Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited rhythm disorder characterized by the occurrence of potentially life-threatening polymorphic ventricular tachyarrhythmias in conditions of physical or emotional stress. The underlying cause is a dysregulation in intracellular Ca handling due to mutations in the sarcoplasmic reticulum Ca release channel. Recent experimental work suggests that the sinus bradycardia that is sometimes observed in CPVT patients may be another primary defect caused by CPVT mutations. Here, we review the pathophysiology of CPVT and discuss the role of sinus node dysfunction as a modulator of arrhythmia risk and potential therapeutic target.
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited rhythm disorder characterized by the occurrence of potentially life-threatening polymorphic ventricular tachyarrhythmias in conditions of physical or emotional stress (1). The underlying cause is a dysregulation in intracellular Ca handling due to mutations in the gene encoding the sarcoplasmic reticulum (SR) Ca release channel (RYR2) (2), or in genes encoding the RyR2 binding proteins cardiac calsequestrin (Casq2) (3), triadin (4, 5) and calmodulin (6) that regulate RyR2 channel openings. In addition, mutations in KCNJ2, which are usually associated with Andersen-Tawil syndrome, have also been identified in patients with a CPVT-like phenotype, although the prognosis of these patients is thought to be more benign that patients with true CPVT (7). In classic CPVT cases, i.e., resulting from dysregulated intracellular Ca handling, release of catecholamines during exercise exacerbates SR dysfunction: beta adrenergic stimulation promotes Ca reuptake in the SR and increases RYR2 permeability to Ca. Moreover, the catecholamine-induced increase in heart rate (HR) further promotes myocyte Ca loading and hence the spontaneous SR Ca release events that trigger CPVT. Interestingly, recent experimental work suggests that the sinus bradycardia that is sometimes observed in CPVT patients may be another primary defect caused by CPVT mutations (8). Furthermore, the sinus node dysfunction may paradoxically favor the initiation of ventricular rhythms as well and could be targeted therapeutically to prevent exercise or stress-induced ventricular arrhythmia in CPVT (9). Here, we review the pathophysiology of CPVT and discuss the role of sinus node dysfunction as a modulator of arrhythmia risk and potential therapeutic target.
Pathophysiology of CPVT
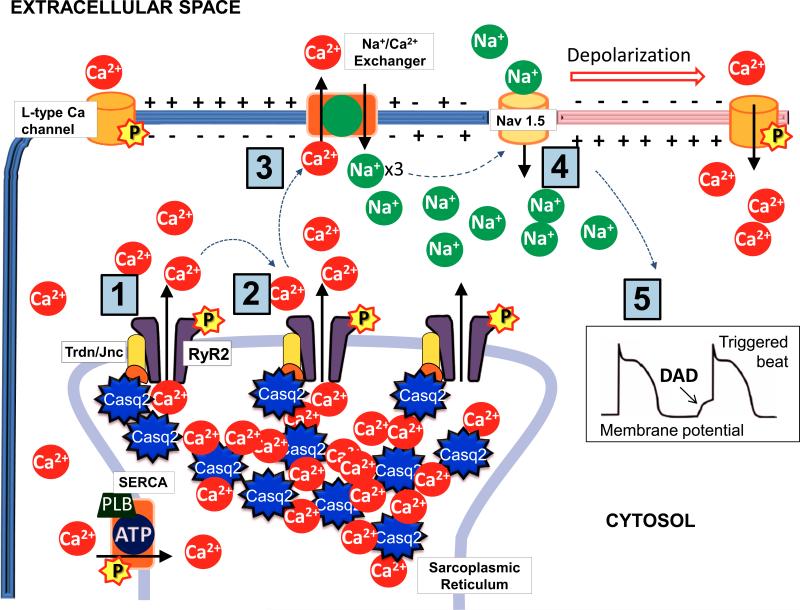
Depending on the CPVT mutation, a number of molecular mechanisms have been suggested by different groups: defective SR luminal Ca sensing (10), defective inter-domain interaction (11), increased cytosolic Ca sensitivity (12), reduced calmodulin binding (13), activation of RyR2 channels by mutant calmodulin (14). Regardless of the specific molecular mechanism, CPVT mutations induce a common dysregulation of intracellular Ca release that is characterized by an increased open probability of RyR2 Ca release channels . The Ca release defect manifests itself primarily when SR Ca content is elevated, which occurs during fast HRs and/or beta-adrenergic receptor stimulation that is part of the physiological fight or flight response. This leads to a cellular chain reaction that is illustrated in the cartoon of Fig. 1. RyR2 channels open spontaneously during diastole in the absence of the physiological trigger provided by L-type Ca channels. Neighboring Ca releasing units are activated through a Ca induced-Ca release mechanism which further increases and spreads the intracellular Ca ions. The high cytosolic Ca concentrations activate the Na/Ca exchanger on the cell membrane, which in turn generates an inward Na current while extruding Ca ions from the cell. This inward current depolarizes the cell membrane during late diastole, generating a phenomenon known as delayed after-depolarization (DAD) that can trigger ectopic activity. DADs are readily observed in isolated ventricular myocytes carrying CPVT mutations, yet theoretical considerations and computer models suggest that the intact heart, a large number of contiguous DAD-susceptible myocytes are necessary to overcome the electrical sink provided by the surrounding hyperpolarized myocardium (15). While that critical cell number might not be achieved at rest, exercise or psychological stress activates a number of signaling pathways that converge to generate the high SR content needed for spontaneous Ca release to occur in a large number of myocytes: Systemic and intracardiac catecholamine released during exercise, via β-adrenergic receptors, activate protein kinase A (PKA). PKA phosphorylation of L-type Ca channels drastically increases Ca influx into the myocyte, and PKA phosphorylation of phospholamban drastically enhances SR Ca uptake, thereby acting in concert to increase SR Ca content. Moreover, RyR2 is also phosphorylated resulting in higher permeability to Ca (16). Independently, the higher HRs during exercise further increases myocyte Ca loading, a phenomenon known as the positive force frequency response (17). As a result, the critical threshold of SR Ca content for spontaneous SR Ca release to occur is reached already during diastole, and it becomes more likely for a cluster of cells to synchronize and trigger a premature ventricular ectopic beat.
Figure 1.
Chain reaction leading to delayed after-depolarizations (DAD) in a cardiac myocyte. Spontaneous diastolic Ca release from the SR can generate Ca induced Ca releases from neighbouring RyR2 channels [1-2]. The resulting increase in cytosolic Ca concentration activates the Na/Ca exchanger with a net inward Na current that can trigger depolarization of the cell membrane [3-4] and potentially an ectopic beat [5]. Beta adrenergic stimulation enhances the likelihood of diastolic Ca releases from the SR and subsequently DADs through a protein kinase A (PKA)-mediated phosphorylation [P] of RyR2, SERCA and L-type Ca channels.
Sinus node dysfunction and atrial tachyarrhythmia – a primary disease manifestation of CPVT mutations?
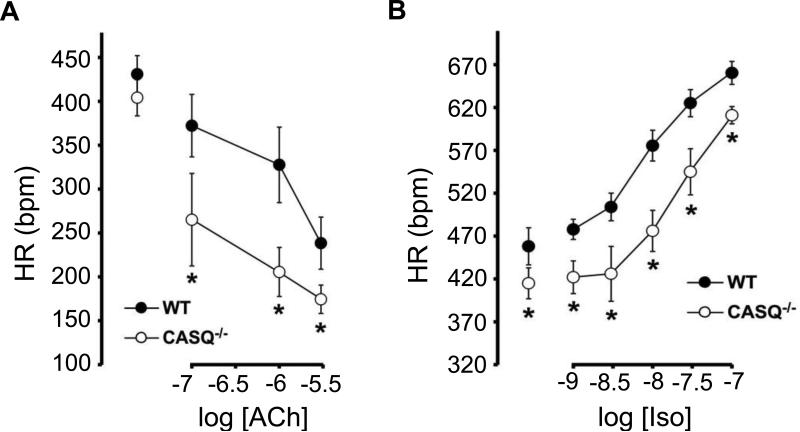
Patients with CPVT typically have a normal EKG at rest. However, the presence of sinus bradycardia in addition to a clinical history of exercise- or emotion-induced cardiac symptoms may provide a clue for the diagnosis of CPVT before exercise testing of pharmacological challenge is performed (18). In a series of carriers of a pathogenic RYR2 mutation who were identified through cascade screening (testing relatives for carriership of the mutation identified in the proband), sinus bradycardia was present in 5-20% of individuals, depending on the definition applied (19). In addition, supraventricular dysrhythmias other than sinus bradycardia (intermittent ectopic atrial rhythm, unspecified supraventricular tachycardia and sick sinus syndrome) were present in 16% of the total population (bradyarrhythmia in 11.3% and tachyarrhythmia in 4.7%) and in 38% of individuals who underwent Holter monitoring. Indeed, electrophysiological studies on patients have confirmed that sinus node dysfunction is present at least in a subpopulation of CPTV patients (20). Supraventricular tachyarrhythmias, such as atrial fibrillation and atrial flutter where also observed (1, 20) and atrial fibrillation has been associated with triggering CPVT clinically (21). In very young children carrying a mutation in RYR2, atrial tachyarrhythmias may even precede the classic ventricular tachyarrhythmias (22). Given that CPVT can be triggered by fast HRs, one might speculate that the sinus bradycardia of CPVT patients may be a protective adaptation in affected individuals. However, recent data from a CPVT mouse model suggest otherwise and indicate that the atrial tachyarrhythmias (23) and sinus node dysfunction (8) likely are primary manifestation of the underlying Ca handling defect caused by CPVT mutations. For example, the Casq2 knockout (Casq2−/−) mouse model of CPVT not only reproduces the resting bradycardia of CPVT patients but also displays a lower peak HR during isoproterenol challenge compared to wild type control animals (9). Sinus node dysfunction is further supported by telemetry EKG recordings of Casq2−/− mice that show periods of atrial rhythm with frequent variation of the p wave morphology and bradycardia (8). Concentration-response curves for the parasympathetic (vagal) neurotransmitter acetylcholine obtained in isolated, and therefore denervated, Casq2−/− hearts show a more prominent response compared to wild type hearts (Fig. 2)(8). In contrast, the HR response to β-adrenergic stimulation with isoproterenol is impaired, as evidenced by the right-shifted concentration-response curve in Casq2−/− hearts (Fig. 2). Taken together, loss of Casq2 causes an intrinsic defect in the sinoatrial node that can explain the sinus bradycardia and lower peak HR in response to catecholamines. Regional microfibrosis in the sinus node of Casq2−/− mice might be responsible for altered generation and propagation of the electrical impulse in the sinus node area and might contribute to sinus node dysfunction and supraventricular arrhythmias (8). The pathophysiology for this remodeling is still not clear and most likely involves multiple molecular pathways. For example, diastolic Ca overload in sinoatrial node myocytes has been shown to promote cell apoptosis and fibrinogenesis (24). Intracellular Ca elevation can activate the Ca-calmodulin kinase II that is involved in several processes including gene transcription and structural remodeling (25). The resulting fibrosis slows down impulse propagation and favors development of a brady/tachy syndrome. Independently, the atrial SR Ca leak can increase the risk for focal atrial tachyarrhythmias and atrial fibrillation (23) and promote the development of atrial fibrosis and reentrant atrial arrhythmias over time (26). In CPVT patients, any form of tachy/brady syndrome might be particularly deleterious as fast rhythm would synchronize cells and load the SR while during sinus pauses ventricular escape beats and malignant arrhythmias might be triggered, as discussed in more detail next.
Figure 2.
Concentration response relationships of the sinus HR response to the muscarinic receptor agonist Acetylcholine (ACh, A) and to the β-adrenergic agonist Isoproterenol (Iso, B). Compared to wild-type (WT) hearts, Casq2 null hearts (CASQ−/−) exhibited an enhanced HR response to ACh, whereas the HR response to β-adrenergic stimulation with Iso was impaired. Figure modified from (8).
With permission: Glukhov AV, Kalyanasundaram A, Lou Q, Hage LT, Hansen BJ, Belevych AE, Mohler PJ, Knollmann BC, Periasamy M, Gyorke S, et al. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex. Eur Heart J. 2013.
Sinus node dysfunction – an independent risk factor and potential therapeutic target for CPVT?
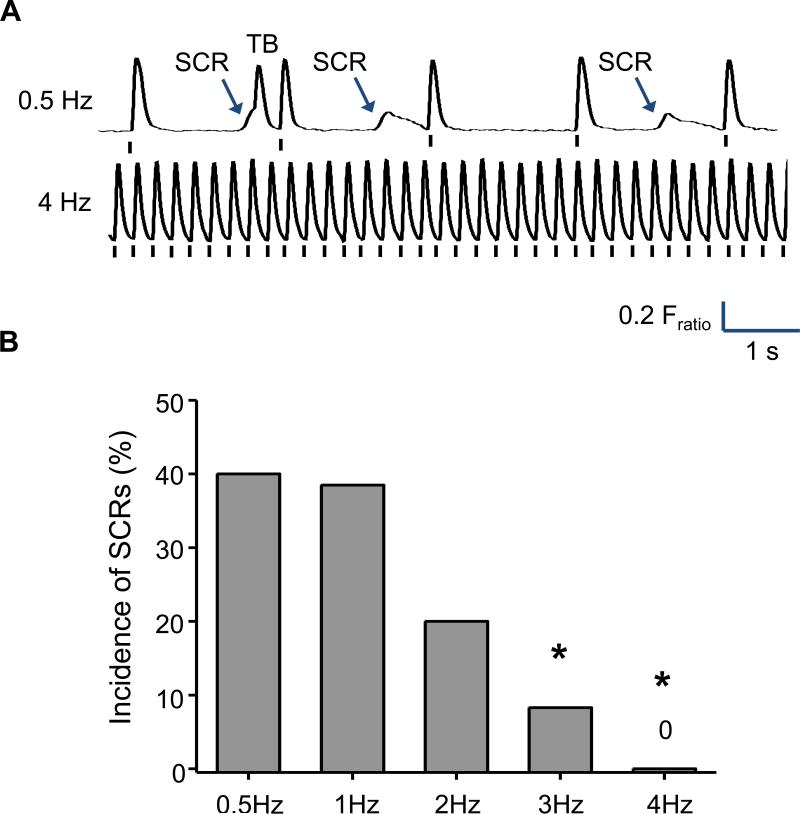
One could speculate that the enhanced responsiveness to vagal stimulation (Fig. 2) should have a protective effect in CPVT by reducing the HR increase and hence the SR Ca loading that occurs during exercise. We recently tested this hypothesis using the Casq2−/− mouse model of CPVT and found that the opposite was the case: The incidence of ventricular tachycardia increased if the sinus node rate was decreased by the muscarinic agonist carbachol. Conversely, raising the sinus node rate by vagolytic treatment with atropine protected against catecholamine-induced CPVT (9). Atrial pacing was similarly effective, demonstrating that a β-adrenergic independent rise in supraventricular rates is sufficient to prevent ventricular arrhythmias in CPVT mice during pharmacological stress. What are possible explanations for this seemingly paradoxical result? As discussed in the pathophysiology section, for a premature ventricular ectopic beat to be generated, the spontaneous Ca release has to occur during diastole, propagate and generate DADs in a cluster of cells (Fig 1). However, if at any time during this process the cells are depolarized by the activation wave arriving from the sinus node via the conduction system, the SR is emptied and cannot open spontaneously. Hence, the occurrence of ventricular arrhythmias is critically dependent on the balance between accelerated SR Ca loading (via β-adrenergic stimulation) and the diastolic interval. The importance of diastolic interval for generating spontaneous Ca release and DADs can be demonstrated experimentally in isolated ventricular Casq2−/− myocytes where a high rate of SR Ca uptake was maintained by exposure to isoproterenol (Fig. 3). Spontaneous Ca releases were present in 40% of Casq2−/− myocytes paced at 0.5 and 1 Hz. Reduction of the diastolic interval by higher pacing frequencies progressively reduced the incidence of SCWs (Fig. 3). We interpret this result as follows: Shortening the diastolic interval by overdrive pacing prevents the SR from reaching the critical threshold for spontaneous firing and thereby could act to suppress the ventricular ectopic activity underlying CPVT. It could be argued that the sinus node dysfunction and impaired sinus HR response caused by CPVT mutations independently contributes to the emergence of ventricular arrhythmia during exercise via the following mechanisms: Not only is there a propensity towards spontaneous Ca release caused by the CPVT mutations, but also the diastolic interval is too long for the level of β-adrenergic stimulation present, allowing the occurrence of premature Ca release in diastole.
Figure 3.
Shortening the diastolic interval by increasing the pacing rate suppresses spontaneous Ca release (SCR) and triggered beats (TB) in isolated ventricular Casq2−/− cardiomyocytes. Figure modified from (9).
With permission: Faggioni M, Hwang HS, van der Werf C, Nederend I, Kannankeril PJ, Wilde AA, and Knollmann BC. Accelerated sinus rhythm prevents catecholaminergic polymorphic ventricular tachycardia in mice and in patients. Circulation research. 2013;112(4):689 97.
β-blockers are used as first-line therapy and reduce the ventricular arrhythmia burden and risk of cardiac events in most patients (27). As expected, β-blockers also reduce resting HR and maximum HR during exercise testing. Interestingly, two studies have reported a lower HR threshold of ventricular arrhythmias on β-blockers (28, 29). This clinical observation may support the previously outlined hypothesis. Although the efficacy of β-blockers in CPVT has clearly been established (30), a reduction of the HR threshold of ventricular arrhythmias may be a potential disadvantage and may explain the significant cardiac event rates on β-blocker therapy (31). In our initial experience, flecainide increased the HR threshold of ventricular arrhythmias, which further underpins the rationale of adding flecainide to β-blockers in insufficiently protected patients (32).
An important caveat is that the experimental studies on the HR dependence of CPVT were done in mice and not humans. Compared to mice, humans exhibit a much stronger force frequency response and frequency-dependent SR Ca loading, which is consistent with the clinical observation of a threshold HR associated with ventricular arrhythmias in CPVT. To evaluate the HR dependence of CPVT in humans, we recently screened a CPVT patient registry for individuals that were off antiarrhythmic drug therapy and reached >85% of their maximum-predicted HR during exercise testing (9). We identified 18 patients that fulfilled those criteria, all of whom developed ventricular arrhythmia upon reaching 87% of their maximum HR. However, in 6 out of 18 patients (33%), ventricular arrhythmias subsided as their sinus HR further increased with continued exercise. In another study that included data on the exercise testing characteristics of CPVT patients prior to β-blockers, mean HR at (ventricular) arrhythmia disappearance was 143 ± 17 beats per minute, while this patients reached a maximum HR of 154 ± 17 beats per minute (29). These data suggest that even in humans, at least in a subset of CPVT patients, ventricular arrhythmias can be paradoxically suppressed by fast sinus HRs. Based on our results with CPVT mouse models, we suggest that the shortened diastolic interval of sinus rhythm (or supraventricular rhythms) obtained during exercise exceeds the frequency of spontaneous Ca release events and DADs in ventricular cells and successfully suppresses ventricular ectopic activity.
Potential clinical implications and caveats
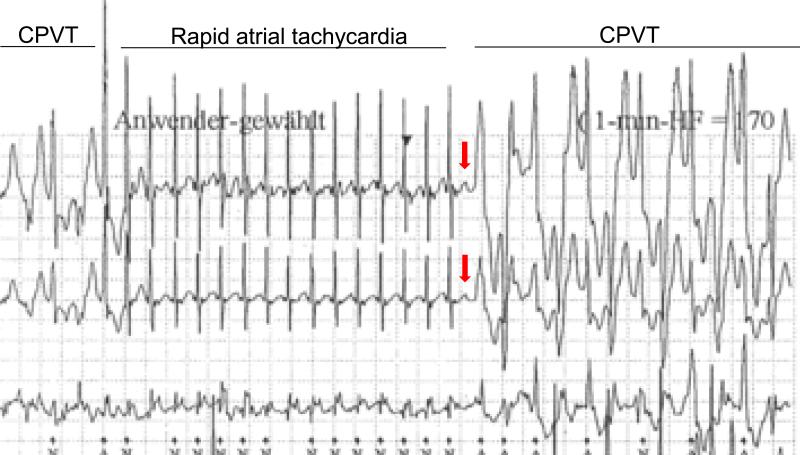
The experimental studies in mice and observations from a cohort of CPVT patients discussed above suggest that sinus node dysfunction may contribute to arrhythmia risk and could be targeted therapeutically. Hypothetically, reducing vagal tone or inhibiting muscarinic receptors in the sinoatrial node of CPVT patients during the early phases of exercise could establish a faster supraventricular rate that mimics the upward shift in the sinus HR obtained in mice with vagolytic drugs. This may be difficult to achieve clinically, given the significant systemic side effects of vagolytic drugs. Another option could be anti-tachycardia pacing therapy conceptually similar to the therapeutic ventricular pacing provided by ICD devices. Although this concept remains to be tested clinically, it is supported by a recent clinical case report of the temporary overdrive suppression of bidirectional VT by a rapid atrial tachycardia in a young CPVT patient (Fig. 4) (33). The EKG record in Fig. 4 also illustrates the focal nature of CPVT in humans, which can be suppressed by shortening of the diastolic interval further. Interestingly, studies on patients with the congenital long-QT syndrome have shown that HR at rest as well as the extent of vagal reflexes correlate with the risk of developing arrhythmic events and could potentially be used for risk stratification (34). One important caveat is that increasing the HR response during exercise will independently load the SR with Ca (35) and may promote the spontaneous Ca release events that trigger CPVT. However, our data in a CPVT cohort demonstrates that at least 30% of CPVT patients tolerated very fast sinus rates during exercise with paradoxical suppression of ventricular arrhythmia (9). To what extent this finding is applicable to other CPVT patients remains to be determined. Another important caveat is the site of overdrive pacing. Given that electrical stimulation by the pacing lead may trigger the release of norepinephrine from sympathetic nerve terminals in the ventricle (36), which in turn could further exacerbate the ventricular ectopic activity, atrial pacing likely will be the preferable approach. Unfortunately, most CPVT patients only have single ventricular lead ICDs (37). Nevertheless, atrial overdrive pacing should be tested in in CPVT patients to evaluate its safety and efficacy. If rapid atrial pacing is shown to prevent exercise-induced VT in CPVT patients, it may be feasible to automatically program high atrial rates during exercise using the rate-response feature (38), and thereby provide a non-pharmacological treatment option for preventing sudden cardiac death in CPVT.
Figure 4.
Example of CPVT overdrive suppression by atrial tachycardia. Holter electrocardiogram recorded during an exercise-related syncopal episode showing a bidirectional ventricular tachycardia typical of CPVT. During atrial tachycardia, heart rate slightly increases (up to approximately 290 bpm) and the atrial rhythm successfully overdrives the first run of ventricular tachycardia. When a “p” wave is not conducted promptly (red arrow), the coupling interval and hence diastolic interval of the ventricular response is increased and ventricular tachycardia resumes. Figure modified from (33).
With permission: Richter S, Gebauer R, Hindricks G, and Brugada P. A classic electrocardiographic manifestation of catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol. 2012;23(5):560.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91(5):1512–9. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103(2):196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 3.Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91(8):e21–6. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 4.Chopra N, Knollmann BC. Triadin regulates cardiac muscle couplon structure and microdomain Ca(2+) signalling: a path towards ventricular arrhythmias. Cardiovascular research. 2013;98(2):187–91. doi: 10.1093/cvr/cvt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, Fauconnier J, Brocard J, Denjoy I, et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21(12):2759–67. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyegaard M, Overgaard MT, Sondergaard MT, Vranas M, Behr ER, Hildebrandt LL, et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91(4):703–12. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vega AL, Tester DJ, Ackerman MJ, Makielski JC. Protein kinase A-dependent biophysical phenotype for V227F-KCNJ2 mutation in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2009;2(5):540–7. doi: 10.1161/CIRCEP.109.872309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glukhov AV, Kalyanasundaram A, Lou Q, Hage LT, Hansen BJ, Belevych AE, et al. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faggioni M, Hwang HS, van der Werf C, Nederend I, Kannankeril PJ, Wilde AA, Knollmann BC. Accelerated sinus rhythm prevents catecholaminergic polymorphic ventricular tachycardia in mice and in patients. Circulation research. 2013;112(4):689–97. doi: 10.1161/CIRCRESAHA.111.300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, et al. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med. 2014;20(2):184–92. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suetomi T, Yano M, Uchinoumi H, Fukuda M, Hino A, Ono M, et al. Mutation-linked defective interdomain interactions within ryanodine receptor cause aberrant Ca(2)(+)release leading to catecholaminergic polymorphic ventricular tachycardia. Circulation. 2011;124(6):682–94. doi: 10.1161/CIRCULATIONAHA.111.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meli AC, Refaat MM, Dura M, Reiken S, Wronska A, Wojciak J, et al. A novel ryanodine receptor mutation linked to sudden death increases sensitivity to cytosolic calcium. Circ Res. 2011;109(3):281–90. doi: 10.1161/CIRCRESAHA.111.244970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, et al. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun. 2010;394(3):660–6. doi: 10.1016/j.bbrc.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang HS, Nitu FR, Yang Y, Walweel K, Pereira L, Johnson CN, et al. Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circulation research. 2014;114(7):1114–24. doi: 10.1161/CIRCRESAHA.114.303391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99(5):1408–15. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashimura TBS, Trafford AW, Napolitano C, Priori SG, Eisner DA, Venetucci LA. In the RyR2(R4496C) mouse model of CPVT, β-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res. 2010;107(12):1483–9. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 17.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87(4):275–81. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 18.Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42(11):863–70. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Werf C, Nederend I, Hofman N, van Geloven N, Ebink C, Frohn-Mulder IM, et al. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ Arrhythm Electrophysiol. 2012;5(4):748–56. doi: 10.1161/CIRCEP.112.970517. [DOI] [PubMed] [Google Scholar]
- 20.Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, et al. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71(10):1606–9. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 21.Sumitomo N, Nakamura T, Fukuhara J, Nakai T, Watanabe I, Mugishima H, Hiraoka M. Clinical effectiveness of pulmonary vein isolation for arrhythmic events in a patient with catecholaminergic polymorphic ventricular tachycardia. Heart Vessels. 2010;25(5):448–52. doi: 10.1007/s00380-009-1214-6. [DOI] [PubMed] [Google Scholar]
- 22.Di Pino A, Caruso E, Costanzo L, Guccione P. A novel RyR2 mutation in a 2-year-old baby presenting with atrial fibrillation, atrial flutter, and atrial ectopic tachycardia. Heart Rhythm. 2014 doi: 10.1016/j.hrthm.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 23.Faggioni M, Savio-Galimberti E, Venkataraman R, Hwang HS, Kannankeril PJ, Darbar D, Knollmann BC. Suppression of spontaneous ca elevations prevents atrial fibrillation in calsequestrin 2-null hearts. Circulation Arrhythmia and electrophysiology. 2014;7(2):313–20. doi: 10.1161/CIRCEP.113.000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121(8):3277–88. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huke S, Knollmann BC. Oxidized CaMKII: a “heart stopper” for the sinus node? J Clin Invest. 2011;121(8):2975–7. doi: 10.1172/JCI58389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129(12):1276–85. doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Haugaa KH, Leren IS, Berge KE, Bathen J, Loennechen JP, Anfinsen OG, et al. High prevalence of exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia mutation-positive family members diagnosed by cascade genetic screening. Europace. 2010;12(3):417–23. doi: 10.1093/europace/eup448. [DOI] [PubMed] [Google Scholar]
- 29.Steriotis AK, Nava A, Rampazzo A, Basso C, Thiene G, Daliento L, et al. Follow-up with exercise test of effort-induced ventricular arrhythmias linked to ryanodine receptor type 2 gene mutations. Am J Cardiol. 2012;109(7):1015–9. doi: 10.1016/j.amjcard.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119(18):2426–34. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 31.van der Werf C, Zwinderman AH, Wilde AA. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace. 2012;14(2):175–83. doi: 10.1093/europace/eur277. [DOI] [PubMed] [Google Scholar]
- 32.van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57(22):2244–54. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter S, Gebauer R, Hindricks G, Brugada P. A classic electrocardiographic manifestation of catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol. 2012;23(5):560. doi: 10.1111/j.1540-8167.2011.02138.x. [DOI] [PubMed] [Google Scholar]
- 34.Crotti L, Spazzolini C, Porretta AP, Dagradi F, Taravelli E, Petracci B, et al. Vagal reflexes following an exercise stress test: a simple clinical tool for gene-specific risk stratification in the long QT syndrome. J Am Coll Cardiol. 2012;60(24):2515–24. doi: 10.1016/j.jacc.2012.08.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 36.Blinks JR. Field stimulation as a means of effecting the graded release of autonomic transmitters in isolated heart muscle. J Pharmacol Exp Ther. 1966;151(2):221–35. [PubMed] [Google Scholar]
- 37.Miyake CY, Webster G, Czosek RJ, Kantoch MJ, Dubin AM, Avasarala K, Atallah J. Efficacy of implantable cardioverter defibrillators in young patients with catecholaminergic polymorphic ventricular tachycardia: success depends on substrate. Circ Arrhythm Electrophysiol. 2013;6(3):579–87. doi: 10.1161/CIRCEP.113.000170. [DOI] [PubMed] [Google Scholar]
- 38.Thomas VC, Law IH, Evans WN. Cardioversion of intraatrial reentrant tachycardia using rate response. Pacing Clin Electrophysiol. 2012;35(7):e199–202. doi: 10.1111/j.1540-8159.2012.03387.x. [DOI] [PubMed] [Google Scholar]