Abstract
BACKGROUND
Major Depressive Disorder (MDD) often begins during adolescence when the brain is still maturing. To better understand the neurobiological underpinnings of MDD early in development, this study examined brain function in response to emotional faces in adolescents with MDD and healthy (HC) adolescents using functional magnetic resonance imaging (fMRI).
METHOD
Thirty-two unmedicated adolescents with MDD and 23 healthy age- and gender-matched controls completed an fMRI task viewing happy and fearful faces. Frontolimbic regions of interest (ROI; bilateral amygdala, insula, subgenual and rostral anterior cingulate cortices) and whole-brain analyses were conducted to examine between-group differences in brain function.
RESULTS
ROI analyses revealed that patients had greater bilateral amygdala activity than HC in response to viewing fearful versus happy faces, which remained significant when controlling for comorbid anxiety. Whole-brain analyses revealed that adolescents with MDD had lower activation compared to HC in a right hemisphere cluster comprised of the insula, superior/middle temporal gyrus, and Heschl's gyrus when viewing fearful faces. Brain activity in the subgenual anterior cingulate cortex was inversely correlated with depression severity.
LIMITATIONS
Limitations include a cross-sectional design with a modest sample size and use of a limited range of emotional stimuli.
CONCLUSIONS
Results replicate previous studies that suggest emotion processing in adolescent MDD is associated with abnormalities within fronto-limbic brain regions. Findings implicate elevated amygdalar arousal to negative stimuli in adolescents with depression and provide new evidence for a deficit in functioning of the saliency network, which may be a future target for early intervention and MDD treatment.
Keywords: Major Depression, adolescence, fMRI, fronto-limbic neural circuitry
BACKGROUND
Major depressive disorder (MDD) is a common and serious mental illness linked to major role impairments (Kessler et al. 2003), reduced life satisfaction (Nes et al. 2013) and high rates of mortality, including suicide (Simon, Hales 2006). Research that furthers our understanding of the neurobiological underpinnings of adolescent MDD is necessary for several reasons. First, MDD often develops during adolescence (Kessler, Avenevoli & Ries Merikangas 2001) and the prognosis for depression is particularly poor when problems are evident early during development (Brent et al. 1998, Zisook et al. 2007). Additionally, neural pruning, maturation and refinement occur throughout this time (Lenroot, Giedd 2006). Lastly, examining biological mechanisms of MDD early in the disease course, uncompromised by the scarring effects of chronic illness, may help us identify neurobiological markers unique to adolescent MDD. This may improve our understanding of treatment targets and lead to the development of innovative early intervention strategies that take advantage of malleable neural systems.
A fronto-limbic dysregulation model of depression proposed by Mayberg (1997) purports that neocortical, subcortical and paralimbic brain structures interact to regulate emotional reactivity and affective behavior. Specifically, neocortial structures (e.g, dorsal-lateral prefrontal cortex) play a regulatory role in emotion processing whereas paralimbic structures (e.g., medial orbital frontal cortex, subgenual cingulate cortex, insula, amygdala) form the foundation of our emotional system responsible for processing emotional cues. For example, the insula is part of a network involved in processing the emotional salience of external and internal stimuli (Seeley et al. 2007). Similarly, the amygdala rapidly reacts to the emotional significance of stimuli (Victor et al. 2010) and is involved in processing facial displays of affect (Fitzgerald et al. 2006).
Abnormal processing of emotional stimuli has been consistently implicated in MDD and is thought to increase risk of disease development, maintenance and relapse (Foland-Ross, Gotlib 2012, Mathews, MacLeod 2005). Mayberg and others have suggested that depressive symptomatology is a consequence of hypo-activity in neocortical structures and hyperactivity in paralimbic structures (Mayberg 1997, Stuhrmann, Suslow & Dannlowski 2011). Numerous neuroimaging studies have provided support for this claim (Drevets, Price & Furey 2008, Price, Drevets 2012, Cullen et al. 2009, Steingard et al. 2002). In particular, since tasks involving emotional facial expressions are efficient at eliciting responses in fronto-limbic areas (Fusar-Poli et al. 2009) and may be particularly sensitive to identifying processing biases in MDD (Stuhrmann, Suslow & Dannlowski 2011), numerous studies have investigated emotional processing using visual face paradigms in conjunction with functional magnetic resonance imaging (fMRI) technology. Although findings within the anterior cingulate cortex (ACC) have been mixed, (Stuhrmann, Suslow & Dannlowski 2011) in adults, fMRI studies of emotion processing in response to affective facial stimuli have generally supported Mayberg's model by showing (a) increased activity in the amygdala when viewing negative (e.g., sad, fearful) emotional faces (Victor et al. 2010, Zhong et al. 2011, Sheline et al. 2001), (b) increased insula activity while viewing sad (Keedwell et al. 2010), fearful, angry (Zhong et al. 2011) and disgusted faces (Surguladze et al. 2010), and (c) decreased functional activity in areas of the dorsal lateral prefrontal cortex and orbital frontal cortex (Zhong et al. 2011, Keedwell et al. 2010).
Compared to the adult literature, face processing in depressed adolescents remains understudied. Using an emotional faces matching task with a sample of medication naïve, depressed adolescents without comorbid psychiatric diagnoses, Yang et al. (2010) reported increased ACC and left amygdala activation in response to fearful, sad, and happy faces as compared to healthy controls. Beesdo et al. (2009) compared amygdala activity in depressed, anxious and healthy youth using an emotional faces task that required varying levels of attentive control. When passively viewing emotional faces, Beesdo et al. (2009) found that adolescents with MDD had decreased (and opposite) bilateral amygdala response compared to both healthy and anxious comparison groups. However, when selectively attending to the emotional valence of images (ensured by having participants rate the severity of the emotion displayed on a Likert scale, e.g., “How fearful is this face?”), MDD and anxious participants both showed greater left amygdala activity than controls. Lastly, Tao et al. (2012) reported that, even after controlling for concurrent anxiety, adolescents with MDD, prior to antidepressant use, had greater brain activation than controls in amygdala, orbitofrontal cortex, and subgenual ACC when passively viewing fearful faces. These three studies suggest that brain activation in fronto-limbic regions, particularly in the amygdala, differentiates depressed from healthy adolescents, although response may vary according to the design of the task and presence of diagnostic comorbidity.
Similar to findings in depression, studies have shown increased amygdala activity to fearful faces in anxiety (Killgore et al. 2005, Thomas et al. 2001a). While anxiety disorders commonly co-occur with depression (Kessler et al. 2003), as noted above, only two studies have examined the influence of co-morbid anxiety on frontolimbic brain function in adolescents with MDD (Beesdo et al. 2009, Tao et al. 2012). The present study aimed to build upon the limited literature by investigating brain function in an inclusive sample of adolescents diagnosed with MDD compared to healthy controls in response to an emotional faces task using fMRI methodology. It was hypothesized that, compared to healthy controls, participants with MDD would show increased amygdala activity in response to fearful facial expressions. With the use of a task similar to that employed by Tao et al. (2012), it was further hypothesized that heightened amygdala response would persist even when controlling for comorbid anxiety. Lastly, it was expected that depressed adolescents would also demonstrate increased activity in secondary limbic structures (e.g., insula, subgenual cingulate cortex) and decreased activity in neocortical regions compared to controls.
Method
Participants
Fifty-five depressed and healthy adolescents (12-19years old) participated in this study. Thirty-two adolescents met criteria for MDD and were recruited through inpatient and outpatient clinics at the University of Minnesota and from community advertisements. Twenty-three healthy controls (HC) were recruited from flyers posted throughout the Twin Cities. This study was approved by the University of Minnesota Institutional Review Board.
Controls had to be free from lifetime psychiatric disorders and could not have a first degree relative with a history of depression. Depressed adolescents not currently on psychotropic medications were included if they met current DSM-IV-TR criteria for a primary diagnosis of MDD. Depressed individuals with a history of bipolar disorder, schizophrenia, or pervasive developmental disorder were excluded. Additional exclusion criteria for all participants included history of serious head injury, medical illness, medical instability, MRI contraindication or full scale IQ less than 80. Six participants out of the full sample (2 HC, 4 MDD) were not able to complete IQ measures but were included in final analyses as there was no reason to suspect the presence of cognitive impairment based on the phone screen or in-person parent/child diagnostic interviews (e.g., parents reported high academic achievement, no evidence of slow development).
Interested participants underwent initial phone screening to assess basic inclusion/exclusion criteria. Those eligible attended a screening visit to obtain informed assent/consent and complete diagnostic (Schedule for Affective Disorders and Schizophrenia for School-Age Children, present and lifetime version, K-SADS-PL; Kaufman et al. 1997), Children's Depression Rating Scale, Revised; CDRS-R (Poznanski, Mokros 1996) and self-reported depression severity (Beck Depression Inventory-II, BDI-II; Beck, Steer & Brown 1996). Trained psychiatrists, clinical psychologists and advanced graduate students conducted separate K-SADS-PL and CDRS-R interviews with the adolescent and one parent. Diagnoses were based on a consensus between the two reports. Case conferences were regularly held and assessments reviewed by the supervising psychologist (BKD) and psychiatrist (KRC) if evaluators had questions about how to achieve consensus between parent and child reports. Enrolled participants completed a subsequent visit consisting of the MRI scan during which they completed an emotional faces task where they were instructed to hold a response pad in their right hand and press a key with their pointer finger whenever the letter ‘o’ appeared on the screen. This was done to ensure that participants remained attentive throughout the task. During scanning acquisition, a small light would illuminate whenever the response pad button was pressed, which allowed investigators to ensure that participants were complying with task instructions. Participants received compensation for completing study procedures.
Behavioral Paradigm (Emotional Faces Task)
All participants completed two 5.2 minute runs of the emotional faces task where standardized grayscale images of adult fearful or happy expressions were presented in a block design format and contrasted with fixation blocks (Ekman, Friesen 1976). Images were projected onto a screen at the back of the scanner and viewed through a mirror attached to a standard head coil. Each run was identical and consisted of 13 24-second blocks (5 fixation (C), 4 happy (H), 4 fearful (F) presented in a counterbalanced fashion: CFHCHFCHFCFHC). During experimental blocks, fearful or happy images were presented for 200 msecs and followed by 1300 msecs of either a fixation cross or an “o”. During control blocks, fixation crosses were presented sequentially for 1500 msecs. While facial expressions presenting numerous negative emotions have been shown to activate the amygdala (Fitzgerald et al. 2006), fearful stimuli were chosen based on research suggesting that amygdala response is more sensitive to viewing fearful faces than sad faces (Fusar-Poli et al. 2009). Similarly, happy faces were chosen as a comparison condition over neutral faces in order to maximize contrast in brain response given research suggesting that youth show exaggerated amygdala response to neutral faces over fearful faces (Thomas et al. 2001b).
Image acquisition
Scanning was conducted using a research-dedicated 3 Tesla Siemens Trio scanner and 12-channel radio-frequency (RF) head coil. A high-resolution T1-weighted anatomical image was acquired for each participant using a magnetization prepared gradient echo sequence (MPRAGE; 224 coronal slices; TR = 2530 msecs; TE = 3.65 msecs; TI = 1100 msecs; flip angle = 7o; FOV=256 mm, voxel size = 1×1×1mm; matrix size=256×256). Functional data were acquired using an echo planar imaging sequence (EPI) where 156 T2*-weighted whole brain functional volumes (34 oblique axial slices aligned with an AC-PC -30°C tilt, interleaved acquisition with a 1 mm skip, TR = 2000 msecs; TE = 28 msecs; flip angle = 80°C, FOV = 200 mm; voxel size = 3.1 × 3.1 × 3.0 mm; matrix = 64x64) were obtained in conjunction with the emotional faces task.
Functional Data Preprocessing and Initial Analysis
Imaging data were processed and analyzed using the FEAT package in FSL 4.1.9 (www.fmrib.ox.ac.uk). Functional data were visually inspected for movement-related artifacts. Volumes with relative displacement from the middle volume in excess of 1.5 mm in any direction were removed. Single volumes preceding and following excess displacement were also excluded. In total, 170 total volumes (1.0%) were excluded due to excessive movement. Images were preprocessed with high-pass temporal filtration (Gaussian-weighted least squares straight line fitting, sigma = 75s), motion and slice timing correction. EPI images were co-registered with each individual's anatomical image, normalized to Montreal Neurological Institute (MNI) standard space, and spatially smoothed (FWHM 6mm). A first-level analysis regressed the model specified by the task onto the blood oxygen level dependent (BOLD) response of each participant. The model yielded four separate contrasts that were then examined in later stages of analysis: fear > fixation, happy > fixation, happy > fear, and fear > happy. The two independent runs of the emotional faces tasks were combined at a second level of analysis using a fixed effects model that allowed for within-subject, multi-session analyses. A third level comparison examining between group differences was then conducted (described below).
Statistical Analysis
Demographic and clinical analyses were conducted using a series of Independent Samples T-tests and Chi-squared tests in SPSS (Version 19.0). Fisher's Exact Test was used to account for low frequency categorical data. Both whole brain and region of interest analyses (ROI) were conducted to address study questions. The primary contrast of interest for the fMRI results was the fear>happy condition, where neural responses to fear were examined and responses to happy stimuli were subtracted to remove effects of processing a face in general. Percent signal change for each ROI (bilateral amygdala (AMG) as defined by the Harvard/Oxford atlas, and bilateral subgenual anterior cingulate cortex (sgACC), rostral anterior cingulate cortex (rACC), and insula (INSL) as defined by Talairach Daemon Labels) was extracted for each participant using Featquery. Independent T-tests were employed to examine differences in BOLD activity between the groups in each ROI. Where group differences were noted, follow-up analyses (ANCOVA) were conducted to rule out possible confounds (i.e., IQ) and to explore the contributions of comorbid conditions (i.e., anxiety disorders). Whole-brain, between group level analyses were conducted using a mixed-effects model and cluster-wise significance testing. Multiple comparison corrections were carried out in this study using Gaussian random field theory (min Z = 1.96; cluster significance = p < 0.05, corrected) with a threshold that has been previously employed in other fMRI research (Filbey et al. 2013, Foland-Ross et al. 2012, Gold et al. 2014) and is more stringent than the uncorrected whole-brain findings reported in published studies of child and adolescent MDD (Tao et al. 2012, Gaffrey et al. 2011). Finally, correlations were conducted to explore possible associations between indices of clinical severity and BOLD response for the MDD participants.
Results
Demographic and Clinical Characteristics
Demographic and clinical information is presented in Tables 1 and 2. Participants were predominately female (74%), Caucasian (70%) and right handed (83%). Fifty-six percent of MDD adolescents had a current co-morbid psychiatric disorder; 18% met criteria for more than one co-morbid disorder. Of those with psychiatric comorbidity, Generalized Anxiety Disorder was endorsed most frequently (n = 9) followed by Social Anxiety Disorder (n = 3). There were no differences in age, gender, race, or handedness between HC and depressed participants. As expected, groups differed significantly in regards to depression severity (BDI-II). Depressed adolescents reported average BDI scores reflective of moderate depression (Beck et al. 1996), which were significantly higher than in HC. Significant group differences were observed in IQ, where MDD participants had lower IQ scores than HC participants. The CDRS was only completed for the MDD participants, and the mean scores exceeded the cutoff for a depression diagnosis (Poznanski, Mokros 1996).
Table 1.
Demographic and clinical data.
| HC (n = 23) | Unmedicated MDD (n=32) | |||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | t | ||
| Age (years) | 16.03 | 2.00 | 15.54 | 1.82 | 0.93 | |
| IQ†a | 111.81 | 11.31 | 102.89 | 16.34 | 2.14* | |
| BDI†b | 2.48 | 3.05 | 28.26 | 12.00 | −10.03** | |
| CDRS-R† | -- | -- | 61.88 | 6.79 | N/A | |
| N | % | N | % | X 2 | ||
| Gender | Male | 7 | 30.4 | 7 | 21.9 | 0.51 |
| Female | 16 | 69.6 | 25 | 78.1 | ||
| Handedness† | Right | 20 | 90.9 | 26 | 92.8 | 0.06 |
| Left | 2 | 0.10 | 2 | 7.14 | ||
| Race | Caucasian | 15 | 65.2 | 24 | 75.0 | 2.84 |
| African American | -- | -- | 2 | 6.3 | ||
| Asian | 2 | 8.7 | 1 | 3.1 | ||
| Biracial | 6 | 26.1 | 5 | 15.6 | ||
HC=Healthy Controls, MDD=Major Depression, BDI=Beck Depression Inventory-II, CDRS-R =Children's Depression Rating Scale, Revised
HC significantly higher than Unmedicated MDD
Unmedicated MDD significantly higher than HC
Based on a subsample of participants: IQ (N = 49), BDI (N = 54), CDRS (N = 26), Handedness (N = 50)
p < 0.05
p < 0.001
Table 2.
Illness Severity and Comorbidity in Depressed Sample (n=32)
| M | SD | |
|---|---|---|
| Age of Onset† (years) | 12.03 | 2.08 |
| Duration of Illness† (months) | 11.16 | 12.44 |
| Comorbid Diagnosesa | N | % |
| Generalized Anxiety Disorder | 9 | 28.1 |
| Social Anxiety Disorder | 5 | 15.6 |
| Posttraumatic Stress Disorder | 1 | 3.12 |
| Obsessive Compulsive Disorder | 1 | 3.12 |
| Panic Disorder | 1 | 3.12 |
| Dysthymia | 1 | 3.12 |
| Attention Deficit Hyperactivity Disorder | 3 | 9.37 |
| Oppositional Defiant Disorder | 2 | 6.25 |
Data unavailable for 1 participant, based on n=31
Number and percentage of depressed adolescents reporting ≥ lconcurrent diagnoses
Imaging Results
ROI Analyses
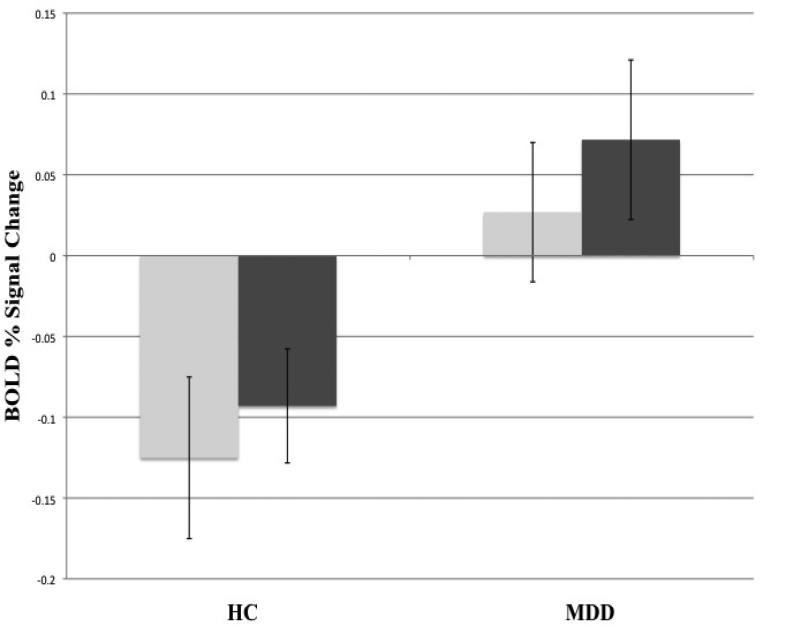
ROI results are presented in Figure 1. Significant group differences in BOLD percent signal change were observed in the right AMG (t(53)= -2.51, p < 0.05) and left AMG (t(53)= -2.49, p < 0.05) in response to the fear>happy contrast, where MDD participants had greater bilateral amygdala activity compared to HC. These findings remained significant when accounting for comorbid anxiety as well as differences in IQ. No differences were observed between groups for the remaining ROIs.
Figure 1.
Between group differences in mean BOLD % signal change in the left (light gray) and right (dark gray) amygdala (bars represent standard error) to the fear > happy contrast; left AMG (t(53)= -2.49, p < 0.05), right AMG (t(53)= -2.51, p < 0.05). Findings remained significant when controlling for age, anxiety status and IQ differences.
Whole Brain Between-Group Analyses
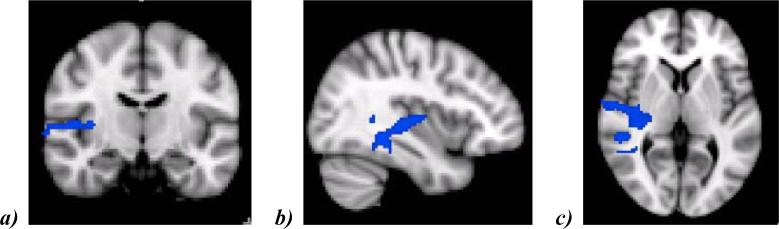
Group comparisons for the fear>happy contrast indicated that MDD participants had lower activation than HC in one right hemisphere cluster covering areas of the superior/middle temporal gyrus, Heschl's gyrus, and insular cortex (z = 1.96, p < 0.05). These findings are illustrated in Figure 2.
Figure 2.
Areas in right hemisphere (depicted via a (a) coronal slice, (b) sagittal slice, and (c) axial slice) that show decreased activation in unmedicated MDD participants compared to healthy controls in response to the fearful > happy contrast. Cluster (1236 voxels, 2x2x2mm) contains regions of the right superior temporal gyrus (MNI coordinates: × = 44, y = -28, z = 0), right middle temporal gyrus (x = 66, y = -30, z = -8), right Heschl's gyrus (x = 48, y = -14, z = 6), and right insula (x = 36, y = -16, z = 6). Voxels exceeded initial threshold, z = 1.96, and survived multiple comparison correction, p < 0.05.
BOLD Response and Clinical Variables
A series of correlation analyses examined how depression severity in the MDD group (assessed by the CDRS-R and BDI-II total score) related to BOLD response within regions of interest and within the significant right hemisphere cluster identified from whole-brain analyses for the fear>happy contrast. No correlations were observed between specified brain regions and interviewer-rated CDRS-R scores. No correlations were observed between depression severity and either the right or left AMG; however, activity in the left sgACC (r(28) = -0.39, p < 0.05) and right sgACC (r(28) = -0.37, p < 0.05) among depressed adolescents inversely correlated with BDI-II total scores. BDI-II total scores also correlated with activity in the right hemisphere cluster (r(28)=0.41, p<0.05).
Discussion
Literature in adults suggests that emotional processing abnormalities in depression are related to alterations in fronto-limbic neurocircuitry. However, relatively few studies have examined this network in response to emotional face processing tasks in naturalistic samples of depressed adolescents. This study aimed to expand previous findings by comparing differences in brain function while viewing fearful and happy faces among a broad sample of clinically depressed and healthy adolescents using fMRI methods. Our findings indicate that unmedicated depressed adolescents have greater functional activity in bilateral amygdala when viewing fearful faces compared to healthy control adolescents, which is unrelated to concurrent psychopathology. Additionally, whole brain analyses showed that unmedicated depressed adolescents have reduced activation in areas of the insular cortex, superior/middle temporal gyrus, and Heschl's gyrus when viewing fearful faces. Finally, evidence suggests that brain activation patterns, specifically in the subgenual ACC, among depressed adolescents correspond to severity of depressive symptoms.
Findings from this study appear consistent with reports that implicate abnormal amygdala hyper-activity in response to negative emotional faces in unmedicated adolescents with MDD (Yang et al. 2010, Beesdo et al. 2009, Tao et al. 2012). Similar to studies among depressed teens, children and adolescents at risk for MDD (i.e. those with depressed parents) have shown greater activation in the amygdala when passively viewing fearful faces compared to low-risk youth (Monk et al. 2008). Unmedicated depressed children (4-6 years of age) have demonstrated heightened right amygdala activity when viewing numerous facial expressions (Gaffrey et al. 2011), and depressed adults have shown left amygdala hyperarousal in response to masked, unconscious displays of fear (Sheline et al. 2001). In addition, amygdala hyperactivity has been shown to normalize following antidepressant treatment (Sheline et al. 2001, Tao et al. 2012), suggesting that altered amygdala response might be a biomarker for depression across development and a potential target for early intervention and MDD treatment.
Although anxiety has also been associated with increased amygdalar and limbic activity in response to negative emotional faces (Wolfensberger et al. 2008), when controlling for the presence of comorbid anxiety, bilateral amygdala hyperactivity in the unmedicated MDD group remained significant. This suggests that, like previous studies (Yang et al. 2010, Beesdo et al. 2009) activity in this area in response to fearful stimuli is not just a consequence of concomitant anxiety. In a gene-brain association study, adolescents diagnosed with anxiety and depression who were homozygons (LA/ LA) for the serotonin transporter gene had increased brain activity in response to fearful faces compared to both healthy controls and patients with either the S/LG allele, where there were no differences in brain response between the two diagnoses (Lau et al. 2009). It is possible that findings of hyper-amygdala activity in this study are not specific to a categorical diagnosis but reflect an underlying genetic vulnerability to internalizing illness that cuts across diagnoses. Future research investigating neurobiological differences, not between discrete diagnoses, but dimensionally (e.g., among individuals with varying levels of depressed mood regardless of psychiatric diagnosis), is needed in order to better understand relationships between brain function and behavior.
While amygdala findings did not persist during whole brain analyses, other findings emerged indicating that depressed adolescents have reduced brain activation in response to fearful faces in the right hemisphere including areas of the insula. Insular and amygdalar regions are thought to process emotional salience and threat, respectively. With resting state neuroimaging techniques, Seeley et al. (2007) have described a salience neural network with primary nodes in insular brain regions in addition to orbitalfrontal and dorsal anterior cingulate areas that possess strong functional connections with subcortical and limbic structures. This network is thought to be involved in automatically processing and regulating interoceptive information where it responds to the emotional significance of painful or pleasurable stimuli (Craig 2002). Additionally, the amygdala, which has interconnections with insular, cingulate and frontal regions plays an integral role in detecting and responding to conscious and unconscious fearful stimuli (Stein et al. 2007). While research in adults has suggested that hyperactivity in insular regions in response to fearful faces is associated with depressive symptomatology (Zhong et al. 2011), this does not appear to be the case for the present sample. Given the pattern of observed findings, it is possible that alterations in insular activity among depressed adolescents compromise communication within this salience network which, when combined with increased neural activity to threatening cues, disrupts the processing of emotional significance and biases younger individuals with depression towards interpreting stimuli as more negatively valenced. More research is needed to determine how amygdala and insular regions interact in MDD and whether abnormalities in the salience network contribute to emotional processing biases observed in depressed adolescents.
BOLD signal within the right and left subgenual ACC was inversely correlated with self-reported depression symptoms. Previous studies have identified alterations among depressed individuals within the subgenual ACC (Drevets, Price & Furey 2008, Gotlib et al. 2008). Most suggest that depression is associated with hyperactivity in this area (Gotlib et al. 2005, Yang et al. 2009) that normalizes with successful treatment (Drevets, Price & Furey 2008, Siegle, Carter & Thase 2006, Holthoff et al. 2004, Mayberg et al. 2000, Keedwell et al. 2009). It is unclear why greater depressive severity was linked to lower activation in the sgACC in this sample; however, Connolly et al. (2013) have demonstrated alterations in functional connectivity between the sgACC, insula and amygdala in medication naive, depressed adolescents, where disruptions in the system more generally may contribute to disturbed salience attribution in depressed youth.
In contrast to previous findings that have implicated more distributed networks (e.g., subgenual and rostral anterior cingulate cortices) in MDD compared to HC on emotional face paradigms (Yang et al. 2010, Tao et al. 2012), no differences in cortical or other paralimbic regions were observed between groups in the present study. It is possible that heterogeneity in reported findings is at least partially accounted for by variation in task presentation. Whereas the present study compared happy and fearful faces viewed in conjunction with a neutral target-response task, other studies in adolescent depression have examined response to other emotions and required different levels of directed attention (Yang et al. 2010, Beesdo et al. 2009, Tao et al. 2012). More research is needed to clarify how subtle variations in emotional stimuli impact brain functioning in adolescent MDD.
The results of this study provide important information about emotional face processing in adolescent MDD; however, there are a number of limitations that should be noted. While relatively sizeable in comparison to several previous fMRI studies in adolescent depression (Yang et al. 2010, Tao et al. 2012), the present sample was still relatively modest. Additionally, this study only examined brain function in response to happy and fearful faces. While happy faces were chosen as a comparison to fearful faces in order to identify neural responses to maximally discrepant emotions, happy faces have also been shown to activate the amygdala (Killgore et al. 2004), which might have impacted the differences in signal observed. Research using other emotions is necessary to further understand the neural correlates of emotion processing in this population. In addition, this study did not ask participants to provide subjective ratings of the emotional value of the stimuli, so we are unable to comment on whether fearful faces were experienced as such. While this study examined neural responses between a discrete, categorical group of individuals with MDD, it may be that approaches examining how brain response relates to narrower domains relevant to depression (e.g., rumination, negative emotion bias, vegetative symptoms, etc.) will be more likely to yield reliable and valid neurobiological markers of psychiatric illness. Future research, examining emotion processing deficits longitudinally, including studies of brain function before and after successful treatment, will further our understanding of state and trait dependant aspects of adolescent MDD in addition to other internalizing disorders. Such information has the potential to advance our understanding of the neurobiological underpinnings of abnormal emotion processing during this critically important developmental period.
In conclusion, findings from this study add to the literature on emotional face processing, replicating findings of amygdala hyperactivity in unmedicated depressed adolescents. In addition, findings provide new evidence for a deficit in functioning in the salience network in response to fearful facial stimuli among adolescents with MDD. While Mayberg (1997) has proposed that depressive symptoms in adult MDD are related to increased limbic and decreased cortical activity, it is possible that adolescent MDD is characterized more by a hyper-limbic activation and corresponding difficulties relaying information about the salience of stimuli to limbic and cortical areas. Directional interactions between activity in insular and amygdala regions during the processing of emotional faces should be studied in future research, perhaps with psychophysiological interaction analyses. This research may have important implications for prevention science. For example, this type of research may ultimately help clinicians predict how functional differences predict treatment response or non-response.
Acknowledgements
This work was supported in part by the University of Minnesota Supercomputing Institute.
Funding Sources: NIMH K23 MH090421, NARSAD, NIH P41 RR008079, Minnesota Medical Foundation, University of Minnesota Office of the Vice President of Research Grant-in-Aid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: All authors declare that they have no competing interests.
Contributors
Leah M.J. Hall conducted all data analyses (in conjunction with Drs. Ruskin Hunt, Kathleen Thomas, and Bryon Mueller). Drs. Bonnie Klimes-Dougan, Katie Cullen, Kelvin Lim contributed to this work by overseeing the study design and implementation. Drs. Bonnie Klimes-Dougan and Katie Cullen as well as Alaa Houri and Emily Noack were instrumental in regards to study recruitment, subject screening and data collection. All authors assisted in drafting the manuscript and have approved the final submission.
References
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX.: 1996. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch. Gen. Psychiat. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, Kolko DJ, Birmaher B, Baugher M, Bridge J, Roth C, Holder D. Predictors of treatment efficacy in a clinical trial of three psychosocial treatments for adolescent depression. J. Am. Acad. Child. Psy. 1998;37:906–914. doi: 10.1097/00004583-199809000-00010. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, Yang TT. Resting-State Functional Connectivity of Subgenual Anterior Cingulate Cortex in Depressed Adolescents. Biol. Psychiat. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, Lim KO, Castellanos FX, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain. Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of facial affect. Consulting Psychologist Press; Palo Alto, CA.: 1976. [Google Scholar]
- Filbey FM, Dunlop J, Myers US, Filbey FM, Dunlop J, Myers US. Neural effects of positive and negative incentives during marijuana withdrawal. PloS one. 2013;8:e61470. doi: 10.1371/journal.pone.0061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J, Torrisi S, Penfold C, Madsen SK, Thompson PM, Altshuler LL. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. NeuroImage. 2012;59:738–744. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Front. Psychol. 2012;3:1–16. doi: 10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatr. Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M, Luby J, Belden A, Hirshberg J, Volsch J, Barch D. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. J. Affect. Disord. 2011;129:364–370. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Morey RA, McCarthy G, Gold AL, Morey RA, McCarthy G. In press. Amygdala - prefrontal cortex functional connectivity during threat- induced anxiety and goal- distraction. Biol. Psychiat. doi: 10.1016/j.biopsych.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP. Neuroimaging and depression: Current status and unresolved issues. Curr. Dir. Psychol. Sci. 2008;17:159–163. [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli J, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Holthoff VA, Beuthien-Baumann B, Zundorf G, Triemer A, Ludecke S, Winiecki P, Koch R, Fuchtner F, Herholz K. Changes in brain metabolism associated with remission in unipolar major depression. Acta. Psychiat. Scand. 2004;110:184–194. doi: 10.1111/j.1600-0447.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child. Psy. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J. Affect. Disord. 2010;20:120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J. Psychopharmacol. 2009;23:775–788. doi: 10.1177/0269881108093589. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol. Psychiat. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-todd DA, Killgore WDS, Yurgelun-Todd D. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA, Killgore WDS, Yurgelun-Todd D. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. NeuroImage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, Monk CS, Nelson EE, Shen P, Pine DS, Ernst M, Lau JYF, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, Monk CS, Nelson EE, Shen P, Pine DS, Ernst M. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol. Psychiat. 2009;65:349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. R. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psycho. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J. Neuropsych. Clin. N. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol. Psychiat. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiat. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Nes RB, Czajkowski NO, Roysamb E, Orstavik RE, Tambs K, Reichborn-Kjennerud T. Major depression and life satisfaction: A population-based twin study. J. Affect. Disord. 2013;144:51–58. doi: 10.1016/j.jad.2012.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Mokros H. Children's Depression Rating Scale–Revised (CDRS-R) WPS; Los Angeles, CA.: 1996. [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. 2012. Trends. Cogn. Sci. 16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol. Psychiat. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am. J. Psychiat. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Simon RI, Hales RE. Suicide Risk: Assessing the Unpredictable. American Psychiatric Publishing, Inc; Arlington, VA.: 2006. [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Steingard RJ, Renshaw PF, Hennen J, Lenox M, Cintron CB, Young AD, Connor DF, Au TH, Yurgelun-Todd DA. Smaller frontal lobe white matter volumes in depressed adolescents. Biol. Psychiat. 2002;52:413–417. doi: 10.1016/s0006-3223(02)01393-8. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol. Mood. Anxiety. Disord. 2011;1:1–17. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, El-Hage W, Dalgleish T, Radua J, Gohier B, Phillips ML. Depression is associated with increased sensitivity to signals of disgust: a functional magnetic resonance imaging study. J. Psychiat. Res. 2010;44:894–902. doi: 10.1016/j.jpsychires.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, Kennard BD, Tamminga CA, Emslie GJ. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am. J. Psychiat. 2012;169:381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch. Gen. Psychiat. 2001a;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol. Psychiat. 2001b;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch. Gen. Psychiat. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-grethe A, Lansing AE, Wu J, Brown GG, Paulus MP, Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, Lansing AE, Wu J, Brown GG, Paulus MP. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009;20:440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, Bischoff-Grethe A, Lansing AE, Brown G, Strigo IA, Wu J, Paulus MP. Adolescents with major depression demonstrate increased amygdala activation. J. Am. Acad. Child. Psy. 2010;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Wang W, Yao S. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol. Psychol. 2011;88:233–242. doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, Gilmer WS, Dresselhaus TR, Thase ME, Nierenberg AA, Trivedi MH, Rush AJ. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiat. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]