.05, 0.91 | 5 | 4, 49 |
| versus SNRIs | ||||||||
| Milnacipran | Gastrointestinal | Vomiting / nausea | 3 | 241 | 1.95 | 1.09, 3.50 | ‐7 | ‐4, ‐83 |
| versus newer ADs | ||||||||
| Mirtazapine | Gastrointestinal | Vomiting / nausea | 1 | 412 | 3.43 | 1.90, 6.19 | ‐7 | ‐4, ‐16 |
| Neuropsychiatric | Sleepiness / drowsiness | 1 | 412 | 0.47 | 0.29, 0.76 | 8 | 6, 19 | |
| Agitation / anxiety | 1 | 412 | 0.17 | 0.05, 0.61 | 16 | 14, 35 | ||
| Moclobemide | Gastrointestinal | Dry mouth | 2 | 191 | 4.73 | 1.14, 19.57 | ‐15 | ‐4, ‐336 |
| Vomiting / nausea | 2 | 170 | 2.01 | 1.03, 3.92 | ‐7 | ‐4, ‐178 | ||
| Only results for statistically significant difference were shown. OR, odds ratio. OR < 1 favours fluvoxamine. | ||||||||
All specific side effects were grouped by organ system, as follows: Cardiovascular: hypertension/tachycardia, hypotension/bradycardia. Dermatological: dermatitis/rash, sweating. Gastrointestinal: increased salivation, dry mouth, oral discomfort/taste disturbance, vomiting/nausea, constipation, diarrhoea, weight gain, weight loss, increased appetite, anorexia. Neruopsychiatric: blurred vision, dizziness/vertigo/faintness, fatigue/tiredness/asthenia, headache, tremor, involuntary movement other than tremor, insomnia, sleepiness/drowsiness, agitation/anxiety, manic symptom, completed suicide, suicide wishes/gestures/attempts. Genitourinary: problems urinating, sexual dysfunction.
1.7.1 Cardiovascular side effects
Reasonable evidence indicated that fluvoxamine was less likely to cause hypotension / bradycardia than was imipramine (OR 0.24, 95%CI 0.10 to 0.62, P=0.003; 4 trials, 560 participants) (Analysis 7.2). No strong evidence suggested that fluvoxamine was more or less likely to cause hypertension/tachycardia than TCAs.
7.2. Analysis.
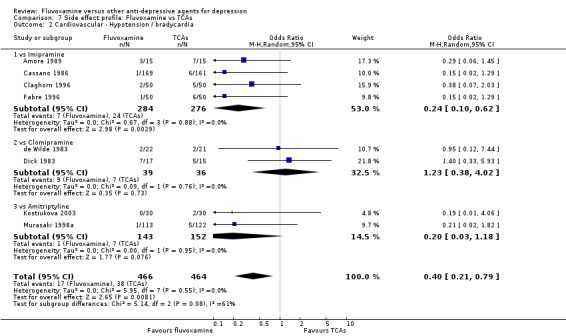
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 2 Cardiovascular ‐ Hypotension / bradycardia.
1.7.2 Dermatological side effects
Sweating was more frequent in imipramine‐treated patients (OR 0.32, 95%CI 0.16 to 0.66, P=0.002; 7 trials, 972 participants) than in fluvoxamine‐treated patients (Analysis 7.4). No strong evidence was apparent to indicate that fluvoxamine was more or less likely to cause dermatitis/rash than were TCAs.
7.4. Analysis.
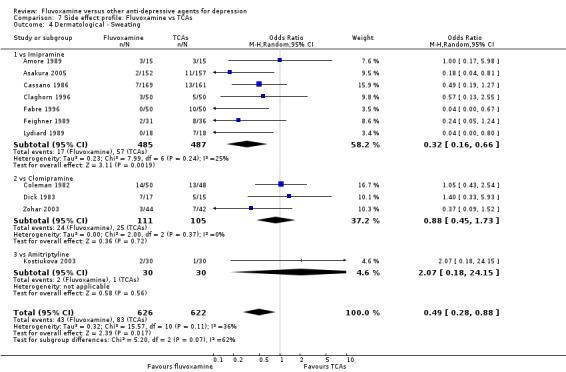
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 4 Dermatological ‐ Sweating.
1.7.3 Gastrointestinal side effects
Dry mouth was more frequent in patients treated with imipramine (OR 0.24, 95%CI 0.16 to 0.34, P<0.001; 9 trials, 1055 participants), clomipramine (OR 0.43, 95%CI 0.22 to 0.81, P=0.009; 7 trials, 972 participants), and dothiepin (OR 0.08, 95%CI 0.01 to 0.70, P=0.02; 1 trial, 972 participants) than in those treated with fluvoxamine (Analysis 7.6). Constipation was more frequent in patients treated with imipramine (OR 0.50, 95%CI 0.27 to 0.93, P=0.03; 8 trials, 1008 participants) (Analysis 7.9).
7.6. Analysis.
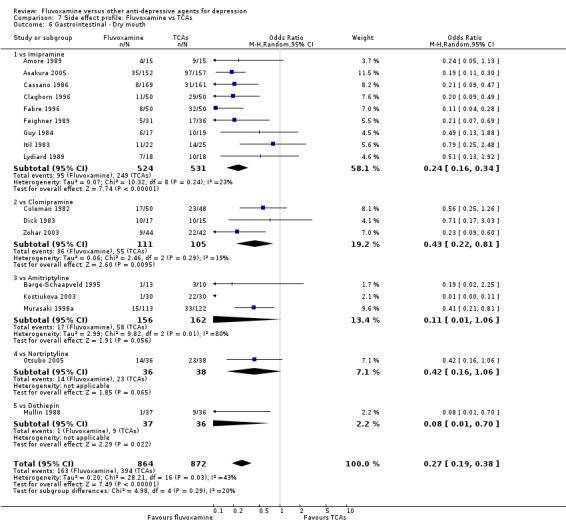
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 6 Gastrointestinal ‐ Dry mouth.
7.9. Analysis.
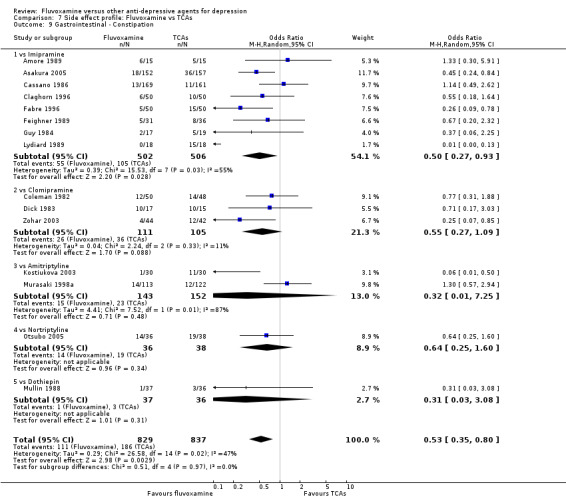
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 9 Gastrointestinal ‐ Constipation.
In contrast, fluvoxamine was associated with higher rates of vomiting/nausea in participants than occurred with imipramine (OR 2.23, 95%CI 1.59 to 3.14, p<0.001; 9 trials, 1055 participants), clomipramine (OR 2.13, 95%CI 1.06 to 4.27, P=0.03; 3 trials, 216 participants), and amitriptyline (OR 2.86, 95%CI 1.31 to 6.23, P=0.008; 4 trials, 387 participants) (Analysis 7.8). Diarrhoea was more frequent in patients treated with fluvoxamine than in those treated with imipramine (OR 6.38, 95%CI 1.27 to 32.04, P=0.02; 2 trials, 136 participants) (Analysis 7.10).
7.8. Analysis.
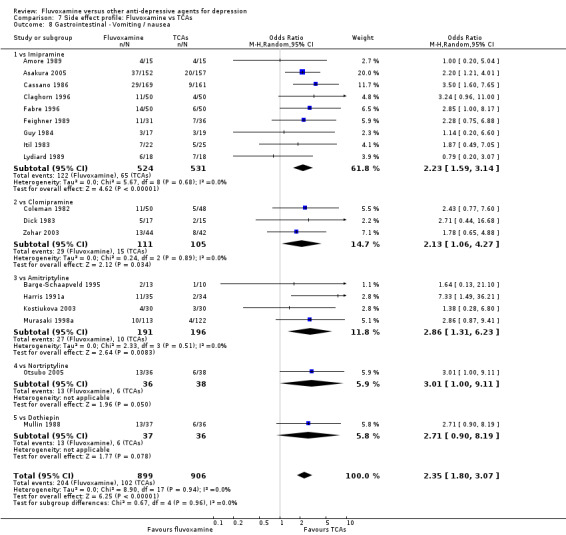
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 8 Gastrointestinal ‐ Vomiting / nausea.
7.10. Analysis.
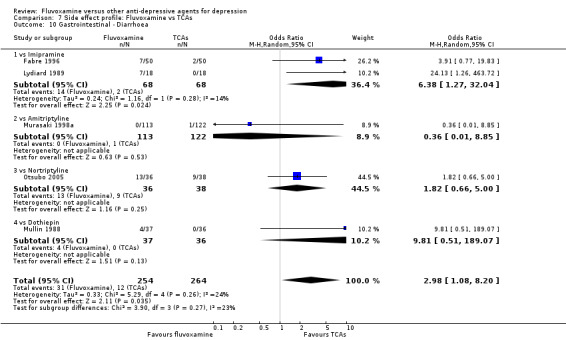
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 10 Gastrointestinal ‐ Diarrhoea.
In terms of the rate of other gastrointestinal side effects (i.e., increased salivation, oral discomfort/taste disturbance, weight gain, weight loss, increased appetite or anorexia), no strong evidence emerged that fluvoxamine was either more or less likely to cause these adverse events than were TCAs.
1.7.4 Neuropsychiatric side effects
Dizziness/vertigo/faintness were less common in recipients of fluvoxamine than in recipients of imipramine (OR 0.24, 95%CI 0.15 to 0.38, p<0.001; 9 trials, 1055 participants), clomipramine (OR 0.21, 95%CI 0.05 to 0.80, P=0.02; 1 trial, 86 participants), amitriptyline (OR 0.31, 95%CI 0.11 to 0.83, P=0.02; 2 trials, 304 participants), nortriptyline (OR 0.22, 95%CI 0.07 to 0.70, P=0.01; 1 trial, 73 participants) (Analysis 7.16).
7.16. Analysis.
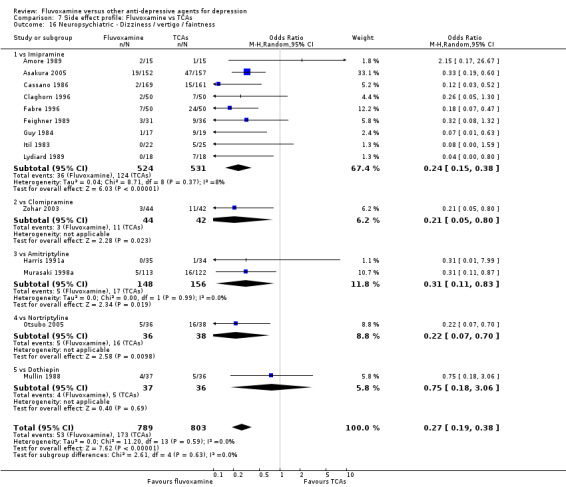
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 16 Neuropsychiatric ‐ Dizziness / vertigo / faintness.
In contrast, fluvoxamine was associated with higher rate of agitation/anxiety in participants than was imipramine (OR 2.24, 95%CI 1.01 to 4.97, P=0.05; 5 trials, 644 participants) (Analysis 7.23).
7.23. Analysis.
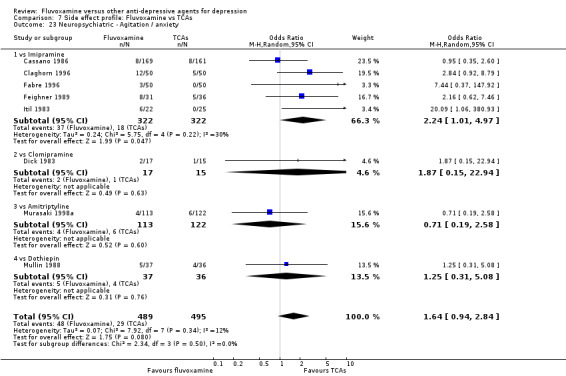
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 23 Neuropsychiatric ‐ Agitation / anxiety.
Recent research has pointed out that some antidepressants, in particular SSRIs, have caused the emergence or worsening of suicidal ideas in vulnerable patients (Barbui 2008; Hammad 2006). Only two trials (Dick 1983; Zohar 2003) among those comparing fluvoxamine with TCAs recorded completed suicide (Analysis 7.25), with two events among 61 patients taking fluvoxamine and no events among 57 those taking TCAs. Suicide wishes/gestures/attempts were reported in only three trials (Cassano 1986; Mullin 1988; Zohar 2003), with 3 events among 250 patients taking fluvoxamine and five among 239 patients taking TCAs.
7.25. Analysis.
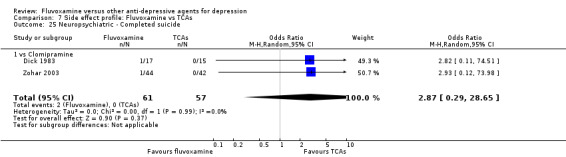
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 25 Neuropsychiatric ‐ Completed suicide.
In terms of the rate of participants experiencing other neuropsychiatric side effects (i.e., blurred vision, fatigue/tiredness/asthenia, headache, tremor, involuntary movement other than tremor, insomnia, sleepiness or manic symptoms), no strong evidence emerged that fluvoxamine was either more or less likely to cause these adverse events than were TCAs.
1.7.5 Genitourinary side effects
Urination problems were less common in recipients of fluvoxamine than in recipients of imipramine (OR 0.18, 95%CI 0.04 to 0.71, P=0.01; 2 trials, 409 participants) and TCAs as a class (OR 0.44, 95%CI 0.23 to 0.83, P=0.01; 6 trials, 818 participants) (Analysis 7.27). In terms of the rate of participants experiencing sexual dysfunction, no strong evidence emerged that fluvoxamine was more or less likely to cause these adverse events than were TCAs.
7.27. Analysis.
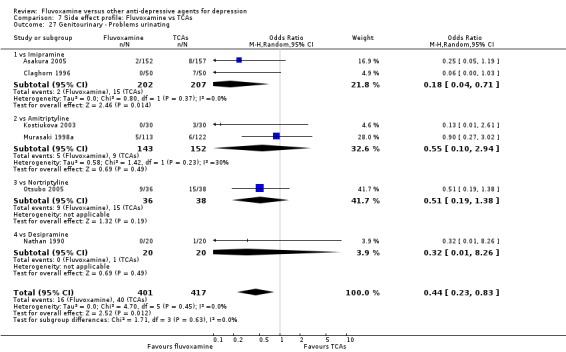
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 27 Genitourinary ‐ Problems urinating.
2. FLUVOXAMINE versus HETEROCYCLICS
Only five RCTs contributed usable data for efficacy and tolerability analyses (versus amineptine: Brunner 1994, versus maprotiline: Kasper 1990; Mendonca Lima 1997, versus mianserin: Moon 1991; Perez 1990). Four trials reported dichotomous data for a number of patients who experienced each side effect (versus maprotiline: Kasper 1990; Mendonca Lima 1997, versus mianserin: Moon 1991; Perez 1990).
2.1 Response ‐ acute phase (between 6 and 12 weeks); Primary outcome
Two trials comparing fluvoxamine with mianserin reported this outcome. Imputation methods were used for Perez 1990. No strong evidence emerged that fluvoxamine was either superior or inferior to mianserin in terms of the response at end of the acute‐phase treatment (Analysis 2.1).
2.1. Analysis.
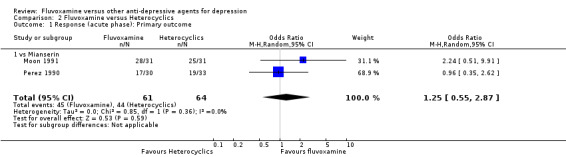
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 1 Response (acute phase): Primary outcome.
2.2 Response ‐ early phase and follow‐up phase
2.2.1 Early phase (between 1 and 4 weeks)
No strong evidence emerged that fluvoxamine was either superior or inferior to amineptine or maprotiline in terms of the response at end of the acute‐phase treatment. No trials comparing fluvoxamine with mianserin reported this outcome. See Analysis 2.2
2.2. Analysis.
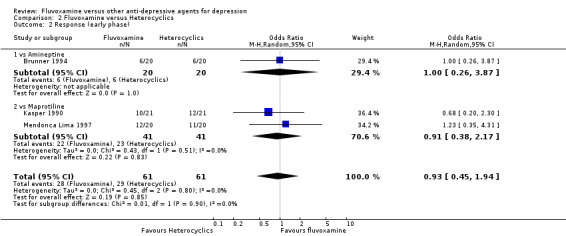
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 2 Response (early phase).
2.2.2 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
2.3 Remission
2.3.1 Early phase (between 1 and 4 weeks)
No strong evidence emerged that fluvoxamine was either superior or inferior to amineptine or maprotiline in terms of the remission . No trials comparing fluvoxamine with mianserin reported this outcome. See Analysis 2.3
2.3. Analysis.
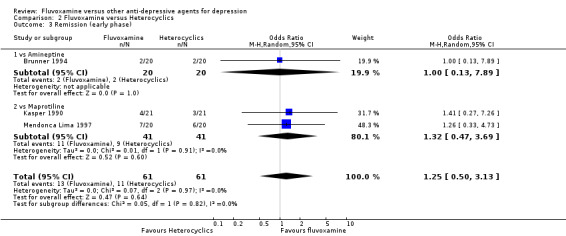
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 3 Remission (early phase).
2.3.2 Acute phase (between 6 and 12 weeks)
Two trials that compared fluvoxamine with mianserin reported this outcome. No strong evidence emerged to indicate that fluvoxamine was either superior or inferior to mianserin in terms of this outcome. See Analysis 2.4.
2.4. Analysis.
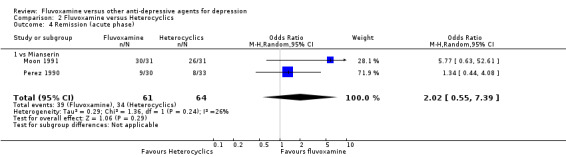
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 4 Remission (acute phase).
2.3.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
2.4 Endpoint score on depression scale
2.4.1 Early phase (between 1 and 4 weeks)
We meta‐analysed non‐skewed data only from 2 trials, and found no strong evidence that fluvoxamine was either superior or inferior to heterocyclics (Analysis 2.5). SDs were missing in one trial and we did not meta‐analyse these data, presenting them descriptively instead (Analysis 2.6).
2.5. Analysis.
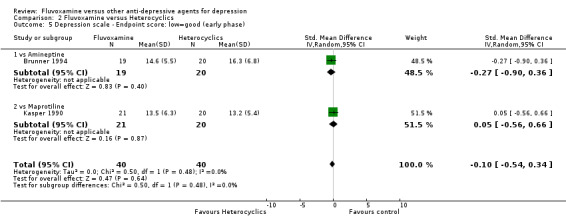
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 5 Depression scale ‐ Endpoint score: low=good (early phase).
2.6. Analysis.
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 6 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Maprotiline | ||||||||
| Mendonca Lima 1997 | MADRS | 13.4 | missing | 20 | 14.0 | missing | 20 | |
2.4.2 Acute phase (between 6 and 12 weeks)
Only one trial (Perez 1990) reported this continuous outcome, but SDs were missing (Analysis 2.7).
2.7. Analysis.
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 7 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Mianserin | ||||||||
| Perez 1990 | MADRS | 11.9 | 7.5 | 22 | 13.6 | 7.5 | 25 | skewed. |
2.4.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
2.5 Change score on depression scale
2.5.1 Early phase (between 1 and 4 weeks)
We meta‐analysed non‐skewed data only from 1 trial and found no strong evidence that fluvoxamine was either superior or inferior to moprotiline (Analysis 2.8). SDs were missing in three trials and we did not meta‐analyse these data, presenting them descriptively instead (Analysis 2.9).
2.8. Analysis.
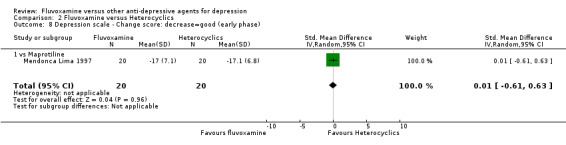
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 8 Depression scale ‐ Change score: decrease=good (early phase).
2.9. Analysis.
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 9 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Amineptine | ||||||||
| Brunner 1994 | HRSD‐17 | ‐9.7 | missing | 19 | ‐8.5 | missing | 20 | |
| vs Maplotiline | ||||||||
| Kasper 1990 | HRSD‐21 | ‐12.5 | missing | 21 | ‐15.4 | missing | 20 | |
| vs Mianserin | ||||||||
| Moon 1991 | MADRS | ‐10 | missing | 28 | ‐11.5 | missing | 21 | |
2.5.2 Acute phase (between 6 and 12 weeks)
Two trials compared fluvoxamine with mianserin and reported this continuous outcome. However, SDs for this outcome were missing. Therefore, we presented the results descriptively (Analysis 2.10).
2.10. Analysis.
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 10 Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Mianserin | ||||||||
| Moon 1991 | MADRS | ‐22 | missing | 28 | ‐24.1 | missing | 21 | |
| Perez 1990 | MADRS | ‐23.9 | missing | 22 | ‐23.6 | missing | 25 | |
2.5.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
2.6 Tolerability
2.6.1 Dropout
We found no strong evidence that fluvoxamine was either more or less acceptable in terms of the total numbers of dropouts for any reason when compared to heterocyclics (i.e., amineptine, maprotiline and mianserin) (Analysis 2.11). Similarly, regarding patients who dropped out because of inefficacy and due to side effects, we found no strong evidence that fluvoxamine was superior or inferior to heterocyclics (Analysis 2.12, Analysis 2.13).
2.11. Analysis.
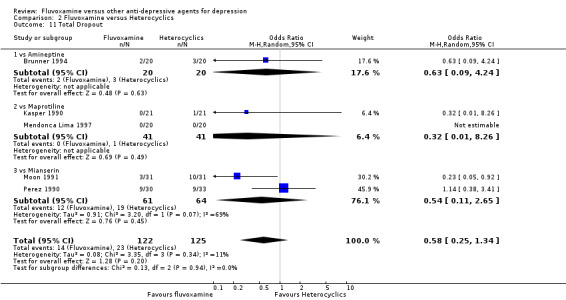
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 11 Total Dropout.
2.12. Analysis.
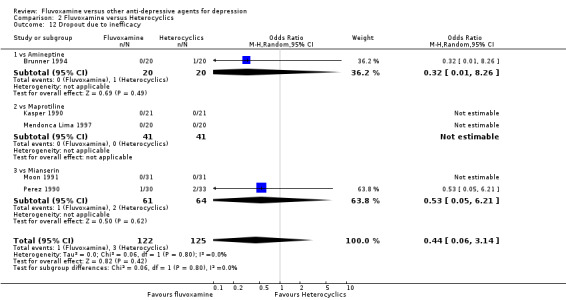
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 12 Dropout due to inefficacy.
2.13. Analysis.
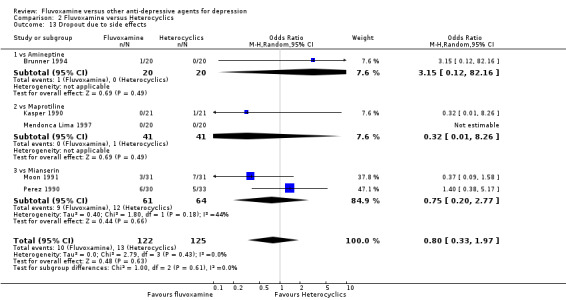
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 13 Dropout due to side effects.
2.6.2 Number of patients experiencing at least one side effect
No strong evidence emerged that fluvoxamine was either superior or inferior to maprotiline or mianserin in terms of this dichotomous outcome (Analysis 2.14).
2.14. Analysis.
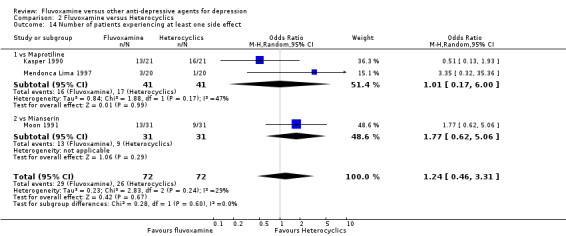
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 14 Number of patients experiencing at least one side effect.
2.7 Side effects profile by body system
No trials comparing fluvoxamine with amineptine reported a number of patients who experienced a specific side effect. Only gastrointestinal side effects, such as dry mouth, vomiting/nausea and dizziness/vertigo/faintness were reported by four RCTs (Kasper 1990; Mendonca Lima 1997; Moon 1991; Perez 1990). See: Table 3.
2.7.1 Cardiovascular side effects
No studies contributed data to this outcome.
2.7.2 Dermatological side effects
No studies contributed data to this outcome.
2.7.3 Gastrointestinal side effects
Reasonable evidence existed that fluvoxamine was associated with a higher rate of vomiting/nausea than was mianserin (OR 9.62, 95%CI 1.96 to 47.30, p=0.009; 4 trials, 207 participants). For further details, see Analysis 8.1, Analysis 8.2, Analysis 8.3). In terms of dry mouth or dizziness/vertigo/faintness, we found no strong evidence that fluvoxamine was either more or less likely to cause these adverse events than were heterocyclics.
8.1. Analysis.
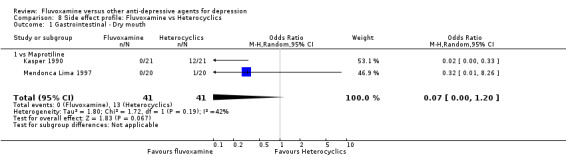
Comparison 8 Side effect profile: Fluvoxamine vs Heterocyclics, Outcome 1 Gastrointestinal ‐ Dry mouth.
8.2. Analysis.
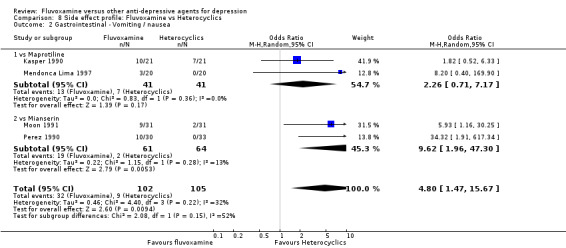
Comparison 8 Side effect profile: Fluvoxamine vs Heterocyclics, Outcome 2 Gastrointestinal ‐ Vomiting / nausea.
8.3. Analysis.
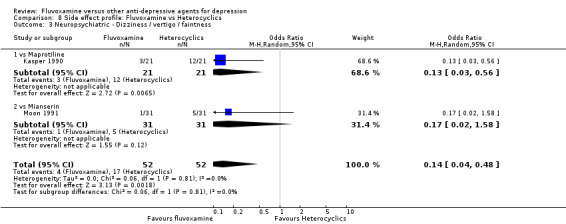
Comparison 8 Side effect profile: Fluvoxamine vs Heterocyclics, Outcome 3 Neuropsychiatric ‐ Dizziness / vertigo / faintness.
2.7.4 Neuropsychiatric side effects
No studies contributed data to this outcome.
2.7.5 Genitourinary side effects
No studies contributed data to this outcome.
3. FLUVOXAMINE versus OTHER SSRIs
Eight RCTs contributed usable data for the efficacy analyses and nine RCTs contributed to the tolerability analyses. Eight trials reported dichotomous data for a number of patients who experienced each side effect.
3.1 Response ‐ acute phase between 6 and 12 weeks; Primary outcome
Eight trials reported this outcome. Imputation methods were used for four trials (Kiev 1997, Nemeroff 1995, Rossini 2005, Rapaport 1996). We found no strong evidence that fluvoxamine was either superior or inferior to other SSRIs (i.e., paroxetine, sertraline, fluoxetine and citalopram) in terms of this dichotomous outcome. See Figure 10.
10.
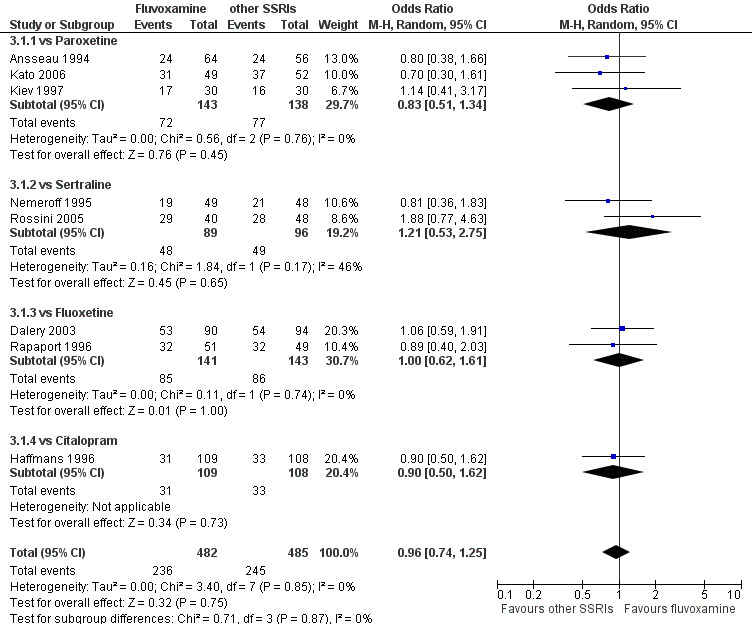
Forest plot of comparison: 3 Fluvoxamine vs other SSRIs, outcome: 3.1 Response (acute phase).
3.2 Response ‐ early phase and follow‐up phase
3.2.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to other SSRIs in terms of this dichotomous outcome. See Analysis 3.2. Substantial heterogeneity existed between trials comparing fluvoxamine to fluoxetine based on two trials, Dalery 2003 and Rapaport 1996 ( I2= 71 %, P = 0.07, Analysis 3.2). However, because of the small number of trials, sources of the heterogeneity cannot be further explained.
3.2. Analysis.
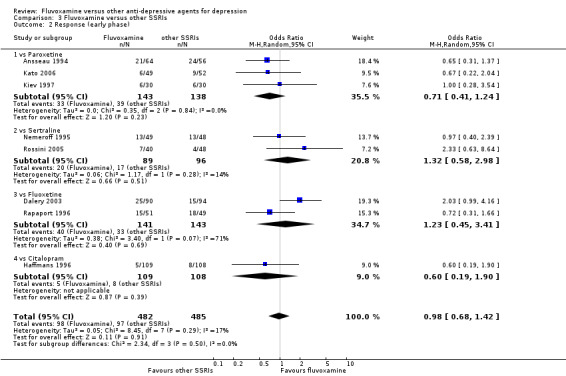
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 2 Response (early phase).
3.2.2 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
3.3 Remission
3.3.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to other SSRIs in terms of remission at the end of early phase. See Analysis 3.3.
3.3. Analysis.
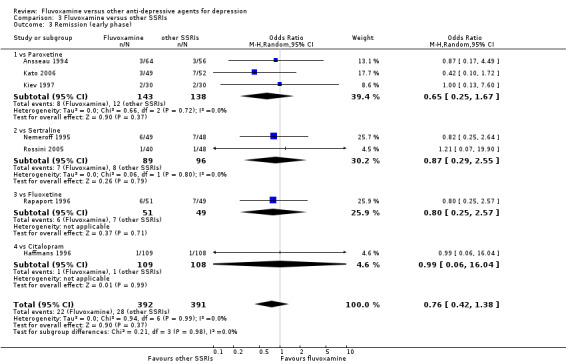
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 3 Remission (early phase).
3.3.2 Acute phase (between 6 and 12 weeks)
No strong evidence emerged that fluvoxamine was either superior or inferior to other SSRIs in terms of remission at the end of acute phase. See Analysis 3.4.
3.4. Analysis.
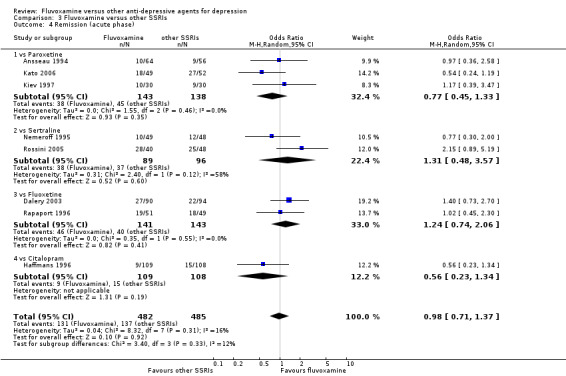
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 4 Remission (acute phase).
3.3.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
3.4 Endpoint score on depression scale
3.4.1 Early phase (between 1 and 4 weeks)
We meta‐analysed non‐skewed data only from 2 trials, and found no strong evidence that fluvoxamine was either superior or inferior to other SSRIs (Analysis 3.5). SDs were missing in five trials and we did not meta‐analyse these data, and presenting them descriptively instead (Analysis 3.6).
3.5. Analysis.
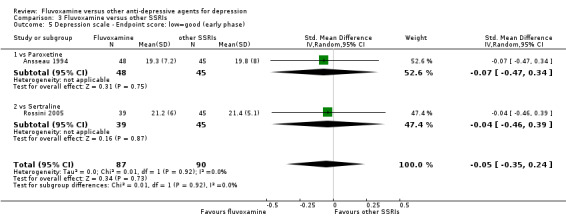
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 5 Depression scale ‐ Endpoint score: low=good (early phase).
3.6. Analysis.
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 6 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Paroxetine | ||||||||
| Kato 2006 | HRSD‐21 | 17.2 | missing | 41 | 13.9 | missing | 39 | |
| Kiev 1997 | HRSD‐21 | 17.1 | missing | 29 | 17.4 | missing | 29 | |
| vs Sertraline | ||||||||
| Nemeroff 1995 | HRSD‐21 | 16.8 | missing | 46 | 15.8 | missing | 46 | |
| vs Citalopram | ||||||||
| Haffmans 1996 | HRSD‐17 | 22.3 | missing | 109 | 21.2 | missing | 108 | |
| vs Fluoxetine | ||||||||
| Rapaport 1996 | HRSD‐21 | 15.5 | missing | 47 | 14.4 | missing | 46 | |
3.4.2 Acute phase (between 6 and 12 weeks)
Eight trials reported this outcome. However, we did not meta‐analyse these data (data were skewed in four trials, and SDs were missing in four trials). We presented them descriptively (Analysis 3.7).
3.7. Analysis.
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 7 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Paroxetine | ||||||||
| Ansseau 1994 | HRSD‐21 | 13.2 | 7.3 | 43 | 13.7 | 7.9 | 38 | skewed. |
| Kato 2006 | HRSD‐21 | 9.0 | missing | 41 | 4.6 | missing | 39 | |
| Kiev 1997 | HRSD‐21 | 10.9 | 7.3 | 29 | 11.5 | 7.4 | 29 | skewed. |
| vs Sertraline | ||||||||
| Nemeroff 1995 | HRSD‐21 | 14.0 | 7.6 | 46 | 12.2 | 6.5 | 46 | skewed. |
| Rossini 2005 | HRSD‐21 | 7.6 | 12.3 | 39 | 11.3 | 11.3 | 45 | skewed. |
| vs Fluoxetine | ||||||||
| Dalery 2003 | HRSD‐17 | 10.0 | missing | 86 | 11.3 | missing | 91 | |
| Rapaport 1996 | HRSD‐21 | 9.6 | missing | 47 | 9.7 | missing | 46 | |
| vs Citalopram | ||||||||
| Haffmans 1996 | HRSD‐17 | 18.0 | missing | 109 | 16.6 | missing | 108 | |
3.4.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
3.5 Change score on depression scale
3.5.1 Early phase (between 1 and 4 weeks)
We meta‐analysed data only from one trial, and found no strong evidence that fluvoxamine was either superior or inferior to paroxetine (Analysis 3.8). SDs were missing in six trials and we did not meta‐analyse these data, and presenting them descriptively instead (Analysis 3.9).
3.8. Analysis.
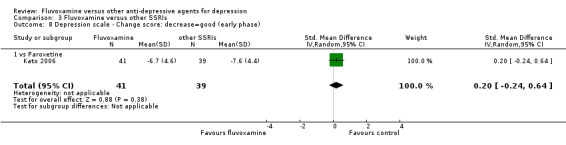
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 8 Depression scale ‐ Change score: decrease=good (early phase).
3.9. Analysis.
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 9 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Paroxetine | ||||||||
| Ansseau 1994 | HRSD‐21 | ‐7.2 | missing | 48 | ‐6.2 | missing | 45 | |
| Kiev 1997 | HRSD‐21 | ‐7.3 | missing | 29 | ‐7 | missing | 29 | |
| vs Sertraline | ||||||||
| Nemeroff 1995 | HRSD‐21 | ‐7.8 | missing | 46 | ‐7.4 | missing | 46 | |
| Rossini 2005 | HRSD‐21 | ‐10.1 | missing | 39 | ‐7.8 | missing | 45 | |
| vs Citalopram | ||||||||
| Haffmans 1996 | HRSD‐17 | ‐2.2 | missing | 109 | ‐3.5 | missing | 108 | |
| vs Fluoxetine | ||||||||
| Rapaport 1996 | HRSD‐21 | ‐9.7 | missing | 47 | ‐11.2 | missing | 46 | |
3.5.2 Acute phase (between 6 and 12 weeks)
We meta‐analysed data only from three trials, and found no strong evidence that fluvoxamine was either superior or inferior to paroxetine or sertraline (Analysis 3.10). SDs were missing in five trials and we did not meta‐analyse these data, presenting them descriptively instead (Analysis 3.11).
3.10. Analysis.
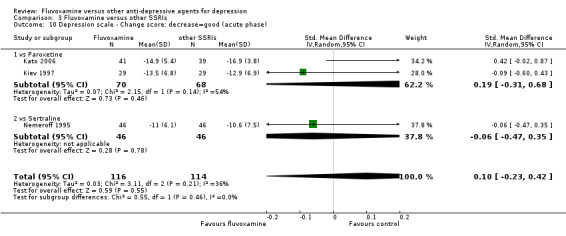
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 10 Depression scale ‐ Change score: decrease=good (acute phase).
3.11. Analysis.
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 11 Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Paroxetine | ||||||||
| Ansseau 1994 | HRSD‐21 | ‐13.3 | missing | 43 | ‐12.3 | missing | 38 | |
| vs Sertraline | ||||||||
| Rossini 2005 | HRSD‐21 | ‐23.7 | missing | 39 | ‐18 | missing | 45 | |
| vs Citalopram | ||||||||
| Haffmans 1996 | HRSD‐17 | ‐6.5 | missing | 109 | ‐8.1 | missing | 108 | |
| vs Fluoxetine | ||||||||
| Dalery 2003 | HRSD‐17 | ‐12.3 | missing | 86 | ‐10.9 | missing | 91 | |
| Rapaport 1996 | HRSD‐21 | ‐15.6 | missing | 47 | ‐15.9 | missing | 46 | |
3.5.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
3.6. Tolerability
3.6.1 Dropout
We found no strong evidence that fluvoxamine was either more or less acceptable than were other SSRIs in terms of withdrawal due to any reason (Analysis 3.12). Similarly, regarding number of patients who dropped out because of inefficacy and because of adverse effects, no strong evidence emerged that fluvoxamine was either superior or inferior to other SSRIs (Analysis 3.13 and Analysis 3.14). We found substantial heterogeneity between trials comparing fluvoxamine to sertraline, based on three trials (Gonul 1999; Nemeroff 1995; Rossini 2005) (I2 = 66 %, P = 0.05, Analysis 3.14.2). Visual inspection revealed that, among these studies, Nemeroff 1995 reported results favourable to sertraline. However, because of the small number of trials, sources of the heterogeneity cannot be further explained.
3.12. Analysis.
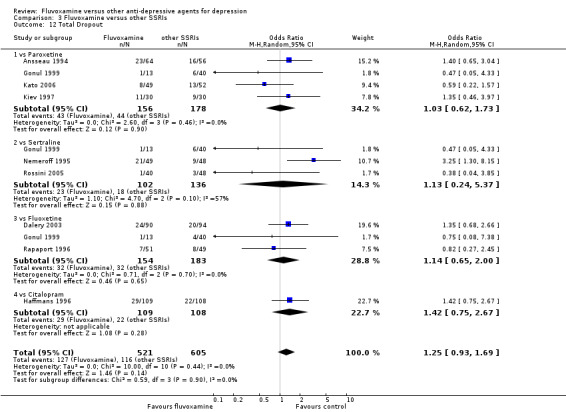
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 12 Total Dropout.
3.13. Analysis.
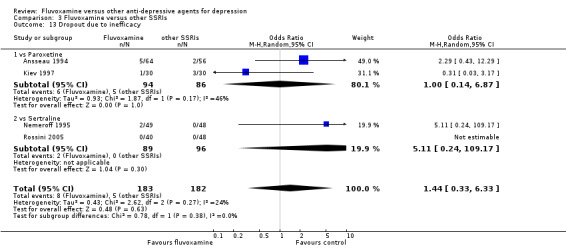
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 13 Dropout due to inefficacy.
3.14. Analysis.
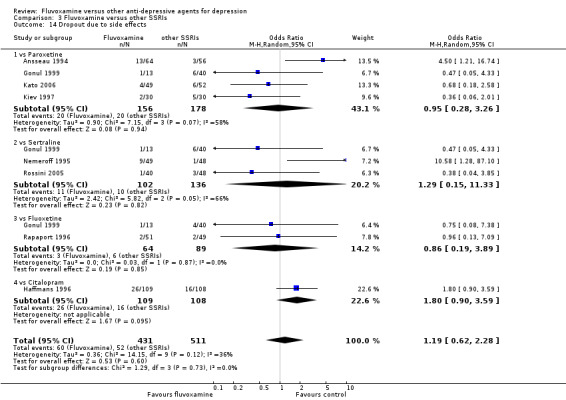
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 14 Dropout due to side effects.
3.6.2 Number of patients experiencing at least one side effect
Only five trials reported this dichotomous outcome, and no strong evidence emerged that fluvoxamine was either superior or inferior to other SSRIs in terms of this outcome. See Analysis 3.15.
3.15. Analysis.
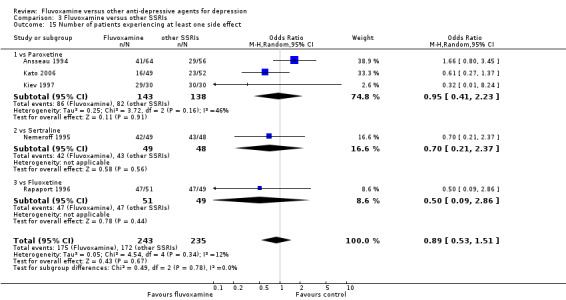
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 15 Number of patients experiencing at least one side effect.
3.7 Side effect profile by body system
Only one trial (Haffmans 1996) compared fluvoxamine with citalopram but did not report the number of patients who experienced specific side effects other than completed suicide or suicide attempts. See: Table 3.
3.7.1 Cardiovascular side effects
In terms of the rate of participants experiencing hypertension/tachycardia or hypotension/bradycardia, we found no strong evidence that fluvoxamine was either superior or inferior to paroxetine (Analysis 9.1, Analysis 9.2).
9.1. Analysis.
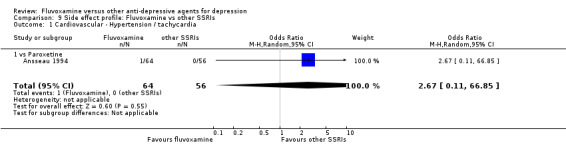
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 1 Cardiovascular ‐ Hypertension / tachycardia.
9.2. Analysis.
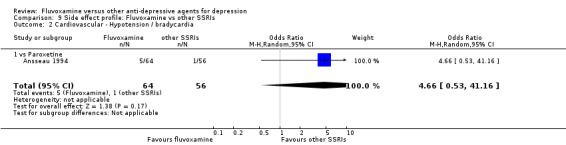
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 2 Cardiovascular ‐ Hypotension / bradycardia.
3.7.2 Dermatological side effects
Sweating was more frequent with paroxetine (OR 0.22, 95%CI 0.05 to 0.91, P=0.04; 1 trial, 60 participants) than in fluvoxamine‐treated patients (Analysis 9.4). In terms of the rate of dermatitis/rash experienced by participants, no strong evidence emerged that fluvoxamine was either superior or inferior to sertraline in this respect (Analysis 9.3).
9.4. Analysis.
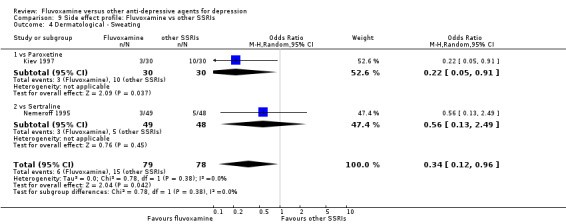
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 4 Dermatological ‐ Sweating.
9.3. Analysis.
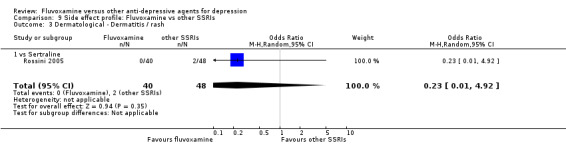
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 3 Dermatological ‐ Dermatitis / rash.
3.7.3 Gastrointestinal side effects
We found no evidence that fluvoxamine was either superior or inferior to other SSRIs in terms of the rate of gastrointestinal side effects experienced by participants (Analysis 9.5, Analysis 9.6, Analysis 9.7, Analysis 9.8 and Analysis 9.9).
9.5. Analysis.
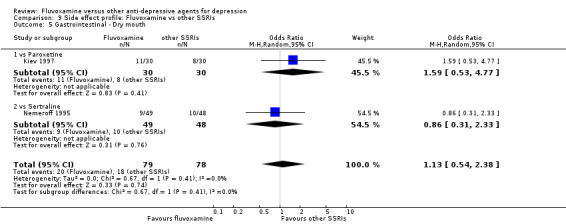
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 5 Gastrointestinal ‐ Dry mouth.
9.6. Analysis.
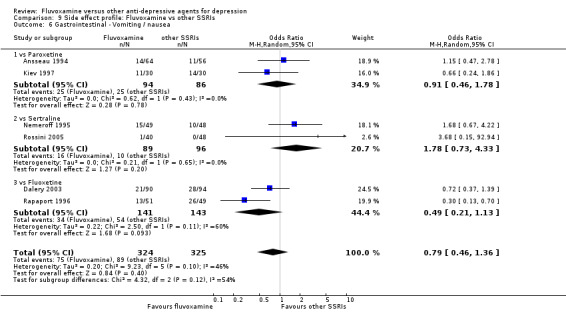
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 6 Gastrointestinal ‐ Vomiting / nausea.
9.7. Analysis.
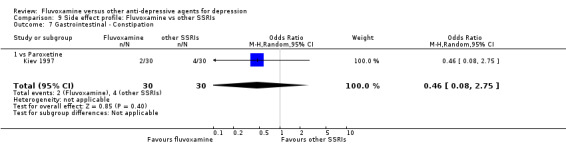
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 7 Gastrointestinal ‐ Constipation.
9.8. Analysis.
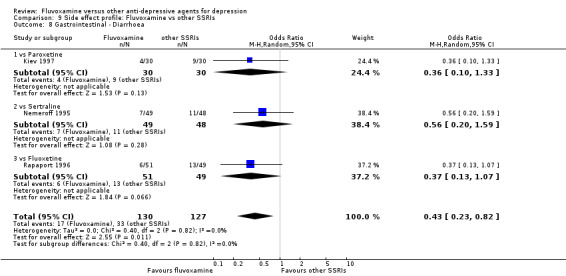
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 8 Gastrointestinal ‐ Diarrhoea.
9.9. Analysis.
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 9 Gastrointestinal ‐ Anorexia.
No studies contributed data regarding increased salivation, oral discomfort/taste disturbance, weight gain, weight loss or increased appetite.
3.7.4 Neuropsychiatric side effects
We found no strong evidence that fluvoxamine, compared with other SSRIS, was either more or less likely to cause headache (Analysis 9.12), insomnia (Analysis 9.14), agitation/anxiety (Analysis 9.16), dizziness/vertigo/faintness (Analysis 9.10), fatigue/tiredness/asthenia (Analysis 9.11), tremor (Analysis 9.13), sleepiness/drowsiness (Analysis 9.15), manic symptom (Analysis 9.17). Only one trial compared fluvoxamine with citalopram (Haffmans 1996) recorded a completed suicide (Analysis 7.25), and cited one event among 108 patients taking citalopram and no events among 109 patients taking fluvoxamine. Suicide attempts/ideation were reported in only four trials (Ansseau 1994; Dalery 2003; Rapaport 1996; Haffmans 1996), with 6 events among 314 patients taking fluvoxamine and 2 patients among 307 patients taking other SSRIs (i.e., paroxetine, fluoxetine and citalopram) (Analysis 9.18, Analysis 9.19). No studies contributed data to blurred vision and involuntary movement other than tremor.
9.12. Analysis.
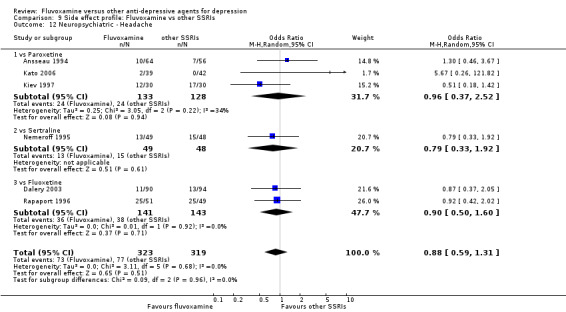
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 12 Neuropsychiatric ‐ Headache.
9.14. Analysis.
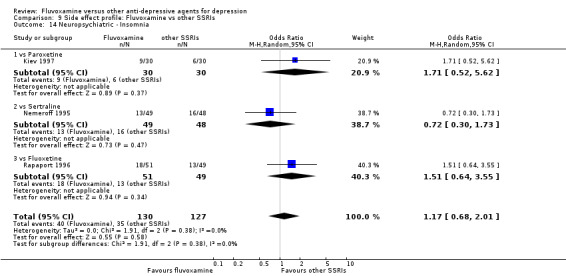
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 14 Neuropsychiatric ‐ Insomnia.
9.16. Analysis.
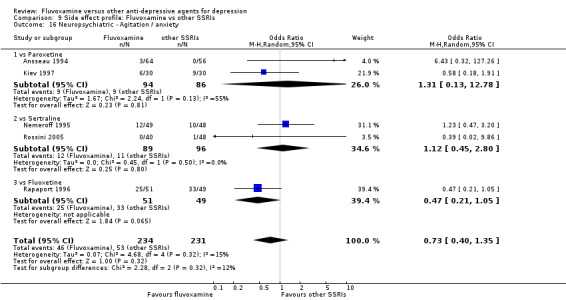
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 16 Neuropsychiatric ‐ Agitation / anxiety.
9.10. Analysis.
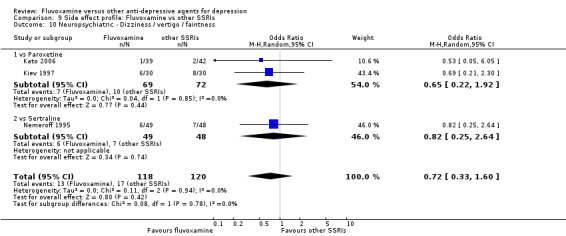
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 10 Neuropsychiatric ‐ Dizziness / vertigo / faintness.
9.11. Analysis.
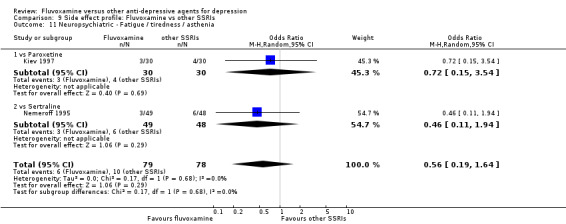
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 11 Neuropsychiatric ‐ Fatigue / tiredness / asthenia.
9.13. Analysis.
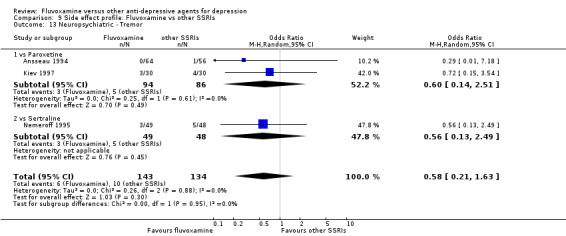
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 13 Neuropsychiatric ‐ Tremor.
9.15. Analysis.
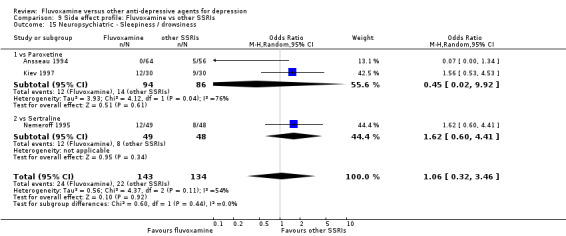
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 15 Neuropsychiatric ‐ Sleepiness / drowsiness.
9.17. Analysis.
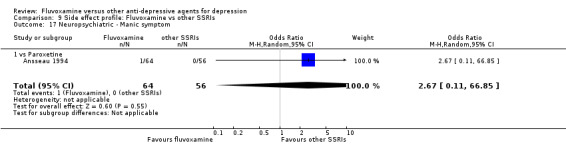
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 17 Neuropsychiatric ‐ Manic symptom.
9.18. Analysis.
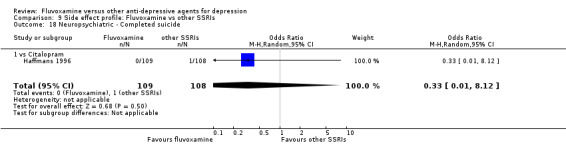
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 18 Neuropsychiatric ‐ Completed suicide.
9.19. Analysis.
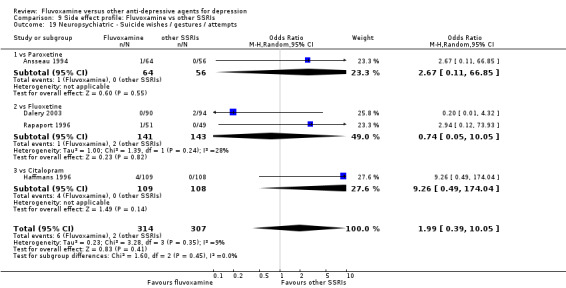
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 19 Neuropsychiatric ‐ Suicide wishes / gestures / attempts.
3.7.5 Genitourinary side effects
Only four trials reported sexual dysfunction as a side effect. Some previous trials have reported that fluvoxamine was associated with a relatively low prevalence of sexual dysfunction compared to other SSRIs (i.e., paroxetine, sertraline and fluoxetine) ( (Mackay 1997; Montejo‐Gonzalez 1997). However, we found no strong evidence that fluvoxamine was either more or less likely to cause sexual dysfunction than were other SSRIs (Analysis 9.20). No studies contributed data to problems urinating.
9.20. Analysis.
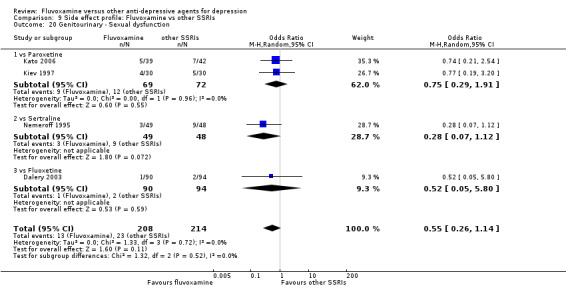
Comparison 9 Side effect profile: Fluvoxamine vs other SSRIs, Outcome 20 Genitourinary ‐ Sexual dysfunction.
4. FLUVOXAMINE versus SNRIs
Three RCTs (five comparisons) contributed usable data for the efficacy analyses and tolerability analyses for milnacipran or venlafaxine (Ansseau 1991a; Ansseau 1991b; Clerc 2001; Hackett 1998a; Hackett 1998b). Two RCTs (three comparisons) reported dichotomous data for a number of patients who experienced specific side effects (Ansseau 1991a; Ansseau 1991b; Clerc 2001).
4.1 Response ‐ acute phase (between 6 and 12 weeks); Primary outcome
Two trials (three comparisons) reported this outcome. Imputation methods were used for Hackett 1998a and Hackett 1998b. Evidence emerged that fluvoxamine was less effective than was venlafaxine, based on one trial (2 comparisons), for which the response had to be calculated based on the imputation method (Furukawa 2005) (OR: 0.40, 95% CI 0.18 to 0.92, P=0.03; 1 trial (2 comparisons), 111 participants) (Hackett 1998a; Hackett 1998b). We found no strong evidence that fluvoxamine was either superior or inferior to milnacipran in terms of this dichotomous outcome. See Figure 11.
11.
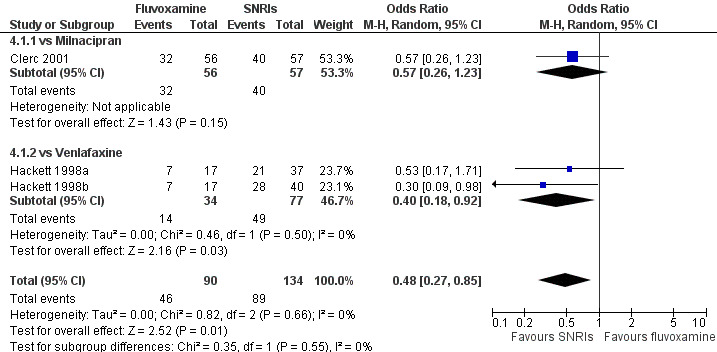
Forest plot of comparison: 4 Fluvoxamine vs SNRIs, outcome: 4.1 Response (acute phase).
4.2 Response ‐ early phase and follow‐up phase
4.2.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to SNRIs in terms of this dichotomous outcome. See Analysis 4.2.
4.2. Analysis.
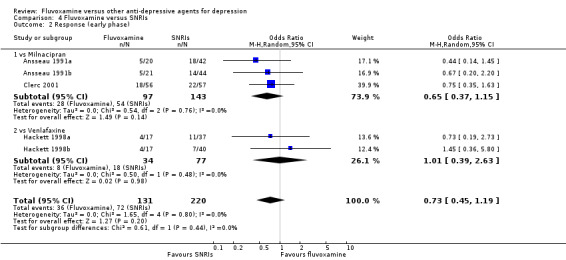
Comparison 4 Fluvoxamine versus SNRIs, Outcome 2 Response (early phase).
4.2.2 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
4.3 Remission
4.3.1 Early phase (between 1 and 4 weeks)
No strong evidence emerged that fluvoxamine was either superior or inferior to SNRIs in terms of this dichotomous outcome. See Analysis 4.3.
4.3. Analysis.
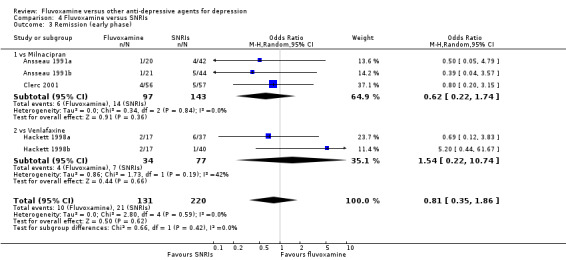
Comparison 4 Fluvoxamine versus SNRIs, Outcome 3 Remission (early phase).
4.3.2 Acute phase (between 6 and 12 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to SNRIs in terms of this dichotomous outcome. See Analysis 4.4.
4.4. Analysis.
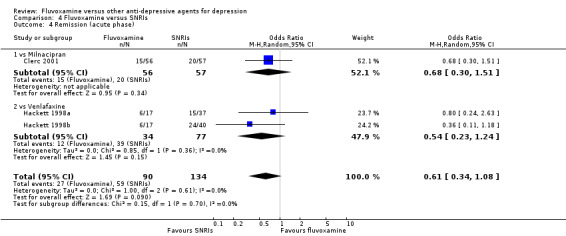
Comparison 4 Fluvoxamine versus SNRIs, Outcome 4 Remission (acute phase).
4.3.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
4.4 Endpoint score on depression scale
4.4.1 Early phase (between 1 and 4 weeks)
We meta‐analysed data only from 3 trials (four comparisons), and found no strong evidence that fluvoxamine was either superior or inferior to mianserin or venlafaxine (Analysis 4.5). SDs were missing in one trial and we did not meta‐analyse the data, and presenting it descriptively instead (Analysis 4.6).
4.5. Analysis.
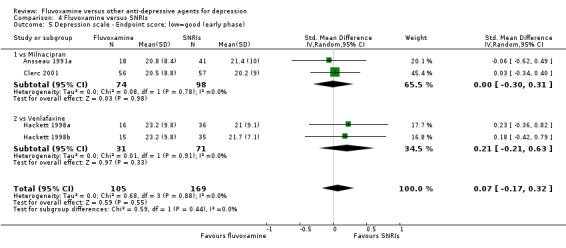
Comparison 4 Fluvoxamine versus SNRIs, Outcome 5 Depression scale ‐ Endpoint score: low=good (early phase).
4.6. Analysis.
Comparison 4 Fluvoxamine versus SNRIs, Outcome 6 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Milnacipran | ||||||||
| Ansseau 1991b | HRSD‐24 | 20.8 | 8.4 | 19 | 20.1 | 10.1 | 42 | skewed. |
4.4.2 Acute phase (between 6 and 12 weeks)
One trial (two comparisons) reported this outcome. However, we did not meta‐analyse these skewed data, instead presenting them descriptively (Analysis 4.7).
4.7. Analysis.
Comparison 4 Fluvoxamine versus SNRIs, Outcome 7 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Venlafaxine | ||||||||
| Hackett 1998a | MADRS | 10.5 | 11.1 | 11 | 7.6 | 6.8 | 31 | skewed. |
| Hackett 1998b | MADRS | 10.5 | 11.1 | 10 | 10.5 | 9.3 | 28 | skewed. |
4.4.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
4.5 Change score on depression scale
4.5.1 Early phase (between 1 and 4 weeks)
Three trials (five comparisons) reported this outcome. However, we did not meta‐analyse these data, since SDs were missing (Analysis 4.8).
4.8. Analysis.
Comparison 4 Fluvoxamine versus SNRIs, Outcome 8 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Milnacipran | ||||||||
| Ansseau 1991a | HRSD‐21 | ‐11.7 | missing | 19 | ‐11.6 | missing | 42 | |
| Ansseau 1991b | HRSD‐21 | ‐11.7 | missing | 18 | ‐12.4 | missing | 41 | |
| Clerc 2001 | HRSD‐21 | ‐10.9 | missing | 56 | ‐12.6 | missing | 57 | |
| vs Venlafaxine | ||||||||
| Hackett 1998a | MADRS | ‐8.4 | missing | 16 | ‐11.7 | missing | 36 | |
| Hackett 1998b | MADRS | ‐8.4 | missing | 15 | ‐10 | missing | 35 | |
4.5.2 Acute phase (between 6 and 12 weeks)
Two trials (three comparisons) reported this outcome. However, we did not meta‐analyse these data, since SDs were missing (Analysis 4.9).
4.9. Analysis.
Comparison 4 Fluvoxamine versus SNRIs, Outcome 9 Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Milnacipran | ||||||||
| Clerc 2001 | HRSD‐21 | ‐15.9 | missing | 56 | ‐20.7 | missing | 57 | |
| vs Venlafaxine | ||||||||
| Hackett 1998a | MADRS | ‐21.1 | missing | 10 | ‐22.2 | missing | 28 | |
| Hackett 1998b | MADRS | ‐21.1 | missing | 11 | ‐24.1 | missing | 31 | |
4.5.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
4.6 Tolerability
4.6.1 Dropout
We found no strong evidence that fluvoxamine was either more or less acceptable in terms of withdrawal due to any reason when compared with milnacipran. Venlafaxine was found to be less likely elicit withdrawal due to any reason (OR: 2.29, 95% CI 0.97 to 5.43, P=0.06; 1 trial (2 comparisons), 111 participants) (Analysis 4.10). Similarly, regarding the number of patients who dropped out because of inefficacy and due to side effects, we found no strong evidence that fluvoxamine was either superior or inferior to milnacipran. (Analysis 4.11, Analysis 4.12).
4.10. Analysis.
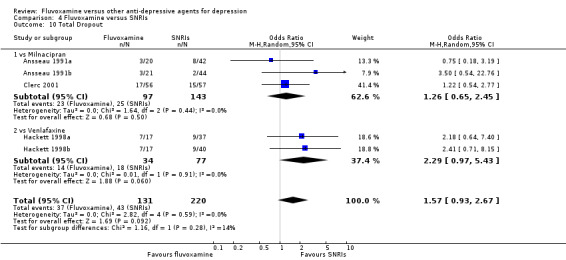
Comparison 4 Fluvoxamine versus SNRIs, Outcome 10 Total Dropout.
4.11. Analysis.
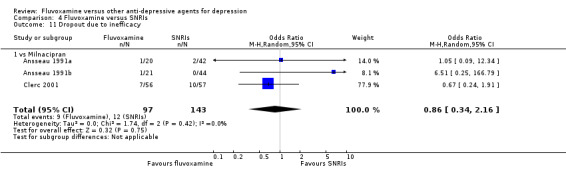
Comparison 4 Fluvoxamine versus SNRIs, Outcome 11 Dropout due to inefficacy.
4.12. Analysis.
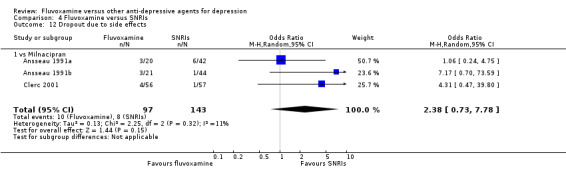
Comparison 4 Fluvoxamine versus SNRIs, Outcome 12 Dropout due to side effects.
4.6.2 Number of patients experiencing at least one side effect
No studies contributed data to this outcome.
4.7 Side effects profile by body system
No studies comparing fluvoxamine to venlafaxine reported any number of patients experiencing specific side effects. See: Table 3.
4.7.1 Cardiovascular side effects
In terms of the rate of hypertension/tachycardia or hypotension/bradycardia, we found no strong evidence that fluvoxamine was either superior or inferior to milnacipran in this respect (Analysis 10.1, Analysis 10.2).
10.1. Analysis.
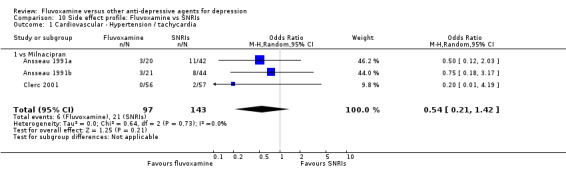
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 1 Cardiovascular ‐ Hypertension / tachycardia.
10.2. Analysis.
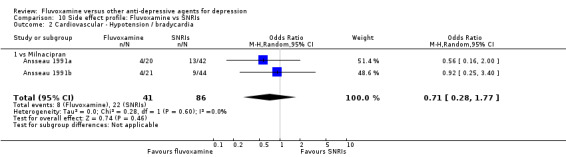
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 2 Cardiovascular ‐ Hypotension / bradycardia.
4.7.2 Dermatological side effects
In terms of the rate of dermatitis/rash or sweating experienced by participants, no strong evidence emerged that fluvoxamine was either superior or inferior to milnacipran. (Analysis 10.3, Analysis 10.4).
10.3. Analysis.
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 3 Dermatological ‐ Dermatitis / rash.
10.4. Analysis.
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 4 Dermatological ‐ Sweating.
4.7.3 Gastrointestinal side effects
We found evidence that fluvoxamine was associated with higher rate of vomiting/nausea experienced by participants than was milnacipran (OR 1.95, 95%CI 1.09 to 3.50, P=0.02; 2 trials (3 comparisons), 240 participants) (Analysis 10.8). In terms of the rate of increased salivation, dry mouth, oral discomfort/taste disturbance, vomiting/nausea, constipation, diarrhoea, weight gain, weight loss and anorexia experienced by participants receiving fluvoxamine compared to milnacipran, no strong evidence emerged to indicate that fluvoxamine was either superior or inferior to milnacipran. See Analysis 10.5 to Analysis 10.13.
10.8. Analysis.
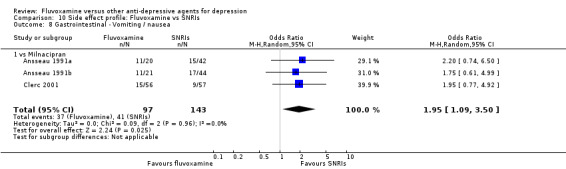
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 8 Gastrointestinal ‐ Vomiting / nausea.
10.5. Analysis.
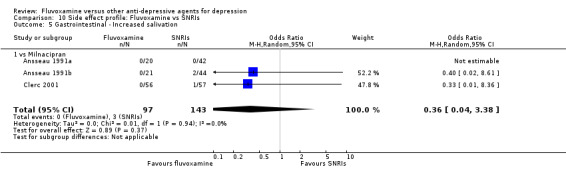
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 5 Gastrointestinal ‐ Increased salivation.
10.13. Analysis.
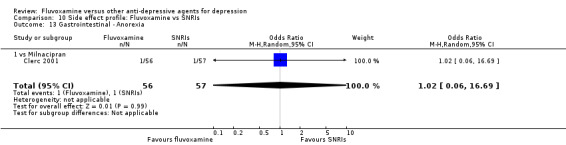
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 13 Gastrointestinal ‐ Anorexia.
No studies contributed data to increased appetite.
4.7.4 Neuropsychiatric side effects
In terms of the rate of blurred vision, dizziness/vertigo/faintness, fatigue/tiredness/asthenia, headache, tremor, involuntary movement other than tremor, insomnia, sleepiness/drowsiness and agitation/anxiety experienced by participants, we found no strong evidence that fluvoxamine was either superior or inferior to milnacipran. See Analysis 10.14 to Analysis 10.22.
10.14. Analysis.
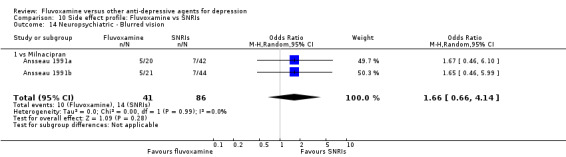
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 14 Neuropsychiatric ‐ Blurred vision.
10.22. Analysis.
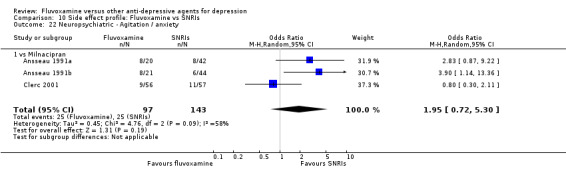
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 22 Neuropsychiatric ‐ Agitation / anxiety.
No studies contributed data to manic symptom, completed suicide and suicide wishes/gestures/attempts.
4.7.5 Genitourinary side effects
We found no strong evidence that fluvoxamine was either more or less likely to cause urination problems than was milnacipran. See Analysis 10.23
10.23. Analysis.
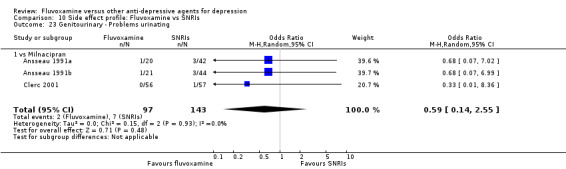
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 23 Genitourinary ‐ Problems urinating.
No studies contributed data to sexual dysfunction.
5. FLUVOXAMINE versus NEWER ANTIDEPRESSANTS
Three RCTs comparing fluvoxamine to moclobemide (Barrelet 1991; Bocksberger 1993; Bougerol 1992) and one RCT comparing fluvoxamine to mirtazapine (Schoemaker 2002) contributed usable data for the efficacy analyses, tolerability analyses and for a number of patients who experienced specific side effects. These two drugs have little chemically in common, we did not pool the results of these two drugs, presenting them separately instead.
5.1 Response ‐ acute phase (between 6 and 12 weeks); Primary outcome
Schoemaker 2002 reported this outcome. Imputation methods were not used for this study. No strong evidence emerged that fluvoxamine was either superior or inferior to mirtazapine in terms of this dichotomous outcome. See Analysis 5.1.
5.1. Analysis.
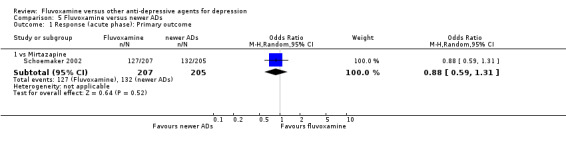
Comparison 5 Fluvoxamine versus newer ADs, Outcome 1 Response (acute phase): Primary outcome.
5.2 Response ‐ early phase and follow‐up phase
5.2.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to moclobemide or mirtazapine in terms of this dichotomous outcome. See Analysis 5.2.
5.2. Analysis.
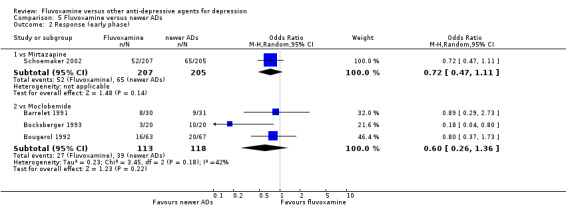
Comparison 5 Fluvoxamine versus newer ADs, Outcome 2 Response (early phase).
5.2.2 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
5.3 Remission
5.3.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to moclobemide or mirtazapine in terms of this dichotomous outcome. See Analysis 5.3.
5.3. Analysis.
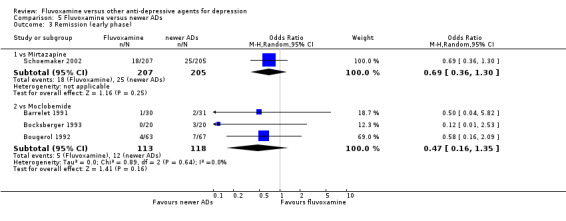
Comparison 5 Fluvoxamine versus newer ADs, Outcome 3 Remission (early phase).
5.3.2 Acute phase (between 6 and 12 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to mirtazapine in terms of this dichotomous outcome. See Analysis 5.4.
5.4. Analysis.
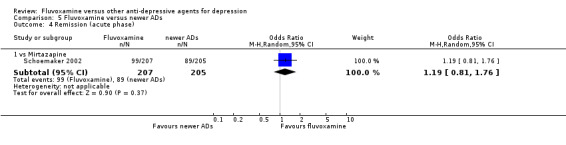
Comparison 5 Fluvoxamine versus newer ADs, Outcome 4 Remission (acute phase).
5.3.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
5.4 Endpoint score on depression scale
5.4.1 Early phase (between 1 and 4 weeks)
Two trials reported this outcome. However, we did not meta‐analyse these data, since SDs were missing (Analysis 5.5).
5.4.2 Acute phase (between 6 and 12 weeks)
Two trials reported this outcome. However, we did not meta‐analyse these data, since SDs were missing (Analysis 5.6).
5.4.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
5.5 Change score on depression scale
5.5.1 Early phase (between 1 and 4 weeks)
One trial that compared fluvoxamine with mirtazapine reported this outcome and showed strong evidence that fluvoxamine was inferior to mirtazapine (SMD 0.32, 95%CI 0.12 to 0.51, P=0.002; 1 trial, n=402) (Analysis 5.7). Three trials that compared fluvoxamine with moclobemide reported this outcome, but we did not meta‐analyse these data since SDs were missing (Analysis 5.8)
5.7. Analysis.
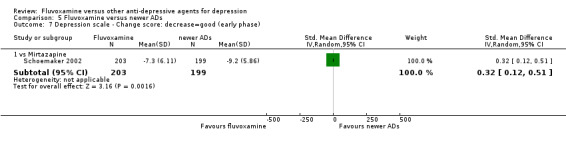
Comparison 5 Fluvoxamine versus newer ADs, Outcome 7 Depression scale ‐ Change score: decrease=good (early phase).
5.8. Analysis.
Comparison 5 Fluvoxamine versus newer ADs, Outcome 8 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs.
| Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Moclobemide | ||||||||
| Barrelet 1991 | HRSD‐17 | ‐10.3 | missing | 25 | ‐10.1 | missing | 26 | |
| Bocksberger 1993 | NADRS | ‐11.8 | missing | 19 | ‐21.9 | missing | 19 | |
| Bougerol 1992 | HRSD‐17 | ‐8.4 | missing | 61 | ‐9.0 | missing | 65 | |
5.5.2 Acute phase (between 6 and 12 weeks)
One trial that compared fluvoxamine with mirtazapine reported this outcome but showed no strong evidence that fluvoxamine was either inferior or superior to mirtazapine (SMD 0.08, 95%CI 0.12 to 0.28, P=0.42; 1 trial, n=402) (Analysis 5.9).
5.9. Analysis.
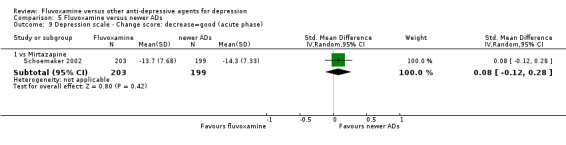
Comparison 5 Fluvoxamine versus newer ADs, Outcome 9 Depression scale ‐ Change score: decrease=good (acute phase).
5.5.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
5.6 Tolerability
5.6.1 Dropout
We found no strong evidence that fluvoxamine was either more or less acceptable in terms of withdrawal due to any reason when compared with the newer ADs (Analysis 5.10). Similarly, regarding number of patients who dropped out because of inefficacy and due to side effects, we found no strong evidence that fluvoxamine was either more or less acceptable than the newer ADs (Analysis 5.11, Analysis 5.12).
5.10. Analysis.
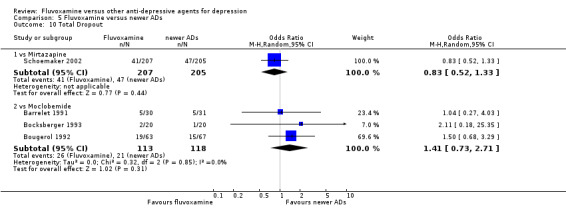
Comparison 5 Fluvoxamine versus newer ADs, Outcome 10 Total Dropout.
5.11. Analysis.
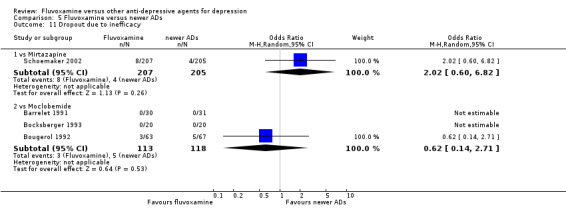
Comparison 5 Fluvoxamine versus newer ADs, Outcome 11 Dropout due to inefficacy.
5.12. Analysis.
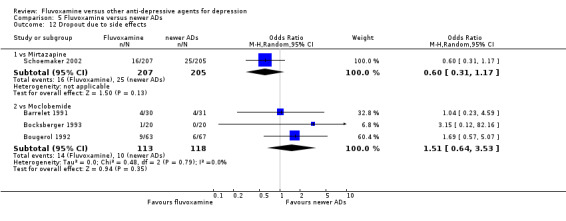
Comparison 5 Fluvoxamine versus newer ADs, Outcome 12 Dropout due to side effects.
5.6.2 Number of patients experiencing at least one side effect
Fluvoxamine did appear to be associated with a higher number of participants experiencing at least one side effect when compared with moclobemide (OR 2.29, 95%CI 1.35 to 3.88, P=0.002; 3 trials, 231 participants) (Analysis 5.13).
5.13. Analysis.
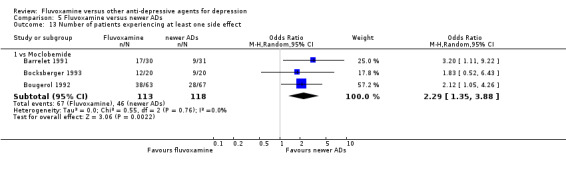
Comparison 5 Fluvoxamine versus newer ADs, Outcome 13 Number of patients experiencing at least one side effect.
5.7 Side effects profile by body system
See: Table 3.
5.7.1 Cardiovascular side effects
In terms of the numbers of participants experiencing hypertension/tachycardia or hypotension/bradycardia, no strong evidence emerged that fluvoxamine was either more or less likely to cause these adverse events than was moclobemide (Analysis 11.1). No studies contributed data to hypertension/tachycardia.
11.1. Analysis.
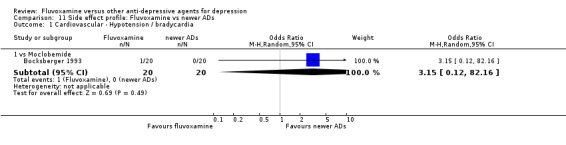
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 1 Cardiovascular ‐ Hypotension / bradycardia.
5.7.2 Dermatological side effects
We found no strong evidence that fluvoxamine was either more or less likely to cause sweating than was moclobemide (Analysis 11.2). No studies contributed data to dermatitis/rash.
11.2. Analysis.
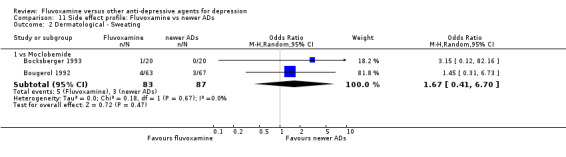
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 2 Dermatological ‐ Sweating.
5.7.3 Gastrointestinal side effects
Fluvoxamine appeared to be associated with a higher number of participants who experienced vomiting/nausea when compared with mirtazapine (OR 3.43, 95%CI 1.90 to 6.19, P<0.001; 1 trial, 412 participants) or moclobemide (OR 2.01, 95%CI 1.03 to 3.92, P=0.04; 2 trials, 170 participants) (Analysis 11.4). We found evidence that fluvoxamine was associated with a higher number of participants who experienced dry mouth when compared with mirtazapine (OR 4.73, 95%CI 1.14 to 19.57, P=0.03; 1 trial, 412 participants) (Analysis 11.3).
11.4. Analysis.
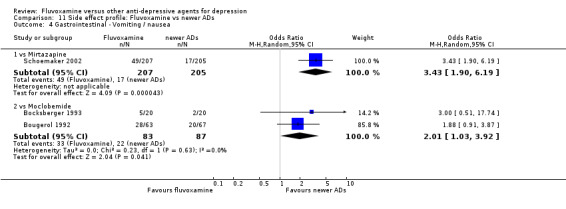
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 4 Gastrointestinal ‐ Vomiting / nausea.
11.3. Analysis.
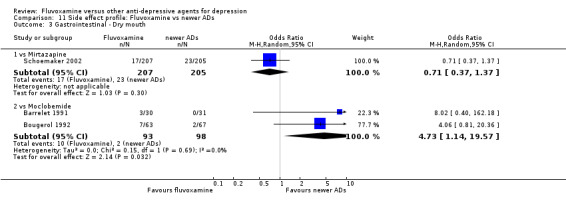
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 3 Gastrointestinal ‐ Dry mouth.
No strong evidence emerged that fluvoxamine, compared with mirtazapine, was either more or less likely to cause constipation, diarrhoea, weight gain or increased appetite. See Analysis 11.5 to Analysis 11.8.
11.5. Analysis.
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 5 Gastrointestinal ‐ Constipation.
11.8. Analysis.
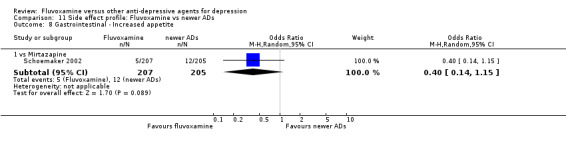
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 8 Gastrointestinal ‐ Increased appetite.
No studies contributed data to increased salivation, oral discomfort/taste disturbance, weight loss or anorexia.
5.7.4 Neuropsychiatric side effects
See Analysis 11.9 to Analysis 11.18.
11.9. Analysis.
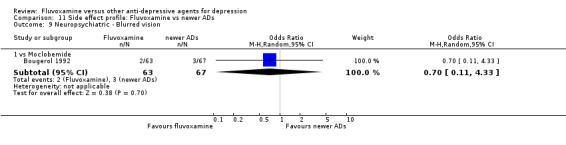
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 9 Neuropsychiatric ‐ Blurred vision.
11.18. Analysis.
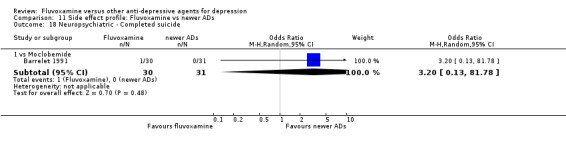
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 18 Neuropsychiatric ‐ Completed suicide.
Fluvoxamine appeared to be associated with lower numbers of participants who experienced sleepiness/drowsiness when compared with mirtazapine (OR 0.47, 95%CI 0.29 to 0.76, P=0.002; 1 trial, 412 participants) (Analysis 11.15). Fluvoxamine was associated with lower numbers of participants who experienced agitation/ anxiety when compared with mirtazapine (OR 0.17, 95%CI 0.05 to 0.61, P=0.03; 1 trial, 412 participants) (Analysis 11.16). Only one trial compared fluvoxamine with moclobemide (Barrelet 1991) and recorded completed suicide (Analysis 11.18), with one event among 30 patients taking fluvoxamine and no events among 31 patients taking moclobemide.
11.15. Analysis.
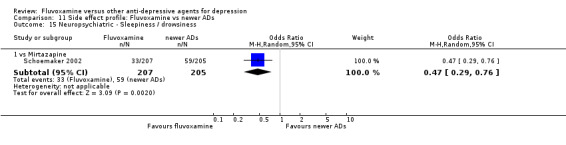
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 15 Neuropsychiatric ‐ Sleepiness / drowsiness.
11.16. Analysis.
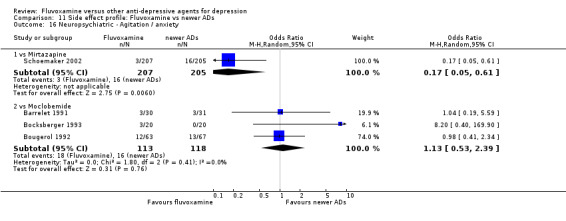
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 16 Neuropsychiatric ‐ Agitation / anxiety.
No studies contributed data to involuntary movements other than tremors or to suicide wishes/gestures/attempts.
5.7.5 Genitourinary side effects
No studies contributed data to genitourinary side effects.
6. FLUVOXAMINE versus OTHER CONVENTIONAL PSYCHOTROPIC DRUGS
Only one 4‐week RCT comparing fluvoxamine to sulpiride (Ueda 2002) contributed usable data for the efficacy analyses and tolerability analyses.
6.1 Response ‐ acute phase (between 6 and 12 weeks); Primary outcome
No studies contributed data to this outcome.
6.2 Response ‐ early phase and follow‐up phase
6.2.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to sulpiride in terms of response at end of the acute‐phase treatment. (Analysis 6.1).
6.1. Analysis.
Comparison 6 Fluvoxamine versus other conventional psychotropic drugs, Outcome 1 Response (early phase).
6.2.2 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
6.3 Remission
6.3.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to sulpiride in terms of remission at end of the early phase treatment. (Analysis 6.2).
6.2. Analysis.
Comparison 6 Fluvoxamine versus other conventional psychotropic drugs, Outcome 2 Remission (early phase).
6.3.2 Acute phase (between 6 and 12 weeks)
No studies contributed data to this outcome.
6.2.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
6.4 Endpoint score on depression scale
6.4.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either superior or inferior to sulpiride in terms of this continuous outcome (Analysis 6.3).
6.3. Analysis.
Comparison 6 Fluvoxamine versus other conventional psychotropic drugs, Outcome 3 Depression scale ‐ Endpoint score: low=good (early phase).
6.4.2 Acute phase (between 6 and 12 weeks)
No studies contributed data to this outcome.
6.4.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
6.5 Change score on depression scale
6.5.1 Early phase (between 1 and 4 weeks)
We found no strong evidence that fluvoxamine was either inferior or superior to sulpiride in terms of this continuous outcome (Analysis 6.4).
6.4. Analysis.
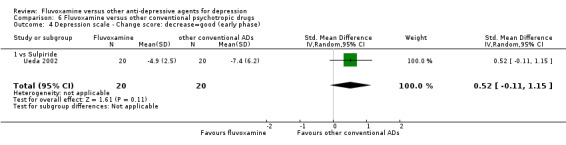
Comparison 6 Fluvoxamine versus other conventional psychotropic drugs, Outcome 4 Depression scale ‐ Change score: decrease=good (early phase).
6.5.2 Acute phase (between 6 and 12 weeks)
No studies contributed data to this outcome.
6.5.3 Follow‐up phase (between 4 and 6 months)
No studies contributed data to this outcome.
6.7 Tolerability
6.7.1 Dropout
We found no strong evidence that fluvoxamine was either more or less acceptable than was sulpiride in terms of withdrawal due to any reason (Analysis 6.5). No studies contributed data to dropout due to inefficacy or due to side effects.
6.5. Analysis.
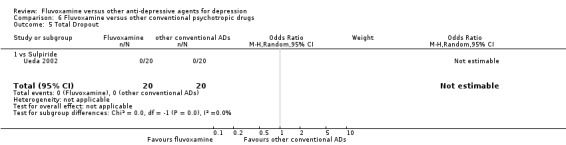
Comparison 6 Fluvoxamine versus other conventional psychotropic drugs, Outcome 5 Total Dropout.
6.7.2 Numbers of patients experiencing at least one side effect
No studies contributed data to this outcome.
6.8 Side effects profile by body system
Ueda 2002 did not report specific side effects.
7. FUNNEL PLOT ANALYSES
Funnel plots were examined only for the comparison between fluvoxamine and TCAs as a class, since there were insufficient trials to allow meaningful formal assessment using funnel plots for other comparisons. Visual inspection did not reveal an asymmetrical appearance of the funnel plot (Figure 12). Tests for funnel plot asymmetry (Egger 1997) did not suggest any strong evidence of asymmetry (Egger's bias coefficient, number of trials= 16, bias = 0.09 (P = 0.94)). However, included trials were of similar size (sample size: 23 to 100) and similar standard errors of OR (SE of ln(OR): 0.40 to 0.89), and the test for funnel plots should not be used (Higgins 2008).
12.
Funnel plot of comparison: 1 Fluvoxamine versus TCAs, outcome: 1.1 Response (acute phase): Primary outcome.
8. SUBGROUP ANALYSES
We conducted subgroup analyses only for fluvoxamine dosing ‐ standard versus high dose (fluvoxamine versus TCAs, fluvoxamine versus other SSRIS), comparator dosing (fluvoxamine versus TCAs, fluvoxamine versus other SSRIS) and treatment settings (fluvoxamine versus TCAs), since there were insufficient number of trials that could produce subgroups and useful findings. Differences in subgroups were reported only for response rates in acute phases.
8.1 Fluvoxamine dosing ‐ standard dose versus high dose
8.1.1 Fluvoxamine versus TCAs
Only 6 trials used standard dose schedules, and 10 trials used high dose schedules. The magnitude of effects in the trials using standard doses of fluvoxamine (OR: 0.83, 95% CI 0.53 to 1.46) and high doses of fluvoxamine (OR: 1.07, 95% CI 0.74 to 1.55) were similar and their CIs were overlapped (Analysis 1.16, Analysis 1.17).
1.16. Analysis.
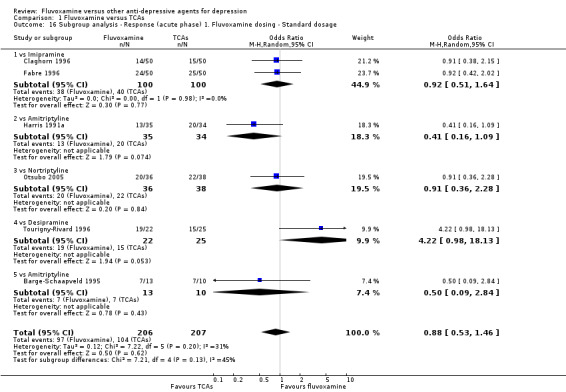
Comparison 1 Fluvoxamine versus TCAs, Outcome 16 Subgroup analysis ‐ Response (acute phase) 1. Fluvoxamine dosing ‐ Standard dosage.
1.17. Analysis.
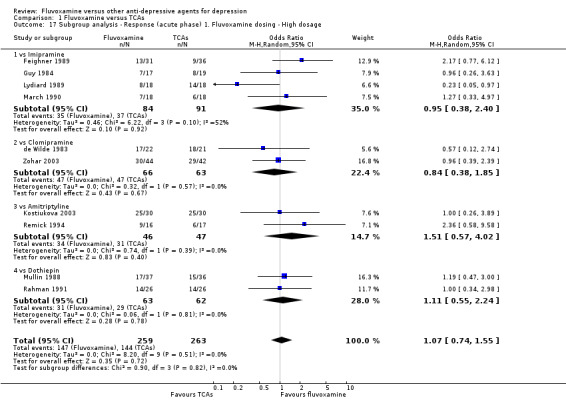
Comparison 1 Fluvoxamine versus TCAs, Outcome 17 Subgroup analysis ‐ Response (acute phase) 1. Fluvoxamine dosing ‐ High dosage.
8.1.2 Fluvoxamine versus other SSRIs
Only 5 trials used standard dose schedules, and 3 trials used high dose schedules. The magnitude of effects in the trials using standard doses of fluvoxamine (OR: 0.92, 95% CI 0.65 to 1.30) and high doses of fluvoxamine (OR: 1.02, 95% CI 0.65 to 1.60) were similar and their CIs were overlapped (Analysis 3.16, Analysis 3.17).
3.16. Analysis.
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 16 Subgroup analysis ‐ Response (acute phase) 1. Fluvoxamine dosing ‐ Standard dosage.
3.17. Analysis.
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 17 Subgroup analysis ‐ Response (acute phase) 1. Fluvoxamine dosing ‐ High dosage.
8.2 Comparator dosing
8.2.1 Fluvoxamine versus TCAs
Only 4 trials used standard dose schedules and 15 trials used high dose schedules of comparator drugs. The magnitude of effects in the trials using standard dose comparator drugs (OR: 1.27, 95% CI 0.64 to 2.50) and high dose comparator drugs (OR: 0.99, 95% CI 0.74 to 1.33) were similar and their CIs were overlapped (Analysis 1.18, Analysis 1.19).
1.18. Analysis.
Comparison 1 Fluvoxamine versus TCAs, Outcome 18 Subgroup analysis ‐ Response (acute phase) 2. Comparator dosing ‐ Standard dosage.
1.19. Analysis.
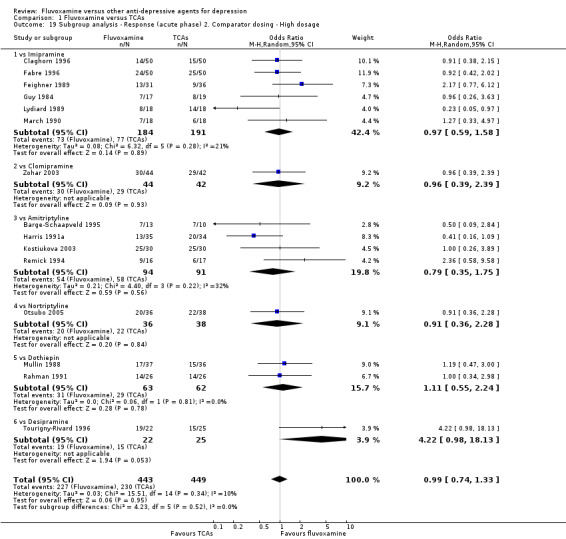
Comparison 1 Fluvoxamine versus TCAs, Outcome 19 Subgroup analysis ‐ Response (acute phase) 2. Comparator dosing ‐ High dosage.
8.2.2 Fluvoxamine versus other SSRIs
Only 2 trials used standard dose schedules, and 6 trials used high dose schedules of comparator drugs. The magnitude of effects in the trials using standard dose comparator drugs (OR: 0.95, 95% CI 0.60 to 1.50) and high dose of comparator drugs (OR: 0.96, 95% CI 0.70 to 1.33) were similar and their CIs were overlapped (Analysis 3.18, Analysis 3.19).
3.18. Analysis.
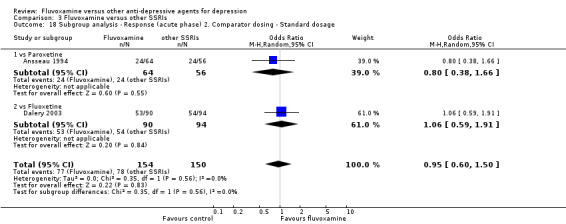
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 18 Subgroup analysis ‐ Response (acute phase) 2. Comparator dosing ‐ Standard dosage.
3.19. Analysis.
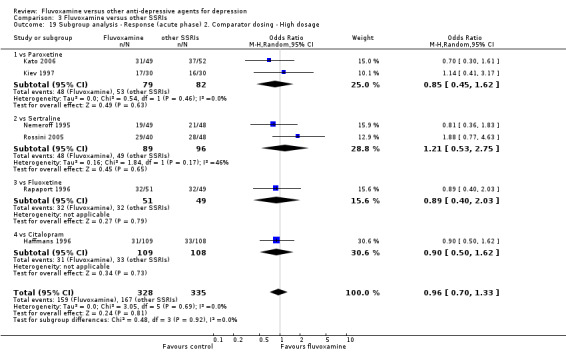
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 19 Subgroup analysis ‐ Response (acute phase) 2. Comparator dosing ‐ High dosage.
8.3 Treatment settings
8.3.1 Fluvoxamine versus TCAs
Only 5 trials were conducted in inpatient settings and 9 trials were conducted in outpatient settings. The magnitude of effects in the trials that were conducted in inpatient settings (OR: 1.17, 95% CI 0.71 to 1.92) and the trials conducted in outpatient settings (OR: 0.84, 95% CI 0.58 to 1.20) were similar and their CIs were overlapped (Analysis 1.20, Analysis 1.21).
1.20. Analysis.
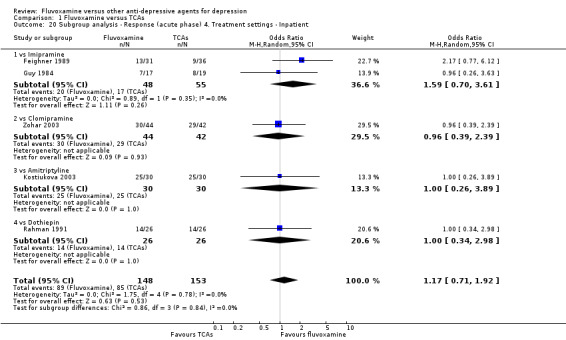
Comparison 1 Fluvoxamine versus TCAs, Outcome 20 Subgroup analysis ‐ Response (acute phase) 4. Treatment settings ‐ Inpatient.
1.21. Analysis.
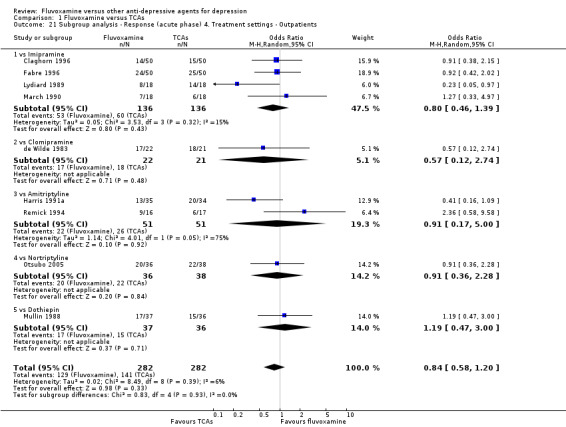
Comparison 1 Fluvoxamine versus TCAs, Outcome 21 Subgroup analysis ‐ Response (acute phase) 4. Treatment settings ‐ Outpatients.
9. SENSITIVITY ANALYSES
We reported the results of sensitivity analyses for efficacy outcome at the acute phase. In the main analyses, we found no evidence that fluvoxamine was either more or less effective than other ADs as a class. In head‐to‐head comparisons, fluvoxamine was more effective than desipramine (response rate at acute phase; OR: 4.22, 95% CI 0.98 to 18.13, P=0.05; 1 trial, 47 participants), while venlafaxine was more effective than fluvoxamine (response rate at acute phase; OR: 0.40, 95% CI 0.18 to 0.92, P=0.03; 1 trial (2 comparisons), 111 participants).
9.1 Excluding trials with unclear concealment of random allocation and/or unclear double blinding
We did not perform sensitivity analyses of excluded trials with unclear concealment of random allocation, as there were no studies that reported details of having conducted allocation concealment. In addition, we did not perform sensitivity analysis of excluding with unclear double blinding as there were only two studies rated as being "low risk of bias" regarding blinding.
9.2 Excluding trials with dropout rates greater than 20%
No substantial change was found in the main findings when fluvoxamine and TCAs (Analysis 1.22) or fluvoxamine and other SSRIs (Analysis 3.20) were compared for sensitivity analysis. We did not perform sensitivity analysis for comparisons between fluvoxamine and heterocyclics, SNRIs or newer ADs because dropout rates greater than 20 % were reported for all of the trials of these comparisons that reported primary outcome.
1.22. Analysis.
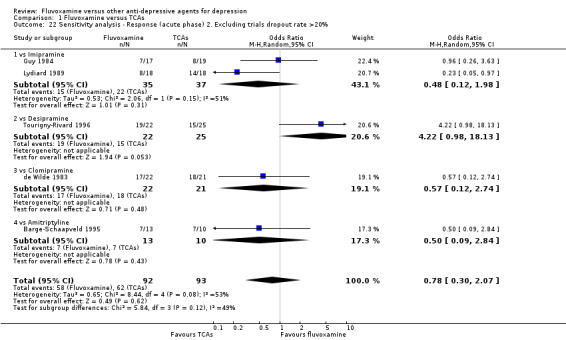
Comparison 1 Fluvoxamine versus TCAs, Outcome 22 Sensitivity analysis ‐ Response (acute phase) 2. Excluding trials dropout rate >20%.
3.20. Analysis.
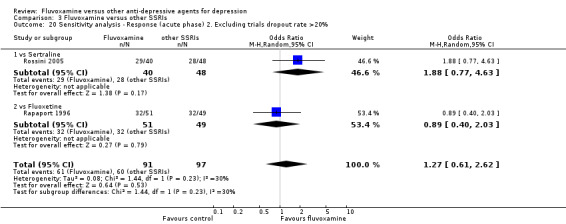
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 20 Sensitivity analysis ‐ Response (acute phase) 2. Excluding trials dropout rate >20%.
9.3 Performing the worst case scenario ITT
Based on sensitivity analysis, fluvoxamine was found to be less effective in terms of response at acute phase than were imipramine (OR 0.60 95%CI 0.39 to 0.90, P=0.01; 6 trials, 375 participants), amitriptyline (OR 0.34 95%CI 0.13 to 0.90, P=0.03; 4 trials, 185 participants), dothiepin (OR 0.34 95%CI 0.16 to 0.72, P=0.005; 2 trials, 125 participants) or venlafaxine (OR 0.11 95%CI 0.04 to 0.29, P<0.001; 1 trial (2 comparisons), 111 participants). See Analysis 1.23, Analysis 4.13.
1.23. Analysis.
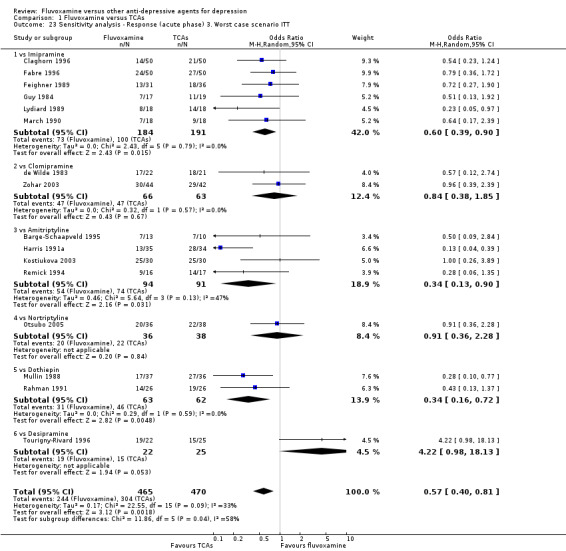
Comparison 1 Fluvoxamine versus TCAs, Outcome 23 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT.
4.13. Analysis.
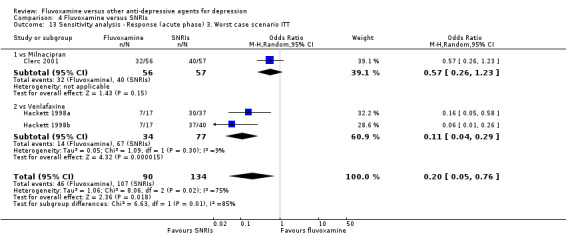
Comparison 4 Fluvoxamine versus SNRIs, Outcome 13 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT.
9.4 Performing the best case scenario ITT
Based on sensitivity analysis, fluvoxamine was found to be more effective in terms of response at acute phase than were dothiepin (OR 4.04 95%CI 1.85 to 8.81, P<0.001; 2 trials, 125 participants) (Analysis 1.24), mianserin (OR 3.04 95%CI 1.20 to 7.67, P=0.02; 2 trials, 125 participants) (Analysis 2.16) or paroxetine (OR 1.77 95%CI 1.08 to 2.92, P=0.02; 3 trials, 281 participants) (Analysis 3.22). In addition, based on this sensitivity analysis, the superiority of venlafaxine over fluvoxamine in terms of response at acute phase was lost ( (OR 2.67 95%CI 0.98 to 7.26, P=0.05; 1 trial (2 comparisons), 111 participants) (Analysis 4.14).
1.24. Analysis.
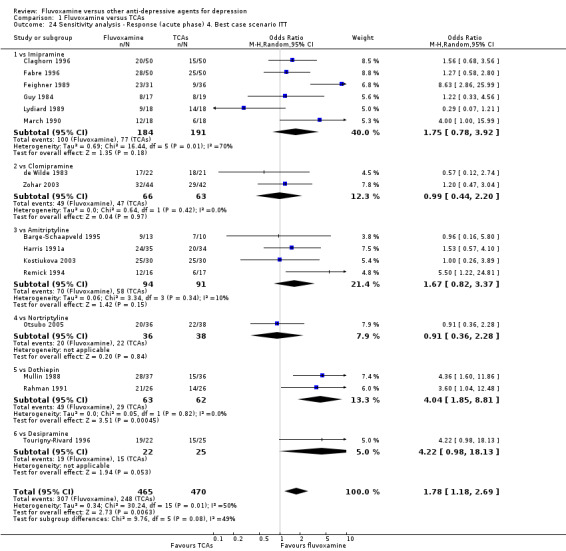
Comparison 1 Fluvoxamine versus TCAs, Outcome 24 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT.
2.16. Analysis.
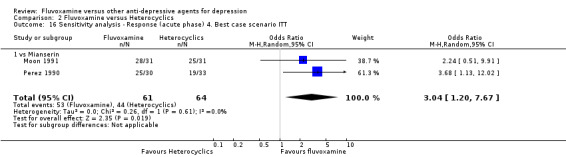
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 16 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT.
3.22. Analysis.
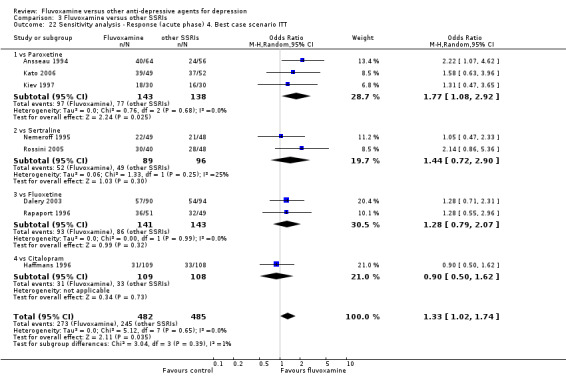
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 22 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT.
4.14. Analysis.
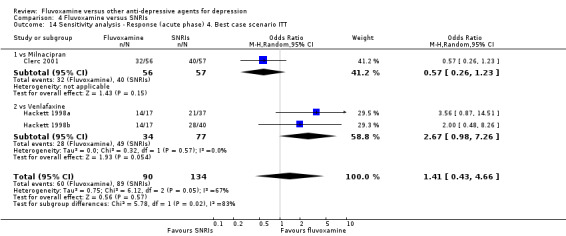
Comparison 4 Fluvoxamine versus SNRIs, Outcome 14 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT.
9.5 Excluding trials for which the response rates had to be calculated based on the imputation method and for which the SD had to be borrowed from other trials
We conducted this sensitivity analysis only for comparison between fluvoxamine and TCAs, and between fluvoxamine and other SSRIs, since there were insufficient numbers of trials that could produce useful findings for other comparisons. No substantial change in the results was found by excluding trials with the imputation method for calculating responses (Analysis 1.25, Analysis 3.23) or by excluding trials that borrowed SDs for imputation (Analysis 1.26, Analysis 3.24).
1.25. Analysis.
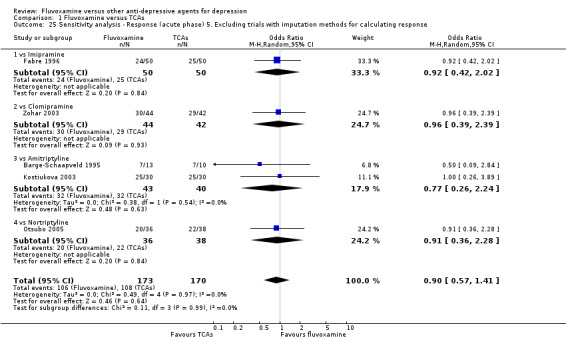
Comparison 1 Fluvoxamine versus TCAs, Outcome 25 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response.
3.23. Analysis.
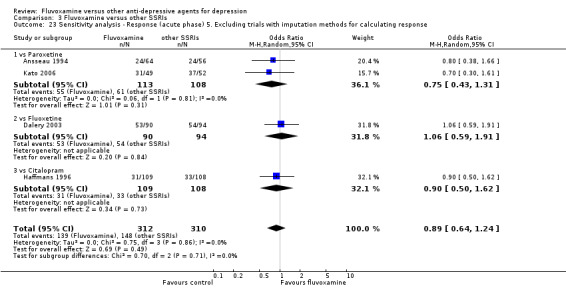
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 23 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response.
1.26. Analysis.
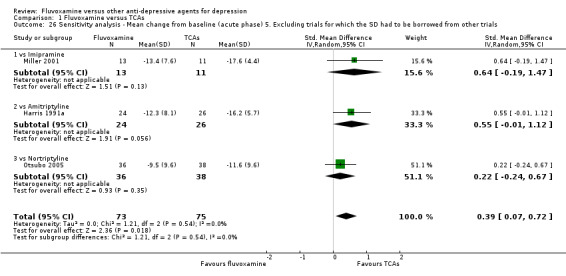
Comparison 1 Fluvoxamine versus TCAs, Outcome 26 Sensitivity analysis ‐ Mean change from baseline (acute phase) 5. Excluding trials for which the SD had to be borrowed from other trials.
3.24. Analysis.
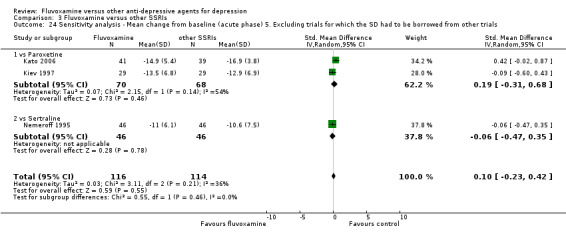
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 24 Sensitivity analysis ‐ Mean change from baseline (acute phase) 5. Excluding trials for which the SD had to be borrowed from other trials.
9.6 Examination of "wish bias" by comparing fluvoxamine as an investigational drug versus fluvoxamine as a comparator
Examination of "wish bias" was impossible because no trials comparing fluvoxamine with TCAs set fluvoxamine as a comparator; among studies comparing fluvoxamine with ADs other than TCAs, only three trials set fluvoxamine as an investigational drug.
9.7 Excluding studies funded by or with at least one author affiliated with a pharmaceutical company marketing fluvoxamine
A sensitivity analysis to investigate the effect of commercial funding, excluding studies sponsored by pharmaceutical companies, was impossible, as almost all of the included trials had been funded by the industry. For example, among 30 trials comparing fluvoxamine with TCAs, there were only two trials free from commercial funding. Therefore, it is impossible to obtain substantial results from this sensitivity analysis.
9.8 Excluding studies that included patients with bipolar depression
No substantial changes in the main findings were noted by these sensitivity analyses (Analysis 1.30, Analysis 2.20, Analysis 3.28, Analysis 4.18).
1.30. Analysis.
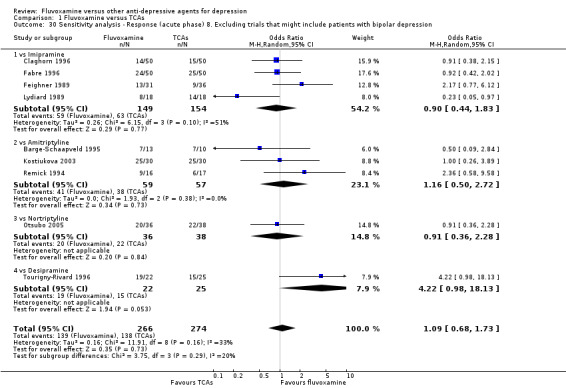
Comparison 1 Fluvoxamine versus TCAs, Outcome 30 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression.
2.20. Analysis.
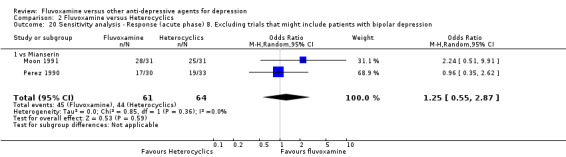
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 20 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression.
3.28. Analysis.
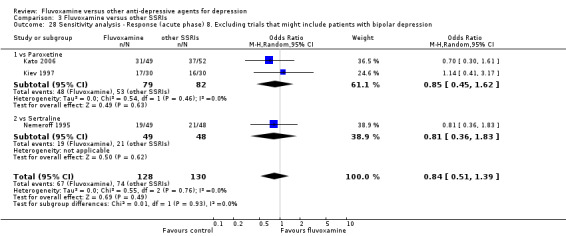
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 28 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression.
4.18. Analysis.
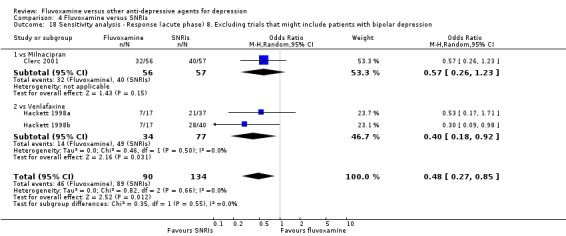
Comparison 4 Fluvoxamine versus SNRIs, Outcome 18 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression.
9.9 Excluding studies that included patients with psychotic features
No substantial changes in the main findings were noted by these sensitivity analyses (Analysis 1.31, Analysis 2.21, Analysis 3.29, Analysis 4.19, Analysis 5.21).
1.31. Analysis.
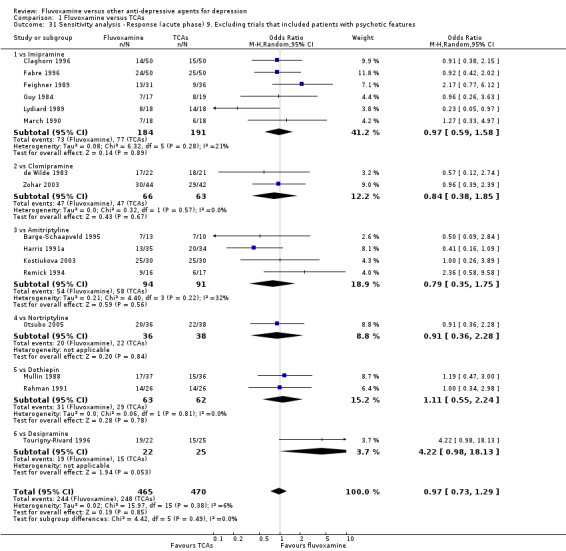
Comparison 1 Fluvoxamine versus TCAs, Outcome 31 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features.
2.21. Analysis.
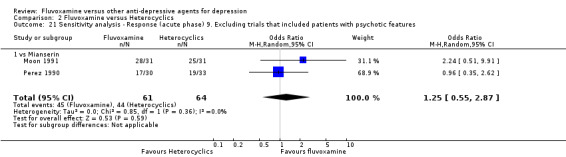
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 21 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features.
3.29. Analysis.
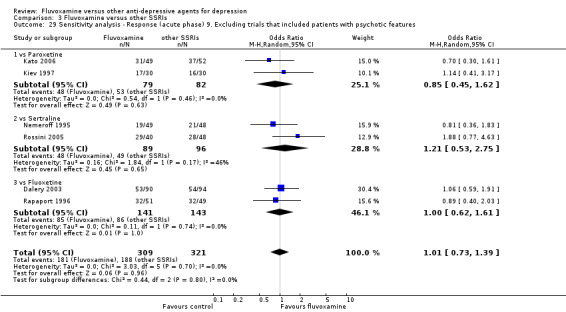
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 29 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features.
4.19. Analysis.
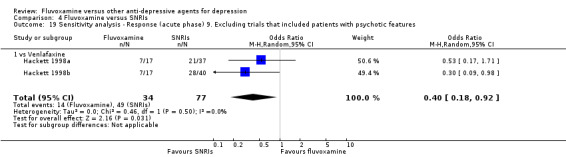
Comparison 4 Fluvoxamine versus SNRIs, Outcome 19 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features.
5.21. Analysis.
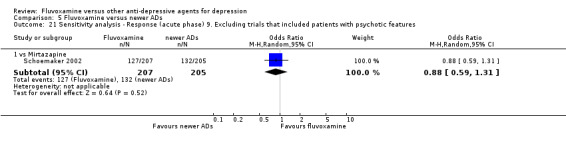
Comparison 5 Fluvoxamine versus newer ADs, Outcome 21 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features.
Discussion
Summary of main results
The main results of our study revealed no strong evidence that fluvoxamine was either inferior or superior to other antidepressants, including TCAs such as amitriptyline or clomipramine, other SSRIs or other forms, in terms of either response or remission in any clinical setting, even though a small number of findings suggested a direction of effect in favour of fluvoxamine (versus desipramine) or in favour of control drug (venlafaxine) in terms of response and remission at acute phase. This was somewhat surprising because TCAs are sometimes believed to be more effective than SSRIs, particularly among hospitalized depressive patients (Anderson 1998).
We were also unable to find differences in dropouts for any reason, including side effects, between fluvoxamine and other antidepressive agents. Although the 95% CI are wide and we cannot exclude possibilities of meaningful differences between fluvoxamine and other antidepressive agents, our findings at least suggest a need for moderation of the general statement that holds that patients tolerate SSRIs better than TCAs. In addition, we included 10 trials involving fluvoxamine in our review, and from the pooled data, we were unable to find any differences in total dropouts or dropouts due to side effects between fluvoxamine and other SSRIs.
The analysis of individual side effects points to evidence of differing side effects profiles, especially when comparing gastrointestinal side effects between fluvoxamine and TCAs. Diarrhoea and weight loss were experienced significantly more frequently with fluvoxamine than with TCAs. Vomiting/nausea and weight loss/anorexia were also experienced significantly more frequently with fluvoxamine than with TCAs and some other antidepressive agents (e.g., mianserin, milnacipran and some newer antidepressants). However, constipation and decreased salivation/dry mouth were more common with TCAs than with fluvoxamine.
Not only are SSRIs chemically different from TCAs, heterocyclics and other antidepressive agents, but considerable structural differences also exist even among the various SSRIs. Therefore, some differential pharmacology between the drugs in the same class may be expected. However, head‐to‐head comparisons found no evidence to suggest side effect profile differences between fluvoxamine and other SSRIs, except for sweating, which was more common in recipients of paroxetine than fluvoxamine in a single trial.
Overall completeness and applicability of evidence
This review has a number of limitations in overall completeness and applicability.
Participants
Severity of depression was believed to be associated with good treatment response in patients taking TCAs compared with those taking SSRIs (Anderson 1994), However, all of the studies included in this review, other than that of Claghorn 1996, were conducted for moderate/mild depression. For this reason, the findings from this review may not be representative of more severely affected patients.
In addition, the findings from this review may not be representative for the depressive elderly, since only one trial was found in which recruitment was wholly conducted for the depressive elderly (Bocksberger 1993). Elder patients with depression are recognized as being more vulnerable to adverse effects of antidepressants (Schneider 1995). If fluvoxamine is more or less acceptable than other antidepressants, this advantage/disadvantage might be obvious in trials focused on geriatric depression.
Twenty‐five out of 54 trials explicitly included or might have included patients with major depressive episodes caused by bipolar disorder. However, we found no heterogeneity in the pooled results.
In many countries, including the UK, many patients with depression are treated solely in primary care (Spijker 2001, Goldberg 1991), and depressive disorder in primary care has a different profile than treated in secondary care (Suh 1997). However, we could find only two articles conducted in primary care settings (Barge‐Schaapveld 1995; Moon 1991), and the relevance of the studies included in this review to primary care settings should be limited.
Interventions
Considering the often chronic and recurrence‐prone presentation of major depression, long‐term or follow‐up interventions are often required for optimal treatment of this disorder. However, we could find no studies that examined the long‐term efficacy of fluvoxamine for major depression.
Outcomes
Treatments for major depression should be assessed not only by psychiatric symptoms, but also by general functioning and/or QOL. However, no trials included in this review incorporated those outcomes. Considering that major depression is associated with a marked personal, social and economic morbidity, the under‐investigation of these outcomes borders on negligence. In addition, no studies included cost to health care services as an outcome. The choice of antidepressants may be influenced by factors such as safety of the medication in overdose and the propensity of the medication to be associated with withdrawal symptoms. However, we found no randomised trials that examined these factors in this review.
Quality of the evidence
Fifty‐four trials with 4353 patients were included in this analysis; 2117 were randomised to fluvoxamine and 2236 to comparator drugs. These included trials had a number of methodological shortcomings.
Randomisation
All of the trials except one (Rossini 2005) failed to describe methods of random sequence generation. In addition, no trials reported the method of allocation concealment. Therefore, it is conceivable that selection bias might have occurred in the trials included in this review.
Blinding
Information on blinding was sought for many trials; however, no test of blinding success was conducted in any study. The use of an independent, blind assessor was explicitly described in only three studies (Miller 2001; Otsubo 2005; Rossini 2005). On the whole, little information was presented on the outcome assessment process, and the extent to which detection bias might have occurred was uncertain.
Selective outcome reporting
Forty‐four out of the 54 included studies used the HRSD as a primary or secondary outcome measure, while a minority of studies used the MADRS and CGI. It is conceivable that the study authors did not report some outcomes in which results failed to show statistical significance. Therefore, the results of our meta‐analyses could overestimate the intervention effect due to outcome reporting bias. In addition, only a few studies reported SDs for a change and endpoint score of any depression scale that we adopted as a secondary outcome, which meant that we had to borrow SDs from other trials that did report SDs for the outcomes.
High discontinuation rate
Twenty‐seven of the 54 studies had dropout rates higher than 20%. This high attrition rate could have influenced treatment outcomes; for two studies with high attrition rate (Mullin 1988 and Rahman 1991), sensitivity analysis performing the worst case scenario revealed the statistically significant results in favour of the comparator and the best case scenario in favour of fluvoxamine.
Sample size
If we assumed that response rate for experimental antidepressants was 50% and that of the control was 35%, 183 participants would be needed in each treatment group to detect this type of difference at 80% power and 95% confidence. However, most trials included in this review were small; the majority of the studies (38 RCTs) recruited less than 100 participants in total. This made it difficult to interpret the negative finding. In addition, most trials did not discuss their low power or did not conduct sample size calculation
Potential biases in the review process
Strength
The comprehensiveness of our study search and the strict quality appraisal we required before any study could be included in the final pooling of the results contributed to the strength of our review. As a result, randomised evidence from unpublished (Schoemaker 2002) as well as published studies was included in the review. Furthermore, our strong efforts made to obtain missing data resulted in additional data from investigators from 15 trials.
We also imputed response and remission outcomes by applying a threshold of the standard depression severity scales, such as the HRSD or MADRS, with a validated statistical method if they were not available in original trials (Furukawa 2005: Furukawa 2006). We believe our methodology can be used in future systematic reviews in order to minimise outcome reporting bias and to make the most use of information from the obtained trials.
Limitations
This systematic review is not without methodological problems.
First of all, although neither the funnel plot nor Egger's test detected small‐study effects, we still cannot rule out the risk of publication bias. There were only 16 studies included in the funnel plots, and all studies were of similar size; therefore, it was difficult to find a meaningful result from funnel plots and their statistical tests. In addition, we have concerns about those five studies that only reported biochemical or physiological outcomes, instead of reporting clinical efficacy and/or tolerability outcomes. For example, one RCT reported prolactin responses to d‐fenfluramine for depressive patients, before and after medication but included no clinical outcome at all (Kavoussi 1999). This trial formed part of an industry drug trial sponsored by Solvay, the primary fluvoxamine marketer. We were unable to locate a trial matching the description in this report, and we strongly suspect that we are missing a large trial sponsored by this company. We excluded a crossover trial that did not report the result of first randomisation period (Emrich 1987) and a trial that only reported the result of a maintenance period (White 1990). We tried to contact the original authors to obtain missing outcomes but our attempts were in vain. We believe that publication bias remained a very real risk in this review.
Second, pharmaceutical companies marketing fluvoxamine sponsored a large majority of the trials comparing fluvoxamine with TCAs; the majority of the authors of these trials set fluvoxamine as an investigational drug. Therefore, comparability between fluvoxamine and TCAs in efficacy and tolerability may not be unconditionally warranted; that is to say, the efficacy and tolerability of fluvoxamine over TCAs might be overestimated (Barbui 2004).
Third, although sensitivity analyses excluding trials for which the response rates had to be calculated based on the imputation method did not find any evidence that it changed the results of the main analyses, this could have happened for many reasons other than validity of the imputation methods. For example, there were few studies included in each analysis and most studies were small‐sized trials that reported null effects; therefore, removing some trials is unlikely to have a large effect.
Finally, very few of the trials employed standardized instruments in the reporting of side effects. Many of the side effects experienced by patients taking antidepressants may be confused with symptoms and signs of depression. Many trials reported the number of patients who experienced unwanted symptoms during trials, but some articles defined side effects strictly as experiences that appeared for the first time during the treatment period, or experiences that appeared between screen and baseline, but increased in severity during the treatment period. Some trials did not report a side effect profile at all. Obviously the emphasis on detecting side effects differs between trials, and this may explain some of the observed differences.
Agreements and disagreements with other studies or reviews
Edwards 1999 conducted a well‐designed systematic review and meta‐analysis of RCTs, involving direct comparisons between five SSRIs in the treatment of major depressive illness. The review reported that significantly more patients on fluvoxamine stopped treatment due to any reason and due to side effects compared with other SSRIs. However, this review included only five trials involving fluvoxamine, and these are outdated. The clinical guidelines released by the same authors (Anderson 2001) suggested that fluvoxamine was not the best SSRI choice in routine practice because of its relatively high discontinuation rate, but the results of our review do not provide any evidence that support or argue against this statement.
We have recently published a multiple‐treatment meta‐analysis (MTM) in which our data for fluvoxamine were merged with those for 11 other new generation antidepressants and both direct and indirect comparisons among them were statistically pooled (Cipriani 2009). The MTM offers a clinically meaningful synthesis when several competing treatments are available for one disease (Lumley 2002; Lu 2006; Salanti 2008), as is the case with major depression, while examining the overall strength and consistency of this network of evidence. The corresponding ORs and their 95%CI for response and total dropout are tabulated in Table 4.
4. Ratio of ORs from MTM and this review.
| Outcomes | OR (95%CI) | Ratio of ORs* | |
| MTM (direct + indirect comparisons) (Cipriani 2009) | Direct comparisons (This review) | ||
| Response | |||
| paroxetine | 0.96 (0.76 to 1.23) | 0.83 (0.51 to 1.34) | 1.16 |
| sertraline | 0.79 (0.61 to 1.01) | 1.21 (0.53 to 2.75) | 0.65 |
| fluoxetine | 0.98 (0.77 to 1.23) | 1.00 (0.62 to 1.61) | 0.98 |
| citalopram | 0.88 (0.68 to 1.16) | 0.90 (0.50 to 1.62) | 0.98 |
| milnacipran | 0.97 (0.68 to 1.37) | 0.57 (0.26 to 1.23) | 1.70 |
| venlafaxine | 0.77 (0.59 to 0.99) | 0.40 (0.18 to 0.92) | 1.93 |
| mirtazapine | 0.71 (0.55 to 0.92) | 0.88 (0.59 to 1.31) | 0.81 |
| Total dropout | |||
| paroxetine | 1.10 (0.84 to 1.47) | 1.08 (0.63 to 1.85)** | 1.02 |
| sertraline | 1.38 (1.03 to 1.89) | 1.46 (0.19 to 11.16)** | 0.95 |
| fluoxetine | 1.22 (0.93 to 1.61) | 1.17 (0.66 to 2.09)** | 1.04 |
| citalopram | 1.37 (1.01 to 1.85) | 1.42 (0.75 to 2.67) | 0.96 |
| milnacipran | 1.18 (0.76 to 1.75) | 1.22 (0.54 to 2.77)** | 0.97 |
| venlafaxine | 1.14 (0.86 to 1.54) | 2.29 (0.97 to 5.43) | 0.50 |
| mirtazapine | 1.18 (0.87 to 1.61) | 0.83 (0.52 to 1.33) | 1.42 |
| MTM, multiple‐treatments meta‐analysis; OR, odds ratio; CI, confidence interval; For response, ORs higher than 1 favor fluvoxamine. For total dropout, ORs lower than one favour fluvoxamine; * ORs of this review as reference; **Data from three comparisons (Ansseau 1991a, Ansseau 1991b, and Gonul 1999) were omitted because these 4‐week trials were not included in MTM (Cipriani 2009). | |||
We note that: 1) all of the confidence intervals overlap widely between direct comparisons and MTM (direct + indirect) comparisons, mainly because the 95%CI for direct comparisons are wide, generally indicating that the network of evidence is consistent. 2) When nominal superiority of fluvoxamine existed in direct comparisons (fluvoxamine over sertraline for response, and fluvoxamine over mirtazapine for dropout), this was lost in the MTM, although again their 95%CI overlapped widely. 3) When the ratio of ORs was greater than 1.5 or smaller than 0.67 (versus sertraline, versus milnacipran and versus venlafaxine for response, and versus venlafaxine for dropout), the MTM results were almost always less extreme (i.e., closer to 1.0) than the direct comparisons, very probably because of cancelling out possible sponsorship, publication and other biases.
The relative merits and demerits of direct versus MTM comparisons are still debatable (Bucher 1997; Song 2003; Ioannidis 2006) and we need to carefully weigh and synthesize the direct with the indirect comparisons.
Authors' conclusions
Implications for practice.
The main finding of the present study is that the present review is based on poor quality evidence of primary studies and we found no strong evidence that fluvoxamine was either superior of inferior to any other antidepressants, including TCAs and SSRIs, in terms of efficacy and tolerability during the treatment of depression in the acute phase. On the other hand, evidence is clear for differing side effect profiles, especially in terms of gastrointestinal side effects between fluvoxamine and TCAs. The results of the study led us to conclude that clinicians should focus on practical or clinically relevant considerations, including these differences in side effect profiles.
Implications for research.
We could have learnt more about the effects of fluvoxamine if the studies included in the review had clearly described sequence generation, allocation concealment, blinding of outcome assessors, and reasons for dropout. In addition to assessment of depressive symptoms and levels of general functioning, QOL and patient satisfaction would be most informative. If continuous rating scales are to be employed, a concerted effort should be made to come up with an agreement as to which measures are the most useful. For analyses of side effect profiles, more reliable and consistent methods of monitoring and reporting side effects during the trial are needed.
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2013 | Amended | Minor error in Additional Table 3 corrected. |
| 1 November 2008 | Amended | Converted to new review format. |
Acknowledgements
This review is one publication of the Meta‐Analyses of New Generation Antidepressants (MANGA) project in which a group of researchers within the Cochrane Collaboration Depression, Anxiety and Neurosis Group agreed to conduct a systematic review of all available evidence for 12 new generation antidepressants to inform clinical practice and mental health policies. We would like to thank Julian Higgins and Georgia Salanti for their helpful comments and feedback on this review. We would like to thank the CCDAN Editorial Team for their support, information and advice.
We also wish to thank Dr Tatsuo Akechi for excellent and thorough comments on all aspects of the review and Dr Nicolai Ostrovischi for generously translating a Russian article into English and extracting data for this review.
We sent several letters asking for extra information about their trials to authors. Dr Alan Feiger, Dr Alessandro Serretti, Dr Ari Kiev, Dr Arif Khan, Dr Burl Daviss, Dr Carlos Augusto de Mendonca Lima, Dr Carmen Lefebvre, Dr Charles B. Nemeroff, Dr Christine Ehlerding, Dr Daniela Q.C.M. Barge‐Schaapveld, Dr David Hackett , Dr Dorotea Muck‐Seler, Dr Doug Keller, Dr Emil Coccaro, Dr Eugene Paykel, Dr Geroge Wan, Dr Jackie Gollan, Dr Jacqueline Strik, Dr James M. Perel, Dr Jean Dalery, Dr Jean‐Pierre Gachoud, Dr Joep Schoemaker, Dr Johan Denollet, Dr L. Lee Tynes, Dr Lev Sverdlov, Dr Marc Ansseau, Dr Marco Mula, Dr Mario Amore, Dr Masaki Kato, Dr Matt Lehman, Dr Michael Mueck‐Weymann, Dr Michael Poole, Dr Nancy A. Nicolson, Dr Nobuhisa Ueda, Dr R. Michael Poole, Dr Raffaella Zanardi, Dr Ram K. Shrivastava, Dr Reiji Yoshimura, Dr Rob Sival, Dr Robert Ferdinand, Dr Robert N. Golden, Dr Roberto Dominguez, Dr Ronald A. Remick, Dr Sandra Kooij, Dr Siegfried Kasper, Dr Tenpei Otsubo, Dr Thomas Rechlin, Dr Victoria J. Grochocinski, Dr Walter Brown, Dr Walter D. Lawhorn, and Dr Yvon.D. Lapierre, were kind enough to respond, for which we are very grateful.
Data and analyses
Comparison 1. Fluvoxamine versus TCAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response (acute phase): Primary outcome | 16 | 935 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.73, 1.29] |
| 1.1 vs Imipramine | 6 | 375 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.59, 1.58] |
| 1.2 vs Clomipramine | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.38, 1.85] |
| 1.3 vs Amitriptyline | 4 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.35, 1.75] |
| 1.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 1.5 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.24] |
| 1.6 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 2 Response (early phase) | 25 | 2148 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.15] |
| 2.1 vs Imipramine | 12 | 1213 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.78, 1.29] |
| 2.2 vs Clomipramine | 5 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.71] |
| 2.3 vs Amitriptyline | 4 | 397 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.13, 1.19] |
| 2.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.58, 4.73] |
| 2.5 vs Desipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.13] |
| 2.6 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.35, 2.68] |
| 3 Remission (early phase) | 25 | 2148 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.73, 1.22] |
| 3.1 vs Imipramine | 12 | 1213 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.71, 1.42] |
| 3.2 vs Clomipramine | 5 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.40, 1.41] |
| 3.3 vs Amitriptyline | 4 | 397 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.10, 1.76] |
| 3.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 2.33 [0.54, 10.14] |
| 3.5 vs Desipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 3.6 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.18, 5.16] |
| 4 Remission (acute phase) | 16 | 935 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.69, 1.45] |
| 4.1 vs Imipramine | 6 | 375 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.59, 1.94] |
| 4.2 vs Clomipramine | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.28, 1.49] |
| 4.3 vs Amitriptyline | 4 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.28, 1.31] |
| 4.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 1.78 [0.67, 4.77] |
| 4.5 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.48, 2.35] |
| 4.6 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.5 [1.31, 15.42] |
| 5 Depression scale ‐ Endpoint score: low=good (early phase) | 5 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.01, 0.57] |
| 5.1 vs Imipramine | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.00, 1.05] |
| 5.2 vs Amitriptyline | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.08, 1.02] |
| 5.3 vs Desipramine | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.49, 0.84] |
| 5.4 vs Dothiepin | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.83, 0.41] |
| 6 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 6.1 vs Imipramine | Other data | No numeric data | ||
| 6.2 vs Clomipramine | Other data | No numeric data | ||
| 6.3 vs Amitriptyline | Other data | No numeric data | ||
| 6.4 vs Nortriptyline | Other data | No numeric data | ||
| 7 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 7.1 vs Imipramine | Other data | No numeric data | ||
| 7.2 vs Clomipramine | Other data | No numeric data | ||
| 7.3 vs Amitriptyline | Other data | No numeric data | ||
| 7.4 vs Nortriptyline | Other data | No numeric data | ||
| 7.5 vs Dothiepin | Other data | No numeric data | ||
| 7.6 vs Desipramine | Other data | No numeric data | ||
| 8 Depression scale ‐ Change score: decrease=good (early phase) | 2 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.33, 1.64] |
| 8.1 vs Amitriptyline | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 1.17 [0.61, 1.73] |
| 8.2 vs Nortriptyline | 1 | 61 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.34, 0.66] |
| 9 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | Other data | No numeric data | ||
| 9.1 vs Imipramine | Other data | No numeric data | ||
| 9.2 vs Clomipramine | Other data | No numeric data | ||
| 9.3 vs Amitriptyline | Other data | No numeric data | ||
| 9.4 vs Desipramine | Other data | No numeric data | ||
| 9.5 vs Dothiepine | Other data | No numeric data | ||
| 10 Depression scale ‐ Change score: decrease=good (acute phase) | 3 | 372 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.22, 0.79] |
| 10.1 vs Imipramine | 2 | 322 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.46, 0.81] |
| 10.2 vs Amitriptyline | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.01, 1.12] |
| 11 Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs | Other data | No numeric data | ||
| 11.1 vs Imipramine | Other data | No numeric data | ||
| 11.2 vs Clomipramine | Other data | No numeric data | ||
| 11.3 vs Amitriptyline | Other data | No numeric data | ||
| 11.4 vs Desipramine | Other data | No numeric data | ||
| 11.5 vs Dothiepine | Other data | No numeric data | ||
| 12 Total Dropout | 28 | 2268 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.18] |
| 12.1 vs Imipramine | 13 | 1263 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.81, 1.41] |
| 12.2 vs Clomipramine | 5 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.58, 2.00] |
| 12.3 vs Amitriptyline | 5 | 420 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.51, 1.17] |
| 12.4 vs Nortriptiyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.41] |
| 12.5 vs Desipramine | 2 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.24, 10.70] |
| 12.6 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.49, 2.25] |
| 13 Dropout due to inefficacy | 22 | 1651 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.68, 1.83] |
| 13.1 vs Imipramine | 10 | 841 | Odds Ratio (M‐H, Random, 95% CI) | 1.48 [0.77, 2.86] |
| 13.2 vs Clomipramine | 3 | 158 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.13, 6.69] |
| 13.3 vs Amitriptyline | 5 | 420 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.20, 1.74] |
| 13.4 vs Nortriptiyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 2.25 [0.39, 13.12] |
| 13.5 vs Desipramine | 2 | 85 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.6 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.08, 2.67] |
| 14 Dropout due to side effects | 24 | 1772 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.60, 1.04] |
| 14.1 vs Imipramine | 11 | 908 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |
| 14.2 vs Clomipramine | 3 | 158 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.24, 1.98] |
| 14.3 vs Amitriptyline | 5 | 420 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.00] |
| 14.4 vs Nortriptiyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.10, 1.70] |
| 14.5 vs Desipramine | 2 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 14.6 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.47, 3.32] |
| 15 Number of patients experiencing at least one side effect | 9 | 663 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.49, 0.98] |
| 15.1 vs Imipramine | 2 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.18, 1.64] |
| 15.2 vs Clomipramine | 2 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.14, 1.43] |
| 15.3 vs Amitriptyline | 3 | 327 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.42, 1.04] |
| 15.4 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.37] |
| 16 Subgroup analysis ‐ Response (acute phase) 1. Fluvoxamine dosing ‐ Standard dosage | 6 | 413 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.53, 1.46] |
| 16.1 vs Imipramine | 2 | 200 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.64] |
| 16.2 vs Amitriptyline | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.09] |
| 16.3 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 16.4 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 16.5 vs Amitriptyline | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.5 [0.09, 2.84] |
| 17 Subgroup analysis ‐ Response (acute phase) 1. Fluvoxamine dosing ‐ High dosage | 10 | 522 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.74, 1.55] |
| 17.1 vs Imipramine | 4 | 175 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.38, 2.40] |
| 17.2 vs Clomipramine | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.38, 1.85] |
| 17.3 vs Amitriptyline | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [0.57, 4.02] |
| 17.4 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.24] |
| 18 Subgroup analysis ‐ Response (acute phase) 2. Comparator dosing ‐ Standard dosage | 4 | 215 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.64, 2.50] |
| 18.1 vs Clomipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.12, 2.74] |
| 18.2 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.24] |
| 18.3 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 19 Subgroup analysis ‐ Response (acute phase) 2. Comparator dosing ‐ High dosage | 15 | 892 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 19.1 vs Imipramine | 6 | 375 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.59, 1.58] |
| 19.2 vs Clomipramine | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.39, 2.39] |
| 19.3 vs Amitriptyline | 4 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.35, 1.75] |
| 19.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 19.5 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.24] |
| 19.6 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 20 Subgroup analysis ‐ Response (acute phase) 4. Treatment settings ‐ Inpatient | 5 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.71, 1.92] |
| 20.1 vs Imipramine | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.70, 3.61] |
| 20.2 vs Clomipramine | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.39, 2.39] |
| 20.3 vs Amitriptyline | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.26, 3.89] |
| 20.4 vs Dothiepin | 1 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.34, 2.98] |
| 21 Subgroup analysis ‐ Response (acute phase) 4. Treatment settings ‐ Outpatients | 9 | 564 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.58, 1.20] |
| 21.1 vs Imipramine | 4 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.46, 1.39] |
| 21.2 vs Clomipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.12, 2.74] |
| 21.3 vs Amitriptyline | 2 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.17, 5.00] |
| 21.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 21.5 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.47, 3.00] |
| 22 Sensitivity analysis ‐ Response (acute phase) 2. Excluding trials dropout rate >20% | 5 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.30, 2.07] |
| 22.1 vs Imipramine | 2 | 72 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.12, 1.98] |
| 22.2 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 22.3 vs Clomipramine | 1 | 43 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.12, 2.74] |
| 22.4 vs Amitriptyline | 1 | 23 | Odds Ratio (M‐H, Random, 95% CI) | 0.5 [0.09, 2.84] |
| 23 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT | 16 | 935 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.81] |
| 23.1 vs Imipramine | 6 | 375 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.39, 0.90] |
| 23.2 vs Clomipramine | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.38, 1.85] |
| 23.3 vs Amitriptyline | 4 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.13, 0.90] |
| 23.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 23.5 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.16, 0.72] |
| 23.6 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 24 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT | 16 | 935 | Odds Ratio (M‐H, Random, 95% CI) | 1.78 [1.18, 2.69] |
| 24.1 vs Imipramine | 6 | 375 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [0.78, 3.92] |
| 24.2 vs Clomipramine | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.44, 2.20] |
| 24.3 vs Amitriptyline | 4 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.82, 3.37] |
| 24.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 24.5 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 4.04 [1.85, 8.81] |
| 24.6 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 25 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response | 5 | 343 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.57, 1.41] |
| 25.1 vs Imipramine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.42, 2.02] |
| 25.2 vs Clomipramine | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.39, 2.39] |
| 25.3 vs Amitriptyline | 2 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.26, 2.24] |
| 25.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 26 Sensitivity analysis ‐ Mean change from baseline (acute phase) 5. Excluding trials for which the SD had to be borrowed from other trials | 3 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.07, 0.72] |
| 26.1 vs Imipramine | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.19, 1.47] |
| 26.2 vs Amitriptyline | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.01, 1.12] |
| 26.3 vs Nortriptyline | 1 | 74 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.24, 0.67] |
| 27 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as an investigational drug | 14 | 860 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.36] |
| 27.1 vs Imipramine | 6 | 375 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.59, 1.58] |
| 27.2 vs Clomipramine | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.38, 1.85] |
| 27.3 vs Amitriptyline | 3 | 162 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.32, 2.50] |
| 27.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 27.5 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.47, 3.00] |
| 27.6 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 28 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Excluding trials funded by the fluvoxamine marketing company | 5 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.82, 2.43] |
| 28.1 vs Imipramine | 2 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.70, 3.61] |
| 28.2 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 28.3 vs Amitriptyline | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.26, 3.89] |
| 28.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 29 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Trials funded by the fluvoxamine marketing company | 11 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.16] |
| 29.1 vs Imipramine | 4 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.46, 1.39] |
| 29.2 vs Clomipramine | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.38, 1.85] |
| 29.3 vs Amitriptyline | 3 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.25, 2.29] |
| 29.4 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.24] |
| 30 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression | 9 | 540 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.68, 1.73] |
| 30.1 vs Imipramine | 4 | 303 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.44, 1.83] |
| 30.2 vs Amitriptyline | 3 | 116 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.50, 2.72] |
| 30.3 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 30.4 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
| 31 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features | 16 | 935 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.73, 1.29] |
| 31.1 vs Imipramine | 6 | 375 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.59, 1.58] |
| 31.2 vs Clomipramine | 2 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.38, 1.85] |
| 31.3 vs Amitriptyline | 4 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.35, 1.75] |
| 31.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.36, 2.28] |
| 31.5 vs Dothiepin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.24] |
| 31.6 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 4.22 [0.98, 18.13] |
1.4. Analysis.
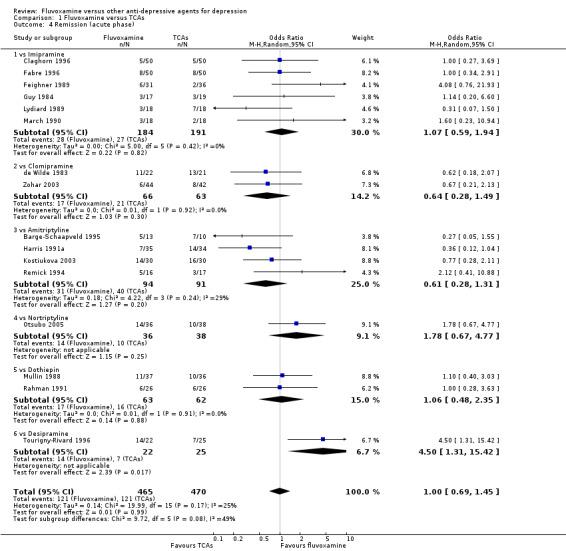
Comparison 1 Fluvoxamine versus TCAs, Outcome 4 Remission (acute phase).
1.12. Analysis.
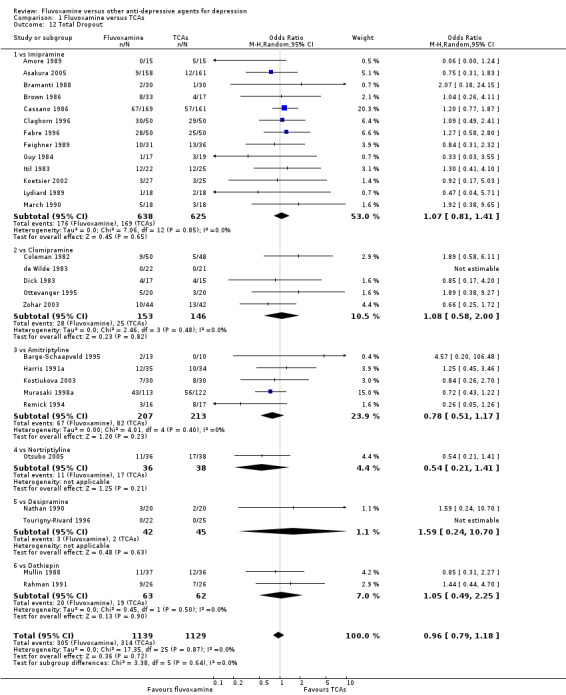
Comparison 1 Fluvoxamine versus TCAs, Outcome 12 Total Dropout.
1.15. Analysis.
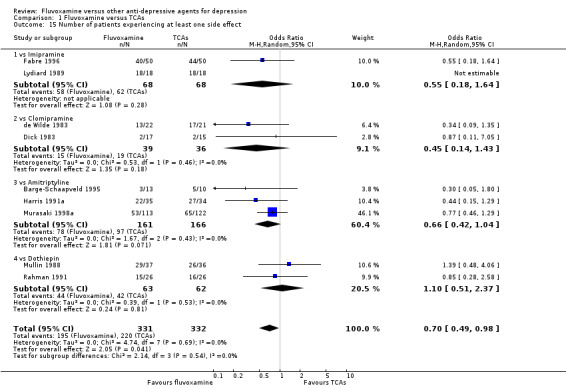
Comparison 1 Fluvoxamine versus TCAs, Outcome 15 Number of patients experiencing at least one side effect.
1.27. Analysis.
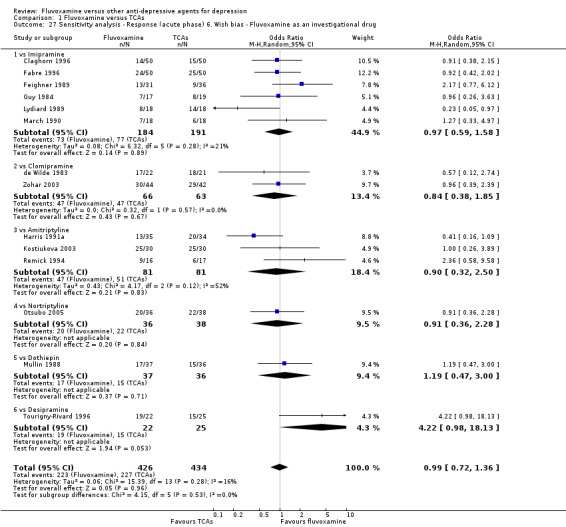
Comparison 1 Fluvoxamine versus TCAs, Outcome 27 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as an investigational drug.
1.28. Analysis.
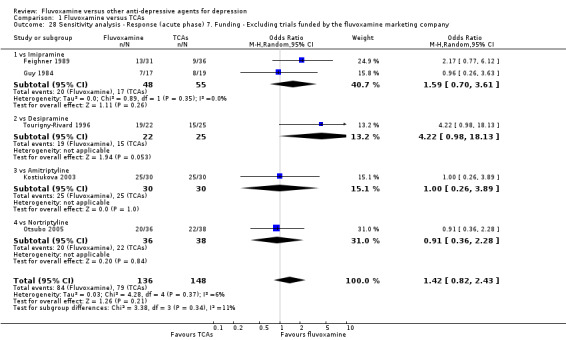
Comparison 1 Fluvoxamine versus TCAs, Outcome 28 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Excluding trials funded by the fluvoxamine marketing company.
1.29. Analysis.
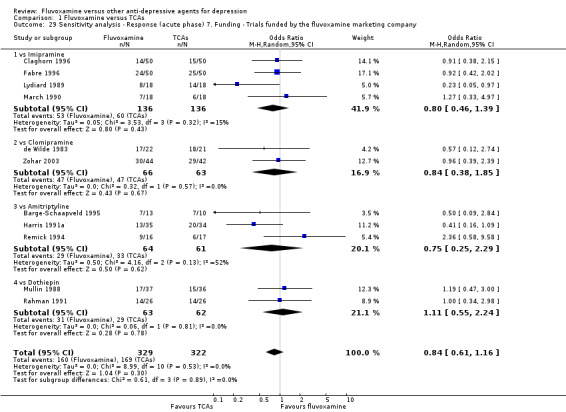
Comparison 1 Fluvoxamine versus TCAs, Outcome 29 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Trials funded by the fluvoxamine marketing company.
Comparison 2. Fluvoxamine versus Heterocyclics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response (acute phase): Primary outcome | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.87] |
| 1.1 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.87] |
| 2 Response (early phase) | 3 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 2.1 vs Amineptine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.26, 3.87] |
| 2.2 vs Maprotiline | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.17] |
| 3 Remission (early phase) | 3 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.50, 3.13] |
| 3.1 vs Amineptine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.13, 7.89] |
| 3.2 vs Maprotiline | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.47, 3.69] |
| 4 Remission (acute phase) | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 2.02 [0.55, 7.39] |
| 4.1 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 2.02 [0.55, 7.39] |
| 5 Depression scale ‐ Endpoint score: low=good (early phase) | 2 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.54, 0.34] |
| 5.1 vs Amineptine | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.90, 0.36] |
| 5.2 vs Maprotiline | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.56, 0.66] |
| 6 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 6.1 vs Maprotiline | Other data | No numeric data | ||
| 7 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 7.1 vs Mianserin | Other data | No numeric data | ||
| 8 Depression scale ‐ Change score: decrease=good (early phase) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.61, 0.63] |
| 8.1 vs Maprotiline | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.61, 0.63] |
| 9 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | Other data | No numeric data | ||
| 9.1 vs Amineptine | Other data | No numeric data | ||
| 9.2 vs Maplotiline | Other data | No numeric data | ||
| 9.3 vs Mianserin | Other data | No numeric data | ||
| 10 Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs | Other data | No numeric data | ||
| 10.3 vs Mianserin | Other data | No numeric data | ||
| 11 Total Dropout | 5 | 247 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.25, 1.34] |
| 11.1 vs Amineptine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.09, 4.24] |
| 11.2 vs Maprotiline | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 11.3 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.11, 2.65] |
| 12 Dropout due to inefficacy | 5 | 247 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.06, 3.14] |
| 12.1 vs Amineptine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 12.2 vs Maprotiline | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.05, 6.21] |
| 13 Dropout due to side effects | 5 | 247 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.33, 1.97] |
| 13.1 vs Amineptine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 13.2 vs Maprotiline | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 13.3 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.77] |
| 14 Number of patients experiencing at least one side effect | 3 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.46, 3.31] |
| 14.1 vs Maprotiline | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.17, 6.00] |
| 14.2 vs Mianserin | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [0.62, 5.06] |
| 15 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.10, 5.62] |
| 15.1 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.10, 5.62] |
| 16 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 3.04 [1.20, 7.67] |
| 16.1 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 3.04 [1.20, 7.67] |
| 17 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 2.24 [0.51, 9.91] |
| 17.1 vs Mianserin | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 2.24 [0.51, 9.91] |
| 18 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as an investigational drug | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 2.24 [0.51, 9.91] |
| 18.1 vs Mianserin | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 2.24 [0.51, 9.91] |
| 19 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Trials funded by the fluvoxamine marketing company | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.87] |
| 19.1 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.87] |
| 20 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.87] |
| 20.1 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.87] |
| 21 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.87] |
| 21.1 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.87] |
2.15. Analysis.
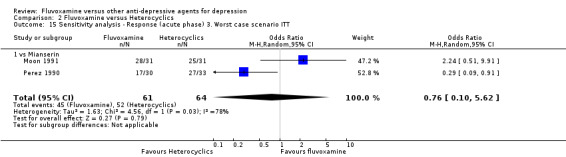
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 15 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT.
2.17. Analysis.
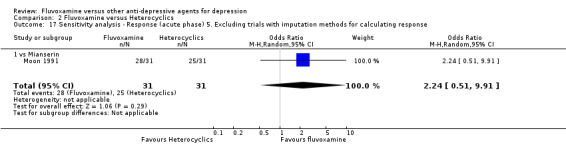
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 17 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response.
2.18. Analysis.
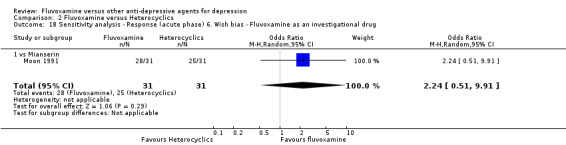
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 18 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as an investigational drug.
2.19. Analysis.
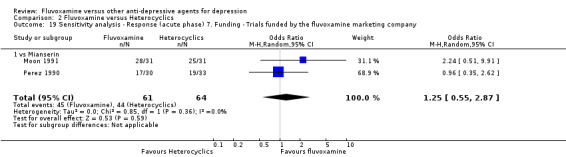
Comparison 2 Fluvoxamine versus Heterocyclics, Outcome 19 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Trials funded by the fluvoxamine marketing company.
Comparison 3. Fluvoxamine versus other SSRIs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response (acute phase): Primary outcome | 8 | 967 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.25] |
| 1.1 vs Paroxetine | 3 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.34] |
| 1.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.53, 2.75] |
| 1.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.62, 1.61] |
| 1.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 2 Response (early phase) | 8 | 967 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.42] |
| 2.1 vs Paroxetine | 3 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.41, 1.24] |
| 2.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.58, 2.98] |
| 2.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.45, 3.41] |
| 2.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.19, 1.90] |
| 3 Remission (early phase) | 7 | 783 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.42, 1.38] |
| 3.1 vs Paroxetine | 3 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.25, 1.67] |
| 3.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.29, 2.55] |
| 3.3 vs Fluoxetine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.25, 2.57] |
| 3.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.06, 16.04] |
| 4 Remission (acute phase) | 8 | 967 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.71, 1.37] |
| 4.1 vs Paroxetine | 3 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.45, 1.33] |
| 4.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.48, 3.57] |
| 4.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.74, 2.06] |
| 4.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.23, 1.34] |
| 5 Depression scale ‐ Endpoint score: low=good (early phase) | 2 | 177 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.35, 0.24] |
| 5.1 vs Paroxetine | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.47, 0.34] |
| 5.2 vs Sertraline | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.46, 0.39] |
| 6 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 6.1 vs Paroxetine | Other data | No numeric data | ||
| 6.2 vs Sertraline | Other data | No numeric data | ||
| 6.3 vs Citalopram | Other data | No numeric data | ||
| 6.4 vs Fluoxetine | Other data | No numeric data | ||
| 7 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 7.1 vs Paroxetine | Other data | No numeric data | ||
| 7.2 vs Sertraline | Other data | No numeric data | ||
| 7.3 vs Fluoxetine | Other data | No numeric data | ||
| 7.4 vs Citalopram | Other data | No numeric data | ||
| 8 Depression scale ‐ Change score: decrease=good (early phase) | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.24, 0.64] |
| 8.1 vs Paroxetine | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.24, 0.64] |
| 9 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | Other data | No numeric data | ||
| 9.1 vs Paroxetine | Other data | No numeric data | ||
| 9.2 vs Sertraline | Other data | No numeric data | ||
| 9.3 vs Citalopram | Other data | No numeric data | ||
| 9.4 vs Fluoxetine | Other data | No numeric data | ||
| 10 Depression scale ‐ Change score: decrease=good (acute phase) | 3 | 230 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.23, 0.42] |
| 10.1 vs Paroxetine | 2 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.31, 0.68] |
| 10.2 vs Sertraline | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.47, 0.35] |
| 11 Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs | Other data | No numeric data | ||
| 11.1 vs Paroxetine | Other data | No numeric data | ||
| 11.2 vs Sertraline | Other data | No numeric data | ||
| 11.3 vs Citalopram | Other data | No numeric data | ||
| 11.4 vs Fluoxetine | Other data | No numeric data | ||
| 12 Total Dropout | 9 | 1126 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.93, 1.69] |
| 12.1 vs Paroxetine | 4 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.62, 1.73] |
| 12.2 vs Sertraline | 3 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.24, 5.37] |
| 12.3 vs Fluoxetine | 3 | 337 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.65, 2.00] |
| 12.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.75, 2.67] |
| 13 Dropout due to inefficacy | 4 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.33, 6.33] |
| 13.1 vs Paroxetine | 2 | 180 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.14, 6.87] |
| 13.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 5.11 [0.24, 109.17] |
| 14 Dropout due to side effects | 8 | 942 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.62, 2.28] |
| 14.1 vs Paroxetine | 4 | 334 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.28, 3.26] |
| 14.2 vs Sertraline | 3 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.15, 11.33] |
| 14.3 vs Fluoxetine | 2 | 153 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.19, 3.89] |
| 14.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [0.90, 3.59] |
| 15 Number of patients experiencing at least one side effect | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.51] |
| 15.1 vs Paroxetine | 3 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.41, 2.23] |
| 15.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.21, 2.37] |
| 15.3 vs Fluoxetine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.5 [0.09, 2.86] |
| 16 Subgroup analysis ‐ Response (acute phase) 1. Fluvoxamine dosing ‐ Standard dosage | 5 | 542 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.30] |
| 16.1 vs Paroxetine | 2 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.62] |
| 16.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.36, 1.83] |
| 16.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.62, 1.61] |
| 17 Subgroup analysis ‐ Response (acute phase) 1. Fluvoxamine dosing ‐ High dosage | 3 | 425 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.60] |
| 17.1 vs Paroxetine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.38, 1.66] |
| 17.2 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 17.3 vs Sertraline | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.77, 4.63] |
| 18 Subgroup analysis ‐ Response (acute phase) 2. Comparator dosing ‐ Standard dosage | 2 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.60, 1.50] |
| 18.1 vs Paroxetine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.38, 1.66] |
| 18.2 vs Fluoxetine | 1 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.59, 1.91] |
| 19 Subgroup analysis ‐ Response (acute phase) 2. Comparator dosing ‐ High dosage | 6 | 663 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.33] |
| 19.1 vs Paroxetine | 2 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.62] |
| 19.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.53, 2.75] |
| 19.3 vs Fluoxetine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.40, 2.03] |
| 19.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 20 Sensitivity analysis ‐ Response (acute phase) 2. Excluding trials dropout rate >20% | 2 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.61, 2.62] |
| 20.1 vs Sertraline | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.77, 4.63] |
| 20.2 vs Fluoxetine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.40, 2.03] |
| 21 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT | 8 | 967 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.05] |
| 21.1 vs Paroxetine | 3 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.15] |
| 21.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.47, 1.99] |
| 21.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.35] |
| 21.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 22 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT | 8 | 967 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [1.02, 1.74] |
| 22.1 vs Paroxetine | 3 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [1.08, 2.92] |
| 22.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.72, 2.90] |
| 22.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.79, 2.07] |
| 22.4 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 23 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response | 4 | 622 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.24] |
| 23.1 vs Paroxetine | 2 | 221 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.43, 1.31] |
| 23.2 vs Fluoxetine | 1 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.59, 1.91] |
| 23.3 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 24 Sensitivity analysis ‐ Mean change from baseline (acute phase) 5. Excluding trials for which the SD had to be borrowed from other trials | 3 | 230 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.23, 0.42] |
| 24.1 vs Paroxetine | 2 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.31, 0.68] |
| 24.2 vs Sertraline | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.47, 0.35] |
| 25 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as an investigational drug | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.77, 4.63] |
| 25.1 vs Sertraline | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.77, 4.63] |
| 26 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Excluding trials funded by the fluvoxamine marketing company | 3 | 425 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.60] |
| 26.1 vs Paroxetine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 0.8 [0.38, 1.66] |
| 26.2 vs Sertraline | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.77, 4.63] |
| 26.3 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.62] |
| 27 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Trials funded by the fluvoxamine marketing company | 5 | 542 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.30] |
| 27.1 vs Paroxetine | 2 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.62] |
| 27.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.36, 1.83] |
| 27.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.62, 1.61] |
| 28 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression | 3 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.51, 1.39] |
| 28.1 vs Paroxetine | 2 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.62] |
| 28.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.36, 1.83] |
| 29 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features | 6 | 630 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.73, 1.39] |
| 29.1 vs Paroxetine | 2 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.45, 1.62] |
| 29.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.53, 2.75] |
| 29.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.62, 1.61] |
3.1. Analysis.
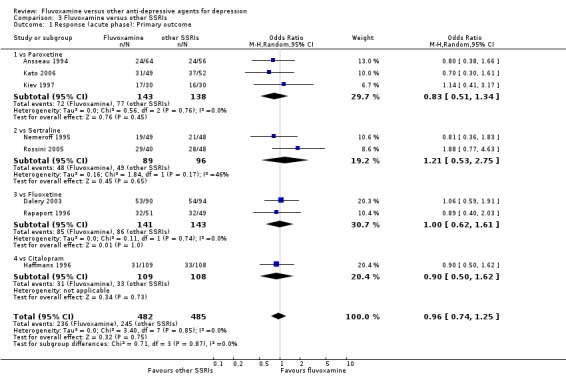
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 1 Response (acute phase): Primary outcome.
3.21. Analysis.
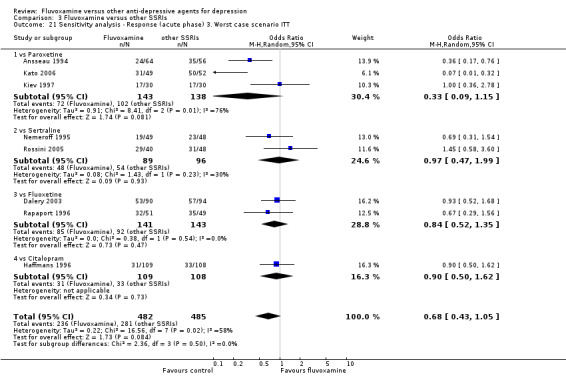
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 21 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT.
3.25. Analysis.
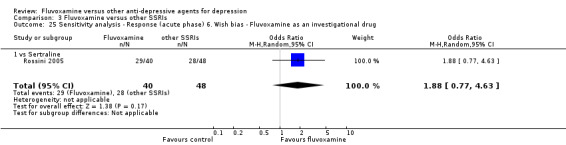
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 25 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as an investigational drug.
3.26. Analysis.
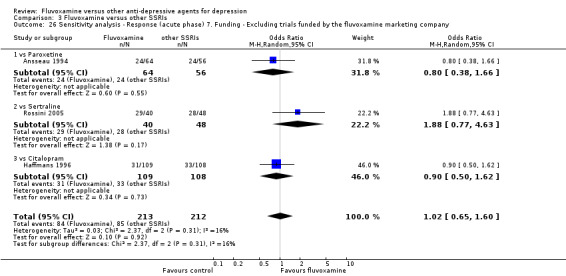
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 26 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Excluding trials funded by the fluvoxamine marketing company.
3.27. Analysis.
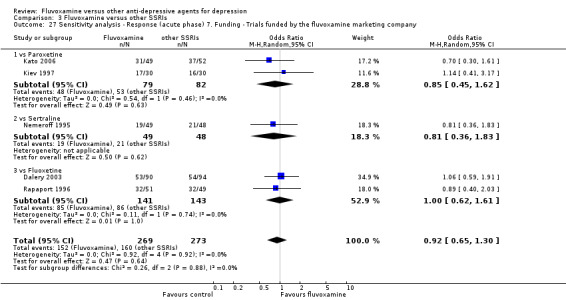
Comparison 3 Fluvoxamine versus other SSRIs, Outcome 27 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Trials funded by the fluvoxamine marketing company.
Comparison 4. Fluvoxamine versus SNRIs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response (acute phase): Primary outcome | 3 | 224 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.85] |
| 1.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.23] |
| 1.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.92] |
| 2 Response (early phase) | 5 | 351 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.45, 1.19] |
| 2.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.15] |
| 2.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.39, 2.63] |
| 3 Remission (early phase) | 5 | 351 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.35, 1.86] |
| 3.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.22, 1.74] |
| 3.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 1.54 [0.22, 10.74] |
| 4 Remission (acute phase) | 3 | 224 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.34, 1.08] |
| 4.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.30, 1.51] |
| 4.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.23, 1.24] |
| 5 Depression scale ‐ Endpoint score: low=good (early phase) | 4 | 274 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.17, 0.32] |
| 5.1 vs Milnacipran | 2 | 172 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.30, 0.31] |
| 5.2 vs Venlafaxine | 2 | 102 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.21, 0.63] |
| 6 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 6.1 vs Milnacipran | Other data | No numeric data | ||
| 7 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 7.1 vs Venlafaxine | Other data | No numeric data | ||
| 8 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | Other data | No numeric data | ||
| 8.1 vs Milnacipran | Other data | No numeric data | ||
| 8.3 vs Venlafaxine | Other data | No numeric data | ||
| 9 Depression scale ‐ Change score: decrease=good (acute phase) ‐ missing SDs | Other data | No numeric data | ||
| 9.1 vs Milnacipran | Other data | No numeric data | ||
| 9.3 vs Venlafaxine | Other data | No numeric data | ||
| 10 Total Dropout | 5 | 351 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.93, 2.67] |
| 10.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.65, 2.45] |
| 10.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 2.29 [0.97, 5.43] |
| 11 Dropout due to inefficacy | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.16] |
| 11.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.16] |
| 12 Dropout due to side effects | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 2.38 [0.73, 7.78] |
| 12.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 2.38 [0.73, 7.78] |
| 13 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT | 3 | 224 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.76] |
| 13.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.23] |
| 13.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.29] |
| 14 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT | 3 | 224 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.43, 4.66] |
| 14.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.23] |
| 14.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 2.67 [0.98, 7.26] |
| 15 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.23] |
| 15.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.23] |
| 16 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as a comparator drug | 3 | 224 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.85] |
| 16.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.23] |
| 16.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.92] |
| 17 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Excluding trials funded by the fluvoxamine marketing company | 3 | 224 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.85] |
| 17.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.23] |
| 17.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.92] |
| 18 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression | 3 | 224 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.85] |
| 18.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.23] |
| 18.2 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.92] |
| 19 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.92] |
| 19.1 vs Venlafaxine | 2 | 111 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.92] |
4.1. Analysis.
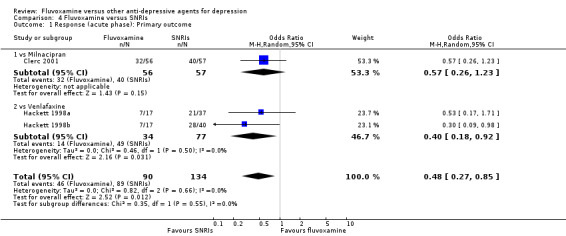
Comparison 4 Fluvoxamine versus SNRIs, Outcome 1 Response (acute phase): Primary outcome.
4.15. Analysis.
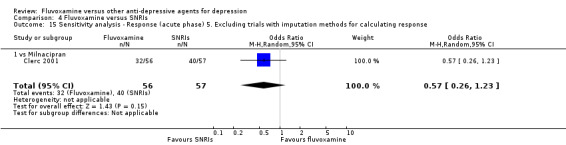
Comparison 4 Fluvoxamine versus SNRIs, Outcome 15 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response.
4.16. Analysis.
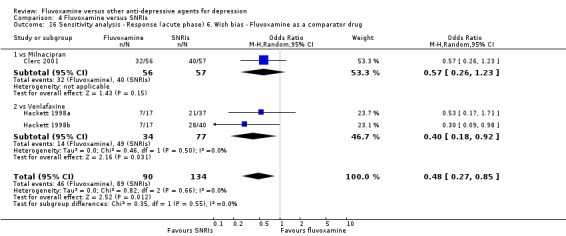
Comparison 4 Fluvoxamine versus SNRIs, Outcome 16 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as a comparator drug.
4.17. Analysis.
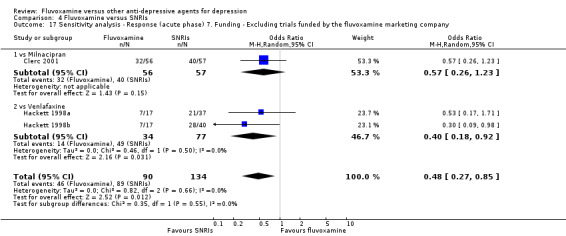
Comparison 4 Fluvoxamine versus SNRIs, Outcome 17 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Excluding trials funded by the fluvoxamine marketing company.
Comparison 5. Fluvoxamine versus newer ADs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response (acute phase): Primary outcome | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 2 Response (early phase) | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.47, 1.11] |
| 2.2 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.26, 1.36] |
| 3 Remission (early phase) | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.30] |
| 3.2 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.16, 1.35] |
| 4 Remission (acute phase) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.81, 1.76] |
| 5 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 5.1 vs Mirtazapine | Other data | No numeric data | ||
| 5.2 vs Moclobemide | Other data | No numeric data | ||
| 6 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | Other data | No numeric data | ||
| 6.1 vs Mirtazapine | Other data | No numeric data | ||
| 6.2 vs Moclobemide | Other data | No numeric data | ||
| 7 Depression scale ‐ Change score: decrease=good (early phase) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 vs Mirtazapine | 1 | 402 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.51] |
| 8 Depression scale ‐ Change score: decrease=good (early phase) ‐ missing SDs | Other data | No numeric data | ||
| 8.2 vs Moclobemide | Other data | No numeric data | ||
| 9 Depression scale ‐ Change score: decrease=good (acute phase) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 vs Mirtazapine | 1 | 402 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.12, 0.28] |
| 10 Total Dropout | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.33] |
| 10.2 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.73, 2.71] |
| 11 Dropout due to inefficacy | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 2.02 [0.60, 6.82] |
| 11.2 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.14, 2.71] |
| 12 Dropout due to side effects | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.31, 1.17] |
| 12.2 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [0.64, 3.53] |
| 13 Number of patients experiencing at least one side effect | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.29 [1.35, 3.88] |
| 14 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.15] |
| 15 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.64, 1.42] |
| 16 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 17 Sensitivity analysis ‐ Mean change from baseline (acute phase) 5. Excluding trials for which the SD had to be borrowed from other trials | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 vs Mirtazapine | 1 | 402 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.12, 0.28] |
| 18 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as a comparator drug | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 19 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 20 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Excluding trials funded by the fluvoxamine marketing company | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 21 Sensitivity analysis ‐ Response (acute phase) 9. Excluding trials that included patients with psychotic features | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
5.5. Analysis.
Comparison 5 Fluvoxamine versus newer ADs, Outcome 5 Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (early phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Mirtazapine | ||||||||
| Schoemaker 2002 | HRSD‐17 | 16.5 | missing | 203 | 14.8 | missing | 199 | |
| vs Moclobemide | ||||||||
| Bocksberger 1993 | MADRS | 31.5 | 9.8 | 19 | 20.0 | 10.8 | 19 | skewed. |
5.6. Analysis.
Comparison 5 Fluvoxamine versus newer ADs, Outcome 6 Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data.
| Depression scale ‐ Endpoint score: low=good (acute phase) ‐ missing SDs or skewed data | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Depression scale | Fluvoxamine; mean | SD | n | comparator; mean | SD | n | Note |
| vs Mirtazapine | ||||||||
| Schoemaker 2002 | HRSD‐17 | 16.5 | missing | 203 | 14.8 | missing | 199 | |
| vs Moclobemide | ||||||||
| Bocksberger 1993 | MADRS | 31.5 | 9.8 | 19 | 20.0 | 10.8 | 19 | skewed. |
5.14. Analysis.
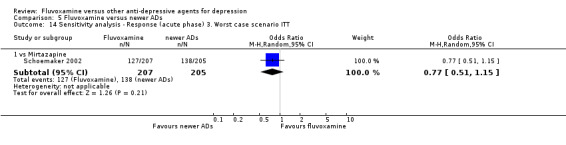
Comparison 5 Fluvoxamine versus newer ADs, Outcome 14 Sensitivity analysis ‐ Response (acute phase) 3. Worst case scenario ITT.
5.15. Analysis.
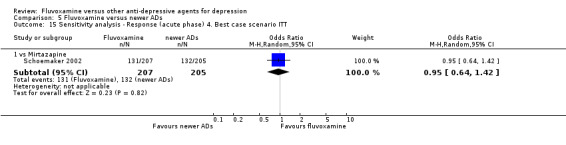
Comparison 5 Fluvoxamine versus newer ADs, Outcome 15 Sensitivity analysis ‐ Response (acute phase) 4. Best case scenario ITT.
5.16. Analysis.
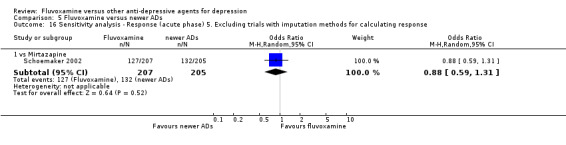
Comparison 5 Fluvoxamine versus newer ADs, Outcome 16 Sensitivity analysis ‐ Response (acute phase) 5. Excluding trials with imputation methods for calculating response.
5.17. Analysis.
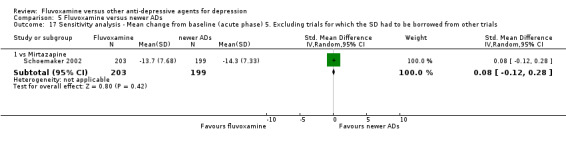
Comparison 5 Fluvoxamine versus newer ADs, Outcome 17 Sensitivity analysis ‐ Mean change from baseline (acute phase) 5. Excluding trials for which the SD had to be borrowed from other trials.
5.18. Analysis.
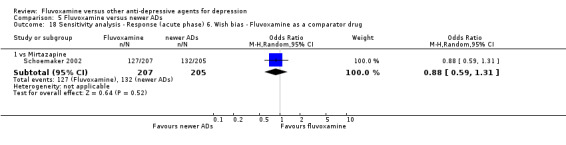
Comparison 5 Fluvoxamine versus newer ADs, Outcome 18 Sensitivity analysis ‐ Response (acute phase) 6. Wish bias ‐ Fluvoxamine as a comparator drug.
5.19. Analysis.
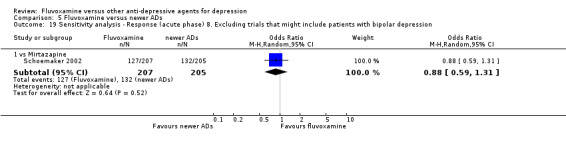
Comparison 5 Fluvoxamine versus newer ADs, Outcome 19 Sensitivity analysis ‐ Response (acute phase) 8. Excluding trials that might include patients with bipolar depression.
5.20. Analysis.
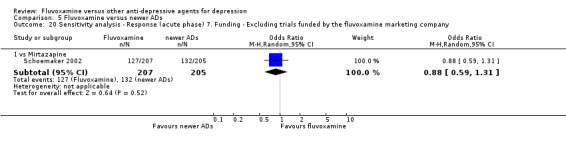
Comparison 5 Fluvoxamine versus newer ADs, Outcome 20 Sensitivity analysis ‐ Response (acute phase) 7. Funding ‐ Excluding trials funded by the fluvoxamine marketing company.
Comparison 6. Fluvoxamine versus other conventional psychotropic drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response (early phase) | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 vs Sulpiride | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Remission (early phase) | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 2.1 vs Sulpiride | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 3 Depression scale ‐ Endpoint score: low=good (early phase) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.16, 1.10] |
| 3.1 vs Sulpiride | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.16, 1.10] |
| 4 Depression scale ‐ Change score: decrease=good (early phase) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.11, 1.15] |
| 4.1 vs Sulpiride | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.11, 1.15] |
| 5 Total Dropout | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 vs Sulpiride | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Side effect profile: Fluvoxamine vs TCAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular ‐ Hypertension / tachycardia | 4 | 363 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.51, 4.78] |
| 1.1 vs Imipramine | 1 | 36 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.31, 8.68] |
| 1.2 vs Clomipramine | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 5.0 [0.22, 112.88] |
| 1.3 vs Amitriptyline | 2 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.19, 5.81] |
| 2 Cardiovascular ‐ Hypotension / bradycardia | 8 | 930 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.79] |
| 2.1 vs Imipramine | 4 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.10, 0.62] |
| 2.2 vs Clomipramine | 2 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.38, 4.02] |
| 2.3 vs Amitriptyline | 2 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.03, 1.18] |
| 3 Dermatological ‐ Dermatitis / rash | 3 | 348 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.17, 4.61] |
| 3.1 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 5.99] |
| 3.2 vs Desipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 3.3 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 5.14 [0.24, 110.89] |
| 4 Dermatological ‐ Sweating | 11 | 1248 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.88] |
| 4.1 vs Imipramine | 7 | 972 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.66] |
| 4.2 vs Clomipramine | 3 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.45, 1.73] |
| 4.3 vs Amitriptyline | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 2.07 [0.18, 24.15] |
| 5 Gastrointestinal ‐ Increased salivation | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 9.33 [0.96, 90.94] |
| 5.1 vs Imipramine | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 9.33 [0.96, 90.94] |
| 6 Gastrointestinal ‐ Dry mouth | 17 | 1736 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.19, 0.38] |
| 6.1 vs Imipramine | 9 | 1055 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.16, 0.34] |
| 6.2 vs Clomipramine | 3 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.81] |
| 6.3 vs Amitriptyline | 3 | 318 | Odds Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.06] |
| 6.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.16, 1.06] |
| 6.5 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.01, 0.70] |
| 7 Gastrointestinal ‐ Oral discomfort / taste disturbance | 2 | 308 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 2.98] |
| 7.1 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.01, 8.85] |
| 7.2 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.45] |
| 8 Gastrointestinal ‐ Vomiting / nausea | 18 | 1805 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [1.80, 3.07] |
| 8.1 vs Imipramine | 9 | 1055 | Odds Ratio (M‐H, Random, 95% CI) | 2.23 [1.59, 3.14] |
| 8.2 vs Clomipramine | 3 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [1.06, 4.27] |
| 8.3 vs Amitriptyline | 4 | 387 | Odds Ratio (M‐H, Random, 95% CI) | 2.86 [1.31, 6.23] |
| 8.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 3.01 [1.00, 9.11] |
| 8.5 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 2.71 [0.90, 8.19] |
| 9 Gastrointestinal ‐ Constipation | 15 | 1666 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.80] |
| 9.1 vs Imipramine | 8 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.27, 0.93] |
| 9.2 vs Clomipramine | 3 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.27, 1.09] |
| 9.3 vs Amitriptyline | 2 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.25] |
| 9.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.25, 1.60] |
| 9.5 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.08] |
| 10 Gastrointestinal ‐ Diarrhoea | 5 | 518 | Odds Ratio (M‐H, Random, 95% CI) | 2.98 [1.08, 8.20] |
| 10.1 vs Imipramine | 2 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 6.38 [1.27, 32.04] |
| 10.2 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.01, 8.85] |
| 10.3 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.66, 5.00] |
| 10.4 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 9.81 [0.51, 189.07] |
| 11 Gastrointestinal ‐ Weight gain | 4 | 425 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.25, 1.09] |
| 11.1 vs Imipramine | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.23, 4.31] |
| 11.2 vs Clomipramine | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.05, 1.22] |
| 11.3 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 3.27 [0.13, 81.01] |
| 11.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.21] |
| 12 Gastrointestinal ‐ Weight loss | 4 | 226 | Odds Ratio (M‐H, Random, 95% CI) | 2.76 [1.20, 6.34] |
| 12.1 vs Imipramine | 2 | 66 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.53, 6.67] |
| 12.2 vs Clomipramine | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 7.76 [0.91, 66.05] |
| 12.3 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 2.83 [0.79, 10.21] |
| 13 Gastrointestinal ‐ Increased appetite | 2 | 335 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.08, 7.04] |
| 13.1 vs Imipramine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 3.18] |
| 13.2 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 3.27 [0.13, 81.01] |
| 14 Gastrointestinal ‐ Anorexia | 6 | 1104 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.54, 2.53] |
| 14.1 vs Imipramine | 5 | 869 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.47, 2.68] |
| 14.2 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [0.19, 24.38] |
| 15 Neuropsychiatric ‐ Blurred vision | 5 | 500 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.23, 0.97] |
| 15.1 vs Clomipramine | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.06, 2.30] |
| 15.2 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.39] |
| 15.3 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.21, 1.56] |
| 15.4 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.45] |
| 16 Neuropsychiatric ‐ Dizziness / vertigo / faintness | 14 | 1592 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.19, 0.38] |
| 16.1 vs Imipramine | 9 | 1055 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.15, 0.38] |
| 16.2 vs Clomipramine | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.05, 0.80] |
| 16.3 vs Amitriptyline | 2 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.83] |
| 16.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.70] |
| 16.5 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.18, 3.06] |
| 17 Neuropsychiatric ‐ Fatigue / tiredness / asthenia | 5 | 565 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.52, 1.73] |
| 17.1 vs Imipramine | 2 | 200 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.25, 4.56] |
| 17.2 vs Clomipramine | 2 | 130 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.40, 1.85] |
| 17.3 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.27, 9.98] |
| 18 Neuropsychiatric ‐ Headache | 16 | 1621 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.73, 1.62] |
| 18.1 vs Imipramine | 8 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.71, 2.01] |
| 18.2 vs Clomipramine | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.5 [0.33, 6.83] |
| 18.3 vs Amitriptyline | 4 | 387 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.07, 1.11] |
| 18.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.66, 5.00] |
| 18.5 vs Desipramine | 1 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.26, 2.79] |
| 18.6 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.5 [0.24, 9.55] |
| 19 Neuropsychiatric ‐ Tremor | 12 | 1247 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.33, 0.75] |
| 19.1 vs Imipramine | 6 | 663 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.32, 1.13] |
| 19.2 vs Clomipramine | 3 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.15, 1.37] |
| 19.3 vs Amitriptyline | 2 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 1.88] |
| 19.4 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.08] |
| 20 Neuropsychiatric ‐ Involuntary movement other than tremor | 3 | 499 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.32, 3.47] |
| 20.1 vs Imipramine | 2 | 430 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.36, 4.58] |
| 20.2 vs Amitriptyline | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.99] |
| 21 Neuropsychiatric ‐ Insomnia | 9 | 1039 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.82, 1.92] |
| 21.1 vs Imipramine | 6 | 674 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.87, 2.39] |
| 21.2 vs Clomipramine | 2 | 130 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.37, 1.81] |
| 21.3 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 5.49 [0.26, 115.67] |
| 22 Neuropsychiatric ‐ Sleepiness / drowsiness | 15 | 1585 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.66, 1.33] |
| 22.1 vs Imipramine | 8 | 1019 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.80, 1.46] |
| 22.2 vs Clomipramine | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.25, 4.71] |
| 22.3 vs Amitriptyline | 4 | 387 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.05, 1.21] |
| 22.4 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.22, 1.42] |
| 22.5 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.40, 6.02] |
| 23 Neuropsychiatric ‐ Agitation / anxiety | 8 | 984 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.94, 2.84] |
| 23.1 vs Imipramine | 5 | 644 | Odds Ratio (M‐H, Random, 95% CI) | 2.24 [1.01, 4.97] |
| 23.2 vs Clomipramine | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [0.15, 22.94] |
| 23.3 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.19, 2.58] |
| 23.4 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.31, 5.08] |
| 24 Neuropsychiatric ‐ Manic symptom | 2 | 565 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.06, 11.58] |
| 24.1 vs Imipramine | 1 | 330 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 2.11] |
| 24.2 vs Amitriptyline | 1 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 3.3 [0.34, 32.19] |
| 25 Neuropsychiatric ‐ Completed suicide | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.29, 28.65] |
| 25.1 vs Clomipramine | 2 | 118 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.29, 28.65] |
| 26 Neuropsychiatric ‐ Suicide wishes / gestures / attempts | 3 | 489 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.17, 2.48] |
| 26.1 vs Imipramine | 1 | 330 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.80] |
| 26.2 vs Clomipramine | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.18, 5.00] |
| 26.3 vs Dothiepin | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.00] |
| 27 Genitourinary ‐ Problems urinating | 6 | 818 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.23, 0.83] |
| 27.1 vs Imipramine | 2 | 409 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.71] |
| 27.2 vs Amitriptyline | 2 | 295 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.10, 2.94] |
| 27.3 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.19, 1.38] |
| 27.4 vs Desipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 28 Genitourinary ‐ Sexual dysfunction | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.29, 1.99] |
| 28.1 vs Nortriptyline | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.29, 1.99] |
7.1. Analysis.
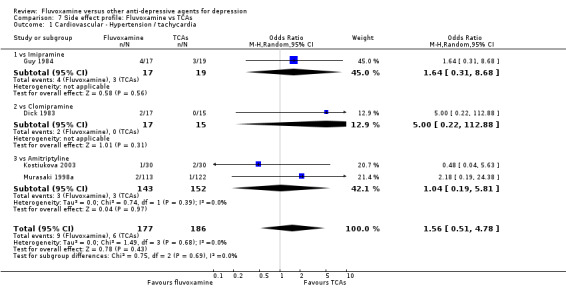
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 1 Cardiovascular ‐ Hypertension / tachycardia.
7.3. Analysis.
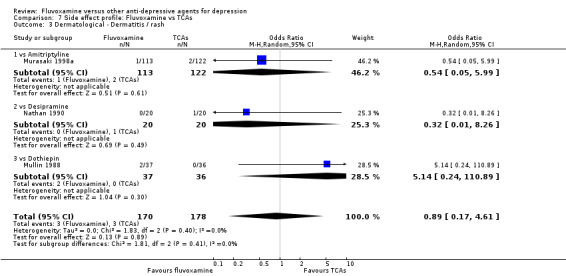
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 3 Dermatological ‐ Dermatitis / rash.
7.5. Analysis.
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 5 Gastrointestinal ‐ Increased salivation.
7.7. Analysis.
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 7 Gastrointestinal ‐ Oral discomfort / taste disturbance.
7.11. Analysis.
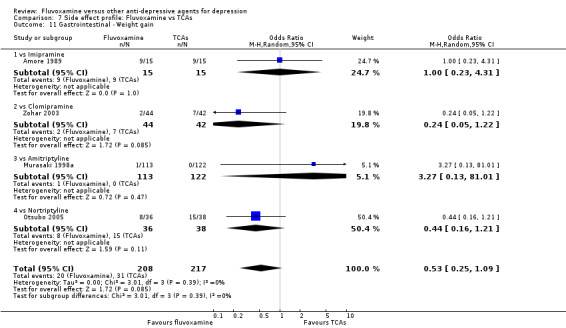
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 11 Gastrointestinal ‐ Weight gain.
7.12. Analysis.
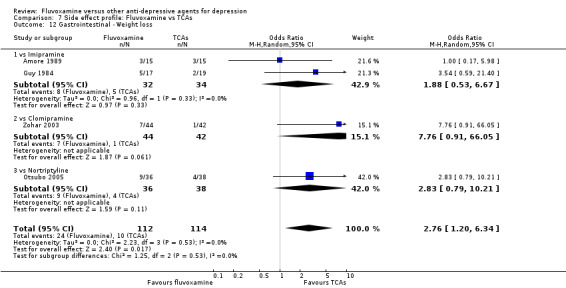
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 12 Gastrointestinal ‐ Weight loss.
7.13. Analysis.
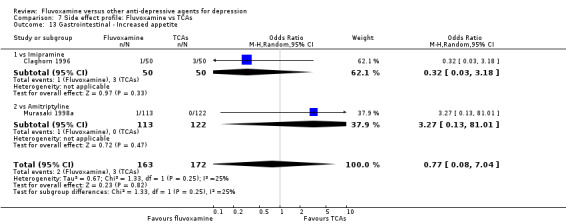
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 13 Gastrointestinal ‐ Increased appetite.
7.14. Analysis.
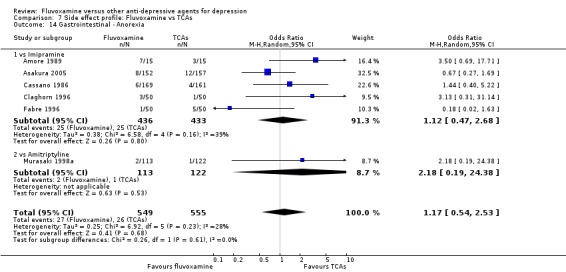
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 14 Gastrointestinal ‐ Anorexia.
7.15. Analysis.
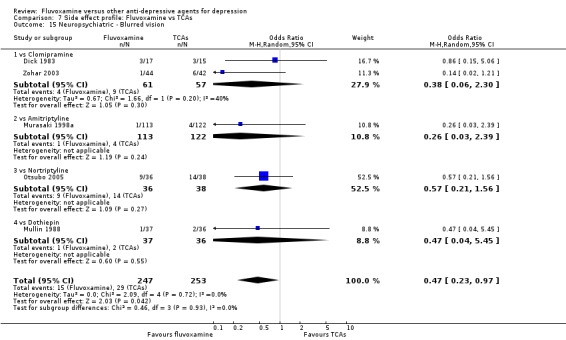
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 15 Neuropsychiatric ‐ Blurred vision.
7.17. Analysis.
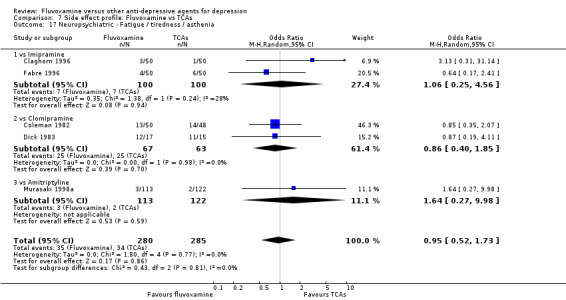
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 17 Neuropsychiatric ‐ Fatigue / tiredness / asthenia.
7.18. Analysis.
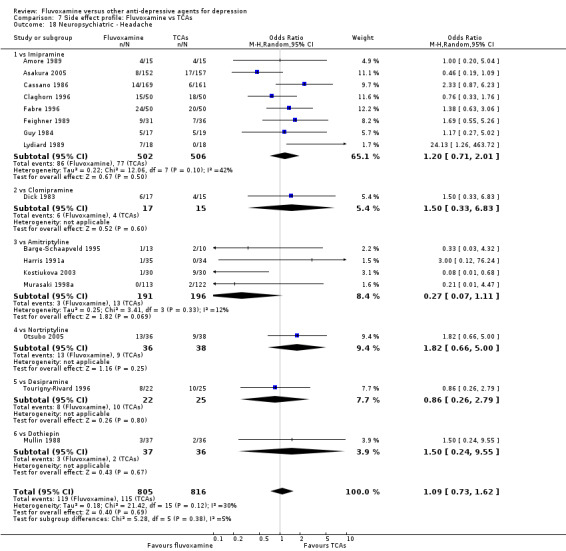
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 18 Neuropsychiatric ‐ Headache.
7.19. Analysis.
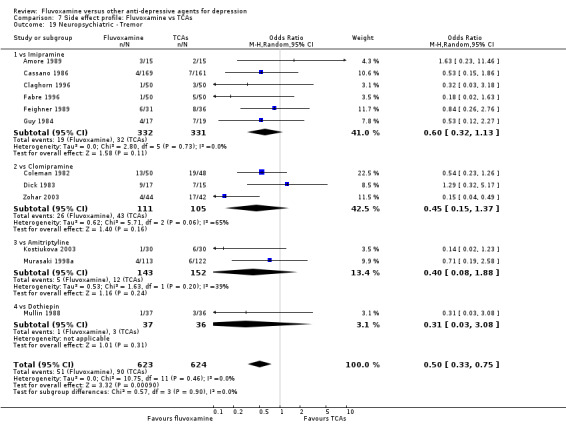
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 19 Neuropsychiatric ‐ Tremor.
7.20. Analysis.
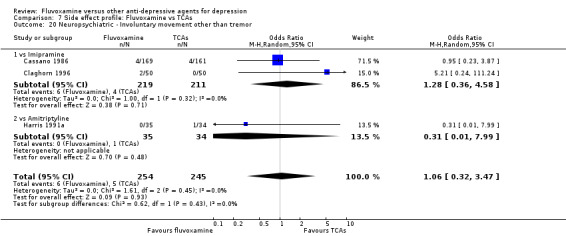
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 20 Neuropsychiatric ‐ Involuntary movement other than tremor.
7.21. Analysis.
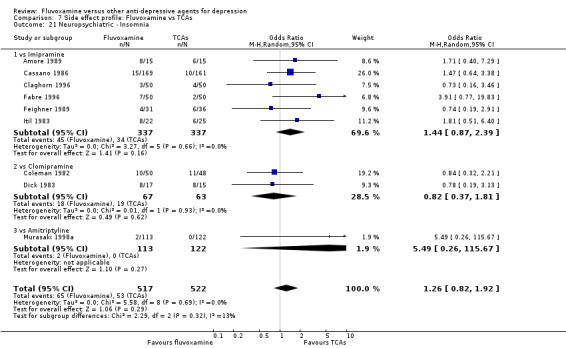
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 21 Neuropsychiatric ‐ Insomnia.
7.22. Analysis.
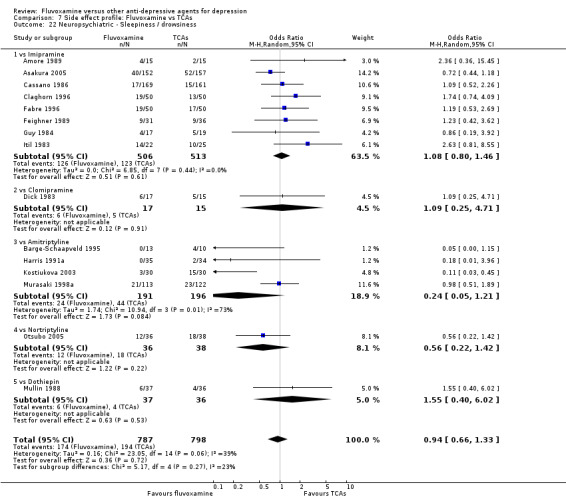
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 22 Neuropsychiatric ‐ Sleepiness / drowsiness.
7.24. Analysis.
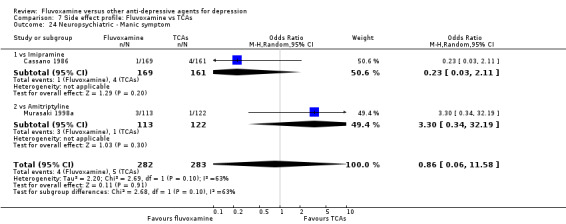
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 24 Neuropsychiatric ‐ Manic symptom.
7.26. Analysis.
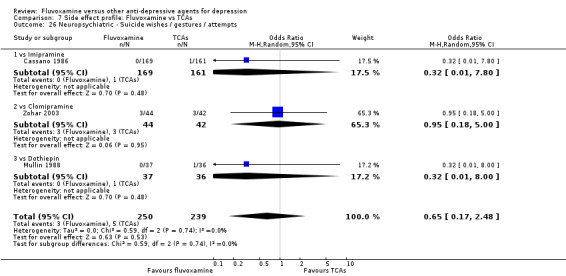
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 26 Neuropsychiatric ‐ Suicide wishes / gestures / attempts.
7.28. Analysis.
Comparison 7 Side effect profile: Fluvoxamine vs TCAs, Outcome 28 Genitourinary ‐ Sexual dysfunction.
Comparison 8. Side effect profile: Fluvoxamine vs Heterocyclics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal ‐ Dry mouth | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.20] |
| 1.1 vs Maprotiline | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.20] |
| 2 Gastrointestinal ‐ Vomiting / nausea | 4 | 207 | Odds Ratio (M‐H, Random, 95% CI) | 4.80 [1.47, 15.67] |
| 2.1 vs Maprotiline | 2 | 82 | Odds Ratio (M‐H, Random, 95% CI) | 2.26 [0.71, 7.17] |
| 2.2 vs Mianserin | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 9.62 [1.96, 47.30] |
| 3 Neuropsychiatric ‐ Dizziness / vertigo / faintness | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.48] |
| 3.1 vs Maprotiline | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 0.13 [0.03, 0.56] |
| 3.2 vs Mianserin | 1 | 62 | Odds Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.58] |
Comparison 9. Side effect profile: Fluvoxamine vs other SSRIs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular ‐ Hypertension / tachycardia | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 2.67 [0.11, 66.85] |
| 1.1 vs Paroxetine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 2.67 [0.11, 66.85] |
| 2 Cardiovascular ‐ Hypotension / bradycardia | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 4.66 [0.53, 41.16] |
| 2.1 vs Paroxetine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 4.66 [0.53, 41.16] |
| 3 Dermatological ‐ Dermatitis / rash | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.92] |
| 3.1 vs Sertraline | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.92] |
| 4 Dermatological ‐ Sweating | 2 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.12, 0.96] |
| 4.1 vs Paroxetine | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.91] |
| 4.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.13, 2.49] |
| 5 Gastrointestinal ‐ Dry mouth | 2 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.54, 2.38] |
| 5.1 vs Paroxetine | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.53, 4.77] |
| 5.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.31, 2.33] |
| 6 Gastrointestinal ‐ Vomiting / nausea | 6 | 649 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.46, 1.36] |
| 6.1 vs Paroxetine | 2 | 180 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.46, 1.78] |
| 6.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.78 [0.73, 4.33] |
| 6.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.13] |
| 7 Gastrointestinal ‐ Constipation | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.08, 2.75] |
| 7.1 vs Paroxetine | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.08, 2.75] |
| 8 Gastrointestinal ‐ Diarrhoea | 3 | 257 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.82] |
| 8.1 vs Paroxetine | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.33] |
| 8.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.20, 1.59] |
| 8.3 vs Fluoxetine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.07] |
| 9 Gastrointestinal ‐ Anorexia | 2 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.95] |
| 9.1 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.16, 2.36] |
| 9.2 vs Fluoxetine | 1 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.56] |
| 10 Neuropsychiatric ‐ Dizziness / vertigo / faintness | 3 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.60] |
| 10.1 vs Paroxetine | 2 | 141 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.22, 1.92] |
| 10.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.25, 2.64] |
| 11 Neuropsychiatric ‐ Fatigue / tiredness / asthenia | 2 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.64] |
| 11.1 vs Paroxetine | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.15, 3.54] |
| 11.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.11, 1.94] |
| 12 Neuropsychiatric ‐ Headache | 6 | 642 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 12.1 vs Paroxetine | 3 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.37, 2.52] |
| 12.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.33, 1.92] |
| 12.3 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.60] |
| 13 Neuropsychiatric ‐ Tremor | 3 | 277 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.21, 1.63] |
| 13.1 vs Paroxetine | 2 | 180 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.14, 2.51] |
| 13.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.13, 2.49] |
| 14 Neuropsychiatric ‐ Insomnia | 3 | 257 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.68, 2.01] |
| 14.1 vs Paroxetine | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [0.52, 5.62] |
| 14.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.30, 1.73] |
| 14.3 vs Fluoxetine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 1.51 [0.64, 3.55] |
| 15 Neuropsychiatric ‐ Sleepiness / drowsiness | 3 | 277 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.32, 3.46] |
| 15.1 vs Paroxetine | 2 | 180 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.02, 9.92] |
| 15.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 1.62 [0.60, 4.41] |
| 16 Neuropsychiatric ‐ Agitation / anxiety | 5 | 465 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.40, 1.35] |
| 16.1 vs Paroxetine | 2 | 180 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.13, 12.78] |
| 16.2 vs Sertraline | 2 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.45, 2.80] |
| 16.3 vs Fluoxetine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.05] |
| 17 Neuropsychiatric ‐ Manic symptom | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 2.67 [0.11, 66.85] |
| 17.1 vs Paroxetine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 2.67 [0.11, 66.85] |
| 18 Neuropsychiatric ‐ Completed suicide | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.12] |
| 18.1 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.12] |
| 19 Neuropsychiatric ‐ Suicide wishes / gestures / attempts | 4 | 621 | Odds Ratio (M‐H, Random, 95% CI) | 1.99 [0.39, 10.05] |
| 19.1 vs Paroxetine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 2.67 [0.11, 66.85] |
| 19.2 vs Fluoxetine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.05, 10.05] |
| 19.3 vs Citalopram | 1 | 217 | Odds Ratio (M‐H, Random, 95% CI) | 9.26 [0.49, 174.04] |
| 20 Genitourinary ‐ Sexual dysfunction | 4 | 422 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.26, 1.14] |
| 20.1 vs Paroxetine | 2 | 141 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.29, 1.91] |
| 20.2 vs Sertraline | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.12] |
| 20.3 vs Fluoxetine | 1 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.05, 5.80] |
Comparison 10. Side effect profile: Fluvoxamine vs SNRIs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular ‐ Hypertension / tachycardia | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.42] |
| 1.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.42] |
| 2 Cardiovascular ‐ Hypotension / bradycardia | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.28, 1.77] |
| 2.1 vs Milnacipran | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.28, 1.77] |
| 3 Dermatological ‐ Dermatitis / rash | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.22, 9.16] |
| 3.1 vs Milnacipran | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.22, 9.16] |
| 4 Dermatological ‐ Sweating | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 3.17 [0.32, 31.43] |
| 4.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 3.17 [0.32, 31.43] |
| 5 Gastrointestinal ‐ Increased salivation | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.04, 3.38] |
| 5.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.04, 3.38] |
| 6 Gastrointestinal ‐ Dry mouth | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.56, 2.36] |
| 6.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.56, 2.36] |
| 7 Gastrointestinal ‐ Oral discomfort / taste disturbance | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.36] |
| 7.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.36] |
| 8 Gastrointestinal ‐ Vomiting / nausea | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.95 [1.09, 3.50] |
| 8.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.95 [1.09, 3.50] |
| 9 Gastrointestinal ‐ Constipation | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.45, 2.76] |
| 9.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.45, 2.76] |
| 10 Gastrointestinal ‐ Diarrhoea | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.59, 3.89] |
| 10.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.59, 3.89] |
| 11 Gastrointestinal ‐ Weight gain | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 4.76] |
| 11.1 vs Milnacipran | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 4.76] |
| 12 Gastrointestinal ‐ Weight loss | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.16] |
| 12.1 vs Milnacipran | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.16] |
| 13 Gastrointestinal ‐ Anorexia | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.06, 16.69] |
| 13.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.06, 16.69] |
| 14 Neuropsychiatric ‐ Blurred vision | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [0.66, 4.14] |
| 14.1 vs Milnacipran | 2 | 127 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [0.66, 4.14] |
| 15 Neuropsychiatric ‐ Dizziness / vertigo / faintness | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.50, 2.52] |
| 15.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.50, 2.52] |
| 16 Neuropsychiatric ‐ Fatigue / tiredness / asthenia | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.5 [0.04, 5.68] |
| 16.1 vs Milnacipran | 1 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.5 [0.04, 5.68] |
| 17 Neuropsychiatric ‐ Headache | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.59, 3.01] |
| 17.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.59, 3.01] |
| 18 Neuropsychiatric ‐ Tremor | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 3.93] |
| 18.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [0.56, 3.93] |
| 19 Neuropsychiatric ‐ Involuntary movement other than tremor | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.87, 9.10] |
| 19.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [0.87, 9.10] |
| 20 Neuropsychiatric ‐ Insomnia | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.62, 4.10] |
| 20.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.62, 4.10] |
| 21 Neuropsychiatric ‐ Sleepiness / drowsiness | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.62, 4.54] |
| 21.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.68 [0.62, 4.54] |
| 22 Neuropsychiatric ‐ Agitation / anxiety | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.95 [0.72, 5.30] |
| 22.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.95 [0.72, 5.30] |
| 23 Genitourinary ‐ Problems urinating | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.55] |
| 23.1 vs Milnacipran | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.55] |
10.6. Analysis.
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 6 Gastrointestinal ‐ Dry mouth.
10.7. Analysis.
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 7 Gastrointestinal ‐ Oral discomfort / taste disturbance.
10.9. Analysis.
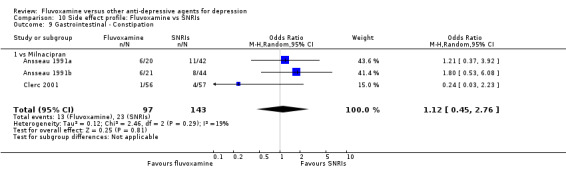
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 9 Gastrointestinal ‐ Constipation.
10.10. Analysis.
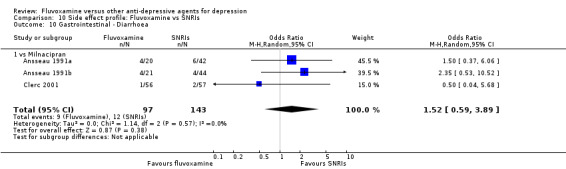
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 10 Gastrointestinal ‐ Diarrhoea.
10.11. Analysis.
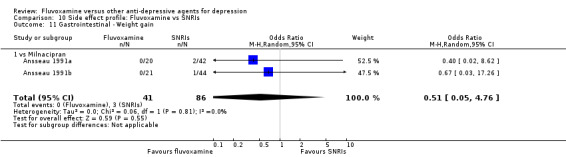
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 11 Gastrointestinal ‐ Weight gain.
10.12. Analysis.
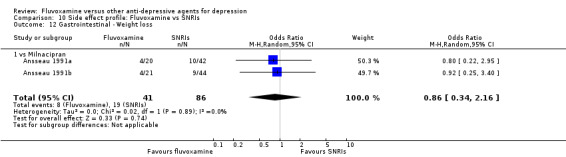
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 12 Gastrointestinal ‐ Weight loss.
10.15. Analysis.
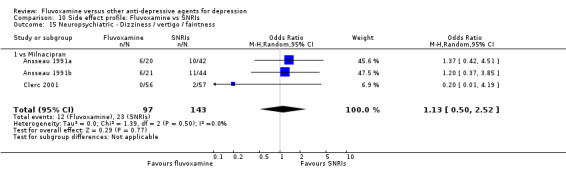
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 15 Neuropsychiatric ‐ Dizziness / vertigo / faintness.
10.16. Analysis.
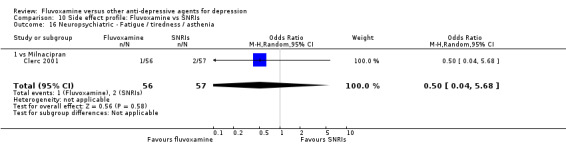
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 16 Neuropsychiatric ‐ Fatigue / tiredness / asthenia.
10.17. Analysis.
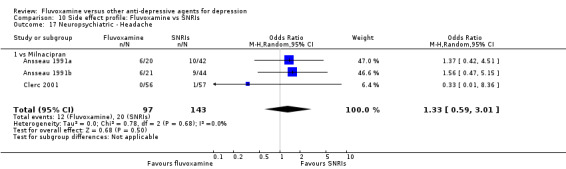
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 17 Neuropsychiatric ‐ Headache.
10.18. Analysis.
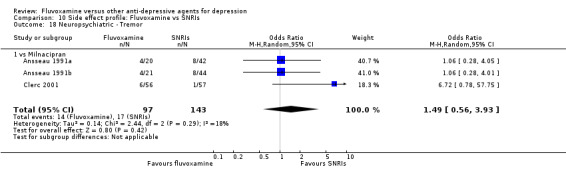
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 18 Neuropsychiatric ‐ Tremor.
10.19. Analysis.
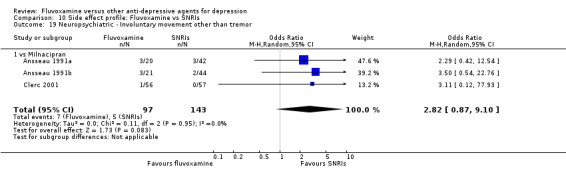
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 19 Neuropsychiatric ‐ Involuntary movement other than tremor.
10.20. Analysis.
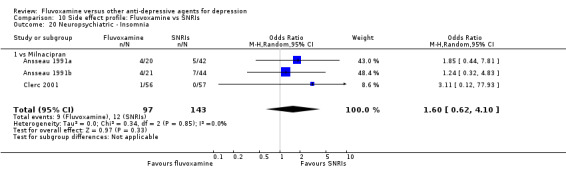
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 20 Neuropsychiatric ‐ Insomnia.
10.21. Analysis.
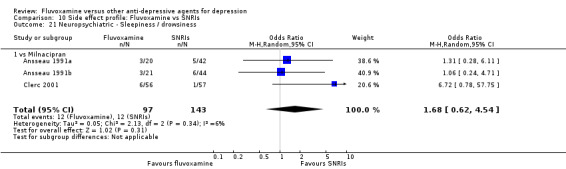
Comparison 10 Side effect profile: Fluvoxamine vs SNRIs, Outcome 21 Neuropsychiatric ‐ Sleepiness / drowsiness.
Comparison 11. Side effect profile: Fluvoxamine vs newer ADs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular ‐ Hypotension / bradycardia | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 vs Moclobemide | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 2 Dermatological ‐ Sweating | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 vs Moclobemide | 2 | 170 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.41, 6.70] |
| 3 Gastrointestinal ‐ Dry mouth | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.37] |
| 3.2 vs Moclobemide | 2 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 4.73 [1.14, 19.57] |
| 4 Gastrointestinal ‐ Vomiting / nausea | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 3.43 [1.90, 6.19] |
| 4.2 vs Moclobemide | 2 | 170 | Odds Ratio (M‐H, Random, 95% CI) | 2.01 [1.03, 3.92] |
| 5 Gastrointestinal ‐ Constipation | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.47, 2.08] |
| 6 Gastrointestinal ‐ Diarrhoea | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.52, 2.15] |
| 7 Gastrointestinal ‐ Weight gain | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.17, 1.03] |
| 8 Gastrointestinal ‐ Increased appetite | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.14, 1.15] |
| 9 Neuropsychiatric ‐ Blurred vision | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 vs Moclobemide | 1 | 130 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.11, 4.33] |
| 10 Neuropsychiatric ‐ Dizziness / vertigo / faintness | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.40, 1.44] |
| 10.2 vs Moclobemide | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.04, 28.71] |
| 11 Neuropsychiatric ‐ Fatigue / tiredness / asthenia | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.50, 2.27] |
| 11.2 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.42, 3.67] |
| 12 Neuropsychiatric ‐ Headache | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.65, 2.19] |
| 12.2 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.23, 6.72] |
| 13 Neuropsychiatric ‐ Tremor | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [0.96, 7.27] |
| 14 Neuropsychiatric ‐ Insomnia | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 vs Moclobemide | 2 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.26, 1.35] |
| 15 Neuropsychiatric ‐ Sleepiness / drowsiness | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.76] |
| 16 Neuropsychiatric ‐ Agitation / anxiety | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 vs Mirtazapine | 1 | 412 | Odds Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.61] |
| 16.2 vs Moclobemide | 3 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.53, 2.39] |
| 17 Neuropsychiatric ‐ Manic symptom | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 vs Moclobemide | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [0.32, 31.64] |
| 18 Neuropsychiatric ‐ Completed suicide | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 vs Moclobemide | 1 | 61 | Odds Ratio (M‐H, Random, 95% CI) | 3.20 [0.13, 81.78] |
11.6. Analysis.
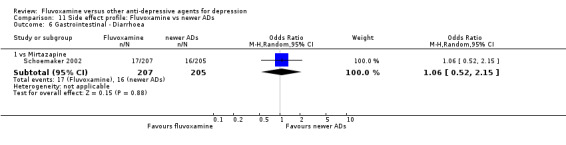
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 6 Gastrointestinal ‐ Diarrhoea.
11.7. Analysis.
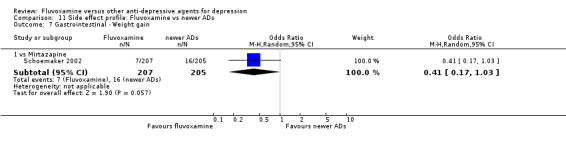
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 7 Gastrointestinal ‐ Weight gain.
11.10. Analysis.
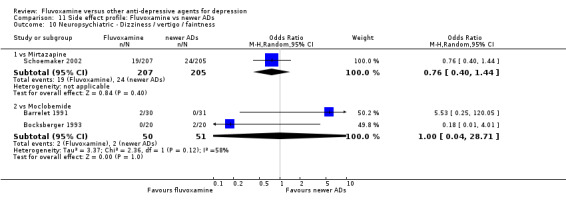
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 10 Neuropsychiatric ‐ Dizziness / vertigo / faintness.
11.11. Analysis.
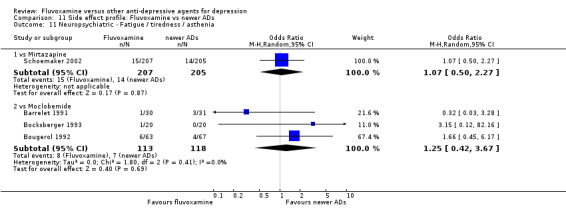
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 11 Neuropsychiatric ‐ Fatigue / tiredness / asthenia.
11.12. Analysis.
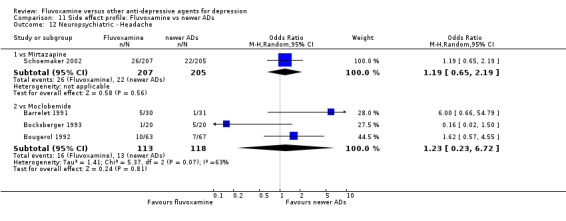
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 12 Neuropsychiatric ‐ Headache.
11.13. Analysis.
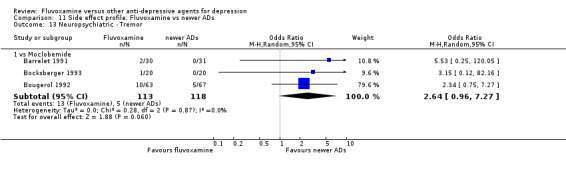
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 13 Neuropsychiatric ‐ Tremor.
11.14. Analysis.
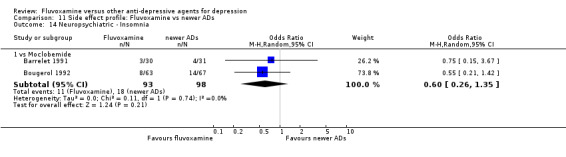
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 14 Neuropsychiatric ‐ Insomnia.
11.17. Analysis.
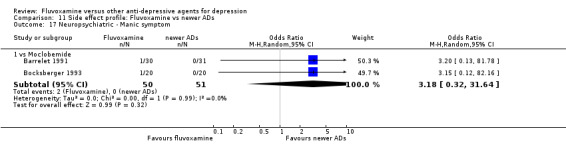
Comparison 11 Side effect profile: Fluvoxamine vs newer ADs, Outcome 17 Neuropsychiatric ‐ Manic symptom.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amore 1989.
| Methods | Four‐week double blind, randomised study. | |
| Participants | Psychiatric inpatients meeting DSM‐III for major depression without psychotic features, with a minimum baseline score of 21 on the HDRS‐21. Age range: 20‐70 years old. Exclusion criteria: during pregnancy, lactation, or being of childbearing potential, serious diseases, alcohol or drug abuse, or treatment with any medications that might interact with antidepressant drugs. | |
| Interventions | Fluvoxamine: 15 participants.
Imipramine: 15 participants.
Fluvoxamine dose range: 100‐150 mg/day.
Imipramine dose range: 100‐150 mg/day. Benzodiazepine were allowed as additional medications. |
|
| Outcomes | Hamilton Rating Scale for Depression (HRSD) ‐21, Zung Self‐Rating Depression Scale, Clinical Global Impression‐severity (CGI‐S) and Clinical Global Impression‐improvement (CGI‐I). Total dropout, dropout due to inefficiency, due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "in a double blind fashion", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 5/15 missing from control group (4 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | Standard deviations (SDs) of endpoint / change score for depression were not reported. |
Ansseau 1991a.
| Methods | Four‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric inpatients meeting RDC for major depressive disorder, endogenous subtype, with a minimum baseline score of 25 on the Montgomery and Asberg Scale for Depression (MADRS). Age range: 20‐70 years old. Exclusion criteria: patients presenting any evidence of contra‐indications for a tricyclic antidepressant, or serious or uncontrolled medical illness. | |
| Interventions | Fluvoxamine: 41 participants.
Milnacipran: 42 participants.
Fluvoxamine dose: 200 mg/day.
Milnacipran dose: 300 mg/day for 2 weeks and 150mg/day during the 2 following weeks) The active period was preceded by a washout period of 4‐7 days on placebo and lorazepam (up to 10 mg/day) and nitrazepam (up to 10 mg/day) if needed. These associated drugs could be maintained during the treatment period if necessary. |
|
| Outcomes | HRSD‐24, CGI‐I, CGI‐S, CGI‐efficacy index, Raskin Scale for Depression. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As a comparator drug. | |
| Notes | 11/127 (8.7%) patients were with bipolar depression and 14/127 (11.0%) patients were with psychotic features. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | no details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Both participants and drug prescribing physicians were blinded. No further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 6/41 missing from fluvoxamine group (1 due to lack of efficacy, 5 due to adverse effects); 10/86 missing from control group (2 due to lack of efficacy, 7 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Ansseau 1991b.
| Methods | =Ansseau 1991a | |
| Participants | =Ansseau 1991a | |
| Interventions | Fluvoxamine: 41 participants. Milnacipran: 44 participants. Fluvoxamine dose: 200 mg/day. Milnacipran dose: 200 mg/day for 4 weeks. The active period was preceded by a washout period of 4‐7 days on placebo and lorazepam (up to 10 mg/day) and nitrazepam (up to 10 mg/day) if needed. These associated drugs could be maintained during the treatment period if necessary. | |
| Outcomes | =Ansseau 1991a | |
| Funded by pharmaceutical companies | =Ansseau 1991a | |
| Fluvoxamine as an investigational or comparator drug | =Ansseau 1991a | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | =Ansseau 1991a |
| Allocation concealment (selection bias) | Unclear risk | =Ansseau 1991a |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | =Ansseau 1991a |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | =Ansseau 1991a |
| Selective reporting (reporting bias) | High risk | =Ansseau 1991a |
Ansseau 1994.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric in‐ and outpatients meeting DSM‐III‐R for major depressive episode, with a minimum baseline score of 18 on the HRSD‐21. Age range: 18‐65 years old. Exclusion criteria: clinically significant co‐existing diseases or other psychiatric disorders, history of alcohol or drug abuse, women of child bearing potential not employing adequate contraception, recent treatment with monoamine oxidase inhibitors, neuroleptics, or lithium, and current treatment with oral anticoagulants and type 1C antiarrythmic. | |
| Interventions | Fluvoxamine: 64 participants.
Paroxetine: 56 participants.
Fluvoxamine dose range: 50‐200 mg/day.
Paroxetine dose range: 20‐30 mg/day. For patients who had received benzodiazepines for at least two weeks prior to continue these agents, providing the dose remained unchanged throughout the study period. In addition, low dose lormethazepam or chloral hydrate were permitted in case of severe insomnia. |
|
| Outcomes | HRSD‐21, CGI‐S, Hamilton Rating Scale for Anxiety (HRSA). Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Patients with major depressive episode (DSM‐III‐R) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "the trial used a double‐blind design", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 23/64 missing from fluvoxamine group (5 due to lack of efficacy, 13 due to adverse effects); 16/56 missing from control group (2 due to lack of efficacy, 3 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Asakura 2005.
| Methods | Four‐week double blind, single‐centre, randomised study. | |
| Participants | Psychiatric in‐ and outpatients meeting major depressive disorder or other affective disorder according to DSM‐IV. Baseline HRSD score: 20.02 (SD 6.60). Age: 20 years old or more, mean 41.3 years old (SD 13.7). Exclusion criteria: treated with any antidepressants for the current depressive episode. | |
| Interventions | Fluvoxamine: 158 participants. Imipramine: 161 participants. Fluvoxamine dose range: 50‐150 mg/day. Imipramine dose range: 75‐150 mg/day. | |
| Outcomes | HRSD‐17, CGI. Total dropout. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical companies market fluvoxamine and comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Almost all the patients were drug naive outpatients. 17% (54/309) of participants were with dysthymic disorder, depressive disorder not otherwise specified, bipolar II disorder or major depressive disorder with psychotic features. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 9/158 missing from fluvoxamine group; 12/161 missing from control group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Barge‐Schaapveld 1995.
| Methods | Six‐week open, multicentre, randomised study. | |
| Participants | Primary care outpatients meeting DSM‐III‐R for major depressive disorder, with a minimum baseline score of 18 on the HRSD‐17. Patients were recruited in five primary care practices. Age range: 18‐65 years old. Exclusion criteria: other psychiatric or medical conditions. | |
| Interventions | Fluvoxamine: 13 participants. Amitriptyline: 10 participants. Fluvoxamine dose: 100 mg/day. Amitriptyline dose: 150 mg/day. | |
| Outcomes | HRSD‐17. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients were blinded. No blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 2/13 missing from fluvoxamine group. |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Barrelet 1991.
| Methods | Four‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric in‐ and outpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 17 on the HRSD‐17. Age: mean 54.2 years old (SD 14.6). Exclusion criteria: organic brain disorders, alcohol dependence, risk of suicide, with schizophrenic symptoms, severe medical illness. | |
| Interventions | Fluvoxamine: 30 participants.
Moclobemide: 31 participants.
Fluvoxamine dose range: 100‐200 mg/day.
Moclobemide dose range: 300‐450 mg/day. Benzodiazepine and lithium were allowed. |
|
| Outcomes | HRSD‐17, HRSA, Widlocher Psychomotor Retardation Scale, Hospital Anxiety and Depression Scale. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As a comparator drug. | |
| Notes | Patients with major affective disorder (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double aveugle", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 5/30 missing from fluvoxamine group (4 due to adverse effects); 5/30 missing from control group (4 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Bocksberger 1993.
| Methods | Four‐week double blind, single‐centre, randomised study. | |
| Participants | Psychiatric inpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 20 on the MADRS. Age range: 65 years old or more. Exclusion criteria: marked suicidal tendency, symptoms of psychosis, severe organic disease, alcoholism, drug abuse. | |
| Interventions | Fluvoxamine: 20 participants.
Moclobemide: 20 participants.
Fluvoxamine dose range: 100‐200 mg/day.
Moclobemide dose range: 300‐450 mg/day. Patients receiving lithium were enrolled in the study, providing treatment had been stabilized for at least four weeks prior to entry. The use of concomitant psychotropic medication was prohibited, with the exception of lithium for patients on a previously established lithium regimen, or one benzodiazepine or chloral hydrate, if judged necessary by the investigator. Drugs that had been given regularly for somatic complaints were allowed to continue if there were no psychotropic effects. |
|
| Outcomes | MADRS, CGI‐I, Widlocher Psychomotor Retardation Scale. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As a comparator drug. | |
| Notes | Patients with major affective disorder (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "two randomized parallel groups", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "a double blind study", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 2/20 missing from fluvoxamine group (1 due to adverse effects); 1/20 missing from control group. |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Bougerol 1992.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric in‐ and outpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 17 on the HRSD‐17. Age range: 18 years old or more. Exclusion criteria: marked suicidal tendency, symptoms of psychosis; severe organic disease, patient undergoing electroshock therapy or structured psychotherapy, alcoholism, drug abuse. | |
| Interventions | Fluvoxamine: 63 participants.
Moclobemide: 67 participants.
Fluvoxamine dose range: 100‐200 mg/day.
Moclobemide dose range: 300‐450 mg/day. Patients receiving lithium were enrolled in the study, providing treatment had been stabilized for at least 4 weeks prior to entry. The use of concomitant psychotropic medication was prohibited, with the exception of lithium for patients on a previously established lithium regimen, or one benzodiazepine, if judged necessary by the investigator. Drugs that had been given regularly for somatic complaints were allowed to continue if there were no psychotropic effects. During the study, patients were not required to avoid tyramine‐rich food. |
|
| Outcomes | HRSD‐17, CGI‐efficacy index, Widlocher Psychomotor Retardation Scale. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As a comparator drug. | |
| Notes | Patients with major affective disorder (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "two randomized parallel groups", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 19/63 missing from fluvoxamine group (3 due to lack of efficacy, 9 due to adverse effects); 15/67 missing from control group (5 due to lack of efficacy, 6 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Bramanti 1988.
| Methods | Four‐week double blind, multicentre, randomised study. | |
| Participants | Patients suffering major depressive disorder according to DSM‐III, with a minimum baseline score of 18 on the HRSD‐21. Age range: 18 years old or more. Exclusion criteria: pregnancy or a risk of pregnancy, lactating women, severe impairment of liver or renal function, treatment with lithium, other antidepressants or MAO inhibitors in the previous two weeks before the study. | |
| Interventions | Fluvoxamine: 30 participants.
Imipramine: 30 participants.
Fluvoxamine dose range: 100‐150 mg/day.
Imipramine dose range: 100‐150 mg/day. Treatments with benzodiazepines was allowed, if necessary. No treatments with barbiturates, other antidepressants, or amphetamine were permitted. |
|
| Outcomes | HRSD‐21, CGI‐I, CGI‐S. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | 5/60 (8%) patients were with psychotic features. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study medicines were dispensed in identical pharmaceutical forms, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 2/30 missing from fluvoxamine group (2 due to lack of efficacy); 1/30 missing from control group (1 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Brown 1986.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for major depression, with a minimum baseline score of 20 on the HRSD‐21. Age range: 21‐60 years old. Exclusion criteria: clinically significant physical illness or were taking medication, such as anticonvulsants. | |
| Interventions | Fluvoxamine: 33 participants. Imipramine: 17 participants. Fluvoxamine dose range: 200‐300 mg/day. Imipramine dose range: 150‐225 mg/day. | |
| Outcomes | HRSD‐21. Total dropout, dropout due to inefficiency, dropout due to side effects. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Study authors examined pituitary‐adrenocortical status in relation to medication. No clinical data were reported for imipramine group. No efficacy data could be entered in a meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 8/33 missing from fluvoxamine group (8 due to adverse effects); 4/17 missing from control group (4 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | No clinical data were reported for imipramine group. |
Brunner 1994.
| Methods | Four‐week open, randomised study. | |
| Participants | Psychiatric inpatients suffering from depression according to Feighner criteria, with a minimum baseline score of 17 on the HRSD‐17. Age range: 18‐70 years old. Exclusion criteria: Woman who were pregnant or not taking adequate contraceptive measures, an initial depressive episode of less than two weeks' duration, depression secondary to another psychiatric condition, severe physical illness, or who had received lithium or shock therapy in the previous four weeks. | |
| Interventions | Fluvoxamine: 20 participants.
Amineptine: 20 participants.
Fluvoxamine dose range: 100‐300 mg/day.
Amineptine dose range: 100‐200 mg/day. Diazepam (sedative), flunitrazepam (hypnotic) and cymemazine (sedative neuroleptic) were allowed. |
|
| Outcomes | HRSD‐17, CGI‐I, CGI‐S. Total dropout, dropout due to inefficiency, dropout due to side effects. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Patients with depression (Feighner criteria) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized study", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "open study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 2/20 missing from fluvoxamine group (1 due to adverse effects); 3/20 missing from control group (4 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Cassano 1986.
| Methods | Four‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric in‐ and outpatients meeting DSM‐III for major affective disorder, with a minimum baseline score of 15 on the HDRS‐17. Age range: 19‐70 years old. Exclusion criteria: child bearing potential or pregnant women; antidepressant therapy in the past 2 weeks; ECT within the last month; depressive symptoms secondary to other psychiatric illness; dependence upon licit or illicit drugs; serious organic diseases; need for concurrent medications which could interact with the study drugs or obscure their effects; patients unwilling or unable to cooperate with the study. | |
| Interventions | Fluvoxamine: 169 participants.
Imipramine: 161 participants.
Placebo: 151 participants.
Fluvoxamine dose range: 50‐300 mg/day.
Imipramine dose range: 50‐300 mg/day. Chloral hydrate or flurazepam as hypnotics were allowed during the trial. |
|
| Outcomes | HDRS‐17, CGI‐I, CGI‐severity, BPRS, SCL‐90, SDS. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Patients with major affective disorder (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 67/169 missing from fluvoxamine group (12 due to lack of efficacy, 19 due to adverse effects); 57/161 missing from control group (10 due to lack of efficacy, 17 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Claghorn 1996.
| Methods | Six‐week double blind, single‐centre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for major depressive disorder (single or recurrent episode without psychotic features or only mood‐congruent psychotic features). Baseline symptom severity (mean (SD) for HRSD‐21) was 26.1 (3.6). Age range: 18‐65years old. Exclusion criteria: any significant health problems, as determined by a physical examination and clinical laboratory tests (blood chemistry, hematology, urinalysis, serum pregnancy test) and electrocardiograms. | |
| Interventions | Fluvoxamine: 50 participants. Imipramine: 50 participants. Placebo: 50 participants. Fluvoxamine dose range: 50‐150 mg/day. Imipramine dose range: 80‐240 mg/day. | |
| Outcomes | HRSD‐21, MADRS, CGI‐I, Raskin Depression Scale and Covi Anxiety Scale, SCL‐56. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 30/50 missing from fluvoxamine group (4 due to lack of efficacy, 12 due to adverse effects); 29/50 missing from control group (3 due to lack of efficacy, 13 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Clerc 2001.
| Methods | Six‐week, multicentre, randomised study. | |
| Participants | Psychiatric in‐ and outpatients suffering from major depression according to DSM‐III‐R, with a minimum baseline score of 25 on the MADRS. Age range: 18‐70 years old. Exclusion criteria: depression was associated with other conditions such as dysthymic disorder or schizophrenia, suicidal ideas or extreme anxiety, bipolar depression treated with lithium within the 3 months prior to the study, pregnancy or breastfeeding, serious medical conditions or treatment with drugs known to interact with fluvoxamine or milnacipran. | |
| Interventions | Fluvoxamine: 56 participants.
Milnacipran: 57 participants.
Fluvoxamine dose: 200 mg/day.
Milnacipran dose: 100 mg/day. No other psychotropic drugs were permitted during the trial and any such drugs were withdrawn during the run‐in period. Tranquilizers or hypnotics (other than nitrazepam or lorazepam) were withdrawn at least 3 days before starting medication, and antidepressants were withdrawn 7 days before the study (15 days for monoamine oxidase inhibitor). Intermediate‐acting neuroleptic agents were withdrawn 7 days before the trial, and long‐acting agents were withdrawn 1 month before. Nitrazepam or lorazepam, at daily doses up to 5 and 10 mg, respectively, or chloral hydrate syrup, could be given as necessary during the run‐in and subsequent phases. |
|
| Outcomes | HRSD‐17, HRSD‐24, MADRS, CGI‐I. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As a comparator drug. | |
| Notes | 2/113 (1.8%) patients were with psychotic features. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Patients were blinded, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 17/56 missing from fluvoxamine group (7 due to lack of efficacy, 4 due to adverse effects); 15/57 missing from control group (10 due to lack of efficacy, 1 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Coleman 1982.
| Methods | Four‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric inpatients suffering from both unipolar and bipolar depression, with the dysphoric mood accompanied by at least five of Feighner criteria. Age range: not stated. Exclusion criteria: not stated. | |
| Interventions | Fluvoxamine: 50 participants. Clomipramine: 48 participants. Fluvoxamine dose range: 150‐300 mg/day. Clomipramine dose range: 150‐300mg/day. | |
| Outcomes | HRSD, CGI‐I, CGI‐S. Total dropout, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Patients with bipolar depression were included, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 9/50 missing from fluvoxamine group; 5/48 missing from control group (due to lack of efficacy, due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Dalery 2003.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for major depressive episode, with a minimum baseline score of 17 on the HRSD‐17. Age range: 18‐70 years old. Exclusion criteria: acute suicidal ideation or a serious suicide attempt in the previous 6 months; dementia; a history of epilepsy or seizures; concurrent or recent (6 months) alcoholism, other psychoactive substance abuse or drug‐induced psychosis, were pregnant, lactating or of childbearing potential and not taking adequate contraceptive measures, or if they had clinically uncontrolled hepatic, renal, pulmonary, endocrine or collagen disease. Also excluded were patients who had previously failed SSRI therapy or who required concomitant lithium, warfarin, hepatically metabolised antivitamine K agents, carbamazepine, theophyline, insulin or hypoglycaemic agents. Patients were required not to receive monoamine oxidase inhibitors or ECT in the 2 weeks prior to the study. | |
| Interventions | Fluvoxamine: 90 participants.
Fluoxetine: 94 participants.
Fluvoxamine dose: 100 mg/day.
Fluoxetine dose: 20 mg/day. Oxazepam or nitrazepam were permitted as necessary for night time sedation; no other psychotherapeutic treatments or ECT were permitted during the study. |
|
| Outcomes | HRSD‐17, CGI‐I, CGI‐S, Clinical Anxiety Scale, Irritability, Depression and Anxiety scale, Beck's scale for suicide ideation score. Total dropout, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Patients with major depressive episode (DSM‐III‐R) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 24/90 missing from fluvoxamine group; 20/94 missing from control group (due to lack of efficacy, due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
de Wilde 1983.
| Methods | Six‐week double blind, randomised study. | |
| Participants | Psychiatric outpatients meeting Feighner criteria for depression, with a minimum baseline score of 16 on the HRSD‐17. Age range: 18‐70 years old. Exclusion criteria: significant concurrent organic disease, depression as a secondary manifestation of other psychiatric illnesses, pregnant or lactating women, patients who had received ECT or lithium within the last 4 weeks, patients with a high suicide risk and those with a history of drug allergy. | |
| Interventions | Fluvoxamine: 22 participants.
Clomipramine: 51 participants.
Fluvoxamine dose range: 100‐300 mg/day.
Clomipramine dose range: 50‐150 mg/day. During the treatment free period and the study period the following concurrent medication was allowed: benzodiazepines as hypnotics, sedatives or tranquillizers, chloral hydrate as a hypnotic, antibiotics, non‐narcotic analgesics, antacids and laxatives. Antihypertensive agents, anticoagulants and alcohol were not allowed. Psychotropic medication other than the benzodiazepines or chloral hydrate were forbidden. |
|
| Outcomes | HRSD‐17, CGI‐S. Total dropout, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | 2/43 (4.7%) patients were with bipolar depression. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | SDs for endpoint score and change score were not reported. |
Dick 1983.
| Methods | Four‐week double blind, randomised study. | |
| Participants | Psychiatric inpatients suffering from persistent alteration of mood (depressed mood), had to be accompanied by at least 5 characteristics as mentioned in the Feighner criteria, with a minimum baseline score of 16 on the HRSD‐17. Age range: 34‐64 years old. Exclusion criteria: not stated. | |
| Interventions | Fluvoxamine: 17 participants.
Clomipramine: 15 participants.
Fluvoxamine dose: 150 mg/day.
Clomipramine dose: 150 mg/day. The protocol did not allow drugs other than the study medication during the study period except, if necessary, limited use of diazepam, levomepromazine (methotrimeprazine) and flunitrazepam. |
|
| Outcomes | HRSD‐17, CGI‐S, CGI‐change in condition score and a self rating scale. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Patients with depression (Feighner criteria) were included, so there might be some bipolar depression, but correct number was not reported. *The HRSD‐17 items 'genital symptoms' and 'loss of weight' were not recorded, study authors analysed left 15 items only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 4/17 missing from fluvoxamine group (2 due to adverse effects); 4/15 missing from control group (1 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Fabre 1996.
| Methods | Six‐week double blind, single‐centre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for major depressive disorder, with a minimum baseline score of 20 on the HRSD‐21. Age range: 18‐65 years old. Exclusion criteria: any other primary psychiatric diagnosis, unstable medical conditions, clinically significant abnormal laboratory findings. | |
| Interventions | Fluvoxamine: 50 participants.
Imipramine: 50 participants.
Placebo: 50 participants.
Fluvoxamine dose range: 50‐150 mg/day.
Imipramine dose range: 80‐240 mg/day. 101 received at least one concomitant medication during the double blind portion of the trial. Concomitant medication was limited, only chloral hydrate was allowed as a hypnotic during the first two weeks of therapy. No sedative hypnotic was permitted after week 2. The most frequently prescribed medications were acetaminophen, aspirin, and other non‐steroidal anti‐inflammatory agents or non‐sedating antihistamines, which should not have influenced the safety or efficacy outcome measures used in this study. |
|
| Outcomes | HRSD‐21, MADRS, CGI‐S, Raskin‐Covi, SCL‐56. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 28/50 missing from fluvoxamine group (7 due to lack of efficacy, 7 due to adverse effects); 25/50 missing from control group (2 due to lack of efficacy, 9 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Feighner 1989.
| Methods | Six‐week double blind, randomised study. | |
| Participants | Psychiatric inpatients meeting DSM‐III for major depression. All but one also quantified for melancholic subtype. Age range: 18‐71 years old. Exclusion criteria: not reported. | |
| Interventions | Fluvoxamine: 31 participants. Imipramine: 36 participants. Placebo: 19 participants. Fluvoxamine dose range: 150‐300 mg/day. Imipramine dose range: 150‐300 mg/day. | |
| Outcomes | HRSD, CGI‐S, BPRS, Zung Depression Scale, SCL‐90. Total dropout, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 10/31 missing from fluvoxamine group (7 due to adverse effects); 13/36 missing from control group (13 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Gonul 1999.
| Methods | Four‐week double blind, randomised study. | |
| Participants | Patients with major depression (DSM‐IV). Age: mean 35.6 years old (SD 11.8). Exclusion criteria: psychotic symptoms, catatonia. | |
| Interventions | Fluvoxamine: 40 participants. Fluoxetine: 40 participants. Paroxetine: 40 participants. Sertraline: 40 participants. Fluvoxamine dose: 150 mg/day. Fluoxetine dose: 20 mg/day. Paroxetine dose: 20 mg/day. Sertraline dose: 50 mg/day. | |
| Outcomes | HRSD. Total dropout, dropout due to side effects. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Patients with "major depression" (DSM‐IV) were included, so there might be some bipolar depression, but correct number was not reported. No clinical efficacy data could be entered in a meta ‐analysis; study authors reported only as "When the number of patients responding therapy was taken into account for the efficacy to each SSRI, we could not find any significant difference among them". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "single blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 3/40 missing from fluvoxamine group (3 due to adverse effects); 4/40 missing from fluoxetine group (4 due to adverse effects); 6/40 missing from paroxetine group (6 due to adverse effects); 4/40 missing from sertraline group (6 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | Results of HRSD were not reported. |
Guy 1984.
| Methods | Six‐week double blind, randomised study. | |
| Participants | Psychiatric inpatients meeting RDC for primary depression (unipolar or bipolar) and a persistent alteration of mood. Age range: 18‐60 years old. Exclusion criteria: females not practicing contraception, significant medical illnesses, continuing alcohol/drug abuse, history of other psychiatric illness in which the depression was secondary. | |
| Interventions | Fluvoxamine: 17 participants.
Imipramine: 19 participants.
Fluvoxamine dose range: 150‐225 mg/day.
Imipramine dose range: 150‐225 mg/day. Remedial medications were administered in at least one occasion to approximately 80 % of the patients in each treatment group. Flurazepam for sleep disturbance and acetaminophen for headache were the most frequently prescribed medications. In both instances, a higher percent of fluvoxamine‐treated patients (64%) received these medications than patients in the imipramine group. Less frequently and non differentially prescribed were medications for constipation, diet supplementation, coughs and colds, and minor infections. |
|
| Outcomes | HRSD‐17, CGI‐I, CGI‐S, BPRS, NOSIE (Nurses' observation scale for independent evaluation), Zung self‐rating depression scale. Total dropout, side effect profile. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | 7/36 (19.4%) bipolar patients were included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "assigned by randomization list", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 1/17 missing from fluvoxamine group; 3/19 missing from control group. |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Hackett 1998a.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III for major depression, with a minimum pre‐study and baseline score of 24 on the MADRS and no more than 20 % decrease in those score from pre‐study to baseline. Age range: not stated. Exclusion criteria: not stated. | |
| Interventions | Fluvoxamine: 34 participants. Venlafaxine (twice a day): 37 participants. Fluvoxamine dose: 200 mg/day. Venlafaxine dose (twice a day): 100 mg/day. | |
| Outcomes | HRSD, MADRS, CGI‐I. Total dropout. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As a comparator drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 13/34 missing from fluvoxamine group; 9/37 missing from venlafaxine (twice a day) group; 9/40 missing from venlafaxine (three times a day) group. |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Hackett 1998b.
| Methods | =Hackett 1998a | |
| Participants | =Hackett 1998a | |
| Interventions | Fluvoxamine: 34 participants. Venlafaxine (three times a day): 40 participants. Fluvoxamine dose: 200 mg/day. Venlafaxine dose (three times a day): 100 mg/day. | |
| Outcomes | =Hackett 1998a | |
| Funded by pharmaceutical companies | =Hackett 1998a | |
| Fluvoxamine as an investigational or comparator drug | =Hackett 1998a | |
| Notes | =Hackett 1998a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | =Hackett 1998a |
| Allocation concealment (selection bias) | Unclear risk | =Hackett 1998a |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | =Hackett 1998a |
| Incomplete outcome data (attrition bias) All outcomes | High risk | =Hackett 1998a |
| Selective reporting (reporting bias) | High risk | =Hackett 1998a |
Haffmans 1996.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for major depression, with a minimum baseline score of 16 on the HRSD‐17. Age range: 18‐70 years old. Exclusion criteria: patients who had been treated with MAO‐I or fluoxetine within the last 3 weeks or with other psychotropic drugs within the last week, with the exception of benzodiazepines; primary psychiatric diagnosis; a history of epilepsy, alcohol and/or drug abuse; pregnant or lactating women and women of childbearing potential failing to use standard birth control methods; renal, hepatic, cardiovascular, neurological or somatic disorders and/or significant abnormal laboratory findings; mental retardation. | |
| Interventions | Fluvoxamine: 109 participants.
Citalopram: 108 participants.
Fluvoxamine dose range: 150‐200 mg/day.
Citalopram dose range: 30‐40 mg/day. Selected benzodiazepines as permitted concomitant medication at baseline could be continued during the study. Patients who were receiving other benzodiazepines were switched to one of the following permitted drugs. The maximum dose of the benzodiazepines which could be given during the study were; oxazepam at 50 mg daily, lormethazepam at 4 mg daily, temazepam at 20 mg daily, lorazepam at 4 mg daily, flurazepam at 15 mg daily. Preferably, the daily dose was stable during the study period. All non psychotropic medication was allowed but the regime was to be kept stable. In case of severe nausea and/or vomiting, domperidone could be given. |
|
| Outcomes | HRSD‐17, CGI‐S, Zung Self‐Rating Depression scale. Total dropout, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | 6/217 (2.8%) patients were with bipolar depression and 4/217 (1.8%) patients with psychotic features. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 29/109 missing from fluvoxamine group (26 due to adverse effects); 22/108 missing from control group (16 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Harris 1991a.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 17 on the HRSD‐17. Age range: 18‐65 years old. Exclusion criteria: pregnant or at risk of pregnancy or breast feeding, epilepsy; patients receiving other antidepressants therapy, adrenergic neuron blockers, clonidine, sympathomimetic or anticholinergic drugs; severe impairment of renal or cardiovascular function, prostatic hypertrophy, urinary retention, narrow angle glaucoma, increased intraocular pressure, hyperthyroidism; severe suicidal risk. | |
| Interventions | Fluvoxamine: 35 participants.
Amitriptyline: 34 participants.
Fluvoxamine dose range: 50‐150 mg/day.
Amitriptyline dose range: 50‐150 mg/day. The use of other antidepressants was prohibited, but benzodiazepine previously described as hypnotics or anxiolytics were permitted. Other concurrent medication had to been kept to a minimum and be fully documented. |
|
| Outcomes | HRSD‐17, CGI‐I. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Patients with major depressive episode (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 12/35 missing from fluvoxamine group (1 due to lack of efficacy, 5 due to adverse effects); 10/34 missing from control group (4 due to lack of efficacy, 6 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint score for depression were not reported. |
Itil 1983.
| Methods | Six‐week double blind, randomised study. | |
| Participants | Psychiatric outpatients meeting RDC for depression, with a minimum baseline score of 15 on the HRSD‐17. Age range: 18‐65 years old. Exclusion criteria: Pregnant women and women of childbearing potential; patients whose depression was secondary to another illness; patients receiving imipramine, MAO inhibitors within 2 weeks of study commencement, ECT within 4 weeks of study commencement, lithium carbonate, or any short or long term medication which might interact with study drug. | |
| Interventions | Fluvoxamine: 22 participants. Imipramine: 25 participants. Placebo: 22 participants. Fluvoxamine dose range: 50‐300 mg/day Imipramine dose range: 50‐300 mg/day. | |
| Outcomes | HRSD‐16, CGI‐I, CGI‐S, SCL‐90, Beck depression inventory (BDI). Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | 3/69 (4.3%) were with bipolar depression. * Study authors stated the using 'HRSD‐16'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 12/22 missing from fluvoxamine group (9 due to adverse effects); 12/25 missing from control group (1 due to lack of efficacy, 7 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Kasper 1990.
| Methods | Six‐week double blind, randomised study. | |
| Participants | Psychiatric inpatients meeting RDC for major depression, with a minimum baseline score of 18 on the HRSD‐21. Age range: 28‐71 years old. Exclusion criteria: medical illness examined by complete medical and neurological evaluations; alcohol and drug abuse; pathological signs of EEG, ECG, and laboratory examinations. | |
| Interventions | Fluvoxamine: 21 participants.
Maprotiline: 21 participants.
Fluvoxamine dose range: 100‐300 mg/day.
Maprotiline dose range: 100‐300 mg/day. Following admission to the study, all psychotropic drugs were discontinued (wash out phase: 7 days) and patients remained drug free except for chlor‐hydrate, which was given for agitation or insomnia. Patients underwent a TSD (total sleep deprivation) at day 0 and day 8. All patients were deprived of sleep for one total night (40 hours). |
|
| Outcomes | HRSD‐21, CGI. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | 2/42 (5%) patients were with bipolar depression and 2/42 (5%) patients were with psychotic features. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", "capsules of either fluvoxamine or maprotiline in identical presentation", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 1/21 missing from control group (due to lack of efficacy, due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Kato 2006.
| Methods | Six‐week open, single‐centre, randomised study. | |
| Participants | Patients meeting DSM‐IV for major depressive disorder. Age: mean 44.8 years old (SD 14.9). Exclusion criteria: additional diagnoses on Axis I and Axis II, pregnancy, and medical and neurological disorders. | |
| Interventions | Fluvoxamine: 49 participants.
Paroxetine: 52 participants.
Fluvoxamine dose range: 50‐150 mg/day.
Paroxetine dose range: 20‐40 mg/day. Patients who had been receiving benzodiazepines for at least 10 days before entering the study were permitted to continue these agents, providing that dose remained unchanged throughout the study period. A low dose sleep inducing hypnotic agent, either brotizolam or triazolam, was permitted for severe insomnia as an additional medication. |
|
| Outcomes | HRSD‐21. Total dropout, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical companies market fluvoxamine and comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Study authors examined the association between 5HTT gene‐linked polymorphic region genotype and SSRI treatment response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "open‐label study", "HRSD score evaluations were administered at weekly intervals by trained raters, who were blind to genotyping". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 8/49 missing from fluvoxamine group (4 due to adverse effects); 13/52 missing from control group (6 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint score for depression were not reported. |
Kavoussi 1999.
| Methods | Eight‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐IV for major depressive disorder, with a minimum baseline score of 20 on the HRSD‐21. Age (mean (SD)): 35.4 (7.7). Exclusion criteria: other Axis I disorders (current or past), any Axis II disorder; had ever been actively suicidal, or had a history of substance dependence within 6 months of the study; had been previously treated with fluoxetine or fluvoxamine. | |
| Interventions | Fluvoxamine: number of participants not stated. Fluoxetine: number of participants not stated. Fluvoxamine dose range: 100‐150 mg/day Fluoxetine dose range: 20‐80 mg/day. | |
| Outcomes | No efficacy and tolerability data could be entered in a meta‐analysis were reported. | |
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Study authors examined prolactin response to d‐fenfluramine challenge before and after antidepressant treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", "the medications were blindly titrated", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details. |
| Selective reporting (reporting bias) | High risk | No clinical outcome data were reported. |
Kiev 1997.
| Methods | Seven‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for recurrent major depressive disorder, with a minimum baseline score of 20 on the HRSD‐21. Age range: 18‐65 years old. Exclusion criteria: woman of childbearing potential were required to use appropriate birth control methods, and no pregnant or nursing patients were included the study; patients who were not fluent in written or oral English; had a history of medication noncompliance or substance abuse within the previous 6 months (other than nicotine); had been treated within 30 days with a drug with anticipated major organ toxicity; had a severe risk of suicide or displayed auto‐aggressive behavior during the current depressive episode; hypersensitivity to SSRIs; participation in previous fluvoxamine studies; significant organic disease; clinically significant laboratory abnormalities; other primary psychiatric diagnoses; patients who would not be able to return for assessment due to transportation difficulties. | |
| Interventions | Fluvoxamine: 30 participants.
Paroxetine: 30 participants.
Fluvoxamine dose range: 50‐150 mg/day.
Paroxetine dose range: 20‐50 mg/day. Sufficient washout from other investigational drugs prior psychotropic drugs, and ECT was assured, and concomitant use of any psychotropic medication was prohibited. While medications to treat gastrointestinal disturbances (antacids, laxatives), and headache (acetaminophen, aspirin, ibuprofen) and to provide nighttime sedation (chloral hydrate only) were permitted, all other medication use was prohibited unless approved by the study physician. |
|
| Outcomes | HRSD‐21, CGI‐I, CGI‐S, HRSA, SCL‐56. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 11/30 missing from fluvoxamine group (1 due to lack of efficacy, 2 due to adverse effects); 9/30 missing from control group (3 due to lack of efficacy, 5 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint score for depression were not reported. |
Koetsier 2002.
| Methods | Four‐week double blind, single‐centre, randomised study. | |
| Participants | Psychiatric inpatients suffering from major depressive disorder according to DSM‐IV, with a minimum baseline score of 14 on the HRSD‐17. Age range: 32‐65 years old. Exclusion criteria: history of a treatment with a TCA with adequate plasma levels during 4 weeks. | |
| Interventions | Fluvoxamine: 27 participants.
Imipramine: 25 participants.
Fluvoxamine dose range: not stated.
Imipramine dose range: not stated. During the treatment period no psychotropic drugs were allowed besides the study medication. |
|
| Outcomes | HRSD‐17, SPRS (Salpetriere retardation rating scale), shortened version of POMS (Profile of mood state). Total dropout, dropout due to inefficiency, dropout due to side effects. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 3/27 missing from fluvoxamine group (2 due to adverse effects); 3/25 missing from control group (2 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Kostiukova 2003.
| Methods | Six‐week, single‐centre, randomised study. | |
| Participants | Psychiatric inpatients meeting ICD‐10 for recurrent depressive disorder without psychotic features, with a minimum baseline score of 19 on the HRSD‐17. Age range: 18‐65 years old. Exclusion criteria: not stated. | |
| Interventions | Fluvoxamine: 30 participants. Amitriptyline: 30 participants. Fluvoxamine dose range: 50‐300 mg/day. Amitriptyline dose range: 50‐250 mg/day. | |
| Outcomes | HRSD‐17. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 7/30 missing from fluvoxamine group (4 due to lack of efficacy, 3 due to adverse effects); 8/30 missing from control group (3 due to lack of efficacy, 5 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Lydiard 1989.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III for major depressive disorder, with a minimum baseline score of 22 on the HRSD‐17. Age range: 23‐80 years old. Exclusion criteria: not physically healthy, were psychotic or had organic brain syndrome, had a history of bipolar affective disorder, exhibited current depressive symptomatology of less than 1 month and greater than 18 month in duration, were currently taking any psychotropic medication, were substance abusers, or exhibited a clear suicidal intent. | |
| Interventions | Fluvoxamine: 18 participants. Imipramine: 18 participants. Placebo: 18 participants. Fluvoxamine dose range: 100‐300 mg/day. Imipramine dose range: 100‐300 mg/day. | |
| Outcomes | HRSD‐17, MADRS, CGI‐I. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 1/18 missing from fluvoxamine group (1 due to adverse effects); 2/18 missing from control group (2 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
March 1990.
| Methods | Six‐week double blind, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III for major affective disorder, with a minimum baseline score of 22 on the HRSD‐17. Age range: 18‐67 years old. Exclusion criteria: pregnant women, lactating women, or women of childbearing potential who were taking inadequate contraceptive measures; patients with schizophrenia, psychotic symptoms, organic dementias, or a diagnosis within 1 year of substance abuse or alcoholism; patients with cardiovascular, hepatic, renal, gastrointestinal, pulmonary, metabolic, or other systematic diseases that could interfere with the diagnosis, treatment, or assessment of depression; patients who required treatment with any concurrent medication that might interact with or obscure the action of the study medications; patients with clinically significant abnormalities in ECG or laboratory results; patients with multiple drug allergies; patients who had received monoamine oxidase inhibitors or lithium in the 2 weeks preceding study entry or who had received any other antidepressant drugs in the preceding 1 week; patients who had received any investigational drug or ECT in the previous 4 weeks. | |
| Interventions | Fluvoxamine: 18 participants. Imipramine: 18 participants. Placebo: 18 participants. Fluvoxamine dose range: 100‐300 mg/day. Imipramine dose range: 100‐300 mg/day. | |
| Outcomes | HRSD‐17. MADRS, CGI‐I, 58 items Hopkins symptom checklist (HSCL). Total dropout, dropout due to inefficiency, dropout due to side effects. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | 1/54 (2%) patients were with bipolar depression. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", "fluvoxamine, imipramine, and placebo were provided in identical gray capsules, "no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 5/18 missing from fluvoxamine group (5 due to adverse effects); 3/18 missing from control group (3 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Mendonca Lima 1997.
| Methods | Four‐week open, single‐centre, randomised study. | |
| Participants | Patients meeting DSM‐III‐R for major depression or dysthymia, with a minimum baseline score of 25 on the MADRS. Age: mean 35.6 years old (SD 8.3). Exclusion criteria: hepatic, renal, endocrine, cardiac disease; taking any medication that could affect thyroid function. | |
| Interventions | Fluvoxamine: 20 participants.
Maprotiline: 20 participants.
Fluvoxamine dose: 100 mg/day
Maprotiline dose: 75 mg/day. Only bromazepam (at most 18 mg/day) were allowed. |
|
| Outcomes | MADRS, Newcastle Diagnostic Scale. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | There were some patients with dysthymia, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "en ouvert" (open study), no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | SDs of endpoint score for depression were not reported. |
Miller 2001.
| Methods | Six‐week double blind, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III for major depression. Age range: not reported. Exclusion criteria: not reported. | |
| Interventions | Fluvoxamine: 13 participants. Imipramine: 11 participants. Placebo: 13 participants. Fluvoxamine dose range: not stated. Imipramine dose range: not stated. | |
| Outcomes | HRSD (change score only). No tolerability data could be entered in a meta‐analysis were reported. | |
| Funded by pharmaceutical companies | No funding by pharmaceutical companies. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind", "HRSD score were obtained by a research clinician unaware of treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Moon 1991.
| Methods | Six‐week double blind, single‐centre, randomised study. | |
| Participants | Primary care outpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 24 on the MADRS. Age range: 18‐65 years old. Exclusion criteria: clinically important impairment of hepatic or renal function; history of epilepsy; severe suicide risk; pregnant females or those at risk of becoming pregnant. | |
| Interventions | Fluvoxamine: 31 participants.
Mianserin: 31 participants.
Fluvoxamine dose range: 100‐300 mg/day.
Mianserin dose range: 60‐180 mg/day. During the study, from entry into the placebo period onwards, no other psychotropic medication (antidepressant, tranquillisers or hypnotics) were allowed, Patients were asked to keep other concurrent drugs to a minimum and to abstain from alcohol. |
|
| Outcomes | MADRS, CGI‐I. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Patients with major depressive episode (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 3/31 missing from fluvoxamine group (3 due to adverse effects); 10/31 missing from control group (7 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Mullin 1988.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric inpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 17 on the HRSD‐17. Age range: 20‐69 years old. Exclusion criteria: pregnant or breast feeding; receiving other anti‐depressive therapy which could not be discontinued for the duration of the trial; severe impairment of liver or renal function; history of recent myocardial infarction, any degree of heart block or any other cardiac arrhythmia, as such conditions contraindicate the use of dothiepin; history of narrow angle glaucoma, prostatic hypertrophy or epilepsy, as dothiepin should be avoided in these patients. | |
| Interventions | Fluvoxamine: 37 participants. Dothiepin: 36 participants. Fluvoxamine dose range: 100‐300 mg/day. Dothiepin dose range: 75‐225 mg/day. | |
| Outcomes | HRSD‐17, CGI‐I. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Patients with major depressive episode (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 11/37 missing from fluvoxamine group (2 due to lack of efficacy, 9 due to adverse effects); 12/36 missing from control group (4 due to lack of efficacy, 7 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs for endpoint score and change score were not reported. |
Murasaki 1998a.
| Methods | Four‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric in‐ and outpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 16 on the HRSD‐17. 186 out of 218 all allocated patients (85%) were with major depressive disorder. Age range: 18‐70 years old. Exclusion criteria: schizophrenia; history of epilepsy; depressive state due to organic brain disorder; treatment with lithium or MAO inhibitors in the previous two weeks before the study; treatment with ECT in the previous three months before the study; dysuria, glaucoma, endocrine disorder including hypothyroidism; history of drug allergy; severe cardiac, hepatic, renal, hematological disorder; pregnant, at risk of pregnant, breastfeeding female. | |
| Interventions | Fluvoxamine: 113 participants. Amitriptyline: 122 participants. Fluvoxamine dose range: 50‐150 mg/day. Amitriptyline dose range: 50‐150 mg/day. | |
| Outcomes | HRSD‐21. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical companies market fluvoxamine and comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | 15/218 (6.9%) patients were with bipolar depression, 11/218 (5.0%) were with dysthymia and 6/218 (2.8%) with depressive disorder not otherwise specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 43/113 missing from fluvoxamine group (1 due to lack of efficacy, 17 due to adverse effects); 56 missing from control group (2 due to lack of efficacy, 28 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Nathan 1990.
| Methods | Four‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric inpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 15 on the HRSD‐17. Age: mean 39.7 years old (SD 13.7). Exclusion criteria: history of bipolar disorder; evidence of psychosis; concurrent medical diseases. | |
| Interventions | Fluvoxamine: 20 participants. Desipramine: 20 participants. Fluvoxamine dose range: not stated. Desipramine dose range: not stated. | |
| Outcomes | HRSD‐17, Raskin, somatic symptoms scale, BDI. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", "patients were rated by an independent clinician", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 3/20 missing from fluvoxamine group (2 due to adverse effects); 2/20 missing from control group (2 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Nemeroff 1995.
| Methods | Seven‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for major depression, with a minimum baseline score of 20 on the HRSD‐21. Age range: 22‐62 years old. Exclusion criteria: pregnant or nursing female; did not have written and oral fluency in the English language; history of noncompliance; had been treated within 30 days with a drug having possible toxic effects on major organs; severe risk of suicide; intolerant to SSRI side effects; had participated in previous fluvoxamine studies; had other significant organic disease or other primary psychotic diagnoses. | |
| Interventions | Fluvoxamine: 49 participants.
Sertraline: 48 participants.
Fluvoxamine dose range: 50‐150 mg/day
Sertraline dose range: 50‐200 mg/day. Concomitant use of any psychotropic medications, other than chloral hydrate for nighttime sedation, was prohibited. While medications to treat gastrointestinal disturbances and headache were permitted, all other medication use was prohibited unless approved by the study physician. Lactobacillus acidophilus was used to treat SSRI‐induced diarrhoea. |
|
| Outcomes | HRSD‐21, CGI‐I, HRSA, Raskin and Covi, SCL‐56. Total dropout, dropout due to inefficiency, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", study drugs were identical in appearance, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 21/49 missing from fluvoxamine group (2 due to lack of efficacy, 9 due to adverse effects); 9/48 missing from control group (1 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint score for depression were not reported. |
Otsubo 2005.
| Methods | Eight‐week open, single‐centre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐IV for major depressive disorder. Age range: 20‐69 years old. Exclusion criteria: patients who had failed to respond to 2 or more prior antidepressant trials in their current episode; medical contraindications to antidepressant therapy; significant hematologic, endocrine, or cardiovascular disease or conditions that might impair the study drug absorption, metabolism, or excretion; acute suicidal tendencies; history of a seizure disorder; history or presence of any psychotic disorder not associated with depression; history of drug or alcohol dependence within the past 2 years; experiences of receiving fluvoxamine or nortriptyline treatment. | |
| Interventions | Fluvoxamine: 36 participants.
Nortriptyline: 38 participants.
Fluvoxamine dose range: 25‐150 mg/day.
Nortriptyline dose range: 25‐150 mg/day. Patients were allowed to take lormethazepam 1‐2 mg/day at bedtime for sleep whenever necessary. |
|
| Outcomes | HRSD‐17, CGI‐I, CGI‐S. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | No funding by pharmaceutical companies. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: ''the patients were able to identify the drugs prescribed from their distinct appearance", however. "the doctor in charge was blind to the medical settings", "the raters were completely blind to the medication settings". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 11/36 missing from fluvoxamine group (4 due to lack of efficacy, 3 due to adverse effects); 17/38 missing from control group (2 due to lack of efficacy, 7 due to adverse effects). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Ottevanger 1995.
| Methods | Four‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric inpatients meeting Feighner criteria for depression, with a minimum baseline score of 17 on the HRSD‐17. Age: mean 54.2 years old (SD 14.0). Exclusion criteria: patients receiving lithium or had undergone shock therapy during the previous 4 weeks; symptoms of depression which were only secondary manifestation of some other psychiatric condition; already on monoamine oxidase inhibitors or other antidepressants. | |
| Interventions | Fluvoxamine: 20 participants.
Clomipramine: 20 participants.
Fluvoxamine dose range: 100‐300 mg/day.
Clomipramine dose range: 50‐150 mg/day. The use of diazepam (5‐35mg/day) for sedation, and flunitrazepam (2 ‐4 mg/day) for insomnia was permitted. |
|
| Outcomes | HRSD‐17, CGI‐S. Total dropout, dropout due to inefficiency, dropout due to side effects. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | 1/40 (3%) patients were with bipolar depression. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 5/20 missing from fluvoxamine group (1 due to lack of efficacy, 2 due to adverse effects); 3/20 missing from control group (2 due to lack of efficacy, 1 due to adverse effects). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Perez 1990.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III for major depressive episode, with a minimum baseline score of 30 on the MADRS. Age range: 18 years old or more; mean 41.6 years old. Exclusion criteria: pregnant or at risk of becoming pregnant, breast feeding; strong suicide potential; major physical disorders such as epilepsy, hepatic or renal disease. | |
| Interventions | Fluvoxamine: 30 participants.
Mianserin: 33 participants.
Fluvoxamine dose range: 100‐300 mg/day
Mianserin dose range: 60‐180 mg/day. No other form of antidepressant medication was allowed during the trial period. |
|
| Outcomes | MADRS, CGI‐I, Leeds Sleep Evaluation Questionnaire. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Patients with major depressive episode (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 9/30 missing from fluvoxamine group (1 due to lack of efficacy, 6 due to adverse effects); 9/33 missing from control group (2 due to lack of efficacy, 5 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Rahman 1991.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Patients meeting DSM‐III for major depressive episode, with a minimum baseline score of 29 on the MADRS. Age range: 61‐86 years old. Exclusion criteria: concurrent depressive delusions or stupor; depression secondary to other psychiatric illness, according to DSM‐III; narrow angle glaucoma; symptoms suggestive of prostatic hypertrophy; history of epilepsy, myocardial infarct within 3 months of entry into the study or any degree of heart block or other clinically significant arrhythmia. | |
| Interventions | Fluvoxamine: 26 participants.
Dothiepin: 26 participants.
Fluvoxamine dose range: 100‐200 mg/day.
Dothiepin dose range: 100‐200 mg/day. Hypnotic and anxiolytic medication was allowed during the study, but other antidepressants were not. |
|
| Outcomes | MADRS, CGI‐I, Newcastle Scale. Total dropout, dropout due to side effects, number of patients experiencing at least one side effect. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Patients with major depressive episode (DSM‐III) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 9/26 missing from fluvoxamine group (2 due to adverse effects); 7/26 missing from control group (2 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs for endpoint score and change score were not reported. |
Rapaport 1996.
| Methods | Seven‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for major depressive episode, with a minimum baseline score of 20 on the HRSD‐21. Age range: 22‐61 years old. Exclusion criteria: pregnant or nursing patients; unstable medical conditions; other axis I diagnoses; acute suicidality; history of substance dependence within 6 months of the study; history of a seizure disorder; had been treated with either fluvoxamine or fluoxetine before enrolment. | |
| Interventions | Fluvoxamine: 51 participants.
Fluoxetine: 49 participants.
Fluvoxamine dose range: 100‐150 mg/day.
Fluoxetine dose range: 20‐80 mg/day. Only chloral hydrate (up to a maximum of 1000 mg/day) was allowed as adjuvant medication for insomnia during the study. |
|
| Outcomes | HRSD‐21, CGI‐I, CGI‐S, HRSA, Raskin‐Covi scale, SCL‐56. Total dropout, dropout due to side effects, number of patients experiencing at least one side effect, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Patients with major depressive episode (DSM‐III‐R) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", "study medication was provided in identical‐appearing green capsules", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 7/51 missing from fluvoxamine group (2 due to adverse effects); 8/49 missing from control group (2 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Rechlin 1994.
| Methods | Two‐week, randomised study. | |
| Participants | Psychiatric inpatients meeting DSM‐III‐R for major depression, with a minimum baseline score of 20 on the HRSD. Age range: 24‐75 years old. Exclusion criteria: diabetes mellitus, polyneuropathy, alcoholism, cardiac diseases, neurologic diseases. | |
| Interventions | Fluvoxamine: 8 participants.
Amitriptyline: 8 participants.
Doxepine: 8 participants.
Paroxetine: 8 participants.
Fluvoxamine dose: 150 mg/day.
Amitriptyline dose: 150 mg/day.
Doxepine dose: 150 mg/day.
Paroxetine dose: 20 mg/day. No other psychopharmacologic drugs were allowed except for the continuation of lithium prophylaxis. |
|
| Outcomes | No efficacy and tolerability data could be entered in a meta‐analysis were reported. | |
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Study authors examined whether or not the application of SSRI in therapeutic doses influence heart rate variability. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details. |
| Selective reporting (reporting bias) | High risk | No clinical outcomes were reported. |
Remick 1994.
| Methods | Seven‐week double blind, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐III‐R for major depressive disorder, with a minimum baseline score of 20 on the HRSD‐17. Age range: 18‐65 years old. Exclusion criteria: any significant abnormalities detected on physical and laboratory examinations (hematology, urinalysis, clinical chemistry, ECG); a diagnosis of schizophrenia, other psychiatric disorders, or a principal diagnosis of panic disorder; history of epilepsy or seizures; history of alcohol or drug abuse within 6 months of study start; a woman who were pregnant or lactating. | |
| Interventions | Fluvoxamine: 16 participants. Amitriptyline: 17 participants. Fluvoxamine dose range: 50‐300 mg/day. Amitriptyline dose range: 50‐300 mg/day. | |
| Outcomes | HRSD‐17, CGI‐I, CGI‐S, CGI‐Efficacy, Patient Global Improvement Scale (PGI), HRSA, Raskin‐Covi scale, BDI. Total dropout, dropout due to inefficiency, dropout due to side effects. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 3/16 missing from fluvoxamine group (3 due to adverse effects); 8/17 missing from control group (2 due to lack of efficacy, 6 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Rossini 2005.
| Methods | Seven‐week double blind, single‐centre, randomised study. | |
| Participants | Psychiatric inpatients suffering from major depressive episode according to DSM‐IV, with a minimum baseline score of 22 on the HRSD‐21. Age range: 59 years old or more. Exclusion criteria: presence of any concomitant axis I diagnosis; presence of psychotic features together with somatic or neurological illnesses impairing psychiatric evaluation; mini mental state examination score less than 23. | |
| Interventions | Fluvoxamine: 40 participants.
Sertraline: 48 participants.
Fluvoxamine dose: 200 mg/day.
Sertraline dose: 150 mg/day. Subjects had not taken nonreversible monoamine oxidase inhibitors or slow release neuroleptics for at least 1 month before entering the study. All bipolar patients were under maintenance with mood stabilizers, the treatment remained unchanged during the present trial. No other psychotropic medication was allowed with the exception of flurazepam (up to 30 mg at bed time) |
|
| Outcomes | HRSD‐21. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | 17/84 (17%) patients were with bipolar depression. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by a computer originated schedule". |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind", outcome assessors were blind to the treatment option. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 1/40 missing from fluvoxamine group (1 due to adverse effects); 3/48 missing from control group (3 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of change score for depression were not reported. |
Rota 2005.
| Methods | Six‐week, randomised study. | |
| Participants | Psychiatric inpatients suffering from major depressive episode according to DSM‐IV, with a minimum baseline score of 17 on the HRSD‐21. Age range: 28‐65 years old. Exclusion criteria: comorbidity with other DSM‐IV axis I disorder; endocrinopathy or major medical illness; ocular glaucoma; prostatic hypertrophy; recent ECT or surgical event; use of psychoactive drugs or hormonal therapies; suicidal ideations. | |
| Interventions | Fluvoxamine: number of participants not stated. Amitriptyline: number of participants not stated. Fluvoxamine dose: 200 mg/day. Amitriptyline dose: 150 mg/day. | |
| Outcomes | HRSD‐21, CGI, BDI. | |
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | Patients with major depressive episode (DSM‐IV) were included, so there might be some bipolar depression, but correct number was not reported. Study authors assessed the time course changes in basal hypothalamic‐pituitary‐adrenocortical axis activity during the trial. Clinical outcome were reported only as "no difference between the two drugs", so those data could not be entered in a meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants not stated. |
| Selective reporting (reporting bias) | High risk | Clinical outcome were reported only as "no difference between the two drugs". |
Schoemaker 2002.
| Methods | Six‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric outpatients meeting DSM‐IV for major depressive disorder, with a minimum baseline score of 18 on the HRSD‐17. Age range: 20‐73 years old. Exclusion criteria: Patients were excluded from the study; if their current depressive episode was present for longer than one year; if they were pregnant or lactating or women of childbearing potential not practicing a reliable method of contraception; had an actual suicide risk; had a history or present condition of: bipolar disorder, schizophrenia or psychotic symptoms, schizotypal or borderline personality disorder, or organic mental disorders; had a present condition of anxiety disorders (according to DSM‐IV), eating disorders, postpartum depression, epilepsy or a history of seizure disorder or ever received treatment with anticonvulsant medication for epilepsy or seizures; alcohol or substance abuse (according to DSM‐IV) during the last 6 months; had any clinically meaningful non‐stable renal, hepatic, cardiovascular respiratory, cerebrovascular disease or other serious, progressive physical disease; had any clinically meaningful abnormal finding uncovered during the physical examination, ECG and/or clinically significant abnormal laboratory results at screening; had participated in other clinical trials within the last three months, suffered from withdrawal symptoms at screening as a result of drug discontinuation; had a body mass index greater than 30 or less than 18, had a known allergy or hypersensitivity to mirtazapine or fluvoxamine; had been treated during the present episode with either mirtazapine or fluvoxamine; required treatment with terfenadine, astemizole, warfarin, theophylline, or cisapride. | |
| Interventions | Fluvoxamine: 207 participants.
Mirtazapine: 205 participants.
Fluvoxamine dose range: 50‐150 mg/day.
Mirtazapine dose range: 15‐45 mg/day. Concomitant intake of psychotropic was prohibited with the exception of a low dose hypnotic or anxiolytic (10 mg diazepam or equivalent dosage). Previous antidepressant treatment, including electroconvulsive therapy (ECT), had to be discontinued well in advance of randomization, excluding interference with study observations. |
|
| Outcomes | HRSD‐17, HRSD‐21, CGI‐I,CGI‐S. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets comparator drug. | |
| Fluvoxamine as an investigational or comparator drug | As a comparator drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 41/207 missing from fluvoxamine group (8 due to lack of efficacy, 16 due to adverse effects); 47/205 missing from control group (4 due to lack of efficacy, 25 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint score for depression were not reported. |
Tourigny‐Rivard 1996.
| Methods | Ten‐week double blind, multicentre, randomised study. | |
| Participants | Patients meeting DSM‐III‐R for major depression, with a minimum baseline score of 18 on the HRSD‐17. Age range: 60‐82 years old. | |
| Interventions | Fluvoxamine: 22 participants. Desipramine: 25 participants. Fluvoxamine dose range: to 150 mg/day. Desipramine dose range: to 150 mg/day. | |
| Outcomes | HRSD, Geriatric Depression Scale, CGI, Brief Symptom Inventory. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Unclear. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Ueda 2002.
| Methods | Four‐week, randomised study. | |
| Participants | Patients meeting DSM‐IV for major depressive disorder, with a minimum baseline score of 17 on the HRSD‐17. Age range: 18‐77 years old. Exclusion criteria: history of alcohol or drug abuse or dependency; physical complications; manic state or psychotic features before study. | |
| Interventions | Fluvoxamine: 24 participants.
Sulpiride: 24 participants.
Fluvoxamine dose range: not stated.
Sulpiride dose range: not stated. None had been administered any antidepressants for at least one month before participating in the study. No drugs were taken other than BZP hypnotics, and the dosages of BZP were kept constant throughout the study period. |
|
| Outcomes | HRSD‐17. Total dropout. |
|
| Funded by pharmaceutical companies | No funding by pharmaceutical companies. | |
| Fluvoxamine as an investigational or comparator drug | Unclear. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study endpoint: 4/24 missing from fluvoxamine group; 4/24 missing from control group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
Zohar 2003.
| Methods | Eight‐week double blind, multicentre, randomised study. | |
| Participants | Psychiatric inpatients suffering from major depressive episode, with or without mood congruent psychotic features not requiring antipsychotic treatment according to DSM‐IV, with a minimum baseline score of 25 on the HRSD‐17. Age range: 18‐70 years old. Exclusion criteria: psychosis, a history of other psychiatric diagnosis; epilepsy or seizures; severe suicide risk; pregnant, lactating or of childbearing potential and not taking adequate contraceptive measures; clinically relevant/unstable disease which could affect the diagnosis and/or treatment of depression; hepatic or renal disease, severe heart disease, glaucoma, adrenal tumor, micturition disturbances, prostate hypertrophy; clinically relevant laboratory test abnormalities; multiple drug allergies; had been treated unsuccessfully with 2 or more antidepressants during the current episode of depression; had been treated with fluvoxamine or clomipramine during the current episode of depression. | |
| Interventions | Fluvoxamine: 44 participants.
Clomipramine: 42 participants.
Fluvoxamine dose range: 100‐250 mg/day.
Clomipramine dose range: 100‐250 mg/day. Patients were required not to have received any antidepressants in the week prior to active treatment (5 weeks in the case of fluoxetine) or lithium, monoamine oxidase inhibitors, antipsychotics or ECT in the 2 weeks prior to active treatment. With the exception of oxazepam, which could be given for night‐time sedation or control of anxiety, no other psychopharmacological treatments or ECT were permitted during the study. |
|
| Outcomes | HRSD‐17, MADRS, CGI‐I, CGI‐S. Total dropout, dropout due to inefficiency, dropout due to side effects, side effect profile. |
|
| Funded by pharmaceutical companies | Funded by pharmaceutical company markets fluvoxamine. | |
| Fluvoxamine as an investigational or comparator drug | As an investigational drug. | |
| Notes | Patients with major depressive episode (DSM‐III‐R) were included, so there might be some bipolar depression, but correct number was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double blind", no further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study endpoint: 10/44 missing from fluvoxamine group (1 due to lack of efficacy, 5 due to adverse effects); 13/42 missing from control group (10 due to adverse effects). |
| Selective reporting (reporting bias) | High risk | SDs of endpoint/change score for depression were not reported. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baischer 1991 | Allocation: randomised. Interventions: fluvoxamine versus placebo |
| Belliini 1992 | Participants: those with bipolar disorder and major depression with psychotic features. |
| Blier 1997 | Allocation: not randomised. |
| Davis 1991 | Interventions: amitriptyline versus fluoxetine. |
| de Jonghe 1991 | Participants: those with major depression (n=22) and dysthymic disorder (n=26). |
| de Kemp 2002 | Participants: those with primary depression (n=40) and primary anxiety disorder (n=38). |
| de Wilde 1982 | Participants: those with unipolar depression (n=21) and bipolar depression (n=9). |
| Emrich 1987 | Allocation: randomised, crossover. Participants: those with major depressive disorder. Interventions: fluvoxamine versus oxaprotilene. Outcome: clinical data not presented for first phase of crossover. |
| Entsuah 2002a | Allocation: not randomised. meta‐analysis. |
| Franchini 1997 | Allocation: randomised. Participants: those with major depression in remission state (not acute phase). |
| Gasperini 1992 | Allocation: randomised. Participants: those with major depression (n=38) and those with bipolar depression (n=18). |
| Gonella 1990 | Allocation: randomised. Participants: those with major depression (n=13), bipolar depressive disorder (n=1) and dysthymia (n=6). |
| Goto 2006 | Allocation: not randomised. |
| Guelfi 1983 | Allocation: randomised. Participants: depressed patients. not presented operational diagnostic criteria. |
| Harris 1991b | Allocation: not randomised. |
| Hewer 1994 | Allocation: not randomised. |
| Hochberg 1995 | Allocation: randomised. Participants: depressed outpatients. Interventions: fluvoxamine versus TCAs versus placebo. Outcomes: clinical data reported for maintenance phase only; no data presented for acute phase. |
| Khan 1989 | Allocation: randomised. Participants: those with major depression. Interventions: fluvoxamine versus imipramine versus placebo. Outcomes: clinical data presented only for the comparison between antidepressants and placebo, no data presented between fluvoxamine and imipramine. |
| Klok 1981 | Allocation: randomised. Participants: those with vital depressive syndromes; not presented operational diagnostic criteria. |
| Lara‐Munoz 1996 | Allocation: not randomised. |
| Manfredonia 1992 | Allocation: randomised. Participants: those with major depression (n=32) and bipolar disorder (n=16). |
| Muck‐Seler 1991 | Allocation: not randomised. |
| Murasaki 1998b | Allocation: not randomised. Participants: those with major depressive disorder (n=129) and those with bipolar disorder (n=15), dysthymic disorder (n=17) and depressive disorder not otherwise specified (n=5). |
| Namiki 1996 | Allocation: not randomised. Participants: those with major depressive disorder (n=101) and those with bipolar disorder (n=8), dysthymic disorder (n=58) cyclothymic disorder (n=1) and depressive disorder not otherwise specified (n=39). |
| Nicolini 1996 | Allocation: not randomised. |
| Nolen 1988 | Allocation: not randomised. Participants: those with major depressive episode without psychotic features (n=51) and those with psychotic features (n=20). |
| Phanjoo 1991 | Allocation: not randomised. |
| Poldinger 1991 | Allocation: randomised. Participants: those with any form of depression as diagnosed by the investigator, not presented any diagnostic criteria. |
| Price 1986 | Allocation: not randomised. |
| Ravindran 1995 | Allocation: randomised. Participants: those with major depression (n=17), dysthymia (n=18) and healthy controls (n=18). |
| Sacchetti 1994 | Allocation: not randomised. |
| Sacchetti 1997 | Allocation: randomised. Participants: those with major depression with mood congruent psychotic features. |
| Schanda 1979 | Allocation: not randomised. Participants: those with depression; not presented operational diagnostic criteria. |
| Serretti 2004 | Allocation: not randomised. |
| Sheline 1997 | Allocation: not randomised. |
| van den Broek 2004 | Allocation: randomised. Participants: those with major depression without psychotic features (n=90), with psychotic features (n=48). |
| Vandel 1995 | Allocation: not randomised. |
| White 1990 | Allocation: randomised, crossover. Participants: those with major depression. Interventions: fluvoxamine versus desipramine versus placebo. Outcome: clinical data not presented for first phase of crossover. |
| Yu 2001 | Allocation: quasi randomised (sequence generated by odd or even hospital record number) Participants: those with major depressive disorder (n=33) and those depressive neurosis (n=27). |
| Zanardi 2000 | Allocation: randomised. Participants: those with major depressive episode with psychotic features. |
Characteristics of studies awaiting assessment [ordered by study ID]
Berlin 1998.
| Methods | Awaiting assessment. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Coleman 1981a.
| Methods | Unpublished. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Coleman 1981b.
| Methods | Unpublished. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Coleman 1983.
| Methods | Unpublished. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Donovan 1993.
| Methods | Awaiting assessment. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Doogan 1981.
| Methods | Unpublished. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Entsuah 2002b.
| Methods | Awaiting assessment. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Faludi 1989.
| Methods | Paper is not yet available. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Mallick 2003.
| Methods | Awaiting assessment. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Naito 2007.
| Methods | Awaiting assessment. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ushiroyama 2004.
| Methods | Awaiting assessment. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
van Beek 1981.
| Methods | Unpublished. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Differences between protocol and review
There are no substantive differences between protocol and review.
Contributions of authors
All reviewers contributed to the production of the protocol. IMO and TAF identified studies for inclusion. IMO and NW extracted data and performed the analyses. IMO and NW wrote the final review, which was approved by the other authors.
Sources of support
Internal sources
Nagoya City University Medical School, Japan.
External sources
No sources of support supplied
Declarations of interest
IMO, AN, AC, CB, HM and RC have nothing to declare. NW has received a speaking fee from GlaxoSmithKline. TAF has received research funds and speaking fees from Dai Nippon Sumitomo, Eli Lilly, GSK, Meiji, Otsuka, and Pfizer. The Japanese Ministry of Education, Science, and Technology and the Japanese Ministry of Health Labor and Welfare have also funded TAF's research.
Edited (no change to conclusions)
References
References to studies included in this review
Amore 1989 {published data only}
- Amore M, Bellini M, Berardi D, Berlinzani L, Cervino G, Cremonini A, Ferrari G, Innamorati A. Double‐blind comparison of fluvoxamine and imipramine in depressed patients. Current Therapeutic Research, Clinical & Experimental 1989;46:815‐20. [Google Scholar]
Ansseau 1991a {published and unpublished data}
- Ansseau M, Frenckell R, Gerard M, Mertens C, Wilde J, Botte L, Devoitille J, Evrard J, Nayer A, Darimont P, Mirel J, Troisfontaines B, Toussaint C, Couzinier JP, Demarez JP, Serre C. Interest of a loading dose of milnacipran in endogenous depressive inpatients. Comparison with the standard regimen and with fluvoxamine. European Neuropsychopharmacology 1991;1:113‐21. [DOI] [PubMed] [Google Scholar]
Ansseau 1991b {published and unpublished data}
- Ansseau M, Frenckell R, Gerard M, Mertens C, Wilde J, Botte L, Devoitille J, Evrard J, Nayer A, Darimont P, Mirel J, Troisfontaines B, Toussaint C, Couzinier JP, Demarez JP, Serre C. Interest of a loading dose of milnacipran in endogenous depressive inpatients. Comparison with the standard regimen and with fluvoxamine. European Neuropsychopharmacology 1991;1:113‐21. [DOI] [PubMed] [Google Scholar]
Ansseau 1994 {published data only}
- Ansseau M, Gabriels A, Loyens J, Bartholome F, Evrard J, Nayer A, Linhart R, Wirtz J, Bruynooghe F, Surinx K, Clarysse H, Marganne R, Papart P. A double‐blind comparison of paroxetine and fluvoxamine in major depression. European Neuropsychopharmacology 1993;3:323‐4. [Google Scholar]
- Ansseau M, Gabriels A, Loyens J, Bartholome F, Evrard JL, Nayer A, Linhart R, Wirtz J, Bruynooghe F, Surinx K, Clarysse H, Marganne R, Papart P. Controlled comparison of paroxetine and fluvoxamine in major depression. Human Psychopharmacology 1994;9:329‐36. [Google Scholar]
Asakura 2005 {published data only}
- Asakura M, Tajima O. A randomized, double‐blind study of fluvoxamine maleate in patients with depression or depressive state: A comparison in anticholinergic effects and cardiovascular adverse reactions between fluvoxamine maleate and imipramine hydrochloride. Japanese Pharmacology and Therapeutics 2005;33(8):773‐87. [Google Scholar]
Barge‐Schaapveld 1995 {published and unpublished data}
- Barge‐Schaapveld DQ, Nicolson NA, Hoop RG, DeVries MW. Changes in daily life experience associated with clinical improvement in depression. Journal of Affective Disorders 1995;34:139‐54. [DOI] [PubMed] [Google Scholar]
Barrelet 1991 {published data only}
- Barrelet L, Blajev B, Bolzani L, Saussure C, Kasas A, Van H, Gachoud JP. Multicenter study comparing efficacy and tolerance of moclobemide and fluvoxamine in hospitalized and ambulatory patients with severe depressive episodes. Schweizerische Rundschau fur Medizin Praxis 1991;80(19):524‐8. [PubMed] [Google Scholar]
Bocksberger 1993 {published data only}
- Bocksberger JP, Gachoud JP, Richard J, Dick P. Comparison of the efficacy of moclobemide and fluvoxamine in elderly patients with a severe depressive episode. European Psychiatry: the Journal of the Association of European Psychiatrists 1993;8(6):319‐24. [Google Scholar]
- Bocksberger P, Gachoud JP, Richard J, Dick P. Comparison of the efficacy and tolerability of moclobemide and fluvoxamine in elderly patients. Clinical Neuropharmacology 1992;15(1 Pt B):146. [Google Scholar]
Bougerol 1992 {published data only}
- Bougerol T, Uchida C, Gachoud JP, Kohler M, Mikkelsen H. Efficacy and tolerability of moclobemide compared with fluvoxamine in depressive disorder (DSM III) A French/Swiss double‐blind trial. Psychopharmacology 1992;106[Suppl]:S102‐8. [DOI] [PubMed] [Google Scholar]
Bramanti 1988 {published data only}
- Bramanti P, Ricci RM, Roncari R, Bilone F, Inga F, Teti V, DeCristofaro A, Ceccarelli G, DiPerri R, Candela L. An Italian multicenter experience with fluvoxamine, a new antidepressant drug, versus imipramine. Current Therapeutic Research, Clinical & Experimental. 1988;43(4):718‐24. [Google Scholar]
Brown 1986 {published data only}
- Brown WA, Arato M, Shrivastava R. Pituitary‐adrenocortical hyperfunction and intolerance to fluvoxamine, a selective serotonin uptake inhibitor. American Journal of Psychiatry 1986;143(1):88‐90. [DOI] [PubMed] [Google Scholar]
Brunner 1994 {published data only}
- Brunner H. A randomised, parallel group comparison of fluvoxamine and amineptine in patients with marked depression. British Journal of Clinical Research 1994;5:101‐9. [Google Scholar]
Cassano 1986 {published and unpublished data}
- Amin MM, Ananth JV, Coleman BS, Darcourt G, Farkas T, Goldstein B, Lapierre YD, Paykel E, Wakelin JS. Fluvoxamine:antidepressant effects confirmed in a placebo‐controlled international study. Clinical Neuropharmacology 1984;7[Suppl 1]:580‐1. [Google Scholar]
- Cassano GB, Conti L, Massimetti G, Mengali F, Waekelin JS, Levine J. Use of a standardized documentation system (BLIPS/BDP) in the conduct of a multicenter international trial comparing fluvoxamine, imipramine, and placebo. Psychopharmacology Bulletin 1986;22:52‐8. [PubMed] [Google Scholar]
- Conti L, Dell'Osso L. Clinical predictors of response to fluvoxamine, imipramine, and placebo. New Trends in Experimental and Clinical Psychiatry 1989;5:221‐9. [Google Scholar]
- Conti L, Placidi GF, Dell'Osso L, Lenzi A, Cassano GB. Therapeutic response in subtypes of major depression. New Trends in Experimental and Clinical Psychiatry 1987;3:101‐7. [Google Scholar]
- Dominguez RA, Goldstein BJ, Jacobson AF, Steinbook RM. A double‐blind placebo‐controlled study of fluvoxamine and imipramine in depression. Journal of Clinical Psychiatry 1985;46:84‐7. [PubMed] [Google Scholar]
- Kasper S, Moller HJ, Montgomery SA, Zondag E. Antidepressant efficacy in relation to item analysis and severity of depression: A placebo‐controlled trial of fluvoxamine versus imipramine. International Clinical Psychopharmacology 1995;9:3‐12. [DOI] [PubMed] [Google Scholar]
- Lapierre YD, Browne M, Horn E, Oyewumi L, Sarantidis D, Roberts N, Badoe K, Tessier P. Treatment of major affective disorder with fluvoxamine. Journal of Clinical Psychiatry 1987;48:65‐8. [PubMed] [Google Scholar]
- Norton KR, Sireling LI, Bhat AV, Rao B, Paykel ES. A double‐blind comparison of fluvoxamine, imipramine and placebo in depressed patients. Journal of Affective Disorders 1984;7:297‐308. [DOI] [PubMed] [Google Scholar]
- Ottevanger EA. The efficacy of fluvoxamine in patients with severe depression. British Journal of Clinical Research 1991;2:125‐32. [DOI] [PubMed] [Google Scholar]
- Ottevanger EA. The efficacy of fluvoxamine in patients with severe depression. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 1994;18:731‐40. [DOI] [PubMed] [Google Scholar]
- Ottevanger EA. The efficacy of fluvoxamine in patients with severe depression. Psychologie Medicale 1991;23:1639‐44. [DOI] [PubMed] [Google Scholar]
- Wagner W, Cimander K, Schnitker J, Koch H‐F. Influence of concomitant psychotropic medication on the efficacy and tolerance of fluvoxamine. Advances in Pharmacotherapy 1986;2:34‐56. [Google Scholar]
- Wakelin J. Fluvoxamine in the treatment of the older depressed patient; double‐blind, placebo‐controlled data. International Clinical Psychopharmacology 1986;1:221‐30. [DOI] [PubMed] [Google Scholar]
Claghorn 1996 {published data only}
- Claghorn JL, Earl CQ, Walczak DD, Stoner KA, Wong LF, Kanter D, Houser VP. Fluvoxamine maleate in the treatment of depression: a single‐center, double‐blind, placebo‐controlled comparison with imipramine in outpatients. Journal of Clinical Psychopharmacology 1996;16(2):113‐120. [DOI] [PubMed] [Google Scholar]
Clerc 2001 {published data only}
- Clerc G, Assicot M, Bouchard JM, Cottin M, Guibert M, Liegaut D, Engel P, May JP, Oules J, Pagot R, Patris MF, Ruimy P, Sombret A, Amerongen AP, Tournoux A. Antidepressant efficacy and tolerability of milnacipran, a dual serotonin and noradrenaline reuptake inhibitor: A comparison with fluvoxamine. International Clinical Psychopharmacology 2001;16(3):145‐51. [DOI] [PubMed] [Google Scholar]
Coleman 1982 {published data only}
- Coleman BS, Block BA. Fluvoxamine maleate, a serotonergic antidepressant; A comparison with chlorimipramine. Progress in Neuro‐Psychopharmacological & Biological Psychiatry 1982;6(4‐6):475‐8. [DOI] [PubMed] [Google Scholar]
Dalery 2003 {published and unpublished data}
- Dalery J. Fluvoxamine and fluoxetine in the treatment of depression; similarities and differences. 11th European College of Neuropsychopharmacology Congress. Paris, France, 31st October to 4th November, 1998.
- Dalery J, Honig A. Fluvoxamine versus fluoxetine in major depressive episode: A double‐blind randomised comparison. Human Psychopharmacology 2003;18(5):379‐84. [DOI] [PubMed] [Google Scholar]
- Honig A. Comparing SSRIs in depression: Fluvoxamine vs fluoxetine. XXIst Collegium Internationale Neuro psychopharmacologicum. Glasgow, Scotland, 12th to 16th July, 1998.
- Strik J, Honig A, Lousberg R, Cheriex EC, van‐Praag HM. Cardiac side effects of two SSRI's in middle‐aged and elderly depressed patients. XXIst Collegium Internationale Neuro psychopharmacologicum. Glasgow, Scotland, 12th to16th July, 1998.
- Strik JJ, Honig A, Lousberg R, Cheriex EC, Praag HM. Cardiac side‐effects of two selective serotonin reuptake inhibitors in middle‐aged and elderly depressed patients. International Clinical Psychopharmacology 1998;13(6):263‐7. [DOI] [PubMed] [Google Scholar]
- Strik JJ, Lousberg R, Cheriex EC, Praag HMV, Honig A. Cardiac side effects of two SSRIs in middle‐aged and elderly depressed patients. 152nd Annual Meeting of the American Psychiatric Association. Washington, DC, 15th to 20th, May, 1999.
- Berg J, Honig A. Fluvoxamine versus fluoxetine ‐ a double‐blind, randomized comparison in major depressive episode. 151st Annual Meeting of the American Psychiatric Association. Toronto, Ontario, Canada, 30th May to 4th June, 1998.
de Wilde 1983 {published data only}
- Wilde JE, Mertens C, Wakelin JS. Clinical trials of fluvoxamine vs chlorimipramine with single and three times daily dosing. British Journal of Clinical Pharmacology 1983;15(Suppl 3):427S‐31S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dick 1983 {published data only}
- Dick P, Ferrero E. A double‐blind comparative study of the clinical efficacy of fluvoxamine and chlorimipramine. British Journal of Clinical Pharmacology 1983;15(Suppl 3):419S‐25S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fabre 1996 {published data only}
- Fabre L, Birkhimer LJ, Zaborny BA, Wong LF, Kapik BM. Fluvoxamine versus imipramine and placebo: A double‐blind comparison in depressed patients. International Clinical Psychopharmacology 1996;11(2):119‐27. [PubMed] [Google Scholar]
- Fabre L, Vettraino J, Birkhimer L, Houser V. Fluvoxamine in the treatment of depression ‐ A double‐blind comparison with imipramine and placebo in outpatients with major depression. Clinical Neuropharmacology 1992;15(Supple.1 Pt B):416. [Google Scholar]
Feighner 1989 {published data only}
- Feighner JP, Boyer WF, Meredith CH, Hendrickson GG. A placebo‐controlled inpatient comparison of fluvoxamine maleate and imipramine in major depression. International Clinical Psychopharmacology 1989;4(3):239‐44. [DOI] [PubMed] [Google Scholar]
- Feighner PJ, Boyer FW. A controlled trial of fluvoxamine in major depression. 141st Annual Meeting of the American Psychiatric Association. Quebec, Canada, 1988.
Gonul 1999 {published data only}
- Gonul AS, Yabanoglu I, Reyhancan M, Oguz A. Selective serotonin reuptake inhibitors: discontinuation rates due to side effects. European Neuropsychopharmacology 1999;9(Suppl 5):215. [Google Scholar]
Guy 1984 {published data only}
- Guy W, Wilson W, Ban TA, King DL, Manov G, Fjetland OK. A double‐blind clinical trial of fluvoxamine and imipramine in patients with primary depression. Psychopharmacology Bulletin 1984;20(1):73‐8. [PubMed] [Google Scholar]
Hackett 1998a {published and unpublished data}
- Hackett D, Salinas E, Desmet A. Efficacy and safety of venlafaxine vs. fluvoxamine in outpatients with major depression. 11th European College of Neuropsychopharmacology Congress. 1998.
Hackett 1998b {published and unpublished data}
- Hackett D, Salinas E, Desmet A. Efficacy and safety of venlafaxine vs. fluvoxamine in outpatients with major depression. 11th European College of Neuropsychopharmacology Congress. 1998.
Haffmans 1996 {published data only}
- Haffmans PM, Timmerman L, Hoogduin C. Efficacy and tolerability of citalopram in comparison with fluvoxamine in depressed outpatients: a double‐blind, multicentre study The lucifer group. International Clinical Psychopharmacology 1996;11(3):157‐64. [DOI] [PubMed] [Google Scholar]
- Timmerman L, Haffmans PMJ, Hoogduin CA. Citalopram in major depression: a comparative study with Fluvoxamine, Preliminary results. Past, Present and Future of Psychiatry (IX World Congress of Psychiatry ‐ 9th 1993 Rio de Janiero, Brazil). River Edge, NJ: World Scientific, 1994:982‐6.
Harris 1991a {published data only}
- Harris B, Szulecka TK, Anstee JA. Fluvoxamine versus amitriptyline in depressed hospital out‐patients: a multicentre double‐blind comparative trial. British Journal of Clinical Research 1991;2:88‐99. [Google Scholar]
- Whitehead AM, Ashford JJ. Fluvoxamine in the treatment of depressive illness. A series of double‐blind hospital based comparative studies carried out in the UK. British Journal of Clinical Practice 1992;46(1):21‐3. [PubMed] [Google Scholar]
Itil 1983 {published data only}
- Itil TM, Shrivastava RK, Mukherjee S, Coleman BS, Michael ST. A double‐blind placebo‐controlled study of fluvoxamine and imipramine in out‐patients with primary depression. British Journal of Clinical Pharmacology 1983;15(Suppl 3):433S‐8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasper 1990 {published and unpublished data}
- Kasper S, Dotsch M, Kick H, Vieira A, Moller HJ. Plasma concentrations of fluvoxamine and maprotiline in major depression: implications on therapeutic efficacy and side effects. European Neuropsychopharmacology 1993;3(1):13‐21. [DOI] [PubMed] [Google Scholar]
- Kasper S, Kick H, Voll G, Vieira A. Therapeutic sleep deprivation and antidepressant medication in patients with major depression. European Neuropsychopharmacology 1991;1(2):107‐11. [DOI] [PubMed] [Google Scholar]
- Kasper S, Lenarz T. Auditory evoked potentials and treatment with specific acting antidepressants. Clinical Neuropharmacology 1992;15(1 Pt B):152. [Google Scholar]
- Kasper S, Lenarz T. Plasma levels of fluvoxamine and maprotiline and clinical response in major depression. Pharmacopsychiatry 1992;25:106. [Google Scholar]
- Kasper S, Voll G, Vieira A, Kick H. Response to total sleep deprivation before and during treatment with fluvoxamine or maprotiline in patients with major depression; results of a double‐blind study. Pharmacopsychiatry 1990;23(3):135‐42. [DOI] [PubMed] [Google Scholar]
- Kasper S, Yieira A. Stimulation with dl‐fenfluramine and antidepressive medication in major depressed inpatients. Pharmacopsychiatry 1989;22:201. [Google Scholar]
Kato 2006 {published and unpublished data}
- Kato M, Fukuda T, Wakeno M, Fukuda K, Okugawa G, Ikenaga Y, Yamashita M, Takekita Y, Nobuhara K, Azuma J, Kinoshita T. Effects of the serotonin type 2A, 3A and 3B receptor and the serotonin transporter genes on paroxetine and fluvoxamine efficacy and adverse drug reactions in depressed Japanese patients. Neuropsychobiology 2006;53(4):186‐95. [DOI] [PubMed] [Google Scholar]
- Kato M, Ikenaga Y, Wakeno M, Okugawa G, Nobuhara K, Fukuda T, Fukuda K, Azuma J, Kinoshita T. Controlled clinical comparison of paroxetine and fluvoxamine considering the serotonin transporter promoter polymorphism. International Clinical Psychopharmacology 2005;20(3):151‐6. [DOI] [PubMed] [Google Scholar]
- Kato M, Wakeno M, Okugawa G, Nagata M, Nobuhara K, Ochi T, Ikenaga Y, Fukuda T, Fukuda K, Azuma J, Kinoshita T. Clinical comparison of paroxetine and fluvoxamine considering of the serotonin transporter promoter polymorphism in patients with affective disorder. International Clinical Psychopharmacology 2004;19(3):175. [DOI] [PubMed] [Google Scholar]
Kavoussi 1999 {published data only}
- Kavoussi RJ, Hauger RL, Coccaro EF. Prolactin response to d‐fenfluramine in major depression before and after treatment with serotonin reuptake inhibitors. Biological Psychiatry 1999;45(3):295‐9. [DOI] [PubMed] [Google Scholar]
Kiev 1997 {published data only}
- Kiev A, Feiger A. A double‐blind comparison of fluvoxamine and paroxetine in the treatment of depressed outpatients. Journal of Clinical Psychiatry 1997;58(4):146‐52. [DOI] [PubMed] [Google Scholar]
- Kiev A, Feiger A. Comparison of fluvoxamine and paroxetine in depression. Xth World Congress of Psychiatry. Madrid, Spain, 1996.
- Kiev A, Feiger AD. A double‐blind comparison of fluvoxamine and paroxetine in major depressive disorder. 149th Annual Meeting of the American Psychiatric Association. New York, NY, 1996.
Koetsier 2002 {published data only}
- Koetsier GC, Volkers AC, Tulen JH, Passchier J, Broek WW, Bruijn JA. CPT performance in major depressive disorder before and after treatment with imipramine or fluvoxamine. Journal of Psychiatric Research 2002;36(6):391‐7. [DOI] [PubMed] [Google Scholar]
- Volkers AC, Tulen JH, Broek WW, Bruijn JA, Passchier J, Pepplinkhuizen L. 24‐Hour motor activity after treatment with imipramine or fluvoxamine in major depressive disorder. European Neuropsychopharmacology 2002;12(4):273‐8. [DOI] [PubMed] [Google Scholar]
- Volkers AC, Tulen JH, Broek WW, Bruijn JA, Passchier J, Pepplinkhuizen L. Effects of imipramine, fluvoxamine,and depressive mood on autonomic cardiac functioning in major depressive disorder. Pharmacopsychiatry 2004;37(1):18‐25. [DOI] [PubMed] [Google Scholar]
Kostiukova 2003 {published data only}
- Kostiukova EG, Granenov GM, Andreichik LA, Serditov OV, Mosolov SN. Comparative efficacy and tolerance of fluvoxamine and amitriptyline in the treatment of moderate and severe depression in mental hospital. Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova 2003;103(1):24‐9. [PubMed] [Google Scholar]
Lydiard 1989 {published data only}
- Brady KT, Lydiard RB, Kellner CH, Joffe R, Laird LK, Morton WA, Steele TE. A comparison of the effects of imipramine and fluvoxamine on the thyroid axis. Biological Psychiatry 1994;36(11):778‐9. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Lydiard RB, Morton WA, Laird LK, Steele TE, Kellner CH, Ballenger JC. Effect of fluvoxamine, imipramine and placebo on catecholamine function in depressed outpatients. Journal of Psychiatric Research 1993;27(2):161‐72. [DOI] [PubMed] [Google Scholar]
- Laird LK, Lydiard RB, Morton WA, Steele TE, Kellner CH, Thompson NM, Ballenger JC. Cardiovascular effects of imipramine, fluvoxamine, and placebo in depressed outpatients. Journal of Clinical Psychiatry 1993;54(6):224‐8. [PubMed] [Google Scholar]
- Lydiard RB, Laird LK, Morton WAJ, Steele TE, Kellner C, Laraia MT, Ballenger JC. Fluvoxamine, imipramine, and placebo in the treatment of depressed outpatients: effects on depression. Psychopharmacology Bulletin 1989;25(1):68‐70. [PubMed] [Google Scholar]
March 1990 {published data only}
- March JS, Kobak KA, Jefferson JW, Mazza J, Greist JH. A double‐blind, placebo‐controlled trial of fluvoxamine versus imipramine in outpatients with major depression. Journal of Clinical Psychiatry 1990;51(5):200‐2. [PubMed] [Google Scholar]
Mendonca Lima 1997 {published data only}
- Mendonca Lima CAd, Vandel S, Bonin B, Bechtel P, Carron R. Maprotiline versus fluvoxamine: comparison of their effects on the hypothalamo‐hypophyseal‐thyroid axis. Encephale 1997;23(1):48‐55. [PubMed] [Google Scholar]
Miller 2001 {published data only}
- Miller HL, Ekstrom RD, Mason GA, Lydiard RB, Golden RN. Noradrenergic function and clinical outcome in antidepressant pharmacotherapy. Neuropsychopharmacology 2001;24(6):617‐23. [DOI] [PubMed] [Google Scholar]
Moon 1991 {published data only}
- Moon CA, Jesinger DK. The effects of psychomotor performance of fluvoxamine versus mianserin in depressed patients in general practice. British Journal of Clinical Practice 1991;45(4):259‐62. [PubMed] [Google Scholar]
Mullin 1988 {published data only}
- Mullin JM, Pandita‐Gunawardena VR, Whitehead AM. A double‐blind comparison of fluvoxamine and dothiepin in the treatment of major affective disorder. British Journal of Clinical Practice 1998;42(2):51‐5. [PubMed] [Google Scholar]
- Whitehead AM, Ashford JJ. Fluvoxamine in the treatment of depressive illness. A series of double‐blind hospital based comparative studies carried out in the UK. British Journal of Clinical Practice 1992;46(1):21‐3. [PubMed] [Google Scholar]
Murasaki 1998a {published data only}
- Murasaki M, Mori A, Miura S. Clinical evaluation of sme3110 (fluvoxamine maleate) in the treatment of depression and depressive state: a double‐blind, comparative study with amitriptyline. Rinsyoiyaku (Journal of clinical therapeutics & medicine) 1998;14(5):951‐80. [Google Scholar]
Nathan 1990 {published and unpublished data}
- Kupfer DJ, Perel JM, Pollock BG, Nathan RS, Grochocinski VJ, Wilson MJ, McEachran AB. Fluvoxamine versus desipramine: Comparative polysomnographic effects. Biological Psychiatry 1991;29(1):23‐40. [DOI] [PubMed] [Google Scholar]
- Nathan RS, Perel JM, Pollock BG, Kupfer DJ. The role of neuropharmacologic selectivity in antidepressant action: fluvoxamine versus desipramine. Journal of Clinical Psychiatry 1990;51(9):367‐72. [PubMed] [Google Scholar]
Nemeroff 1995 {published and unpublished data}
- Nemeroff C, Ninan P, Ballenger J, Feighner J, Greist J, Patterson W. Comparison of the safety and tolerance of fluvoxamine and sertraline in depressed outpatients. 8th ECNP (European College of Neuropsychopharmacology) Congress. Venice, Italy, 1995.
- Nemeroff CB, Ninan PT, Ballenger J, Lydiard RB, Feighner J, Patterson WM, Greist JH. Double‐blind multicenter comparison of fluvoxamine versus sertraline in the treatment of depressed outpatients. Depression 1995;3(4):163‐9. [Google Scholar]
Otsubo 2005 {published and unpublished data}
- Otsubo T, Akimoto Y, Yamada H, Koda R, Aoyama H, Tanaka K, Mimura M, Nakagome K, Kamijima K. A comparative study of the efficacy and safety profiles between fluvoxamine and nortriptyline in Japanese patients with major depression. Pharmacopsychiatry 2005;38(1):30‐5. [DOI] [PubMed] [Google Scholar]
Ottevanger 1995 {published data only}
- Ottevanger EA. Fluvoxamine and clomipramine in depressed hospitalised patients: results from a randomised, double‐blind study. Encephale 1995;21(4):317‐21. [PubMed] [Google Scholar]
Perez 1990 {published data only}
- Perez A, Ashford JJ. A double‐blind, randomized comparison of fluvoxamine with mianserin in depressive illness. Current Medical Research and Opinion 1990;12(4):234‐41. [DOI] [PubMed] [Google Scholar]
- Whitehead AM, Ashford JJ. Fluvoxamine in the treatment of depressive illness. A series of double‐blind hospital based comparative studies carried out in the UK. British Journal of Clinical Practice 1992;46(1):21‐3. [PubMed] [Google Scholar]
Rahman 1991 {published data only}
- Rahman MK, Akhtar MJ, Savla NC, Sharma RR, Kellett JM, Ashford JJ. A double‐blind, randomised comparison of fluvoxamine with dothiepin in the treatment of depression in elderly patients. British Journal of Clinical Practice 1991;45(4):255‐8. [PubMed] [Google Scholar]
- Whitehead AM, Ashford JJ. Fluvoxamine in the treatment of depressive illness. A series of double‐blind hospital based comparative studies carried out in the UK. British Journal of Clinical Practice 1992;46(1):21‐3. [PubMed] [Google Scholar]
Rapaport 1996 {published data only}
- Rapaport M, Coccaro E, Sheline Y, Holland P, Perse T, Fabre L, Bradford D. Comparison of fluvoxamine and fluoxetine in major depression. 8th ECNP (European College of Neuropsychopharmacology) Congress. Venice, Italy, 1995.
- Rapaport M, Coccaro E, Sheline Y, Perse T, Holland P, Fabre L, Bradford D. A comparison of fluvoxamine and fluoxetine in the treatment of major depression. Journal of Clinical Psychopharmacology 1996;16(5):373‐8. [DOI] [PubMed] [Google Scholar]
Rechlin 1994 {published and unpublished data}
- Rechlin T. The effect of amitriptyline, doxepin, fluvoxamine, and paroxetine treatment on heart rate variability. Journal of Clinical Psychopharmacology 1994;14(6):392‐5. [PubMed] [Google Scholar]
Remick 1994 {published and unpublished data}
- Remick RA, Reesal R, Oakander M, Allen J, Claman J, Ramirez CE, Perry K, Keller FD. Comparison of fluvoxamine and amitriptyline in depressed outpatients. Current Therapeutic Research, Clinical and Experimental 1994;55(3):243‐50. [Google Scholar]
Rossini 2005 {published and unpublished data}
- Rossini D, Franchini L, Smeraldi E, Zanardi R. Sertraline versus fluvoxamine in the treatment of elderly patients with major depression: A double‐blind, randomised trial. European Neuropsychopharmacology 2002;12(Suppl 3):S251. [DOI] [PubMed] [Google Scholar]
- Rossini D, Serretti A, Franchini L, Mandelli L, Smeraldi E, Ronchi D, Zanardi R. Sertraline versus fluvoxamine in the treatment of elderly patients with major depression: A double‐blind, randomized trial. Journal of Clinical Psychopharmacology 2005;25(5):471‐5. [DOI] [PubMed] [Google Scholar]
Rota 2005 {published data only}
- Rota E, Broda R, Cangemi L, Migliaretti G, Paccotti P, Rosso C, Torre E, Zeppegno P, Portaleone P. Neuroendocrine (HPA axis) and clinical correlates during fluvoxamine and amitriptyline treatment. Psychiatry Research 2005;133(2‐3):281‐4. [DOI] [PubMed] [Google Scholar]
Schoemaker 2002 {published and unpublished data}
- Schoemaker J, Gailledreau J, Hoyberg OJ. First, randomized, double‐blind comparison of mirtazapine (15‐45 mg) and fluvoxamine (50‐150 mg) in the treatment of depression. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):140. [Google Scholar]
- Shoemaker JH, The Mirtazapine bridging study group. Double‐blind comparison of mirtazapine versus fluvoxamine in patients with major depressive disorder (DSM‐IV). Unpublished data.
Tourigny‐Rivard 1996 {published data only}
- Tourigny‐Rivard M, Nair N, Vincent P. Fluvoxamine versus desipramine in elderly patients with major depression: A double‐blind comparison. 9th ECNP (European College of Neuropsychopharmacology) Congress. Amsterdam, Netherlands, 1996.
- Tourigny‐Rivard M‐F, Nair VK, Vincent P. Fluvoxamine vs desipramine in elderly depressed patients. Xth World Congress of Psychiatry. Madrid, Spain, 1996.
Ueda 2002 {published and unpublished data}
- Ueda N, Yoshimura R, Shinkai K, Nakamura J. Plasma levels of catecholamine metabolites predict the response to sulpiride or fluvoxamine in major depression. Pharmacopsychiatry. 2002;35(5):175‐81. [DOI] [PubMed] [Google Scholar]
Zohar 2003 {published data only}
- Bnnter M, Barrelet L. Severe depression: Fluvoxamine vs clomipramine. XXIst Collegium Internationale Neuro psychopharmacologicum. Glasgow, Scotland, 1998.
- Katz IR. Drug treatment of depression in the frail elderly, discussion of the NIH consensus development conference on the diagnosis and treatment of depression late‐life. Psychopharmacology Bulletin 1993;29:101. [PubMed] [Google Scholar]
- Berg J, Vekens Cl. Fluvoxamine is as effective as clomipramine in severe depression. 151st Annual Meeting of the American Psychiatric Association. Toronto, Ontario, Canada, 1998.
- Wagner W, Zaborney BA, Gray TE. Fluvoxamine. A review of its safety profile in world‐wide studies. International Clinical Psychopharmacology 1994;9:222. [DOI] [PubMed] [Google Scholar]
- Zohar J, Keegstra H, Barrelet L. Fluvoxamine as effective as clomipramine against symptoms of severe depression: results from a multicentre, double‐blind study. Human Psychopharmacology 2003;18(2):113‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baischer 1991 {published data only}
- Baicsher W, Schonbeck G, Chaunhry HR, Aschauer H, Berger P, Dezwaan M. Clinical use of fluvoxamine in selected outpatients with depression, anxiety, and eating disorders. Pakistan Journal of Clinical Psychiatry 1991;3:143‐50. [Google Scholar]
Belliini 1992 {published data only}
- Bellini L, Gatti F, Macciardi F, Lucca A, Della Maggiore P, Gasperini M, Smeraldi E. A standardized treatment for delusional depression: Preliminary results. Clinical Neuropharmacology 1992;15(1 Pt B):413. [Google Scholar]
Blier 1997 {published data only}
- Blier P, Bergeron R, Montigny C. Selective activation of postsynaptic 5‐HT(1A) receptors induces rapid antidepressant response. Neuropsychopharmacology 1997;16(5):333‐8. [DOI] [PubMed] [Google Scholar]
Davis 1991 {published data only}
- Davis BA, Boulton AA, Yu PH, Durden DA, Bowen RC, Keegan DL, Blackshaw S, D'Arcy C, Remillard AJ, Dayal N, Shrikhande S, Saleh S. Deuterium‐labelled p‐tyramine challenge test and phenolsulfotransferase activity in depressed patients‐ Failure to replicate decreased p‐tyramine conjugation in depression. Progress in Neuro‐psychopharmacology & Biological Psychiatry 1991;15(2):241‐7. [DOI] [PubMed] [Google Scholar]
de Jonghe 1991 {published data only}
- Jonghe F, Swinkels J, Tuynman‐Qua H. Randomized double‐blind study of fluvoxamine and maprotiline in treatment of depression. Pharmacopsychiatry 1991;24(1):21‐7. [DOI] [PubMed] [Google Scholar]
de Kemp 2002 {published data only}
- Kemp EC, Moleman P, Hoogduin CA, Broekman TG, Goedhart A, Schaap CP, Berg PC. Diagnosis at the first episode to differentiate antidepressant treatment responses in patients with mood and anxiety disorders. Psychopharmacology 2002;160(1):67‐73. [DOI] [PubMed] [Google Scholar]
de Wilde 1982 {published data only}
- Wilde JEM, Doogan DP. Fluvoxamine and chlorimipramine in endogenous depression. Journal of Affective Disorders 1982;4(3):249‐59. [DOI] [PubMed] [Google Scholar]
Emrich 1987 {published data only}
- Emrich HM, Berger M, Riemann D, Zerssen D. Serotonin reuptake inhibition vs norepinephrine reuptake inhibition: a double‐blind differential‐therapeutic study with fluvoxamine and oxaprotiline in endogenous and neurotic depressives. Pharmacopsychiatry 1987;20(2):60‐3. [DOI] [PubMed] [Google Scholar]
Entsuah 2002a {published data only}
- Entsuah R, Shaffer M, Zhang J. A critical examination of the sensitivity of unidimensional subscales derived from the Hamilton Depression Rating Scale to antidepressant drug effects. Journal of Psychiatric Research 2002;36(6):437‐48. [DOI] [PubMed] [Google Scholar]
Franchini 1997 {published data only}
- Franchini L, Gasperini M, Zanardi R, Smeraldi E. Four‐year follow‐up study of sertraline and fluvoxamine in long‐term treatment of unipolar subjects with high recurrence rate. Journal of Affective Disorders 1997;58(3):233‐6. [DOI] [PubMed] [Google Scholar]
Gasperini 1992 {published data only}
- Gasperini M, Gatti F, Bellini L, Anniverno R, Smeraldi E. Perspectives in clinical psychopharmacology of amitriptyline and fluvoxamine A double‐blind study in depressed inpatients. Neuropsychobiology 1992;26(4):186‐92. [DOI] [PubMed] [Google Scholar]
Gonella 1990 {published data only}
- Gonella G, Baignoli G, Ecari U. Fluvoxamine and imipramine in the treatment of depressive patients: a double‐blind controlled study. Current Medical Research and Opinion 1990;12(3):177‐84. [DOI] [PubMed] [Google Scholar]
Goto 2006 {published data only}
- Goto F, Araki Y, Saito A, Kunihiro T, Ogawa K. Treatment of anxiety and depression‐related vertigo and dizziness with SSRIs and SNRIs. Equilibrium Research 2006;65(1):17‐23. [Google Scholar]
Guelfi 1983 {published data only}
- Guelfi JD, Dreyfus JF, Pichot P. A double‐blind controlled clinical trial comparing fluvoxamine with imipramine. British Journal of Clinical Pharmacology 1983;15(Suppl 3):411‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfi JD, Dreyfus JF, Pichot P. Fluvoxamine and imipramine: results of a long‐term controlled trial. International Clinical Psychopharmacology 1987;2(2):103‐9. [DOI] [PubMed] [Google Scholar]
Harris 1991b {published data only}
- Harris B, Ashford J. Maintenance antidepressants and weight gain: A comparison of fluvoxamine and amitriptyline. British Journal of Clinical Research 1991;2:81‐8. [Google Scholar]
Hewer 1994 {published data only}
- Hewer W, Rost W, Gattaz WF. Cardiovascular effects of fluvoxamine and maprotiline in depressed patients. European Archives of Psychiatry and Clinical Neuroscience 1995;246(1):1‐6. [DOI] [PubMed] [Google Scholar]
- Hewer W, Rost W, Gattaz WF. Effects of fluvoxamine and maprotiline on ECG parameters. Nervenheilkunde 1994;13(8):375‐80. [Google Scholar]
Hochberg 1995 {published data only}
- Hochberg HM, Kanter D, Houser VP. Electrocardiographic findings during extended clinical trials of fluvoxamine in depression: one year's experience. Pharmacopsychiatry 1995;28(6):253‐6. [DOI] [PubMed] [Google Scholar]
Khan 1989 {published data only}
- Khan A, Cohen S, Dager S, Avery D, Dunner D. Onset of response in relation to outcome in depressed outpatients with placebo and imipramine. Journal of Affective Disorders 1989;17(1):33‐8. [DOI] [PubMed] [Google Scholar]
- Khan A, Dager SR, Cohen S, Avery DH, Scherzo B, Dunner DL. Chronicity of depressive episode in relation to antidepressant‐placebo response. Neuropsychopharmacology 1991;4(2):125‐30. [PubMed] [Google Scholar]
Klok 1981 {published data only}
- Klok CJ, Brouwer GJ, Praag HM, Doogan D. Fluvoxamine and clomipramine in depressed patients: A double blind clinical study. Acta Psychiatrica Scandinavica 1981;64(1):1‐11. [DOI] [PubMed] [Google Scholar]
Lara‐Munoz 1996 {published data only}
- Lara C, Heinze G, Fuente J. The quality‐of‐life of depressed patients. Xth World Congress of Psychiatry. Madrid, Spain, 1996.
- Lara‐Munoz C, Heinze G, Fuente JR. Do the antidepressants have effects on quality‐of‐life independently of their antidepressant actions?. 149th Annual Meeting of the American Psychiatric Association. New York, NY, 1996.
Manfredonia 1992 {published data only}
- Manfredonia MG, Gasperini M, Franchini L, Smeraldi E. Memory impairments in depression. Clinical Neuropharmacology 1992;15(1 Pt B):568. [Google Scholar]
Muck‐Seler 1991 {published and unpublished data}
- Muck‐Seler D, Jakovljevic M, Deanovic Z. Effect of antidepressant treatment on platelet 5‐HT content and relation to therapeutic outcome in unipolar depressive patients. Journal of Affective Disorders 1991;23(3):157‐64. [DOI] [PubMed] [Google Scholar]
Murasaki 1998b {published data only}
- Murasaki M, Mori A, Yamashita I. A late clinical phase ii study of sme3110 (fluvoxamine maleate), a selective serotonin reuptake inhibitor, in the treatment of depression and depressive state: an investigation of an optimal dose range using imipramine as a comparator. Rinsyoiyaku (Journal of clinical therapeutics & medicine) 1998;14(5):919‐49. [Google Scholar]
Namiki 1996 {published data only}
- Namiki M, Muto E, Minemoto H. A clinical phase iii study of sme3110 (fluvoxamine maleate) in depressed patients at the department of internal medicine: a double‐blind, comparative study with trazodone hydrochloride. Rinsyoiyaku 1996;12(4):651‐77. [Google Scholar]
Nicolini 1996 {published data only}
- Nicolini H, Herrera K, Pez F, Camarena B, Guajardo R, Fuente JR. Temperament, character, and molecular genotypes as predictors of antidepressant response. XXth Collegium Internationale Neuro psychopharmacologicum. Melbourne, Australia;, 1996.
Nolen 1988 {published data only}
- Nolen WA, Putte JJ, Dijken WA, Kamp JS, Blansjaar BA, Kramer HJ, Haffmans J. Treatment strategy in depression I Non‐tricyclic and selective reuptake inhibitors in resistant depression: a double‐blind partial crossover study on the effects of oxaprotiline and fluvoxamine. Acta Psychiatrica Scandinavica 1998;78(6):668‐75. [DOI] [PubMed] [Google Scholar]
Phanjoo 1991 {published data only}
- Phanjoo AL, Wonnacott S, Hodgson A. Double‐blind comparative multicentre study of fluvoxamine and mianserin in the treatment of major depressive episode in elderly people. Acta Psychiatrica Scandinavica 1991;83(6):476‐9. [DOI] [PubMed] [Google Scholar]
Poldinger 1991 {published data only}
- Poldinger W, Calanchini B, Schwarz W. A functional‐dimensional approach to depression: serotonin deficiency as a target syndrome in a comparison of 5‐hydroxytryptophan and fluvoxamine. Psychopathology 1991;24(2):53‐81. [DOI] [PubMed] [Google Scholar]
Price 1986 {published data only}
- Price LH, Charney DS, Delgado PL, Anderson GM, Heninger GR. Effects of desipramine and fluvoxamine treatment on the prolactin response to tryptophan. Serotonergic function and the mechanism of antidepressant action. Archives of General Psychiatry 1989;46(7):625‐31. [DOI] [PubMed] [Google Scholar]
- Price LH, Charney DS, Heninger GR. Variability of response to lithium augmentation in refractory depression. American Journal of Psychiatry 1986;143(11):1387‐92. [DOI] [PubMed] [Google Scholar]
Ravindran 1995 {published data only}
- Ravindran A, Griffiths J, Waddell C, Anisman H. Stressful life events and coping styles in relation to dysthymia and major depressive disorder: Variations associated with alleviation of symptoms following pharmacotherapy. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 1995;19(4):637‐53. [DOI] [PubMed] [Google Scholar]
Sacchetti 1994 {published data only}
- Sacchetti E, Conte G, Guarneri L. Are SSRI antidepressants a clinically homogeneous class of compounds?. Lancet 1994;344(8915):126‐7. [PubMed] [Google Scholar]
Sacchetti 1997 {published data only}
- Sacchetti E, Conte G, Guarneri L, Calzeroni A, Bertini M, Panariello A. Effectiveness of fluvoxamine and paroxetine in major depressives with psychotic features. Human Psychopharmacology 1997;12:277‐8. [Google Scholar]
Schanda 1979 {published data only}
- Schanda H, Saletu B. Repolarisation disturbances in the ECG under antidepressant drugs. A comparison of two drugs, differing in chemical structure and pharmacological profile. Pharmakopsychiatrie, Neuro‐Psychopharmakologie 1979;12(4):338‐45. [DOI] [PubMed] [Google Scholar]
Serretti 2004 {published data only}
- Serretti A, Cusin C, Rossini D, Artioli P, Dotoli D, Zanardi R. Further evidence of a combined effect of SERTPR and TPH on SSRIs response in mood disorders. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 2004;129(1):36‐40. [DOI] [PubMed] [Google Scholar]
Sheline 1997 {published data only}
- Sheline YI, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5‐HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. Journal of Clinical Psychopharmacology 1997;17(1):11‐4. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proceedings of the National Academy of Sciences of the United States of America 1998;95(6):3239‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandel 1995 {published data only}
- Vandel S, Bertschy G, Baumann P, Bouquet S, Bonin B, Francois T, Sechter D, Bizouard P. Fluvoxamine and fluoxetine: Interaction studies with amitriptyline, clomipramine and neuroleptics in phenotyped patients. Pharmacological Research 1995;31(6):347‐53. [DOI] [PubMed] [Google Scholar]
van den Broek 2004 {published data only}
- Birkenhager TK, W.W. vdB, Mulder PG, Bruijn J, Moleman P. Comparison of two‐phase treatment with imipramine or fluvoxamine, both followed by lithium addition, in inpatients with major depressive disorder. American Journal of Psychiatry 2004;161(11):2060‐5. [DOI] [PubMed] [Google Scholar]
- Broek WW, Birkenhager TK, Mulder PG, Bruijn JA, Moleman P. A double‐blind randomized study comparing imipramine with fluvoxamine in depressed inpatients. Psychopharmacology 2004;175(4):481‐6. [DOI] [PubMed] [Google Scholar]
White 1990 {published data only}
- White K, Wykoff W, Tynes L, Schneider L, Zemansky M. Fluvoxamine in the treatment of tricyclic‐resistant depression. Psychiatric Journal of the University of Ottawa 1990;15(3):156‐8. [PubMed] [Google Scholar]
Yu 2001 {published data only}
- Yu G, Liang S, Wu F. A comparative study of Luvox and amitriptyline in patients with major depression. Medical Journal of Chinese Civil Administration 2001;13(4):227‐8. [Google Scholar]
Zanardi 2000 {published data only}
- Zanardi R, Franchini L, Serretti A, Perez J, Smeraldi E. Venlafaxine versus fluvoxamine in the treatment of delusional depression: a pilot double‐blind controlled study. Journal of Clinical Psychiatry 2000;61(1):26‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Berlin 1998 {published data only}
- Berlin I, Lavergne F. Early predictors of two month response with mianserin and selective serotonin reuptake inhibitors and influence of definition of outcome on prediction. European Psychiatry 1998;13(3):138‐42. [DOI] [PubMed] [Google Scholar]
Coleman 1981a {published data only}
- Coleman BS, Doogan DP. Medical report on a double‐blind comparative study of fluvoxamine and chlorimipramine in institutionalized patients with bipolar and unipolar depression. Unpublished 1981.
Coleman 1981b {published data only}
- Coleman B, Block B. A double‐blind placebo‐controlled randomized comparative study of fluvoxamine and imipramine in out‐patients with primary depression. Unpublished 1981. [DOI] [PMC free article] [PubMed]
Coleman 1983 {published data only}
- Coleman BS, Block BA, Vause, EW. A double‐blind placebo‐controlled randomized multicenter comparison of fluvoxamine and imipramine in patients with primary depression. Unpublished 1983. [DOI] [PMC free article] [PubMed]
Donovan 1993 {published data only}
- Donovan S. The efficacy and tolerability of dothiepin versus serotonin specific reuptake inhibitors in the treatment of depression. European Neuropsychopharmacology 1993;3(3):331‐332. [Google Scholar]
Doogan 1981 {unpublished data only}
- Doogan DP, Beek AA. Fluvoxamine (DU23000) and chlorimipramine (Anafranil) in hospitalized patients with depressive syndromes. A double‐blind, randomized, comparative clinical study. Unpublished 1981.
Entsuah 2002b {published data only}
- Entsuah R, Kelsey J. Venlafaxine offers significant therapeutic benefits over existing SSRI treatments irrespective of the patient's depressive duration. European Neuropsychopharmacology 2002;12 Suppl 3:S238. [Google Scholar]
Faludi 1989 {published data only}
- Faludi G, Magyar I, Antalics E, Mod L. Fluvoxamine versus maprotiline (Ludiomil) in the treatment of depression. IDeggyogyaszati Szemle 1989;46:116‐22. [Google Scholar]
Mallick 2003 {published data only}
- Mallick R, Chen J, Entsuah AR, Schatzberg AF. Depression‐free days as a summary measure of the temporal pattern of response and remission in the treatment of major depression: a comparison of venlafaxine, selective serotonin reuptake inhibitors, and placebo. Journal of clinical psychiatry 2003;64(3):321‐30. [DOI] [PubMed] [Google Scholar]
Naito 2007 {published data only}
- Naito S, Sato K, Yoshida K, Higuchi H, Takahashi H, Kamata M. Gender differences in the clinical effects of fluvoxamine and milnacipran in Japanese major depressive patients. Psychiatry and Clinical Neurosciences 2007;61(4):421‐27. [DOI] [PubMed] [Google Scholar]
Ushiroyama 2004 {published data only}
- Ushiroyama T. Ikeda A. Ueki M. Evaluation of double‐blind comparison of fluvoxamine and paroxetine in the treatment of depressed outpatients in menopause transition. Journal of Medicine 2004;35:151‐62. [PubMed] [Google Scholar]
van Beek 1981 {published data only}
- Beek AA, Sharp D. Medical report fluvoxamine and imipramine in hospitalized patients and depressive syndromes. A double‐blind, randomized, multicentre clinical study. Unpublished 1981.
Additional references
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. JAMA 2003;290(7):921‐8. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. British Medical Journal 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 1994
- Anderson IM, Tomenson BM. The efficacy of selective serotonin reuptake inhibitors in depression: a meta‐analysis of studies against tricyclic antidepressants. J Psychopharmacol 1994;8:238‐249. [DOI] [PubMed] [Google Scholar]
Anderson 1998
- Anderson I M. SSRIS versus tricyclic antidepressants in depressed inpatients: a meta‐analysis of efficacy and tolerability. Depression and Anxiety 1998;7 Suppl 1:11‐7. [PubMed] [Google Scholar]
Anderson 2000
- Anderson IM, Nutt DJ, Deakin JF. Evidence‐based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines.British Association for Psychopharmacology. Journal of Psychopharmacology 2000 Mar;14(1):3‐20. [DOI] [PubMed] [Google Scholar]
Anderson 2001
- Anderson I M, Edwards J G. Guidelines for choice of selective serotonin reuptake inhibitor in depressive illness. Advances in psychiatric treatment 2001;7:170‐80. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, D.C: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). American Psychiatric Association. American Journal of Psychiatry 2000;157(4 Suppl):1‐45. [PubMed] [Google Scholar]
Barbui 2000
- Barbui C, Hotopf M, Freemantle N, Boynton J, Churchill R, Eccles MP, Geddes JR, Hardy R, Lewis G, Mason JM. Selective serotonin reuptake inhibitors versus tricyclic and heterocyclic antidepressants: comparison of drug adherence. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: ] [DOI] [PubMed] [Google Scholar]
Barbui 2004
- Barbui C, Cipriani A, Brambilla P, Hotopf M. "Wish bias" in antidepressant drug trials?. Journal of Clinical Psychopharmacology 2004 Apr;24(2):126‐30. [DOI] [PubMed] [Google Scholar]
Barbui 2008
- Barbui C, Furukawa T A, Cipriani A. Effectiveness of paroxetine in the treatment of acute major depression in adults: a systematic re‐examination of published and unpublished data from randomized trials. Canadian Medical Association Journal 2008;178(3):296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. [DOI] [PubMed] [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, Mears D, Schemitsch EH, Heels‐Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170(4):477‐80. [PMC free article] [PubMed] [Google Scholar]
Bollini 1999
- Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta‐analysis of dose‐effect relationships in randomised clinical trials. British Journal of Psychiatry 1999;174:297‐303. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of clinical epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Buchkowsky 2004
- Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Annals of Pharmacotherapy 2004;38(4):579‐85. [DOI] [PubMed] [Google Scholar]
Burton 1991
- Burton S W. A review of fluvoxamine and its uses in depression. International Clinical Psychopharmacology 1991;6 Suppl 3:1‐17; discussion 17‐21. [DOI] [PubMed] [Google Scholar]
Churchill 2009
- Churchill R, Caldwell D, McGuire H, Barbui C, Cipriani A, Furukawa TA, Watanabe N, Lewis G. Reboxetine versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007852] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2005
- Cipriani A, Brambilla P, Furukawa T, Geddes J, Gregis M, Hotopf M, Malvin L, Barbui C. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2006
- Cipriani A, Barbui C, Brambilla P, Furukawa T A, Hotopf M, Geddes J R. Are all antidepressants really the same? The case of fluoxetine: a systematic review. Journal of Clinical Psychiatry 2006;67(6):850‐64. [DOI] [PubMed] [Google Scholar]
Cipriani 2007b
- Cipriani A, Furukawa TA, Veronese A, Watanabe N, Churchill R, McGuire H, Barbui C, Meta‐Analysis of New Generation Antidepressants (MANGA) Study Group. Paroxetine versus other anti‐depressive agents for depression. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006531] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2007c
- Cipriani A, Signoretti A, Furukawa TA, Churchill R, Tomelleri S, Omori I, McGuire H, Barbui C, Meta‐Analysis of New Generation Antidepressants (MANGA) Study Group. Venlafaxine versus other anti‐depressive agents for depression. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006530] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2009
- Cipriani A, Furukawa TA, Salanti G, Geddes J R, Higgins J P, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. Lancet 2009;373(9665):746‐58. [DOI] [PubMed] [Google Scholar]
Cipriani 2009a
- Cipriani A, Santilli C, Furukawa TA, Signoretti A, Nakagawa A, McGuire H, Churchill R, Barbui C. Escitalopram versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006532.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2009b
- Cipriani A, Ferla T, Furukawa TA, Signoretti A, Nakagawa A, Churchill R, McGuire H, Barbui, C. Sertraline versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006117.pub2] [DOI] [PubMed] [Google Scholar]
Ciuna 2004
- Ciuna A, Andretta M, Corbari L, Levi D, Mirandola M, Sorio A, Barbui C. Are we going to increase the use of antidepressants up to that of benzodiazepines?. European Journal of Clinical Pharmacology 2004;60(9):629‐34. [DOI] [PubMed] [Google Scholar]
Claassen 1977
- Claassen V, Davies J E, Hertting G, Placheta P. Fluvoxamine, a specific 5‐hydroxytryptamine uptake inhibitor. British Journal of Pharmacology 1977;60(4):505‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Depression Guideline Panel 1993
- Depression Guideline Panel. Depression in primary care: Vol 2. Treatment of major depression, Clinical Practice Guideline, Number 5, AHCPR Publication No. 93‐0551. Rockville, MD: U. S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, 1993. [Google Scholar]
Dozois 2004
- Dozois D J A, Dobson K S. Depression. In: Antony M M, Barlow D H editor(s). Handbook of Assessment and Treatment Planning for Psychological Disorders. New York: Guilford Press, 2004:259‐99. [Google Scholar]
Edwards 1999
- Edwards J G, Anderson I. Systematic review and guide to selection of selective serotonin reuptake inhibitors. Drugs 1999;57(4):507‐33. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] [DOI] [PubMed] [Google Scholar]
Ellis 2004
- Ellis P. Australian and New Zealand clinical practice guidelines for the treatment of depression. Australian and New Zealand Journal of Psychiatry 2004;38(6):389‐407. [DOI] [PubMed] [Google Scholar]
Feighner 1972
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26:57‐63. [DOI] [PubMed] [Google Scholar]
Fuller 1987
- Fuller R W, Wong D T. Serotonin reuptake blockers in vitro and in vivo. Journal of Clinical Psychopharmacology 1987;7(6 Suppl):36S‐43S. [PubMed] [Google Scholar]
Furukawa 2002a
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. [DOI] [PubMed] [Google Scholar]
Furukawa 2002b
- Furukawa TA, McGuire H, Barbui C. Meta‐analysis of effects and side effects of low dosage tricyclic antidepressants in depression: systematic review. British Medical Journal 2002;325(7371):991‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20(1):49‐52. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa T A, Watanabe N, Omori I M, Montori V M, Guyatt G H. Association between unreported outcomes and effect size estimates in Cochrane meta‐analyses. JAMA 2007;297(5):468‐70. [DOI] [PubMed] [Google Scholar]
Geddes 2000
- Geddes JR, Freemantle N, Mason J, Eccles MP, Boynton J. SSRIs versus other antidepressants for depressive disorder. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: ] [DOI] [PubMed] [Google Scholar]
Goldberg 1991
- Goldberg David, Huxley Peter. Common mental disorders : a bio‐social model. Tavistock/Routledge, 1991. [Google Scholar]
Greenberg 2003
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey‐Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000?. Journal of Clinical Psychiatry 2003;64(12):1465‐75. [DOI] [PubMed] [Google Scholar]
Greenberg 2005
- Greenberg PE, Birnbaum HG. The economic burden of depression in the US: societal and patient perspectives. Expert Opinion on Pharmacotherapy 2005;6(3):369‐76. [DOI] [PubMed] [Google Scholar]
Guaiana 2005
- Guaiana G, Andretta M, Corbari L, Mirandola M, Sorio A, D'Avanzo B, Barbui C. Antidepressant drug consumption and public health indicators in Italy, 1955 to 2000. Journal of Clinical Psychiatry 2005;66(6):750‐5. [DOI] [PubMed] [Google Scholar]
Guaiana 2007
- Guaiana G, Barbui C, Hotopf M. Amitriptyline for depression. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004186.pub2] [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions ‐ ECDEU Asessment Manual Psychopharmacology (DHEW Publ No ADM 76‐338). Revised. Rockville MD: U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH, 1976. [Google Scholar]
Hall 1995
- Hall RC. Global Assessment of Functioning ‐ a modified scale. Psychosomatics 1995;36:267‐75. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton MTI. A rating scale of depression. Journal of Neurology 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammad 2006
- Hammad T A, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Archives of General Psychiatry 2006;63(3):332‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. [The Cochrane Collaboration, 2008.]. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. [Google Scholar]
Imperadore 2007
- Imperadore G, Cipriani A, Signoretti A, Furukawa TA, Watanabe N, Churchill R, McGuire H, Barbui C, Meta‐Analysis of New Generation Antidepressants (MANGA) Study Group. Citalopram versus other anti‐depressive agents for depression. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006534] [DOI] [Google Scholar]
Ioannidis 2006
- Ioannidis JP. Indirect comparisons: the mesh and mess of clinical trials. Lancet 2006;368(9546):1470‐2. [DOI] [PubMed] [Google Scholar]
Khan 2003
- Khan A, Khan SR, Walens G, Kolts R, Giller EL. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacology 2003;28(3):552‐7. [DOI] [PubMed] [Google Scholar]
Lecrubier 1996
- Lecrubier Y, Pletan Y, Solles A, Tournoux A, Magne V. Clinical efficacy of milnacipran: placebo‐controlled trials. International Clinical Psychopharmacology 1996 Sep;11(Suppl 4):29‐33. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. British Medical Journal 2003;326(7400):1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2008
- Linde K, Berner MM, Kriston L. St John's wort for major depression. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000448.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez‐Ibor 1996
- Lopez‐Ibor J, Guelfi JD, Pletan Y, Tournoux A, Prost JF. Milnacipran and selective serotonin reuptake inhibitors in major depression. International Clinical Psychopharmacology 1996;11(Suppl 4):41‐6. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidenceinconsistency in mixed treatmentcomparisons. Journal of American StatisticalAssociation 2006;101:447–59. [Google Scholar]
Lumley 2002
- Lumley T. Network meta‐analysis for indirect treatment comparisons. Statistics in medicine 2002;21(16):2313‐24. [DOI] [PubMed] [Google Scholar]
Mackay 1997
- Mackay F J, Dunn N R, Wilton LV. A comparison if fluvoxamine, fluoxetine, sertraline and paroxetine examined by observational cohort studies. Pharmacoepidemiology and Drug Safety 1997;6:235–246. [DOI] [PubMed] [Google Scholar]
Montejo‐Gonzalez 1997
- Montejo‐Gonzalez A L, Llorca G, Izquierdo J A. SSRI‐induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. Journal of Sexual and Marital Therapy 1997;23:176–194. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Montgomery 2004
- Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F, Holland R. An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Controlled clinical trials 2004;25(6):598‐612. [DOI] [PubMed] [Google Scholar]
Mottram 2006
- Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003491.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2003
- Muller M J, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery‐Asberg depression rating scale (MADRS). Journal of Affective Disorders 2003;77(3):255‐60. [DOI] [PubMed] [Google Scholar]
Nakagawa 2007
- Nakagawa A, Watanabe N, Omori I, Cipriani A, Barbui C, McGuire H, Churchill R, Furukawa TA, Meta‐Analysis of New Generation Antidepressants (MANGA) Study Group. Milnacipran versus other anti‐depressive agents for depression. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006529] [DOI] [Google Scholar]
NICE 2004
- NICE. Depression: management of depression in primary and secondary care. London: National Institute for Clinical Excellence, 2004. [Google Scholar]
Nose 2007
- Nose M, Cipriani A, Furukawa TA, Omori I, Churchill R, McGuire H, Barbui C, Meta‐Analysis of New Generation Antidepressants (MANGA) Study Group. Duloxetine versus other anti‐depressive agents for depression. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006533] [DOI] [Google Scholar]
Okamura 2006
- Okamura K, Furukawa TA. Remission rates with milnacipran 100mg/day and 150 mg/day in the long‐term treatment of major depression. Clinical Drug Investigation 2006;26(3):135‐142. [DOI] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78‐84. [DOI] [PubMed] [Google Scholar]
Perlis 2005
- Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, Nierenberg AA. Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. American Journal of Psychiatry 2005 Oct;162(10):1957‐60. [DOI] [PubMed] [Google Scholar]
Perucca 1994
- Perucca E, Gatti G, Spina E. Clinical pharmacokinetics of fluvoxamine. Clinical Pharmacokinetics 1994;27(3):175‐90. [DOI] [PubMed] [Google Scholar]
Procyshyn 2004
- Procyshyn RM, Chau A, Fortin P, Jenkins W. Prevalence and outcomes of pharmaceutical industry‐sponsored clinical trials involving clozapine, risperidone, or olanzapine. Canadian Journal of Psychiatry 2004;49(9):601‐6. [DOI] [PubMed] [Google Scholar]
Puech 1997
- Puech A, Montgomery SA, Prost JF, Solles A, Briley M. Milnacipran, a new serotonin and noradrenaline reuptake inhibitor: an overview of its antidepressant activity and clinical tolerability. International Clinical Psychopharmacology 1997;12(2):99‐108. [DOI] [PubMed] [Google Scholar]
Salanti 2008
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279‐301. [DOI] [PubMed] [Google Scholar]
Schneider 1995
- Schneider LS, Olin JT. Efficacy of acute treatment for geriatric depression. International Psychogeriatrics 1995;7(Suppl):7‐25. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Smith 2002
- Smith D, Dempster C, Glanville J, Freemantle N, Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta‐analysis. British Journal of Psychiatry 2002;180:396‐404. [DOI] [PubMed] [Google Scholar]
Song 2003
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. British Medical Journal 2003;326(7387):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spigset 1995
- Spigset O, Carleborg L, Hedenmalm K, Dahlqvist R. Effect of cigarette smoking on fluvoxamine pharmacokinetics in humans. Clin Pharmacol Ther 1995;58:399‐403. [DOI] [PubMed] [Google Scholar]
Spijker 2001
- Spijker J, Bijl R V, Graaf R, Nolen W A. Care utilization and outcome of DSM‐III‐R major depression in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand 2001;104(1):19‐24. [DOI] [PubMed] [Google Scholar]
Spitzer 1972
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives General Psychiatry 1978;35(6):773‐82. [DOI] [PubMed] [Google Scholar]
Sterne 2000
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of clinical epidemiology 2000;53(11):1119‐29. [PUBMED: 11106885] [DOI] [PubMed] [Google Scholar]
Suh 1997
- Suh T, Gallo J J. Symptom profiles of depression among general medical service users compared with specialty mental health service users. Psychological Medicine 1997;27(5):1051‐63. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor M J, Freemantle N, Geddes J R, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta‐analysis. Archives of General Psychiatry 2006;63(11):1217‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Harten 1995
- Harten J. Overview of the pharmacokinetics of fluvoxamine. Clinical Pharmacokinetics 1995;29 Suppl 1:1‐9. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short form health survey (SF‐36). Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
Ware 1997
- Ware MR. Fluvoxamine: a review of the controlled trials in depression. Journal of Clinical Psychiatry 1997;58 Suppl 5:15‐23. [PubMed] [Google Scholar]
Ware 1998
- Ware JE, Kosinski M, Keller SD. SF‐12: How to Score the SF‐12.Physical and Mental Health Summary Scales. Lincoln RI: QualityMetric Inc, 1998. [Google Scholar]
Watanabe 2008
- Watanabe N, Omori I M, Nakagawa A, Cipriani A, Barbui C, McGuire H, Churchill R, Furukawa TA. Mirtazapine versus other antidepressants in the acute‐phase treatment of adults with major depression: systematic review and meta‐analysis. Journal of Clinical Psychiatry 2008;69(9):1404‐15. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organisation. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10). Geneva: World Health Organisation, 1992. [Google Scholar]
WHO 2006
- WHO. Revised Global Burden of Disease 2002 estimates.. http://www.who.int/healthinfo/bodgbd2002/en/index. WHO, 2006. [Google Scholar]
WHO 2009
- WHO Collaborative Centre for Drug Statistics Methodology. ATC/DDD Index 2009. http://www.whocc.no/atcddd/ 2009.
WHOQOL Group 1998
- WHOQOL Group. The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Social Science and Medicine 1998;46(12):1569‐65. [DOI] [PubMed] [Google Scholar]
Williams 2000
- Williams JW, Jr, Mulrow CD, Chiquette E, Noel PH, Aguilar C, Cornell J. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Annals of Internal Medicine 2000;132(9):743‐56. [DOI] [PubMed] [Google Scholar]
Wing 1998
- Wing JK, Beevor AS, Curtis RH, Park SBG, Burns A. Health of the nation outcome scales (HoNOS): Research and development. British Journal of Psychiatry 1998;172:11‐18. [DOI] [PubMed] [Google Scholar]


