Abstract
Background
Melanoma cell lines treated with decitabine show upregulation of cancer antigens, and interferon-α upreglates MHC Class I antigens in cancer cells, leading to enhanced T-cell recognition and T-cell mediated tumor apoptosis. We evaluated the synergy between the hypomethylating effects of decitabine and the immunomodulatory effects of interferon in a combination regimen administered to advanced melanoma patients in a phase 1 trial.
Methods
Patients with one prior systemic therapy were eligible. Using a modified 3+3 design, patients received escalating doses of decitabine and pegylated interferon α-2b (PEG-IFN) during every 28-day treatment cycle. Global DNA methylation was measured on days 1 and 5 of cycles 1 and 3. Cytokine profiling and quantification of T-cell subpopulations by FACS were performed at baseline and cycle 3.
Results
Seventeen patients were assigned to one of four dose levels. Decitabine 15 mg/m2/d + PEG-IFN 3 µg/kg was the maximum tolerated dose (MTD). Grade 3/4 cytopenias were seen across all dose levels: anemia (1), neutropenia (7), and thrombocytopenia (2). One patient remained progression-free for 37 weeks. The other 16 patients progressed at or before 12 weeks. Median overall survival was 39 weeks. Hypomethylation was seen at all dose levels. Due to treatment-induced lymphocytopenia, absolute changes in T-cell populations post-treatment were too small to be meaningfully interpreted.
Conclusions
The response to this combination regimen was characterized by significant myelosuppression, particularly neutropenia. Although disappointing efficacy and slow accrual led to early closure of the trial, hypomethylation showed pharmacodynamic evidence of a therapeutic effect of decitabine at all dose levels.
INTRODUCTION
Metastatic melanoma has a 2-year survival rate of less than 10–20%.[1] In 2011, the U.S. Food and Drug Administration (FDA) approved ipilimumab, a monoclonal antibody against the cytotoxic T lymphocyte antigen 4 (CTLA 4) receptor found on melanoma cells, and vemurafenib, a signal inhibitor for mutated BRAF, for the treatment of metastatic melanoma. Both therapies showed improved progression-free survival and overall survival when compared to chemotherapy in phase III trials. [2,3] Previously, treatment consisted of dacarbazine, high-dose interleukin-2, interferon alpha, temozolomide, imatinib for tumors with c-KIT mutations, the bacillus Calmette Guerin vaccine, and paclitaxel with the option of carboplatin, all with marginal efficacy. Presently, metastatic melanoma remains incurable, and rationally designed clinical trials of immunotherapy and targeted agents represent the greatest hope to change the course of this otherwise fatal disease.
Decitabine is a DNA methyltransferase inhibitor that is approved for the treatment of leukemia and myelodysplastic syndrome. Through steric inhibition of DNA methyltransferase, decitabine reduces the transfer of methyl groups during cell division, theoretically reversing methylation-induced gene silencing.[4] In a phase I study in which 20 melanoma patients were treated with decitabine, one patient achieved a near-complete remission for 116 days.[5] More recently, Tawbi et al conducted a phase I/II trial that showed the combination of decitabine and temozolomide for metastatic melanoma led to a 12.4-month median overall survival, with 2 complete responses and 4 partial responses. The most significant side effect in this study was grade 3/4 neutropenia.[6]
Pegylated interferon alpha-2b (PEG-IFN) has been approved for high-risk melanoma in the adjuvant setting. Dummer et al conducted a 150-patient study using 3 different doses of pegylated interferon (180 ug/week, 360 ug/week, or 450 ug/week) and found response rates of 6%, 8%, and 12% for the 3 doses, respectively[7]. These differences were not statistically significant, indicating that dose level did not correlate with response, and lower doses were as effective as higher doses. The most common adverse effects were fatigue, pyrexia and nausea. [7] In a phase II study of temozolomide plus low-dose PEG-IFN (0.5 mcg/kg/week) in 35 patients with treatment-naïve metastatic melanoma, 11 patients (31%) had an objective tumor response, including 3 with complete response and 8 partial response. The median survival was 12 months, with a median follow-up of 16 months. Hematologic toxicity consisted mainly of lymphopenia (31% grade 2 and 37% grade 3) and leukopenia (17% grade 2 and 3% grade 3); no Grade 4 hematologic toxicity was observed.[8]
The combination of decitabine and pegylated interferon was rationally selected for clinical study based on preclinical data showing synergistic antitumor activity by combining the two agents. [9] In addition to mediating apoptosis by inducing direct cytotoxic mechanisms, interferon can enhance immune recognition of tumor cells by upregulating antigen-presenting molecules such as the MHC family while stimulating multiple cytokine pathways that function to increase immune surveillance.[10,11] In preclinical in vitro studies, decitabine treatment led to upregulation of MHC class I antigens as well as cancer-testis antigens (CTAs). [12] Examples of CTAs that are upregulated by decitabine are the MAGE family, GAGE 1–6, NY-ESO-1, RAGE-1, and SSX. Our central hypothesis was that combination therapy with decitabine and interferon would enhance tumor cell death through two distinct pathways. First, decitabine would increase tumor antigenicity and enhance interferon-induced immune activation to trigger more efficient immune-mediated cell death. Second, decitabine would synergize with interferon, through upregulation of interferon-stimulated genes, to induce apoptosis through a direct cytotoxic mechanism. We proposed that these dual modes of attack would result in consequent durable clinical benefit in patients with advanced melanoma.
PATIENTS AND METHODS
Patient Selection
We designed this phase I trial to test the primary objectives of safety and tolerability of the combination of decitabine and pegylated interferon and to find the maximum tolerated dose (MTD) of this combination in patients with advanced melanoma. We also sought to characterize antitumor activity and changes in global methylation and T-cell subsets as a result of this combination therapy. Patients eligible for this study were required to have pathologically confirmed, unresectable stage III or stage IV malignant melanoma. Prior therapy was limited to one prior cytotoxic regimen. Measurable disease as defined by RECIST 1.0 was required. Other key inclusion criteria included age > 18 years, ECOG performance status 0 or 1, and adequate organ and marrow function defined by absolute neutrophil count > 1500 uL, platelets > 100,000 uL, creatinine (serum) < 2.0 mg/dL, total bilirubin < 1.5 mg/dL, and AST/ALT < 2.5 × institutional upper limit of normal. Key exclusion criteria included active autoimmune disorders, previous treatment with adjuvant high-dose interferon, and known brain metastases.
Study Design
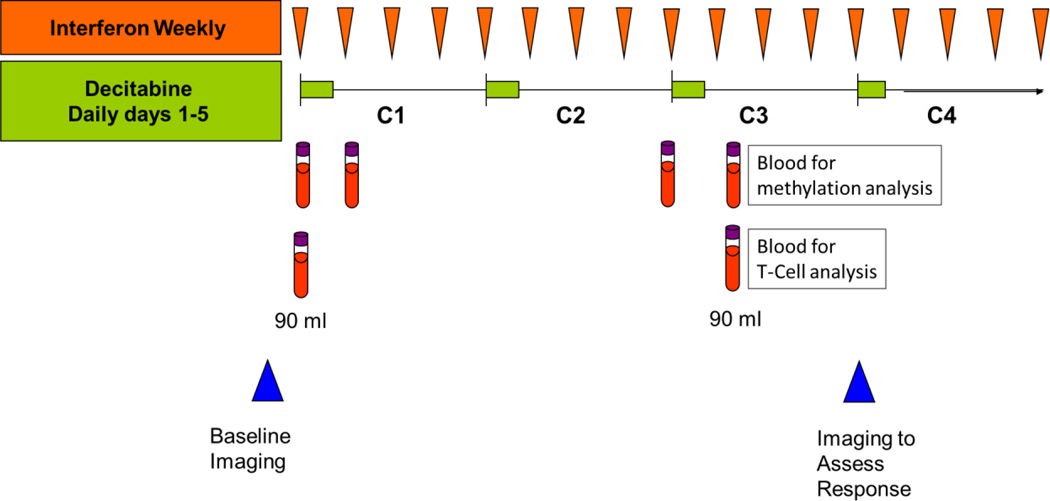
This study consisted of a Phase I dose escalation part, followed by a Phase II randomized part. In Phase I, decitabine and pegylated interferon were administered in a modified 3 + 3 design to determine the admissible doses. Decitabine was given intravenously over 1 hour daily for 5 days, on days 1–5 during a 28-day treatment cycle, and pegylated interferon was given by subcutaneous injection once weekly during the same cycle (Figure 1). Blood samples were obtained on days 1 and 5 of cycles 1 and 2 and analyzed for changes in methylation and T-cell subsets as outlined below. Doses were escalated according to standard 3 + 3 dose escalation criteria to establish admissible dose levels, defined as any dose level where 0/3 or 1/6 patients experienced DLT during cycle 1. The novel design of this study allowed for testing of two cohorts simultaneously. In this study, if 1 out of 3 patients experienced a DLT at dose level 1, 3 more patients were assigned to dose level 1. If 1/6 of those patients had DLT, then dose levels 2 and 3 were opened for testing. If 2/6 patients experienced DLT, then dose level 1 was considered toxic and dose level -1 was opened for testing. If 0/3 experienced DLT, then doses 2 and 3 were opened for testing. In a similar fashion, dose 5 was opened after dose 3 was admissible; dose 4 was opened after both doses 2 and 3 were admissible; and dose 6 was opened after both doses 4 and 5 were admissible. After the admissible dose levels were defined in the Phase I part of the study, patients were to be randomized to these levels adaptively in Phase II. However, only one patient had been randomized when decision was made to close the study due to lack of observed responses. DLT was defined as one or more of the following using the NCI Common Toxicity Criteria (CTC) Version 3.0 grading system: any grade 3 or 4 nonhematologic toxicity regardless of duration except grade 3 nausea or vomiting without maximal antiemetic therapy and grade 3 diarrhea without adequate loperamide therapy; grade 4 thrombocytopenia; grade 4 neutropenia lasting > 2 weeks or associated infection; any toxicity that results in treatment delay of >4 weeks. Due to concern regarding toxicity in cohort 1, the protocol was amended to add a cohort at dose level −1. Dose levels are listed in Table 1.
Figure 1.
Treatment Schema
Table 1.
Phase I Dose Levels
| Dose Level | Dose of Decitabine (days 1–5 every 28 days) |
Dose of Pegylated Interferon α-2b (subcutaneous weekly) |
|---|---|---|
| −1 | 5 mg/m2/day | 1.5 µg/kg |
| 1 | 10 mg/m2/day | 3 µg/kg |
| 2 | 15 mg/m2/day | 3 µg/kg |
| 3 | 10 mg/m2/day | 4.5 µg/kg |
Pretreatment and follow-up studies
CBC and serum chemistry studies were monitored prior to each decitabine treatment. Patients were imaged with CT scan and disease response assessments per RECIST 1.0 performed at week 12 of therapy and every 8 weeks thereafter. Patients were given supportive care with antiemetics. No steroids or hematopoietic growth factors were used out of concern that these would undermine the effects of the study therapy. Treatment was continued until disease progression, DLT, withdrawal of consent for study treatment or intercurrent illness.
PBMC collection and processing
Blood specimens (60 mL; 6 × 10 mL heparizined tubes) were taken for PBMC isolation and T-cell number determination. Samples were collected using standard techniques for “Green Top” Vaccutainer™ blood collection tubes. Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Hypaque gradient, resuspended in AB serum with 10% DMSO, frozen at −80 degrees Celsius overnight, and then transferred to liquid nitrogen. For analysis, PBMC were removed from liquid nitrogen and thawed in a 37-degree Celsius water bath. Thereafter, cells were washed with 20 mL of complete medium (RPMI 1640 plus 10% AB serum), centrifuged at 1,500 rpm for 10 minutes, and suspended in complete medium.
DNA methylation analysis
DNA extracted from PBMC was treated with bisulfite, as follows. Two micrograms of genomic DNA were denatured by 0.2 mol/L NaOH at 37°C for 10 minutes followed by incubation with freshly prepared 30 µL of 10 mmol/L hydroquinone and 520 µL of 3 mol/L sodium bisulfite (pH 5.0) at 50°C for 16 hours. DNA was purified with a Wizard Miniprep Column (Promega, Madison, WI), desulfonated with 0.3 mol/L NaOH at 25°C for 5 minutes, precipitated with ammonium acetate and ethanol, and dissolved in 50 µL of TE buffer (Tris-HCl 10 mmol/L, EDTA 1 mmol/L, pH 8.0). Bisulfite-treated DNA (40–80 ng) was amplified with primers specific for the LINE-1 repetitive element, forward 5'-TTTTGAGTTAGGTGTGGGATATA and reverse primer 5’-biotin- AAAATCAAAAAATTCCCTTTC. PCR conditions were as follows: initial denaturation 95°C for 5 minutes, then 40 cycles (94°C/15 seconds, 56°C for 30 seconds, 72°C for 30 seconds), final extension at 72°C for 5 minutes. Each PCR was performed in a total volume of 20 µL of 67 mmol/L Tris-HCl (pH=8.8), 16 mmol/L ammonium sulfate, 2 mmol/L MgCl2, 0.125 mmol/L dNTPs, 1 unit of Taq polymerase, and 100 nmol/L PCR primers. We measured levels of DNA methylation as the percentage of bisulfite-resistant cytosines at CpG sites by pyrosequencing with the PSQ HS 96 Pyrosequencing System (Biotage, Charlottesville, VA), Pyro Gold CDT Reagents (Biotage) and the sequencing primer 5'-AGTTAGGTGTGGGATATAGT.
Flow Cytometric Analysis
Multi-parameter flow cytometry analysis of different cell subsets was performed using PBMC from patients at baseline and at cycle 3. Cells were stained with different antibody panels to study different lymphocyte populations. To study T regulatory (Treg) cells we used the following panel: CD3-AmCyan, Foxp3-efluor450 (eBioscience, CA), CD127-FITC, ICOS-PE (eBioscience, CA), CD4-PerCP-Cy5.5, CD39-APC, CD25-PE-Cy7 and CD8-APC-H7. For the proliferation panel we stained for CD3-AmCyan, Foxp3-efluor450, KI-67-Alexa Fluor 488, ICOS-PE, CD4-PerCP-Cy5.5, CD39-APC, CD25-PE-Cy7 and CD8-APC-H7. The frequencies of the different populations were translated into cell numbers (# cells/µl of blood) using Absolute Lymphocyte Counts (ALC). All antibodies are from BD Biosciences, unless otherwise indicated. The acquisition was carried out on a FACS Canto II flow cytometer (BD Biosciences, CA). All analysis was done with the software FlowJo (Tree Star, OR).
Statistical Analysis
The method of Kaplan and Meier was used to estimate the distribution of progression-free survival from the start of the study. Spearman rank correlation methods were used to assess the association between continuous variables. No adjustment was made for the multiplicity of testing.
RESULTS
Patient Demographics and Treatment
Patient demographics are summarized in Table 2. A total of 17 patients were enrolled in this study. Nine had received prior chemotherapy, nine had prior immunotherapy, and five had prior radiation therapy, with some patients receiving multiple therapies. Seven patients had no prior systemic therapy. All 17 patients received a cumulative total of 40 cycles of decitabine/PEG-IFN. The median number of completed cycles was 3 (range 0–6).
Table 2.
Patient Demographics
| Demographic or Clinical Characteristic | No of Patients (N = 17) |
|
|---|---|---|
| Age (years) | ||
| Median | 65 | |
| Range | 45–74 | |
| Gender | ||
| Male | 12 | |
| Female | 5 | |
| Prior therapies | ||
| Chemotherapy | 9 | |
| Immunotherapy | 9 | |
| Radiation therapy | 5 | |
| No prior systemic therapy | 7 | |
| Treated at Dose Level | ||
| −1 | 5 | |
| 1 | 4 | |
| 2 | 3 | |
| 3 | 5 | |
Toxicity
The first cohort of three patients was treated at dose level 1. One patient in this group developed fever and infection after day 2 of study treatment and was withdrawn from the study. Initially this was felt to be possibly treatment-related, hence dose level 1 was closed and dose level −1 added and opened. The attribution of the infection was later revised to unlikely related; this patient was determined to be inevaluable, and was replaced with a new patient at dose level 1. When there were no DLTs at dose levels 1 or −1, dose levels 2 and 3 opened concurrently as per study design. Three patients experienced dose-limiting toxicities at levels 2 and 3. At dose level 2, one patient had a pulmonary embolism that prevented progression to the next cycle of therapy. Before the pulmonary embolism, the patient had grade 2 leukopenia, thrombocytopenia and neutropenia. At dose level 3, one patient developed an intracranial hemorrhage on day 11 of cycle 1 consistent with DLT. This patient had fevers and failure to thrive and was found to have a urinary tract infection. He consequently had altered mental status and a CT scan showed hydrocephalus and hemorrhaging in the third and fourth ventricles and no metastases in the brain. A third patient had DLT in dose level 3 on day 15 of cycle 1 with grade 4 thrombocytopenia, grade 3 leukopenia, and grade 2 anemia that was treated with transfusion of 6 units of platelets and 2 units of packed red blood cells. On day 20, the thrombocytopenia was grade 2 and neutropenia grade 4 treated with one dose of pegfilgrastim. The patient was subsequently dose reduced for myelosuppression.
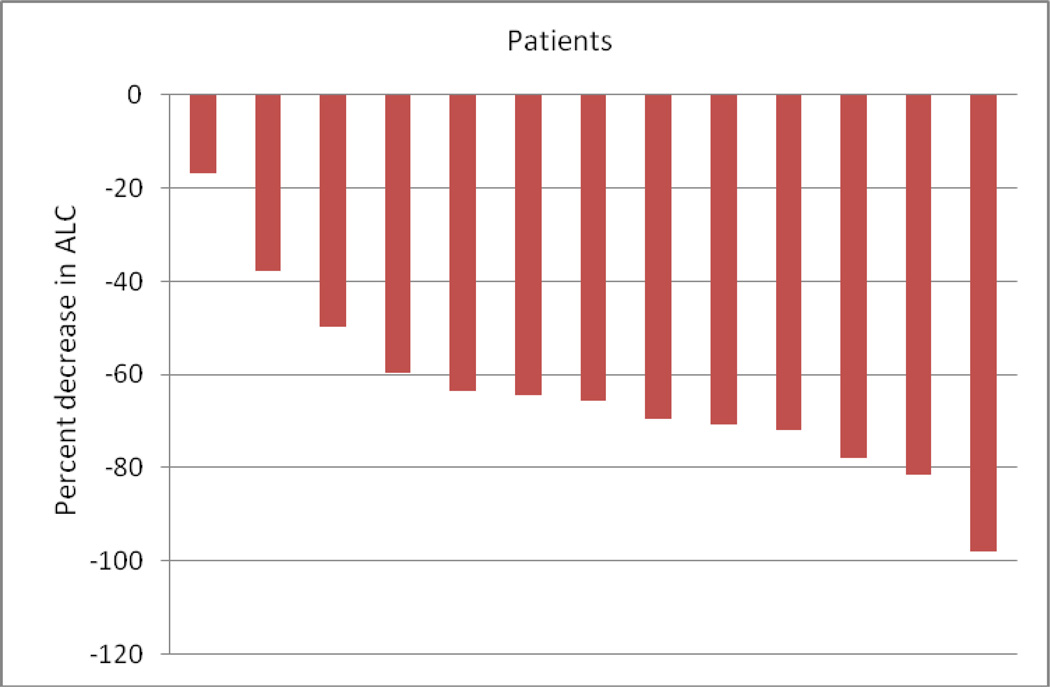
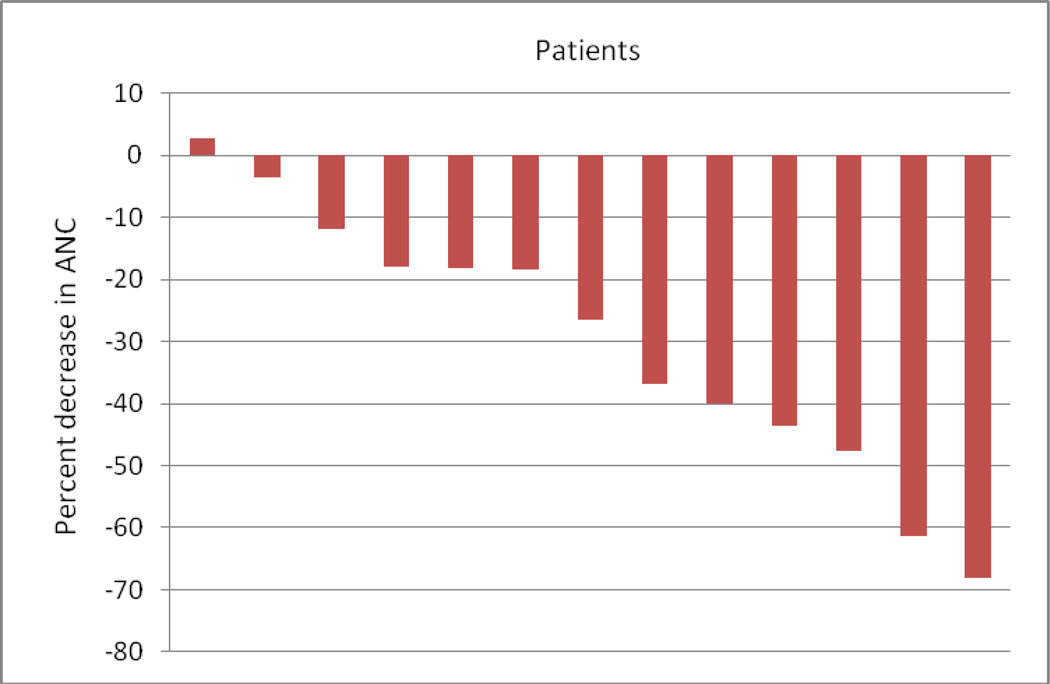
Most of the patients on the study developed myelosuppression while on treatment. Two patients had grade 3 anemia in cycle 1. Six patients had grade 3 or grade 4 neutropenia develop during cycle 1, and an additional two patients developed grade 3 neutropenia in later cycles. The decreases in absolute lymphocytes and absolute neutrophils are depicted in Figures 2 and 3, respectively. Four patients had grade 3 or grade 4 thrombocytopenia in cycle 1. An additional patient developed grade 4 thrombocytopenia in cycle 3.
Figure 2.
Treatment-related decreases in Absolute Lymphocyte Count (ALC) from cycle 1 day 1 to cycle 3 day 5.
Figure 3.
Treatment-related Decreases in Absolute Neutrophil Count (ANC) from cycle 1 day 1 to cycle 1 day 15.
Response and Survival
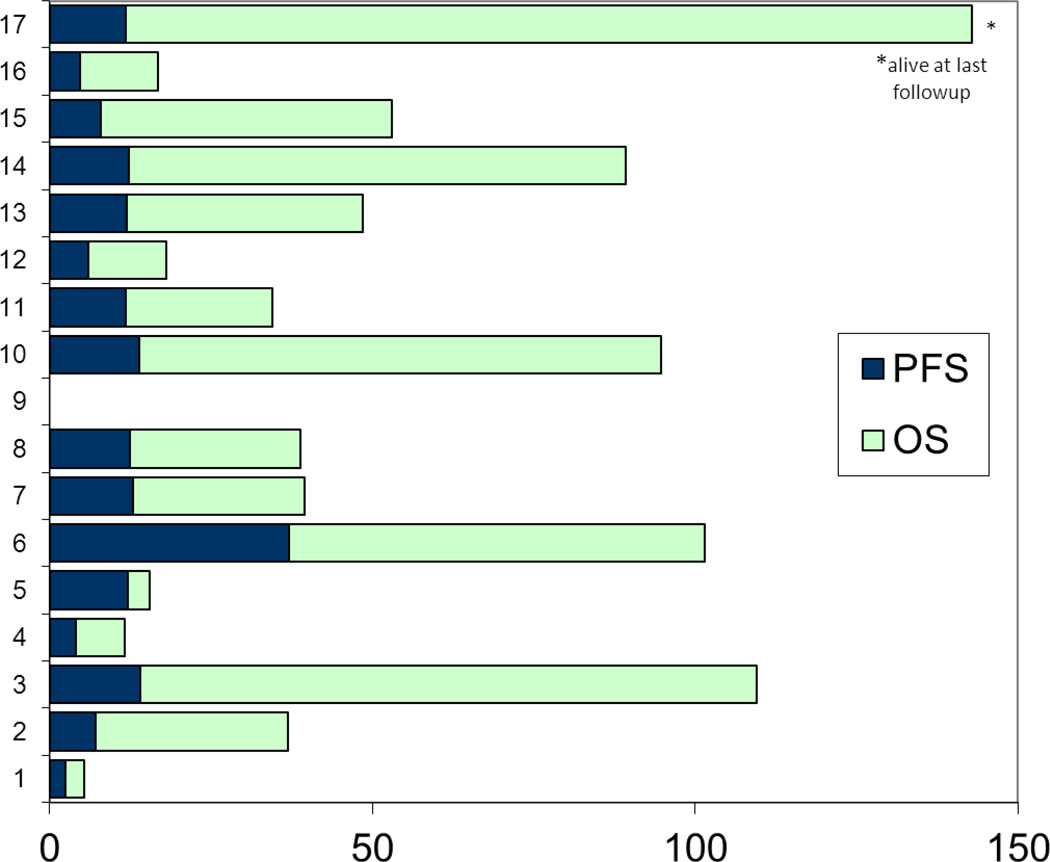
Of the 17 patients treated in this study, one had stable disease at first response assessment imaging at 12 weeks. The other 16 patients progressed either at or prior to first imaging at 12 weeks. No objective responses were seen. The median progression-free survival (PFS) was 12 weeks, ranging from 2 weeks to 37 weeks. The median overall survival was 39 weeks, ranging from 5 weeks to 110+ weeks (Figure 4). One patient who was found to have progression at 12 weeks was still alive at the time of data cutoff, having received subsequent treatment with talimogene laherparepvec (T-VEC), an oncolytic virus. One patient developed brain metastasis after 4 weeks of study treatment. Six additional patients were found to have brain metastases at the time of the 12-week restaging scan.
Figure 4.
Progression free survival and overall survival.
Methylation
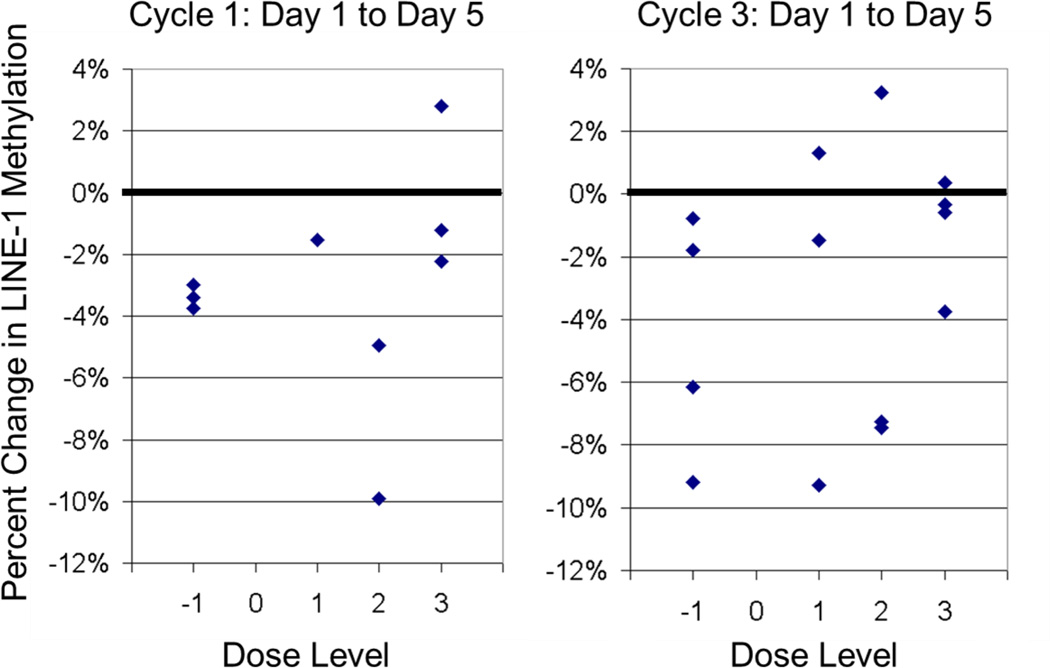
LINE-1 Methylation was measured in PBMCs as outlined above and previously described, providing an estimate of global methylation.[13] Hypomethylation ranging from 0.3% – 9.9% at day 5 was seen in 14/15 patients for whom data is available. As shown in Figure 5, hypomethylation between days 1 and 5 was seen during both cycle 1 and cycle 3 and at all dose levels, consistent with pharmacodynamic effect of decitabine. There was no correlation between percent hypomethylation and dose level, decitabine dose, or number of cycles completed.
Figure 5.
LINE-1 methylation in PBMCs
T-cell Populations
To test the hypothesis that decitabine and interferon would result in a shift in T-cell populations from regulatory to effector, T-cell subsets were measured. Unfortunately, due to the marked decline in all leukocytes (leukopenia) as a result of study therapy, the absolute differences in T-cell populations post-treatment were too small to be meaningfully interpreted.
Discussion
This study tested the safety and tolerability of the combination of decitabine and PEG-IFN in patients with advanced melanoma. The treatment was notable for significant declines in lymphocytes and neutrophils as a result of treatment (Figures 2 and 3). The significant hematological toxicity combined with lack of efficacy led to the decision to terminate the study after the dose-finding portion was completed; hence, the planned phase II portion of the study was not conducted.
It is likely that our inability to reproduce in the clinic the efficacy of this combination seen in cell lines and xenografts resulted from the fact that these preclinical models do not reflect the clinical setting due to the lack of functioning lymphocytes in vitro and in the nude mouse models.[9] In clinical use, any potential benefit that decitabine may have had via increased expression of cancer antigens was likely offset by the depletion of lymphocytes as a result of therapy, eliminating the interaction required for immune-mediated cytotoxicity. Since the initiation of our study, further data have emerged, suggesting that decitabine may upregulate regulator T-cell activity by decreasing promoter methylation of Foxp3.[14] Thus, increased regulatory T-cell activity represents another potential explanation of our failure to see clinical benefit with this combination.
We chose to administer decitabine and PEG-IFN concurrently in this trial, based on evidence from clinical trials in both solid tumors and leukemia that decreases in LINE-1 methylation in response to decitabine were short-lived, returning to baseline levels within a few weeks.[15,16] Our goal was to treat with PEG-IFN during the methylation nadir, presuming that any antigen upregulation as a result of hypomethylation would peak at that time. However, newer evidence has emerged suggesting that sequential administration may be preferable.[17] Triozzi et al showed that decitabine in combination with IL-2 in immune-competent melanoma xenograft models yielded tumor responses when given in sequence, but not concurrently.
While no evidence of response or clinical benefit with decitabine and PEG-IFN was seen while patients were on study, several patients derived benefit from subsequent therapies, with one patient still alive at last follow-up. While this is likely a reflection of patients receiving more active subsequent treatments such as BRAF and PD-1 inhibitors and other novel therapies, it is possible that priming with a hypomethylating agent potentiates responses to additional conventional and investigational therapies. Such a “memory” phenomenon was seen in patients with lung cancer treated with low-dose azacitadine and entinostat who, after progressing on study therapy, demonstrated a longer-than-expected duration of disease control on subsequent cytotoxic therapy.[18] One proposed mechanism for this is that prolonged low-dose treatment with hypomethylating agents can lead to sustained alteration in signaling pathways involved in tumorigenesis, rendering the tumor cells more sensitive to conventional cytotoxic chemotherapy and potentially immunotherapy as well.[19] These emerging data underscore the value of further clinical trials sequencing epigenetic therapy and cytotoxic chemotherapy or immunotherapy in melanoma and other solid tumors.
Acknowledgments
Dr. Plimack has received research funding from Merck. Dr. Issa has received research funding from Merck and honoraria from Eisai. Dr. Hwu has received research funding from Merck and served as Advisory Board consultant for Merck.
Footnotes
CONFLICT OF INTEREST STATEMENT
The other authors have no conflicts of interest to declare.
REFERENCES
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Hu W, Jin L, Jiang CC, Long GV, Scolyer RA, Wu Q, Zhang XD, Mei Y, Wu M. AEBP1 upregulation confers acquired resistance to BRAF (V600E) inhibition in melanoma. Cell death & disease. 2013;4:e914. doi: 10.1038/cddis.2013.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Merkel DG, Nagler A. The role of hypomethylating agents in myelodysplastic syndrome: changing the management paradigm. Expert review of hematology. 2013 doi: 10.1586/17474086.2013.854699. [DOI] [PubMed] [Google Scholar]
- 5.Abele R, Clavel M, Dodion P, Bruntsch U, Gundersen S, Smyth J, Renard J, van Glabbeke M, Pinedo HM. The EORTC Early Clinical Trials Cooperative Group experience with 5-aza-2'-deoxycytidine (NSC 127716) in patients with colo-rectal, head and neck, renal carcinomas and malignant melanomas. European journal of cancer & clinical oncology. 1987;23(12):1921–1924. doi: 10.1016/0277-5379(87)90060-5. [DOI] [PubMed] [Google Scholar]
- 6.Tawbi HA. Safety and efficacy of decitabine in combination with temozolomide in metastatic melanoma: a phase I/II study and pharmacokinetic analysis. Annals of Oncology. 2013;24(4):8. doi: 10.1093/annonc/mds591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dummer R, Garbe C, Thompson JA, Eggermont AM, Yoo K, Maier T, Bergstrom B. Randomized dose-escalation study evaluating peginterferon alfa-2a in patients with metastatic malignant melanoma. J Clin Oncol. 2006;24(7):1188–1194. doi: 10.1200/JCO.2005.04.3216. [DOI] [PubMed] [Google Scholar]
- 8.Guillot B, Khamari A, Cupissol D, Delaunay M, Bedane C, Dreno B, Picot MC, Dereure O. Temozolomide associated with PEG-interferon in patients with metastatic melanoma: a multicenter prospective phase I/II study. Melanoma research. 2008;18(2):141–146. doi: 10.1097/CMR.0b013e3282f6309c. [DOI] [PubMed] [Google Scholar]
- 9.Reu FJ, Bae SI, Cherkassky L, Leaman DW, Lindner D, Beaulieu N, MacLeod AR, Borden EC. Overcoming resistance to interferon-induced apoptosis of renal carcinoma and melanoma cells by DNA demethylation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(23):3771–3779. doi: 10.1200/JCO.2005.03.4074. [DOI] [PubMed] [Google Scholar]
- 10.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6(1):34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 11.Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ. Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res. 1999;5(10):2726–2734. [PubMed] [Google Scholar]
- 12.Fonsatti E, Nicolay HJ, Sigalotti L, Calabro L, Pezzani L, Colizzi F, Altomonte M, Guidoboni M, Marincola FM, Maio M. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2'-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(11):3333–3338. doi: 10.1158/1078-0432.CCR-06-3091. [DOI] [PubMed] [Google Scholar]
- 13.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32(3):e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE, Bromberg JS. Epigenetic Regulation of Foxp3 Expression in Regulatory T Cells by DNA Methylation. The Journal of Immunology. 2009;182(1):259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, Faderl S, Bueso-Ramos C, Ravandi F, Estrov Z, Ferrajoli A, Wierda W, Shan J, Davis J, Giles F, Saba HI, Issa JP. Results of a randomized study of three schedules of low-dose decitabine in higher risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2006 doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 16.Stewart DJ, Issa JP, Kurzrock R, Nunez MI, Jelinek J, Hong D, Oki Y, Guo Z, Gupta S, Wistuba II. Decitabine effect on tumor global DNA methylation and other parameters in a phase I trial in refractory solid tumors and lymphomas. Clin Cancer Res. 2009;15(11):3881–3888. doi: 10.1158/1078-0432.CCR-08-2196. [DOI] [PubMed] [Google Scholar]
- 17.Triozzi P, Aldrich W, Achberger S, Ponnazhagan S, Alcazar O, Saunthararajah Y. Differential effects of low-dose decitabine on immune effector and suppressor responses in melanoma-bearing mice. Cancer Immunology, Immunotherapy. 2012;61(9):1441–1450. doi: 10.1007/s00262-012-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, Sebree R, Rodgers K, Hooker CM, Franco N, Lee B, Tsai S, Delgado IE, Rudek MA, Belinsky SA, Herman JG, Baylin SB, Brock MV, Rudin CM. Combination Epigenetic Therapy Has Efficacy in Patients with Refractory Advanced Non–Small Cell Lung Cancer. Cancer Discovery. 2011 doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai H-C, Li H, Van Neste L, Cai Y, Robert C, Rassool Feyruz V, Shin James J, Harbom Kirsten M, Beaty R, Pappou E, Harris J, Yen R-Whay C, Ahuja N, Brock Malcolm V, Stearns V, Feller-Kopman D, Yarmus Lonny B, Lin Y-C, Welm Alana L, Issa J-P, Minn I, Matsui W, Jang Y-Y, Sharkis Saul J, Baylin Stephen B, Zahnow Cynthia A. Transient Low Doses of DNA-Demethylating Agents Exert Durable Antitumor Effects on Hematological and Epithelial Tumor Cells. Cancer Cell. 2012;21(3):430–446. doi: 10.1016/j.ccr.2011.12.029. doi: http://dx.doi.org/10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]