Editorial
Nonalcoholic steatohepatitis (NASH) is one of the most common causes of chronic liver disease in the Western world 1. Currently, there are no approved therapies for the treatment of NASH 1. Lifestyle interventions, Vitamin E, and pioglitazone have been shown to improve liver histology and reverse NASH 2. Nonetheless, their widespread acceptability has been limited, because of limited efficacy, side-effect profile, and lack of commercial sponsorship. Therefore, the search for better treatments for NASH is one the hottest areas in the field of liver disease. It is arguably the last big, billion dollar market in liver disease that remains untapped.
In this issue, Ratziu and colleagues report a phase I as well as a phase II randomized controlled clinical trial (RCT) that aimed to examine the safety and efficacy of a phosphodiesterase-4 inhibitor, ASP9831, for the treatment of NASH 3. After assessing the therapeutic window in the phase I trial, the investigators randomized 96 patients with biopsy-proven NASH and elevated serum ALT into three groups (1:1:1) including placebo (30 patients), 50 mg ASP9831 (33 patients) and 100 mg ASP9831 (33 patients) twice daily for 12 weeks. The primary outcome was the mean difference in serum ALT value between the baseline value and week-12 value between the treatment-arm versus the placebo-arm. The authors reported that there was no significant difference in serum ALT between the baseline and the week-12 value between the treatment-arm and the placebo-arm, and concluded that ASP9831 although safe in a 12-week study did not improve liver disease in patients with biopsy-proven NASH.
A key question is whether a decrease in serum ALT is an accurate indicator of treatment response in NASH. If so, should one stop drug development if there is no serum ALT improvement in a NASH clinical trial? These key issues will be discussed hereafter in this piece. A strength of the phase II trial was its design -- randomized, double-blind, parallel-group, placebo-controlled clinical trial. The authors carefully and rightly chose their patient population to include patients with both biopsy-proven NAFLD and increased serum ALT (≥1.5 × upper limit of normal [ULN; 41 U/L for male and 31 U/L for female]. Given that serum ALT reduction was the primary outcome of the study it was important to include patients whose ALT values were sufficiently high that a true decrease could be detected. Based upon a systematic review of placebo-arm data derived from RCTs, it is known that a 20 IU/L absolute decline (on an average) is seen in serum ALT in the placebo-treated patients 4. Therefore, the trial needed to be powered to show the additional incremental serum ALT reduction on top of what would be expected in the placebo-treated patients. Indeed, this trial was appropriately designed and had adequate power to detect a clinically meaningful difference in serum ALT.
Hoofnagle and colleagues have recently shown that decline in serum ALT matters in NASH because it is associated with improvement in liver histology 5. They reported that normalization of serum ALT in patients with biopsy-proven NASH who have elevated serum ALT at baseline is usually associated with improved histological activity. Their data support the view that serum ALT may be used as a treatment end-point in phase IIa trials as a convenient and inexpensive surrogate for histologic improvement. However, it can be argued that serum ALT is rather nonspecific. Serum ALT levels can be influenced by injury to tissues other than the liver, and even when serum ALT elevations do indicate liver disease, they give little specific information as to the type of liver injury.
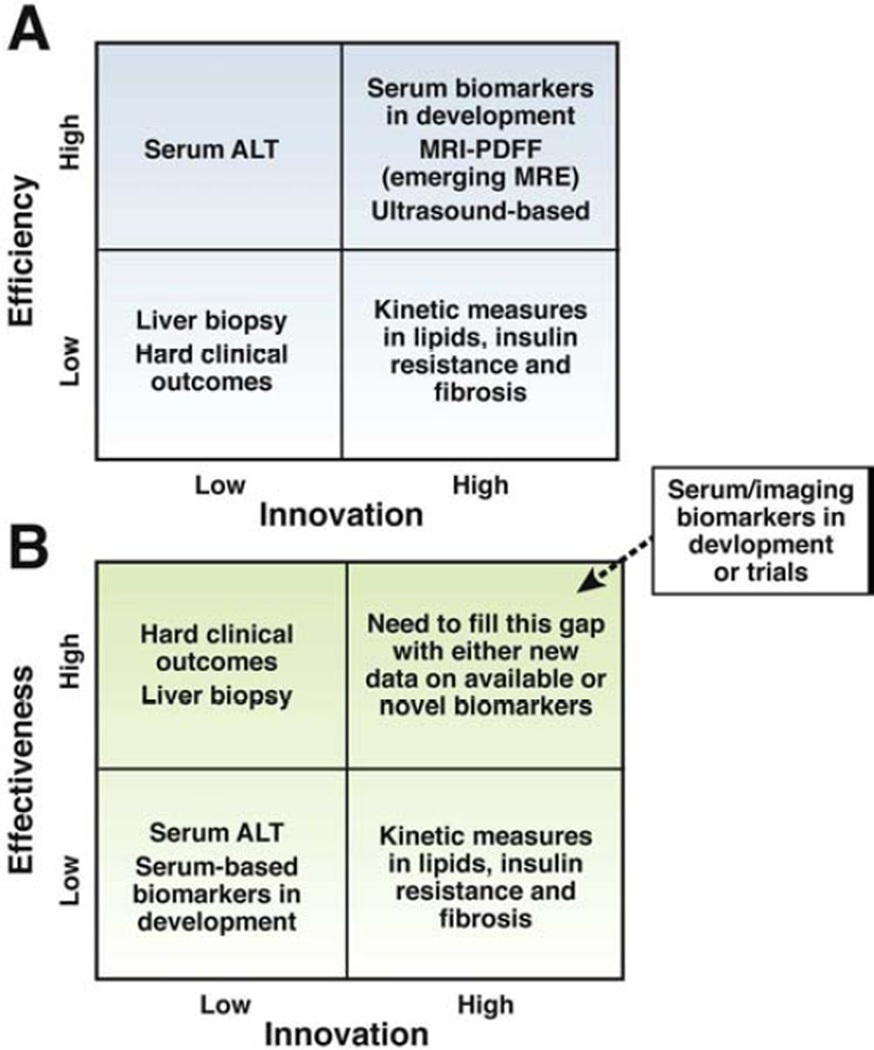
Until we develop a more robust biomarker (or a panel of biomarkers) to comprehensively assess NASH, a piecemeal approach might be taken such as targeting assessment of steatosis, inflammation and/or ballooning degeneration. This has led to introduction and now more widespread utilization of techniques for quantifying hepatic steatosis using magnetic resonance imaging (MRI) or spectroscopy (MRS) with the aim of using these to efficiently screen for improvement in clinical trials in NASH 6–9. Recent studies have shown that MRI using proton-density-fat-fraction (MRI-PDFF), an advanced imaging method for quantification of liver fat, corrects for biases seen with conventional MRI techniques 6–8. MRI-PDFF has a robust correlation with MRS-PDFF, and both of these are more sensitive than liver histology-determined steatosis-grade to assess quantitative changes in liver fat in a clinical trial6, 8. As opposed to MRS-PDFF, MRI-PDFF provides a fat map of the liver that allows for more efficient co-localization of liver fat before and after treatment 6, 8. As with ALT, when therapies improve NASH they are also likely to lead to reduction in liver fat content as quantified by MRI or MRS. Figure 1a and 1b show a proposed model of how the field is rapidly evolving and aiming to maximize efficiency in phase 1 and 2, and effectiveness in phase 3 and 4 NASH clinical trials.
Figure 1.
Figure A (footnote): Efficiency is needed for the conduct of phase 1 and phase 2 trials. Therefore, noninvasive biomarkers are needed. Serum ALT is an efficient biomarker for assessment of treatment response but is non-specific. Using innovative technologies, we need to strive, develop and incorporate more innovative ways of assessing treatment response in NASH to improve efficiency of early phase trials. Liver histology assessment and hard clinical outcomes such as hepatic decompensation or liver mortality are important but not practical for efficient screening for agents for drug development in early phase trials. Magnetic resonance imaging-derived proton density fat fraction, magnetic resonance elastography, and emerging ultrasound-based methods as well as emerging serum/plasma based biomarkers may be utilized for assessment of treatment response in NASH in future. High degree of innovation and high degree of efficiency needs to be matched to lead the field towards an efficient biomarker for assessing treatment response in phase 1 and 2 trials.
Figure B (footnote): Effectiveness is desirable for the conduct of phase 3 and phase 4 trials so one can credibly conclude that the therapy has unquestionable benefit in improving health in patients with NASH. Hepatic decompensation or liver mortality are clinically important end-points. However, they are not ideal because it would require a very large sample size and prolonged follow-up to show a significant benefit of treatment versus placebo due to low event rates. Therefore, innovative non-invasive biomarkers or panel of biomarkers (serum/plasma and/or imaging-based) are needed that can act as surrogate for the key hard clinical outcomes (hepatic decompensation or liver mortality) in phase 3 and 4 trials. It is advisable to incorporate these biomarkers into ongoing phase 3 trials, and future phase 4 trials in NASH.
This trial illustrates that serum ALT is an efficient tool for assessment of treatment response in NASH10. Therefore, it is reasonable to assume non-response to an agent if there is no significant decline in serum ALT in a NASH treatment trial. But biomarkers that are more accurate and precise, and biologically informative, are emerging and may be utilized along with serum ALT in future trials.
Acknowledgement
Author is grateful to Drs. Jay Hoofnagle and Alan Hofmann for their insightful comments.
Funding support: RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303.
Abbreviations
- ALT
Alanine aminotransferase
- MRI
magnetic resonance imaging
- PDFF
proton-density-fat-fraction
- MRE
magnetic resonance elastography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential competing interests: none
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837–858. doi: 10.1016/j.cgh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Ratziu V, Bedossa P, Francque SM, et al. Lack of Efficacy of an Inhibitor of PDE4 in Phase 1 and 2 Trials of Patients with Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Wesley R, Pucino F, et al. Placebo in nonalcoholic steatohepatitis: insight into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol. 2008;6:1243–1248. doi: 10.1016/j.cgh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoofnagle JH, Van Natta ML, Kleiner DE, et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang A, Tan J, Sun M, et al. Nonalcoholic Fatty Liver Disease: MR Imaging of Liver Proton Density Fat Fraction to Assess Hepatic Steatosis. Radiology. 2013 doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]