Abstract
The alpha7 nicotinic acetylcholine receptor (nAChR) is a potential target in neuroinflammation. Screening a plant extract library identified Solidago nemoralis as containing methyl-quercetin derivatives that are relatively selective ligands for the alpha7 nAChR. Flavonoids are not known for this activity, so we screened a small library of pure flavonoids to confirm our findings. Some flavonoids, e.g. rhamnetin, displaced a selective alpha7 nAChR radioligand from rat brain membranes whereas similar structures e.g. sakuranetin, did not. To evaluate the contribution of this putative nAChR activity to the known anti-inflammatory properties of these flavonoids, we compared their effects on lipopolysaccharide induced release of inflammatory mediators from BV2 microglia. Both rhamnetin and sakuranetin reduced mediator release, but differed in potency (rhamnetin>sakuranetin) and the Hill slope of their concentration response curves. For rhamnetin the Hill coefficient was >3.0 whereas for sakuranetin the coefficient was 1.0, suggesting that effects of rhamnetin are mediated through more than one mechanism, whereas sakuranetin has a single mechanism. nACHR antagonists decreased the Hill coefficient for rhamnetin toward unity, which suggests that a nAChR-mediated mechanism contributes cooperatively to its overall anti-inflammatory effect. In contrast nAChR antagonists had no effect on the potency or Hill coefficient for sakuranetin, but a concentration of nicotine (1μM) that had no effect alone, significantly increased the Hill coefficient of this flavonoid. In conclusion, the anti-inflammatory effects of rhamnetin benefit cooperatively from a nAChR-mediated mechanism. This action, together with potent free radical scavenging activity, suggests that flavonoids with alpha7 nAChR activity have therapeutic potential in neuroinflammatory conditions.
Keywords: Solidago nemoralis, flavonoids, drug discovery, nicotinic acetylcholine receptors, neuroinflammation
1. Introduction
In the last decade, plant natural products have emerged as a valuable source for neuroprotective compounds [1]. Polyphenols (flavonoids, anthocyanins, chalcones, curcuminoids, stilbenoids) in particular have been widely investigated in this area [2] and they generally not only exhibit great antioxidant properties [3] but they also exhibit potent anti-inflammatory properties [4]. Therefore, they have come to light as valuable multifunctional compounds targeting two neurotoxic mechanisms (oxidative stress and neuroinflammation) associated with neurodegeneration.
Neuroinflammation has become known as a central mechanism of neurodegeneration in diseases such as Alzheimer's disease [5], Parkinson's disease [6] or alcohol induced neurotoxicity [7]. It is mediated primarily by microglia, the resident immune cells of the central nervous system (CNS). As a result of acute neuronal damage, microglia become alternatively activated resulting in tissue repair and clean-up [8]. However, when the neuronal insult becomes chronic, as observed in neurodegenerative diseases, microglia become classically activated ultimately leading to additional toxicity [9]. Therefore, the primary aim when targeting neuroinflammation is inhibiting the neurotoxic effects of classical microglial activation. Common, well-known anti-inflammatory drugs, such as ibuprofen, are currently being studied successfully for the treatment of neurodegenerative diseases [10]. However, the discovery of novel anti-inflammatory compounds specific to neuroinflammation has become pivotal in discovering effective treatments for neurodegeneration.
Nicotinic acetylcholine receptors (nAChRs) are emerging as very interesting molecular targets for attenuating neuroinflammation specifically. The homomeric alpha7 nAChR subtype in particular is heavily implicated both in the vagus nerve anti-inflammatory cholinergic pathway [11] and in brain tissues [12]. Wang et al [13] show in an elegant study using an alpha7 nAChR specific antisense oligonucleotide that nicotine inhibits tumor necrosis factor synthesis in activated macrophage cultures through alpha7 nAChRs activation. This finding was then extended to microglia where nicotine and acetylcholine attenuate both microglial activation and LPS-induced tumor necrosis factor alpha (TNF-alpha) release in a dose dependent manner through an alpha7 nAChR dependent pathway [14]. Therefore, an alpha7 nAChR selective agonist would be valuable for targeting neuroinflammation specifically. Moreover, it could have the potential to inhibit excitotoxicity, another primary mechanism of injury observed in neurodegeneration [15].
Although there has been considerable effort toward synthesizing alpha7 nAChR selective ligands in the last decade [16], few of them have made it to market. Interestingly, most of these synthetic compounds are derived from alkaloids originally discovered primarily in plants [17]. The majority of these are toxic or have abuse liability (like nicotine) due partly to their high affinity for alpha4beta2 nAChRs [18], the other major nAChR subtype in the brain. However, not all plant alkaloids have this selectivity. Thus, methyllycaconitine (MLA) is a relatively selective ligand for the alpha7 nAChR subtype that is isolated from the seeds of Delphinium brownii, a North American wildflower [19]. MLA has been used extensively in research for the study of alpha7 nAChRs but presents little therapeutic use as it is an antagonist [20]. With the exception of nicotine [21], few of these plant metabolites have been tested against neuroinflammation. However, plants may contain novel, non-toxic, and alpha7 nAChR selective agonists that would have therapeutic value for the treatment of neurodegenerative diseases by attenuating neuroinflammation.
In order to find plants containing metabolites with selective activity at alpha7 nAChRs we developed a differential screening approach and applied this to a large native plant species extract library [22]. These extracts were first screened for the presence of metabolites which bound to nAChRs in rat brain homogenates using a non-subtype selective ligand, [3H]-epibatidine. Of the 1000 extracts tested, about 350 species showed significant displacement of this ligand suggesting that metabolites which interact with nAChRs are common in this sample. Extracts from these 350 species were then compared for their ability to displace an alpha7 selective ligand, [3H]-MLA, or an alpha4beta2 selective ligand, [3H]-cytisine, from rat brain homogenates. Extracts which displaced the alpha7 selective ligand at lower concentrations than they displaced the alpha4beta2 selective ligand were considered likely to contain compounds with relative selectivity for alpha7 nAChRs. The great majority of plant extracts showed the reverse selectivity, suggesting that the majority of plant metabolites in this library are, like nicotine, relatively selective for alpha4beta2 nAChRs. However, 8 species extracts showed relative selectivity for alpha7 nAChRs [22] and these are now under investigation to determine the active metabolites which they contain.
One of the first sources of alpha7 selective binding activity to be investigated was Solidago nemoralis which, to our knowledge, has not been reported to contain bioactive alkaloids, the major class of natural products from plants which act on nAChRs. Assay-guided fractionation followed by preparative HPLC and mass spectrometry identified specific methyl-quercetin derivatives as responsible for the displacement of [3H]-MLA binding. This was surprising for two reasons. First, similar flavonoids are widespread in plants, so it seemed likely that many other plant species should contain alpha7 selective binding activity based on this type of compound. However, our identification of S. nemoralis as “positive” was based not only on binding to alpha7 nAChRs, but also on the relative absence of binding to alpha4beta2 nAChRs. Since alkaloids which bind to alpha4beta2 nAChRs are widespread in plants [22] this activity will mask the presence of alpha7 selective compounds in many species. Thus it may be precisely because S. nemoralis contains no bioactive alkaloids that we were able to identify the active flavonoids in this species using our differential screen. The second surprising aspect of this discovery is that flavonoids have never previously been reported to interact directly with nAChRs. However, closely following our observation, electrophysiological studies indicated that quercetin has co-agonist effects on the alpha7 nAChR expressed in Xenopus oocytes [23]. This activity of flavonoids at alpha7 nAChRs is potentially important in several of their known therapeutic effects. For example, flavonoids have been extensively investigated for their anti-inflammatory properties peripherally [24] and in the CNS [25] as well as for their neuroprotective effects in models of neurodegeneration [26]. Many of these effects could be mediated through alpha7 nAChRs but this has never previously been investigated. It will depend on which specific flavonoids have this activity, and the extent to which alpha7 nAChRs are involved in the pathological response. The main aim of this study is therefore to assess the alpha7 nAChR selectivity of a small library of pure flavonoids and whether this activity translates to enhanced anti-inflammatory properties on activated microglia.
2. Materials and methods
2.1. Chemicals, reagents and kits
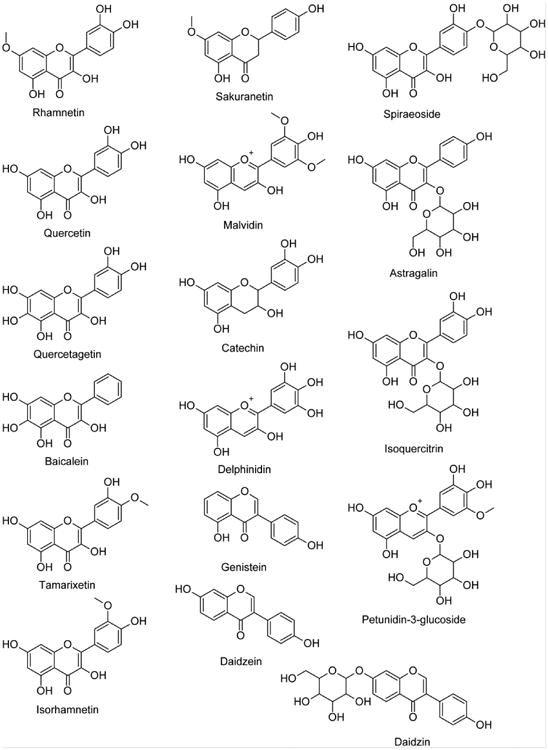
Methanol, hexane, chloroform, ethyl acetate, butanol, lipopolysaccharide (LPS) from Escherichia coli serotype 026:B6 (Lot# 021M4072V), (-)-nicotine, methyllycaconitine citrate salt hydrate (MLA) from Delphinium brownii seeds, mecamylamine hydrochloride and 7-hydroxy-3H-phenoxazin-3-one-10-oxide sodium salt (resazurin) were purchased from Sigma-Aldrich (St Louis, MO, USA). Astragalin, baicalein, catechin, daidzin, daidzein, delphinidin, genistein, isoquercitrin, isorhamnetin, malvidin, petunidin-3-glycoside, quercetagetin, quercetin, rhamnetin, sakuranetin, spiraeoside and tamarixetin (Fig. 1) were purchased from Chromadex (Irvine, CA, USA). [3H]-MLA (∼60Ci/mmol), [3H]-cytisine (∼16Ci/mmol) and [3H]-epibatidine (∼30Ci/mmol) were purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO, USA). Antibiotics (10000U/mL penicillin and 10000ug/mL streptomycin), 2.5% trypsin (10×), fetal bovine serum (FBS), Dulbeccos' Modified Eagle Medium (DMEM), DMEM:Nutrient mixture F-12 (DMEM/F-12) and Hanks Balanced Salt Solution (HBSS) were purchased from Life Technologies Corporation (Grand Island, NY, USA). Griess reagent system was purchased from Promega Corporation (Madison, WI, USA). TNF-alpha ELISA Ready-SET-Go!® was purchased from eBioscience Inc. (San Diego, CA, USA).
Figure 1. Pure flavonoids screened for [3H]-MLA displacement in rat hippocampal membranes.
2.2. Solidago nemoralis extraction and chemical identification
2.2.1. Assay guided fractionation
The aerial parts of Solidago nemoralis, including stems and flowers, were dried, ground and suspended in methanol for 24 hours. The methanol extract was decanted and the resulting plant material was re-suspended in fresh methanol for another 24 hours. Methanol extracts were then pooled and dried in a rotary evaporator. The dry methanol extract was re-suspended in water and subsequently extracted in hexane, chloroform, ethyl acetate and butanol. Resulting fractions were assayed for [3H]-MLA displacement on rat hippocampal membranes (see below).
2.2.2. Preparative high performance liquid chromatography (HPLC)
Preparative HPLC was performed with a Waters XBridge preparative c18 column (5μm particles, 19×150mm) and a gradient analysis method (flow rate= 7mL/min) where methanol was gradually increased and water proportionally decreased. Detection was performed with the Waters 2998 photodiode array detector and fractions were collected with the Waters 2767 sample manager. Resulting fractions were assayed for [3H]-MLA displacement on rat hippocampal membranes (see below).
2.2.3. Electrospray ionization mass spectrometry (ESI-MS/MS)
Preparative HPLC samples were dried and re-suspended in methanol/MilliQ water and analyzed at the University of Kentucky Mass Spectrometry Facility using ESI-MS/MS in both positive and negative ion ESI modes.
2.3. Radioligand binding studies
2.3.1. Rat brain membrane preparation
Hippocampal and cortical tissues were removed from adult male Sprague-Dawley rats (225-250g) and homogenized in sucrose buffer (0.32 M sucrose, 1mM EDTA, 0.1mM phenylmethylsulfonyl fluoride, 0.01% w/v sodium azide, pH adjusted to 7.4), centrifuged at 1,000g for 10min at 4°C and the supernatant reserved on ice. The pellet was re-suspended and centrifuged once more at 1,000g for 10min at 4°C. The supernatant was combined with the previously reserved supernatant and centrifuged at 50,000g for 10min at 4°C. The resulting supernatant was discarded and the pellet washed twice by re-suspension and centrifugation in binding buffer (50mM Tris, 144mM NaCl, 1.5mM KCl, 2mM CaCl2, 1mM MgSO4.7H2O, 20mM HEPES, pH adjusted to 7.4) at 50,000g for 10min at 4°C. Total protein content was measured using the Bicinchoninic Acid Kit (Sigma-Aldrich), adjusted to 3mg/ml protein and frozen at -80°C for future experimentation.
2.3.2. General radioligand binding method
Solutions to be tested were mixed with membranes (final protein content = 1mg/ml) and radioligand (2nM [3H]-MLA or 2nM [3H]-cytisine for displacement studies) in individual wells on 96 well plates. Reactions were allowed to reach equilibrium (2h-3h) and the plates were harvested by vacuum filtration onto GF/B filter plate (Perkin Elmer Inc., Waltham, MA, USA) followed rapidly by 3 washes with 50mM Tris-HCl buffer (pH adjusted to 7.4). Filter plates were dried overnight and scintillation counting was performed for 2min per well in a Packard TopCount® NXT™ microplate scintillation and luminescence counter following the addition of 35μL scintillation fluid (Microscint 20, Packard Inc) to each filter well. For each assay plate, non-specific binding was measured in the presence of excess nicotine (300μM final concentration) and specific binding was calculated by subtracting non-specific binding from total binding of radioligand alone. Specific binding in the presence of competitors was converted to percentage of total specific binding of radioligand alone
2.3.3. Pure flavonoid competition binding experiments
Flavonoids were solubilized at high concentration (100mM) in 100% DMSO and diluted in binding buffer to obtain stock solutions of 30μM. The stock solutions were subjected to 1:10 serial dilutions to obtain a wide range of flavonoid concentrations. Using the general radioligand binding method described above, flavonoids were assessed for [3H]-cytisine and [3H]-MLA displacement.
2.4. BV2 microglia studies
2.4.1. BV2 microglia cell culture
BV2 murine microglia (kindly provided by Dr. Linda Van Eldik) were cultured in DMEM/F-12 supplemented with 10% FBS and antibiotics (penicillin 100U/ml, streptomycin 100ug/ml). Cells were kept at 37°C in a humidified atmosphere of air and 5% CO2. For membrane preparation and RLB studies, cells were propagated in T75 flasks, harvested by cell scraping upon confluency, centrifuged (1200 rpm, swinging bucket) for 4 min, re-suspended in sucrose buffer and frozen for future use. Membranes were then prepared as described above for animal tissue. Membranes were adjusted to a final concentration of 3mg/ml and frozen at -80°C for future experimentation. For LPS elicited inflammatory mediator release, cultured cells were detached upon confluency with 0.25% trypsin (2min at 37°C), seeded in 24 well plates at densities of 5×105 cells/well and allowed to adhere overnight. Cells were then pretreated with test compounds (serum-free media) for 1h and subjected to LPS challenge (10μg/ml in serum-free media) for 24h.
2.4.2. Saturation binding with BV2 membranes
Using the general radioligand binding method described above, saturation binding experiments were undertaken as follows: BV2 membranes were mixed in individual wells of a 96 well plate with increasing concentrations of radioligand ([3H]-MLA and [3H]-epibatidine) in the presence or absence of excess nicotine (300μM). Specific binding at each concentration was calculated by subtracting non-specific binding to total binding.
2.4.3. Measurement of cell viability, nitric oxide (NO) content and TNF-alpha release
Cell-free culture supernatants were collected, assayed for nitrite content immediately to avoid the effects of freeze-thawing [27] and subsequently frozen for future TNF-alpha content analysis. Each well was then filled with fresh media containing 100μM resazurin and allowed to incubate for 4h. Fluorescence (Excitation = 560nm, emission = 590nm) was then measured using a Wallac 1420 VICTOR plate reader (PerkinElmer, MA, USA) and cell viability was normalized to percentage control (no LPS group). Nitrite levels were measured using the Griess Reagent System (Promega) in accordance with the manufacturer's instructions. Briefly, 50μL of experimental samples were plated in a 96 well plate, 50μL sulphanilamide and 50μL N-1-napthylethylenediamine dihydrochloride were then added sequentially with a 5min incubation interval. Absorbance was measured at 550nm using a Wallac 1420 VICTOR plate reader (PerkinElmer, MA, USA) and the amount of nitrite was calculated from a NaNO2 standard curve. TNF-alpha levels were measured by ELISA using the READY-SET-GO! Mouse TNF-alpha kit (eBioscience, CA, USA) in accordance to the manufacturer's instruction. When necessary, sample dilutions were performed in order to fall within the concentration range of the standard curve.
2.5. Statistical analysis and graphical presentation
Statistical analysis and graphical presentation were done in GraphPad Prism 4.03 for Windows (GraphPad Software, CA, USA). Displacement binding data was fitted with non-linear regression using the sigmoidal dose-response with variable slope and with the top and bottom constrained at 100% and 0% respectively. Binding parameters were extrapolated from the curves and flavonoids were ranked in ascending pIC50 values for prioritization into functional studies. For saturation binding experiments, total and specific binding were fitted with a one site binding hyperbola and non-specific binding was fitted linearly. Binding parameters were extrapolated from specific binding. For dose response curves in functional assays, inflammatory mediator release (NO or TNF-alpha) was normalized by cell viability when statistical differences were found in cell viability between treatment groups by one way ANOVA. Two non-linear regression models (sigmoidal dose-response and sigmoidal dose-response with variable slope) were then compared using an F-test prior to fitting. Response parameters were extrapolated from the best fit. IC50 values were statistically compared using unpaired t-tests on the corresponding pIC50 values.
3. Results
3.1. [3H]-MLA displacing fractions from Solidago nemoralis contain methyl-quercetin derivatives
Solidago nemoralis was previously identified as containing selective binding activity for the alpha7 nAChR relative to beta2-containing nAChRs using our radioligand binding differential smart screen [22]. Therefore, we sought to identify the compound(s) responsible for this activity using [3H]-MLA displacement guided fractionation, pHPLC and ESI-MS/MS. [3H]-MLA displacement on Solidago nemoralis extract solvent partitions indicated that the activity was in the ethyl acetate. Further separation of the ethyl acetate fraction using preparative HPLC yielded a single [3H]-MLA displacing fraction. This fraction was sent for ESI-MS/MS analysis which produced 3 major peaks at m/z787, m/z657 and m/z515. Based on a literature search, their fragmentation patterns are indicative of three distinct compounds: a quercetin derivative, a quercetagetin derivative and a dicaffeoylquinic acid (Table 1).
Table 1. MS/MS fragments of major MS peaks and compound identification from the literature.
A small library of pure dicaffeoylquinic acid derivatives was compiled and tested for [3H]-MLA displacement. None of the compounds tested displaced [3H]-MLA (data not shown) suggesting that the nAChR activity present in the extract is attributable to the methyl-quercetin derivatives identified. We were unable to purify large enough amounts of the active fractions for definitive identification using NMR. Instead, we compiled a small library of pure flavonoids to be screened for [3H]-cytisine and [3H]-MLA displacement.
3.2. Flavonoids differentially displace [3H]-MLA from hippocampal rat membranes
Pure flavonoids were tested for their ability to displace [3H]-cytisine and [3H]-MLA from rat cortical and hippocampal membranes respectively. Most compounds tested did not displace [3H]-cytisine or did so minimally preventing the extrapolation of any kind of binding parameters from fitted displacement curves. However, the majority of compounds tested, but not all, dose dependently inhibited specific [3H]-MLA binding resulting in a wide range of IC50 values (Table 2). Therefore, [3H]-MLA displacement is not a general property of flavonoids and while some structures displace [3H]-MLA (rhamnetin, spiraeoside, quercetagetin), others do not (genistein, daidzein, sakuranetin). The lack of [3H]-cytisine displacement, a ligand selective for beta2-containing nAChRs, in conjunction with the differential displacement of [3H]-MLA observed suggests that these compounds may be more selective for alpha7 nAChRs than for alpha4beta2 nAChR.
Table 2. [3H]-MLA displacement parameters of pure flavonoids from hippocampal rat membranes.
| Flavonoid | pIC50 | IC50 (μM) | Subclass |
|---|---|---|---|
| Rhamnetin | 5.953 ± 0.15 | 1.11 | Flavonol |
| Spiraeoside | 5.078 ± 0.13 | 8.35 | Flavonol |
| Quercetin | 4.940 ± 0.05 | 11.4 | Flavonol |
| Quercetagetin | 4.846 ± 0.21 | 14. 2 | Flavonol |
| Isoquercitrin | 4.667 ± 0.22 | 21.5 | Flavonol |
| Isorhamnetin | 4.332 ± 0.17 | 46.5 | Flavonol |
| Baicalein | 4.18 ± 0.09 | 66.1 | Flavonol |
| Malvidin | 4.046 ± 0.11 | 90.0 | Anthocyanidin |
| Daidzin | 3.922 ± 0.20 | 119.8 | Isoflavone |
| Tamarixetin | 3.623 ± 0.23 | 238.4 | Flavonol |
| Astragalin | 3.305 ± 0.78 | 495.6 | Flavonol |
| Petunidin-3-glucoside | 2.464 ± 0.95 | 3434 | Anthocyanidin |
| Catechin | 0.3036 ± 1.7 | 497100 | Flavan-3-ol |
| Delphinidin | 0.8436 ± 4.1 | 6976000 | Anthocyanidin |
| Genistein | N/A | N/A | Isoflavone |
| Daidzein | N/A | N/A | Isoflavone |
| Sakuranetin | N/A | N/A | Flavanone |
3.3. BV2 microglia exhibit functional nicotinic acetylcholine receptors
Microglial cultures purified from rat brain express functional nAChRs and activation of the alpha7 subtype exerts anti-inflammatory effects [33]. However, and as far as the authors know, there is only indirect evidence in the literature for the presence of functional nicotinic receptors in the BV2 immortalized microglial cell line [34, 35]. Therefore, we sought to evaluate the presence of functional nAChRs in BV2 by identifying membrane expressed nAChR with [3H]-MLA saturation binding on BV2 membranes and by assessing the functional anti-inflammatory effects of nicotine on LPS stimulated live cells.
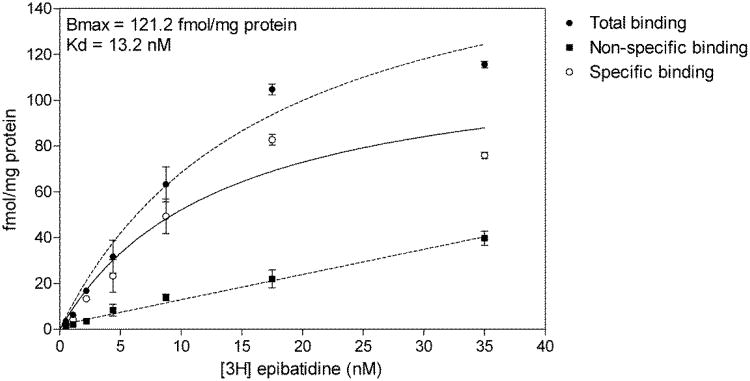
We obtained evidence for [3H]-MLA specific binding in BV2 microglia membranes. However, we were unable to reach saturation, and thus extrapolate binding parameters, due to a low apparent affinity. The low affinity of [3H]-MLA (nM range) [36] relative to that of [3H]-epibatidine (pM range) [37] for alpha7 nAChRs and the expenses associated with carrying out [3H]-MLA saturation binding prompted the alternative use of [3H]-epibatidine. BV2 microglia membranes were found to be saturable with [3H]-epibatidine (Fig. 2 – Bmax = 121.2fmol/mg protein; Kd = 6.5nM) indicating that nicotinic binding sites are present on BV2 membranes.
Figure 2.
[3H]-epibatidine saturation binding on BV2 membranes. BV2 membranes were incubated with increasing concentrations of [3H]-epibatidine in the presence or absence of 300μM nicotine. Specific binding was calculated by subtracting non-specific binding from total binding. Non-specific binding was fit with linear regression while total and specific binding were fit with non-linear one site binding hyperbola.
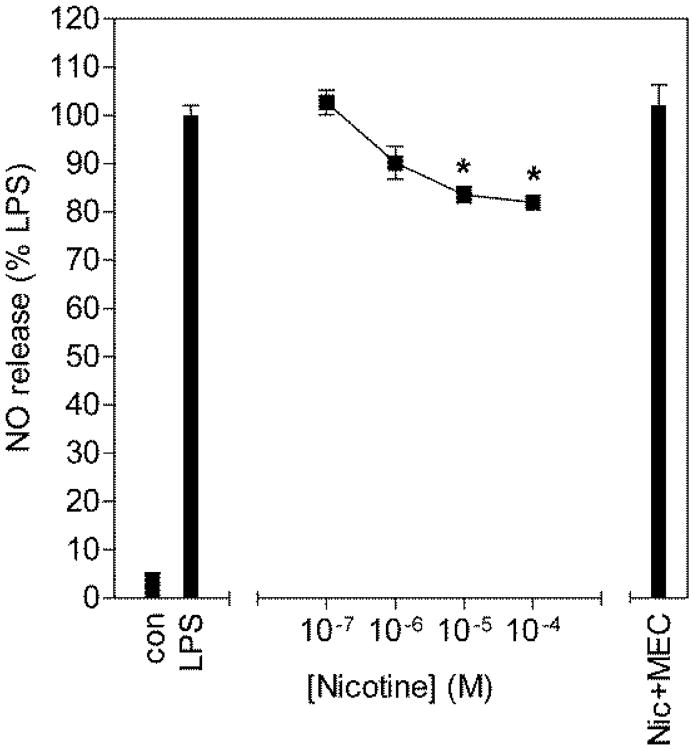
In cultured BV2 cells, nicotine dose dependently inhibited LPS elicited release of NO with a maximal inhibition of ∼20% (Fig. 3). Furthermore, this effect was blocked by 10μM mecamylamine, a non-selective nicotinic antagonist, suggesting that nicotinic receptors present on BV2 microglia are functional and can be pharmacologically manipulated.
Figure 3.
Inhibition of NO release from LPS stimulated BV2 microglia by nicotine. BV2 microglia were pretreated for 1h with increasing concentrations of nicotine and subsequently challenged with 10μg/ml LPS for 24h. Culture media was collected and assayed for nitrite content using the Griess reaction. NO release is expressed as percentage of NO release from LPS alone. * p<0.001 compared to LPS alone and Nic+MEC by Tukey's multiple comparison test following a one-way ANOVA.
3.4. [3H]-MLA displacement predicts functional anti-inflammatory nicotinic mediated effects
To assess the functional significance of [3H]-MLA displacement studies, rhamnetin, which potently displaces [3H]-MLA with the lowest IC50, and sakuranetin, which does not displace [3H]-MLA and therefore constitutes a structurally related negative control, were tested for their anti-inflammatory properties on LPS stimulated BV2 microglia. Flavonoids are well known to be anti-inflammatory in this cell line [38, 39]. Therefore, to evaluate the contribution of putative nicotinic activity, their effects were blocked with MLA.
3.4.1. Rhamnetin is anti-inflammatory and benefits from a nicotinic mediated mechanism
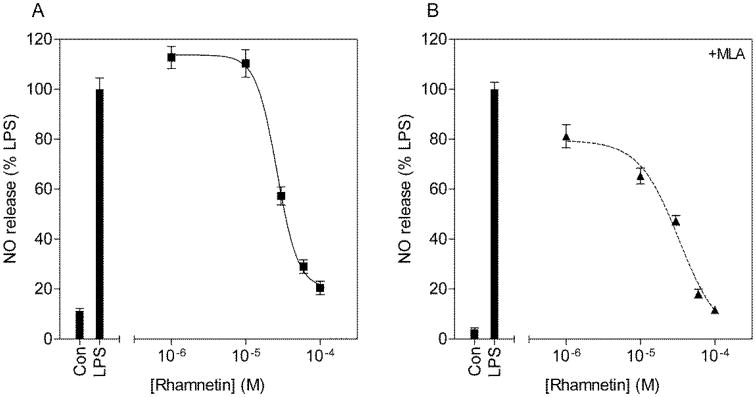
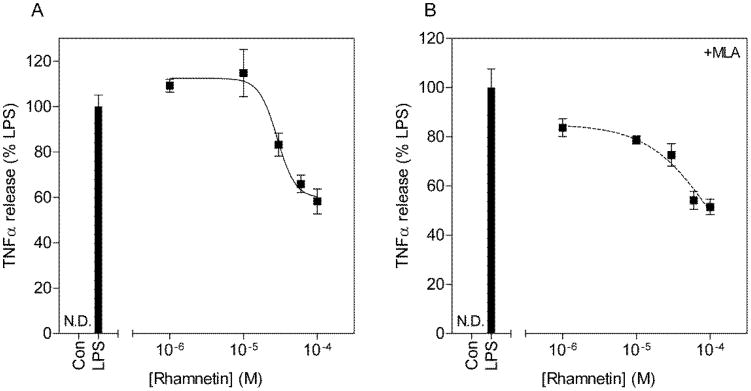
Rhamnetin dose dependently inhibited NO (IC50 = 26.3μM) and TNF-alpha (IC50 = 28.7μM) release (Fig. 4 & Fig. 5). The addition of MLA increased the IC50s for both inhibition of NO release (IC50 = 33.1μM) and inhibition of TNF-alpha release (IC50 = 105.3μM) although this did not reach statistical significance (t(6) = 0.94, p = 0.37 for NO; t(6) = 1.3, p = 0.22 for TNF-alpha). Furthermore, close examination of the concentration response curves without MLA revealed Hill coefficients of 3.0±0.9 and 3.5±4.1 respectively (Table 3). The addition of MLA decreased the Hill coefficients to 1.6±0.4 for inhibition of NO release and the concentration response curve for inhibition of TNF-alpha release best fit the sigmoidal dose response model with no variable slope (F(1,16) = 2.14, p = 0.16). These data collectively suggest that rhamnetin mediates its anti-inflammatory effects in part through nAChRs.
Figure 4.
Concentration response curves for the inhibition of NO release from LPS stimulated BV2 microglia by rhamnetin (A) and with the addition of MLA (B). BV2 microglia were pretreated with increasing concentrations of rhamnetin, in the presence or absence of 10μM MLA for 1h and subsequently challenged with 10μg/ml LPS for 24h. Culture media was collected and assayed for nitrite content using the Griess reaction. NO release is expressed as percentage of NO release from LPS alone.
Figure 5.
Concentration response curves for the inhibition of TNF-alpha release from LPS stimulated BV2 microglia by rhamnetin (A) and with the addition of MLA (B). BV2 microglia were pretreated with increasing concentrations of rhamnetin, in the presence or absence of 10μM MLA for 1h and subsequently challenged with 10μg/ml LPS for 24h. Culture media was collected and assayed for TNF-alpha content by ELISA. TNF-alpha release is expressed as percentage of TNF-alpha release from LPS alone.
Table 3. Concentration response curve parameters for the inhibition of NO release and TNF-alpha release by rhamnetin.
|
|
|||
|---|---|---|---|
| Rhamnetin | IC50 (μM) | pIC50 | Hill slope |
| NO release | 26.3 | 4.579 ± 0.03 | -3.0 ± 0.9 |
| + MLA (10μM) | 33.1 | 4.48 ± 0.10 | -1.6 ± 0.4 |
|
| |||
| TNF-alpha release | 28.7 | 4.541 ± 0.06 | -3.5 ± 4.1 |
| + MLA (10μM) | 105.3 | 3.978 ± 0.41 | -1.0* |
best fit the sigmoidal dose response curve with no variable slope
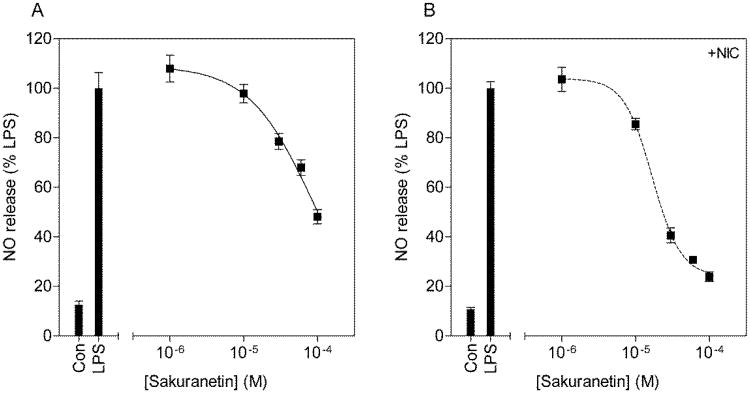
3.4.2. The anti-inflammatory activity of sakuranetin does not benefit from a nicotinic mediated mechanism
Sakuranetin dose dependently inhibited NO (IC50 = 85.8μM) and TNF-alpha (IC50 = 158μM) release (Fig. 6 & Fig. 7), albeit with lower relative potency compared to rhamnetin. Close examination of the dose response curves indicated Hill coefficients at unity (Table 4) since both curves fit the sigmoidal dose response model with no variable slope (F(1,16) = 0.0009, p = 0.97 for NO; F(1,16) = 3.5, p = 0.07 for TNF-alpha). The addition of MLA had no effect on the anti-inflammatory properties of sakuranetin. However, the addition of a below threshold concentration of nicotine (1μM) decreased IC50s for both NO (IC50 = 16.9μM) and TNF-alpha inhibition (IC50 = 22.3μM). The difference was found to be statistically significant for NO (t(6) = 2.9, p < 0.05) but not for TNF-alpha inhibition (t(6) = 1.2, p = 0.25). Furthermore, the Hill coefficients were significantly increased to 2.2±0.3 for inhibition of NO release (F(1,16) = 15.54, p < 0.01) and 2.7±1.0 for inhibition of TNF-alpha release (F(1,16) = 6.7, p < 0.05). These data suggest that sakuranetin does not mediate its anti-inflammatory effects through nAChRs but that a below threshold concentration of nicotine can enhance its pharmacological profile.
Figure 6.
Concentration response curves for the inhibition of NO release from LPS stimulated BV2 microglia by sakuranetin (A) and with the addition of nicotine (B). BV2 microglia were pretreated with increasing concentrations of sakuranetin, in the presence or absence of 1μM nicotine for 1h and subsequently challenged with 10μg/ml LPS for 24h. Culture media was collected and assayed for nitrite content using the Griess reaction. NO release is expressed as percentage of NO release from LPS alone.
Figure 7.
Concentration response curves for the inhibition of TNF-alpha release from LPS stimulated BV2 microglia by sakuranetin (A) and with the addition of nicotine (B). BV2 microglia were pretreated with increasing concentrations of sakuranetin, in the presence or absence of 1μM nicotine for 1h and subsequently challenged with 10μg/ml LPS for 24h. Culture media was collected and assayed for TNF-alpha content by ELISA. TNF-alpha release is expressed as percentage of TNF-alpha release from LPS alone.
Table 4. Concentration response curve parameters for the inhibition of NO release and TNF-alpha release by sakuranetin.
|
|
|||
|---|---|---|---|
| Sakuranetin | IC50 (μM) | pIC50 | Hill slope |
| NO release | 85.8 | 4.06 ± 0.24 | -1.0** |
| + Nicotine (1μM) | 16.9 | 4.77 ± 0.04* | -2.2 ± 0.3 |
|
| |||
| TNF-alpha release | 158.0 | 3.80 ± 0.67 | -1.0** |
| + Nicotine (1μM) | 22.3 | 4.65 ± 0.06 | -2.7 ± 1.0 |
p<0.05 compared to NO release of sakuranetin alone by t-test
best fit the sigmoidal dose response curve with no variable slope
4. Discussion
Preliminary studies in our laboratory using a radioligand binding differential smart screen on a large plant extract library found that Solidago nemoralis extracts produce selective [3H]-MLA displacement relative to [3H]-cytisine displacement suggesting it contains compounds selective for the alpha7 nAChR [22]. Chemical analysis of a semi-pure [3H]-MLA displacing fraction indicated the presence of methyl-quercetin-like flavonoids and a dicaffeoylquinic acid. Lack of [3H]-MLA displacement from a small library of pure dicaffeoylquinic acid derivatives suggested that flavonoids may be responsible for the nAChR activity of the plant extract. Thus, the present studies were conducted to test the hypothesis that specific flavonoid structures selectively bind alpha7 nAChR relative to alpha4beta2 nAChRs. Therefore, pure flavonoids were evaluated for their ability to displace [3H]-MLA, a relatively selective ligand for the alpha7 nAChR, and [3H]-cytisine, selective for beta2-containing nAChRs. Our original predictions were that some flavonoids would displace [3H]-MLA only, some flavonoids would displace [3H]-cytisine only and some would displace both with varying degrees of potency.
Specific flavonoid structures, such as rhamnetin, specifically displaced [3H]-MLA dose dependently while other specific structures, such as sakuranetin, did not. Therefore, [3H]-MLA displacement is not a general property of flavonoids despite structural similarities. For example, rhamnetin only has 2 additional hydroxyl groups (3-hydroxyl and 3′-hydroxyl) and an unsaturated B ring in comparison to sakuranetin. Interestingly, it appears that flavonols, as a specific subclass, exhibit overall more potent [3H]-MLA displacement in comparison to other subclasses such as anthocyanidins, isoflavones and flavan-3-ols. Therefore, flavonol specific functional groups, such as 4-carbonyl group and 3-hydroxyl group, which are inexistent in other classes, may be contributing to selective [3H]-MLA displacement. Despite relatively low potencies for [3H]-MLA displacement, those for rhamnetin, spiraeoside, and quercetin for example, fall within the same range as physiological concentrations of flavonoids reported in plasma [40] and reported anti-inflammatory potencies in vitro [41]. Therefore, specific structures displace [3H]-MLA specifically at physiological relevant concentrations. On the other hand, none of the flavonoids tested appeared to displace [3H]-cytisine. Collectively, these results support our hypothesis that certain flavonoid structures specifically bind alpha7 nAChRs relative to alpha4beta2 nAChRs. A recent report by Lee et al [23] shows that quercetin is capable of enhancing acetylcholine induced inward currents through human alpha7 nAChRs expressed on xenopus oocytes, potentially through interaction with the Ca2+ binding site. Therefore, we cannot exclude the possibility that flavonoids may displace [3H]-MLA non-specifically through an allosteric mechanism at the Ca2+ binding site. In turn, this could explain the lack of [3H]-cytisine displacement as beta2-containing nAChR are less permeable to Ca2+ than alpha7 nAChRs [42]. Nevertheless, [3H]-MLA displacement may have functional significance for the anti-inflammatory properties of these flavonoids which may translate to added value as pharmacotherapies for neuroinflammation specifically. Therefore, we decided to evaluate and compare rhamnetin and sakuranetin as prototypical [3H]-MLA-displacing and non-[3H]-MLA-displacing flavonoids against LPS elicited inflammatory mediator release from BV2 microglia.
Flavonoids are plant secondary metabolites that are involved in attracting pollinators, deterring herbivorous insects, and allelopathy [43]. They are found in a variety of plant species as well as throughout the human diet in foods such as fruits and vegetables, tea, and cocoa and have been described for their various health benefits [44]. Sakuranetin is the major flavonoid found in rice [45] and it has been primarily studied for its anti-inflammatory [46], anti-diabetic [47], and anti-bacterial [48] properties. Rhamnetin is found in most buckthorns (rhamnus genus) [49] and has been primarily studied for its anti-cancer [50], antioxidant [51] and anti-inflammatory properties [41]. Thus, both rhamnetin and sakuranetin have been found to be anti-inflammatory. Therefore, we hypothesized that rhamnetin, which displaces [3H]-MLA, would benefit from enhanced anti-inflammatory effects, via a nicotinic mediated mechanism, in comparison to sakuranetin, which does not displace [3H]-MLA. The anti-inflammatory properties of both compounds were evaluated by their ability to dose dependently inhibit NO and TNF-alpha release from LPS stimulated BV2 microglia. Additionally, to assess their putative nicotinic activity, they were pharmacologically manipulated with nicotinic agonists and antagonists. We predicted that (1) rhamnetin would be more potent than sakuranetin, (2) rhamnetin would be partially inhibited by nAChR antagonists and (3) sakuranetin would become more potent with the addition of nAChR agonists.
Prior to examining the anti-inflammatory properties of rhamnetin and sakuranetin, BV2 microglia were validated for cell surface presence of functional nAChRs. There is little evidence in the literature for functional nAChRs in this cell line. Mencel et al [35] report that the alpha7 nAChR subtype can be detected by western blot in BV2 microglia lysates. However, no pharmacological manipulation of the nicotinic receptors in BV2 microglia has been reported to date despite an attempt to block the anti-inflammatory effects of donepezil with MLA and DHBE in these cells, which did not produce an effect [34]. In our hands, BV2 microglia membranes exhibited [3H]-MLA specific binding and were saturable with [3H]-epibatidine. Furthermore, nicotine was found to inhibit NO release from LPS stimulated cells. Therefore, the BV2 microglia cell line appears to have functional nAChRs that can be pharmacologically manipulated to produce anti-inflammatory effects.
Rhamnetin and sakuranetin both dose-dependently inhibited NO and TNF-alpha release with relatively similar potencies but close examination of their concentration-response curves revealed differences in IC50s and Hill coefficients. Rhamnetin exhibited lower IC50s and higher Hill coefficients than sakuranetin. Additionally, pharmacological blockade of rhamnetin with MLA increased IC50s and decreased Hill coefficients. Furthermore, a non-effective concentration of nicotine (1μM) was able to decrease IC50s and increase Hill coefficients for sakuranetin. The Hill coefficient, first described by Archibald Hill in 1910 [52], is a well known parameter in the field of biochemistry where it is extensively used to describe cooperative binding of a ligand or substrate to a receptor or enzyme. However, in the fields of cellular biology, it is used to describe the ultrasensitivity of a system, such as a signal transduction pathway [53]. In our study, we interpret it to describe the activation of numerous mechanisms that cooperate towards a common outcome, namely inhibition of inflammatory mediator release. Therefore, these data collectively support our hypothesis that [3H]-MLA-displacing flavonoids benefit from a nicotinic mediated anti-inflammatory mechanism.
The alpha7 nAChR selective activity of rhamnetin has important implications not only for enhancement of anti-inflammatory effects but also for its potential as an alpha7 nAChR selective agonist. Indeed, activation of the alpha7 nAChR selectively has the potential to inhibit excitotoxicity mediated through NMDA receptors. Nicotine has been found to be neuroprotective against NMDA induced excitotoxicity through alpha7 nAChR activation both in rat primary hippocampal cultures [15] and hippocampal slice cultures [54]. This activity of nicotine has been proposed as an explanation for the negative association of cigarette smoking with the incidence of AD and PD [55]. Therefore, rhamnetin, as an alpha7 nAChR selective agonist would not only attenuate neuroinflammation but also inhibit excitotoxicity simultaneously. Together with its well-known antioxidant properties, rhamnetin is potentially a multifunctional neuroprotective agent that can simultaneously attenuate neuroinflammation, oxidative stress and excitotoxicity observed in neurodegenerative diseases.
Acknowledgments
This work was supported in part by NIAAA (National Institute on Alcohol Abuse and Alcoholism) grants (R21-AA020188, R42-AA014555, and R42-AA015475) awarded to Dr. Littleton as Principal Investigator. The authors would like to thank Dr. Linda Van Eldik from the Sanders Brown Center on Ageing at the University of Kentucky for providing BV2 microglia.
Abbreviations
- nAChR
nicotinic acetylcholine receptor
- MLA
methyllycaconitine
- NO
nitric oxide
- TNF-alpha
tumor necrosis factor alpha
- IC50
50% inhibitory concentration
Footnotes
Chemical compounds studied in this article: Rhamnetin (PubChem CID: 5281691); Sakuranetin (PubChem CID: 73571); Spiraeoside (PubChem CID: 5320844); Quercetin (PubChem CID: 5280343); Quercetagetin (PubChem CID: 5281680); Isoquercitrin (PubChem CID: 5280804); Isorhamnetin (PubChem CID: 5281654); Baicalein (PubChem CID: 5281605); Malvidin (PubChem CID: 159287); Daidzin (PubChem CID: 107971)
Conflict of interest: Dr. Littleton owns stock in Naprogenix™ and functions as Chief Scientific Officer. The remaining authors have no other conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levi MS, Brimble MA. A review of neuroprotective agents. Curr Med Chem. 2004;11:2383–97. doi: 10.2174/0929867043364522. [DOI] [PubMed] [Google Scholar]
- 2.Albarracin SL, Stab B, Casas Z, Sutachan JJ, Samudio I, Gonzalez J, Gonzalo L, Capani F, Morales L, Barreto GE. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci. 2012;15:1–9. doi: 10.1179/1476830511Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 3.Choi DY, Lee YJ, Hong JT, Lee HJ. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Res Bull. 2012;87:144–53. doi: 10.1016/j.brainresbull.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Yoon JH, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. 2005;46:585–96. doi: 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Curr Pharm Des. 2010;16:2766–78. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S210–2. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- 7.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer's disease brain. Arch Immunol Ther Exp (Warsz) 2012;60:251–66. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res. 2005;2:355–65. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- 11.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–84. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 12.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–29. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 14.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by α7 nicotinic receptors. Journal of Neurochemistry. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 15.Dajas-Bailador FA, Lima PA, Wonnacott S. The [alpha]7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca2+ dependent mechanism. Neuropharmacology. 2000;39:2799–2807. doi: 10.1016/s0028-3908(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 16.Mazurov A, Hauser T, Miller CH. Selective alpha7 nicotinic acetylcholine receptor ligands. Curr Med Chem. 2006;13:1567–84. doi: 10.2174/092986706777442011. [DOI] [PubMed] [Google Scholar]
- 17.Breining SR. Recent Developments in the Synthesis of Nicotinic Acetylcholine Receptor Ligands. Current Topics in Medicinal Chemistry. 2004;4:609–629. doi: 10.2174/1568026043451131. [DOI] [PubMed] [Google Scholar]
- 18.Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med. 2013;3:a012112. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Molecular Pharmacology. 1992;41:802–808. [PubMed] [Google Scholar]
- 20.Drasdo A, Caulfield M, Bertrand D, Bertrand S, Wonnacott S. Methyl lycaconitine: A novel nicotinic antagonist. Molecular and Cellular Neuroscience. 1992;3:237–243. doi: 10.1016/1044-7431(92)90043-2. [DOI] [PubMed] [Google Scholar]
- 21.Park HJ, Lee PH, Ahn YW, Choi YJ, Lee G, Lee DY, Chung ES, Jin BK. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 2007;26:79–89. doi: 10.1111/j.1460-9568.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- 22.Littleton J, Rogers T, Falcone D. Novel approaches to plant drug discovery based on high throughput pharmacological screening and genetic manipulation. Life Sciences. 2005;78:467–475. doi: 10.1016/j.lfs.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Lee BH, Choi SH, Shin TJ, Pyo MK, Hwang SH, Kim BR, Lee SM, Lee JH, Kim HC, Park HY, Rhim H, Nah SY. Quercetin enhances human alpha7 nicotinic acetylcholine receptor-mediated ion current through interactions with Ca(2+) binding sites. Mol Cells. 2010;30:245–53. doi: 10.1007/s10059-010-0117-9. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez R, Ballester I, Lopez-Posadas R, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51:331–62. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- 25.Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Dajas F, Andres AC, Florencia A, Carolina E, Felicia RM. Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features. Cent Nerv Syst Agents Med Chem. 2013;13:30–5. doi: 10.2174/1871524911313010005. [DOI] [PubMed] [Google Scholar]
- 27.Daiber A, Bachschmid M, Kavaklí C, Frein D, Wendt M, Ullrich V, Munzel T. A new pitfall in detecting biological end products of nitric oxide—nitration, nitros(yl)ation and nitrite/nitrate artefacts during freezing. Nitric Oxide. 2003;9:44–52. doi: 10.1016/j.niox.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Hong YJ, Tomas-Barberan FA, Kader AA, Mitchell AE. The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix dactylifera) J Agric Food Chem. 2006;54:2405–11. doi: 10.1021/jf0581776. [DOI] [PubMed] [Google Scholar]
- 29.Lin LZ, Chen P, Ozcan M, Harnly JM. Chromatographic profiles and identification of new phenolic components of Ginkgo biloba leaves and selected products. J Agric Food Chem. 2008;56:6671–9. doi: 10.1021/jf800488x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho MJ, Howard LR, Prior RL, Morelock T. Flavonoid content and antioxidant capacity of spinach genotypes determined by high-performance liquid chromatography/mass spectrometry. Journal of the Science of Food and Agriculture. 2008;88:1099–1106. [Google Scholar]
- 31.Vilegas W, Nehme CJ, Dokkeddal AL, Piacente S, Rastrelli L, Pizza C. Quercetagetin 7-methyl ether glycosides from Paepalanthus vellozioides and Paepalanthus latipes. Phytochemistry. 1999;51:403–409. [Google Scholar]
- 32.Lin LZ, Harnly JM. Identification of Hydroxycinnamoylquinic Acids of Arnica Flowers and Burdock Roots Using a Standardized LC-DAD-ESI/MS Profiling Method. Journal of Agricultural and Food Chemistry. 2008;56:10105–10114. doi: 10.1021/jf802412m. [DOI] [PubMed] [Google Scholar]
- 33.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–43. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 34.Hwang J, Hwang H, Lee HW, Suk K. Microglia signaling as a target of donepezil. Neuropharmacology. 2010;58:1122–1129. doi: 10.1016/j.neuropharm.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Mencel M, Nash M, Jacobson C. Neuregulin Upregulates Microglial α7 Nicotinic Acetylcholine Receptor Expression in Immortalized Cell Lines: Implications for Regulating Neuroinflammation. PLoS ONE. 2013;8:e70338. doi: 10.1371/journal.pone.0070338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies AR, Hardick DJ, Blagbrough IS, Potter BV, Wolstenholme AJ, Wonnacott S. Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling alpha 7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology. 1999;38:679–90. doi: 10.1016/s0028-3908(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 37.Peng JH, Fryer JD, Hurst RS, Schroeder KM, George AA, Morrissy S, Groppi VE, Leonard SS, Lukas RJ. High-affinity epibatidine binding of functional, human alpha7-nicotinic acetylcholine receptors stably and heterologously expressed de novo in human SH-EP1 cells. J Pharmacol Exp Ther. 2005;313:24–35. doi: 10.1124/jpet.104.079004. [DOI] [PubMed] [Google Scholar]
- 38.Lau FC, Bielinski DF, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res. 2007;85:1010–7. doi: 10.1002/jnr.21205. [DOI] [PubMed] [Google Scholar]
- 39.Park HY, Kim GY, Choi YH. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-kappaB and inhibiting mitogen-activated protein kinases. Int J Mol Med. 2012;30:204–10. doi: 10.3892/ijmm.2012.979. [DOI] [PubMed] [Google Scholar]
- 40.Morand C, Crespy V, Manach C, Besson C, Demigné C, Rémésy C. Plasma metabolites of quercetin and their antioxidant properties. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1998;275:R212–R219. doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- 41.Jnawali HN, Lee E, Jeong KW, Shin A, Heo YS, Kim Y. Anti-inflammatory Activity of Rhamnetin and a Model of Its Binding to c-Jun NH2-Terminal Kinase 1 and p38 MAPK. Journal of Natural Products. 2014;77:258–263. doi: 10.1021/np400803n. [DOI] [PubMed] [Google Scholar]
- 42.Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Iwashina T. Flavonoid function and activity to plants and other organisms. Biol Sci Space. 2003;17:24–44. doi: 10.2187/bss.17.24. [DOI] [PubMed] [Google Scholar]
- 44.Xiao ZP, Peng ZY, Peng MJ, Yan WB, Ouyang YZ, Zhu HL. Flavonoids health benefits and their molecular mechanism. Mini Rev Med Chem. 2011;11:169–77. doi: 10.2174/138955711794519546. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu T, Lin F, Hasegawa M, Okada K, Nojiri H, Yamane H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J Biol Chem. 2012;287:19315–25. doi: 10.1074/jbc.M112.351270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toledo AC, Sakoda CP, Perini A, Pinheiro NM, Magalhaes RM, Grecco S, Tiberio IF, Camara NO, Martins MA, Lago JH, Prado CM. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br J Pharmacol. 2013;168:1736–49. doi: 10.1111/bph.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenman SW, Poulev A, Struwe L, Raskin I, Ribnicky DM. Qualitative variation of anti-diabetic compounds in different tarragon (Artemisia dracunculus L.) cytotypes. Fitoterapia. 2011;82:1062–74. doi: 10.1016/j.fitote.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Kong Y, Wu D, Zhang H, Wu J, Chen J, Ding J, Hu L, Jiang H, Shen X. Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008;17:1971–8. doi: 10.1110/ps.036186.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozipek M, Calis I, Ertan M, Ruedi P. Rhamnetin 3-p-coumaroylrhamninoside from Rhamnus petiolaris. Phytochemistry. 1994;37:249–53. doi: 10.1016/0031-9422(94)85035-6. [DOI] [PubMed] [Google Scholar]
- 50.Kim YJ. Rhamnetin attenuates melanogenesis by suppressing oxidative stress and pro-inflammatory mediators. Biol Pharm Bull. 2013;36:1341–7. doi: 10.1248/bpb.b13-00276. [DOI] [PubMed] [Google Scholar]
- 51.Jiang H, Zhan WQ, Liu X, Jiang SX. Antioxidant activities of extracts and flavonoid compounds from Oxytropis falcate Bunge. Nat Prod Res. 2008;22:1650–6. doi: 10.1080/14786410701875686. [DOI] [PubMed] [Google Scholar]
- 52.PROCEEDINGS OF THE PHYSIOLOGICAL SOCIETY: January 22, 1910. The Journal of Physiology. 1910;40:i–vii. doi: 10.1113/jphysiol.1886.sp000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang CY, Ferrell JE. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proceedings of the National Academy of Sciences. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prendergast MA, Harris BR, Mayer S, Holley RC, Pauly JR, Littleton JM. Nicotine exposure reduces N-methyl-D-aspartate toxicity in the hippocampus: relation to distribution of the alpha7 nicotinic acetylcholine receptor subunit. Med Sci Monit. 2001;7:1153–60. [PubMed] [Google Scholar]
- 55.Fratiglioni L, Wang HX. Smoking and Parkinson's and Alzheimer's disease: review of the epidemiological studies. Behav Brain Res. 2000;113:117–20. doi: 10.1016/s0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]