Abstract
Background
Type 2 diabetes (diabetes) and its complications can sometimes be prevented, if identified and treated early. One fifth of diabetics in the U.S. remain undiagnosed. Commonly used screening guidelines are inconsistent.
Purpose
To examine the optimal age cut-point for opportunistic universal screening, compared to targeted screening, which is recommended by U.S. Preventive Services Task Force (USPSTF) and American Diabetes Association (ADA) guidelines.
Methods
Cross-sectional analysis of a nationally representative sample from the National Health and Nutrition Examination Survey, 2007–2010. Number of people needed to screen (NNS) to obtain one positive test result was calculated for different guidelines. Sampling weights were applied to construct national estimates. The 2010 Medicare fee schedule was used for cost estimation. Analysis was conducted in January 2014.
Results
NNS, under universal screening, drops sharply at age 35 years, from 80 (30–34-year-olds) to 31 (35–39-year-olds). Opportunistic universal screening of eligible people aged ≥35 years would yield a NNS of 15, translating to $66 per positive test. Among people aged 35–44 years (who are not recommended for universal screening by ADA), most (71%) were overweight or obese and all had at least one other ADA risk factor. Only 34% of individuals aged ≥35 years met USPSTF criteria. Strictly enforcing USPSTF guidelines would have resulted in a majority (61%) of potential positive tests cases being missed (5,508,164 cases nationwide).
Conclusions
Opportunistic universal screening among individuals aged ≥35 years could greatly reduce the national prevalence of undiagnosed pre-diabetes or diabetes at relatively low cost.
Introduction
There were 18.8 million people diagnosed with type 2 diabetes (diabetes) in the U.S. in 2010,1 and an estimated additional 7 million individuals with diabetes remain undiagnosed.1,2 Diabetes can be a preventable disease.3,4 Early glucose screening can help identify individuals with pre-diabetes, and early treatment of pre-diabetes can in some cases result in prevention of progression to diabetes.3,4 Further, when detected and treated early, complications of diabetes can sometimes be prevented.5 However, diabetes may be asymptomatic for as many as 7 years,6 and many cases are likely to remain undetected in the absence of screening.
The global discussion about whom to screen for diabetes began over 20 years ago, and remains a topic of debate amidst recent calls for increased healthcare affordability and reform. The screening debate has produced a number of screening guidelines,6–10 which differ widely in whom they identify as appropriate for screening.11 The U.S. Preventive Services Task Force (USPSTF) recommends screening a targeted high-risk population (asymptomatic adults with sustained blood pressure >135/80 mmHg only8). The American Diabetes Association (ADA) recommends screening asymptomatic younger adults (aged <45 years) with a BMI ≥25 kg/m2 and at least one additional diabetes risk factor, and all individuals aged ≥45 years irrespective other risk factors.7 There are 11 additional risk factors that clinicians are asked to assess per the ADA guidelines (Table 1).7 Given the current increasing prevalence of diabetes, decreasing age at onset,12 and complexity of assessing a myriad risk factors based on inconsistent guidelines, a more simple universal approach to screening may now be warranted.
Table 1.
Inclusion criteria and clinical characteristics of the study population
| Inclusion criteria | n | % |
|---|---|---|
| Age ≥18 years | 12,355 | |
| Not pregnant and no evidence of existing diabetesa | 10,703 | 86.6 |
|
| ||
| Clinical characteristics of included people | n=10,703 | %b |
|
| ||
| Eligible based on ADA guideline | 8,738 (81.6%) | |
| Age ≥45 years | 5,378 | |
| Age 18–44 years | 5,768 | |
| Overweight or obese (BMI ≥25kg/m2) | 3,522 (65.1%) | |
| Additional risk factors, if overweight or obese | 3,522 | 100% |
| Racial/ethnic minority (not non-Hispanic white) | 1,419 | 61.3% |
| Hypertensionc | 416 | 11.6% |
| Low HDL cholesterol (<25 mg/dL) | 204 | 15.9% |
| High triglycerides (>250 mg/dL) | 115 | 4.1% |
| History of insulin resistanced | 96 | 2.8% |
| History of cardiovascular diseasee | 50 | 1.5% |
| Family (first-degree relatives) history of diabetesf | 1,185 | 35.8% |
| Physical inactivityg | 603 | 19.7% |
| History of gestational diabetesh | 87 | 2.8% |
| Giving birth baby of more than 9 pounds | 165 | 5.1% |
| Eligible based on USPSTF guideline | ||
| Average blood pressure of ≥135/80mmHg | 3,021 (28.2%) | |
Yes to any of the following questions: Have you ever been told by a doctor or other health professional that you have diabetes or sugar diabetes?, Are you now taking insulin?, and Are you now taking diabetic pills to lower your blood sugar? These are sometimes called oral agents or oral hypoglycemic agents.
Numbers presented below indicate % of people who had each of the additional risk factors (numerator) among those who were aged between 18 and 44 years and overweight or obese (denominator). Percent of respondents who did not provide valid response to each survey question (e.g., do not know or refused) ranged between 0% and 1.8% (family history of diabetes). For triglycerides, blood examination was conducted only for people who took the exam in the morning while fasting, thus 1,792 (55.5%) did not have available laboratory values. Missing information for this criterion, however, did not affect identifying ADA eligibility. All of those without triglyceride values had at least one other additional risk factor, and thus were classified as meeting ADA guidelines.
Met one of following: average blood pressure (average of three consecutive blood pressure readings during the examination) ≥140/90 mmHg, yes to the question: Are you currently taking medication to lower your blood pressure?, or yes to the question: Have you ever been told by a doctor or other health professional that you have hypertension or high blood pressure?
Yes to the question: Have you ever been told by a doctor or other health professional that you have any of following: prediabetes, impaired fasting glucose, impaired glucose tolerance, borderline diabetes or your blood sugar is higher than normal but not high enough to be called diabetes or sugar diabetes?
Yes to one of the following questions: … have you ever had heart failure (coronary heart disease/angina/heart attack/stroke)?
Yes to the question: Including living and deceased, were any of your biological relatives, that is, blood relatives, including grandparents, parents, brothers, and sisters, ever told by a health professional that they had diabetes?
No to all of the four following questions: …Does your work involve vigorous-intensity that causes large increase in breathing or heart rate like carrying or lifting heavy loads, digging, or construction work for at least 10 minutes continuously?, …Does your work involve moderate-intensity activity that causes small increase in breathing or heart rate such as brisk walking or carrying light loads for at least 10 minutes continuously?, Do you do any vigorous-intensity sports, fitness, or recreational activities that cause large increases in breathing or heart rate like running or basketball for at least 10 minutes continuously?, and …Do you do any moderate-intensity sports, fitness, or recreational activities that cause a small increase in breathing or heart rate such as brisk walking, bicycling, swimming, or golf for at least 10 minutes continuously?
Yes to the question: …were you ever told by a doctor or other health professional that you had diabetes, sugar diabetes, or gestational diabetes [during the pregnancy]? This question was asked of women aged ≥20 years.
ADA, American Diabetes Association; HDL, high-density lipoprotein; USPSTF, U.S. Preventive Services Task Force
In this study, we investigated the implications of opportunistic universal diabetes screening for individuals aged ≥18 as compared to targeted screening recommended by clinical guidelines. Opportunistic screening is defined as screening during a healthcare visit for another purpose, as opposed to adding an additional medical visit purely for the purposes of screening. Using data from the National Health and Nutrition Examination Survey (NHANES), we estimated: (1) the proportion of the U.S. population who would be eligible for screening when applying recommended screening criteria defined by the two most referenced guidelines—USPSTF (post-2008) and the ADA; and (2) the number of diabetes-free individuals who need to be tested to detect one positive test (number needed to screen [NNS]). Given the current trends of increasing overweight/obesity, increasing prevalence of diabetes, and decreased age of onset of diabetes, our hypotheses are that: (1) a large proportion of the U.S. population already meets ADA screening criteria; and (2) the NNS for a positive test result is low for opportunistic universal screening.
Methods
Study Populations and Data Sources
Two recent NHANES cycles, conducted in 2007–2008 and 2009–2010, were used. NHANES uses a complex sampling design and constructs sample weights to produce nationally representative data. NHANES samples about 5,000 persons each year, with oversampling of persons aged ≥60 years, African Americans, and Hispanics to produce reliable statistics for these subpopulations. Data are collected through interviews and physical examinations. All NHANES respondents received at least one of the following screening glucose tests: fasting blood glucose (FBG), oral glucose tolerance test (OGTT), or glycosylated hemoglobin (HbA1c). Procedures for blood collection and processing are described elsewhere (cdc.gov/nchs/nhanes/about_nhanes.htm).
We applied appropriate sampling weights to combine multiple waves and compute weighted statistics. As two waves of the surveys were combined, a half of sampling weight for each individual was used to derive U.S. national estimates (cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/Task2.htm).
Between 2007 and 2010, 12,355 individuals aged ≥18 years (5,172 from 2007–2008 and 5,531 from 2009–2010) completed the survey. Of these individuals, 1,652 were excluded: 125 individuals were pregnant and additional 1,527 individuals were identified as currently having diabetes. Identification of diabetes was based on responding yes to any of the following questions: Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?, Are you now taking insulin?, and “Are you now taking diabetic pills to lower your blood sugar? These are sometimes called oral agents or oral hypoglycemic agents. The remaining 10,703 individuals were determined to be non-pregnant and diabetes free, and served as the study sample considered eligible for diabetes screening.
Identification of Eligibility for Screening Based on ADA and USPSTF Guidelines
We identified people eligible for screening based on ADA7 and USPSTF8 guidelines, following the specified criteria. The ADA recommends screening for adults aged <45 years who are overweight or obese (BMI ≥25 kg/m2) and have at least one additional risk factor (Table 1), and for any individual ≥45 years regardless of other risk factors.7 Risk factors, in addition to being overweight or obese, in the ADA guidelines include being a member of a racial/ethnic minority group, hypertension, low high-density lipoprotein cholesterol, high triglycerides, history of insulin resistance, history of cardiovascular disease, family history of diabetes, physical inactivity, history of gestational diabetes, history of giving birth to a baby of >9 pounds (macrosomia), and history of polycystic ovarian syndrome (PCOS). The USPSTF recommends screening only for asymptomatic adults with sustained blood pressure ≥135/80 mmHg.8
All information on ADA and USPSTF risk factors, except for the history of PCOS, is available in the NHANES survey, either self-reported or through examination. When values to each risk factor was missing (i.e., refused or don’t know for an interview question, or no test value), we used a conservative approach. That is, we regarded one as having the risk factor only if there was confirmatory information (i.e., yes to the relevant question or positive on clinical exam), thereby treating missing information as not having the risk factor. Nevertheless, everyone with missing risk factor information had at least one other risk factor that met ADA criteria. Implications of missing values are discussed in footnote b in Table 1.
Identification of Positive Test Result and NNS
A positive diabetes test result was determined based on ADA guidelines for diagnosing diabetes7 and included at least one occurrence of the following: FBG ≥126mg/dL, 2-hour oral OGTT ≥200 mg/dL, or HbA1c ≥6.5% (48 mmol/mol). About half (48%) of the participants had more than one of these tests. In a few cases (3% of the participants) there was a conflict (one positive and one negative test), and this was considered a positive test in our analyses. The three tests produced similar rates of positive results: 2.0% (FBG), 2.2% (OGTT), and 2.4% (HbA1c) among who were self-reported as diabetes-free. Actual diagnosis of diabetes generally requires confirmation of elevated glycemic levels by repeat testing.7 Therefore, for the purposes of this study, a positive screening test is not considered a definitive diagnosis of diabetes. Based on the rate of positive test results, NNS was computed (e.g., a positive test rate of 2% translates to NNS of 50=1/0.02).
Statistical Analysis
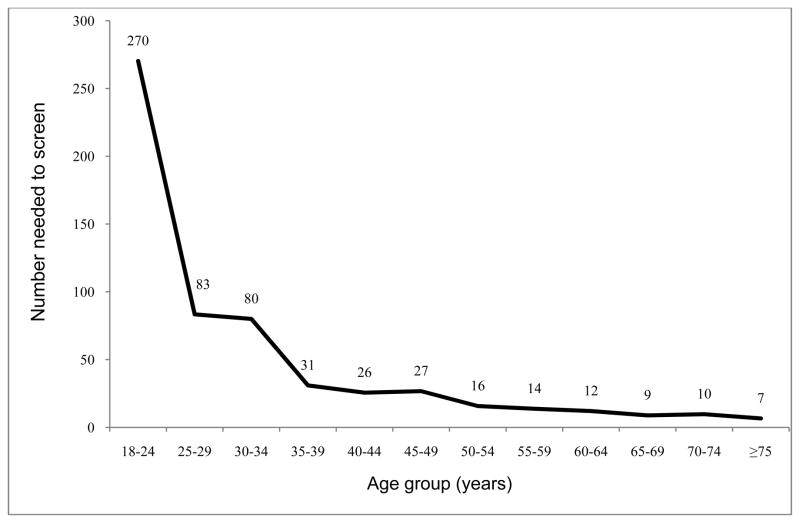
We assessed NNS with universal screening in 12 age categories (Figure 1). Based on these results, we then grouped people into three age categories: 18–34 years, 35–44 years, and ≥45 years. The cut-point of 45 years was chosen to compare statistics for age 35–44 years based on universal screening versus ADA guidelines. We estimated positive test rates, NNS, and costs for screening for people in each of three age groups, when applying: (1) opportunistic universal screening; (2) screening based on ADA guidelines; and (3) screening based on USPSTF guidelines. Cost estimates used the 2010 Medicare fee schedule ($4.54 for a blood glucose test, [Current Procedural Terminology=82947]).13 For our analysis, we assumed an opportunistic screening approach in which diabetes screening could be added for patients who come to the clinic for other reasons such as a routine physical exam, monitoring hypertension, or another acute condition. Thus, we did not include costs for office visits here because a separate medical visit solely for diabetes screening does not commonly occur in real-world clinical practice. Based on the proportion of the population eligible for screening and positive test rates, we computed potentially missed positive cases in the U.S. for each guideline. All statistical analyses were conducted in January 2014 using Stata, version 11.2 (Stata Corp, College Station TX).
Figure 1.
Number needed to screen by age group
Results
Table 1 shows the clinical and demographic risk factors of diabetes to be considered when determining whether a person would be recommended for screening based on ADA or USPSTF guidelines. Among adult survey respondents who were diabetes-free and non-pregnant, 81.6% met ADA criteria and 28.2% met USPSTF criteria for diabetes screening (Table 1).
Among those who aged <45 years, a majority was overweight or obese (65.1%). Among those who were aged <45 years and were overweight or obese, all had at least one additional ADA risk factor, and thus the additional risk factor did not serve as a criterion for narrowing the eligibility for screening. Therefore, missing values on some of the additional risk factors did not affect the classification of individuals meeting ADA eligibility, as those who had missing data in some criteria already met at least one another criterion.
We next applied sampling weights to compute national estimates for the population meeting each screening guideline and potentially missed cases when applying each guideline (Table 2, column 1). The ADA recommends universal screening for individuals aged ≥45 years. On the other hand, the proportion of people recommended for screening based on USPSTF guidelines is relatively small: 26% (age ≥18 years), 34% (age ≥35 years), and 37% (age ≥45 years).
Table 2.
Positive detection rates by screening eligibility criteria, weighted national estimates
| %Eligible | %Positive | NNS | Cost per 1 positive test | Positive tests, n | Potentially missed cases, n | % Positive tests missed | |
|---|---|---|---|---|---|---|---|
| Age ≥18 years | |||||||
| Universal screening | 100% | 5.0% | 20 | $91.11 | 9,598,214 | ||
| ADA guideline | 80% | 6.0% | 17 | $75.47 | 9,292,119 | 306,095 | 3.2% |
| USPSTF guideline | 26% | 7.4% | 14 | $61.36 | 3,717,445 | 5,880,770 | 61.3% |
| Age ≥35 years | |||||||
| Universal screening | 100% | 6.8% | 15 | $66.37 | 9,075,042 | ||
| ADA guideline | 91% | 7.3% | 14 | $62.10 | 8,868,586 | 206,455 | 2.3% |
| USPSTF guideline | 34% | 8.0% | 12 | $56.58 | 3,566,877 | 5,508,164 | 60.7% |
| Age 18–34 years | |||||||
| Universal screening | 100% | 0.9% | 115 | $520.27 | 523,173 | ||
| ADA guideline | 55% | 1.3% | 78 | $355.42 | 423,533 | 99,640 | 19.0% |
| USPSTF guideline | 10% | 2.6% | 38 | $174.54 | 150,567 | 372,606 | 71.2% |
| Age 35–44 years | |||||||
| Universal screening | 100% | 3.6% | 28 | $127.07 | 1,374,725 | ||
| ADA guideline | 71% | 4.3% | 23 | $105.42 | 1,168,270 | 206,455 | 15.0% |
| USPSTF guideline | 25% | 6.8% | 15 | $67.13 | 662,145 | 712,580 | 51.8% |
| Age ≥45 years | |||||||
| Universal screening | 100% | 8.2% | 12 | $55.53 | 7,700,317 | ||
| ADA guideline | 100% | 8.2% | 12 | $55.53 | 7,700,317 | ||
| USPSTF guideline | 37% | 8.4% | 12 | $54.17 | 2,904,732 | 4,795,585 | 62.3% |
ADA, American Diabetes Association; NNS, number needed to screen; USPSTF, U.S. Preventive Services Task Force
If universally screened, 5% of people aged ≥18 years in the U.S. would have had a positive test result (NNS=20) (Table 2, columns 2–3). When broken down by age group with 5-year intervals, positive test rates increased substantially between age 30–34 years (1.3%; NNS=80) and 35–39 years (3.4%; NNS=31) (Figure 1). Then, NNS declined steadily with advancing age, except for a relatively large decline at age 50 years (NNS=27 for age 45–49 years vs 16 for 50–54 years), and reached NNS=7 for those aged ≥75 years. When applying guidelines, the NNS for people aged ≥18 years was 17 (ADA) and 14 (USPSTF) as compared to 20 with universal screening (Table 2, column 3). For people aged ≥35 years, the NNS with universal screening (15) is closer to that based on selected screening according to the ADA (14) or USPSTF (12). The estimated cost of universal screening for individuals aged ≥35 years, assuming opportunistic screening, would be $66 per each positive test result as compared to $62 (screening ADA-eligible individuals only) and $57 (screening USPSTF-eligible individuals only).
Based on these rates, if ADA screening guidelines were strictly followed, an estimated 306,095 individuals or 3.2% of positive tests among people aged ≥18 years would have been missed (Table 2, column 6). For individuals aged 35–44 years, an estimated 206,455 (or 14.6% of positive tests) would have been missed when applying ADA guidelines. If USPSTF screening guidelines were strictly enforced, owing to their strict eligibility criteria and not substantially better detection rate, an estimated 5,880,770 individuals or 61.3% of positive tests among people aged ≥18 years would have been missed, and an estimated 5,508,164 individuals or 60.7% of positive tests among people aged ≥35 years would have been missed.
Discussion
This study examined the application of two of the most widely used screening guidelines (ADA and USPSTF) in a large nationally representative U.S. sample Existing guidelines vary in defining screening eligible individuals. Among those identified as eligible for screening, the positive result rate was similar for ADA, USPSTF, and universal screening guidelines for people aged ≥35 years. However, strictly enforcing the USPSTF guidelines would have resulted in almost 6 million people potentially being missed. Other studies have also demonstrated the limitations of the USPSTF screening recommendations for diabetes.14 Use of ADA guidelines would result in fewer missed cases, but fully ascertaining the presence of any of the 12 additional risk factors may be cumbersome, time intensive, and unnecessary. Further, the ADA acknowledges that recent mathematical models suggest that screening independent of risk factors is highly cost-effective.7
Our examination of 5-year age groups identifies age 35 years as an important threshold above which the NNS declines by more than half. When ADA guidelines are implemented among those aged 35–44 years, the NNS for one positive test did not differ substantially from a universal approach (NNS=23 vs 28). For the younger age group, 18–34 years, the NNS is much higher and the difference between the ADA guidelines and universal screening is greater (NNS=78 vs 115). Universal screening of individuals aged ≥35 years would simplify the current complex criteria for assessment for screening eligibility in primary care and maintain a low NNS. For individuals aged 35–44 years, 71% qualify for screening based on ADA guidelines owing to the high prevalence of overweight or obesity. Everyone who is overweight or obese has at least one additional risk factor for ADA eligibility criteria. This means that the 11 additional ADA risk factors provide no additional information beyond BMI level in determining ADA guideline eligibility. The ADA guidelines can be simplified, without any effect on eligibility, to use age and overweight/obesity as the sole screening criteria.
Current practice patterns from two sites in the Midwest15 and California16 suggest that nearly universal opportunistic screening is already in place. At a large ambulatory care organization in California, diabetes screening rates among diabetes-free individuals aged ≥35 years were reported to be 80% (N=109,351).16 Another study from a Midwest organization (N=46,991) reported a comparable rate, 85%.15 The NNS in this Midwestern study ranged from 13–20 when applying USPSTF versus ADA criteria,15 which are similar to the NNSs derived from the universal screening of the NHANES sample in our study.
The cost-effectiveness of optimal age to start screening for diabetes remains uncertain.17 In a recent simulation study, Kahn et al. (2010)18 provided compelling evidence for the cost-effectiveness of screening for diabetes in the U.S. at age 30 years. They concluded that with an opportunistic screening approach screening should start between the ages of 30–45 years and be repeated every 3–5 years to be most cost-effective.18 Opportunistic screening is a viable approach even in younger age groups, given that that most (78%) people aged 25–44 years have visited a healthcare professional at least once in the past year.19 The utilization rate in this age group is expected to further increase with expanded insurance coverage under the Affordable Care Act. In addition, the recent endorsement by the ADA of using a single HbA1c test to diagnose diabetes7 is also helpful in implementing universal opportunistic screening, as HbA1c can be obtained without fasting and therefore can be done at any office visit.
Diagnosis of diabetes requires confirmation of a positive test result with a follow-up laboratory test, and not all of those identified with one positive test in our analysis would eventually meet the formal diagnostic criteria for diabetes. Any screening program for diabetes will inevitably identify those at risk for diabetes (potential false positives), but the evidence for prevention of diabetes in this pre-diabetic group is very strong.20–22 In other conditions (e.g., HIV,23 prostate-specific antigen,24 mammography25) false positives can lead to unnecessary workups, stigma, and potential harmful treatments. In contrast, false positives in diabetes testing most likely lead to the diagnosis of pre-diabetes, which has all of the same initial treatment recommendations (diet, lifestyle, medication) as diabetes.26 Treatment for both diabetes and pre-diabetes can involve lifestyle change, which is not harmful and has been shown to be beneficial for many disorders.27 Metformin, which is often the first drug prescribed for treatment of diabetes, has also been proven effective for the treatment of pre-diabetes.26
A growing body of evidence indicates that earlier detection and treatment of pre-diabetes or diabetes can be beneficial.18,22,28 Good glucose control early in the course of diabetes can reduce microvascular and cardiovascular consequences in subsequent years.29–31 Further, early detection at a younger age represents a wider window of opportunity for behavior change, lifestyle intervention, and prevention. The age at diabetes diagnosis appears to be decreasing12,32–34 and is thought to reflect a true population trend in earlier onset, which makes earlier screening and intervention more important.
Several limitations of our study, mostly due to the nature of the data, warrant discussion. First, the absence of diabetes is identified based on self-report. Some people may not report that they have diabetes, because of fear or perceived stigma, even when they were aware of the problem. Second, this study does not address repeat testing and interval for rescreening, which are both important clinical questions. Third, a positive screening test result is based on one abnormal test rather than two, which are required for definitive diabetes diagnosis. Although we are not able to identify true positive cases of diabetes, a positive screening test result does indicate dysglycemia, which in a clinical setting would merit further investigation; this is another purpose of screening—to catch potential cases.
Conclusions
Our findings suggest that a more universal approach to diabetes screening in all people aged ≥35 years is not only simpler to implement, but can greatly reduce the national prevalence of undiagnosed diabetes. Detection/positive test rates are generally high regardless of guideline screening eligibility, with an NNS of 12 (USPSTF), 13 (ADA), and 14 (universal screening) for individuals aged ≥35 years. Given the substantial benefit of early detection35 with small incremental costs for extending diabetes screening,18 and evidence that nearly universal screening is already practiced,18,19 it may be time to harmonize guidelines and recognize universal screening for diabetes in adults aged ≥35 years as formal policy.
Acknowledgments
Dr. Sukyung Chung had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of analysis. This study was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. 1R01DK081371-01A1) and Agency for Healthcare Research and Quality (grant no. K01 HS019815). The authors would like to thank Mr. Eric C. Wong, MS, for his feedback regarding this manuscript.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Diabetes Association. Data from the 2011 National Diabetes Fact Sheet. 2011 diabetes.org/diabetes-basics/diabetes-statistics/?print=t.
- 2.National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics. 2011 diabetes.niddk.nih.gov/dm/pubs/statistics/#fast.
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Colagiuri S, Cull CA, Holman RR. Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes?: U.K. prospective diabetes study 61. Diabetes Care. 2002;25(8):1410–17. doi: 10.2337/diacare.25.8.1410. [DOI] [PubMed] [Google Scholar]
- 6.Patel P, Macerollo A. Diabetes mellitus: diagnosis and screening. Am Fam Physician. 2010;81(7):863–70. [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(S1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults. U.S Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(11):846–54. doi: 10.7326/0003-4819-148-11-200806030-00007. [DOI] [PubMed] [Google Scholar]
- 9.Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan: executive summary. Endocr Pract. 2011;17(2):287–302. doi: 10.4158/ep.17.2.287. [DOI] [PubMed] [Google Scholar]
- 10.Pottie K, Jaramillo A, Lewin G, et al. Recommendations on screening for type 2 diabetes in adults. CMAJ. 2012;184(15):1687–96. doi: 10.1503/cmaj.120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutten G. Screening for type 2 diabetes—where are we now? Lancet. 2010;375(9723):1324–6. doi: 10.1016/S0140-6736(10)60455-2. [DOI] [PubMed] [Google Scholar]
- 12.Koopman RJ, Mainous AG, 3rd, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the U.S. 1988 to 2000. Ann Fam Med. 2005;3(1):60–3. doi: 10.1370/afm.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule. 2010 cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html.
- 14.Casagrande SS, Cowie CC, Fradkin JE. Utility of the U.S. Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45(2):167–74. doi: 10.1016/j.amepre.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehy AM, Flood GE, Tuan WJ, Liou JI, Coursin DB, Smith MA. Analysis of guidelines for screening diabetes mellitus in an ambulatory population. Mayo Clin Proc. 2010;85(1):27–35. doi: 10.4065/mcp.2009.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung S, Azar KMJ, Zhao B, Lauderdale DS, Palaniappan L. Diabetes screening and detection in an ambulatory clinical population. Proceedings of the 19th annual HMO Research Network Conference; 2013 April 16–18; San Francisco CA. [Google Scholar]
- 17.Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010;33(8):1872–94. doi: 10.2337/dc10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn R, Alperin P, Eddy D, et al. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet. 2010;375(9723):1365–74. doi: 10.1016/S0140-6736(09)62162-0. [DOI] [PubMed] [Google Scholar]
- 19.CDC. National Health Interview Survey. 2009 census.gov/compendia/statab/cats/health_nutrition/health_care_utilization.html.
- 20.Khunti K, Davies M. Should we screen for type 2 diabetes: yes. BMJ. 2012;345:e4514. doi: 10.1136/bmj.e4514. [DOI] [PubMed] [Google Scholar]
- 21.Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334(7588):299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shanks L, Klarkowski D, O’Brien DP. False positive HIV diagnoses in resource limited settings: operational lessons learned for HIV programmes. PLoS One. 2013;8(3):e59906. doi: 10.1371/journal.pone.0059906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013;1:CD004720. doi: 10.1002/14651858.CD004720.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerlikowske K, Zhu W, Hubbard RA, et al. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–16. doi: 10.1001/jamainternmed.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;(3S2):S211–S216. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 28.Simmons RK, Echouffo-Tcheugui JB, Griffin SJ. Screening for type 2 diabetes: an update of the evidence. Diabetes Obes Metab. 2010;12(10):838–44. doi: 10.1111/j.1463-1326.2010.01244.x. [DOI] [PubMed] [Google Scholar]
- 29.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 30.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. New Engl J Med. 2010;362(9):800–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackermann RT, Cheng YJ, Williamson DF, Gregg EW. Identifying adults at high risk for diabetes and cardiovascular disease using hemoglobin A1c National Health and Nutrition Examination Survey 2005–2006. Am J Prev Med. 2011;40(1):11–17. doi: 10.1016/j.amepre.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Brosnan CA, Upchurch S, Schreiner B. Type 2 diabetes in children and adolescents: an emerging disease. J Pediatr Health Care. 2001;15(4):187–93. doi: 10.1067/mph.2001.111250. [DOI] [PubMed] [Google Scholar]
- 33.Mayer-Davis EJ. Type 2 diabetes in youth: epidemiology and current research toward prevention and treatment. J Am Diet Assoc. 2008;108(4S1):S45–S51. doi: 10.1016/j.jada.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Pettitt DJ, Talton J, Dabelea D, et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37(2):402–8. doi: 10.2337/dc13-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a U.S. perspective. Diabetes Metab Res Rev. 2000;16(4):230–6. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]