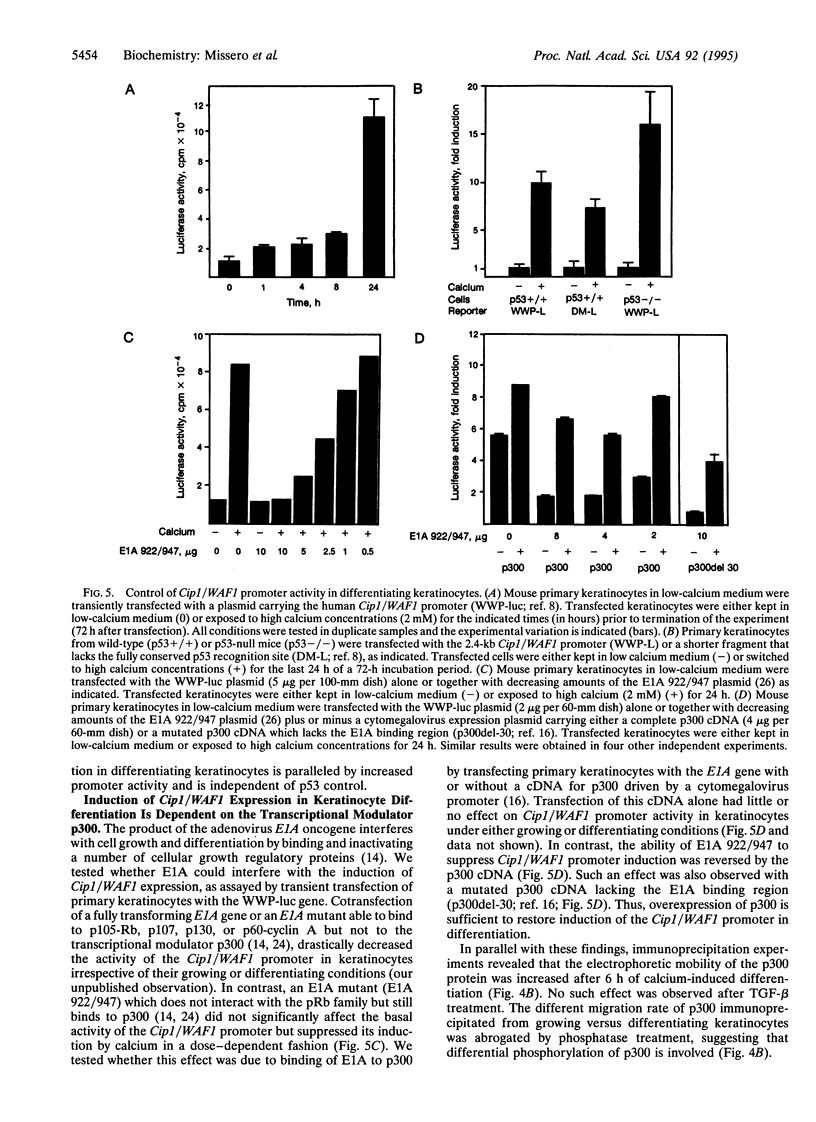
Abstract
The mechanism of cell cycle withdrawal during terminal differentiation is poorly understood. We report here that the cyclin-dependent kinase (CDK) inhibitor p21Cip1/WAF1 is induced at early times of both keratinocyte and myoblast differentiation. p21Cip1/WAF1 induction is accompanied by a drastic inhibition of total Cdk2, as well as p21Cip1/WAF1-associated CDK kinase activities. p21Cip1/WAF1 has been implicated in p53-mediated G1 arrest and apoptosis. In keratinocyte differentiation, Cip1/WAF1 induction is observed even in cells derived from p53-null mice. Similarly, keratinocyte differentiation is associated with induction of Cip1/WAF1 promoter activity in both wild-type and p53-negative keratinocytes. Induction of the Cip1/WAF1 promoter upon differentiation is abolished by expression of an adenovirus E1A oncoprotein (d1922/947), which is unable to bind p105-Rb, p107, or cyclin A but which still binds the nuclear phosphoprotein p300. Overexpression of p300 can suppress the E1A effect, independent of its direct binding to E1A. Thus, terminal differentiation-induced growth arrest in both keratinocyte and myoblast systems is associated with induction of Cip1/WAF1 expression. During keratinocyte differentiation, Cip1/WAF1 induction does not require p53 but depends on the transcriptional modulator p300.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arany Z., Sellers W. R., Livingston D. M., Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994 Jun 17;77(6):799–800. doi: 10.1016/0092-8674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Banerjee A. C., Recupero A. J., Mal A., Piotrkowski A. M., Wang D. M., Harter M. L. The adenovirus E1A 289R and 243R proteins inhibit the phosphorylation of p300. Oncogene. 1994 Jun;9(6):1733–1737. [PubMed] [Google Scholar]
- Brasier A. R., Tate J. E., Habener J. F. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. Biotechniques. 1989 Nov-Dec;7(10):1116–1122. [PubMed] [Google Scholar]
- Brissette J. L., Kumar N. M., Gilula N. B., Dotto G. P. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol Cell Biol. 1991 Oct;11(10):5364–5371. doi: 10.1128/mcb.11.10.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Sipes N. J., Bascom C. C., Graves-Deal R., Pennington C. Y., Weissman B. E., Moses H. L. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res. 1988 Mar 15;48(6):1596–1602. [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Eckner R., Ewen M. E., Newsome D., Gerdes M., DeCaprio J. A., Lawrence J. B., Livingston D. M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994 Apr 15;8(8):869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- Filvaroff E., Calautti E., McCormick F., Dotto G. P. Specific changes of Ras GTPase-activating protein (GAP) and a GAP-associated p62 protein during calcium-induced keratinocyte differentiation. Mol Cell Biol. 1992 Dec;12(12):5319–5328. doi: 10.1128/mcb.12.12.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Novitch B. G., Spicer D. B., Skapek S. X., Rhee J., Hannon G. J., Beach D., Lassar A. B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995 Feb 17;267(5200):1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Halevy O. p53 gene is up-regulated during skeletal muscle cell differentiation. Biochem Biophys Res Commun. 1993 Apr 30;192(2):714–719. doi: 10.1006/bbrc.1993.1473. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harvey M., Sands A. T., Weiss R. S., Hegi M. E., Wiseman R. W., Pantazis P., Giovanella B. C., Tainsky M. A., Bradley A., Donehower L. A. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene. 1993 Sep;8(9):2457–2467. [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hunter T. Braking the cycle. Cell. 1993 Dec 3;75(5):839–841. doi: 10.1016/0092-8674(93)90528-x. [DOI] [PubMed] [Google Scholar]
- Jiang H., Lin J., Su Z. Z., Collart F. R., Huberman E., Fisher P. B. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene. 1994 Nov;9(11):3397–3406. [PubMed] [Google Scholar]
- Johnson M., Dimitrov D., Vojta P. J., Barrett J. C., Noda A., Pereira-Smith O. M., Smith J. R. Evidence for a p53-independent pathway for upregulation of SDI1/CIP1/WAF1/p21 RNA in human cells. Mol Carcinog. 1994 Oct;11(2):59–64. doi: 10.1002/mc.2940110202. [DOI] [PubMed] [Google Scholar]
- Kiess M., Gill R. M., Hamel P. A. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation of myoblasts into quiescent myotubes. Oncogene. 1995 Jan 5;10(1):159–166. [PubMed] [Google Scholar]
- Li R., Waga S., Hannon G. J., Beach D., Stillman B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994 Oct 6;371(6497):534–537. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994 Mar;14(3):2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Missero C., Filvaroff E., Dotto G. P. Induction of transforming growth factor beta 1 resistance by the E1A oncogene requires binding to a specific set of cellular proteins. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3489–3493. doi: 10.1073/pnas.88.8.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E. DNA tumor virus transforming proteins and the cell cycle. Curr Opin Genet Dev. 1993 Feb;3(1):63–70. doi: 10.1016/s0959-437x(05)80342-9. [DOI] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci. 1993 Jun;18(6):195–197. doi: 10.1016/0968-0004(93)90185-p. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Steinman R. A., Hoffman B., Iro A., Guillouf C., Liebermann D. A., el-Houseini M. E. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994 Nov;9(11):3389–3396. [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Hannon G. J., Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994 Aug 1;8(15):1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]