Abstract
Background
Cognitive impairment in individuals with multiple sclerosis (MS) is now well recognized. One of the most common cognitive deficits is found in memory functioning, largely due to impaired acquisition.
Objective
Examine functional brain activity 6 months after memory retraining in individuals with MS.
Methods
The current report presents long term follow-up results from a randomized clinical trial on a memory rehabilitation protocol known as the modified Story Memory Technique. Behavioral memory performance and brain activity of all participants were evaluated at baseline, immediately after treatment, and 6 months after treatment.
Results and Conclusions
Results revealed that previously observed increases in patterns of cerebral activation during learning immediately after memory training were maintained 6 months post training.
Keywords: fMRI, memory rehabilitation, clinical trial
Multiple sclerosis is a neurodegenerative disorder leading to inflammation and demyelination of the central nervous system that leads to physical, sensory and cognitive deficits. One of the most prominent cognitive deficits is in learning and memory functioning. DeLuca et al. (1998) demonstrated that memory deficits in MS are associated with impaired information acquisition, which subsequently leads to poor recall and recognition (DeLuca, Gaudino, Diamond, Christodoulou, & Engel, 1998). The identification of an effective intervention for improving the acquisition of information into long term memory would therefore likely be of great benefit to persons with MS. Demonstrated long term efficacy is particularly important given the time and expense of such treatments. The current study examines the long-term mainenance of the neurofunctional effects of cognitive rehabiliation in individuals with MS with documented memory impairment.
We previously reported results of a randomized clinical trial (Chiaravalloti, Moore, Nikelshpur, & Deluca, 2013) assessing the efficacy of the modified Story Memory Technique (mSMT) (Allen, Goldstein, Heyman, & Rondinelli, 1998) to improve learning and memory in individuals with MS. We demonstrated that the mSMT, a 10-session treatment paradigm in which patients are taught context and imagery to faciltate leraning, leads to significant improvements in new learning in the treatment group, with no changes noted in the placebo control group. Increased functional brain activity during learning was evident following treatment in the medial temporal lobe (MTL), inferior parietal lobule (IPL), visual and prefrontal regions, with no changes noted in the control group (Chiaravalloti, Wylie, Leavitt, & Deluca, 2012). In the current follow-up investigation, we hypothsized that the significant treatment effects of the mSMT at immediate follow-up in the treatment group would be evident 6 months after the completion of the mSMT. To test this hypothesis, we examined the mSMT treatment effect from baseline to immediate follow-up and the maintenance of the treatment effect from immediate follow-up to 6-month follow-up on both memory performance and patterns of cerebral activation during an fMRI verbal encoding task. Based on the results of our previous investigation, we expected to detect a time x group interaction in regions responsible for memory encoding including medial preforntal cortex, MTL, IPL and areas within the visual cortex. We used these regions of interest (ROIs) to guide our analyses.
Methods
Participants
Eight individuals (3 males) with clinically definite MS (McDonald et al., 2001) (relapsing remitting = 7, primary progressive =1) completed fMRI before, immediately after and 6 months after the mSMT (treatment: n=4, Mage = 40; SD = 5.66; placebo-control n=4, Mage = 46; SD = 1.53). There were no significant differences between the groups in age, education, ambulation index, or months since MS diagnosis (see Supplementary Materials). Neuropsychological test data were available for 6 of the 8 participants in the current study (2 treatment, 4 control). Specifically, neuropsychological test data was not available for two treatment group participant due to an exacerbation between the long-term fMRI scan and the long term neuropsychological assessment. The study was approved by the Kessler Foundation IRB. All participants provided informed consent prior to enrollment and were compensated for participation.
Procedure
Memory performance and brain activity was examined at three time points: before, immediately after and 6 months following mSMT. Participants in the treatment group completed 10 sessions of mSMT, while control participants underwent memory exercises at the same frequency and duration as the treatment group (see Supplementary Materials for a complete decsription of the treatment procedures). All participants had objective memory impairment at baseline, defined as performance at least 1.5 standard deviations below the mean of a normative sample on the Open-Trial Selective Reminding Test (Chiaravalloti, Balzano, Moore, & Deluca, 2009). There were no significant differences in memory performance between the groups at baseline. Memory improvement was operationally defined as a 10% improvemnet on the CVLT Short Delaye Free Recall (SDFR). Due to the small sample size, the CVLT data were examined non-parametrically (χ2-test).
During the fMRI, participants performed a word encoding task due to the fact that the mSMT is designed to treat deficits in encoding and the memory deficit observed in MS has been determined to be due to deficient encoding (DeLuca et al., 1998). Two types of imaging analyses were performed: the ROI analysis, to specifically examine changes in cerebral activation in ROIs over time, and a whole brain analysis, in order to avoid overlooking potentially important brain activity.
Results
Behavioral data was missing for 2 participants. Thus, data was analysed with intent-to-terat analysis where values were computed by averaging the scores from all other subjects and imputing the missing values for these subjects. Results indicate that the treatment effect observed immediately after treatment in the treatment group relative to the placebo control group on the CVLT Short Delay Free Recall (χ2 (1)=4.8, p<.05) was maintained at the long-term follow-up evaluation (χ2 (1)=.000, p=1.0).
ROI analysis
As our primary anaylsis, we performed an ROI analysis on the regions that showed an interaction effect in Chiaravalloti et al. (2012). Mean parameter estimates from each region in each participant was subjected to a 2×3 fixed effects ANOVA with group (treatment vs. control) as a between-subject variable and time (baseline vs. immediate follow-up vs. 6 months follow-up) as a within-subject variable. Based of our previous work (Chiaravalloti et al. 2012), an alpha level of p<0.05 and a cluster size of at least 25 contiguous voxels was used as a technique to minimize Type I error. Four regions showed the group x time interaction: the IPL, medial occipital gyrus, cerebellum and medial prefrontal cortext (Table 1).
Table 1.
p < 0.05, cluster corrected for multiple comparisons.
| Region of Interest Analysis | ||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Voxel Size | Peak x | Peak y | Peak z | Peak F |
| Middle Frontal Gyrus | L | 60 | 38 | −55 | 8 | 4.94 |
| Inferior Parietal Lobule | L | 52 | 42 | 65 | 40 | 4.77 |
| Cerebellum/Cerebellar Tonsils | R | 35 | −6 | 41 | −32 | 4.48 |
| Middle Occipital Gyrus | L | 25 | 50 | 77 | 0 | 4.72 |
| Whole brain analysis | ||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Voxel Size | Peak x | Peak y | Peak z | Peak F |
| Inferior Occipital Gyrus | R | 853 | −6 | 97 | 12 | 7.77 |
| Medial Temporal Lobe | L | 250 | 66 | 17 | −4 | 7.17 |
| Inferior Parietal Lobule | L | 201 | 6 | 77 | 48 | 7.69 |
| Medial Temporal Lobe | R | 185 | −66 | 13 | 12 | 8.48 |
Whole brain analysis
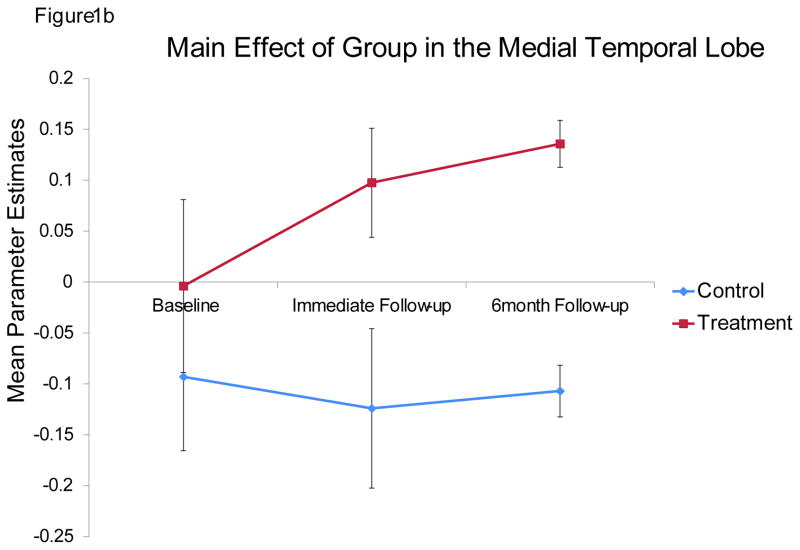
For completeness, we performed a whole-brain analysis to examine the impact of the mSMT on other brain regions. Because this was not an ROI analysis, it was necessary to increase the cluster size to minimize Type I error, which was set to 170 contiguous voxels at alpha level of p<0.05, as determined by the Monte Carlo simulations. Four regions showed a main effect of group: the lingual gyrus, bilateral MTL/insula, and the IPL (Table 1; Figure 1a). Specifically, compared to the control group, the treatment group showed significantly greater activation in these brain regions (Figure 1b) across the two follow-up periods. The effect size for all four regions was also large: inferior occipital gyrus: η2 =.9; IPL: η2 = 0.85; right MTL: η2 = .87; left MTL: η2 = .81.
Figure 1.
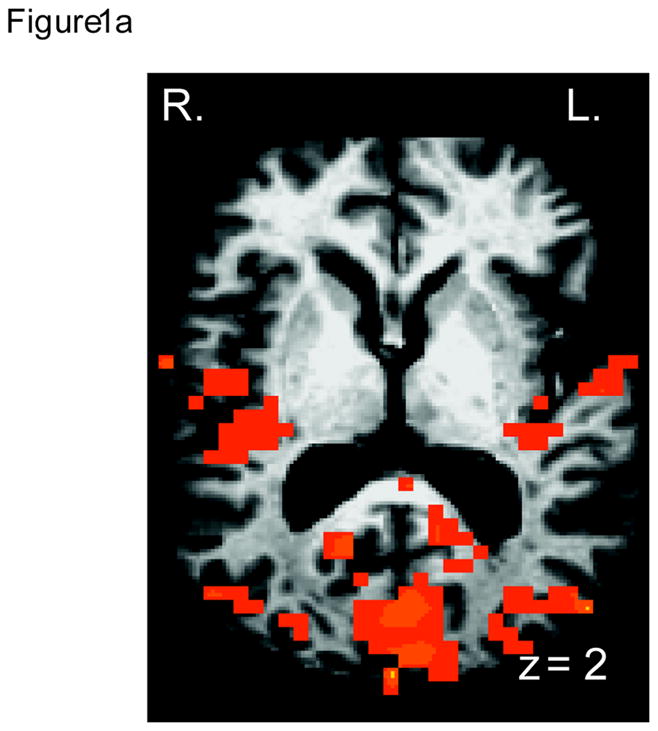
A. Medial temporal lobe, the insula and the visual cortex areas showing a main effect of treatment, with treatment group showing more activation than the placebo control group. B. Mean parameter estimates from the left medial temporal lobe, showing the main effect of group. Other three regions showed the same pattern of results. Error bars represent standard error of the mean.
Discussion
The mSMT trains the patient to apply context and imagery to facilitate learning. Previous research has demonstrated that treatment with the mSMT results in behavioral improvement in memory ability (Chiaravalloti et al., 2013, 2012), as well as significant increases in cerebral activation during learning in regions associated with imagery and verbal learning (Chiaravalloti et al., 2012). Our results additionally demonstrate that memory improvement and increased cerebral activation observed in the treatment group immediately after training is maintained 6 months later. Analysis of neuroimaging data during encoding revealed a group x time interaction in brain regions known to be responsible for visualization and information acquisition. Specifically, from baseline to immediate and long-term follow-up, the treatment group demonstrated increased activation of the visual cortex, that has been previously shown to be associated with memory formation (Ganis, 2004; Kim, 2011). Similarly, the treatment group, as compared to the placebo group, demonstrated increased activity in the MTL (from baseline to immediate and long-term follow-up), consistent with previous studies that note MTL activation in association with information acquisition (Gabrieli, Brewer, & Poldrack, 1998).
The rehabilitation protocol used in the current study effectively improves performance on new learning tasks (Chiaravalloti et al., 2013). The mechanism underlying this behavioral improvement is represented by the increased use of brain regions know to underlie imagery and contextual processing strategies that were taught during the treatment (Chiaravalloti et al., 2012). The current findings extend our previous work, suggesting that the observed change is maintained over time.
Several limitations of the current work restrict the conclusion that we can draw. Specifiaclly, the sample size is small, limiting the generalizability of the results. Similar studies should be repeated with larger samples. We also did not track the activities of our participants between the immediate and long-term follow-up. Therefore, it is possible that some participants may have engaged in more cognitively demanding activities (i.e. reading) on a daily basis and potentially applied the techniques taught in treatment (i.e. imagery and context) more that others. However, the inclusion of the control group in the current study minimizes the possibility that such random error could account for the observed results.
Despite these limitations, our findings are consistent with other cognitive rehabilitation studies that demonstrate changes in patterns of cerebral activation corresponding with post-treatment improvements in cognitive functioning (Cerasa et al., 2013; Ernst et al., 2012; Parisi et al., 2013; Rosti-Otajärvi, Mäntynen, Koivisto, Huhtala, & Hämäläinen, 2013). Our findings suggest that behavioral and neural changes following treatment with the mSMT are maintained long-term and highlight the effectiveness of the mSMT in this population.
Supplementary Material
Acknowledgments
The authors would like to acknowledge grant support from the National Institute of Health (NCMRR) to N. D. Chiaravalloti (R01 HD045798S, R01 HD045798) and Kessler Foundation. The contents of this article were also developed under the NIDRR grant # H133P090009 to N.D. Chiaravalloti. However, these contents do not necessarily represent the policy of the Department of Education, and endorsement by the Federal Government should not be assumed.
Footnotes
The authors have no conflicts of interest to report.
References
- Allen DN, Goldstein G, Heyman RA, Rondinelli T. Teaching memory strategies to persons with multiple sclerosis. Journal of Rehabilitation Research and Development. 1998;35(4):405–10. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10220218. [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Valentino P, Nisticò R, Chiriaco C, Pirritano D, Quattrone A. Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with FMRI correlates. Neurorehabilitation and Neural Repair. 2013;27(4):284–95. doi: 10.1177/1545968312465194. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti N, Balzano J, Moore NB, Deluca J. The Open-Trial Selective Reminding Test (OT-SRT) as a Tool for the Assessment of Learning and Memory. The Clinical Neuropsychologist. 2009;23:231–254. doi: 10.1080/13854040802121158. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti N, Moore NB, Nikelshpur OM, Deluca J. An RCT to treat learning impairment in multiple sclerosis: The MEMREHAB trial. Neurology. 2013 doi: 10.1212/01.wnl.0000437295.97946.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti N, Wylie G, Leavitt V, Deluca J. Increased cerebral activation after behavioral treatment for memory deficits in MS. Journal of Neurology. 2012;259(7):1337–46. doi: 10.1007/s00415-011-6353-x. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Gaudino EA, Diamond BJ, Christodoulou C, Engel RA. Acquisition and storage deficits in multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 1998;20(3):376–90. doi: 10.1076/jcen.20.3.376.819. [DOI] [PubMed] [Google Scholar]
- Ernst A, Botzung A, Gounot D, Sellal F, Blanc F, de Seze J, Manning L. Induced brain plasticity after a facilitation programme for autobiographical memory in multiple sclerosis: a preliminary study. Multiple Sclerosis International. 2012;2012:820240. doi: 10.1155/2012/820240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Poldrack RA. Images of medial temporal lobe functions in human learning and memory. Neurobiology of Learning and Memory. 1998;70(1–2):275–83. doi: 10.1006/nlme.1998.3853. [DOI] [PubMed] [Google Scholar]
- Ganis G. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Cognitive Brain Research. 2004 Jul 1;20(2) doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuro Image. 2011;54(3):2446–61. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- McDonald WI, Compston a, Edan G, Goodkin D, Hartung HP, Lublin FD, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology. 2001;50(1):121–7. doi: 10.1002/ana.1032. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11456302. [DOI] [PubMed] [Google Scholar]
- Parisi L, Rocca MA, Mattioli F, Copetti M, Capra R, Valsasina P, Filippi M. Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2013 doi: 10.1177/1352458513505692. [DOI] [PubMed] [Google Scholar]
- Rosti-Otajärvi E, Mäntynen A, Koivisto K, Huhtala H, Hämäläinen P. Neuropsychological rehabilitation has beneficial effects on perceived cognitive deficits in multiple sclerosis during nine-month follow-up. Journal of the Neurological Sciences. 2013;334(1–2):154–60. doi: 10.1016/j.jns.2013.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.