Abstract
Tetrandrine, a constituent of Chinese herb Stephania tetrandra, causes cell death in prostate cancer, but the molecular mechanisms leading to apoptosis is not known. Here we demonstrated that tetrandrine selectively inhibits the growth of prostate cancer PC3 and DU145 cells compared to normal prostate epithelial PWR-1E cells. Tetrandrine-induced cell death in prostate cancer cells is caused by reactive oxygen species (ROS)-mediated activation of c-Jun NH2-terminal kinase (JNK1/2). JNK1/2-mediated proteasomal degradation of c-FLIPL/S and Bcl2 proteins are key events in the sensitization of prostate cancer cells to Fas- and mitochondria-mediated apoptosis by tetrandrine. Tetrandrine-induced JNK1/2 activation caused the translocation of Bax to mitochondria by disrupting its association with Bcl2 which was accompanied by collapse of mitochondrial membrane potential (MMP), cytosolic release of cytochrome c and Smac, and apoptotic cell death. Additionally, tetrandrine-induced JNK1/2 activation increased the phosphorylation of Bcl2 at Ser70 and facilitated its degradation via the ubiquitin-mediated proteasomal pathway. In parallel, tetrandrine-mediated ROS generation also caused the induction of ligand-independent Fas-mediated apoptosis by activating procaspase-8 and Bid cleavage. Inhibition of procaspase-8 activation attenuated the cleavage of Bid, loss of MMP and caspase-3 activation suggest that tetrandrine-induced Fas-mediated apoptosis is associated with the mitochondrial pathway. Furthermore, most of the signaling effects of tetrandrine on apoptosis were significantly attenuated in the presence of antioxidant N-acetyl-L-cysteine, thereby confirming the involvement of ROS in these events. In conclusion, the results of the present study indicate that tetrandrine-induced apoptosis in prostate cancer cells is initiated by ROS generation and that both intrinsic and extrinsic pathway contributes to cell death.
Keywords: Tetrandrine, prostate cancer, apoptosis, reactive oxygen species, ubiquitination
1. Introduction
Prostate cancer is one of the most prevalent malignancies affecting men of all ages worldwide and is the second leading cause of cancer-related death in American men [1]. Prostate cancer development is initially androgen dependent, so the basic therapeutic strategy has been the deprivation of androgen [2]. Although most patients initially respond to androgen deprivation therapy by showing low prostate-specific antigen values, they invariably relapse with a more aggressive form of prostate cancer termed androgen-independent or castration-resistant prostate cancer (CRPC) [3,4]. Yet, the molecular mechanisms that promote the development of CRPC are not fully understood. Prostate tumors that recur after androgen deprivation therapy have been shown to have amplified androgen receptor gene, resulting in increased androgen receptor expression and hypersensitivity to low levels of circulating androgens [5-7]. Certain growth factors such as epidermal growth factor and insulin-like growth factor-I have been shown to activate androgen receptor in the absence of androgen [8]. Overexpression of antiapoptotic proteins such as Bcl2 and c-FLIP are seen in CRPC, which have been implicated in promoting tumor survival and reducing sensitivity to chemotherapy [9,10]. Currently there is no effective therapy for CRPC and the median survival after the development of CRPC is 20 to 24 months [11,12]. The CRPC is an invariably lethal condition, which frequently metastasize and is associated with a significant morbidity and mortality. Therefore, identification of agents that will selectively kill or sensitize castration-resistant tumor cells with no additional toxicity to normal tissues would have significant impact on existing therapies.
Tetrandrine, a benzylisoquinoline alkaloid, is a calcium channel blocker isolated from the Chinese herb Stephania tetrandra S. Moore [13-15]. Tetrandrine is used in traditional Chinese medicine as an antirheumatic, anti-inflammatory, and antihypertensive agent for the past several years [16-19]. Tetrandrine has been used as an antifibrotic drug to treat the lesions of silicosis in China since the 1960s. Tetrandrine has been shown to be a potent inhibitor of P-glycoprotein drug efflux [20-22]. Compared to verapamil, etoposide and cytarabine, tetrandrine was more effective in reversing drug resistance to daunorubicin, vinblastine and doxorubicin in leukemia cells [21,22]. Tetrandrine exerts cytotoxic effect by inhibiting cell proliferation and inducing apoptosis in various cancer cells including breast cancer, lung cancer, hepatoma, glioma, leukemia and colon cancer [23-29]. In addition, tetrandrine modulates many cellular signaling events, including cell cycle arrest, mitogen-activated protein kinase activation, NF-κB signaling, Wnt/β-catenin signaling, and the transforming growth factor-β signaling pathway [24,27,28,30-32]. Recent studies have indicated that tetrandrine used alone can exhibit significant anti-cancer activity against cancer cells by inhibiting pathways involved in cell proliferation, migration and angiogenesis [26,28]. Despite its potential as an anti-cancer agent, the effects of tetrandrine on prostate cancer have not been studied. In the present study, we elucidate the mechanism through which tetrandrine induces proapoptotic effect in androgen-independent prostate cancer PC3 and DU145 cells. The results of these studies show that tetrandrine-induced apoptosis in prostate cancer cells is dependent on reactive oxygen species (ROS) generation and that contributes to cell death. Furthermore, we demonstrate for the first time that ROS-mediated activation of JNK1/2 leads to ubiquitin-mediated proteasomal degradation of c-FLIPL/S and Bcl2, and sensitize prostate cancer cells to Fas- and mitochondria-mediated apoptosis by tetrandrine.
2. Materials and methods
2.1 Cell lines and culture Conditions
Human prostate carcinoma cell lines, PC3 and DU145, and the normal epithelial prostate cell line, PWR-1E, were obtained from the American Type Culture Collection (Rockville, MD). The prostate cancer cell lines were cultured in RPMI-1640 (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 50 mg/ml penicillin and 50 mg/ml streptomycin (Invitrogen, Carlsbad, CA), and maintained in an incubator with a humidified atmosphere of 95% air and 5% CO2 at 37°C. The PWR-1E cells were cultured in keratinocyte growth medium supplemented with 5 ng/ml human recombinant epidermal growth factor and 0.05 mg/ml bovine pituitary extract (Invitrogen, Carlsbad, CA) and maintained in an incubator under the conditions described above.
2.2 Materials
Tetrandrine was purchased from Enzo Life Sciences (Farmingdale, NY). The cell fractionation kit was purchased from MitoScience Inc. (Eugene, OR), protein A/G-agarose from Santa Cruz Biotechnology (Santa Cruz, CA), and MG132 and z-DEVD-FMK from Cayman Chemical (Ann Arbor, MI). Antibodies against Bax, Bcl2, Apaf-1, cytochrome c, c-Jun, Fas, FADD, FasL, c-FLIPL/S and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), whereas ubiquitin, PARP, Bid, p-JNK Thr183/Tyr185, JNK1/2, p-Bcl2 Ser70, p-ASK1 Thr845, ASK1, p-SEK1 Thr261, SEK1, p-c-Jun Ser63, Smac and caspase-3, -8 and -9 were from Cell Signaling Technology (Danvers, MA). The cell culture medium RPMI-1640 and fetal bovine serum were from GIBCO (Invitrogen, Carlsbad, CA). All other reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
2.3 Cytotoxicity assay
Proliferation of the PWR-1E, PC-3 or DU145 cells in the presence of tetrandrine was measured by the MTT assay [33]. Briefly, 2 × 104 cells in an aliquot of 190 μl of full serum medium were seeded in 96-well flat bottomed plates for 24 h to allow attachment to the culture plates. After confirming cell attachment, 10 μl of PBS containing various amounts of tetrandrine (final concentration 0 to 80 μM) were added in each well and incubated for different time intervals. After 12, 24 or 48 h of incubation at 37 °C, 10 μl of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium Bromide (MTT) solution (5 mg/ml in PBS) was added to each well, and the plate was further incubated for 4 h at 37°C. The plate was then centrifuged at 2,000 g for 10 min and the medium was aspirated from each well. Dimethylsulfoxide (100 μl) was added to each well and the formazan dye crystals formed in cells were dissolved by shaking the plates at room temperature for 1 h. The absorbance of formazan at 562 nm was measured using a plate reader (Synergy 2, BioTek Instruments, Inc.).
2.4 Preparation of cell extracts and Western blot analysis
After treatment, cells were collected, washed with cold PBS and then incubated in 150 μl of radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM sodium chloride; 0.5% sodium deoxycholate; 1% Nonidet P-40; 0.1% sodium dodecyl sulfate; 1 mg/ml aprotinin; 1 mg/ml leupeptin; 1 mM Sodium orthovanadate; 1 mM phenylmethanesulfonyl fluoride) at 4°C for 30 min. After sonication on ice, cell debris was removed by centrifugation at 12,000 g for 10 min at 4°C. Protein concentrations were determined by Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). Cell extracts were separated on 4-20% Bis-Tris Nu-PAGE gel (Invitrogen Corporation, CA) using MES buffer and transferred onto nitrocellulose membrane (Bio-Rad). Membranes were blocked with 5% fat-free milk in Tris-buffered saline containing 0.05% Tween 20 (TBST) at room temperature for 60 min, and incubated overnight at 4°C with the appropriate primary antibody in 5% milk in TBST. After three washings with TBST, the membrane was incubated with the appropriate secondary antibody (Promega, WI) at room temperature for 2 h. After washing again with TBST, the membranes were developed using ECL plus (Amersham Pharmacia Biotech, IL), and the image was captured using alpha-imager Fluoretech HD2. Isolation of mitochondrial and cytoplasm enriched fractions was by the MitoSciences cell fractionation kit as per the manufacturer's instructions (MitoSciences Inc., Eugene, OR).
2.5 Immunoprecipitation
After treatment, normalized amounts of cell lysate (400 μg of proteins) were incubated with the appropriate primary antibodies (2 μg) at 4°C overnight and shaken in a 500 μl of lysis buffer, followed by incubation with 50 μl of the protein A/G-PLUS agarose beads (SantaCruz Biotechnology) for 4 h at 4°C. The agarose beads were collected, washed 3 times with lysis buffer, resuspended in 60 μl of 2 × SDS sample buffer, and boiled for 10 min to dissociate the immunocomplexes from the beads. The supernatant collected after centrifugation was subjected to Western blot analysis. For validation of conformational change in Bax, normalized amounts of cell lysates were prepared in zwitterionic detergent CHAPS {3-[(3-Cholamidopropyl)-dimethylammonio]-1- propanesulfonate} lysis buffer (150 mM NaCl, 10 mM HEPES, pH 7.4, 1% CHAPS) containing protease inhibitors and subjected to immunoprecipitation with anti-Bax (BH3 domain specific) antibody (Abegent Inc., San Diego, CA).
2.6 Apoptosis Assay
After tetrandrine treatment, apoptosis was analyzed by Vybrant apoptosis assay kit (Invitrogen Corporation, Carlsbad, CA) in accordance with the manufacturer's protocol. In brief, treated and untreated cells (∼ 105 cells) were suspended in 1 ml of PBS. Reagents A (YO-PRO-1, 100 μM) and B (propidium iodide, 1 mg/ml) from the kit were added to each well (1 μl/ml) and the plates were left to incubate for 30 min on ice. After incubation, cells were analyzed using the Beckman Cytomics FC500 flow cytometry analyzer. Live cells do not exhibit any fluorescence because the dyes are impermeable to living cells, dead and necrotic cells exhibit red fluorescence (PI staining), and apoptotic cells fluoresce green (YO-PRO-1 staining). Live vs apoptotic or dead cells were counted and the percentage of cells exhibiting apoptosis was calculated.
2.7 RNA Interference
Small interfering RNA (siRNA) against JNK1 (Catalog No. L-003514-00-0005), JNK2 (Catalog No. L-003505-00-0005), and scrambled nonspecific siRNA (control, Catalog No. D-001810-10-05) were purchased from Dharmacon (ON-TARGET plus SMARTpool, Dharmacon, Lafayette, CO). To obtain effective silencing of protein expression, we utilized the SMARTpool siRNA reagent, which is a combination of four SMART selection-designed siRNAs in a single pool. Transfection was done following the manufacturer's instruction.
2.8 In Situ Caspase-3 Assay for Apoptosis
PC-3 cells were grown to 60% confluence on glass coverslips in 12-well plates. The cells were treated with 0-15 μM tetrandrine for 12 h at 37°C. Apoptotic cells were detected by staining with an in situ marker (10 μM, CaspACE FITC-VAD-FMK, Promega) for 30 min in the dark. The slides were fixed with 4% paraformaldehyde for 30 min, rinsed with PBS twice, mounted in a medium containing 1.5 μg/ml DAPI and observed under an Olympus AX70 fluorescence microscope.
2.9 Flow cytometry
Briefly, PC3 or DU145 cells at 70% confluence were treated or untreated with tetrandrine (15μM) for 24 h. After treatment, cells were trypsinized, washed, and fixed with 4% paraformaldehyde in PBS at room temperature for 10 min, and then permeabilized with 0.2% CHAPS for 10 min if required. The cells were suspended in PBS with 5% goat serum for 20 min at room temperature. Cells were then incubated with anti-Fas (CD95), anti-Bax (BH3 domain specific) or with a non-specific rabbit or mouse IgG for 2 h at room temperature, washed three times with PBS, and incubated with Alexa fluor 488-conjugated anti-rabbit or anti-mouse IgG for 30 min at room temperature. After washing with PBS three times, cells were analyzed by flow cytometry using the FITC signal detector (FL1) in Beckman Coulter FC500 flow cytometer. For each measurement, 10,000 cells were counted.
2.10 Mitochondrial membrane potential (MMP) assay
JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra-ethylbenzimidazolocarbocyanine iodide; Cayman Chemical, USA) was used to visualize changes in MMP of DU145 cells after treatment with tetrandrine. Briefly, treated and untreated DU145 cells were further incubated with JC-1 staining solution (1 μM) for 20 min at 37°C in a CO2 incubator protected from light. After incubation, the cells were washed with PBS and fluorescence intensity were analyzed by confocal microscopy using standard filter sets for 488 nm excitation (529 nm emission) and 549 nm excitation (590 nm emission). After fluorescence quantification, the MMP was determined from the ratio of red fluorescence (i.e. aggregates) to green fluorescence (i.e. monomers).
2.11 Measurement of intracellular reactive oxygen species (ROS)
Intracellular ROS such as hydrogen peroxide (H2O2), and hydroxyl radical (•OH) were detected by using oxidation-sensitive fluorescent dye, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). As H2DCFDA is poorly selective for superoxide anion (O2•−), dihydroethidium (DHE), which is highly selective for O2•−, was used for its detection. Briefly, the cells were grown on glass cover slips and treated with tetrandrine for 6 h. After treatment, cells were further incubated with H2DCFDA (5 μM) or DHE (5 μM) for 30 min at 37°C. Cells were washed with serum free medium and 2′,7′-dichlorofluorescein (DCF) and DHE fluorescence intensities were detected using a fluorescence microscope (Olympus). The fluorescence of cells was viewed using laser wavelength settings of excitation 488 nm and emission 525 nm for DCF, and excitation 530 nm and emission 590 nm for DHE. Intensity of fluorescence was quantified and bar graphs were plotted as fold change to control.
2.12 Statistical Analysis
The data are expressed as the mean ± S.E. for each group. The statistical significance was determined by Student's t test and was set at p < 0.05.
3 Results
3.1 Tetrandrine causes apoptosis in prostate cancer cells
The antiproliferative effects of tetrandrine on prostate cancer cell lines, PC3 and DU145, and normal prostate epithelial cell line, PWR-1E cells, were determined by an MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium Bromide) assay [33]. Results presented in Fig. 1 showed that at concentrations ranging from 0 to 80 μM, tetrandrine gradually decreased the viability of PC3 (Fig. 1A), DU145 (Fig. 1B) and PWR-1E (Fig.1C) cells in a dose- and time-dependent manner. The results of MTT assay showed that PWR-1E cells were more resistant to tetrandrine treatment than PC3 and DU145 cells. To address the question whether growth-suppressive effects of tetrandrine is associated with apoptosis, flow cytometric analysis was performed using Yo-PRO-1 and PI double-staining method in PC3, DU145 and PWR-1E cells. Results presented in Fig. 1D showed that after treatment with different concentrations of tetrandrine ranging from 0 to 30 μM for 24 h in PC3 cells, the viability of cells decreased from 92% to 26% with an increase in the percent of apoptotic cells from 7.1% to 48% in a dose dependent manner. A significant increase in dead cell population i.e. 15.6% and 25.4%, was observed in cells treated with 20 and 30 μM of tetrandrine, respectively. Under identical conditions, similar apoptotic effects were also observed on treatment of DU145 cells with tetrandrine (Fig. 1E). Unlike prostate cancer cells, PWR-1E cells showed less apoptosis even at higher concentration of tetrandrine exposure. A non-significant increase in apoptotic cells (2.9 to 9.5%) was observed only at 30 μM concentration of tetrandrine treatment compared to control in PWR-1E cells (Fig. 1F). These results indicated that initial response to sub-lethal doses of tetrandrine (<15 μM) treatment predominantly caused apoptosis in PC3 and DU145 cells that ultimately proceeded to cell death at lethal concentration of tetrandrine (>30 μM).
Fig. 1.
The cytotoxic effect of tetrandrine in normal and prostate cancer cells. (A) PC3, (B) DU145 and (C) PWR-1E cells (2 × 104) were plated in 96-well plates in complete growth medium for 24 h to allow for complete attachment to the culture plates. After incubation, solutions containing various amounts of tetrandrine were added to achieve final concentrations of 0-80 μM tetrandrine. After 12, 24 or 48 h of treatment, MTT assay was performed as described under “Materials and methods.” Results presented are percentage of cell survival in tetrandrine-treated groups with respect to control cells (mean ± S.E. (error bars); n = 8). (D) PC3, (E) DU145 and (F) PWR-1E cells were treated with 0 to 30 μM tetrandrine for 24 h at 37°C. Both floating and attached cells were collected and live vs apoptotic or dead cells were determined by flow cytometry after Yo-PRO-1 and PI staining, as described under “Materials and methods.” Represented histograms are shown. Results presented are percentage of cells present in apoptosis or dead in control or tetrandrine-treated cells (mean ± S.E. (error bars); n = 3; *, p < 0.05; °, p < 0.01; •, p < 0.001; versus control).
Based on these results, tetrandrine concentrations of 0-15 μM were used to examine its effect on proteolytic cleavage of caspase-3, -8, and -9 and poly (ADP-ribose) polymerase (PARP) in PWR-1E, PC3 and DU145 cells by Western blotting. As seen in Fig. 2A, treating the cells with tetrandrine induced the cleavage of caspase-3, -8, and -9 and PARP in both PC3 and DU145 cells in a concentration-dependent manner. However, we could not detect any cleaved forms of caspase-3, -8, and -9 and PARP in response to tetrandrine treatment in PWR-1E cells. The cleavage of caspase-8 and -9 suggest the involvement of both extrinsic and intrinsic pathways, respectively, in the induction of tetrandrine-induced apoptosis in prostate cancer cells. To investigate further whether tetrandrine induced apoptosis is caspase-dependent, the effect of z-DEVD-FMK, a caspase-3 inhibitor, was analyzed. As shown in Fig. 2B and 2C, pretreatment of z-DEVD-FMK completely blocked the tetrandrine-induced apoptosis in PC3 cells. Preincubation with z-DEVD-FMK significantly decreased the proportion of apoptotic cells from 38% to 12%, the levels found in untreated cells (Fig. 2B). In addition, the effect of pretreatment of z-DEVD-FMK on tetrandrine-induced cleavage of caspase-3 and PARP was further examined by Western blot analysis in PC3 cells. Our results presented in Fig. 2C clearly showed that pretreatment of z-DEVD-FMK completely inhibited the cleavage of PARP and procaspase-3 compared to the cells treated with tetrandrine alone.
Fig. 2.
Tetrandrine induced caspase-dependent apoptosis in prostate cancer cells. (A) Western blot analysis showing the PARP, caspase-3, caspase-8 and caspase-9 cleavage after 24 h of treatment of tetrandrine in PWR-1E, PC3 and DU145 cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control. (B) PC3 cells were pretreated with 50 μM z-DEVD-FMK for 1 h, followed by 24 h of tetrandrine treatment. After treatment, live vs apoptotic or dead cells were determined by Yo-PRO-1 and PI staining followed by flow cytometry. The data are expressed as means ± S.E. of three separate experiments (°, p < 0.01; versus control). (C) Western blots analysis showing the abrogation of PARP cleavage and caspase-3 activation by z-DEVD-FMK inhibitor.
3.2 Tetrandrine causes Fas-mediated apoptosis
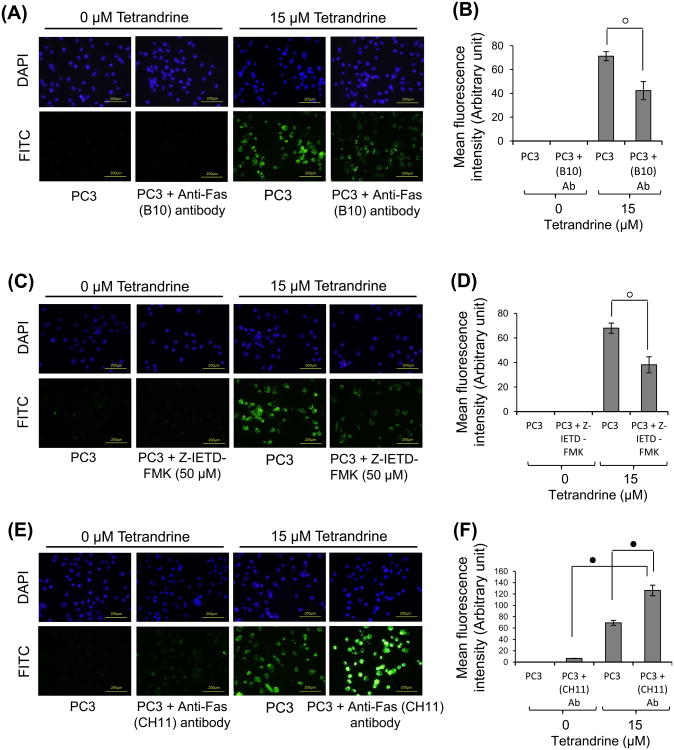
Since tetrandrine exposure causes the cleavage of procaspase-8, we considered that its proapoptotic response, at least in part, may be mediated via the death receptor-signaling pathway [34]. As shown in Fig. 3A and 3B, tetrandrine treatment at 15 μM was found to cause a time-dependent upregulation of Fas (CD95 or Apo-1) protein without a significant change in the expression of FasL in PC3 and DU145 cells. However, under the same conditions, the expression of DR4 and DR5 was not affected by tetrandrine treatment in both cell types (data not shown). To further confirm whether tetrandrine treatment increases the cell surface expression of Fas, we performed flow cytometry analysis in PC3 and DU145 cells without cell permeabilization. As shown in Fig. 3C, tetrandrine treatment in both cell types showed a significant increase in the levels of Fas expression on the cell surface. To address the question as to whether the upregulation of Fas at cell surface was associated with tetrandrine-induced apoptosis, PC3 cells were pre-coated with the antagonistic Fas (B10) monoclonal antibodies and examined their effects using CaspACE FITC-VAD-FMK in situ marker. Tetrandrine-induced apoptosis was significantly inhibited in B10 coated cells suggesting that upregulation of Fas is associated with apoptosis in prostate cancer cells (Fig. 4A and 4B). It may be noted that tetrandrine-induced apoptosis was not completely inhibited in B10 coated DU145 cells. Although, cells coated with B10 antibodies were resistant to tetrandrine-induced apoptosis, a small population of cells still underwent apoptosis suggesting the involvement of other signaling pathways. Initiation of cell death by death receptors such as Fas requires recruitment of the adaptor protein FADD to the death-inducing signaling complex (DISC) [34]. FADD in turn mediates recruitment of initiator procaspase-8 in the DISC, then procaspase-8 gets activated and apoptosis is executed [34]. Our results clearly demonstrated that addition of tetrandrine significantly increased the expression of FADD in a time-dependent manner in both cell types (Fig. 3A). To establish that tetrandrine treatment activates the Fas-FADD-procaspase-8 associated DISC formation, we performed the immunoprecipitation experiments using anti-Fas and anti-FADD antibodies. The result of Western blot analysis showed that FADD interacts with Fas and procaspase-8 upon tetrandrine treatment in DU145 cells (Fig. 3D). When the immunoprecipitates obtained using anti-Fas antibodies were probed with FADD and FasL antibody, the results further confirmed binding of Fas with FADD, but not with FasL. Together, the results of these experiments indicate that tetrandrine-induced DISC was formed by the recruitment of Fas, FADD and procaspase-8. Interestingly, Fas-mediated DISC formation was found to be independent of FasL recruitment, which suggests that FasL does not play any role in tetrandrine-induced Fas-mediated apoptosis.
Fig. 3.
Effect of tetrandrine on Fas-mediated apoptosis. (A) PC3 and DU145 cells were treated with 15 μM of tetrandrine at for 0, 6, 12, 18, and 24 h at 37°C. The protein lysates (25 μg of protein) were analyzed by Western blotting for the expression of Fas, FADD, FasL, c-FLIPL/S Bid and tBid. GAPDH was used as a loading control. (B) A bar graph showing densitometric analysis of bands (using image J analysis software) of protein used in (A). Data were collected from 2 independent experiments, and the mean ± S.E. (error bars) was calculated (*, p < 0.05; °, p < 0.01; •, p < 0.001; versus control). (C) Tetrandrine-induced cell surface expression of Fas was measured by flow cytometry in PC3 and DU145 cells. The cells were treated with tetrandrine (15 μM) for 24 h. After fixation, cells were stained with anti-Fas antibody without permeabilization, followed by Alexa fluor 488-conjugated anti-mouse IgG. After washing, cells were analyzed by flow cytometry using the FITC signal detector (FL1) in Beckman Coulter FC500 flow cytometer. The data are plotted for cell numbers versus the intensity of fluorescence. Results are representative of 2 independent experiments. (D) Total protein lysates were collected from the control and tetrandrine-treated (15 μM for 24 h) DU145 cells. Normalized amounts of lysate were processed for immunoprecipitation (IP) by using either anti-Fas or anti-FADD antibodies followed by Western blotting to check the expression of different proteins as indicated in the panel. IP with normal mouse or rabbit IgG was used as the negative control. (E) Control (without z-IETD-FMK) and pretreated (with z-IETD-FMK; 50 μM for 2 h) DU145 cells were treated with 0 or 15 μM tetrandrine for 24 h at 37°C. Total protein lysates (25 μg) were analyzed by Western blotting for the expression of Bid and tBid. GAPDH was used as a loading control.
Fig. 4.
Effect of tetrandrine on Fas-mediated apoptosis in PC3 cells. Control (without anti-Fas B10 antibody) and pretreated (with anti-Fas B10 antibody; 500ng/ml for 2 h) PC3 cells (A) were treated with 0 or 15 μM tetrandrine for 24 h at 37°C. The activation of caspase-3 in these cells was examined by staining with CaspACE FITC-VAD-FMK in situ marker (10 μM) according to the manufacturer's instructions (Promega Inc., Madison, WI). The slides were fixed and mounted with ProLong Gold antifade reagent containing DAPI and observed with a fluorescence microscope (Olympus) using the standard filter sets for DAPI and FITC. The photographs were taken at ×200 magnification. (B) Bar graph showing mean fluorescence intensity of CaspACE FITC-VAD-FMK in situ marker presented in (A). Data were collected from three independent experiments, and the mean, ± S.E. (error bars) was calculated. (C) Control (without z-IETD-FMK) and pretreated (with z-IETD-FMK; 50 μM for 2 h) PC3 cells were treated with 0 or 15 μM tetrandrine for 24 h at 37°C followed by incubation with CaspACE FITC-VAD-FMK in situ marker as described earlier and mean fluorescence intensity (D) was quantified (mean ± S.E. (error bars) n = 3). (E) Control (without anti-Fas CH11 antibody) and pretreated (with anti-Fas CH11 antibody; 50 ng/ml for 1 h) PC3 cells were treated with 0 or 15 μM tetrandrine for 24 h at 37°C followed by incubation with CaspACE FITC-VAD-FMK in situ marker (10 μM) as described earlier and mean FITC fluorescence intensity (F) was quantified (mean ± S.E. (error bars); n = 3) (°, p < 0.01; •, p < 0.001; versus control).
Previous studies have shown that prostate cancer cells are least sensitive to Fas-mediated apoptosis in vitro and in vivo due to overexpression of c-FLIPL/S, a catalytically inactive homolog of procaspase-8, which inhibits binding of procaspase-8 to FADD, and prevents DISC formation [10,35,36]. The results of the present study demonstrated that tetrandrine treatment significantly reduced both isoforms of c-FLIP in a time-dependent manner in PC3 and DU145 cells (Fig. 3A and 3B). Our immunoprecipitation results further confirmed that both isoforms of c-FLIP are present in a complex with FADD in untreated DU145 cells (Fig. 3D). Upon tetrandrine treatment, the association of c-FLIP with FADD was significantly decreased and subsequently induces the binding of procaspase-8 to FADD. Additionally, our results showed that tetrandrine exposure to these cells caused the proteolytic cleavage of Bid to truncated Bid (tBid) (Fig 3A and 3B). The cleavage of Bid to tBid is considered to be the molecule responsible for crosstalk between the death receptor-mediated apoptotic signaling and the mitochondria [37,38]. Our results further confirmed that preincubation of cells with caspase-8 inhibitor z-IETD-FMK completely blocked the tetrandrine induced Bid cleavage in DU145 cells (Fig. 3E). To further confirm that tetrandrine-induced downregulation of Bid is associated with the activation of procaspase-3, we performed immunofluorescence study using the CaspACE FITC-VAD-FMK in situ marker in PC3 cells. Results presented in Fig. 4C and 4D clearly showed that preincubation with z-IETD-FMK significantly inhibited the tetrandrine-induced caspase-3 activation compared to the cells treated with tetrandrine alone. However, a significant number of cells are still undergoing apoptosis suggesting the involvement of other apoptotic signaling pathways. Moreover, tetrandrine treated prostate cancer cells became more sensitive to apoptosis caused by agonistic anti-Fas (CH11) antibodies [39], as indicated by the onset of increased caspase-3 activation in PC3 cells treated with both tetrandrine and CH11 antibodies (Fig. 4E and 4F). These results suggest that treatment of prostate cancer cells with tetrandrine induces apoptosis through extrinsic pathway by involving the death receptor Fas, FADD and caspase-8.
3.3 Tetrandrine alters the levels of Bcl2 family proteins
Members of the Bcl2 protein family play a critical role through their proapoptotic (e.g. Bax) or antiapoptotic (e.g. Bcl2) activities [40-43]. Results presented in Fig. 5A and 5B showed that the expression of Bax protein was significantly increased in a time-dependent manner after tetrandrine treatment at 15 μM in PC3 and DU145 cells, whereas the expression of Bcl2 was significantly decreased in both cell types. Thus, tetrandrine treatment was found to result in alteration in Bax/Bcl2 ratio in favor of apoptosis that was ∼3.5-fold higher in PC3 cells and ∼4.9-fold higher in DU145 cells after 24 h, compared with their respective controls. It has been reported that the phosphorylation of Bcl2 at Ser70 suppresses its antiapoptotic activity by losing its ability to heterodimerize with Bax [44,45]. Therefore, we examined the effect of tetrandrine on Ser70 phosphorylation of Bcl2. As shown in Fig. 5A and 5B, the phosphorylation of Bcl2 at Ser70 was significantly increased at early time point of tetrandrine exposure. An optimal induction in Ser70 phosphorylation was seen at the 12 h time points in both cell types and it significantly declined at 24 h. This reduction in Ser70-phosphorylated Bcl2 at late time points was consistent with relatively more reduction in the expression of total Bcl2 protein at the 24 h time point. To determine if tetrandrine treatment affected the Bax/Bcl2 interaction, untreated and treated DU145 cells were immunoprecipitated with anti-Bax antibody and subjected to Western blotting using anti-Bcl2 antibody. As shown in Fig. 5C, a noticeable decrease in Bcl2 protein level was observed in tetrandrine treated cell lysates compared with control. Likewise, immunoprecipitates obtained with anti-Bcl2 antibody showed significantly low expression of Bax in tetrandrine-treated lysates compared with control. These results indicated that tetrandrine treatment reduced the complex formation between Bcl2 and Bax. Next, we explored the possibility whether reduced Bax/Bcl2 interaction resulted in activation of mitochondrial apoptotic pathway. As shown in Fig. 5A and 5B, tetrandrine treatment significantly increases the expression of cytochrome c and Apaf-1 in a time-dependent manner in both cell types. However no significant change was observed in the expression of Smac in control and tetrandrine treated cells (Fig. 5A and 5B).
Fig. 5.
Effect of tetrandrine on mitochondrial apoptotic pathway. (A) PC3 and DU145 cells were treated with 15 μM of tetrandrine for 0, 6, 12, 18, and 24 h at 37°C. Cell extracts (25 μg of protein) were resolved on 4-20% SDS-PAGE and immunoblotted using the anti-Bax (N20), anti-Bcl2, anti-phospho Bcl2 Ser70, anti-cytochrome c, anti-Apaf-1 and anti-Smac antibodies. Anti-GAPDH was used as a loading control. (B) A bar graph showing densitometric analysis of bands used in (A). Data were collected from 2 independent experiments, and the mean ± S.E. (error bars) was calculated (*, p < 0.05; °, p < 0.01; •, p < 0.001; versus control). (D) Cell extracts were collected from the control and tetrandrine treated (15 μM for 24 h) DU145 cells. Equal amounts of lysate were processed for immunoprecipitation (IP) by using anti-Bax, or anti-Bcl2, antibodies followed by Western blotting to check the expression of different proteins as indicated in the panel. IP with normal rabbit IgG was used as the negative control.
3.4 Tetrandrine causes mitochondrial translocation of Bax and tBid, collapse of the mitochondria membrane potential and release of cytochrome c and Smac from mitochondria
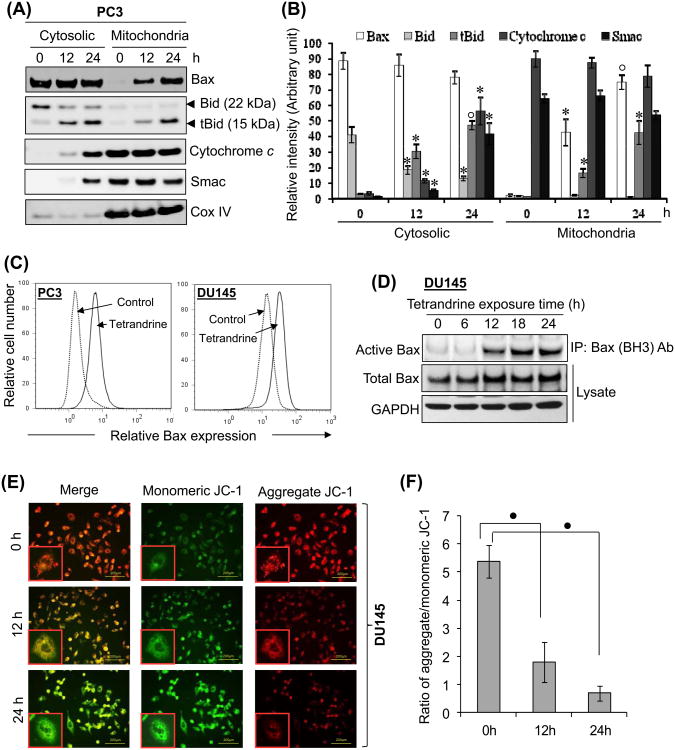
In the normal state, the proapoptotic protein Bax resides in the cytosol in association with Bcl2 but translocates to the outer mitochondrial membrane upon apoptotic stimuli [41,43]. Additionally, Bid is also primarily localized in the cytosol, but upon apoptotic stimuli it is cleaved by caspase-8, and the resulting tBid translocates to the mitochondrial membrane, where it interacts with Bax, enhancing their oligomerization and leading to outer membrane permeabilization, loss of membrane potential and release of mitochondrial apoptogens [37,38,46]. The effect of tetrandrine on the mitochondrial accumulation of Bax and tBid was therefore, examined by Western blot analysis of the cytosolic and mitochondrial fractions prepared from the control and tetrandrine treated DU145 cells. Our observation revealed that Bax level in mitochondria was significantly increased after 12 and 24 h of tetrandrine treatment, (Fig. 6A and 6B). Moreover, intact Bid of 22 kDa was mainly detected in the cytosol, whereas tBid of 15 kDa showed both cytosolic and mitochondrial distribution upon tetrandrine treatment. Previous reports have demonstrated that alteration in protein conformation of Bax is necessary steps in cells undergoing apoptosis [41,43]. The activity-related conformational changes in Bax was analyzed by flow cytometic analysis using immunostaining with specific anti-Bax (BH3 domain specific) antibody, which recognize the BH3 domain epitope of the active conformation of Bax, which is hidden in undamaged cells and becomes exposed upon a conformational change in the Bax protein in response to apoptotic signals [43,47]. The fluorescence histograms shown in Fig. 6C, demonstrate that exposure to 15 μM tetrandrine induces a shift to the right of the Bax fluorescence curves compared with control in both cell types, indicating that tetrandrine leads to Bax activated conformational change. To further validate these observation, we used the zwitterionic detergent CHAPS, which has been proven to retain the Bax at native conformation [48], to prepare cell lysate and then to immunoprecipitate total Bax protein with anti-Bax (BH3 domain specific) antibody. As shown in Fig. 6D, the signal of active Bax was barely detectable at time 0, was first detected at 12 h of tetrandrine treatment and gradually increased with time. Taken together, the above results raised the possibility that tetrandrine treatment could result in the loss of MMP in these cells. Hence, JC-1 staining was performed to demonstrate changes in the MMP in DU145 cells. In non-apoptotic cells JC-1 dye accumulates and aggregates within the mitochondria, resulting in bright red staining. In apoptotic cells, due to the collapse of the membrane potential, the JC-1 cannot accumulate within the mitochondria and remains in the cytoplasm in its green-fluorescent monomeric form [49]. As shown in Fig. 6C and 6D, tetrandrine treatment at 15 μM for 12 and 24 h led to 42.3% and 26.0% of membrane potential collapse, respectively in DU145 cells. The loss of mitochondrial potential is known to be associated with the subsequent release of mitochondrial apoptogenic factors from mitochondria to cytoplasm of the cells. Western blot analysis showed that treatment of DU145 cells with tetrandrine (15 μM) resulted in a time-dependent enhancement in release of cytochrome c and Smac from mitochondria to the cytosol (Fig. 6A and 6B), which suggests disruption of the mitochondrial membrane potential.
Fig. 6.
Effect of tetrandrine on mitochondrion-mediated apoptosis. (A) PC3 cells were treated with 15 μM tetrandrine for 0, 12 and 24 h at 37°C. Mitochondrial and cytosolic extracts of the pelleted cells were prepared. The extracts were subjected to Western blot analysis for the detection of Bax, Bid, tBid, cytochrome c, Smac and Cox IV. Antibody against Cox IV was used to determine the purity of the mitochondrial fractions. (B) A bar graph showing densitometric analysis of bands of Bax, cytochrome c, Smac, Bid and tBid used in (A). Data were collected from 2 independent experiments, and the mean ± S.E. (error bars) was calculated (*, p < 0.05; °, p < 0.01; versus control). (C) Tetrandrine-induced conformational change of Bax was measured by flow cytometry in PC3 and DU145 cells. The cells were grown on 6-well plates, and untreated and treated (15 μM tetrandrine for 24 h) cells were fixed, permeabilized, and incubated with anti-Bax (BH3 domain specific) antibody, followed by Alexa fluor 488-conjugated anti-rabbit IgG. After washing, cells were analyzed by flow cytometry using the FITC signal detector (FL1) in Beckman Coulter FC500 flow cytometer. The data are plotted for cell numbers versus the intensity of fluorescence. Results are representative histograms of 2 independent experiments. (D) Tetrandrine-induced activation of Bax conformation was determined by immunoprecipitation (IP). DU145 cells were treated with 15μM tetrandrine for the indicated time. After treatment, cells were harvested, and cell pellets were divided into two aliquots. One aliquot was used for the preparation of IP with anti-Bax (BH3 domain specific antibody (Abgent Inc.) as described under “Materials and methods.” The other cell aliquot was lysed with RIPA lysis buffer. The level of Bax was determined by Western blotting using Bax (D2E11) antibody (Cell signaling technology). (E) DU145 cells treated with tetrandrine (15 μM) for 0, 12, and 24 h were assayed for MMP by staining with JC-1 dye. MMP was determined using a fluorescence microscope (magnification, ×200) and representative images are shown. (F) Quantification of MMP expressed as a ratio of JC-1 aggregate to JC-1 monomer (red : green) fluorescence intensity. Each bar is the mean ± S.E. derived from three independent experiments (•, p < 0.001; versus control).
3.5 Sustained activation of JNK by tetrandrine is crucial for the initiation of mitochondrial apoptotic signaling
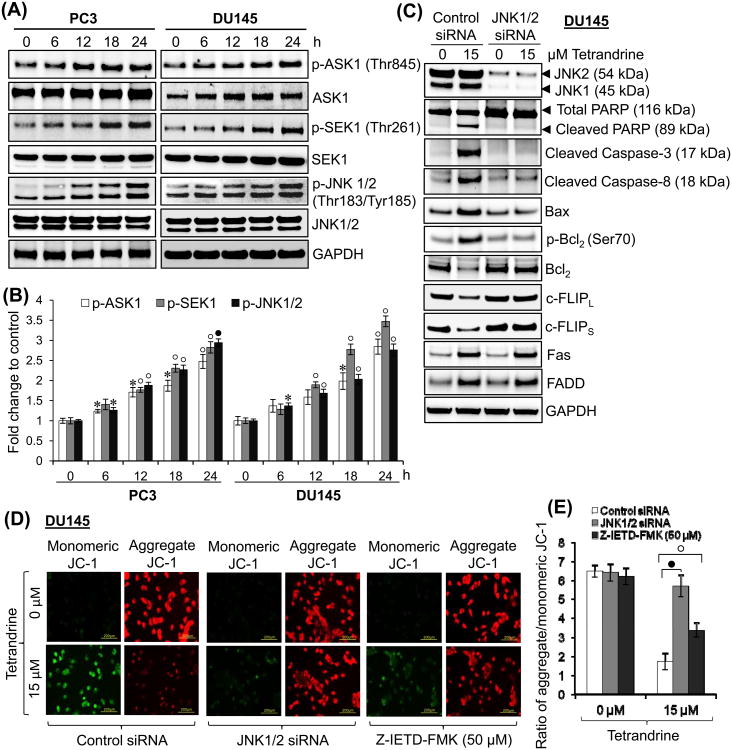
Previous studies have shown that tetrandrine exposure to cells causes the generation of ROS [25,27,50]. Because it has been shown that ROS-mediated cell death triggers activation of apoptosis signal-regulating kinase 1 (ASK1) [51,52], we assessed the status of ASK1 signaling pathway after tetrandrine treatment in prostate cancer cells. Results presented in Fig. 7A and 7B showed that tetrandrine exposure significantly increased the phosphorylation of ASK1 at Thr845 in PC3 and DU145 cells. However, we could not observe any significant change in total ASK1 expression in both cell types. Furthermore, ASK1 kinase activation is known to activate SEK1-JNK1/2 and MKK3/MKK6-p38 signaling cascades [51,52]. Our results showed that exposure of PC3 or DU145 cells to tetrandrine resulted in a rapid and sustained phosphorylation of SEK1 at Thr-261 and JNK1/2 at Thr183/Tyr185 (Fig. 7A and 7B). On the other hand, the expression of total SEK1 and JNK1/2 was not altered by tetrandrine treatment. Moreover, tetrandrine exposure does not activate the phosphorylation of MKK3 at Ser189, MKK6 at Ser207 and p38 at Thr180/Tyr182 in either PC3 or DU145 cells at any time points (data not shown). The observed activation of ASK1, SEK1, and JNK1/2 by tetrandrine in the present studies was rapid and sustained suggesting that a sustained activation of JNK1/2 may be needed for tetrandrine-induced apoptosis of prostate cancer cells. This prediction was supported by the results of the experiments showing that depletion of JNK1/2 in DU145 cells made these cells resistant to apoptosis by tetrandrine as observed by decrease in PARP and caspase-3 cleavage (Fig. 7C). Taken together, these data strongly suggest that the activation of ASK1-SEK1-JNK1/2 plays an important role in tetrandrine-induced apoptosis in prostate cancer cells.
Fig. 7.
Effect of tetrandrine treatment on ASK1/SEK1/JNK pathway. (A) PC3 and DU145 cells were treated with tetrandrine (15 μM) for 0, 6, 12, 18, and 24 h at 37°C. Total protein lysates (25 μg) were analyzed by Western blotting for the expression of proteins indicated in the panel. GAPDH was used as a loading control. (B) A bar graph showing densitometric analysis of bands of protein used in (A). Data were collected from 2 independent experiments, and the mean ± S.E. (error bars) was calculated (*, p < 0.05; °, p < 0.01; •, p < 0.001; versus control). (C) Silencing of JNK1/2 was performed by the ON-TARGET plus SMART pool JNK1 and JNK2 siRNA as per the manufacturer's instructions (Thermo Scientific Dharmacon) and control cells were treated with ON-TARGET plus Non-targeting siRNA in a similar way. After 48 h of transfection, DU145 cells were treated with 0 or 15 μM tetrandrine for 24 h at 37°C. Total protein lysates (25 μg) were analyzed by Western blotting for the expression of proteins as indicated in the panel. GAPDH was used as a loading control. (D) After 2 h pretreatment with or without z-IETD-FMK inhibitor, or 48 h of control and JNK1/2 siRNA transfection, DU145 cells were treated with tetrandrine (15 μM) for 24 h and assayed for MMP by JC-1 dye. MMP was determined using a fluorescence microscopy and representative images are shown. (E) Quantification of MMP was expressed as a ratio of J-aggregate to JC-1 monomer (red: green) fluorescence intensity. Each bar is the mean ± S.E. derived from three independent experiments (°, p < 0.01; •, p < 0.001).
To find out the role of JNK activation by tetrandrine in the sensitization of prostate cancer cells, we evaluated the effect of JNK1/2 depletion on tetrandrine-induced pro- and anti-apoptotic proteins in DU145 cells. Most importantly, the depletion of JNK1/2 completely prevented the loss of Bcl2 and c-FLIPL/S proteins caused by tetrandrine (Fig. 7C). Furthermore, tetrandrine-induced phosphorylation of Bcl2 at Ser70 was markedly inhibited in the absence of JNK1/2. Interestingly, depletion of JNK1/2 in DU145 cells also inhibited the tetrandrine-induced upregulation of Bax suggesting that JNK1/2 activation is essential for the upregulation of Bax [53]. Additionally, JNK1/2 depletion effectively attenuated the procaspase-8 cleavage caused by tetrandrine but had no effect on Fas and FADD induction (Fig. 7C). However, inhibition of procaspase-8 activation in JNK depleted cells is expected due to inhibition of c-FLIPL/S downregulation, which significantly inhibits the binding of procaspase-8 to FADD, and prevents DISC formation [35]. To further assess the role of JNK activation and caspase-8 mediated Bid cleavage induced by tetrandrine on the mitochondria apoptotic pathway, the MMP was measured. As shown in Fig. 7D and 7E, tetrandrine treatment resulted in a significant decrease in the ratio of the red fluorescence to green fluorescence, suggesting the loss of MMP. Furthermore, depletion of JNK1/2 or preincubation with z-IETD-FMK significantly, but not entirely, prevented the loss of MMP induced by tetrandrine. These results revealed that tetrandrine-induced mitochondrial apoptotic pathway was significantly regulated by JNK activation and partially by procaspase-8 activation.
3.6 Tetrandrine causes the ubiquitin-mediated proteasomal degradation of Bcl2 and c-FLIPL/S proteins
Expression of Bcl2 and c-FLIPL/S has been shown to be regulated by ubiquitin-mediated proteasomal degradation under diverse apoptotic stimuli [36,54-56]. To determine whether this pathway is involved in the downregulation of Bcl2 and c-FLIPL/S induced by tetrandrine, cells were treated with tetrandrine in the presence or absence of MG132, a specific proteasome inhibitor, and Bcl2 and c-FLIPL/S expression was determined by Western blotting. Fig. 8A shows that tetrandrine-mediated decline in Bcl2 and c-FLIPL/S proteins level in DU145 cells was nearly completely blocked in the presence of MG132, suggesting proteasomal degradation as the primary mechanism of Bcl2 and c-FLIPL/S downregulation caused by tetrandrine. In addition, higher molecular weight polyubiquitin conjugates appeared to be increased in the lane containing lysate from cells treated with tetrandrine and MG132 and tetrandrine alone compared to the control DU145 cells (Fig. 8A, last panel). To further confirm tetrandrine-mediated ubiquitination of Bcl2 and c-FLIPL/S, we immunoprecipitated Bcl2, c-FLIPL/S or ubiquitinated proteins from the lysates of DU145 cells treated with or without tetrandrine in the presence of MG132. Western blot analysis presented in Fig. 8B demonstrated the binding of ubiquitin with Bcl2 or c-FLIPL/S upon tetrandrine treatment. Together, these results suggest that tetrandrine-induced decline in the level of Bcl2 and c-FLIPL/S proteins was due to its degradation in proteasomes and that it seems to play an important role in the mechanisms through which tetrandrine sensitizes prostate cancer cells to apoptosis.
Fig. 8.
Tetrandrine induces the ubiquitin-mediated proteasomal degradation of Bcl2 and c-FLIPL/S in prostate cancer cells. (A) DU145 cells were pretreated with 20 μM MG132 for 2 h, followed by treatment with 0 or 15 μM tetrandrine for an additional 24 h. Cell lysates were prepared and subjected to Western blot (WB) for Bcl2 and c-FLIPL/S protein. The Bcl2 Western blot membrane was stripped and reprobed with anti-ubiquitin antibody. GAPDH was used as a loading control. (B) DU145 cells were pretreated with 20 μM MG132 for 2 h and then either left untreated or treated with 15 μM tetrandrine for an additional 24 h. Equal amount of cell lysates were processed for immunoprecipitation (IP) by using either anti-Bcl2/anti-c-FLIPL/S or anti-ubiquitin antibody, followed by Western blotting with the anti-ubiquitin antibody or anti-Bcl2/anti-c-FLIPL/S antibody, respectively.
3.7 Tetrandrine-induced reactive oxygen species causes apoptosis in prostate cancer
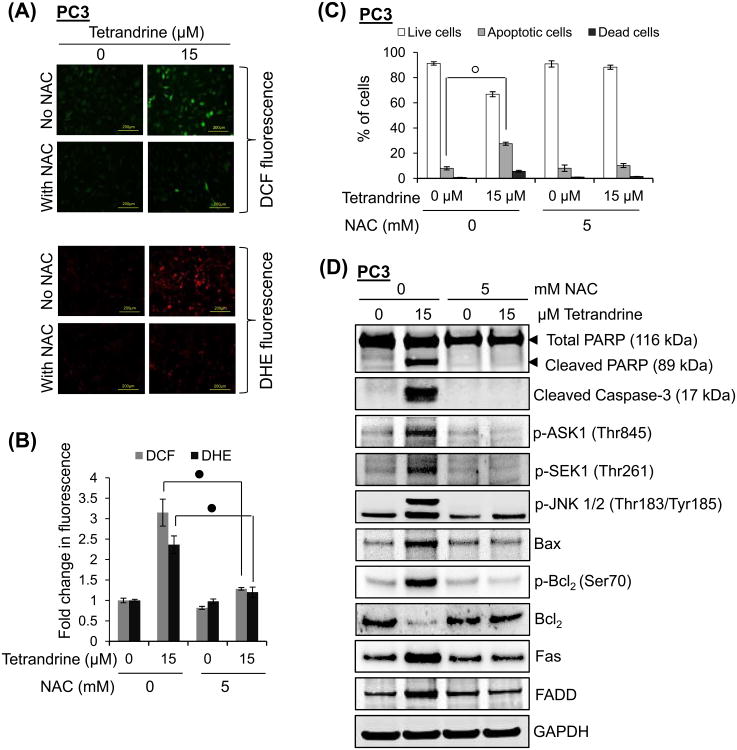
Tetrandrine was shown to stimulate ROS generation in different cell types [25,27,50]. Therefore, we investigated whether tetrandrine leads to generation of ROS in PWR-1E, PC3 and DU145 and whether generation of ROS is responsible for induction of tetrandrine-mediated apoptosis in prostate cancer cells. To determine intracellular ROS, cells were treated with tetrandrine for 4 h and ROS production was measured by fluorescence microscopy using the specific ROS-detecting fluorescent dye H2DCF-DA and DHE [57,58]. As shown in Fig. 9A to 9D, exposure of PC3 and DU145 cells to tetrandrine significantly enhanced the DCF and DHE fluorescence intensity in a concentration-dependent manner in both cell types, indicating an increase in H2O2 and •OH, and O2•− generation, respectively. However, unlike prostate cancer cells, PWR-1E cells generate fewer amounts of ROS even at higher concentration of tetrandrine treatment (Fig. 9B and 9D). In addition, when PC3 cells were pretreated with free radical scavenger, N-acetyl-L-cysteine (NAC) 1 h prior to the treatment of tetrandrine, the fluorescence intensity of DCF and DHE significantly decreased compared with the levels found in cells without NAC pretreatment (Fig. 10A and 10B). To determine whether ROS production plays a role in tetrandrine-induced apoptosis, cells were treated with or without NAC before tetrandrine treatment and apoptosis was measured by flow cytometry, PARP cleavage, and caspase-3 activation. As shown in Fig. 10C, the number of apoptotic cells induced by tetrandrine was significantly reduced from 28% to 10% when PC3 cells were pretreated with NAC. Furthermore, the increase in PARP cleavage and caspase-3 activation induced by tetrandrine was almost completely reversed when cells were pretreated with NAC (Fig. 10D). Because ROS has been implicated in the induction of ASK1, Fas and its associated downstream signaling, we investigated whether tetrandrine-induced up-regulation of apoptotic signaling requires ROS. Results presented in Fig. 10D showed that pretreatment of cells with NAC resulted in the inhibition of phosphorylation of ASK1, SEK1 and JNK1/2, and abrogation of tetrandrine-mediated phosphorylation of Bcl2 at Ser70, degradation of Bcl2 and upregulation of Bax. Likewise, tetrandrine-mediated activation of Fas and FADD was also inhibited by the pretreatment of cells with NAC (Fig. 10D). Collectively, these results suggest that tetrandrine-induced apoptosis in prostate cancer cells is mediated by intracellular ROS generation, and the ROS generation is an upstream event of all apoptotic signaling and caspase activation.
Fig. 9.
Effect of tetrandrine on ROS generation in cells. PWR-1E, PC3 and DU145 cells were treated with tetrandrine (0-30 μM for 4 h) and ROS generation were evaluated by the oxidation of (A) H2DCF-DA to DCF and (C) DHE. Intracellular ROS was determined by fluorescence microscopy and representative images are shown. Quantification of fluorescence intensity of DCF (B) and DHE (D) are expressed as the mean ± S.E. from three independent experiments (*, p < 0.05; °, p < 0.01; •, p < 0.001; versus control).
Fig. 10.
Effect of antioxidative agents on tetrandrine-induced ROS generation. (A) Control (without NAC) and pretreated (with NAC; 5 mM for 1 h) PC3 cells were treated with 0 or 15 μM tetrandrine for 4 h at 37°C and ROS generation was determined by the oxidation of H2DCF-DA to DCF and DHE. Representative images are shown. (B) The bar graph represents the fold increase in DCF and DHE fluorescence in tetrandrine-treated cells compared with control cells. Each bar represents the mean ± S.E. of three independent experiments (•, p < 0.001; versus control). (C) Control (without NAC) and pretreated (with NAC; 5 mM for 1 h) PC3 cells were treated with 0 or 15 μM tetrandrine for 14 h at 37°C. Both floating and attached cells were collected and processed for analysis of live vs apoptotic cell distribution by flow cytometry as described under “Materials and methods.” The bar graph showing the percent of cells are live or apoptotic before and after tetrandrine treatment. Bar graph was expressed as means ± S.E. of three independent experiments (°, p < 0.01; versus control). (D) Control (without NAC) and pretreated (with NAC; 5 mM for 1 h) PC3 cells were treated with 0 or 15 μM tetrandrine for 24 h at 37°C. Total protein lysates (25 μg) were analyzed by Western blotting for the expression of proteins as indicated in the panel. GAPDH was used as a loading control.
4 Discussion
The pharmacologic action of tetrandrine has gathered increased attention in recent years in view of its potential as a novel anti-cancer drug either alone or in combination with generic drugs [13,14,23-32]. Previous studies have shown that tetrandrine causes cell cycle arrest and apoptosis in cancer cell lines of different tissue origins in vitro and in some tumor xenograft models in vivo, and modulates many genes and proteins involved in cancer cell survival and proliferation [21-32]. The mechanisms through which tetrandrine exerts such a wide variety of effects are largely unknown. The results of the present study demonstrated that tetrandrine decreased the viability of prostate cancer cells in a dose-dependent manner. However, the normal prostate epithelial cells were resistant to tetrandrine-induced lethality at a dose which was still effective in inhibiting cancer cell growth. The growth-suppressive effects of tetrandrine correlated with apoptosis induction, which is a widely accepted mechanism for antiproliferative activity of many chemotherapeutic drugs [59]. Previously, it was reported that cancer chemotherapeutic agents, including tetrandrine, induce apoptosis at least in part through ROS generation and disruption of redox homeostasis [25,27]. We also found that induction of ROS is critical for the activation of Fas- and mitochondria-mediated apoptosis by tetrandrine in prostate cancer cells. Our results show that first, tetrandrine induced ROS in a dose-dependent manner; second, quenching of ROS by NAC abolished the tetrandrine-induced cell death; third, quenching of ROS also abrogated the effect of tetrandrine on induction of Fas- and mitochondria-mediated apoptotic signaling pathway. Thus all of these evidences suggest that oxidative stress plays a major role as a mediator of cell death in action of tetrandrine. These results are in agreement with those reported previously on induction of apoptosis and the associated signaling events by curcumin, proteasome inhibitors, phenethyl isothiocyanate, sulforaphane, and zerumbone [60-65].
Over-expression of constitutively active ASK1 has been shown to induce apoptosis in various cancer cells through mitochondria-dependent caspase activation, and the activation of ASK1 is required for oxidative stress-induced apoptosis [51,52]. In the presence of any oxidative stress stimuli, ASK1 activates SEK1, which in turn activates JNK1/2. The present study indicates that the cell death caused by tetrandrine in prostate cancer cells is tightly linked to activation of JNK1/2. This conclusion is based on the following observations: (a) tetrandrine-induced JNK1/2 activation causes the degradation of c-FLIPL/S thereby sensitizing the prostate cancer cells to Fas-mediated apoptosis. (b) tetrandrine-induced apoptosis is nearly completely attenuated by JNK1/2 inhibitor. (c) tetrandrine-induced JNK1/2 activation contributes to the initiation of mitochondria-mediated apoptosis via phosphorylation of Bcl2 at Ser70. (d) tetrandrine-induced JNK1/2 activation causes the upregulation of proapoptotic protein Bax. Furthermore, pretreatment of PC3 cells with NAC attenuates the tetrandrine-induced phosphorylation of ASK1, SEK1 and JNK1/2, which suggest that ROS may act upstream of ASK1 activation. Our results are in agreement with other studies that reported that ASK1 is activated mostly by ROS and plays a critical role in oxidative stress-mediated apoptosis through the activation of SEK1-JNK1/2-signaling pathway [51-53]. The mechanisms through which JNK1/2 upregulates tetrandrine-induced Bax expression is not clear but these results are in agreement with previous studies where it has been shown that UV radiation caused Bax activation in wild-type fibroblasts but not in JNK-deficient (Jnk1−/− Jnk2−/−) fibroblasts [53]. However, tetrandrine-mediated JNK activation was also observed in U937 leukemia cells [25]. Unlike the results of the present study, pharmacological inhibition of JNK1/2 did not show any protection against tetrandrine-induced apoptosis in U937 cells [25]. Even though, the JNK activation was significantly inhibited by tetrandrine treatment in hepatocellular carcinoma [27]. Our data clearly indicate that tetrandrine-mediated phosphorylation of JNK1/2 in prostate cancer cells leads to the activation of Fas- and mitochondria-mediated apoptotic pathway to trigger caspase cascade.
The Bcl2 family proteins have emerged as critical regulators of the mitochondria-mediated apoptosis by functioning as either promoter (e.g. Bax) or inhibitor (e.g. Bcl2) of the cell death process [40-43]. In unstimulated conditions, Bcl2 present in the cytosol in complex with Bax and prevents apoptosis by inhibiting Bax translocation to the mitochondria, where it forms a channel for the exit of cytochrome c and loss of MMP. As the level of cytochrome c increases in cytosol, it interacts with Apaf-1 and forms a complex with procaspase-9, leading to sequential activation of caspase-9, -3 and PARP [41-43,66]. Consistent with these reports, in the present study, we found that treatment of prostate cancer cells with tetrandrine resulted in a time-dependent increase in the expression of Bax and a decrease in the expression of Bcl2 protein and increased the ratio of Bax/Bcl2. In addition, tetrandrine also induced the translocation of Bax from cytosol to mitochondria, Bax protein conformational change and lead to the disruption of MMP and increased the release of cytochrome c and Smac to cytosol. Further, this effect of tetrandrine leads to the activation of Apaf-1, caspase-9, -3 and PARP. These results are consistent with previously reported mitochondria mediated apoptosis in hepatocarcinoma and gastric cancer cells by tetrandrine [31,67]. In addition, phosphorylation of Bcl2 has also emerged as an important mechanism for regulation of mitochondria-mediated apoptosis [44,45,68-70]. Bcl2 binds to and inactivates Bax function, but whether or not Bcl2 phosphorylation affects its interaction with Bax is still controversial. In some reports, phosphorylated Bcl2 loses its ability to form heterodimer with Bax [44,45]. Consistent with these findings, we observed that tetrandrine induces the phosphorylation of Bcl2 at Ser70 as early as 6 h and persists at least until 24 h. Moreover, we found that Bcl2 phosphorylation in response to tetrandrine is closely associated with JNK activation, as its depletion leads to suppression of tetrandrine-induced Bcl2 phosphorylation at Ser70 and downregulation of Bcl2 levels. Furthermore, it has been reported that JNK-mediated phosphorylation of Bcl2 leads to ubiquitin-mediated proteasomal degradation of Bcl2 [54,55,71,72]. We provide further evidence that tetrandrine treatment induces the downregulation of Bcl2 via ubiquitin-mediated proteasomal system. Thus, the Bcl2 phosphorylation at Ser70 and ubiquitin-mediated proteasomal degradation of Bcl2 appears to be the main mechanism of mitochondria-mediated apoptosis by tetrandrine.
Numerous studies have shown that c-FLIPL/S overexpression confers resistance to Fas-mediated apoptosis in prostate tumors [10,35]. In our study, treatment of prostate cancer cells with tetrandrine caused a marked reduction in the expression of both c-FLIPL and c-FLIPS proteins. JNK activation by ROS has been shown to reduce the stability of c-FLIPL/S by a mechanism involving JNK-mediated phosphorylation and activation of the E3 ubiquitin ligase, which ubiquitinates c-FLIPL/S and induces its degradation via proteasomal pathway [36,56]. Our results show that in prostate cancer cells tetrandrine treatment leads to the ubiquitin-mediated proteasomal degradation of both isoforms of c-FLIP through JNK-dependent mechanism. However, downregulation of c-FLIPL/S has been shown to cause apoptosis by inducing procaspase-8 activation via association of DISC formation [35,36,56]. Consistent with previous studies, our results further confirmed that depletion of JNK1/2 significantly inhibits the tetrandrine-induced activation of procaspase-8 which makes these cells resistant to apoptosis. The present study revealed that the activation of caspase-8 in tetrandrine-treated cells is preceded by its association with FADD and their subsequent recruitment by Fas. These results are consistent with other studies that have shown that tetrandrine-induced apoptosis is significantly attenuated by caspase-8 inhibitor [67]. A number of chemotherapeutic drugs activate the Fas-mediated apoptosis via enhancement of FasL expression which facilitates the DISC formation [34]. Surprisingly, the protein level of FasL was found to be slightly reduced in prostate cancer cells exposed to tetrandrine and does not form a complex with FADD, which suggests that tetrandrine-induced Fas-mediated apoptosis is FasL independent. These finding are consistent with reported evidence that FasL is not required for drug-induced apoptosis because apoptosis is not suppressed by inhibition of Fas/FasL interaction [73,74]. In addition, tetrandrine-mediated activation of Fas and FADD is significantly attenuated in the presence of NAC, which suggest that ROS is upstream of Fas activation. Furthermore, caspase-8 activation can cause cleavage of Bid to tBid, which is a BH3-only proapoptotic Bcl2 family member that is exclusively localized in the cytoplasm [37,38]. The truncated Bid, however, translocates to the mitochondrial membrane, where it interacts with Bax, enhancing their oligomerization by altering the protein conformation and triggers cytochrome c and Smac release by reducing MMP, activation of caspase-9/3 and cell death [37,38,46,47,66]. In the present study, we found that caspase-8 inhibitor completely blocked tetrandrine-induced Bid processing, loss of MMP and activation of caspase-3, suggesting that Bid regulates tetrandrine-mediated release of cytochrome c and Smac to the cytosol. Based on these observations, we conclude that Bid protein, at least in part, is involved in the regulation of tetrandrine-induced cell death.
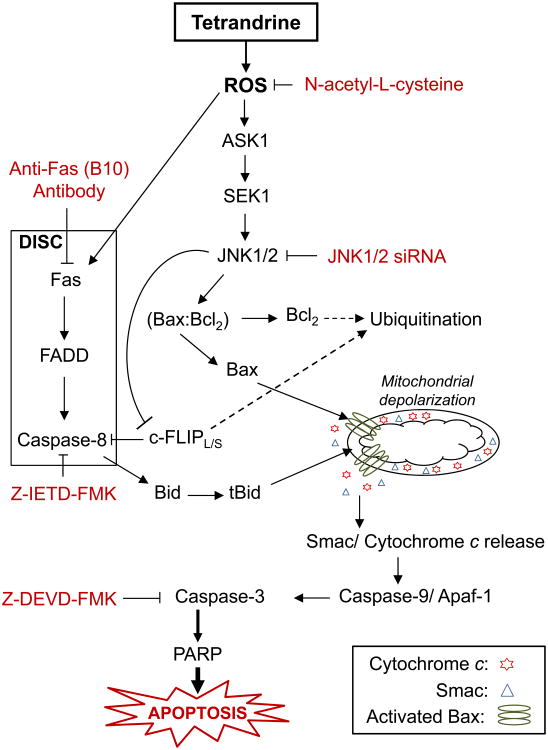
In conclusion, as summarized in Fig. 11, the present study provides the first mechanistic evidence that prostate cancer cells undergo apoptosis in response to tetrandrine treatment through ROS-mediated activation of JNK pathway. Furthermore, we provide evidence for the first time that JNK-mediated proteasomal degradation of c-FLIPL/S and Bcl2 proteins are key events in the sensitization of prostate cancer cells to Fas- and mitochondria-mediated apoptosis by tetrandrine. The results of the present study established that JNK activation is one of the major mechanisms through which tetrandrine sensitize prostate cancer cells to apoptosis. Taken together, our data provide a novel insight into the potential application of tetrandrine for the treatment of prostate cancer.
Fig. 11.
Proposed model of tetrandrine-induced apoptosis in prostate cancer. Proposed model for the activation of Fas-mediated extrinsic and mitochondria-mediated intrinsic apoptotic signaling pathway induced by tetrandrine.
Acknowledgments
This work was supported by National Institutes of Health Grant 1P20 MD006882.
Abbreviations
- ASK1
Apoptosis signal-regulating kinase 1
- Bax
Bcl2-associated X protein
- Bcl2
B-cell lymphoma 2
- Bid
BH3 interacting-domain death agonist
- CRPC
castration-resistant prostate cancer
- DAPI
4′,6-diamidino-2-phenylindole
- DHE
Dihydroethidium
- DISC
death-inducing signaling complex
- DMSO
Dimethylsulfoxide
- FADD
Fas-Associated protein with Death Domain
- FasL
Fas ligand
- FITC
fluorescein isothiocyanate
- H2DCF-DA
2′,7′-dichlorodihydrofluorescein diacetate
- IP
immunoprecipitation
- JNK
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinases
- MMP
mitochondrial membrane potential
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium Bromide
- NAC
N-acetyl-L-cysteine
- PARP
Poly (ADP-ribose) polymerase
- PI
propidium iodide
- ROS
reactive oxygen species
- S.E.
standard error of the mean
- Smac
second mitochondria-derived activator of caspases
- z-DEVD-FMK
Z-Asp-Glu-Val-Asp fluoromethylketone
- z-IETD-FMK
Z-Ile-Glu(O-ME)-Thr-Asp(O-Me) fluoromethylketone
Footnotes
Conflict of interest: The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Semin Surg Oncol. 2000;18:52–74. doi: 10.1002/(sici)1098-2388(200001/02)18:1<52::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Isaacs JT, Furuya Y, Berges R. The role of androgen in the regulation of programmed cell death/apoptosis in normal and malignant prostatic tissue. Semin Cancer Biol. 1994;5:391–400. [PubMed] [Google Scholar]
- 4.Lamont KR, Tindall DJ. Minireview: alternative activation pathways for the androgen receptor in prostate cancer. Mol Endocrinol. 2011;25:897–907. doi: 10.1210/me.2010-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 6.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 7.Crawford ED, Petrylak D. Castration-resistant prostate cancer: descriptive yet pejorative? J Clin Oncol. 2010;28:e408. doi: 10.1200/JCO.2010.28.7664. [DOI] [PubMed] [Google Scholar]
- 8.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 9.Colombel M, Symmans F, Gil S, O'Toole KM, Chopin D, Benson M, Olsson CA, Korsmeyer S, Buttyan R. Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- 10.McCourt C, Maxwell P, Mazzucchelli R, Montironi R, Scarpelli M, Salto-Tellez M, O'Sullivan JM, Longley DB, Waugh DJ. Elevation of c-FLIP in castrate-resistant prostate cancer antagonizes therapeutic response to androgen receptor-targeted therapy. Clin Cancer Res. 2012;18:3822–3833. doi: 10.1158/1078-0432.CCR-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 12.Shamash J, Dancey G, Barlow C, Wilson P, Ansell W, Oliver RT. Chlorambucil and lomustine (CL56) in absolute hormone refractory prostate cancer: re-induction of endocrine sensitivity an unexpected finding. Br J Cancer. 2005;92:36–40. doi: 10.1038/sj.bjc.6602263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YJ. Potential role of tetrandrine in cancer therapy. Acta Pharmacol Sin. 2002;23:1102–1106. [PubMed] [Google Scholar]
- 14.Wang G, Lemos JR, Iadecola C. Herbal alkaloid tetrandrine: from an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol Sci. 2004;25:120–123. doi: 10.1016/j.tips.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.King VF, Garcia ML, Himmel D, Reuben JP, Lam YK, Pan JX, Han GQ, Kaczorowski GJ. Interaction of tetrandrine with slowly inactivating calcium channels. Characterization of calcium channel modulation by an alkaloid of Chinese medicinal herb origin. J Biol Chem. 1988;263:2238–2244. [PubMed] [Google Scholar]
- 16.Li SY, Ling LH, Teh BS, Seow WK, Thong YH. Anti-inflammatory and immunosuppressive properties of the bis-benzylisoquinolines: in vitro comparisons of tetrandrine and berbamine. Int J Immunopharmacol. 1989;11:395–401. doi: 10.1016/0192-0561(89)90086-6. [DOI] [PubMed] [Google Scholar]
- 17.Liu QY, Li B, Gang JM, Karpinski E, Pang PK. Tetrandrine, a Ca++ antagonist: effects and mechanisms of action in vascular smooth muscle cells. J Pharmacol Exp Ther. 1995;273:32–39. [PubMed] [Google Scholar]
- 18.Pang L, Hoult JR. Cytotoxicity to macrophages of tetrandrine, an antisilicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem Pharmacol. 1997;53:773–782. doi: 10.1016/s0006-2952(96)00817-9. [DOI] [PubMed] [Google Scholar]
- 19.Lai JH. Immunomodulatory effects and mechanisms of plant alkaloid tetrandrine in autoimmune diseases. Acta Pharmacol Sin. 2002;23:1093–1101. [PubMed] [Google Scholar]
- 20.Chambers TC, McAvoy EM, Jacobs JW, Eilon G. Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J Biol Chem. 1990;265:7679–7686. [PubMed] [Google Scholar]
- 21.Liu ZL, Hirano T, Tanaka S, Onda K, Oka K. Persistent reversal of P-glycoprotein-mediated daunorubicin resistance by tetrandrine in multidrug-resistant human T lymphoblastoid leukemia MOLT-4 cells. J Pharm Pharmacol. 2003;55:1531–1537. doi: 10.1211/0022357022115. [DOI] [PubMed] [Google Scholar]
- 22.Xu WL, Shen HL, Ao ZF, Chen BA, Xia W, Gao F, Zhang YN. Combination of tetrandrine as a potential-reversing agent with daunorubicin, etoposide and cytarabine for the treatment of refractory and relapsed acute myelogenous leukemia. Leuk Res. 2006;30:407–413. doi: 10.1016/j.leukres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Fu LW, Zhang YM, Liang YJ, Yang XP, Pan QC. The multidrug resistance of tumour cells was reversed by tetrandrine in vitro and in xenografts derived from human breast adenocarcinoma MCF-7/adr cells. Eur J Cancer. 2002;38:418–426. doi: 10.1016/s0959-8049(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Kang GH, Kim KC, Kim KM, Park DI, Choi BT, Kang HS, Lee YT, Choi YH. Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int J Oncol. 2002;21:1239–1244. [PubMed] [Google Scholar]
- 25.Jang BC, Lim KJ, Paik JH, Cho JW, Baek WK, Suh MH, Park JB, Kwon TK, Park JW, Kim SP, Shin DH, Song DK, Bae JH, Mun KC, Suh SI. Tetrandrine-induced apoptosis is mediated by activation of caspases and PKC-delta in U937 cells. Biochem Pharmacol. 2004;67:1819–1829. doi: 10.1016/j.bcp.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Chen JC, Tseng SH. Tetrandrine suppresses tumor growth and angiogenesis of gliomas in rats. Int J Cancer. 2009;124:2260–2269. doi: 10.1002/ijc.24208. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Gong K, Mao X, Li W. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int J Cancer. 2011;129:1519–1531. doi: 10.1002/ijc.25817. [DOI] [PubMed] [Google Scholar]
- 28.He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim SH, Huang E, Gao Y, Yang K, Wagner ER, Wang L, Tang N, Luo J, Liu X, Li M, Bi Y, Shen J, Luther G, Hu N, Zhou Q, Luu HH, Haydon RC, Zhao Y, He TC. Tetrandrine inhibits Wnt/β-catenin signaling and suppresses tumor growth of human colorectal cancer. Mol Pharmacol. 2011;79:211–219. doi: 10.1124/mol.110.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W, Debeb BG, Lacerda L, Li J, Woodward WA. Tetrandrine, a compound common in chinese traditional medicine, preferentially kills breast cancer tumor initiating cells (TICs) in vitro. Cancers (Basel) 2011;3:2274–2285. doi: 10.3390/cancers3022274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng LH, Zhang H, Hayward L, Takemura H, Shao RG, Pommier Y. Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S-specific cyclin-dependent kinases and by inducing p53 and p21Cip1. Cancer Res. 2004;64:9086–9092. doi: 10.1158/0008-5472.CAN-04-0313. [DOI] [PubMed] [Google Scholar]
- 31.Qin R, Shen H, Cao Y, Fang Y, Li H, Chen Q, Xu W. Tetrandrine induces mitochondria-mediated apoptosis in human gastric cancer BGC-823 cells. PLoS One. 2013;8:e76486. doi: 10.1371/journal.pone.0076486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen XL, Ren KH, He HW, Shao RG. Involvement of PI3K/AKT/GSK3beta pathway in tetrandrine-induced G1 arrest and apoptosis. Cancer Biol Ther. 2008;7:1073–1078. doi: 10.4161/cbt.7.7.6142. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 34.Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- 35.Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- 36.Wilkie-Grantham RP, Matsuzawa S, Reed JC. Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-FLIP protein. J Biol Chem. 2013;288:12777–12790. doi: 10.1074/jbc.M112.431320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fadeel B, Thorpe CJ, Yonehara S, Chiodi F. Anti-Fas IgG1 antibodies recognizing the same epitope of Fas/APO-1 mediate different biological effects in vitro. Int Immunol. 1997;9:201–209. doi: 10.1093/intimm/9.2.201. [DOI] [PubMed] [Google Scholar]
- 40.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 41.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 42.Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 43.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic Bax and Bak: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srivastava RK, Mi QS, Hardwick JM, Longo DL. Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci USA. 1999;96:3775–3780. doi: 10.1073/pnas.96.7.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 49.Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 50.Gong K, Chen C, Zhan Y, Chen Y, Huang Z, Li W. Autophagy-related gene 7 (ATG7) and reactive oxygen species/extracellular signal-regulated kinase regulate tetrandrine-induced autophagy in human hepatocellular carcinoma. J Biol Chem. 2012;287:35576–35588. doi: 10.1074/jbc.M112.370585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda K, Matsuzawa A, Nishitoh H, Ichijo H. Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct. 2003;28:23–29. doi: 10.1247/csf.28.23. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dimmeler S, Breitschopf K, Haendeler J, Zeiher A. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J Exp Med. 1999;189:1815–1822. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breitschopf K, Haendeler J, Malchow P, Zeiher AM, Dimmeler S. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol Cell Biol. 2000;20:1886–1896. doi: 10.1128/mcb.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 57.Rothe G, Valet GJ. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J Leukoc Biol. 1990;47:440–448. [PubMed] [Google Scholar]
- 58.Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- 59.Ferreira CG, Epping M, Kruyt FA, Giaccone G. Apoptosis: target of cancer therapy. Clin Cancer Res. 2002;8:2024–2034. [PubMed] [Google Scholar]
- 60.Ling YH, Liebes L, Zou Y, Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 61.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 62.Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;26:1905–1913. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- 63.Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone Enhances TRAIL-Induced Apoptosis through the Induction of Death Receptors in Human Colon Cancer Cells: Evidence for an Essential Role of Reactive Oxygen Species. Cancer Res. 2009;69:6581–6589. doi: 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao D, Powolny AA, Moura MB, Kelley EE, Bommareddy A, Kim SH, Hahm ER, Normolle D, Van Houten B, Singh SV. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem. 2010;285:26558–26569. doi: 10.1074/jbc.M109.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma R, Sharma A, Chaudhary P, Pearce V, Vatsyayan R, Singh SV, Awasthi S, Awasthi YC. Role of lipid peroxidation in cellular responses to D,L-sulforaphane, a promising cancer chemopreventive agent. Biochemistry. 2010;49:3191–3202. doi: 10.1021/bi100104e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 67.Oh SH, Lee BH. Induction of apoptosis in human hepatoblastoma cells by tetrandrine via caspase-dependent Bid cleavage and cytochrome c release. Biochem Pharmacol. 2003;66:725–731. doi: 10.1016/s0006-2952(03)00397-6. [DOI] [PubMed] [Google Scholar]
- 68.May WS, Tyler PG, Ito T, Armstrong DK, Qatsha KA, Davidson NE. Interleukin-3 and bryostatin-1 mediate hyperphosphorylation of BCL2 alpha in association with suppression of apoptosis. J Biol Chem. 1994;269:26865–26870. [PubMed] [Google Scholar]
- 69.Ito T, Deng X, Carr B, May WS. Bcl-2 Phosphorylation Required for Anti-apoptosis Function. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 70.Ruvolo PP, Deng X, Carr BK, May WS. A Functional Role for Mitochondrial Protein Kinase Cα in Bcl2 Phosphorylation and Suppression of Apoptosis. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 71.Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C, Gillieron C, Boschert U, Vial-Knecht E, Martinou JC, Arkinstall S. Bcl-2 Undergoes Phosphorylation by c-Jun N-terminal Kinase/Stress-activated Protein Kinases in the Presence of the Constitutively Active GTP-binding Protein Rac1. J Biol Chem. 1997;272:25238–25242. doi: 10.1074/jbc.272.40.25238. [DOI] [PubMed] [Google Scholar]
- 72.Geng F, Tang L, Li Y, Yang L, Choi KS, Kazim AL, Zhang Y. Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via Bcl-2 protein phosphorylation. J Biol Chem. 2011;286:32259–32267. doi: 10.1074/jbc.M111.278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT. Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem. 1999;274:7987–7992. doi: 10.1074/jbc.274.12.7987. [DOI] [PubMed] [Google Scholar]
- 74.Shao RG, Cao CX, Nieves-Neira W, Dimanche-Boitrel MT, Solary E, Pommier Y. Activation of the Fas pathway independently of Fas ligand during apoptosis induced by camptothecin in p53 mutant human colon carcinoma cells. Oncogene. 2001;20:1852–1859. doi: 10.1038/sj.onc.1204264. [DOI] [PubMed] [Google Scholar]