Abstract
AIM: To report 13 patients with benign esophageal stenosis treated with the biodegradable stent.
METHODS: We developed a Ultraflex-type stent by knitting poly-l-lactic acid monofilaments.
RESULTS: Two cases were esophageal stenosis caused by drinking of caustic liquid, 4 cases were due to surgical resection of esophageal cancers, and 7 cases were patients with esophageal cancer who received the preventive placement of biodegradable stents for post-endoscopic mucosal dissection (ESD) stenosis. The preventive placement was performed within 2 to 3 d after ESD. In 10 of the 13 cases, spontaneous migration of the stents occurred between 10 to 21 d after placement. In these cases, the migrated stents were excreted with the feces, and no obstructive complications were experienced. In 3 cases, the stents remained at the proper location on d 21 after placement. No symptoms of re-stenosis were observed within the follow-up period of 7 mo to 2 years. Further treatment with balloon dilatation or replacement of the biodegradable stent was not required.
CONCLUSION: Biodegradable stents were useful for the treatment of benign esophageal stenosis, particularly for the prevention of post-ESD stenosis.
Keywords: Stents, Esophageal cancer, Endoscopic submucosal dissection
INTRODUCTION
The placement of self-expanding metallic stents is routinely used to maintain esophageal patency in patients with malignancy that either have non-resectable disease or are poor candidates for surgery[1-3]. Many reports have documented the clinical effectiveness of these tools, particularly covered metallic stents[2,3]. However, the usefulness of metallic stents for benign stenosis is limited due to relatively little information regarding their long-term complications, including migration, the formation of new strictures, fistula formation and hyperplastic tissue reactions[1,4,5].
Since the cause of benign esophageal stenosis does not directly affect the patients’ prognosis, it is more important to prevent the incidence of long-term complications[4]. It is highly desirable to develop a stent that could be kept in the proper position during the repair process, and then be easily removed, thus avoiding re-stenosis. In other words, if a stent could be constructed from a biodegradable material, then a subsequent stent removal operation would not be necessary[6,7]. The degradable nature of the stent would prevent serious long-term complications.
In Japan, the majority of esophageal cancers are squamous cell carcinomas[8]. Since it has been reported that lymph node metastasis is rare in esophageal mucosal cancers, endoscopic treatment for esophageal mucosal cancers such as endoscopic submucosal dissection (ESD) is recommended in Japan[8]. ESD is a recently-developed technique, and makes it possible to perform an en bloc resection of a mucosal lesion. The quality of life after an endoscopic resection is also much better than that after an esophagectomy. However, a mucosal resection over 75% of the circumference sometimes causes stenotic changes, and requires balloon dilatation[8].
Recently, we developed an Ultraflex-type biodegradable stent by knitting poly-l-lactic acid (PLLA) monofilaments together for the treatment of patients with benign gastrointestinal tract stenosis[9]. PLLA has been used for orthopedic applications in humans, and has generally been found to be bio-absorbed over a few months[7,10-13]. The radial force of this biodegradable stent was comparable to that of commercially available metallic stents[9]. Here, we report 13 cases with benign esophageal stenosis that were treated with biodegradable stents. These cases include benign esophageal stenosis due to drinking of caustic liquid in an attempt to commit suicide and anastomosis after surgical resection for esophageal cancer. Seven cases of preventive placement of biodegradable stents for post-ESD stenosis were also included.
MATERIALS AND METHODS
Description of the stent and endoscopic implantation
The PLLA esophageal stent (Tanaka-Marui stent; Marui Textile Machinery Co., Ltd., Osaka, Japan) is composed of a PLLA monofilament (molecular mass 183 kD, diameter 0.23 mm) with a machine knitted design like an ultra flex metallic stent (Figure 1A). The length and diameter of the PLLA stent was designed according to the esophageal lesion of each individual patient. One end of the PLLA stent was tied into a 5 mm diameter by silk sutures, and the PLLA stent was fitted over an endoscope (Figure 1B). The radial force of the biodegradable stent was 117 gf[9], and this is comparable to commercially available metallic stents[9].
Figure 1.
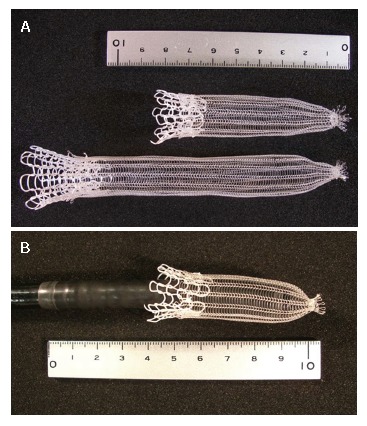
The PLLA esophageal stent (Tanaka-Marui stent; Marui Textile Machinery Co., Ltd., Osaka, Japan). A: One end of the PLLA stent was tied into a 5 mm diameter by silk sutures; B: The stent was fitted over an endoscope.
The ethics committee of the Shiga University of Medical Science approved this project, and written informed consent was obtained from each patient.
Procedure of biodegradable stent placement
A 64-year-old man was admitted to the Shiga University Hospital (Otsu, Japan) due to an early squamous esophageal cancer in the middle esophagus. The lesion was 40 mm in diameter on the posterior wall (Figure 2A). The lesion occupied 75% of the circumference, and endoscopic ultrasound sonography indicated that the depth of invasion did not reach the muscularis mucosa. According to the patient’s request, ESD was performed[8]. The lesion was resected en bloc, and the mucosal defect was seven eighths of the circumference (Figure 2B). As shown in Figure 2C, to prevent an occurrence of stenosis, the PLLA esophageal stent was placed on the next day. The PLLA esophageal stent was fitted over a fiberscope and inserted through the resected portion. When the tip of the endoscope reached 20 mm from the caudal side of the resected portion, the PLLA stent was released by pulling it out from the fiberscope (Figure 2C). The rostral end of the PLLA stent was fixed to the esophageal wall by 5 endoscopic clips (HX-110LR; Olympus medical System Corp. Tokyo, Japan) (Figure 2D), and then the silk suture at the distal margin was cut by scissor forceps (FS-3L-1; Olympus). A follow-up study 6 month later showed sufficient patency in the resected portion (Figure 2E).
Figure 2.
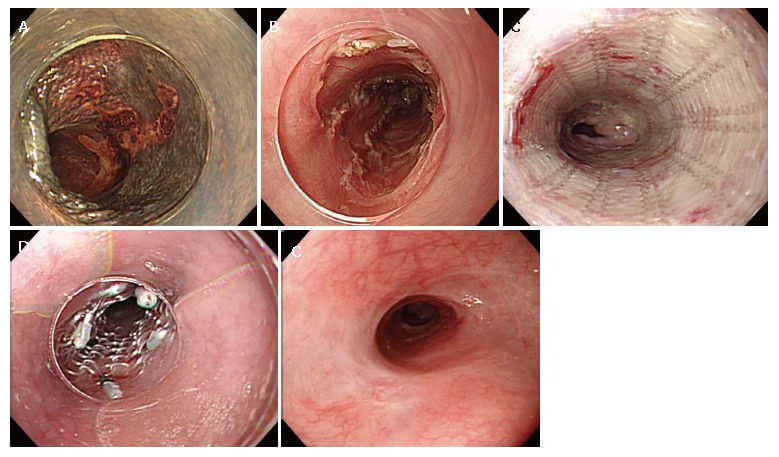
Endoscopic findings of the PLLA stent. A: Endoscopic findings of early esophageal cancer with iodine staining; B: The mucosal defect after ESD; C: The released PLLA stent; D: Fixation of the rostral side by endoscopic clips; and E: The view at 6 mo of follow-up.
RESULTS
As shown in Table 1, 13 patients with benign esophageal stenosis were treated with biodegradable stent. Case 1 and 2 were esophageal stenosis caused by the drinking of caustic liquid. Case 3 to 6 were esophageal stenosis caused by surgical resection for esophageal cancer. These 6 cases (case 1 to 6) received a repeated balloon dilatation before treatment with the biodegradable stent. Cases 7 to 13 were preventive placement of the biodegradable stent for post ESD stenosis. In these patients mucosal defect by ESD was over 75% of the circumference, and stenotic changes were highly supposed. The preventive placement of biodegradable stent was performed with 2 to 3 d after ESD. In 10 of the 13 cases, spontaneous migration of stents occurred between 10 to 21 d after placement. In these cases, the migrated stents were excreted with feces, and no obstructive complication was experienced. In 3 cases, the stents remained at their proper position until 21 d after placement. The follow-up period of these patients was between 7 mo to 2 years, and no patient complained of symptoms of re-stenosis. In cases 1 to 6, further treatment with balloon dilatation was not required.
Table 1.
Cases of benign esophageal stenosis
| No | Initial | Age | Sex | Cause of esopahgeal stenosis | Observed period (yr) |
| 1 | KK | 19 | F | Drinking of caustic liquid | 1.7 |
| 2 | HT | 15 | M | Drinking of caustic liquid | 1.0 |
| 3 | OM | 64 | M | Surgical resection of esophageal cancer | 1.2 |
| 4 | KG | 67 | M | Surgical resection of esophageal cancer | 2.0 |
| 5 | TH | 61 | M | Surgical resection of esophageal cancer | 0.6 |
| 6 | YT | 51 | M | Surgical resection of esophageal cancer | 0.6 |
| 7 | MT | 62 | M | Post ESD for esophageal cancer | 1.2 |
| 8 | NK | 77 | M | Post ESD for esophageal cancer | 1.2 |
| 9 | KM | 77 | M | Post ESD for esophageal cancer | 1.0 |
| 10 | IY | 75 | M | Post ESD for esophageal cancer | 0.7 |
| 11 | SN | 65 | M | Post ESD for esophageal cancer | 0.6 |
| 12 | NS | 64 | M | Post ESD for esophageal cancer | 1.4 |
| 13 | YT | 61 | M | Post ESD for esophageal cancer | 0..6 |
ESD: endoscopic submucosal dissection.
DISCUSSION
Metallic stents are effective for treating malignant stenoses of various tract organs. Metallic stents are less invasive, but various complications such as perforation, bleeding, migration, tumor in-growth and foreign body reactions have been reported[1,2]. In cases with serious complications, surgical removal of the metallic stent is required[14]. Based on this notion, considerable effort should be devoted to avoiding serious complications in patients with benign esophageal stenosis, because their prognoses are good with an expected long-term survival. If the stents gradually degrade while maintaining their dilatation force, they would be ideal treatments for patients with benign esophageal stenosis.
Recently, stents composed of biodegradable materials such as polylactic acids have been developed for treating patients with benign stenosis[10,11,15]. The aforementioned biodegradable materials have proven to be biologically safe and have been used in various medical devices such as surgical sutures and bone nails. Experimental and clinical studies have also confirmed that dilation using biodegradable stents is effective for treating various stenotic lesions[13,15-17]. The characteristic features of biodegradable stents, their solubility and natural absorption after a certain time period, may prevent the serious complications reported for the use of metallic stents[2]. Furthermore, biodegradable stents are expected to be used as drug delivery systems[18,19] and numerous studies regarding drug delivery by biodegradable stents are in progress[15,18,19].
Benign gastrointestinal strictures are usually managed by maintaining an unobstructed passage with periodic balloon dilatation. If the strictures do not respond to these therapies, surgical resection is considered. With advances in endoscopic intervention, a variety of new techniques have been developed. For examples the injection of corticosteroids into the stenotic portion under endoscopic guidance has been used to prevent stenosis by blocking the progression of fibrosis[20,21]. An endoscopic incision of the stenotic wall has also been attempted[21]. However, metallic stents are not suitable for benign stenotic lesions in the gastrointestinal tract because they cannot be easily removed, and therefore may cause serious unavoidable complications during long-term use.
In this study, we presented 13 cases treated with the biodegradable stent. Six cases (cases 1 to 6) received a repeated balloon dilatation before placement of the biodegradable stent, but no further treatment was required after biodegradable stent implantation. In 10 of the 13 cases, spontaneous migration of the stents occurred between 10 to 21 d after placement. This may be due to a loss of patency as the stents degrade. In these cases, the migrated stent was excreted with the feces, and no obstructive complication was experienced. However, as one possible complication careful attention should be paid to potential bowel obstruction caused by the migrated stent. The follow-up period of these patients was between 6 mo to 2 years, and no patients complained of symptoms of re-stenosis. These findings support the effectiveness of this novel developed biodegradable stent for benign esophageal stenosis.
There are an increasing number of patients with early esophageal cancers who receive complete resection by endoscopic procedures such as ESD[8]. This treatment is less invasive than traditional surgical approaches, but when the mucosal defect covers over 75% of the circumference, then stenosis frequently occurs[8]. A mucosal defect that covers the entire circumference always causes stenosis. Thus, the number of patients with esophageal stenosis after ESD is increasing, and these patients are sometimes resistant to repeated balloon dilatation. In these patients, the application of biodegradable esophageal stents as a tool for preventing of stenosis seems to be reasonable. We usually place the stents in patients with the mucosal defect of over 75% of the circumference. Our data showed that placement of the biodegradable stents with 2 to 3 d after ESD effectively prevented ESD-related stenosis. This suggests that early phase of repair process plays an important role in the progression of stenosis. A detailed clinical follow-up of the biodegradable stent should be performed over a longer period.
In conclusion, this study demonstrated the usefulness of biodegradable stents for treating patients with benign esophagal stenoses. The biodegradable stents exerted sufficient dilation force and were safe for clinical use. In particular, these were useful for the prevention of post ESD stenosis. A long-term follow-up with more patients should be performed to assess the effectiveness of these biodegradable stents in the future.
Footnotes
S- Editor Zhu LH L- Editor Alpini GD E- Editor Liu Y
References
- 1.Ackroyd R, Watson DI, Devitt PG, Jamieson GG. Expandable metallic stents should not be used in the treatment of benign esophageal strictures. J Gastroenterol Hepatol. 2001;16:484–487. doi: 10.1046/j.1440-1746.2001.02367.x. [DOI] [PubMed] [Google Scholar]
- 2.Song HY, Do YS, Han YM, Sung KB, Choi EK, Sohn KH, Kim HR, Kim SH, Min YI. Covered, expandable esophageal metallic stent tubes: experiences in 119 patients. Radiology. 1994;193:689–695. doi: 10.1148/radiology.193.3.7526414. [DOI] [PubMed] [Google Scholar]
- 3.Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc. 2004;60:1010–1017. doi: 10.1016/s0016-5107(04)02276-x. [DOI] [PubMed] [Google Scholar]
- 4.Song HY, Park SI, Do YS, Yoon HK, Sung KB, Sohn KH, Min YI. Expandable metallic stent placement in patients with benign esophageal strictures: results of long-term follow-up. Radiology. 1997;203:131–136. doi: 10.1148/radiology.203.1.9122381. [DOI] [PubMed] [Google Scholar]
- 5.Evrard S, Le Moine O, Lazaraki G, Dormann A, El Nakadi I, Devière J. Self-expanding plastic stents for benign esophageal lesions. Gastrointest Endosc. 2004;60:894–900. doi: 10.1016/s0016-5107(04)02278-3. [DOI] [PubMed] [Google Scholar]
- 6.Böstman OM. Absorbable implants for the fixation of fractures. J Bone Joint Surg Am. 1991;73:148–153. [PubMed] [Google Scholar]
- 7.Knutson T, Pettersson S, Dahlstrand C. The use of biodegradable PGA stents to judge the risk of post-TURP incontinence in patients with combined bladder outlet obstruction and overactive bladder. Eur Urol. 2002;42:262–267. doi: 10.1016/s0302-2838(02)00314-7. [DOI] [PubMed] [Google Scholar]
- 8.Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Takahashi M, Nitta N, Furukawa A, Andoh A, Saito Y, Fujiyama Y, Murata K. Newly developed biodegradable stents for benign gastrointestinal tract stenoses: a preliminary clinical trial. Digestion. 2006;74:199–205. doi: 10.1159/000100504. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966;93:839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 11.Schakenraad JM, Dijkstra PJ. Biocompatibility of poly (DL-lactic acid/glycine) copolymers. Clin Mater. 1991;7:253–269. doi: 10.1016/0267-6605(91)90067-p. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji T, Tamai H, Igaki K, Kyo E, Kosuga K, Hata T, Okada M, Nakamura T, Komori H, Motohara S, et al. Biodegradable Polymeric Stents. Curr Interv Cardiol Rep. 2001;3:10–17. [PubMed] [Google Scholar]
- 13.Schakenraad JM, Hardonk MJ, Feijen J, Molenaar I, Nieuwenhuis P. Enzymatic activity toward poly(L-lactic acid) implants. J Biomed Mater Res. 1990;24:529–545. doi: 10.1002/jbm.820240502. [DOI] [PubMed] [Google Scholar]
- 14.Wang MQ, Sze DY, Wang ZP, Wang ZQ, Gao YA, Dake MD. Delayed complications after esophageal stent placement for treatment of malignant esophageal obstructions and esophagorespiratory fistulas. J Vasc Interv Radiol. 2001;12:465–474. doi: 10.1016/s1051-0443(07)61886-7. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Minami K, Kobayashi M, Nakao Y, Omiya H, Imamura H, Sakaida N, Okamura A. New tubular bioabsorbable knitted airway stent: biocompatibility and mechanical strength. J Thorac Cardiovasc Surg. 2002;123:161–167. doi: 10.1067/mtc.2002.118503. [DOI] [PubMed] [Google Scholar]
- 16.Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S, Uehata H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 17.Robey TC, Eiselt PM, Murphy HS, Mooney DJ, Weatherly RA. Biodegradable external tracheal stents and their use in a rabbit tracheal reconstruction model. Laryngoscope. 2000;110:1936–1942. doi: 10.1097/00005537-200011000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Saito Y, Minami K, Kaneda H, Okada T, Maniwa T, Araki Y, Imamura H, Yamada H, Igaki K, Tamai H. New tubular bioabsorbable knitted airway stent: feasibility assessment for delivery and deployment in a dog model. Ann Thorac Surg. 2004;78:1438–1440. doi: 10.1016/S0003-4975(03)01408-5. [DOI] [PubMed] [Google Scholar]
- 19.Yamawaki T, Shimokawa H, Kozai T, Miyata K, Higo T, Tanaka E, Egashira K, Shiraishi T, Tamai H, Igaki K, et al. Intramural delivery of a specific tyrosine kinase inhibitor with biodegradable stent suppresses the restenotic changes of the coronary artery in pigs in vivo. J Am Coll Cardiol. 1998;32:780–786. doi: 10.1016/s0735-1097(98)00312-x. [DOI] [PubMed] [Google Scholar]
- 20.Zein NN, Greseth JM, Perrault J. Endoscopic intralesional steroid injections in the management of refractory esophageal strictures. Gastrointest Endosc. 1995;41:596–598. doi: 10.1016/s0016-5107(95)70198-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Kubik CM, Polhamus CD, Brady CE, Kadakia SC. Preliminary experience with endoscopic intralesional steroid injection therapy for refractory upper gastrointestinal strictures. Gastrointest Endosc. 1995;41:598–601. doi: 10.1016/s0016-5107(95)70199-0. [DOI] [PubMed] [Google Scholar]


