Abstract
The enzyme 5-lipoxygenase (5LO) is up-regulated in Alzheimer’s disease (AD), and its pharmacological blockade with zileuton slows down the development of the AD-like phenotype in young AD mice. However, its efficacy after the AD pathology is established is unknown. To this end, starting at 12-months of age triple transgenic mice (3xTg) received zileuton, a selective 5LO inhibitor, or placebo for 3 months, and then the effect of this treatment on behavior, amyloid and tau pathology assessed. While mice on placebo showed worsening of their memory, treated mice performed even better than at baseline. Compared with placebo, treated mice had significant less Aβ deposits and tau phosphorylation secondary to reduced γ-secretase and CDK-5 activation, respectively. Our data provide novel insights into the disease-modifying action of pharmacologically inhibiting 5LO as a viable AD therapeutic approach. They represent the successful completion of preclinical studies for the development of this class of drug as clinically applicable therapy for the disease.
Keywords: Alzheimer’s disease, amyloid beta, tau protein, transgenic mouse models, 5-lipoxygenase, memory
1. Introduction
Alzheimer’s disease (AD) is an irreversible age-associated neurodegenerative condition characterized by progressive memory loss and cognitive decline. Although we know that in the early-onset the disease is secondary to gene mutations, the initiating events for the most common form of AD, the sporadic/late-onset, are still unknown. As result, recent research in AD has moved more and more toward prevention and away from treatment, leaving five millions U.S. AD patients with little hope for new, effective drugs. In recent years, we have identified a novel pathway, the 5-Lipoxygenase (5LO), as a therapeutic target that has the potential to fill this gap.
The 5LO is an enzyme that catalyzes the conversion of arachidonic acid to 5-hydroxy-peroxy-eicosatetraenoic acid (5-HPETE) and subsequently to hydroxyl-eicotetraenoic acid (5-HETE), which can be then metabolized in different leukotrienes (Radmark et al., 2007). It is widely expressed in the neuronal cells of the central nervous system, where its level increases in an age-dependent manner in the hippocampus and cortex, two brain regions prone to neurodegenerative insults (Chinnici et al., 2007). Previous works have shown that this pathway is up-regulated in AD patients (Ikonomovic et al., 2008; Firuzi et al., 2008), and that its genetic absence or over-expression modulates Aβ levels and tau metabolism in transgenic mouse models of the disease (Chu and Praticò, 2006; Chu et al., 2013). Moreover, recent studies show that treatment with an orally available and specific inhibitor of 5LO, zileuton, at an early stage of the development of the AD- like phenotype delays cognition impairments, reduces amyloid beta (Aβ) levels, and tau phosphorylation in the same mouse models of AD (Chu et al., 2013). However, the suitability of this pharmacological approach as an effective therapy for AD is still unclear, since its efficacy and real disease-modifying property at a later stage of the disease phenotype development remain to be fully demonstrated.
With this goal in mind, in the present paper we investigated the effect of 5LO pharmacological inhibition on the AD-like phenotype of the 3xTg mouse model of AD starting at 12 months of age when amyloid plaques and tau neuropathology are well established (Oddo et al.). After 3 months of treatment, we observed that compared with untreated mice, the ones receiving the 5LO blocker showed complete restoration of their cognitive abilities, as well as a significant reduction in Aβ deposition and decrease in tau phosphorylation, secondary to a γ-secretase down-regulation and a decrease in the activation of CDK-5, respectively.
2. Methods
2.1 Animals
All animal procedures were approved by the Animal Care and Usage Committee and were in accordance with the National Institute of Health guidelines. The 3xTg mice harboring a mutant amyloid precursor protein (APP; KM670/671NL), a human mutant PS1 (M146V0) knockin, and tau (P301L) transgenes were used in this study. Mice were kept in a pathogen-free environment, on a 12-hour light/dark cycle and had access to food and water ad libitum. Starting at twelve months of age, after baseline behavioral assessment, mice were randomized to two groups, one receiving zileuton in their drinking water (200mg/L) (n=6) (ZIL), the other vehicle (n=6) (CTR) for 3 months until they were 15 months of age. At 15 months of age all the mice were tested again in the same behavioral paradigms and subsequently sacrificed. At sacrifice, the animals were perfused with ice-cold 0.9% phosphate-buffered saline (PBS) containing ethylene-diamine-tetra-acetic acid (EDTA, 2mmol/l), pH 7.4. Brains were removed, gently rinsed in cold 0.9% PBS and immediately dissected in 2 halves. One half was stored at −80°C for biochemistry assays, the other half was fixed in 4% paraformaldehyde in PBS, pH7.4 for immunohistochemistry studies.
2.2 Behavioral Paradigms
Mice were assessed in the following behavioral paradigms: Y-maze and fear conditioning, as previously described (Chu et al. 2013; Joshi and Praticò, 2013). Briefly, for the Y-maze each mouse was placed in the center of the maze and allowed to explore freely during a 5-min session for the assessment of spontaneous alternating behavior. An alternation was defined as three consecutive entries in three different arms (i.e. 1, 2, 3 or 2, 3, 1, etc). The percentage alternation score was calculated using the following formula: Total alternation number/total number of entries-2)*100. An entry was counted when the mouse had all four paws in an arm of the maze.
The fear conditioning tests were conducted in a chamber equipped with black methacrylate walls, a transparent front door, a speaker and grid floor. During the training phase, each mouse was placed in the chamber and underwent three cycles of thirty seconds sound/ten seconds electric shock, within a six minutes time span. The following day, the mouse spent five minutes in the chamber without getting shocked or hearing the sound (contextual recall). Two hours later the animal spent six minutes in the same chamber but with different flooring, walls, smell and lighting and heard the cued sound for thirty seconds (cued recall). For each phase, the freezing activity of the mouse was recorded.
2.3 Biochemical analysis
Mouse brain homogenates were sequentially extracted first in radioimmunoprecipitation assay buffer (RIPA) for the soluble and then in formic acid (FA) for the insoluble protein fraction as previously described (Firuzi et al., 2008; Giannopoulos et al., 2013). Aβ1-40 and Aβ1-42 levels were assayed by a sensitive sandwich ELISA kits (WAKO Chem., Richmond, VA). LTB4 levels were assayed by a colorimetric competitive enzyme immunoassay ELISA kit (ENZO Life Sciences, Farmingdale, NY). Analyses were always performed in duplicate and in a coded fashion.
2.4 Immunoblotting
Mouse brain homogenates were extracted in RIPA buffer as previously described (Chu et al. 2013; Joshi and Praticò, 2013). Briefly, total protein concentration was measured with BCA Protein Assay Kit (Pierce, Rockford, IL). Samples were separated on SDS page by using a 10% Bis-Tris gel and then transferred onto nitro-cellulose membranes (Bio-Rad, Richmond, CA), blocked with Odyssey blocking buffer for 1h at room temperature and incubated with primary antibodies overnight at 4°C. After three washing cycles in T-TBS, membranes were incubated with IRDye 800CW labeled secondary antibody (LI-COR Bioscience) for 1 hr at room temperature and developed with Odyssey Infrared Imaging System (LI-COR Bioscience). The following primary antibodies were used: 5-LO (1:200, BD Biosciences), APP (1:200 Millipore), ADAM10 (1:500 Millipore), BACE-1 (1:200 Millipore), Nicastrin (1:200 Cell Signaling), PS-1 (1:200 Cell Signaling), APH-1 (1:200 Millipore), PEN-2 (1:200 Invitrogen), HT7 (1:200 Thermo), AT180 (1:200; Thermo), AT270 (1:200; Thermo), PHF-1 (1:20; generous gift of Dr. P. Davies), PHF-13 (1:200; Cell Signaling), cdk-5 (1:800; Santa Cruz Biotechnology), MC1 (1:100; generous gift of Dr. P. Davies), P35/25 (1:400; Santa Cruz Biotechnology), GSK3α/β (1:200; Santa Cruz Biotechnology), pGSK3α/β (1:200 Cell Signaling), PP-2A (1:200; Thermo), synaptophysin (1:400; Santa Cruz Biotechnology), PSD-95 (1:200; Cell Signaling), GFAP (1:200 Santa Cruz Biotechnology), IBA-1 (1:200; Millipore), β-actin (1:1000; Santa Cruz Biotechnology). Actin was always used as an internal loading control.
2.5 Immunohistochemistry
Mouse brains were prepared for immunohistochemistry as previously described (Chu et al. 2013; Joshi and Praticò, 2013). Briefly, serial brain sections were cut throughout each brain and mounted on 3-aminopropyl-triethoxysaline-coated slides. Sections were deparaffinized, hydrated, rinsed with PBS, and pretreated with citric acid for 5 minutes for antigen retrieval for tau or formic acid (88%) for Aβ, then with 3% H2O2 in methanol for 30 minutes to eliminate endogenous peroxidase activity in the tissue, and with blocking solution (5% normal serum in Tris buffer, pH7.6). Subsequently, sections were incubated overnight at 4°C with the following primary antibodies: Aβ-4G8 (1:300 Covance) HT7, MC1, AT8, AT180, PHF-1 and PHF-13, then incubated with secondary antibody and developed using the avidin-biotin complex method (Vector Laboratories) with 3,3diaminobenzidine as chromogen.
2.6 Statistical Analyses
All data are expressed as mean ± standard error of the mean. The two-tailed Student’s t-test was used to determine statistical significance, with significance set at p<0.05.
Results
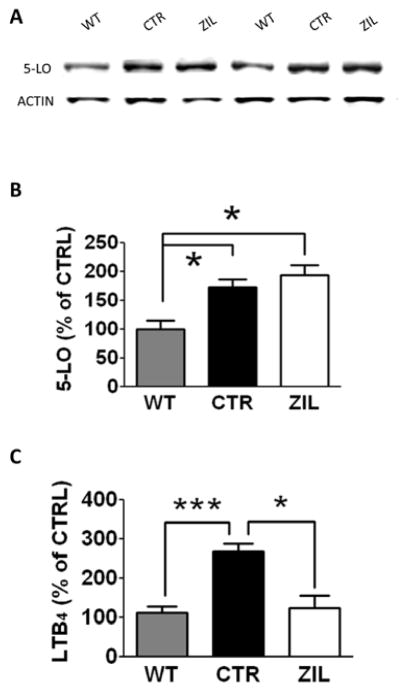
3.1. Zileuton blocks 5-lipoxygenase activity in the brain of 3xTg mice
To verify whether zileuton administration had effectively inhibited its target, 5LO, we assayed the brain levels of one of the principal leukotrienes produced by its activation, the leukotriene B4 (LTB4) (Radmark, 2007). As shown in figure 1A, we found that compared with controls, zileuton-treated mice had a significant reduction in brain levels of LTB4 supporting the compliance with the therapy and the specific action of the drug. Interestingly, we also observed that 3xTg mice on placebo had a significant activation of 5LO enzyme as indicated by LTB4 levels when compared with matched wild type controls (Figure 1A). This observation was further corroborated by western blot analysis showing an up-regulation of the 5LO steady state protein levels in 15-month old 3xTg compared with wild type which was unaffected by zileuton (Figure 1B, C).
Figure 1. Zileuton inhibits brain 5-lipoxygenase activity.
A. Levels of leukotriene B4 (LTB4) in brain homogenates of 15 months old wild type mice (WT), 3xTg mice treated with placebo (CTR) and 3xTg mice treated with zileuton (ZIL) (*p<0.05, ***p<0.001). B. Representative Western blot analyses of 5-lipoxygenase (5-LO) in brain homogenates of 15 months old wild type mice (WT), 3xTg mice placebo treated (CTR) and 3xTg mice zileuton treated (ZIL). C. Densitometric analyses of the immunoreactivities shown in the previous panel (*p<0.05). Results are mean ± sem.
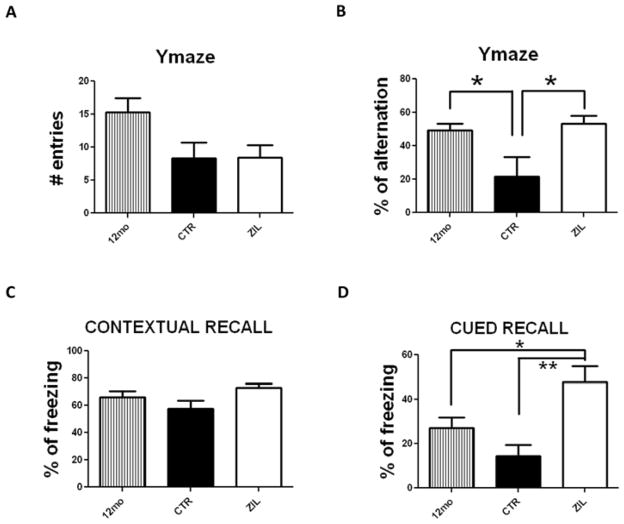
3.2. Zileuton treatment restores memory impairments of aged 3xTg mice
To assess whether zileuton treatment could rescue the cognitive decline that occurs between 12 and 15 months of age in the 3xTg, mice were tested initially when they were 12-month old. As shown in figure 2A, while we observed that compared with baseline (12 months) the mice at 15 months had a reduction in the number of entries, we did not observe any significant difference for this parameter between the treated and untreated group at 15 months. Conversely, when we assessed the percentage of alternation, which measures working memory, we found that the drug had a significant impact. Thus, as shown in figure 2B, we observed that the predicted decrease in this parameter between 12 and 15 months of age in the control group was completely reversed in the zileuton-treated mice.
Figure 2. Zileuton restores memory impairments of aged 3xTg mice.
A. Total number of entries in the Y-maze for 3xTg 12 months old (12mo) and 3xTg 15 months old receiving placebo (CTR) or zileuton (ZIL) (*p<0.05). B. Percentage of alternation in the Y-maze for the same three groups of mice (*p<0.05). C. Contexual fear conditioning memory responses for the same three groups of mice (*p<0.05). D. Cued fear conditioning memory responses for the same three groups of mice (*p<0.05, ** p<0.01). Results are mean ± sem.
Next, mice underwent fear conditioning testing for both the cued and contextual form. No significant differences were observed between baseline and 15 months of age among the 3 groups in the training session (not shown). Despite some trends, a similar result was obtained when we compared baseline with the untreated and treated group in the contextual recall phase (Figure 2C). By contrast, compared with the measurement at 12 months of age, 15 month-old mice receiving placebo showed a statistically significant decrease in the freezing time in the cued recall phase (Figure 2D). However, zileuton treatment reversed the worsening of this measurement, and mice in this group actually displayed a statistically significant increase in the freezing time compared to both 12 and 15 months old placebo groups (Figure 2D).
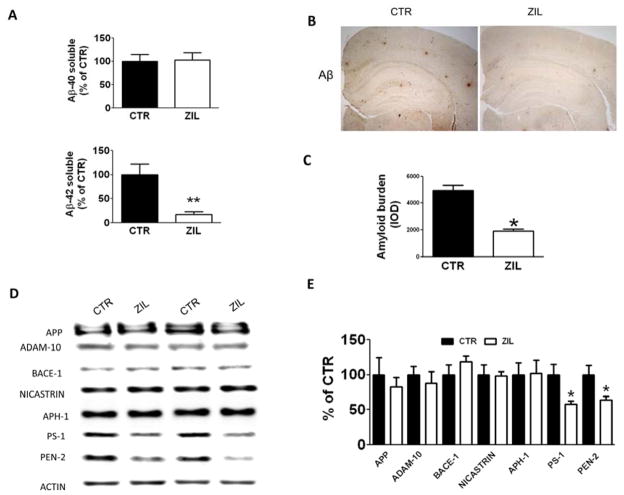
3.3. Zileuton treatment reduces Aβ deposition in aged 3xTg mice
In order to investigate whether Zileuton treatment had any effect on the levels and deposition of the Aβ peptides, brain homogenates were assayed for Aβ1-40 and Aβ1-42 levels. As shown in figure 3A, while we did not observe any significant changes for RIPA-soluble Aβ1-40 levels, we found that Aβ1-42 were significantly lower in the brains of the zileuton-treated animals. Next, we investigated the effect of the treatment on Aβ deposition, by analyzing the areas occupied by 4G8-immunopositive reactions using an immunohistochemistry approach. Compared with mice on placebo, we found that zileuton-treated animals displayed a significant reduction in the amount of Aβ peptides deposited as measured by the amyloid burden (Figure 3B, C).
Figure 3. Zileuton reduces Aβ levels and deposition in aged 3xTg mice.
A. RIPA-soluble (RIPA) Aβ1-40 and Aβ1-42 levels in brain homogenates of 15 months old 3xTg mice receiving placebo (CTR) or zileuton (ZIL) were measured by sandwich ELISA (** p<0.01). B. Representative sections of brains from the same two groups of mice immunostained with 4G8 antibody. C. Quantification of the area occupied by Aβ immunoreactivity in brains of the same two groups of mice (*p<0.05). D. Representative western blots analyses of APP, ADAM-10, BACE-1, Nicastrin, APH-1, PS1 and Pen-2 levels in brain homogenates from15 months old 3xTg mice receiving placebo (CTR) or zileuton (ZIL). E. Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (*p<0.05). Results are mean ± sem.
To elucidate the mechanisms responsible for the effect of zileuton treatment on the amyloid pathology, we assayed steady state levels of Aβ precursor protein (APP) and the different proteases involved in its metabolism. Compared with placebo, zileuton-treated mice had no significant changes in APP, α-secretase (ADAM-10), or β-secretase (BACE-1) levels (Figure 3D, E). By contrast, when we looked at the four components of the γ-secretase complex we observed a statistically significant decrease in the levels of PS-1 and PEN-2, but no differences were detected in the other two components (i.e., nicastrin and APH-1) (Figure 3D, 3).
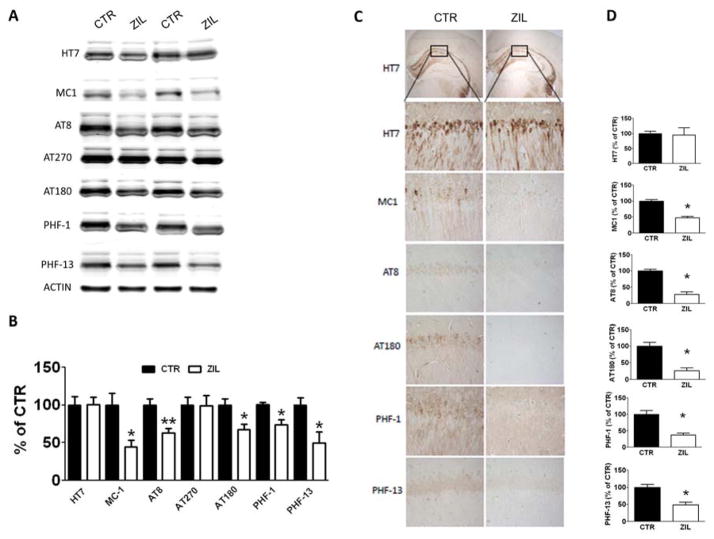
3.4. Zileuton decreases tau phosphorylation via a cdk-5-dependent mechanism
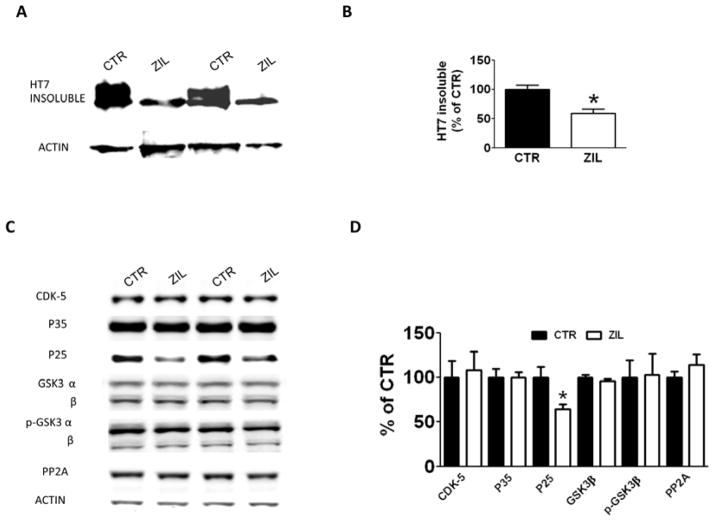
To test the effect of zileuton on tau metabolism, we assayed the levels of total tau and several AD-relevant phosphorylated tau epitopes between the two groups of mice. As shown in figure 4A and 4B, the levels of soluble total tau, recognized by the antibody HT7, and tau phosphorylated at thr181, as recognized by the antibody AT270, did not change between the two groups. However, zileuton-treated animals displayed a decrease in tau phosphorylation at the following epitopes: ser202/thr205, as recognized by the antibody AT8, thr231/ser235, as recognized by the antibody AT180, ser396/404, as recognized by the antibody PHF-1 and ser396, as recognized by the antibody PHF-13. To confirm our results we performed immunohistochemistry analysis on the brains of both experimental groups. As shown in figure 4C, 4D we observed a significant decrease in the immunostaining for the same epitopes as observed in the Western blot analyses. By contrast, the immunostaining for HT7 was similar in both experimental conditions. Since we observed changes in tau metabolism in the brains of zileuton-treated mice, we investigated the potential mechanism involved by assaying AD-relevant kinases and phosphatases that are considered major regulators of tau post-translational modifications. Immunoblot analysis of kinases, such as cyclin dependent kinase 5 (CDK-5), total GSK3α/β, and their activated forms phosphorylated GSK3α/β (pGSK3α/β), as well as phosphatase PP-2A, did not show any significant differences between the two groups (Figure 4C, 4D). On the other hand, while no changes were observed for P35, a significant decrease in the levels of P25, another co-activator of CDK-5, was detected in the zileuton-treated group.
Figure 4. Zileuton reduces tau phosphorylation in aged 3xTg mice.
A. Representative Western blot analyses of soluble total tau (HT-7), tau N-terminal amino acids 7–9 and C-terminal amino acids 312–322 (MC-1), and phosphorylated tau at residues S202/T205 (AT8), T181 (AT270), T231/S235 (AT180), S396/S404 (PHF-1), and S396 (PHF-13) in brain homogenates of 15 months old 3xTg mice receiving placebo (CTR) or zileuton (ZIL). B. Densitometric analyses of the immunoreactivities shown in the previous panel (*p<0.05, **p<0.01). C. Representative images of immunohistochemistry analyses for HT7, MC-1, AT8, AT180, PHF-1 and PHF-13 in brain sections of the same two groups of mice. D. Densitometric analyses of the immunoreactivities shown in the previous panel (*p<0.05). Results are mean ± sem.
Finally, we assessed changes in tau solubility and conformation by biochemistry and immunohistochemistry. Compared with controls, brain homogenates from mice receiving zileuton had a significant decrease in the level of total insoluble tau fraction, as recognized by the antibody HT7 (Figure 5A, 5B). Because we observed a decrease in the insoluble fraction of tau, we assessed whether the same animals had change in its conformational status by using the antibody MC-1, which recognizes N-terminal amino acids 7–9 and C-terminal amino acids 312–322. As shown in figure 4A and 4B, we found a significant decrease in the MC1 immuno-reactivity in brain homogenates of zileuton-treated mice when compared to the control animals. To further confirm the conformational modification of tau, the areas occupied by MC1-immunopositive reactions were analyzed by immunohistochemistry. As show in Figure 4C and 4D, zileuton-treated mice displayed a statistically significant decrease in the immunostaining for MC1 compared to controls.
Figure 5. The effect zileuton on tau solubility and tau metabolism.
A. Representative Western blot analyses of formic acid-soluble total tau (HT-7) in brain homogenates of 15 months old 3xTg mice receiving placebo (CTR) or zileuton (ZIL). B. Densitometric analyses of the immunoreactivities shown in the previous panel (*p<0.05). C. Representative Western blot analyses of CDK-5, P35, P25, GSK-3α and GSK-3β, p-GSK-3α and p-GSK3β, and PP-2A in brain homogenates from the same two groups of mice. D. Densitometric analyses of the immunoreactivities shown in the previous panel. (*p<0.05). Results are mean ± sem.
3.5. The effect of zileuton on synaptic integrity and neuroinflammation
As changes in tau solubility and conformation have been implicated in alterations of synaptic integrity and neuroinflammation in AD brains, next we assessed whether these aspects of the AD phenotype were influenced by the treatment. To this end, we assayed the levels of synaptophysin and post-synaptic density protein 95 (PSD-95) indices of pre-synaptic and post-synaptic integrity, respectively, but no significant differences between the two groups were observed (Figure 5A). In addition, under our experimental conditions, we did not observe any differences in the levels of ionized calcium-binding adapter molecule 1 (IBA-1), a marker of microglia activation, and in the levels of glial fibrillary acidic protein (GFAP), a marker for astrocytosis (Figure 5B) between the two groups of mice.
4. Discussion
In this study, we provide experimental evidence that pharmacological blockade of 5LO restores behavioral and cognitive function, and significantly reduces Aβ deposition and tau hyper-phosphorylation in aged 3xTg mice.
This is the first preclinical evidence of the suitability of zileuton, an orally available and specific 5LO inhibitor, as a potentially truly disease-modifying therapeutic approach in AD models since its beneficial effects were observed in the animals at a stage when memory is already impaired and the for Aβ and tau neuropathology well-established. It is known that the clinical signs of AD, in most cases, manifest years after the pathology of the disease is developed. In this sense our findings have translational relevance since the age of our mice when the treatment was started most likely reflects a real-life scenario of an individual who gets his or her first diagnosis of the disease. Thus, while previous studies have shown that pharmacological inhibition of 5LO when started at a young age, 2/3 months, delays the development of the early functional and pathological AD-like phenotype in two different transgenic mouse models of the disease, no data are available on the effect that the same therapy may have after the disease is developed.
To fill this gap we embarked in the current study in which we sought to assess the therapeutic effect of zileuton in aged 3xTg mice, starting the treatment when they were 12 months old. At the end of the study, as predicted, we observed that three months after their first memory and learning evaluation mice without treatment performed worse than at baseline. This effect was not observed in mice treated with zileuton, which actually performed better than at baseline, suggesting a functional restoration of their behavioral deficits.
In association with the improvement of this functional aspect of their AD-like phenotype, we observed that the same mice had a significant reduction in their brain Aβ deposition. In particular, we found a significant reduction of the levels of Aβ-42 upon zileuton treatment but no changes in Aβ-40 levels. The biological importance of this finding lies in the fact that numerous studies have clearly indicated that Aβ-42 is more prone to aggregation compared to Aβ-40, and data from familiar forms of AD show that the genetic mutations underlying them result in increased Aβ-42/Aβ-40 ratio. Based on this evidence, we speculate that zileuton treatment influences amyloid deposition at the later stages of the AD-like amyloidotic phenotype by specifically reducing the levels of the more aggregation-prone Aβ peptide isoform. In an effort to elucidate the mechanism responsible for the reduction of the amyloid burden, we assayed the steady levels of APP and the most important proteases responsible for its processing. However, we did not find any changes in APP, ADAM-10 and BACE-1 levels, suggesting the independence of the biological effect of zileuton from the Aβ precursor or the α- or β-secretase proteolytic pathways. By contrast, we found that PS1 and PEN-2, two of the four components of γ-secretase complex were significantly reduced, thus providing evidence of an effect of zileuton on this pathway.
In addition to Aβ pathology, AD is characterized by the progressive accumulation of intracellular neurofibrillary tangles, formed by abnormally hyper-phosphorylated microtubule-associated protein tau and increasing evidence shows a direct correlation between this type of pathology with cognitive impairments (Querfurth and La Ferla, 2010; Wyss-Coray and Mucke, 2002; Santacruz et al., 2005; Oddo et al., 2006; Wallin et al., 2006).
The use of aged 3xTg mice offered a unique opportunity to investigate the effect of 5LO inhibition on this very important aspect of AD neuropathology. Here we show that while pharmacological blockade of 5LO did not alter levels of soluble total tau, it significantly reduced the phosphorylation levels of several epitopes, which have been implicated in the development of neurofibrillary tangles (Martin et al., 2011). To elucidate the mechanism for the zileuton-dependent reduction of tau phosphorylation, we assayed the protein level of putative kinases which are considered major regulators of its post-translational modifications. Our data point out that zileuton reduces tau phosphorylation by modulating the CDK-5-kinase pathway. In particular, the total levels of CDK-5 were not affected by the treatment but the levels of P25, a CDK-5 activator, were significantly reduced by the treatment. Further supporting an ameliorative role of zileuton treatment on tau pathology, we observed a significant decrease in the insoluble fraction of total tau compared to placebo group. Moreover, the immunoreactivity for the antibody MC-1, which is known to specifically detect pathogenic tau conformational changes (Cripps et al., 2006), was significant lower upon zileuton treatment, demonstrating an effect of the drug on tau conformation.
Aside from amyloid and tau pathologies, activation of microglia and astrocytes is another feature found consistently in the AD brain, and some works suggest that they play a functional role in AD pathogenesis ((Querfurth and La Ferla, 2010; Wyss-Coray and Mucke, 2002). Interestingly, in our study we observed that the beneficial effect of 5LO pharmacological inhibition was not dependent on a modulatory action of zileuton on the neuroinflammatory responses, since no changes were observed when markers of microglia and astrocytes activation were assayed. Our findings support the hypothesis that this aspect of the AD phenotype does not play a functional role in the behavioral deficits, Aβ and tau pathology at the later stages of AD pathology development.
In conclusion, our study provides further insights on the efficacy of 5LO pharmacological inhibition as viable therapeutic approach for AD patients since it demonstrates that it has the potential to reverse all three most important aspects of the disease phenotype, such as memory decline, amyloid burden and neurofibrillary tangles, after they are established.
It represents the successful completion of the preclinical studies for the development and implementation of this class of drug as an attractive and clinically applicable therapy for the disease.
Figure 6. The effect of Zileuton on synaptic integrity and neuroinflammation in aged 3xTg mice.
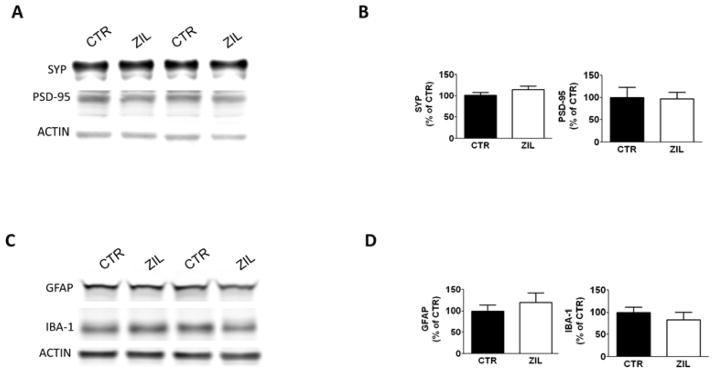
A. Representative Western blot analyses of synaptophysin (SYP) and post-synaptic protein-95 (PSD-95) in brain homogenates of 15 months old 3xTg mice receiving placebo (CTR) or zileuton (ZIL). B. Densitometric analyses of the immunoreactivities shown in the previous panel. (*p<0.05). C. Representative Western blot analyses of glial fibrillary acidic protein (GFAP) and ionized calcium-binding adapter molecule 1 (IBA-1) in brain homogenates of 15 months old 3xTg mice receiving placebo (CTR) or zileuton (ZIL). D. Densitometric analyses of the immunoreactivities shown in the previous panel. (*p<0.05). Results are mean ± sem.
Research highlights.
Pharmacological blockade of 5Lipoxygenase with zileuton restores memory impairments in aged 3xTg mice.
In aged 3xTg mice zileuton reduces Aβ peptides deposition by modulating the γ-secretase pathway, and decreases tau phosphorylation via a cdk-5-mediated mechanism.
Both biological actions are independent from an effect on neuroinflammation.
The study represents the successful completion of preclinical investigation on 5LO inhibition as viable AD therapy.
Acknowledgments
This study was supported in part by grants from the National Institute of Health (AG3368) and the Alzheimer Art Quilt Initiative. We thank Dr. Peter Davies for kindly donating us the PHF-1 and MC-1 antibodies. Special thanks to Dr. Jian-Guo Li and Dr. Jin Chu for the technical assistance.
Footnotes
Disclosure statement
All authors report no actual or potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007;28:1457–1462. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Chu J, Li JG, Ceballos-Diaz C, Golde T, Praticò D. The influence of 5-lipoxygenase on Alzheimer’s disease-related tau pathology: in vivo and in vitro evidence. Biol Psychiatry. 2013;74:321–328. doi: 10.1016/j.biopsych.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Li JG, Praticò D. Zileuton improves memory deficits, amyloid and tau pathology in a mouse model of Alzheimer’s disease with plaques and tangles. PLoS One. 2013 doi: 10.1371/journal.pone.0070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Praticò D. 5-lipoxygenase as an endogenous modulator of amyloid β formation in vivo. Ann Neurol. 2011;69:34–46. doi: 10.1002/ana.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps D, Thomas ST, Jeng Y, Yang F, Davies P, Yang AJ. Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-tau is polyubiquitinated through Lys-48, Lys-11, and Lys-6 ubiquitin conjugation. J Biol Chem. 2006;281:10825–10838. doi: 10.1074/jbc.M512786200. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Praticò D. 5-Lipoxygenase gene disruption reduces amyloid-β pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008;22:1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopoulos PF, Chu J, Joshi YB, Sperow M, Li JG, Kirby LG, et al. Gene knockout of 5-lipoxygenase rescues synaptic dysfunction and improves memory in the triple-transgenic model of Alzheimer’s disease. Mol Psych. 2014;19:511–518. doi: 10.1038/mp.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST. Increased 5-Lipooxygenase immunoreactivity in hippocampus of patients with Alzheimer’ diseases. J Histochem Cytochem. 2008;56:1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi YB, Praticò D. The involvement of 5-lipoxygenase activating protein in anxiety-like behavior. J Psychiatr Res. 2013;47:694–698. doi: 10.1016/j.jpsychires.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Latypova X, Terro F. Post-translational modifications of tau protein: Implications for Alzheimer’s disease. Neurochem Int. 2011;58:458–471. doi: 10.1016/j.neuint.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, La Ferla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Aβ and Synaptic Dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, La Ferla FM. Alzheimer’s Disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek K, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin AK, Blennow K, Andreasen N, Minthon L. CSF biomarkers for Alzheimer’s disease: levels of beta-amyloid, tau, phosphorylated tau relate to clinical symptoms and survival. Dement Geriatr Cogn Disord. 2006;21:131–138. doi: 10.1159/000090631. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease – a double-edge sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]