Abstract
Three hundred and fifty million people worldwide are estimated to be chronically infected with hepatitis B virus. 15%-40% of these subjects will develop cirrhosis, liver failure or hepatocellular carcinoma during their life. The treatment of chronic hepatitis B has improved dramatically over the last decade merits to the advent of nucleoside/nucleotide analogues and the use of pegylated interferons. Approved drugs for chronic hepatitis B treatment include: standard interferon-alpha 2b, pegylated interferon-alpha 2a, lamivudine, adefovir dipivoxil, and entecavir. Unfortunately, these agents are not effective in all patients and are associated with distinct side effects. Interferons have numerous side effects and nucleoside or nucleotide analogues, which are well tolerated, need to be used for prolonged periods, even indefinitely. However, prolonged treatment with nucleoside or nucleotide analogues is associated with a high rate of resistance. Telbivudine is a novel, orally administered nucleoside analogue for use in the treatment of chronic hepatitis B. In contrast to other nucleoside analogues, Telbivudine has not been associated with inhibition of mammalian DNA polymerase with mitochondrial toxicity. Telbivudine has demonstrated potent activity against hepatitis B with a significantly higher rate of response and superior viral suppression compared with lamivudine, the standard treatment. Telbivudine has been generally well tolerated, with a low adverse effect profile, and at its effective dose, no dose-limiting toxicity has been observed. Telbivudine is one of the most potent antiviral agents for chronic hepatitis B virus and was approved by the FDA in late 2006.
Keywords: Telbivudine, Chronic hepatitis B, Hepatitis B virus, Nucleoside analogue, Antiviral agents, Pegylated interferons, Lamivudine, Adefovir dipivoxil, Entecavir
INTRODUCTION
Hepatitis B virus (HBV) infection is a significant health problem worldwide. Of the 6 billion worldwide populations, an estimated 2 billion have been infected by HBV[1]. It is estimated that 350-400 million people have chronic hepatitis B (CHB) infection[2]. There is clear epidemiologic evidence that chronic HBV infection can result in the development of hepatocellular carcinoma (HCC) and cirrhosis[3,4]. Approximately 15%-40% of HBV carriers develop cirrhosis, liver failure, and HCC; worldwide, more than 50% of primary HCC is related to chronic HBV infection[5]. Each year, 500 000 deaths are expected because of complications related to hepatitis B[6].
EPIDEMIOLOGY IN INDIA
In India nearly 3%-4% of the population is infected by the virus, and chronic hepatitis B constitutes more than 50% of the chronic hepatitis cases in the country[7]. The prevalence ranges from 1.1% to 12.2% with maximum incidence in Madhya Pradesh, Arunachal Pradesh and South India and least in Kashmir and Kerala[8]. There is a peak prevalence after the second decade of life. Most (90%) of these HBV infected subjects are HBeAg negative; the majority (80%) have normal ALT[9]. The prevalence of HBeAg among asymptomatic HBsAg positive persons varies from 9%-20%[9,10].
This, in the context of a large population and absence of a national immunization program would spell off a projected increasing burden of infection and liver disease due to HBV in this country in the years to come. In this perspective, the HBV epidemiology in India becomes relevant not only nationally, but also internationally, because of the possibility that India may soon have the largest HBV infection pool in the world. Economic burden is high and management is affected by cost and availability of diagnostic modalities and patients’ awareness and compliance of various treatment options available. Though there have been several guidelines published by various organizations including the Indian association of Study of Liver, the management of HBV infection varies widely in the country. Many physicians in India still find it difficult to make satisfactory management decisions[11].
COST EFFECTIVENESS OF TREATMENT
The main obstacle to treatment in developing countries like India is the expenditure of drug therapy. Cost effective studies have shown savings in countries with intermediate or low endemicity[11]. In India cost of treatment with oral drugs like lamivudine and adefovir range from Rs. 3000 to Rs. 7000/year, a drastic reduction in cost compared to subcutaneous therapy. Additionally the orally used drugs are associated with a good response rate, excellent safety profile and making the overall treatment with oral drugs cost effective. However, the main limitation of these drugs is the emergence of drug-resistant viral strains during the course of treatment.
HBV INFECTION - NATURAL HISTORY
The natural history of CHB is complex and understanding it is important for the selection of patients for treatment. Infected individuals can go through four phases of infection: the immune tolerance phase, the immune reaction or clearance phase (HBeAg-positive, chronic HBV), the inactive carrier (low replication) phase, and the reactivation phase (HBeAg-negative chronic HBV)[1].
In the natural history of HBV infection, the most important event is HBeAg seroconversion characterized by loss of HBeAg and development of antibody to HBeAg (Anti HBe)[12]. The prognosis of chronic HBV infection is dependent upon the amount of inflammation, necrosis and fibrosis in the liver at this point of seroconversion. If significant liver damage is already present at this point, then the prognosis after seroconversion, spontaneous or treatment related is unlikely to be good, despite suppression of viral replication. On the other hand, if the seroconversion has occurred early and is maintained, then the long-term prognosis is excellent. In a subset of persons, this relationship between seroconversion and suppression of viral replication does not hold true. In them, despite anti-HBe positivity, active viral replication persists due to the emergence of mutants in the ‘precore’ and basal core promoter regions of HBV. This state, characterized by continuing viral replication despite anti HBe positivity has been termed as HBeAg negative CHB[13-15]. Compared with HBeAg-positive CHB, HBeAg negative CHB can follow an aggressive course, requires long or even infinite treatment, and leads to the rapid development of cirrhosis and HCC.
Goals of treatment
The ultimate goal of treatment is the eradication of HBV before it causes irreversible damage including cirrhosis and/or HCC. The eradication of HBV is impossible with currently approved drugs. This is because of extrahepatic reservoirs of HBV, integration of HBV DNA into host DNA, and the presence of covalently closed circular DNA (cccDNA) in the hepatocyte nucleus. Such cccDNA serves as a transcriptional template for HBV replication without the need for reinfection[16,17]. Current antiviral agents have little inhibitory effect on cccDNA, leading to high relapse rates after discontinuation of treatment.
The more realistic goals of therapy are early and prolonged viral suppression, remission of chronic liver disease, a decreased rate of cirrhosis, liver failure, HCC, and reduced morbidity and mortality.
TREATMENT OF CHB
The treatment of CHB has undergone tremendous change and continues to evolve with the advent of potent antiviral agents. The FDA currently approves interferon alpha-2b, pegylated interferon alpha-2a (PEG-IFN-α2a), and four oral agents, adefovir dipivoxil, entecavir, lamivudine and telbivudine as monotherapeutic agents[18].
IFN-α was the first FDA-approved medication for the treatment of CHB. Polyethylene glycol, an inert water soluble molecule when attached to standard interferon to form pegylated interferon decreases the clearance and antigenicity of interferon, thus extending and sustaining its activity in vivo allowing for once-weekly administration.
IFN-α and PEG-IFN-α2a have significant side effects, require injection therapy and are less efficacious in patients acquiring CHB during early childhood, for example Asian patients. Furthermore IFN treatment fails to decrease the chance of development of cirrhosis-related complications and HCC[19].
Lamivudine, an oral nucleoside analogue, was the second FDA-approved medication for the treatment of CHB. Viral breakthrough remains a problem with lamivudine with incidence of resistance ranging from 16%-32% after 1 year of therapy to as high as 58% with 2-3 years of therapy[20]. Furthermore, disease progression has been shown to resume following viral breakthrough.
Adefovir dipivoxil, an oral nucleotide analogue, was approved by the FDA in September 2003. Although resistance is less frequent with adefovir compared to lamivudine, this agent may be restricted by nephrotoxicity and a relatively modest potency[18].
Entecavir, a deoxyguanosine nucleoside analog, has recently been licensed by the FDA. Early trials have shown it to be more potent than lamivudine. However, care must be taken when using entecavir in individuals with renal dysfunction[18].
The newest antiviral nucleoside analogues like telbivudine was approved by the FDA based on the results of a Phase III clinical trial for the treatment of CHB. The aim of this review article is to introduce and review current information on telbivudine for the treatment of CHB.
TELBIVUDINE
Telbivudine (LdT) is a novel agent for the treatment of CHB. It is an HBV-specific L-nucleoside analogue of thymidine. The chemical name of telbivudine is β-L-2-deoxythymidine (LdT). Telbivudine is an unsubstituted, unmodified β-L-2-nucleoside and the first compound of this series.
Mechanism of action
Telbivudine must be activated by phosphorylation and is efficiently metabolized to 5-triphosphate derivative. 5-triphosphate metabolite of β-L-2-deoxynucleosides interacts with the viral polymerase and inhibits viral replication and results in obligate chain termination of DNA synthesis[21]. This inhibition occurs mainly in the synthesis of the second strand of DNA (DNA-to-DNA transcription) for telbivudine (in contrast to lamivudine which strongly inhibited first strand DNA synthesis; RNA-to-DNA reverse transcription). Since transcription fidelity is higher in DNA-to-DNA synthesis than RNA-to-DNA synthesis, telbivudine treatment may have a slower rate of emergence of drug-resistant virus when compared to lamivudine[21].
Preclinical studies
There is no demonstrable toxic effect on human DNA polymerases α, β and γ by telbivudine after phospho-rylation in the experiments using hepatoma cell lines, primary human peripheral blood monocyte cells and human foreskin fibroblasts and other cell types of mammalian origin[21].
In preclinical studies, telbivudine was investigated in rats and monkeys at concentrations substantially greater than the anticipated dose in humans. No significant toxic effects were observed in animal models, suggesting a minimal risk of cumulative, carcinogenic or reproductive toxicity in humans[22].
Pharmacokinetics
A phase I/II a dose-escalation study in HBV-infected patients showed that telbivudine is rapidly absorbed with the peak concentration reached within approximately 1-3 h. The plasma concentration of telbivudine increased proportionally with increasing doses in the 25-800 mg/d range studied. There were no serious adverse events in all the subjects either receiving telbivudine or placebo[23].
Systemic telbivudine is predominantly cleared unchanged by the kidneys with minimal metabolism and elimination via the hepatic route. Zhou et al[24] studied the pharmacokinetic profile of telbivudine administered to patients with moderate to severe renal impairment and hepatic impairment with regards to peak concentration and overall drug exposure [area under the curve (AUC)]. In patients with renal impairment, the AUC was two to three-fold higher in subjects with moderate renal impairment when dosing was normalized to 600 mg/d, suggesting that telbivudine dosage needs to be adjusted in renally impaired patients preferably by reducing the daily dose. Pharmacokinetic profiles were comparable in subjects with normal and impaired hepatic function. The pharmacokinetics are not altered by the food intake[24].
CLINICAL STUDIES
Dose ranging study
The excellent results achieved in the early human PK and safety studies led to the phase I/II clinical trial in patients with CHB[25]. In this first clinical study of telbivudine, safety, antiviral activity, and pharmacokinetics were assessed in 43 adults with hepatitis Be antigen-positive chronic hepatitis B. This placebo-controlled dose-escalation trial investigated 6 telbivudine daily dosing levels (25, 50, 100, 200, 400, and 800 mg/d); treatment was given for 4 wk, with a 12 wk follow-up. Serum HBV DNA levels were monitored via quantitative polymerase chain reaction (PCR). The results indicate that telbivudine was well tolerated at all dosing levels, with no dose-related or treatment-related clinical or laboratory adverse events. Telbivudine plasma pharmacokinetics was dose-proportional within the studied dose range. Marked dose-related antiviral activity was evident, with a maximum of telbivudine doses of 400 mg/d or more. In the 800 mg/d cohort, the mean HBV DNA reduction was 3.75 log10 copies/mL at wk 4, comprising a 99.98% reduction in serum viral load. Correspondingly, post treatment return of viral load was slowest in the high-dose groups[25].
Phase IIb studies
Owing to the encouraging results in the phase I/II study, a multicenter, international phase IIb trial was initiated. This randomized, double-blind trial evaluated the efficacy and safety of telbivudine 400 or 600 mg/d and telbivudine 400 or 600 mg/d plus lamivudine 100 mg/d (Comb400 and Comb600) compared with lamivudine 100 mg/d in hepatitis Be antigen (HBeAg)-positive adults with compensated chronic hepatitis B[26].
A total of 104 patients were randomized 1:1:1:1:1 among the 5 groups. Median reductions in serum hepatitis B virus (HBV) DNA levels at wk 52 (log10 copies/mL) are shown in Figure 1.
Figure 1.
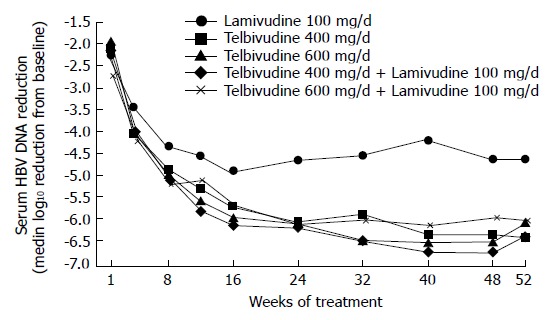
Median reductions in serum hepatitis B virus (HBV) DNA levels at wk 52 (log10 copies/mL) in all treatment groups.
At wk 52, telbivudine monotherapy showed a significantly (P < 0.05 for each comparison) greater mean reduction in HBV DNA levels (Figure 1), clearance of polymerase chain reaction-detectable HBV DNA, and normalization of alanine aminotransferase (ALT) levels compared with lamivudine monotherapy, with proportionally greater HBeAg seroconversion and less viral breakthrough (Figure 2). Combination treatment was not better than telbivudine alone. All treatments were well tolerated[26]. This study also examined the prognostic significance of the magnitude of HBV DNA reduction at wk 24 during therapy. For patients with HBV DNA less than 3 log at wk 24 none developed viral breakthrough at wk 52. More importantly 100% of these patients had undetectable HBV DNA by PCR assay at wk 52. In addition, these patients had a higher rate of loss of HBeAg and higher chances of ALT normalization at wk 52. These important findings emphasize that treatment should target a rapid and maximal suppression of viral replication[26].
Figure 2.
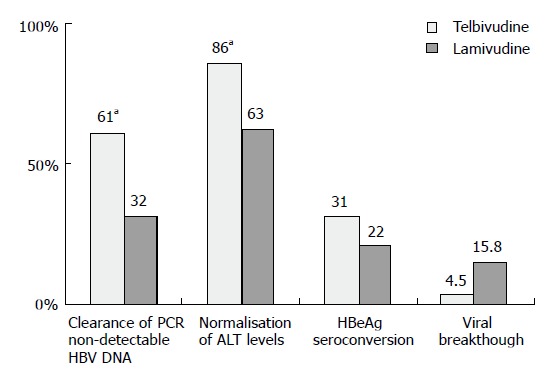
Comparison of telbivudine and lamivudine monotherapy. aP < 0.05.
Phase III studies
Efficacy vs lamivudine: Globe is a phase III, double-blinded, randomized, international, multicenter clinical trial designed to compare telbivudine vs lamivudine in over 1369 individuals with CHB over a 2 years period[18].
Individuals entered into the study were screened for HBeAg positivity, and required HBV DNA more than 6 log10 copies/mL by Roche COBAS® Amplicor PCR assay, an ALT greater or equal to 1.3-10 times the upper limit of normal and compensated liver disease. They were stratified for HBeAg status (positive or negative) and ALT value (less or greater than 2.5 times the upper limit of normal). Individuals were randomized to receive 2 years of either: (1) Lamivudine 100 mg qd or (2) Telbivudine 600 mg qd.
The primary end-point of Globe was ‘Therapeutic Response’, a composite serological end-point comprising suppression of serum HBV DNA to below 5 log10 copies/mL.
Secondary end-points included reduction in serum HBV DNA, normalization of serum ALT, HBeAg loss, seroconversion, and safety[18].
At 1 year[18], a significant reduction in HBV DNA in the telbivudine exposed individuals compared with lamivudine was observed, there being a greater HBV clearance to PCR non-detectable levels in this group also (Table 1). Telbivudine was associated with fewer flares of serum ALT levels when compared to lamivudine. On reviewing histological findings, 65% of HBeAg positive individuals exposed to telbivudine had a significant improvement in histology vs 56% of those exposed to lamivudine (P < 0.01)[18].
Table 1.
Efficacy at 1 yr in HBeAg positive and HBeAg negative individuals in the Globe study receiving telbivudine or lamivudine
| Telbivudine | Lamivudine | P value | |
| HBeAg positive patients | |||
| HBV DNA fall (mean log10) | -6.5 | -5.5 | < 0.01 |
| HBV DNA non-detectable by PCR (%) | 60 | 40 | < 0.01 |
| Treatment failure (%)1 | 5 | 13 | < 0.01 |
| HBeAg negative patients | |||
| HBV DNA fall (mean log10) | -5.2 | -4.4 | < 0.01 |
| HBV DNA non-detectable by PCR (%) | 88 | 71 | < 0.01 |
| Treatment failure (%)1 | < 1 | 3 | NS |
Defined as HBV DNA > 5 log10 copies/mL.
For all clinical and virological efficacy parameters, efficacy at 1 year was proportional to HBV DNA level at wk 24 (Table 2). Achieving HBV DNA < 300 copies/mL at wk 24 with lamivudine or telbivudine was highly predictive of not developing resistance at wk 52. Overall significantly less resistance was seen in the telbivudine arm than the lamivudine arm (2%-3% vs 7%-8%). The ability to predict subsequent outcomes at 24 wk enables clinicians to estimate a response and plan future therapeutic interventions.
Table 2.
Efficacy at 1 yr against virological suppression at 24 wk in HBeAg positive and negative individuals in the Globe study
|
Virological suppression at wk 24 (%) |
||||
| < 300 copies/mL | 300-1000 copies/mL | > 1000-10 000 copies/mL | > 10 000 copies/mL | |
| HBeAg positive patients (wk 52) | ||||
| HBV DNA (< 300 copies/mL) | 90 | 70 | 30 | 5 |
| ALT normalization | 90 | 89 | 80 | 54 |
| Viral breakthrough | 1 | 3 | 8 | 11 |
| HBeAg negative patients (wk 52) | ||||
| HBV DNA (< 300 copies/mL) | 93 | 66 | 38 | 10 |
| ALT normalization | 83 | 74 | 63 | 36 |
| Viral breakthrough | 0 | 9 | 18 | 32 |
The 2 years results of this study[27] were presented recently at the AASLD annual meeting at Boston. At 2 years telbivudine showed significantly greater therapeutic response, a greater reduction in HBV DNA from baseline and greater HBV DNA clearance to PCR negative. Telbivudine showed significantly less primary and secondary failure, breakthrough and resistance. Clinical adverse event profiles were similar between the two treatment groups (Table 3). Both study drugs were generally well-tolerated, with similar patterns of clinical adverse events. Clinical and virological efficacy at 2 years was also linked to magnitude of HBV suppression at wk 24 (Table 4)[28].
Table 3.
Efficacy at 2 yr in HBeAg positive and HBeAg negative individuals in the Globe study receiving Telbivudine or lamivudine
| Telbivudine | Lamivudine | P value | |
| HBeAg positive patients | |||
| HBV DNA fall (mean log10) | -5.7 | -4.4 | < 0.05 |
| HBV DNA non-detectable by PCR (%) | 54 | 38 | < 0.05 |
| Treatment failure (%)1 | 4 | 12.3 | < 0.05 |
| HBeAg negative patients | |||
| HBV DNA fall (mean log10) | -5 | -4.2 | < 0.05 |
| HBV DNA non-detectable by PCR (%) | 79 | 53 | < 0.05 |
| Treatment failure (%)1 | 0 | 3 | < 0.05 |
Defined as HBV DNA > 5 log10 copies/mL.
Table 4.
Efficacy at 2 yr against virological suppression (PCR negative) at 24 wk in HBeAg positive and negative individuals in the Globe study
| Yr 2 outcome | Probability (%) | |
| HBeAg positive | HBeAg seroconversion | 45 |
| ALT normalization | 79 | |
| HBV DNA non-detectable by PCR | 77 | |
| HBeAg negative | ALT normalization | 77 |
| HBV DNA non-detectable by PCR | 74 |
Another phase III trial compared telbivudine with lamivudine in Chinese individuals with CHB[29], 87% of whom were HBeAg positive. At 52 wk, 70% of telbivudine exposed individuals had undetectable HBV DNA levels (defined as < 300 copies/mL by PCR) compared with only 43% of lamivudine treated individuals (P < 0.001). Telbivudine was superior to lamivudine in normalizing ALT levels (89% vs 76%, respectively, P < 0.005). In telbivudine exposed individuals who were HBeAg positive, on entering the study, 25% had seroconverted at 52 wk, compared with 18% of those treated with lamivudine. Both Telbivudine and lamivudine were equally well tolerated.
Efficacy vs adefovir: A third major phase III study has compared the use of telbivudine and adefovir in HBeAg positive individuals with CHB for 24 wk[30]. At the end of the study, a significantly greater HBV DNA reduction was seen in those individuals exposed to telbivudine (6.37 vs 5.11 log10 copies/mL; P < 0.01). In individuals exposed to adefovir, 42% failed to reach a HBV DNA below 5 log10 copies/mL, compared with 5% in the telbivudine arm (P < 0.01). There was no significant difference in HBeAg loss or normalization of ALT levels between the two arms. There was no difference in adverse events between the two arms.
Resistance: Of the 1367 individuals included in the Globe ITT analysis, 81 patients experienced viral breakthrough[18]. Among these, genotypic resistance was confirmed in 69:17 telbivudine-treated patients and 52 lamivudine recipients. Following sequencing, all resistance was associated with M204 variants in the YMDD motif of the genome. Individuals failing lamivudine had acquired either M204I, M204V or a mixed picture of M204M/I/V. In those exposed to telbivudine, M204I was the only mutation detected in 16 of 17 telbivudine patients with resistance; the other patient carried a mixture of M204M/I/V.
The M204V lamivudine resistant mutation was associated with the 180M compensatory mutation, thus forming a double mutant, whereas the M204I telbivudine mutation was not. These results imply that telbivudine may suppress the emergence of fully resistant HBV via the M204V pathway that is dominant with lamivudine.
FUTURE TRENDS IN THE MANAGEMENTOF CHRONIC HEPATITIS B
Emerging data suggest that the magnitude of reduction in serum HBV DNA levels that are achieved early in the course of therapy with nucleos(t)ides predicts the likelihood of subsequent efficacy outcomes. Data from the GLOBE study suggest that patients who have rapid and profound viral response to treatment (serum HBV DNA < 300 copies/mL at 24 wk) tend to have a higher probability of maintaining the response and a lower probability of resistance over time. Conversely, patients who have a lower initial viral response tend to have a higher probability of low rates of response and resistance over time.
Understanding the factors that predict poor resp-onse and resistance can potentially permit treatment modification to optimize response to treatment. Therefore, the response to a single agent after 24 wk of therapy may be used effectively by the clinician to determine whether the patient is likely to benefit from continued monotherapy or if the addition of another agent may offer a better probability for improved long term outcomes.
A clinical trial to assess this approach with telbivudine will be conducted in India. The trial design will involve assessment of response, based on serum HBV DNA levels, at specific early time points starting at 24 wk. Based on the degree of viral suppression at these time points, telbivudine may be continued or another agent could be added.
CONCLUSION
Owing to its good safety profile and high antiviral potency, telbivudine is one of the most promising drugs for the treatment of CHB. Telbivudine is one of the new β-L-nucleoside analogues with potent antiviral activity against HBV. Telbivudine is an obligate chain terminator, incorporating into HBV DNA, and exerts a preferential effect on second strand DNA synthesis. The promising results of the early in vitro and animal studies paved the way for phase I/II human clinical trials. Phase IIB human clinical studies demonstrated superior antiviral efficacy of telbivudine, significantly better ALT normalization and better HBeAg loss as compared with lamivudine. Further large international multicenter phase III studies have confirmed these results with telbivudine in comparison not only to lamivudine but also to adefovir.
Overall, telbivudine appears to be efficacious, easy to take with a good safety profile, proving to be a valuable therapeutic option in the management of hepatitis B.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Lu W
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 2.Mohanty SR, Kupfer SS, Khiani V. Treatment of chronic hepatitis B. Nat Clin Pract Gastroenterol Hepatol. 2006;3:446–458. doi: 10.1038/ncpgasthep0550. [DOI] [PubMed] [Google Scholar]
- 3.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759–768. doi: 10.7326/0003-4819-135-9-200111060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 6.Fung SK, Lok AS. Drug insight: Nucleoside and nucleotide analog inhibitors for hepatitis B. Nat Clin Pract Gastroenterol Hepatol. 2004;1:90–97. doi: 10.1038/ncpgasthep0056. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury A. Epidemiology of hepatitis B virus infection in India. Hep B Annual. 2004;1:17–24. [Google Scholar]
- 8.Thyagrajan SP, Jayaram S, Hari R, Mohan KVK, Murugavel KG. Epidemiology of hepatitis B in India – A comprehensive analysis. In: Sarin SK, Okuda K, editors. Hepatitis B and C carrier to cancer. 1st ed. New Delhi: Harcourt India Private Ltd; 2002. pp. 25–39. [Google Scholar]
- 9.Tandon BN, Acharya SK, Tandon A. Epidemiology of hepatitis B virus infection in India. Gut. 1996;38 Suppl 2:S56–S59. doi: 10.1136/gut.38.suppl_2.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury A, Santra A, Pal S, Chakravarty R, Banerji A, Pal S, Dhali GK, Datta S, Banerji S, Manna B, Roy Chowdhury S, Bhattacharya SK, Guha Mazumder D. Community based epidemiological study of Hepatitis B virus infection (HBV) Indian Journal Gastroenterol. 2001;20 Suppl 2:A2. doi: 10.1111/j.1440-1746.2005.04070.x. [DOI] [PubMed] [Google Scholar]
- 11.Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, Gane E, Kao JH, Omata M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25:472–489. doi: 10.1111/j.1478-3231.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 12.Lok AS. Natural history and control of perinatally acquired hepatitis B virus infection. Dig Dis. 1992;10:46–52. doi: 10.1159/000171343. [DOI] [PubMed] [Google Scholar]
- 13.Hadziyannis SJ, Bramou T, Alexopoulou A, Makris A. Immunopathogenesis and natural course of anti-HBe positive chronic hepatitis with replicating B virus. In: Hollinger FB, Lemon SM, Margolis HS, editors. Viral Hepatitis and Liver Disease. Baltimore: Williams and Wilkins; 1991. pp. 673–676. [Google Scholar]
- 14.Hunt CM, McGill JM, Allen MI, Condreay LD. Clinical relevance of hepatitis B viral mutations. Hepatology. 2000;31:1037–1044. doi: 10.1053/he.2000.6709. [DOI] [PubMed] [Google Scholar]
- 15.Miyakawa Y, Okamoto H, Mayumi M. The molecular basis of hepatitis B e antigen (HBeAg)-negative infections. J Viral Hepat. 1997;4:1–8. doi: 10.1046/j.1365-2893.1997.00101.x. [DOI] [PubMed] [Google Scholar]
- 16.Newbold JE, Xin H, Tencza M, Sherman G, Dean J, Bowden S, Locarnini S. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J Virol. 1995;69:3350–3357. doi: 10.1128/jvi.69.6.3350-3357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 18.Jones R, Nelson M. Novel anti-hepatitis B agents: A focus on telbivudine. Int J Clin Pract. 2006;60:1295–1299. doi: 10.1111/j.1742-1241.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- 19.Yuen MF, Hui CK, Cheng CC, Wu CH, Lai YP, Lai CL. Long-term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: The effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology. 2001;34:139–145. doi: 10.1053/jhep.2001.25273. [DOI] [PubMed] [Google Scholar]
- 20.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 21.Standring DN, Bridges EG, Placidi L, Faraj A, Loi AG, Pierra C, Dukhan D, Gosselin G, Imbach JL, Hernandez B, et al. Antiviral beta-L-nucleosides specific for hepatitis B virus infection. Antivir Chem Chemother. 2001;12 Suppl 1:119–129. [PubMed] [Google Scholar]
- 22.Bridges E. Telbivudine preclinical safety studies suggest minimal risk of chronic toxicity, reproductive toxicity, or carcinogenicity [Abstract]. 41st Annual Meeting of the European Association for the Study of the Liver, Vienna, Austria. J Hepatol. 2006;44(suppl 2):S147. [Google Scholar]
- 23.Zhou XJ, Lim SG, Lloyd DM, Chao GC, Brown NA, Lai CL. Pharmacokinetics of telbivudine following oral administration of escalating single and multiple doses in patients with chronic hepatitis B virus infection: pharmacodynamic implications. Antimicrob Agents Chemother. 2006;50:874–879. doi: 10.1128/AAC.50.3.874-879.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou XJ, Myers M, Chao GC, Dubuc G, Brown NA. Clinical pharmacokinetics of Telbivudine, a potent antiviral for hepatitis B, in subjects with impaired hepatic or renal function. J Hepatol. 2004;40:(Abstract). [Google Scholar]
- 25.Lai CL, Lim SG, Brown NA, Zhou XJ, Lloyd DM, Lee YM, Yuen MF, Chao GC, Myers MW. A dose-finding study of once-daily oral telbivudine in HBeAg-positive patients with chronic hepatitis B virus infection. Hepatology. 2004;40:719–726. doi: 10.1002/hep.20374. [DOI] [PubMed] [Google Scholar]
- 26.Lai CL, Leung N, Teo EK, Tong M, Wong F, Hann HW, Han S, Poynard T, Myers M, Chao G, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005;129:528–536. doi: 10.1016/j.gastro.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 27.Lai CL, Gane E, Hsu CW, Thongsawat S, Wang Y, Chen Y. Two year results from the Globe trial in patients with Hepatitis B: Greater clinical and antiviral efficacy for Telbivudine vs lamivudine. Hepatology. 2006;44 Suppl 1:222A. [Google Scholar]
- 28.DiBisceglie A, Lai CL, Gane E. Telbivudine GLOBE Trial: Maximal Early HBV Suppression is Predictive of Optimal Two-Year Efficacy in Nucleoside-Treated Hepatitis B Patients. Hepatology. 2006;44 Suppl 1:230A. [Google Scholar]
- 29.Hou J, Yin Y, Xu DZ, Tan D, Niu J, Zhou XQ, Wang Y, Zhu L, He Y, Ren H, et al. A phase III comparative trial of Telbivudine and lamivudine for treatment of chronic hepatitis B in Chinese patients: first year results (Abstract) J Gastroenterol Hepatol. 2006;21 suppl 2:A128–A129. [Google Scholar]
- 30.Heathcote E, Chan HL, Cho M, Lai C, Moon Y, Chao Y, Myers R, Minuk G, Marcellin P, Jeffers L, Sievert W, Kaiser R, Chao G, Brown N, 018 Study Group. A randomised trial of Telbivudine (LdT) vs adefovir for HBeAg positive chronic hepatitis B: results of the primary week 24 analysis. Gastroenterology. 2006;130 suppl 2:A765 (Abstract). [Google Scholar]


