Abstract
AIM: To characterize and evaluate DNA alterations among intrahepatic cholangiocarcinoma (ICC) patients.
METHODS: DNA from tumor and corresponding normal tissues of 52 patients was amplified with 33 arbitrary primers. The DNA fragment that alters most frequently in ICC was cloned, sequenced, and identified by comparison with known nucleotide sequences in the genome database (www.ncbi.nlm.nih.gov). The DNA copy numbers of the allelic alterations in cholangiocarcinoma were determined by quantitative real-time PCR and interpreted as allelic loss or DNA amplification by comparison with the reference gene. Associations between allelic imbalance and clinicopathological parameters of ICC patients were evaluated by χ2-test. The Kaplan-Meier method was used to analyze survival rates.
RESULTS: From 33 primers, an altered DNA fragment (518 bp) amplified from BC17 random primer was found frequently in the tumors analyzed and mapped to chromosome 17p13.2. Sixteen of 52 (31%) cases showed DNA amplification, while 7 (13%) showed allelic loss. Interestingly, DNA amplification on chromosome 17p13.2 was associated with a good prognosis, median survival time (wk) of amp vs no amp was 44.14 vs 24.14, P = 0.002; whereas allelic loss of this DNA sequence corresponded with a poor prognosis, median survival time (wk) of loss vs no loss was 18.00 vs 28.71, P = 0.019). Moreover, Kaplan-Meier curves comparing the DNA alterations with survival depicted highly significant separation that the median survival time equal to DNA amplification, allelic loss, and normal was 44.14 wk, 18.00 wk, and 24.29 wk, respectively (P = 0.005).
CONCLUSION: Alterations in the DNA sequence on chromosome 17p13.2 may be involved in cholangio-carcinogenesis, and could be used as a prognostic marker in the treatment of ICC patients.
Keywords: Intrahepatic cholangiocarcinoma, 17p13.2, Allelic imbalance, Prognosis
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is predominantly found in Thai patients with bile duct cancer[1,2] and its incidence is much higher in the northeastern region of Thailand, where it is closely associated with liver-fluke infestation[3-7] and the consumption of carcinogen-contaminated daily foods[8]. Data from the World Health Organization (WHO) database, and national registries worldwide, show an increase in ICC-related mortality[9,10].
Recent investigations into the underlying molecular mechanisms involved in cholangiocarcinogenesis and tumor growth have contributed greatly to understanding the disease. Significant progress has been made over the past decade in defining molecular alterations associated with cholangiocarcinoma (CCA). Alterations in p53[11-13] and p16INK4α[12] are frequently detected in CCA and likely contribute to oncogenesis in the biliary tract. Other alterations that seem to occur early in cholangiocarcinogenesis include over-expression of the receptor tyrosine kinases (RTKs), ErbB-2, and Met[14] as well as the up-regulation of COX-2[15]. Likewise, WISP1v expression has been found to be associated with lymphatic and perineural spread of CCA and poor clinical outcome[16]. Human telomerase reverse transcriptase (hTERT) has also been detected in a high percentage of analyzed cases of ICC, irrespective of tumor grade and subtype, as well as heterogeneously in dysplastic lesions, suggesting that acquired hTERT activity may reflect an early stage leading to CCA development[17]. However, the clinical value of established survival predictors seems limited, since they fail to reliably predict post-resection survival.
With regards to genome scanning method, arbitrarily primed polymerase chain reaction (AP-PCR)[18], also called random amplified polymorphic DNA (RAPD)[19], has enormously wide application, since it can be used to study virtually any nucleic acid entity, whether previously characterized or not. The semi-quantitative native of AP-PCR has proven a promising technique for identifying novel gene alterations in many human cancers[20].
In this study, we evaluated the DNA alteration as a prognostic factor for survival of ICC patients post-resection. The localization of an altered DNA fragment on chromosome 17p13.2, which significantly predicted overall survival of ICC patients is reported here for the first time. Furthermore, the finding of aberrations in this DNA region, correlating with clinicopathological parameters of patients with ICC, was also demonstrated.
MATERIALS AND METHODS
Subjects and tissue samples
Samples from 52 cases with primary ICC (42 males and 10 females) and corresponding normal tissues were obtained from Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand, during surgical resection. The median age at diagnosis was 56 (range 26-75) years. This work was approved by the Ethics Committee of the Faculty of Medicine, Khon Kaen University, Thailand (HE471214). Samples were frozen after resection and stored at -80°C until DNA extraction. None of the patients received radiation or chemotherapy before surgery. All patients were residents of northeast Thailand, where liver-fluke infections are highly endemic. Hematoxylin-eosin stained sample sections from each tumor block were examined microscopically to confirm the presence of > 80% cancer cells. Paired normal tissues from the same patient were used as controls and showed normal histological features.
DNA preparation
Genomic DNA was isolated from fresh cancerous and normal counterpart tissues by proteinase K digestion and salting-out method[21], with some modifications. The cancerous and normal tissues were embedded in optimum cutting temperature (OCT) and cryostat tissue sections were performed (10 μm). The tissue sections were washed out 3 times from OCT with normal saline, then incubated in lysis buffer (10 mmol/L Tris-HCl, pH 8.0, 400 mmol/L NaCl, 2 mmol/L EDTA), 200 μL of 10% sodium dodecyl sulfate (SDS) and 10 mg/mL proteinase K at 60°C for 3 h. The solutions were mixed with 6 mmol/L NaCl, shaken and centrifuged at 10 000 × g for 10 min at 4°C. DNA was precipitated by absolute ethanol and washed 3 times with 70% ethanol. The DNA pellet obtained was dissolved in TE buffer. DNA concentration was determined by spectrophotometry at A260 nm. The paired DNA was equalized by comparison with PCR product amplified from exon 1 of β-globin, a housekeeping gene (110 bp).
AP-PCR analysis
Thirty-three arbitrary decamer primers: AA14, AB19, AD10, AO5, AO10, AO16, AO19, AP19, AT11, AT17, AU1, AY19, AZ2, BB3, BB13, BC17, BF12, BG4, F2, G14, H8, J3, L1, M7, M19, N20, O15, Q7, S3, S13, U8, Y7, and Y19 (Operon, USA) were used in screening the ICC genome for alterations. DNA isolated from ICC and corresponding normal tissues of the same patients was used as template. AP-PCR was performed in a thermal cycler (GeneAmp 9700, Perkin-Elmer, USA) for 45 cycles based on the method described by Williams et al[18]. The total volume of 25 μL contained 100 ng of genomic DNA extracted from carcinoma or corresponding normal tissues, 1× PCR buffer, 200 μmol/L each of dATP, dCTP, dGTP, dTTP, 2.5 mmol/L MgCl2, 20 μmol/L of each random ten-mer primer and 1 unit of Taq DNA polymerase (Pharmacia Biotech, USA). Each cycle consisted of denaturation at 95°C for 1 min, annealing at 36°C for 1 min, and extension at 72°C for 2 min. Generally, we adjusted the working DNA concentration at 20 ng/μL. The AP-PCR products were resolved by electrophoresis on 1.4% agarose gels. The gels were stained with ethidium bromide for photography under UV light. By direct visualization, the amplified DNA fragments exhibited presence/absence, or increase/decrease, in ICC sample intensity when compared case-by-case with the rest of the bands. The DNA content in tumor and normal tissues was normalized using β-globin gene amplification with primer sequences 5'ACACAACTGTGTTCACTAGCA 3' (forward primer) and 5'GGTGAACGTGGATGAAGTTG 3' (reverse primer).
Cloning and sequencing of aberrant DNA fragment
The altered band (518 bp) employing the primer BC17 was excised from the 0.8% agarose gels and purified with DNA purifying kit (Nucleospin, Machery-Nagel GmbH & Co. KG, Germany). The eluted DNA was confirmed for purity and quantity on 0.8% agarose gels. The 518 bp fragment was cloned using a TA cloning kit (Invitrogen, USA) following the manufacturer’s instructions. Plasmid DNA was isolated from each bacterial colony using a QIAGEN plasmid kit (QIAGEN, USA). Restriction analysis of the recombinant plasmid DNA was carried out by EcoRI digestion. The plasmid DNA containing DNA fragment insertion was further nucleotide-sequenced with either forward or reverse M13 primer as the sequencing primer (customized by Macrogen Inc., Korea).
Bioinformatic analysis
The nucleotide sequences obtained from each clone were identified by comparison with known nucleotide sequences in the human genome database (http://www.ncbi.nlm.nih.gov/blastn) via BLASTn program.
Real-time quantitative PCR
The 3 loci on chromosomes 2q24, 17p13.2, and 18p11 were identified from the altered band. Specific primers were designed using the GeneFisher program (http://www.genefisher.com), i.e. 5'TGGACAAACAGGCTCCA 3' (forward primer) and 5'CTGGCTTCTCGCGAGA 3' (reverse primer) for DNA alteration on chromosome 2q24, 5'CATGCCAACTGCATCCA 3' (forward primer) and 5'TCTCCAGTGGTTTCCCAA 3' (reverse primer) for detection of allelic imbalance on chromosome 17p13.2 and 5'GAGTTGGACCTTTCCAGA 3' (forward primer) and 5'TGCTTGCACAGATGTGA 3' (reverse primer) for determination of DNA alteration on chromosome 18p11. The DNA alterations on these chromosomal loci were detected by real-time PCR using the specific primers (BioService Unit, Thailand). The β-globin gene amplified from DNA extracted from cancerous and corresponding normal tissues was normalized as a reference gene. PCR was performed in a total volume of 20 μL in each LightCycler glass capillary, containing 40 ng genomic DNA, 3 mmol/L MgCl2, 5 μmol/L of each primer and 1 × LightCycler FastStart DNA Master SYBR Green I(Roche Diagnostic, Germany). The PCR condition consisted of an initial denaturation step at 95°C for 15 s, at 67°C-57°C (step delay 15 cycles, touchdown PCR) for 5 s, and at 72°C for 20 s. Thermal cycling and fluorescent monitoring were performed using a LightCycler (Roche Applied Science, Germany). The point at which the PCR product is first detected above the fixed threshold, termed the cycle threshold (Ct), was determined for each sample. The relative concentrations were determined employing Ct values, which are equivalent to the cycle number at which the PCR product is first detected above a fixed threshold. The Ct values obtained from the analyzer were then calculated for DNA copy number utilizing the delta-delta-Ct method[22]. Samples were run in duplicate. In this study, a sample with an amplification ≥ 1.5 fold was interpreted as having DNA amplification, otherwise ≤0.5 was interpreted as having allelic loss, and the rest was interpreted as no aberration[23].
Statistical analysis
Clinicopathological features of patients with ICC, including patient’s age at initial diagnosis, gender, histological type, tumor size, lymph node and/or intrahepatic metastasis, were correlated with the alterations in the distinct region. Results were evaluated by χ2-test. Survival analysis was carried out with patients who were followed up for at least 200 wk, or until death, after surgery. Three patients who died in the post-operative period were excluded and 4 cases were lost to follow-up. Thus, only 45 patients were available for the survival study. Overall survival distributions were calculated by Kaplan-Meier method and analyzed by log-rank test. P < 0.05 was considered statistically significant.
RESULTS
DNA alterations detected by AP-PCR and quantitated by real-time PCR
In this study, all genomic DNAs from 52 Thai patients with ICC were analyzed using 33 different arbitrary primers. Twenty-one of 33 decamer primers revealed genetic aberrations. The highest frequency of altered band (518 bp) was from a DNA fingerprint amplified from the BC17 primer (Figure 1). The discriminative band was cloned, sequenced, and identified by BLASTn program for locus identification. The results revealed 3 different sequences deposited in NCBI, including clone RP11-357L2 on chromosome 2q24 (AC009961.11) and clone RP11-931H21 on chromosome 17p13.2 (AP005900.1) as well as clone RP11-459C13 on chromosome 18p11 (AC067815.5). The changes in DNA copy number at the 3 definite regions were quantitated by real-time PCR with specific primers (Figure 2). Their reliability was confirmed by running amplicons at cycle 33, the mid log phase of fluorescence graph, on 0.8% agarose gel and stained with ethidium bromide (Figure 3). The results indicated that, of all 52 patients with ICC, aberrations in the DNA fragment on chromosome 2q24 were observed in 9 cases (18%), including 6 (12%) with DNA amplification and 3 (6%) with allelic loss; 23 cases (44%) had allelic imbalance on chromosome 17p13.2, of these, 16 (31%) showed DNA amplification and 7 (13%) allelic loss; 27 cases (52%) presented DNA alteration on chromosome 18p11, 25 (48%) had DNA amplification and 2 (4%) allelic loss.
Figure 1.
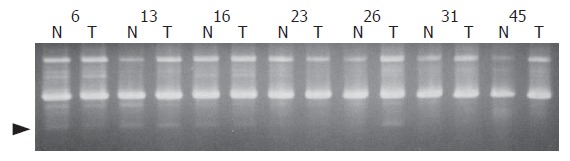
Representative AP-PCR fingerprints of ICC (T) and corresponding normal tissue DNA (N), with primer BC17. Arrowhead indicates the position of altered DNA fragments.
Figure 2.
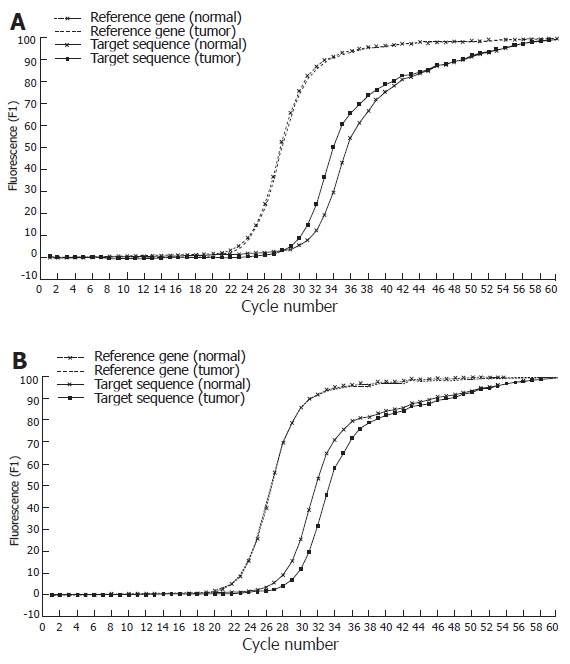
Real-time PCR SYBR Green I fluorescence record versus cycle number of target fragment (518bp) and reference gene (β-globin) in tumor DNA and corresponding normal DNA, in case of DNA amplification (A) and allelic loss (B).
Figure 3.
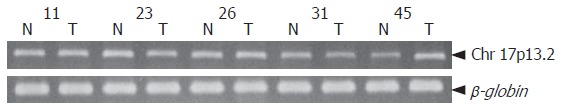
Changes in DNA copy number of ICC (T) and corresponding normal tissue DNA (N) compared with reference gene (β-globin). Case 11 showed no change. Cases 23 and 31 showed loss of DNA copy number, whereas cases 26 and 45 showed DNA amplification at chromosome 17p13.2.
Association between allelic imbalance and patient clinicopathological parameters
Statistical analysis showed that DNA amplification on 17p13.2 was associated with a good prognosis, median survival time (wk) of amp vs no amp was 44.14 vs 24.14 (P = 0.002); whereas allelic loss on this chromosomal region corresponded with a poor prognosis, median survival time (wk) of loss vs no loss was 18.00 vs 28.71 (P = 0.019). Moreover, Kaplan-Meier survival curves showed significant correlation with allelic aberrations on chromosome 17p13.2, the median survival time equal to DNA amplification, allelic loss, and normal was 44.14 wk, 18.00 wk, and 24.29 wk, respectively (P = 0.005 for all, by log-rank test) (Figure 4). However, the DNA alterations on chromosome 17p13.2 did not correlate with the clinicopathological data (Tables 1 and 2).
Figure 4.
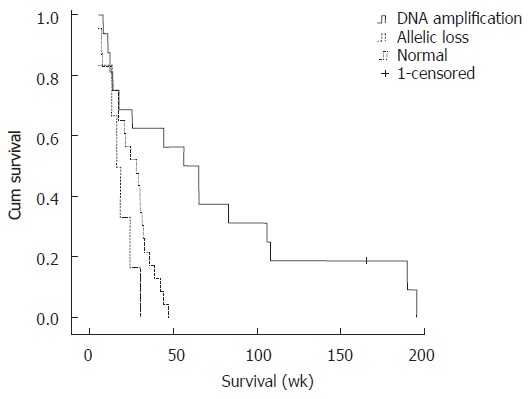
Kaplan-Meier estimated survival rates according to the altered DNA fragment on chromosome 17p13.2.
Table 1.
Clinocopathological parameters and DNA amplification of the target fragment (518 bp) on chromosome 17p13.2 in ICC patients
| Parameters |
DNA amplification |
P | OR (95% CI) | |
| + | - | |||
| n (%) | n (%) | |||
| Age (yr) | 0.771 | |||
| ≤ 50 | 6 (37.5) | 10 (62.5) | 1.00 (referent) | |
| > 50 | 12 (33.3) | 24 (66.7) | 0.83 (0.24-2.84) | |
| Gender | 0.69 | |||
| Male | 14 (33.3) | 28 (66.7) | 1.00 (referent) | |
| Female | 4 (40.0) | 6 (60.0) | 1.33 (0.32-5.51) | |
| Histological type | 0.61 | |||
| Well-differentiated | 7 (29.2) | 17 (70.8) | 1.00 (referent) | |
| Moderately differentiated | 7 (50.0) | 7 (50.0) | 2.43 (0.51-12.04) | |
| Poorly differentiated | 5 (45.5) | 6 (54.5) | 2.02 (0.37-11.45) | |
| Tumor size (cm) | 0.843 | |||
| ≤ 7 | 3 (20.0) | 12 (80.0) | 1.00 (referent) | |
| > 7 | 3 (23.1) | 10 (76.9) | 1.20 (0.20-7.31) | |
| Metastasis | 0.368 | |||
| Positive | 14 (40.0) | 21 (60.0) | 1.83 (0.41-8.58) | |
| Negative | 4 (26.7) | 11 (73.3) | 1.00 (referent) | |
| Lymph node metastasis | 0.197 | |||
| Positive | 8 (53.3) | 7 (46.7) | 2.29 (0.54-9.88) | |
| Negative | 10 (33.3) | 20 (66.7) | 1.00 (referent) | |
Table 2.
Clinocopathological parameters and allelic loss of the target fragment (518 bp) on chromosome 17p13.2 in ICC patients
| Parameters |
Allelic loss |
P | OR (95% CI) | |
| + | - | |||
| n (%) | n (%) | |||
| Age (yr) | 0.456 | |||
| ≤ 50 | 3 (18.8) | 13 (81.3) | 1.00 (referent) | |
| > 50 | 4 (11.1) | 32 (88.9) | 0.54 (0.11-2.76) | |
| Gender | 0.721 | |||
| Male | 6 (14.3) | 36 (85.7) | 1.00 (referent) | |
| Female | 1 (10.0) | 9 (90.0) | 0.67 (0.07-6.26) | |
| Histological type | 0.726 | |||
| Well-differentiated | 6 (25.0) | 18 (75.0) | 1.00 (referent) | |
| Moderately differentiated | 6 (42.85) | 8 (57.15) | 2.25 (0.45-11.62) | |
| Poorly differentiated | 3 (27.27) | 8 (72.73) | 1.13 (0.17-7.25) | |
| Tumor size (cm) | 0.63 | |||
| ≤ 7 | 2 (13.3) | 13 (86.7) | 1.00 (referent) | |
| > 7 | 1 (7.7) | 12 (92.3) | 0.54 (0.04-6.77) | |
| Metastasis | 0.447 | |||
| Positive | 5 (14.3) | 30 (85.7) | 2.33 (0.22-57.93) | |
| Negative | 1 (6.7) | 14 (93.3) | 1.00 (referent) | |
| Lymph node metastasis | 0.737 | |||
| Positive | 2 (13.3) | 13 (86.7) | 1.38 (0.14-12.27) | |
| Negative | 3 (10.0) | 27 (90.0) | 1.00 (referent) | |
DISCUSSION
The modified AP-PCR technique is a useful method for screening genetic alterations in various cancers, such as novel gene alteration on chromosome 11q23.2 in Wilms tumor[24], gene amplification on chromosome 10q24.3 in ovarian cancer[25], and DNA amplification on chromosomes 2p25.3 and 7q11.23 in CCA[26]. In addition, amplification of the DNA on chromosome 2p25.3 has been predominantly observed in poorly differentiated tumors of cholangiocarcinoma patients[26].
In this study, we used this AP-PCR technique to detect and characterize genomic instability in primary ICC. According to the delta-delta-Ct method[22], the DNA copy number could be detected by real-time PCR, calculated and compared with an internal control[27,28]. The result revealed allelic imbalance on chromosome 17p13.2 in 44% of ICC patients and was prognostic for these patients. BLAST analysis showed several candidate genes at this chromosomal region, such as cyb5d2 (cytochrome b5 domain containing 2), zzef1 (zinc finger, ZZ-type with EF-hand domain 1), atp2a3 (ATPase, Ca++ transporting, ubiquitous), p2rx1 (purinergic receptor P2X, ligand-gated ion channel, 1), and camkk1 (calcium/calmodulin-dependent protein kinase kinase 1, alpha). Of those, variants of camkk1 gene confer susceptibility to lung cancer[29].
In addition, this DNA fragment was located 3.5 Mbp from p53 (17p13.1), a generally altered tumor-suppressor gene that controls cell proliferation and survival through several coordinated pathways, and this gene mutation plays a key role in the development of several cancers, including CCA[30-32].
Deletion of the 17p13.2 locus has also been connected to breast cancer[33], brain malignancy[34] and gastric adenocarcinoma[35]. Likewise, the allelic imbalance on chromosome 17p13 presented in various malignancies, such as non-small-cell lung cancer (NSCLC)[36], natural killer (NK) cell lymphomas/leukemias[37], and sporadic breast cancer[38], indicating that the aberration imbalance on chromosome 17p13-13.2 might play an important role in cancer development.
In our series of 45 ICCs, Kaplan-Meier survival analysis (Figure 4) showed that the altered DNA sequence on chromosome 17p13.2 affected patient survival (P = 0.005), with DNA amplification patients having longer lives (44 wk) than those with allelic loss in the particular region (18 wk), showing that DNA alterations on chromosome 17p13.2 could serve as prognostic indicators for cholangiocarcinoma.
In keeping with the theory that pathological parameters are not prognostic for ICC patients, age, gender, tumor size, and metastasis all failed to correlate with outcome. Lymph node metastasis is the strongest factor for ICC after surgery[39-41]. Although previous reports suggested that some types of ICC might be less aggressive, even if lymph node metastasis is present[42-44], some ICC tumors might produce lymph node metastasis at an early stage, suggesting that surgery might control lymph node metastasis in selected ICC patients.
In conclusion, aberrations in DNA amplification on chromosome 17p13.2 provide an independent biological predictor of survival for ICC patients. Thus, assessment of this specific region could help identify patients who might benefit from particularly aggressive surgical strategies, and could also be useful for planning adjuvant therapies during follow-up.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Phaibul Punyarit and his staff at the Pathology Institute, Phramongkutklao Hospital, for their support with facilities for cryostat tissue section. The authors thank Mr. Paul Adams for his critical reading of this manuscript.
Footnotes
Supported by Rajabhat University Suan Dusit Fund; Faculty of Tropical Medicine, Mahidol University; DAAD scholarship through Southeast Asian Ministers of Education Organization, Regional Tropical Medicine and Public Health Network
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
References
- 1.Haswell-Elkins MR, Satarug S, Elkins DB. Opisthorchis viverrini infection in northeast Thailand and its relationship to cholangiocarcinoma. J Gastroenterol Hepatol. 1992;7:538–548. doi: 10.1111/j.1440-1746.1992.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Ohshima H, Srivatanakul P, Vatanasapt V. Cholangiocarcinoma: epidemiology, mechanisms of carcinogenesis and prevention. Cancer Epidemiol Biomarkers Prev. 1993;2:537–544. [PubMed] [Google Scholar]
- 3.Srivatanakul P, Ohshima H, Khlat M, Parkin M, Sukaryodhin S, Brouet I, Bartsch H. Opisthorchis viverrini infestation and endogenous nitrosamines as risk factors for cholangiocarcinoma in Thailand. Int J Cancer. 1991;48:821–825. doi: 10.1002/ijc.2910480606. [DOI] [PubMed] [Google Scholar]
- 4.Watanapa P. Cholangiocarcinoma in patients with opisthorchiasis. Br J Surg. 1996;83:1062–1064. doi: 10.1002/bjs.1800830809. [DOI] [PubMed] [Google Scholar]
- 5.Shimonishi T, Sasaki M, Nakanuma Y. Precancerous lesions of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:542–550. doi: 10.1007/s005340070002. [DOI] [PubMed] [Google Scholar]
- 6.Watanapa P, Watanapa WB. Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002;89:962–970. doi: 10.1046/j.1365-2168.2002.02143.x. [DOI] [PubMed] [Google Scholar]
- 7.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [Google Scholar]
- 9.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 10.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 11.Petmitr S, Pinlaor S, Thousungnoen A, Karalak A, Migasena P. K-ras oncogene and p53 gene mutations in cholangiocarcinoma from Thai patients. Southeast Asian J Trop Med Public Health. 1998;29:71–75. [PubMed] [Google Scholar]
- 12.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 13.Berthiaume EP, Wands J. The molecular pathogenesis of cholangiocarcinoma. Semin Liver Dis. 2004;24:127–137. doi: 10.1055/s-2004-828890. [DOI] [PubMed] [Google Scholar]
- 14.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 15.Nakanuma Y, Harada K, Ishikawa A, Zen Y, Sasaki M. Anatomic and molecular pathology of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:265–281. doi: 10.1007/s00534-002-0729-3. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Sugimachi K, Kameyama T, Maehara S, Shirabe K, Shimada M, Wands JR, Maehara Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122–1129. doi: 10.1053/jhep.2003.50187. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki S, Harada K, Sanzen T, Watanabe K, Tsui W, Nakanuma Y. In situ nucleic acid detection of human telomerase in intrahepatic cholangiocarcinoma and its preneoplastic lesion. Hepatology. 1999;30:914–919. doi: 10.1002/hep.510300419. [DOI] [PubMed] [Google Scholar]
- 18.Williams JG, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro JM, Jorcano JL. The use of arbitrarily primed polymerase chain reaction in cancer research. Electrophoresis. 1999;20:283–290. doi: 10.1002/(SICI)1522-2683(19990201)20:2<283::AID-ELPS283>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Kaushik N, Fear D, Richards SC, McDermott CR, Nuwaysir EF, Kellam P, Harrison TJ, Wilkinson RJ, Tyrrell DA, Holgate ST, et al. Gene expression in peripheral blood mononuclear cells from patients with chronic fatigue syndrome. J Clin Pathol. 2005;58:826–832. doi: 10.1136/jcp.2005.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh KP, Roy D. SKCG-1: a new candidate growth regulatory gene at chromosome 11q23.2 in human sporadic Wilms tumours. Br J Cancer. 2006;94:1524–1532. doi: 10.1038/sj.bjc.6603090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pongstaporn W, Rochanawutanon M, Wilailak S, Linasamita V, Weerakiat S, Petmitr S. Genetic alterations in chromosome 10q24.3 and glutathione S-transferase omega 2 gene polymorphism in ovarian cancer. J Exp Clin Cancer Res. 2006;25:107–114. [PubMed] [Google Scholar]
- 26.Chariyalertsak S, Khuhaprema T, Bhudisawasdi V, Sripa B, Wongkham S, Petmitr S. Novel DNA amplification on chromosomes 2p25.3 and 7q11.23 in cholangiocarcinoma identified by arbitrarily primed polymerase chain reaction. J Cancer Res Clin Oncol. 2005;131:821–828. doi: 10.1007/s00432-005-0031-2. [DOI] [PubMed] [Google Scholar]
- 27.Bodin L, Beaune PH, Loriot MA. Determination of Cytochrome P450 2D6 (CYP2D6) Gene Copy Number by Real-Time Quantitative PCR. J Biomed Biotechnol. 2005;2005:248–253. doi: 10.1155/JBB.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prior FA, Tackaberry ES, Aubin RA, Casley WL. Accurate determination of zygosity in transgenic rice by real-time PCR does not require standard curves or efficiency correction. Transgenic Res. 2006;15:261–265. doi: 10.1007/s11248-005-4024-3. [DOI] [PubMed] [Google Scholar]
- 29.Rudd MF, Webb EL, Matakidou A, Sellick GS, Williams RD, Bridle H, Eisen T, Houlston RS. Variants in the GH-IGF axis confer susceptibility to lung cancer. Genome Res. 2006;16:693–701. doi: 10.1101/gr.5120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang YK, Kim YI, Kim WH. Allelotype analysis of intrahepatic cholangiocarcinoma. Mod Pathol. 2000;13:627–631. doi: 10.1038/modpathol.3880108. [DOI] [PubMed] [Google Scholar]
- 31.Limpaiboon T, Krissadarak K, Sripa B, Jearanaikoon P, Bhuhisawasdi V, Chau-in S, Romphruk A, Pairojkul C. Microsatellite alterations in liver fluke related cholangiocarcinoma are associated with poor prognosis. Cancer Lett. 2002;181:215–222. doi: 10.1016/s0304-3835(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 32.Liengswangwong U, Nitta T, Kashiwagi H, Kikukawa H, Kawamoto T, Todoroki T, Uchida K, Khuhaprema T, Karalak A, Srivatanakul P, et al. Infrequent microsatellite instability in liver fluke infection-associated intrahepatic cholangiocarcinomas from Thailand. Int J Cancer. 2003;107:375–380. doi: 10.1002/ijc.11380. [DOI] [PubMed] [Google Scholar]
- 33.Varma G, Varma R, Huang H, Pryshchepava A, Groth J, Fleming D, Nowak NJ, McQuaid D, Conroy J, Mahoney M, et al. Array comparative genomic hybridisation (aCGH) analysis of premenopausal breast cancers from a nuclear fallout area and matched cases from Western New York. Br J Cancer. 2005;93:699–708. doi: 10.1038/sj.bjc.6602784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Smaele E, Di Marcotullio L, Ferretti E, Screpanti I, Alesse E, Gulino A. Chromosome 17p deletion in human medulloblastoma: a missing checkpoint in the Hedgehog pathway. Cell Cycle. 2004;3:1263–1266. doi: 10.4161/cc.3.10.1200. [DOI] [PubMed] [Google Scholar]
- 35.Byun DS, Cho K, Ryu BK, Lee MG, Kang MJ, Kim HR, Chi SG. Hypermethylation of XIAP-associated factor 1, a putative tumor suppressor gene from the 17p13.2 locus, in human gastric adenocarcinomas. Cancer Res. 2003;63:7068–7075. [PubMed] [Google Scholar]
- 36.Hilbe W, Auberger J, Dirnhofer S, Schmid T, Erdel M, Duba HC. High rate of molecular alteration in histologically tumour-free bronchial epithelium of NSCLC patients detected by multicolour fluorescence in situ hybridisation. Oncol Rep. 2006;15:1233–1240. [PubMed] [Google Scholar]
- 37.Nakashima Y, Tagawa H, Suzuki R, Karnan S, Karube K, Ohshima K, Muta K, Nawata H, Morishima Y, Nakamura S, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosomes Cancer. 2005;44:247–255. doi: 10.1002/gcc.20245. [DOI] [PubMed] [Google Scholar]
- 38.Roncuzzi L, Brognara I, Baiocchi D, Amadori D, Gasperi-Campani A. Loss of heterozygosity at 17p13.3-ter, distal to TP53, correlates with negative hormonal phenotype in sporadic breast cancer. Oncol Rep. 2005;14:471–474. [PubMed] [Google Scholar]
- 39.Uenishi T, Hirohashi K, Kubo S, Yamamoto T, Yamazaki O, Kinoshita H. Clinicopathological factors predicting outcome after resection of mass-forming intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:969–974. doi: 10.1046/j.0007-1323.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 40.Inoue K, Makuuchi M, Takayama T, Torzilli G, Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Konishi M, Kinoshita T, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127:498–505. doi: 10.1067/msy.2000.104673. [DOI] [PubMed] [Google Scholar]
- 41.Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:1463–1466. doi: 10.1046/j.0007-1323.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- 42.Murakami Y, Yokoyama T, Takesue Y, Hiyama E, Yokoyama Y, Kanehiro T, Uemura K, Matsuura Y. Long-term survival of peripheral intrahepatic cholangiocarcinoma with metastasis to the para-aortic lymph nodes. Surgery. 2000;127:105–106. doi: 10.1067/msy.2000.99057. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto M, Takasaki K, Imaizumi T, Ariizumi S, Matsumura N, Nakano M. A long-term survivor of intrahepatic cholangiocarcinoma with lymph node metastasis: a case report. Jpn J Clin Oncol. 2002;32:206–209. [PubMed] [Google Scholar]
- 44.Uenishi T, Yamazaki O, Horii K, Yamamoto T, Kubo S. A long-term survivor of intrahepatic cholangiocarcinoma with paraaortic lymph node metastasis. J Gastroenterol. 2006;41:391–392. doi: 10.1007/s00535-006-1757-6. [DOI] [PubMed] [Google Scholar]


