Abstract
AIM: To assess the diagnostic accuracy of endoscopic ultrasound (EUS), fluid tumor markers and cytology in distinguishing benign from (pre)malignant pancreatic cystic lesions.
METHODS: 46 consecutive patients, referred to a gastroenterologist and surgeon for a symptomatic or incidental pancreatic cyst, were reviewed. EUS, cytology, and carcinoembryonic antigen (CEA) and carbohydrate antigen (CA 19-9) levels were compared with the final diagnosis, based on surgical pathology and/or imaging follow-up of at least 12 mo. Cysts were classified as benign (pseudocyst, serous cystadenoma) or malignant/pre-malignant (mucinous cystic neoplasm). Receiver-operator characteristics (ROC) curve analysis was performed.
RESULTS: The mean age was 56 years; 29% were male and median cyst diameter was 3 cm. Final outcome was obtained in 41 (89%) patients. Twenty-three (56%) of these 41 had surgical pathology. Twenty-three (56%) had benign lesions and 18 (44%) had malignant/pre-malignant lesions. Sensitivity, specificity and positive and negative predictive value of EUS alone to distinguish benign from malignant/premalignant pancreatic cystic lesions were 50%, 56%, 36% and 54% and for cytology were 71%, 96%, 92% and 85%, respectively. The corresponding values for the ROC-derived ideal cutoffs were 75%, 90%, 75%, 90% for CA 19-9 (> 37 U/mL) and 70%, 85%, 79% and 78% for CEA (> 3.1 ng/mL). Subgroup analysis of those with surgical pathology yielded almost identical performance and cutoffs.
CONCLUSION: Cytology and cyst fluid tumor marker analysis is a very useful tool in distinguishing benign from (pre)malignant pancreatic cystic lesions.
Keywords: Endoscopic ultrasound, Carcinoembryonic antigen, CA 19-9, Pancreatic cystic lesions, Fine needle aspiration
INTRODUCTION
Most pancreatic cysts are either inflammatory or neoplastic and pseudocysts account for the majority of pancreatic cystic lesions[1]. “Simple” or congenital pancreatic cysts, in contrast with those in the liver and kidney, are rare. Other types of pancreatic cysts may be broadly classified as benign or pre-malignant and malignant cystic neoplasms. The former include serous cystadenomas, lymphangioma, cystic teratoma, hemangioma, or paraganglioma. Malignant cysts include mucinous cystic neoplasms (mucinous cystadenoma or cystadenocarcinoma)[2,3]. Since some of these lesions may harbor occult malignancy or may develop into malignancy, differentiation between benign (serous), malignant/premalignant, and inflammatory (pseudocysts) cystic lesions is important[4]. Unfortunately, there are yet no reliable clinical or radiological criteria that allow accurate differentiation among the different types of pancreatic cystic lesions.
Endoscopic ultrasound (EUS) is rapidly becoming the imaging modality of choice for a variety of pancreatic lesions; however the role of the endosonographic features alone is controversial and EUS alone may not be sufficiently reliable in differentiating between serous and mucinous lesions[5,6]. Analysis of the cyst fluid for CEA, CA 19-9 and cytology may help in differentiating pancreatic cystic lesions[7-11]. However, data to support the role of cytology and tumor markers of the cystic fluid in differentiating benign from premalignant and malignant pancreatic cystic lesions is still limited.
The aim of this analysis was to assess the diagnostic accuracy of EUS without fine needle aspiration (FNA), as well as fluid tumor markers and cytology in differentiating inflammatory cyst and cysts of low malignant potential from malignant and premalignant pancreatic cystic lesions.
MATERIALS AND METHODS
Subjects
The clinic charts of 46 consecutive patients with pancreatic cystic lesions referred to a gastroenterologist (JR) and pancreatic surgeon (FS) from February 1999 to April 2003 were reviewed. The results of EUS, fluid cytology, and fluid CEA and CA 19-9 levels were compared with the final diagnosis. Based on surgical histopathology and/or imaging follow-up of at least 12 mo, cysts were classified as benign (pseudocysts, serous cystadenoma) versus malignant or pre-malignant (mucinous cystadenoma, intraductal papillary mucinous neoplasms, solid-pseudopapillary tumors, and cystadenocarcinomas). Demographics and symptoms were recorded. History of acute or chronic pancreatitis was also recorded in addition to other risk factors of pancreatic disease (e.g. alcohol).
Patients who did not undergo resection (because of patient preference or due to comorbidities) but had significant changes (increase in size more than 1cm or development of an associated solid mass) of their primary cystic lesion on follow-up imaging of at least 12 mo[12], and patients who had a surgical pathological specimen indicating a malignant or mucinous tumor were defined as having a “malignant/pre-malignant” lesion; otherwise, the lesion was considered to be a benign pancreatic cystic lesion (pseudocyst or serous cystadenoma).
Imaging
The EUS and CT radiologists were effectively blinded to the fluid analysis as this data is not available to either when these tests are being performed in routine practice. The following data regarding the description of the pancreatic cystic lesions were abstracted from EUS and CT reports: size, location and number of the cysts, wall thickness (< 3 mm or ≥ 3 mm), septation (presence and thickness of the septae), presence or absence of solid component and presence or absence of chronic pancreatitis. Cyst sizes (largest dimension, including all cystic compartments if present). According to endosonographic features alone, all pancreatic cystic lesions were classified as either benign or malignant/premalignant by EUS (prior to knowledge of FNA results). The lesion was considered suspicious of being malignant or premalignant if one or more of the following criteria were met: wall thickness of 3 mm or greater, thick septae (> 2 mm), macrocystic (> 2 cm) component(s), or presence of a solid adjacent or intracystic mass or nodule. The presence of chronic pancreatitis was felt to favor a pseudocyst unless one or more of the above criteria were met. A microcystic pattern and the presence of a stellate scar or central calcification were considered suggestive of a serous cystadenoma. The finding of mobile echogenic debri was considered compatible with a pseudocyst.
All EUS procedures were performed with conscious sedation with a radial scanning echoendoscope (GFU-130, Olympus America, Inc., Melville, NY or GF-3060, Pentax). FNA was performed using a linear array echoendoscope (EG-3630U Pentax Medical Co, Montvale, NJ) and a 22 gauge, 8 cm needle (Echotip, Wilson-Cook Medical, Inc., Winston-Salem, NC). Cyst aspiration was accomplished by 1 needle pass in almost all cases. Antibiotics were routinely administered prophylactically (oral ciprofloxacin or levafloxacin for 3-5 d, starting one day before the procedure). When FNA was performed, the largest cyst component was targeted. To reduce the risk of infection, an attempt was made to completely drain all contaminated components. The wall of the cyst was not specifically biopsied/aspirated unless there was wall thickening or an adjacent mass. All EUS procedures were done by the same gastroenterologist (JR) with the assistance of an advanced endoscopy fellow (AA, RP).
Pancreatic cyst fluid analysis
All cytology specimens were collected in Cytolyte (Cytyc Corporation, Boxborough, MA), and spun down in the cytology lab. The median volume of the cyst fluid aspirated was 3 mL. If the volume of the aspirate was small (< 1 mL), the fluid was sent only for cytological evaluation alone, otherwise, CEA, CA19-9, and cytology were requested. Aspirated cystic fluid was sent to lab in a 10 mL syringe. Cytologic material was expressed onto a frosted glass slide immersed in 95% ethanol.
Cytology was considered positive (malignant or pre-malignant) if the cytological evaluation showed features of a mucinous cystadenoma (e.g. mucin) or if malignant cells were identified.
Statistical analysis
Test performances of EUS, cytology, CEA, and CA 19-9 fluid levels in predicting the final diagnosis were analyzed with two-by-two contingency tables. Sensitivity, specificity, positive and negative predictive values and the diagnostic accuracy with their 95% binomial confidence intervals were calculated. Chi-square univariate analysis was performed to compare the frequency of various factors between the benign or inflammatory and the malignant or premalignant groups. The Wilcoxon rank-sum test (Mann-Whitney) was used to compare continuous variables (e.g. the tumor marker levels) among these two groups. Receiver-operator characteristic (ROC) curves were constructed to determine the optimal cutoff values of CEA and CA 19-9 levels in predicting the final diagnosis of a pancreatic cystic lesion. An ROC curve displaying the false positive rate on the x-axis (1-specificity) and the true positive rate on the y-axis (sensitivity) for varying test thresholds was constructed[13]. The ideal cut-off values for the CEA and CA 19-9 were chosen by determining the cutoff closest to an ideal test (the upper left corner of the graph)[14]. Quantitative ROC curve analysis included calculation of areas under each curve (AUC) with 95% confidence intervals (MedCalc, v.7.3.0).
RESULTS
Patient and cyst characteristics
Final diagnosis was obtained in 41 (89%) of these 46 patients, 23 of whom (56%) had surgical pathology. Twenty-three (56%) patients had benign lesions and 18 (44%) had malignant/premalignant lesions (Table 1). The mean (SD) age of the patients was 56.2 (18) years; 71% were female. Sixty-three percent of patients (n = 26) had abdominal pain, 22% had a previous history of acute pancreatitis, and 20% had features of chronic pancreatitis on imaging; 24% of patients were asymptomatic (incidental cyst). Median cyst diameter was 3 (range: 0.5-15) cm, with 68% measuring under 5 cm in diameter. The cyst was located in the head or uncinate in 39% of cases.
Table 1.
Patient and cyst characteristics n (%)
| Entire group (n = 41) | Benign (n = 23) | Malignant/ pre-malignant (n = 18) | P values1 | |
| Age (mean, yr) | 56 | 62 | 51 | 0.03 |
| Male | 12 (29) | 5 (22) | 7 (39) | 0.4 |
| Symptoms | ||||
| Abdominal pain | 26 (63) | 12 (52) | 13 (72) | 0.3 |
| Other symptoms | 5 (12) | 4 (17) | 2 (11) | 0.9 |
| Asymptomatic | 10 (24) | 7 (30) | 3 (17) | 0.6 |
| Pancreatitis | ||||
| Previous acute pancreatitis | 9 (22) | 7 (30) | 2 (11) | 0.3 |
| Chronic pancreatitis | 8 (20) | 5 (22) | 3 (17) | 0.9 |
| Cyst diameter | ||||
| < 1 cm | 3 (7) | 3 (13) | 0 (0) | 0.3 |
| 1-1.9 cm | 7 (17) | 5 (22) | 2 (11) | 0.6 |
| 2.0-5 cm | 18 (44) | 9 (39) | 8 (44) | 0.9 |
| > 5 cm | 13 (32) | 5 (22) | 8 (44) | 0.2 |
| Mean (cm) | 3.0 | 3.4 | 5.6 | 0.006 |
| Location | ||||
| Head | 13 (32) | 9 (39) | 4 (22) | 0.4 |
| Uncinate | 3 (7) | 1 (4) | 2 (11) | 0.8 |
| Body | 14 (34) | 7 (30) | 7 (39) | 0.8 |
| Tail | 11 (27) | 5 (22) | 5 (28) | 0.9 |
| Investigations | ||||
| Surgery | 23 (56) | 8 (35) | 14 (78) | 0.02 |
| Cytology | 41 (100) | 23 (100) | 17 (94) | 0.8 |
| CEA | 23 (56) | 12 (52) | 11 (61) | 0.8 |
| CA 19-9 | 22 (54) | 10 (44) | 12 (67) | 0.2 |
| EUS | 26 (63) | 13 (57) | 9 (39) | 0.4 |
Comparison between benign and malignant/premalignant columns.
Eight (35%) of 23 patients with benign lesions underwent surgical resection because of their symptoms and/or because of suspicious features of imaging, cytology and/or cyst tumor markers. Four (22%) of 18 patients with growth on imaging follow-up did not undergo surgery because of high operative risk, refusal of surgery or metastases. Final diagnosis included mucinous cystadenoma (n = 8), mucinous cystadenocarcinoma (n = 6), serous cystadenoma (n = 15), pseudocyst (n = 7), solid pseudopapillary tumors (n = 2) and others (n = 3).
The patient characteristics (demographics, presentation, and history of acute or chronic pancreatitis) and imaging features did not differ significantly between the two groups, except that the malignant/premalignant group was slightly younger and had slightly bigger cysts (Table 1).
Diagnostic accuracy of EUS and fluid analysis
Twenty-six patients underwent EUS. Thirty-three percent were under 2 cm in diameter, 17% had calcifications or a central scar, 50% had at least one septation, 17% had a thick septum or wall and 25% had 4 or more EUS criteria for chronic pancreatitis. Sensitivity, specificity and positive and negative predictive values of EUS (without fluid analysis) to distinguish benign from malignant/premalignant pancreatic cystic lesions were 50%, 56%, 36% and 69%. These are summarized in Table 2.
Table 2.
Diagnostic performance of EUS, cytology, CEA and CA 19-9 fluid levels in identifying premalignant of malignant cystic lesions mean 95% CI (%)
| EUS alone | Cytology | CEA > 3.1 ng/mL1 | CA 19-9 > 37 U/mL1 | |
| Sensitivity | 50 (16-84) | 71 (49-92) | 70 (42-98) | 75 (33-75) |
| Specificity | 56 (30-80) | 96 (87-99) | 85 (65-99) | 90 (71-99) |
| Negative predictive value | 69 (39-91) | 82 (62-94) | 79 (57-99) | 75 (43-95) |
| Positive predictive value | 36 (11-69) | 92 (64-99.8) | 78 (51-99) | 90 (56-99.8) |
| Accuracy | 54 (34-74) | 85 (74-96) | 78 (61-95) | 82 (66-98) |
| Area under curve | 0.53 (0.32-0.74) | 0.83 (0.68-0.93) | 0.78 (0.54-0.93) | 0.8 (0.57-0.94) |
Optimal cut-offs for CEA and CA 19-9 as determined by receiver-operator characteristic (ROC) curve analysis.
The mean CA 19-9 level was 16.8 U/mL for benign lesions, and 3624 U/mL for premalignant/malignant ones (P = 0.055). For CEA, the mean was 1.3 ng/mL for benign lesions, and 64 U/mL for premalignant/malignant ones (P = 0.02). Using quantitative ROC curve analysis, 37 U/mL was found to be the ideal cut-off (i.e. best balance of sensitivity and specificity) for CA 19-9 and 3.1 ng/mL was best for CEA (Figure 1A and B).
Figure 1.
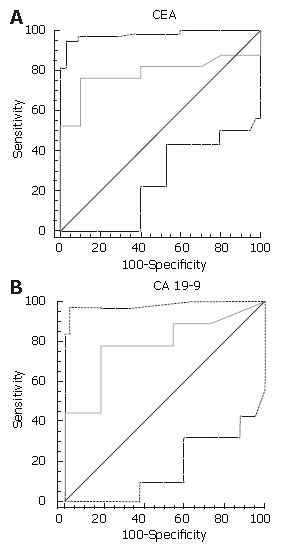
A receiver operator characteristic (ROC) curve of fluid CEA or CA 19-9 with respect to diagnosing malignant/premalignant pancreatic cystic neoplasms. A: CEA > 3.1 ng/mL had a 70% sensitivity and 85% specificity; B: CA 19-9 > 37 U/mL had a 75% sensitivity and 90% specificity.
A cyst fluid CA 19-9 over 37 U/mL had 75% sensitivity, 90% specificity and 82% diagnostic accuracy, whereas CEA over 3.1 ng/mL had 70% sensitivity, 85% specificity and 78% diagnostic accuracy. Very high levels of CEA (> 100 ng/mL) were never seen in benign lesions (100% specific; 100% positive predictive value), but were seen in only 33% of malignant/premalignant ones (67% sensitivity). Interestingly, the two patients with solid pseudopapillary epithelial neoplasms, with positive cytology, had low tumor cyst marker levels (CEA levels were 0.5 and < 0.5 ng/mL, and the CA19-9 levels were 1 and 10 U/mL, respectively). The subgroup of patients (n = 41) who had surgical pathology had comparable results, with accuracies of 89% and 77% for CA19-9 and CEA, respectively.
Sensitivity, specificity and diagnostic accuracy for cytology to distinguish benign from malignant/premalignant pancreatic cystic lesions were 71%, 96% and 85%, respectively (Table 2). Cytology specimens were acellular in 9 patients, one of which had debris consistent with a pseudocyst. Six (67%) of these 9 turned out to be benign lesions, and of the other three, two were positive for mucin; none were malignant. Eleven specimens were called “non-malignant, hypocellular.” Nine (82%) turned out to be benign lesions, and 1 of the other 2 was positive for mucin; none were malignant. Again the subgroup that had surgical pathology had very comparable cytology accuracy (83%).
Complications from cyst aspiration
Of the 41 patients who underwent FNA, two had complications (4.8%): one had minor self-limited intracystic bleeding (no pain, no significant hemoglobin drop, no transfusion, kept overnight for observation) and another one had severe pancreatitis that resolved with supportive therapy. There was no documented infection, leaks, or perforation.
DISCUSSION
With improved and more frequent imaging, incidental pancreatic cysts are increasingly recognized, and in contrast to other organs, the majority of incidental non-inflammatory pancreatic cysts are neoplastic. Predicting the pathology and prognosis of these cysts is difficult because of tremendous overlap in imaging features, but is very important, as benign low-malignant potential lesions (serous cystadenomas) and inflammatory cysts (pseudocysts) do not generally require resection in asymptomatic or minimally symptomatic individuals. EUS is the ideal tool for the evaluation of cystic lesions of the pancreas because of its ability to image these lesions with high resolution (due to close proximity, high ultrasound frequency), and its ability to easily and safely sample the fluid from these lesions during the same session[15].
Almost all previous studies restrict the patient cohort to those patients with surgical pathology. While ensuring the accuracy of the gold standard, that design can result in a considerably biased selection of lesions and patients (larger lesions, younger patients fit for surgery, and probably more concerning cyst features on imaging). Our series of patients was consecutive, and the final diagnosis was made based on the surgical specimen in those operated on (almost half of our cohort), and included one year of radiological and clinical follow-up in those followed conservatively.
One limitation of our design is that the natural history of a mucinous cystadenoma is not well known. Small benign mucinous lesions grow slowly and develop malignancy at a very low rate[16]. It is possible that one year of follow-up is insufficient; however it is unlikely that frankly malignant or high-grade dysplastic lesions would be missed with this approach. Other series have used follow-up of 12-31 mo, in those who did not undergo resection[17,18], to confirm their benign status. In support of our design, our findings regarding test performance and ideal cutoffs were nearly identical in those with and without surgical pathology.
In our series, the sensitivity and specificity of cytology and mucin staining to distinguish benign from malignant/premalignant pancreatic cystic lesions were 71% and 96%, respectively, with a negative predictive value of 82% and a diagnostic accuracy of 85%. Although, the specificity in other studies is comparable to that in our series, there is lower agreement in the literature regarding sensitivity for specimens obtained under CT or ultrasound (range 49% to 98%)[19-21], usually because of variable frequency of specimens reported as hypocellular or acellular. Pinto et al[22] and Brandwein et al[23] both reported lower sensitivities than ours (50%-65%)[11]. In a retrospective study of 34 patients, Sedlack et al[11], found that fluid cytology had a sensitivity of only 27% with a specificity of 100%. This study had few cytology specimens (n = 18) and only included those patients undergoing surgical resection[11]. In keeping with our results, a recent prospective study[24] of 67 patients who were all surgical candidates and referred for surgical resection, cytology had a sensitivity of 97% and specificity of 100% in predicting whether a lesion needed surgery. Recently, Brugge et al[25] reported the results of a multi-center trial of fluid analysis in 109 patients that had surgery (33% of initial cohort). The accuracy of cytology was 59% (64/109) in differentiating between mucinous and non-mucinous cystic lesions[25]. It is not clear whether a positive mucin stain and/or a hypocellular or acellular specimen were classified as positive or negative in that study.
One practical limitation for small cysts is the lack of sufficient fluid to perform both cytology and tumor marker analysis. We, in cooperation with our cytology department, found that sending a tumor marker requisition to the cytology lab, accompanying the low-volume fluid specimen, can help. The cytology technicians can spin down a cellular pellet for cytologic interpretation; the supernatant can then be sent directly for tumor marker measurement from the cytology lab.
While we and others[7,25] have shown very promising results with cyst fluid tumor marker analysis, others have shown poorer performance, reporting accuracies as low as 27%[11,24]. CEA sensitivity has generally been found to be very good in most studies, albeit with some variability in the optimal cut-off value. CA 19-9 has generally been found to be less specific. Similarly, Hammel et al[7] showed that CA 19-9 levels had a 75% sensitivity with a 90% specificity for distinguishing mucinous tumors from other cystic lesions, however the cutoff he used was very different (> 50 000 U/mL). We agree with Frossard et al[24], who showed that CA 19-9 > 50 000 U/mL had a sensitivity of only 15%, that this high threshold for CA19-9 is likely reasonable for detecting malignancy but would be insensitive for premalignant lesions.
With respect to the accuracy of EUS without FNA, our results are in keeping with Brugge et al[25], who showed that the accuracy of CEA (88/111, 79%) was significantly greater than the accuracy of EUS morphology alone (57/112, 51%) in differentiating between mucinous and non-mucinous cystic lesions. Song et al[6], showed that pseudocysts exhibited echogenic debris and parenchymal changes more often than cystic tumors did (29% vs 6% and 65% vs 4%, respectively), but these features were neither specific nor sensitive. Mural nodules were also insensitive but moderately specific in that study (56% in cystic neoplasms vs 12% in pseudocysts. Differentiating cystic neoplasms (of any type) from pseudocysts is arguably an easier task.
In conclusion, tumor marker analysis of cyst fluid was found to be a safe and useful adjunct in distinguishing inflammatory and low-malignant potential benign neoplasms from malignant or premalignant pancreatic cystic lesions, and appears similar in performance to cytology. Although fluid CEA is likely most reliable, CA19-9 also appears to have very good performance. Cytology has reasonable diagnostic performance when acellular and hypocellular specimens without mucin are considered “benign” and mucin positivity is considered to be “suspicious” for a premalignant or malignant process, but its performance is considerably lower when specimens without adequate cellularity are treated as non-diagnostic.
Footnotes
Supported by funds from the Alberta Heritage Foundation of Medical Research
S- Editor Zhu LH L- Editor Alpini GD E- Editor Liu Y
References
- 1.Warshaw AL, Rutledge PL. Cystic tumors mistaken for pancreatic pseudocysts. Ann Surg. 1987;205:393–398. doi: 10.1097/00000658-198704000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balthazar EJ, Chako AC. Computed tomography of pancreatic masses. Am J Gastroenterol. 1990;85:343–349. [PubMed] [Google Scholar]
- 3.Van Dam J. EUS in cystic lesions of the pancreas. Gastrointest Endosc. 2002;56:S91–S93. doi: 10.1067/mge.2002.127702. [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR. Evaluation of pancreatic cystic lesions with EUS. Gastrointest Endosc. 2004;59:698–707. doi: 10.1016/s0016-5107(04)00175-0. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad NA, Kochman ML, Lewis JD, Ginsberg GG. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am J Gastroenterol. 2001;96:3295–3300. doi: 10.1111/j.1572-0241.2001.05328.x. [DOI] [PubMed] [Google Scholar]
- 6.Song MH, Lee SK, Kim MH, Lee HJ, Kim KP, Kim HJ, Lee SS, Seo DW, Min YI. EUS in the evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2003;57:891–896. doi: 10.1016/s0016-5107(03)70026-1. [DOI] [PubMed] [Google Scholar]
- 7.Hammel P, Levy P, Voitot H, Levy M, Vilgrain V, Zins M, Flejou JF, Molas G, Ruszniewski P, Bernades P. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology. 1995;108:1230–1235. doi: 10.1016/0016-5085(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 8.Young NA, Villani MA, Khoury P, Naryshkin S. Differential diagnosis of cystic neoplasms of the pancreas by fine-needle aspiration. Arch Pathol Lab Med. 1991;115:571–577. [PubMed] [Google Scholar]
- 9.Lewandrowski KB, Southern JF, Pins MR, Compton CC, Warshaw AL. Cyst fluid analysis in the differential diagnosis of pancreatic cysts. A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217:41–47. doi: 10.1097/00000658-199301000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperti C, Pasquali C, Guolo P, Polverosi R, Liessi G, Pedrazzoli S. Serum tumor markers and cyst fluid analysis are useful for the diagnosis of pancreatic cystic tumors. Cancer. 1996;78:237–243. doi: 10.1002/(SICI)1097-0142(19960715)78:2<237::AID-CNCR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Sedlack R, Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56:543–547. doi: 10.1067/mge.2002.128106. [DOI] [PubMed] [Google Scholar]
- 12.Walsh RM, Vogt DP, Henderson JM, Zuccaro G, Vargo J, Dumot J, Herts B, Biscotti CV, Brown N. Natural history of indeterminate pancreatic cysts. Surgery. 2005;138:665–670; discussion 670-671. doi: 10.1016/j.surg.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 13.McNeil BJ, Keller E, Adelstein SJ. Primer on certain elements of medical decision making. N Engl J Med. 1975;293:211–215. doi: 10.1056/NEJM197507312930501. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 15.Brugge WR. The role of EUS in the diagnosis of cystic lesions of the pancreas. Gastrointest Endosc. 2000;52:S18–S22. doi: 10.1067/mge.2000.110720. [DOI] [PubMed] [Google Scholar]
- 16.Handrich SJ, Hough DM, Fletcher JG, Sarr MG. The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol. 2005;184:20–23. doi: 10.2214/ajr.184.1.01840020. [DOI] [PubMed] [Google Scholar]
- 17.Walsh RM, Henderson JM, Vogt DP, Baker ME, O'malley CM, Herts B, Zuccaro G, Vargo JJ, Dumot JA, Conwell DL, et al. Prospective preoperative determination of mucinous pancreatic cystic neoplasms. Surgery. 2002;132:628–633; discussion 633-634. doi: 10.1067/msy.2002.127543. [DOI] [PubMed] [Google Scholar]
- 18.Allen PJ, Jaques DP, D'Angelica M, Bowne WB, Conlon KC, Brennan MF. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003;7:970–977. doi: 10.1016/j.gassur.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Linder S, Blåsjö M, Sundelin P, von Rosen A. Aspects of percutaneous fine-needle aspiration biopsy in the diagnosis of pancreatic carcinoma. Am J Surg. 1997;174:303–306. doi: 10.1016/s0002-9610(97)00106-2. [DOI] [PubMed] [Google Scholar]
- 20.Tillou A, Schwartz MR, Jordan PH. Percutaneous needle biopsy of the pancreas: when should it be performed? World J Surg. 1996;20:283–286; discussion 287. doi: 10.1007/s002689900045. [DOI] [PubMed] [Google Scholar]
- 21.Athlin L, Blind PJ, Angström T. Fine-needle aspiration biopsy of pancreatic masses. Acta Chir Scand. 1990;156:91–94. [PubMed] [Google Scholar]
- 22.Pinto MM, Meriano FV. Diagnosis of cystic pancreatic lesions by cytologic examination and carcinoembryonic antigen and amylase assays of cyst contents. Acta Cytol. 1991;35:456–463. [PubMed] [Google Scholar]
- 23.Brandwein SL, Farrell JJ, Centeno BA, Brugge WR. Detection and tumor staging of malignancy in cystic, intraductal, and solid tumors of the pancreas by EUS. Gastrointest Endosc. 2001;53:722–727. doi: 10.1067/mge.2001.114783. [DOI] [PubMed] [Google Scholar]
- 24.Frossard JL, Amouyal P, Amouyal G, Palazzo L, Amaris J, Soldan M, Giostra E, Spahr L, Hadengue A, Fabre M. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol. 2003;98:1516–1524. doi: 10.1111/j.1572-0241.2003.07530.x. [DOI] [PubMed] [Google Scholar]
- 25.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]


