Abstract
Ultrashort pulsed laser irradiation is a new method for virus reduction in pharmaceuticals and blood products. Current evidence suggests that ultrashort pulsed laser irradiation inactivates viruses through an impulsive stimulated Raman scattering process, resulting in aggregation of viral capsid proteins. However, the specific functional defect(s) in viruses inactivated in this manner have not been demonstrated. This information is critical for the optimization and the extension of this treatment platform to other applications. Toward this goal, we investigated whether viral internalization, replication, or gene expression in cells were altered by ultrashort pulsed laser irradiation. Murine Cytomegalovirus (MCMV), an enveloped DNA virus, was used as a model virus. Using electron and fluorescence microscopy, we found that laser-treated MCMV virions successfully internalized in cells, as evidenced by the detection of intracellular virions, which was confirmed by the detection of intracellular viral DNA via PCR. Although the viral DNA itself remained polymerase-amplifiable after laser treatment, no viral replication or gene expression was observed in cells infected with laser-treated virus. These results, along with evidence from previous studies, support a model whereby the laser treatment stabilizes the capsid, which inhibits capsid uncoating within cells. By targeting the mechanical properties of viral capsids, ultrashort pulsed laser treatment represents a unique potential strategy to overcome viral mutational escape, with implications for combatting emerging or drug-resistant pathogens.
Keywords: Ultrashort pulsed lasers, pathogen reduction, pathogen inactivation, murine cytomegalovirus
Introduction
Ultrashort pulsed (USP) lasers are an attractive, chemical-free method to inactivate pathogens in pharmaceuticals and blood products1. Visible USP laser irradiation is a unique strategy to overcome the limitations of conventional antiviral agents due to its physical mechanism of action – the excitation of molecular vibrations within viral capsids through impulsive stimulated Raman scattering (ISRS)2. This strategy targets fundamental mechanical properties of viruses that may be difficult to escape through genetic mutation. Additionally, visible light shows negligible intrinsic absorption by nucleic acids and proteins in the absence of chromophores. Furthermore, unlike ultraviolet (UV) or gamma radiation, visible light does not cause molecular ionization. These properties could enable the use of USP lasers to selectively inactivate pathogens without harming desired biological materials such as mammalian proteins in blood products. Visible USP lasers have shown broad spectrum efficacy against both DNA and RNA viruses2–9, including non-enveloped viruses that are conventionally difficult to inactivate. Under these laser treatment conditions, the structure of a mammalian protein was well preserved7.
A previous study demonstrated the effects of visible USP laser irradiation on the molecular structure of Murine Cytomegalovirus (MCMV), an enveloped DNA virus9. This work showed that USP laser treatment causes aggregation of viral capsid proteins. It was proposed that the laser treatment, through ISRS, transiently induces partial unfolding of tertiary protein structures leading to density-dependent protein aggregation through exposed hydrophobic regions of proteins (Figure 1). Under this model, the high density capsid proteins of virions are more susceptible to laser-induced aggregation relative to free proteins in solution. The study demonstrated that the laser-induced aggregation effect increased with the concentration of a purified protein9.
Figure 1. Proposed model for USP laser-induced protein aggregation.

USP laser light scattering leads to transient, partial unfolding of protein structures, due to disruption of electrostatic interactions through ISRS. In cases where the protein is at high density, aggregation will occur between exposed hydrophobic patches on nearby proteins. In cases where the protein is at low density, the proteins rapidly recover by regaining their native conformation. Filled spheres indicate hydrophilic regions of the protein; hollow spheres indicate hydrophobic regions of the protein.
To date, however, functional studies of USP laser-treated viruses in cells are lacking. These data are needed to establish the molecular and functional basis for the abovementioned mechanistic hypotheses. In addition, such data should prove valuable for the optimization of the treatment process and for the extension of the technology to other applications. To this end, we have investigated the effects of visible USP laser treatment on the viral trafficking and functional state in cells.
Methods
Cells and viruses
MEF 10.1 murine embryonic fibroblast cells10 were cultured in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% fetal calf serum, 1mM sodium pyruvate, and nonessential amino acids. Balb/3T3 murine embryonic fibroblast cells were cultured in RPMI medium, supplemented with 10% fetal calf serum and antibiotics. GFP-expressing MCMV virus (hereafter referred to as “MCMV”) was generated as previously described11,12. To produce viral stocks, MEF 10.1 cells were infected with MCMV at a low multiplicity of infection. Cell supernatants were harvested 5 d post-infection after 100% cytopathic effect and cleared of cell debris by centrifugation. Extracellular virions were pelleted by ultracentrifugation with sorbitol cushion and resuspended in phosphate-buffered saline (PBS). Viral titers were determined using a median tissue culture infectious dose (TCID50) assay, as described below.
Femtosecond laser irradiation
The excitation source employed in this work was a diode-pumped continuous wave mode-locked Ti-sapphire laser. The laser produced a continuous train of 60 fs pulses at a repetition rate of 80 MHz. The output of the second harmonic generation (SHG) system of the Ti-sapphire laser was used to irradiate the sample. The excitation laser was chosen to operate at a wavelength of λ = 425 nm and with an average power of approximately 120 mW. It has a pulse width of full-width at half maximum = 100 fs. A lens was used to focus the laser beam into a spot within the sample volume. MCMV virus was irradiated at a final concentration of about 5×106 TCID50/ml. A magnetic stirring device was used to facilitate exposure of the sample to the laser beam. Irradiation was carried out at 22°C and with the single laser beam excitation. After laser irradiation, samples were immediately stored at −80°C.
TCID50 assays
TCID50 assays were performed to determine reduction in viral titers following laser irradiation. MEF 10.1 cells were seeded into 96 well plates at a density of 6×104 cells/mL and incubated overnight. Cells were approximately 100% confluent at the time of infection. Control (untreated) or laser-treated viruses were serially diluted and added to cells, which were incubated for 4 days. Viral titers were determined on day 4 post-infection by scoring each well for GFP-positive cells using a fluorescent microscope.
Fluorescence imaging
For tracking of viral internalization, laser-treated or control MCMV virions were labeled with PKH26 dye (Sigma) according to the manufacturer’s instructions. Balb/3T3 cells were infected with PKH26-labeled MCMV at a multiplicity of infection (MOI) of ~ 100 TCID50/cell for 2 h, washed three times in PBS, and fixed with Vectashield mounting medium with DAPI (Vector Laboratories, Inc). For the time course imaging of viral GFP expression, Balb/3T3 cells were infected with control or laser-treated MCMV at an MOI of ~100 TCID50/cell and imaged at 24 h, 48 h, and 72 h post-infection. Samples were visualized with a Zeiss Axioskop 2 Mot Plus fluorescence microscope equipped with an Axiocam MRm monochrome camera and a 10X, 0.3 numerical aperture Zeiss Plan Neo-Fluar objective or a 63X, 1.4 numerical aperature Zeiss Plan Apochromat oil objective. Images were acquired using Axiovision 4.6 software (Carl Zeiss Inc., Thornwood, NY).
Electron microscopy
MEF 10.1 cells were infected with control or laser-treated MCMV at an MOI of ~20 TCID50/cell for 2 h. For ultrastructural analysis, infected cells were fixed in 2% paraformaldehyde/2.5% glutaraldehyde (Polysciences Inc., Warrington, PA) in 100 mM cacodylate buffer, pH 7.2 for 1 h at room temperature. Samples were washed in cacodylate buffer and postfixed in 1% osmium tetroxide (Polysciences Inc.)/1.5% potassium ferricyanide (Sigma, St Louis, MO) for 1 h. Samples were then rinsed extensively in dH20 prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 h. Following several rinses in dH20, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc). Sections of 95 nm were cut with a Leica Ultracut UCT ultramicrotome (Leica Microsystems Inc., Bannockburn, IL), stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA Inc., Peabody, MA) equipped with an AMT 8 megapixel digital camera (Advanced Microscopy Techniques, Woburn, MA).
PCR
Intracellular viral DNA within MCMV-infected cells and virion-associated DNA were quantified by PCR amplification followed by agarose gel analysis. For detection of intracellular viral DNA, cells were infected with either control or laser-treated MCMV for 18 h. Cells were then washed in PBS, trypsinized, washed again in PBS, pelleted by centrifugation, and lysed. Cell lysates were used directly as PCR template. For detection of virion-associated DNA, virions were mixed in lysis buffer and either pre-digested with proteinase K for 2 h at 37°C or used directly as PCR template. In all PCR experiments, a primer pair specific for the MCMV IE1 gene was used11 (forward CAGGGTGGATCATGAAGCCT, reverse AGCGCATCGAAAGACAACG), which yields a 300-bp product. Gels were stained with SYBR Safe Green reagent (Invitrogen) and visualized with a Kodak Multispectral Imaging System (Eastman Kodak).
Western blotting analysis
For detection of IE1 protein in MCMV-infected samples, MEF 10.1 cells were either mock-infected or infected with control or laser-treated MCMV at an MOI of ~20 TCID50/cell for 4 h, 12 h, or 24 h. Samples were lysed, vortexed, and boiled for 5 min. Cell lysates were separated by SDS-PAGE and transferred onto nitrocellulose filters. Filters were incubated with anti-MCMV IE1 monoclonal antibody (CROMA101) (a generous gift from Stipan Jonjic, University of Rijeka, Croatia) or anti-mouse actin monoclonal antibody (Sigma). As a secondary antibody, horseradish peroxidase-conjugated anti-mouse IgG (Sigma) was used. Blots were developed by autoradiography.
Results
USP laser-treated virus is internalized by host cells
For all experiments, we used a GFP-expressing MCMV described in a previous report9,12. Samples of MCMV were irradiated using a 425nm femtosecond laser. Laser-inactivated virus showed an approximate 5-log reduction in viral titers by TCID50 assay, to a level near the limit of detection (Supplementary Figure 1). To determine whether virions were internalized by host cells, we used electron microscopy to view viral particles within murine embryonic fibroblast cells that had been infected with control (untreated) MCMV or USP laser-treated MCMV for 2 h. In both control and laser-treated groups, we observed the presence of MCMV virions within cells (Figure 2). To confirm these findings, we labeled control or laser-treated MCMV with a fluorescent dye, PKH26. Murine fibroblast cells were infected with PKH26-labeled control or laser-treated MCMV for 2 h before imaging by fluorescence microscopy. In both control and laser-treated groups, we found fluorescent virus particles within cells (Figure 3). These results were further confirmed by PCR detection of MCMV genomic DNA in cells infected with both control and laser-treated MCMV (Figure 4). Therefore, laser treatment does not alter the ability of MCMV virions to be internalized by cells.
Figure 2. Electron microscopy shows cellular internalization of USP laser-treated MCMV.
Control or laser-treated MCMV were infected into murine embryonic fibroblast cells, and the cells were harvested, fixed, and sectioned for imaging. Images show cellular internalization of control MCMV or USP laser-treated MCMV at 2h post-infection. Arrows indicate intracellular virions.
Figure 3. Fluorescence microscopy shows cellular internalization of USP laser-treated MCMV.

PKH26-labeled control or laser-treated MCMV were infected into murine embryonic fibroblast cells. Images show combined bright field and fluorescence depicting cellular internalization of control MCMV or USP laser-treated MCMV at 2h post-infection.
Figure 4. MCMV DNA is present in cells infected with USP laser-treated MCMV.

Murine embryonic fibroblast cells were infected with control or laser-treated MCMV. PCR analysis was performed at 18h post-infection with primers specific for the MCMV IE1 gene. M: molecular weight DNA ladder; 1: cells infected with control MCMV; 2: cells infected with laser-treated MCMV.
USP laser-treated virus cannot express virus-encoded genes in cells
We used GFP-expressing MCMV as an indicator of viral DNA replication in host cells. MCMV replicates and produces progeny virus over the course of 18–36 h post-infection13,14. To assess the replication of MCMV in host cells, murine fibroblast cells were infected with either control or laser-treated MCMV and imaged for GFP fluorescence at 24, 48, and 72h post-infection (Figure 5). In cells infected with control MCMV, we observed strong GFP signal in the majority of cells at 24, 48, and 72 h (in addition to cytopathic effect). In contrast, cells infected with laser-treated MCMV did not exhibit observable GFP signal even after 72 h post-infection. This result demonstrates that laser-treated MCMV virus does not replicate when infected into cells.
Figure 5. USP laser-treated MCMV cannot replicate in cells.
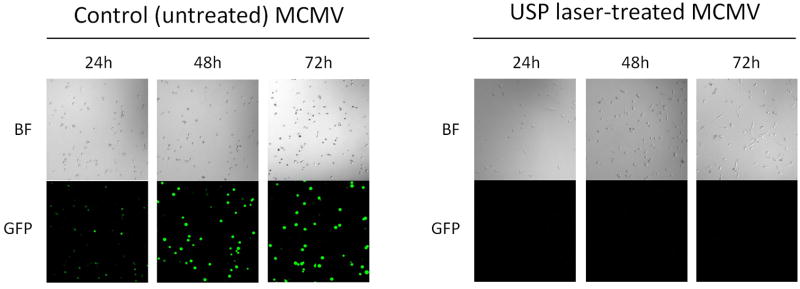
Control GFP-expressing MCMV or laser-treated GFP-expressing MCMV virus were infected into murine embryonic fibroblast cells and imaged for GFP expression 24, 48, and 72h post-infection using fluorescence microscopy. Images show GFP expression in cells infected with either control MCMV or laser-treated MCMV at 24, 48, and 72h post-infection. BF, bright field; GFP, green fluorescent protein.
To further confirm the effect of USP laser treatment on viral gene expression, we assessed the expression of the MCMV immediate early 1 (IE1) protein within infected cells, which is one of the initial viral proteins expressed by the cell upon MCMV infection13,14. We infected murine fibroblast cells with control or laser-treated MCMV virions for 4 h, 12 h, or 24 h, and the cell lysates were then immunoblotted for IE1 (Figure 6). We observed expression of IE1 protein in cells infected with control MCMV at all three time points tested. In contrast, we did not detect any IE1 protein in cells infected with laser-treated MCMV. Since IE1 is an important gene that activates downstream virus transcription, these results suggest that many MCMV genes are likely not expressed during infection with laser-treated virus.
Figure 6. IE1 protein is absent in cells infected with USP laser-treated MCMV.

Murine embryonic fibroblasts were either mock-infected or infected with control (untreated) MCMV or laser-treated MCMV. At the time points after infection indicated, cells were harvested and lysed, subjected to SDS-PAGE electrophoresis analysis, and immunoblotted with anti-IE1 or anti-actin monoclonal antibodies.
Based on our observations, there was the possibility that the USP laser treatment may be damaging the MCMV genomic DNA. However, this effect is unlikely because USP laser treatment at 425 nm lacks sufficient energy to disrupt covalent bonds in DNA. A previous study also failed to detect any effect of USP laser treatment on MCMV DNA9. To provide further evidence that USP laser treatment does not damage MCMV genomic DNA, we showed that the MCMV IE1 gene can be PCR-amplified from both control and laser-treated MCMV virions (Figure 7). Therefore, the MCMV DNA remains sufficiently intact to be polymerase-amplifiable (i.e., it can still be utilized as a template for DNA polymerases) after laser treatment. These results indicate that in the context of cellular infection, the functional defect of the laser-treated virus lies upstream of viral gene expression.
Figure 7. DNA from USP laser-treated MCMV virions is PCR-amplifiable.

Control or laser-treated MCMV virions were subjected to PCR analysis with primers specific for the MCMV IE1 gene. M: molecular weight DNA marker; 1: control MCMV; 2: laser-treated MCMV; 3: control MCMV pre-digested with proteinase K; 4: laser-treated MCMV pre-digested with proteinase K.
Discussion
In a previous study, USP laser irradiation was shown to cause the aggregation of MCMV capsid proteins, resulting in stabilization of the viral capsid against detergent dissociation9. In this report, we found indirect evidence for a functional defect in capsid function of USP laser-treated virions. Our data indicate that the defect in USP laser-treated virus occurs downstream of cell internalization but upstream of viral replication/gene expression. The results support a model whereby the USP laser treatment aggregates the viral capsid, thus inhibiting intracellular capsid uncoating and preventing the viral DNA from undergoing replication or transcription in the host cell nucleus. The precise mechanisms governing capsid uncoating in MCMV are poorly understood, thereby hampering more detailed analyses of the behavior of USP laser-treated capsids in cells. A likely scenario is one whereby the virus is defective in either intracellular capsid uncoating or translocation. The previous report on USP laser-induced aggregation of viral capsid proteins9 suggests that the laser may stabilize the viral capsid, preventing it from disassembling properly within cells and thus “trapping” viral DNA so that it is unable to be replicated or transcribed in the nucleus (Figure 6). Based on the available evidence, this model provides the most plausible functional explanation for the USP laser inactivation of viruses. It is worth noting that in certain herpes viruses, the minor capsid protein UL25 is required for viral DNA release at the nuclear pore15. A previous study showed that MCMV UL25 was among the proteins that were affected in USP laser- treated MCMV virions9. Therefore, in addition to the resistance of aggregated capsid complexes to dissociation, laser-induced damage to UL25 might also contribute to impaired capsid uncoating.
While many physical methods such as ultraviolet (UV) light and gamma radiation cause covalent damage to proteins and nucleic acids, visible USP laser irradiation lacks the energy to perturb covalent structures in viruses. Instead, the USP laser treatment relies on disruption of noncovalent electrostatic interactions within and between proteins, leading to density-dependent aggregation of capsid proteins through ISRS to inactivate viruses9. Unlike heating (which causes widespread denaturing of proteins), the postulated USP laser mechanism is expected to damage proteins in high-density microenvironments while leaving other proteins relatively unaffected. We note that both heat and UV treatment lead to extensive denaturation of proteins, and therefore these methods cannot be used for sterilization of biologicals. On the other hand, we have demonstrated that the USP laser technique preserves the structure of proteins7. Therefore, in contrast to heat and UV techniques, the USP laser method is novel and has applications in pathogen reduction of blood products and other biologicals.
We note that at this time the precise mechanism of MCMV entry into cells, including critical receptor(s) involved, is not entirely clear. Therefore, a receptor blocking study was not feasible. However, we have previously shown that the structure of proteins such as bovine serum albumin is retained after laser treatment7. Along with these data, the cellular internalization studies in this report support a model in which laser treatment does not alter the structure of viral envelope proteins, and thus does not inhibit viral entry.
The results of this study also indicate a potential application of the USP laser method in vaccine production. The data suggest that USP laser-inactivated viruses retain their ability to enter cells by the normal infection route. If this is the case, then a USP laser-inactivated virus vaccine might be capable of eliciting the strong immune responses normally associated with live vaccines, but with the safety profile of a killed vaccine. Furthermore, as USP laser treatment preserves the structure of proteins, a vaccine prepared by USP laser treatment may lead to improved antibody responses compared to vaccines treated with heat or UV, which cause extensive protein damage.
Specific targeting of viral capsids through ISRS would make the USP laser technology unique among physical pathogen inactivation methods. From an evolutionary standpoint it is difficult to envision how viruses could alter fundamental aspects of their structure, namely the capsid protein density and/or vibrational frequencies of their capsids, through genetic mutation. Therefore, the USP laser method may be effective against newly emerging or rapidly mutating viral pathogens. In addition, the capsid is a universal feature of viruses. This would explain the broad spectrum virus inactivation which has been observed for USP lasers2–9. Finally, USP laser irradiation may be an effective way to generate whole virus vaccines with preserved antigenic/immunogenic structures.
To date, the functional aspects of USP laser-inactivated viruses have not been fully explored. The data presented in this manuscript are critical for the optimization and eventual clinical translation of the technology. In addition, the functional studies in this report have yielded considerable insight toward the possible application of the laser technology towards vaccine development. Therefore, virus inactivation by USP lasers represents a unique and significant new field of study that warrants further investigation.
Conclusions
We reveal evidence for a functional capsid defect in USP laser-inactivated virus. This information will be indispensable for the translation of this platform technology toward clinical applications. Development of this USP laser technology for pathogen reduction of pharmaceuticals, antiviral therapies, and viral vaccine development is warranted.
Supplementary Material
Viral titers of control or laser-treated MCMV were assessed by TCID50 assay. Results are representative of duplicate experiments and error bars indicate SEM.
Figure 8. Proposed model for USP laser-induced viral capsid defect.

USP laser treatment causes aggregation of viral capsid proteins9. The laser-treated virus is internalized by cells, but the aggregated viral capsid cannot uncoat and thus the viral genome remains “trapped” and cannot replicate or express viral genes.
Highlights.
We reveal evidence supporting a functional capsid defect in viruses inactivated by ultrashort pulsed laser treatment.
USP laser targeting of capsids is a unique mechanism with advantages against rapidly mutating or drug-resistant pathogens.
USP laser treatment has applications in pathogen reduction, antiviral treatments, and vaccine development.
Acknowledgments
This work was supported in part by the Mallinckrodt Institute of Radiology Development Fund, NIH grants R01 EB008111, R33 CA123537 (SA), NHLBI Ruth L. Kirschstein NRSA F30 grant HL116183-01 (SDT), and Public Health Service grant R01CA120768 (DY).
Glossary
- ISRS
impulsive stimulated Raman scattering
- USP
ultrashort pulsed laser
Footnotes
Authorship Contributions
SDT conceived of the study, participated in design and execution of experiments, and drafted the manuscript. TC assisted with TCID50 assays and in vitro infection analysis. WB assisted in electron microscopic analysis. BX assisted with western blot analysis. KTT provided technical assistance in laser irradiation. SA helped conceive of the study, participated in design and coordination, and helped draft the manuscript. All authors read and approved of the final manuscript.
Disclosure of Conflicts of Interest
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsen SW, Wu TC, Kiang JG, Tsen KT. Prospects for a novel ultrashort pulsed laser technology for pathogen inactivation. Journal of biomedical science. 2012;19:62. doi: 10.1186/1423-0127-19-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsen KT, et al. Inactivation of viruses by laser-driven coherent excitations via impulsive stimulated Raman scattering process. Journal of biomedical optics. 2007;12:064030. doi: 10.1117/1.2821713. [DOI] [PubMed] [Google Scholar]
- 3.Tsen K, et al. Inactivation of viruses with a very low power visible femtosecond laser. Journal of Physics: Condensed Matter. 2007;19:322102. doi: 10.1186/1743-422X-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsen K, Tsen SWD, Hung CF, Wu T, Kiang JG. Selective inactivation of human immunodeficiency virus with subpicosecond near-infrared laser pulses. Journal of Physics: Condensed Matter. 2008;20:252205. [Google Scholar]
- 5.Tsen KT, et al. Inactivation of viruses by coherent excitations with a low power visible femtosecond laser. Virology journal. 2007;4:50. doi: 10.1186/1743-422X-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsen KT, et al. Photonic approach to the selective inactivation of viruses with a near-infrared subpicosecond fiber laser. Journal of biomedical optics. 2009;14:064042. doi: 10.1117/1.3275477. [DOI] [PubMed] [Google Scholar]
- 7.Tsen KT, et al. Studies of inactivation of encephalomyocarditis virus, M13 bacteriophage, and Salmonella typhimurium by using a visible femtosecond laser: insight into the possible inactivation mechanisms. Journal of biomedical optics. 2011;16:078003. doi: 10.1117/1.3600771. [DOI] [PubMed] [Google Scholar]
- 8.Tsen KT, Tsen SWD, Sankey OF, Kiang JG. Selective inactivation of micro-organisms with near-infrared femtosecond laser pulses. Journal of Physics: Condensed Matter. 2007;19:472201. [Google Scholar]
- 9.Tsen SW, et al. Inactivation of enveloped virus by laser-driven protein aggregation. Journal of biomedical optics. 2012;17:128002. doi: 10.1117/1.JBO.17.12.128002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey DM, Levine AJ. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes & development. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 11.Qian Z, Xuan B, Chapa TJ, Gualberto N, Yu D. Murine cytomegalovirus targets transcription factor ATF4 to exploit the unfolded-protein response. Journal of virology. 2012;86:6712–6723. doi: 10.1128/JVI.00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapa TJ, et al. Murine cytomegalovirus protein pM79 is a key regulator for viral late transcription. Journal of virology. 2013;87:9135–9147. doi: 10.1128/JVI.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shellan GR, Redwood AJ, Smith LM, Gorman S. The mouse in biomedical research. Academic Press; 2006. [Google Scholar]
- 14.Britt WJ, Alford CA. Fields Virology. Lippincott-Raven; 1996. pp. 2493–2523. [Google Scholar]
- 15.Preston VG, Murray J, Preston CM, McDougall IM, Stow ND. The UL25 gene product of herpes simplex virus type 1 is involved in uncoating of the viral genome. Journal of virology. 2008;82:6654–6666. doi: 10.1128/JVI.00257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Viral titers of control or laser-treated MCMV were assessed by TCID50 assay. Results are representative of duplicate experiments and error bars indicate SEM.



