Abstract
Cancer occurs when cells acquire genomic instability and inflammation, produce abnormal levels of epigenetic factors/proteins and tumor suppressors, reprogram the energy metabolism and evade immune destruction, leading to the disruption of cell cycle/normal growth. An early event in carcinogenesis is loss of polarity and detachment from the natural basement membrane, allowing cells to form distinct three-dimensional (3D) structures that interact with each other and with the surrounding microenvironment. Although valuable information has been accumulated from traditional in vitro studies in which cells are grown on flat and hard plastic surfaces (2D culture), this culture condition does not reflect the essential features of tumor tissues. Further, fundamental understanding of cancer metastasis cannot be obtained readily from 2D studies because they lack the complex and dynamic cell-cell communications and cell-matrix interactions that occur during cancer metastasis. These shortcomings, along with lack of spatial depth and cell connectivity, limit the applicability of 2D cultures to accurate testing of pharmacologically active compounds, free or sequestered in nanoparticles. To recapitulate features of native tumor microenvironments, various biomimetic 3D tumor models have been developed to incorporate cancer and stromal cells, relevant matrix components, and biochemical and biophysical cues, into one spatially and temporally integrated system. In this article, we review recent advances in creating 3D tumor models employing tissue engineering principles. We then evaluate the utilities of these novel models for the testing of anticancer drugs and their delivery systems. We highlight the profound differences in responses from 3D in vitro tumors and conventional monolayer cultures. Overall, strategic integration of biological principles and engineering approaches will both improve understanding of tumor progression and invasion and support discovery of more personalized first line treatments for cancer patients.
Keywords: 3D tumor models, Bioreactors, Microfluidic devices, Hydrogels, Scaffolds, Cancer therapeutics, Drug delivery, Drug resistance
1. Introduction
Cancer is the major cause of death worldwide, and one in every four deaths in the United States is due to cancer-related diseases (Siegel et al., 2012). While cells in normal tissue reside in defined locations and maintain steady numbers, cancer cells remove these constraints through mutations in oncogenes and tumor suppressor genes (Esmaeilsabzali et al., 2013; Joyce and Pollard, 2009). Consequently, cells in the tumor tissues can sustain proliferative signaling, evade growth suppressors, resist cell death, enable replicative immortality, induce angiogenesis, and activate invasion and metastasis (Hanahan and Weinberg, 2011). During cancer progression and metastasis, malignant cells maintain their close interactions with surrounding cells and the stromal extracellular matrices (ECM) (Fig. 1) (DelNero et al., 2013; Hanahan and Weinberg, 2011; Infanger et al., 2013; Nyga et al., 2011; Seo et al., 2013). Numerous stromal cells, including endothelial cells of the blood and lymphatic circulation, stromal fibroblasts, and innate and adaptive infiltrating immune cells together comprise the complex tumor microenvironment (Hanahan and Weinberg, 2011; Joyce and Pollard, 2009; Koontongkaew, 2013). The stromal ECM is composed of complex assemblies of collagens, glycosaminoglycans and proteoglycans and the molecules that bind to them (Jain, 1999; 2012). Tumor cells interact with those stromal components dynamically through growth factor-mediated tumor-stromal cell crosstalk (Murata et al., 2011) and integrin-mediated tumor-ECM interactions (Desgrosellier and Cheresh, 2010). Moreover, these interactions evolve along with the progression of the disease (Tlsty and Coussens, 2006), where the stromal microenvironment can initially exert inhibitory effects on even aggressive malignant tumor cells (Bissell and Hines, 2011; Joyce and Pollard, 2009; Xu et al., 2012a). However, as the disease progresses, cancer cells exploit and modify their surroundings to facilitate the inappropriate growth, angiogenesis, invasion and ultimately metastasis in a secondary site (Chung et al., 2012; Joyce and Pollard, 2009; Psaila and Lyden, 2009). In general, tumor growth and progression requires intricate interactions between cancer cells and their surrounding microenvironment.
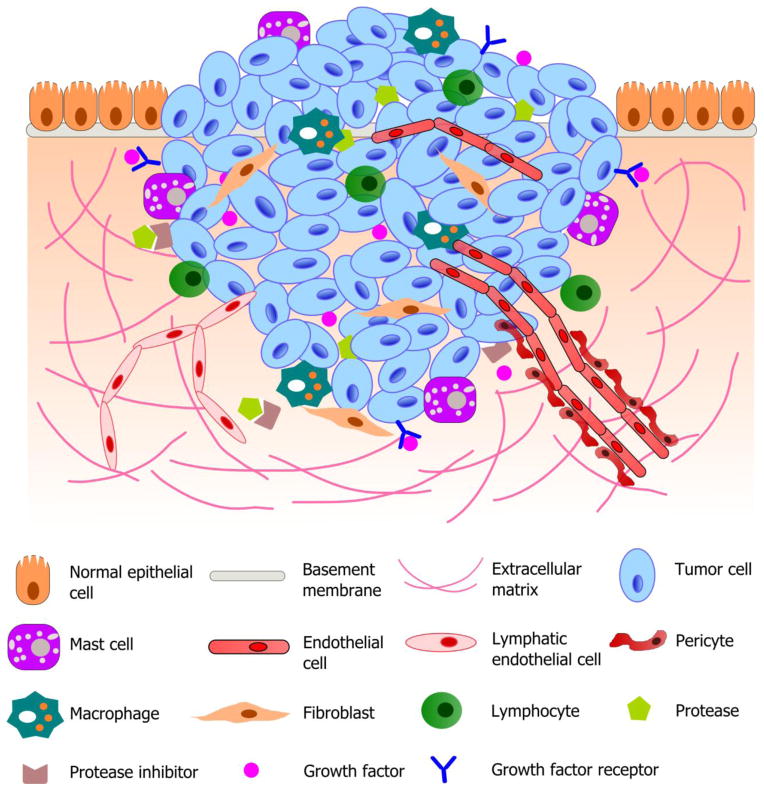
Fig. 1.
Schematic illustration of a typical tumor microenvironment. Cancer cells reside in a complex microenvironment containing various supporting cells, extracellular matrix (ECM) and a suite of signaling molecules. These environmental components collectively contribute to the tumor-stromal interaction and tumor progression. Adapted from (Joyce and Pollard, 2009; Koontongkaew, 2013).
In vitro studies aimed at gaining molecular understanding of cancer progression or the identification of effective anti-cancer therapeutics rely on the availability of a versatile platform that closely recapitulates pathophysiological features of the native tumor tissue and its surrounding microenvironment. Conventional two dimensional (2D) platforms (Hutmacher et al., 2010) are well established and straightforward to use. However, the absence of the third dimension can obscure the experimental observations, generating misleading and contradictory results (Hutmacher, 2010; Hutmacher et al., 2010). Additionally, screening in 2D may miss promising lead compounds whose actions are suppressed when cells are adhered to plastic. Often, promising results obtained from 2D cannot be translated similarly into in vivo settings (Goodman et al., 2008). Whereas cells on 2D are exposed to a uniform environment with sufficient oxygen and nutrients, cells in solid tumors are exposed to gradients of critical chemical and biological signals (Mehta et al., 2012), which can exert both stimulatory and inhibitory effects on tumor progression (Mehta et al., 2012). Intriguingly, certain tumor cells from cancer patients are intrinsically resistant to a broad spectrum of chemotherapeutic drugs without any previous exposure to those cytotoxic agents (Sanchez et al., 2009; Zhu et al., 2005; Zhu et al., 2012). This intrinsic drug resistance has been attributed to the overexpression of the multidrug resistance (MDR) proteins by tumor cells (Sanchez et al., 2009; Wartenberg et al., 1998; Zhu et al., 2012). Characteristics of the tumor tissue, namely hypoxia (Milane et al., 2011; Zhu et al., 2012), low nutrient supply (Zhu et al., 2012) and low pH (Webb et al., 2011; Wei and Roepe, 1994; Xu et al., 2014), all have been suggested to upregulate the expression of MDR proteins through specific cellular signaling pathways. Although one can partially recreate a MDR-conducive environment in 2D cultures, the lack of a three dimensional (3D) architectural context precludes the recapitulation and the maintenance of MDR behaviors (Correia and Bissell, 2012; Faute et al., 2002). Finally, the lack of the complex 3D ECM network structures in monolayer cultures can affect drug testing results. While anti-cancer agents applied to a monolayer cell culture typically reach cells without physical barriers, the same therapeutics delivered in vivo encounter an entirely different environment that significantly restricts the partition of the drugs throughout the entire tumor (Goodman et al., 2008). The 3D organization of the tumor mass, as well as the associated stroma, fundamentally alters the diffusion profile for drugs, both through the cell-cell contacts and cell-matrix interactions (Chauhan et al., 2011). Detailed descriptions on physicochemical properties of native tumor microenvironments including cell-cell and cell-matrix interactions, tissue structure and mechanics, as well as juxtacrine and soluble factor signaling, can be found in recent reviews by Fischbach and coworkers (DelNero et al., 2013; Infanger et al., 2013; Seo et al., 2013).
Realizing the limitations of monolayer cultures, and inspired by the complexity of the native tumor microenvironment, researchers have developed various 3D models that recapitulate certain features of solid tumor tissues, such as tumor morphology (Gurski et al., 2012), gradient distribution of chemical and biological factors (Fracasso and Colombatti, 2000), expression of pro-angiogenic and MDR proteins (Fischbach et al., 2009; Xu et al., 2014), dynamic and reciprocal interactions between tumor and its stroma (Xu et al., 2012a). Moreover, compared to 2D monolayer cultures, cells in 3D generally exhibit a reduced sensitivity to some chemotherapeutic agents (Fong et al., 2013). This review adds to the existing literature by summarizing recent advances in ex vivo assembly of pathologically-relevant 3D tumor models using custom-designed culture devices and biologically-derived or biomimetic matrices. Critical assessments are provided to highlight the applications of these models toward a mechanistic understanding of cancer biology and in therapeutic evaluations of anticancer drugs, both free and nanoencapsulated. Possible mechanisms for the altered drug sensitivity observed in 3D culture conditions as compared to the corresponding 2D systems also are discussed.
2. Device-assisted assembly of tumor models
Three-dimensional multicellular tumoroids can be grown using engineered devices that maximize cell-cell interactions and solute transport without the undesirable interference from scaffolding materials. Outlined below are two types of devices employed in cancer research: tissue engineering bioreactors and microfluidic systems.
2.1. Tissue engineering bioreactors
The multicellular tumor spheroid (MCTS) model, developed by Sutherland and collaborators (Sutherland et al., 1970) in the 1970s, has become a classic model system for cancer research. The straightforward procedure and the reliable reproducibility have contributed to the success and wide-spread usage of this model (LaBarbera et al., 2012). Using traditional cell culture plates or spinner flasks, MCTS can be produced by various techniques, such as liquid overlay, hanging drop or suspension culture (Page et al., 2013). Depending on the cell types, their substrate consumption rates and cell packing densities, tumor spheroids as large as 600 μm can be generated (Kunz-Schughart et al., 1998) and under certain conditions, MCTS with a necrotic core and gradient distributions of critical metabolites and growth factors have been obtained (Grimes et al., 2014).
Culturing cancer cells in an aggregated state statically does not always lead to the rapid formation of complex 3D structures. In tissue engineering applications, bioreactors are designed to overcome mass transfer limitations associated with traditional static cultures (Wendt et al., 2009) and/or to introduce physiologically relevant biomechanical and biophysical stimulations to cultured cells (Tandon et al., 2013; Tong et al., 2013; Tong et al., 2014). By maintaining culture parameters with tissue-specific biological, chemical, or physical cues, these bioreactors (Martin et al., 2004) have facilitated the engineering of complex and functional tissue constructs (Wendt et al., 2009). In the context of cancer progression, invasion and metastasis, bioreactors have been utilized both to miniaturize the natural counterparts, to introduce relevant forces or to create a controlled environment to foster the assembly tumor-like tissues (Hutmacher et al., 2010).
To facilitate accelerated cell growth and to maintain unrestricted cell-cell interactions, a rotating wall vessel (RWV) bioreactor, originally designed to recapitulate certain conditions that occur in the microgravity environment of space, was adapted for the culture of cancer cells, with the goal of generating 3D tumor aggregate structures that simulate the native tumor tissue (Becker and Souza, 2013; Zhau et al., 1997). As shown in Fig. 2A, RWV is designed as a horizontally rotating vessel without internal mechanical agitator. Additionally, the vessel is completely filled with cell culture media; therefore there is no air-liquid interface in the device (Ingram et al., 1997). Within the RWV, microcarrier beads are utilized to provide the solid support for adherent cells to attach and aggregate. As the RWV rotates, culture fluid can reach a near laminar flow condition and the cellular aggregates cultured in the fluid are in a state of free fall, but never reach the bottom of the vessel because of the constant rotation of the device (Becker and Souza, 2013; Goodwin et al., 1993). As the tumor aggregates grow, multiple cell-covered microcarrier beads coalesce, undergo cellular bridging and as a result, form high-density, 3D aggregate structures. Of note, when microcarrier beads are included as an integral component of a RWV bioreactor, the resultant tumor construct will contain a non-degradable, bio-inert core that compromises the overall biological integrity of the engineered tumor. The removal of the solid core is not straightforward and can results in the dissociation of the cell aggregates (Skardal et al., 2010).
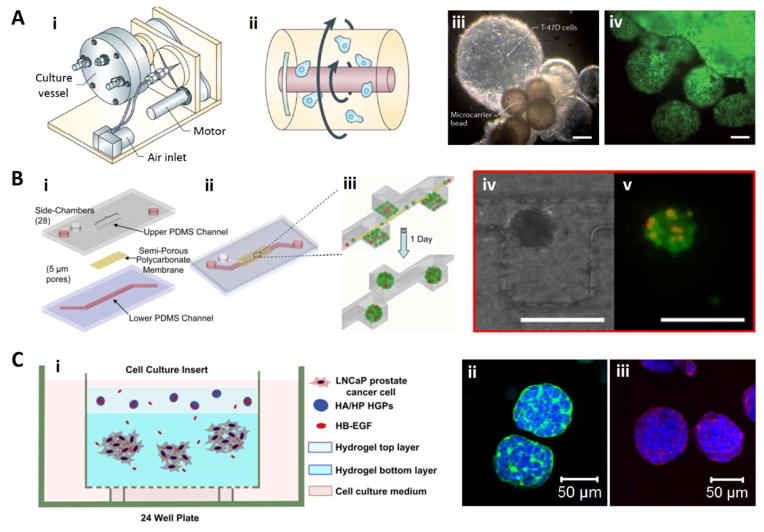
Fig. 2.
Representative examples of culture platforms employed for the growth of 3D tumoroids. (A) Rotating wall vessel (RWV) bioreactors (i, ii) have been used for the 3D culture of human breast ductal carcinoma cells (T-47D, iii) and glioblastoma cells (labeled with green fluorescent protein, iv). Cells cultured in a state of free fall with minimal shear (ii) organized into millimeter sized aggregates with multiple layers of cells (iii and iv, scale bar: 200 μm). Reproduced with permission (Becker and Souza, 2013), Copyright 2013, Macmillan Publishers Limited. (B) A microfluidic device (i–iii) has been designed for the 3D culture of PC-3 prostate cancer cells. The device is composed of upper and lower channels separated by a non-adhesive, semi-permeable membrane. PC-3 cells, mixed with pre-osteoblasts and endothelial cells and introduced to the device as a monolayer, self-organized into 3D spheroids in one day (iv: optical image; v: fluorescent image, red-PC-3 cells, green-live cells; scale bar: 200 μm). Reproduced with permission (Hsiao et al., 2009), Copyright 2009, Elsevier Ltd. (C) A bilayer hydrogel platform (i) for the 3D culture of prostate cancer cells. LNCaP cells culture in the bottom layer, receiving heparin-binding epidermal growth factor-like growth factor (HB-EGF) released from the top layer, grew into spheroids (ii, iii, nuclei: blue) displaying cortical F-actin (green) and expressing E-cadherin (red). Reproduced with permission (Xu et al., 2012), Copyright 2012, Elsevier Ltd.
The RWV cell culture technique has enabled the production of large batches (10 to 500 mL) of 3D multicellular aggregates (Hammond and Hammond, 2001) in millimeter scale (Goodwin et al., 1992). Tumor agglomerates cultured in this type of bioreactor develop small areas of necrosis during continued growth (Becker and Souza, 2013). Therefore, these tumor constructs can be used to model bulky tumors with regions of low proliferative activity and increased drug resistance (Becker and Souza, 2013). Various tumor models, including hepatocellular carcinoma (Chang and Hughes-Fulford, 2009), neuroblastoma (Redden and Doolin, 2011), breast adenocarcinoma (Kaur et al., 2011), and melanoma (Marrero et al., 2009), have been successfully engineered using RWV-based bioreactors. This type of bioreactor also has been utilized for the ex vivo culture of tissue explants. For example, bone marrow biopsies from multiple myeloma patients cultured in an RWV bioreactor exhibited viable myeloma cells inside the bone microenvironment and overall well preserved histo-architecture. This system then was applied successfully to evaluate the cytotoxic effects exerted by a proteasomal inhibitor not only on myeloma cells but also on angiogenic blood vessels (Ferrarini et al., 2013).
While the RWV system minimizes physical forces, other bioreactors are designed to model naturally occurring forces in the tumor microenvironment. Owing to tumor-associated angiogenesis and lymphangiogenesis, as well as to changes in the tumor stroma, tumor tissues exhibit elevated interstitial fluid flow (Munson et al., 2013; Shields et al., 2007) Using a model system that incorporates Matrigel™ with entrapped cancer cells and interstitial flow, established by a pressure head of 1 cm water at an average velocity of 0.2 μm/s, researchers examined effects of flow on tumor cell migration, with and without lymphatic endothelial cells. Overall, physiological levels of interstitial flow strongly enhance tumor cell polarization and migration. Moreover, tumor cells utilize interstitial flow to create and amplify autologous transcellular chemokine gradients and thus are chemotactic toward the draining lymphatic (Shieh and Swartz, 2011).
Bioreactors also have been designed to enable the investigation of cancer cell interactions with stromal cells (Krishnan et al., 2011; Yates et al., 2007). For example, a compartmentalized bioreactor was utilized for the growth of osteoblastic tissue (OT) and the co-culture of OT with metastatic breast cancer cells (Dhurjati et al., 2008). The bioreactor contains two compartments separated by a dialysis membrane (6–8 KDa cutoff) (Dhurjati et al., 2006). One of the compartments was used for cell culture and the other one as a medium reservoir. The purpose of the dialysis membrane is to retain all the growth factors and cytokines (with molecular weights larger than the dialysis membrane cutoff) that are secreted into the cell growth compartment, because it is known that the spatially and temporally ordered sequence of those secreted growth stimuli is closely aligned with specific stages in both the osteoblast development and the metastatic cancer colonization. The bioreactor-based co-culture system allows for the mechanistic study of the early stages of metastatic colonization of breast cancer cells to the bone tissue. In this work, sequential stages of the interaction between invasive cancer cells and the OT, including cancer cell adhesion, penetration and colony formation, reflected some of the features involved in breast cancer bone metastasis as observed in clinic (Dhurjati et al., 2008).
2.2. Microfluidic devices
Microfluidic technology was introduced as a tool for biological analysis in the early 1990s (Hong and Quake, 2003). It processes or manipulates small (10−9 to 10−18 liters) amounts of fluids, using microchannels with dimensions of 1 to 1000 micrometers (Zhang and Nagrath, 2013). In such regime, fluid flow within the microfluidic devices is strictly laminar, therefore, the concentrations of molecules can be well controlled both in space and time (Whitesides, 2006). Microfluidic technologies offer a number of useful capabilities for analysis including the ability to use very small quantities of sample and reagent and to perform experiments with short processing times, high resolution and sensitivity (Whitesides, 2006). Recently, microfluidics also has been used to simulate the physiological cues in cellular environment by precise spatial and temporal control over gradients of soluble biological factors and cell-cell contacts (El-Ali et al., 2006). Components for downstream cellular analysis (e.g. imaging or molecular characterization) also can be connected with the microfluidic cell culture device to form an integrated system (Zhang and Nagrath, 2013). These attractive features make microfluidics a powerful tool with which to study tumor progression, invasion and angiogenesis (Zhang and Nagrath, 2013).
For instance, a two-layer microfluidic system was developed for the culture of 3D multi-cell type spheroids of fluorescently labeled metastatic prostate cancer cells (PC-3 cell line), osteoblasts and endothelial cells (Fig. 2B) (Hsiao et al., 2009). The system enables the formation of uniformly-sized spheroids, and also ensures the uniform distribution of PC-3 cells as well as the other two co-cultured cell types across all spheroids within the device. The engineered 3D microfluidic tumor model mimics the bone microenvironment in which the metastatic prostate cancer cells reside. This microfluidic device maintained high cell viability and permitted prostate cancer cells to grow at a physiologically relevant rate (Hsiao et al., 2009). These 3D microscale tumor tissue constructs have been used both for drug testing and for gaining in-depth understanding of cancer biology (Liu et al., 2010; Wu et al., 2008).
In another example, a microfluidic system was developed for the co-culture of tumor and endothelial cells under varying flow shear stress conditions (Buchanan et al., 2014). The system enables the study of the shear force effect on endothelial organization and paracrine signaling during tumor angiogenesis. Specifically, in this study, a central microchannel (711 μm) embedded within a collagen I-based hydrogel matrix serves as a neovessel through which tumor-relevant hydrodynamic stresses are introduced. Green fluorescent protein (GFP)-labeled breast cancer cells (MDA-MB-231) were seeded in the bulk of the collagen hydrogel, which surrounded endothelial cells lining the lumenal surface of the central microchannel. The authors demonstrated that endothelial cells assemble a confluent endothelium on the microchannel lumen, maintaining the channel integrity under physiological flow shear stresses. The cancer cells co-cultured with endothelial cells under low flow conditions significantly enhanced the expression of pro-angiogenic factors (Buchanan et al., 2014).
In the native tumor microenvironment, an intricate network of chemokines and growth factors facilitates the crosstalk between cancer cells and stromal cells. Such intimate communications are conducive to cancer progression, through the recruitment of the immune system and new blood vessels (Mantovani et al., 2010; Roussos et al., 2011). Both tumor cells and stromal cells exhibit directional migration towards a chemokine source during disease progression and tumor metastasis (Mantovani et al., 2010; Roussos et al., 2011). To investigate cancer migration and invasion, researchers have relied on microfluidic devices to generate stable and quantifiable concentration gradients of chemokines. For example, U87MG brain cancer cells seeded on channels in a microfluidic device were found to invade a type I collagen-based matrix in response to a gradient of epidermal growth factor (EGF). Specifically, cancer cells were guided towards higher EGF level, and the directional bias relied on both the gradient magnitude and EGF concentration (Zervantonakis et al., 2010) In addition to providing a versatile and powerful tool for cancer research, microfluidic technology also has been utilized for the isolation of circulating tumor cells (Nagrath et al., 2007), molecular diagnosis (Pekin et al., 2011) and high-throughput screening of anti-cancer drugs (Kim et al., 2012). The readers are referred to a recent review article for in-depth discussions of these applications (Zhang and Nagrath, 2013).
3. Matrix-assisted assembly of 3D tumor models
The intricate molecular network of tumor-associated stromal ECM is an important component of the tumor microenvironment, and plays crucial roles in cancer progression and invasion (Dutta and Dutta, 2009; Weaver et al., 1997). Studies have shown that the blockage of ECM-integrin interactions led to death of highly metastatic breast cancer cells, thereby restoring morphologically normal breast structures (Wang et al., 2002). In general, association of cancer cells with the ECM produce survival or death signals at cellular levels to influence the efficacy of chemotherapeutic agents (Fracasso and Colombatti, 2000). Because tumor-associated stromal cells exerts either positive or negative effects on tumor growth and propagation, the absence of the stromal components in MCTS models limits the utility of such models in cancer research (Joyce and Pollard, 2009). Advanced technologies developed in the field of tissue engineering have enabled cancer researchers to create matrix-derived 3D tumor models that more closely recapitulate pathophysiological features of native tumor tissues (Hutmacher et al., 2010). These 3D systems have improved our understanding of the dynamic and reciprocal interactions between the solid tumor and their surrounding microenvironment, including the stromal ECM molecules, immune systems, stromal cells, as well as growth factors and cytokines (LaBarbera et al., 2012). In this section, we summarize various types of matrices (Table 1) that have been utilized for the assembly of 3D tumor models.
Table 1.
Naturally derived and synthetic matrices for 3D tumor engineering.
| Type of materials | Properties | Tumor types/Cell lines | References |
|---|---|---|---|
| Fibroblast-derived matrices | Produced mainly by cancer associated fibroblasts. Mimic microenvironment in advanced carcinomas. | Human colorectal carcinoma/HCT116 Human pancreatic carcinoma/PANC-1 |
Serebriiskii et al. (2008) |
| Matrigel™ or Cultrex® | Basement membrane extracts consisting of collagen type IV, laminin, perlecan, etc. | Human colon adenocarcinoma/SW480 Human breast adenocarcinoma/MDA-MB-231 |
Price et al. (2012) Poincloux et al. (2011) |
| Type I collagen | Native ECM component produced by cells in the stromal environment. Influences the growth and metastasis of resident tumor cells. | Human breast carcinoma/MCF-7 Human hepatocellular liver carcinoma/HepG2 |
Chen et al. (2012) Yip and Cho (2013) |
| Silk fibroin | Unique mechanical properties, good biocompatibility, well-controlled degradability and versatile processability. | Human breast adenocarcinoma/MDA-MB-231 | Talukdar and Kundu (2012) |
| Alginate | Biodegradable hydrogels with controlled mechanical properties and pore sizes. Non-adhesive to cells. | Human hepatocellular carcinoma/MHCC97L, HCCLM3 Oral squamous cell carcinoma/OSCC-3 |
Xu et al. (2013) Fischbach et al. (2009) |
| Hyaluronic acid | Essential ECM component in the tumor microenvironment. Interacts with its cell surface receptors, such as CD44 or RHAMM. Involved in tumor expansion, angiogenesis and metastasis. | Human prostate cancer/LNCaP Human glioma/U87MG |
Xu et al. (2012a) Xu et al. (2014) Pedron et al. (2013) |
| PEG | Synthetic matrices with controllable biochemical and mechanical properties. Specific biological functionalities can be covalently incorporated into the PEG hydrogels. | Human epithelial ovarian cancer/OV-MZ-6 Human pancreatic ductal adenocarcinoma/PANC-1 |
Loessner et al. (2010) Ki et al. (2013) |
| PLGA | Porous biodegradable synthetic scaffolds. Convenient to handle and amenable to large-scale use. | Oral squamous cell carcinoma/OSCC-3 Human glioma/U251 |
Fischbach et al. (2007) Kim et al. (2011) |
| PCL | Biologically inert synthetic scaffolds. Medical grade PCL-tricalcium phosphate (mPCL-TCP) scaffolds can be used to study bone metastasis. | Ewing sarcoma/TC-71 Prostate cancer/PC3 and LNCaP |
Fong et al. (2013) Sieh et al. (2010) |
| Synthetic peptides | Defined amino acid composition for facile incorporation of specific biological relevant ligands. | Human ovarian carcinoma/SK-OV-3 Human breast carcinoma/MCF-7 |
Yang and Zhao (2011) Huang et al. (2013) |
3.1. Naturally derived matrices
Three-dimensional tumor models have been created using fibroblast-derived matrices (Lee et al., 2011). This matrix, produced predominately by cancer associated fibroblasts, resembles the mesenchymal microenvironment typically associated with advanced carcinomas in vivo (Serebriiskii et al., 2008). Compared to 2D plastic culture, cancer cells cultured in the fibroblast-derived matrices exhibited distinctly different cell morphology, aggregation pattern, proliferation profile and invasive potential (Serebriiskii et al., 2008). Disadvantages of this type of cell-derived matrices include lengthy sample preparation and batch-to-batch composition variations. In addition, the membrane-like structures have limited thickness and cannot be physically manipulated without compromising their structural integrity. Finally, fibroblast associated matrices do not fully represent the composition and structure of the tumor microenvironment (Nyga et al., 2011).
Separately, basement membrane extracts have been widely utilized in cancer research (Hutmacher et al., 2010; Poincloux et al., 2011). The utility of reconstituted basement membranes from mouse tumor as cell culture substrate was first demonstrated in the mid-1980s (Kleinman et al., 1986). The product, known as Matrigel™ or Cultrex® (Fridman et al., 2012; Sasser et al., 2007) consisting of collagen type IV, perlecan/HSPG2 and laminin, has been considered for a long time as the material of choice for 3D cancer cell culture. For example, Price et al (Price et al., 2012) demonstrated the influence of Matrigel™ culture on the expression of microRNA (miRNAs), non-encoding RNAs thought to be involved in cancer cell adhesion, proliferation and invasion. These authors discovered that tissue-like miRNA expression levels and patterns were found when colon cancer cells were cultured in Matrigel™, but not on 2D plastic (Price et al., 2012). Although critical microenvironmental cues are restored in Matrigel™, this type of matrix often contains residual heparan sulfate growth factors, lacks human motifs, and contains undefined substances, and suffers from considerable batch-to-batch variation (Zaman, 2013). These shortcomings make it difficult to compare and analyze results from various research groups (Hutmacher et al., 2010). The identification of specific matrix components and soluble factors that contribute to the observed cell behaviors also is challenging.
Collagen type I, a native ECM component produced by cells in the stromal environment, influences the growth and metastasis of resident cancer cells (Moss et al., 2009). Collagen can be collected from various biological sources including bovine skin, rat tail and human placenta (Elsdale and Bard, 1972; Pacak et al., 2014). Collagen-based hydrogels have been widely used for the construction of 3D tumor models (Chen et al., 2012; Ingber, 2008; Szot et al., 2011; Yip and Cho, 2013). MCF-7 breast cancer cells cultured in collagen gels exhibited stronger invasive and metastatic propensities than did those in 2D cultures (Chen et al., 2012), as evidenced by the increased expression of pro-angiogenic growth factors, matrix metalloproteinases (MMPs) and epithelial mesenchymal transition (EMT) markers (Chen et al., 2012). Although collagen gels can be manufactured in a more reproducible manner than Matrigel™, their microstructure nevertheless is affected by a number of factors, including tissue source, assembly conditions and crosslinking density. These variations may render inconsistent results (Hutmacher et al., 2010). Most importantly, the hydrogel properties cannot be altered easily to interrogate the contributions of specific environmental cues to tumor assembly.
Owing to its unique mechanical properties, good biocompatibility, well-controlled degradability and versatile processability, silk fibroin biomaterials have been designed for tissue regeneration (Kundu et al., 2013). In a recent study, non-mulberry A. mylitta silk fibroin protein scaffolds, prepared by surfactant assisted dissolution, followed by dialysis and lyophilization, were used for 3D culture of breast cancer cell line MDA-MB-231 cells (Talukdar and Kundu, 2012). The study showed that the silk-based 3D microenvironment provided a biocompatible niche for cancer cell attachment, growth and tumor tissue formation. The engineered tumor exhibited tissue-like heterogeneity with various zones of cell proliferation.
Various biopolymers originally developed for tissue engineering applications also have been employed for the in vitro construction of tumor models. In the presence of divalent cations (e.g. Ca2+), alginates form hydrogels with controlled mechanical properties and pore sizes (Fischbach et al., 2009; Xu et al., 2013). Calcium crosslinked alginate gels degrade slowly under physiological conditions through the gradual exchange of calcium (Shoichet et al., 1996). Because alginate is typically non-adhesive to cells, RGD peptides have been conjugated covalently to the polymeric matrix to facilitate cell adhesion (Fischbach et al., 2009; Ruoslahti and Pierschbacher, 1987). Oral squamous cell carcinoma cells (OSCC-3) cultured in RGD modified, alginate hydrogels secreted a higher level of interleukin 8 (IL-8), presumably as a consequence of integrin engagement. In contrast, substrate adhesion did not significantly affect the cellular production of vascular endothelial growth factor (VEGF). This study highlights the importance of tumor microenvironment in regulating cancer cell angiogenic signaling (Fischbach et al., 2009).
Hyaluronic acid (HA) is another biopolymer widely exploited for the construction of 3D tumor models (Dicker et al., 2013), and unlike alginate, is structurally identical to its human counterpart that is intimately involved in cancer growth and progression (Dicker et al., 2013). HA is highly expressed in tumors and is an necessary component of the microenvironment of cancer cells (Franzmann et al., 2003; Kosaki et al., 1999; Lokeshwar et al., 1997; Lokeshwar et al., 2001). The metastasis of tumor cells can be directed by the amount of HA on the cell surface (Naor et al., 1997; Zhang et al., 1995). Through the interaction with its cell surface receptors, HA can alter the biological activity of cancer cells by triggering transforming growth factor β (TGF-β), Rho GTPase, and FAK signaling pathways (Bourguignon et al., 2003; Bourguignon et al., 2002; Fujita et al., 2002). In tumor tissues, HA facilitates the migration of invasive cancer cells through the expansion upon hydration and their interaction with HA via certain cell surface receptors (Toole and Hascall, 2002). The biodegradation of HA by hyaluronidase (HAase) also helps tumor cells to escape from the primary tumor mass and metastasize to secondary sites (Stern, 2008b). Inhibition of hyaluronidase in 3D HA hydrogels is shown to block the formation of “invadopodia” (see below) (Gurski et al., 2012). HA oligomers trigger angiogenesis and induce inflammatory cytokine production, which activates various signaling mechanisms for cancer progression (Franzmann et al., 2003). Hence, tumor progression and angiogenesis depend on HA and hyaluronidase levels, as well as the degradation profile of HA (Stern, 2008a; b; Toole and Hascall, 2002). High concentrations of HA sometimes are observed at tumor invasion sites, and it has been shown that the HA coating around tumor cells effectively protects these cells from immune system surveillance (Lokeshwar et al., 1997).
We have developed several types of chemically crosslinked HA hydrogels that are hierarchically structured, mechanically robust and biologically active (Xu et al., 2012a). These synthetic matrices have been used to investigate prostate cancer progression and metastasis (Gurski et al., 2009; Gurski et al., 2012; Xu et al., 2012a; Xu et al., 2014). Hydrogels with controllable mechanical stiffness (140 – 230 Pa) and pore sizes (70–100 nm) were prepared using HA derivatives carrying complementary reactive groups (Gurski et al., 2009; Xu et al., 2012b). Of note, the storage modulus of the elastic-solid-like hydrogel is comparable to that of the native lymph tissue through which PCa frequently metastasizes (storage modulus: 330 Pa) (Levental et al., 2007), and the pore size of the hydrogel is also relevant to the spacing (20–130 nm) found in tumor ECMs (Pluen et al., 2001). When cultured in 3D HA hydrogel environments, prostate cancer cells formed distinct clustered tumoroid structures that grew and merged, reminiscent of native tumors. Compared to cells cultured on 2D, the engineered tumoroids in 3D significantly increased the expression of pro-angiogenic factors and MDR proteins, both at mRNA and protein levels. To mimic the tumor/stroma interaction, a HA-based bilayer hydrogel system that not only supports the tumoroid formation from prostate cancer (PCa) cells, but also simulates their reciprocal interactions with the tumor-associated stroma, was engineered (Xu et al., 2012a). As shown in Fig. 2C, PCa cells embedded in the bottom hydrogel layer received the stromal growth factor signals from the top, and in response formed enlarging tumoroids that exhibited biological features reminiscent of native tumor tissues (Xu et al., 2012a). Our HA hydrogel system also provides a flexible, quantifiable, and physiologically relevant platform to study prostate cancer metastasis. Metastatic prostate cancer cells in these hydrogels develop fingerlike structures, “invadopodia”, consistent with their invasive properties. The number of invadopodia, as well as cluster size, shape, and convergence, can provide a quantifiable measure of invasive potential. Prostate cancer cell invasion through the HA hydrogel depends on HA interaction with its cell surface receptor RHAMM/CD168 and requires hyaluronidase activity. The engineered PCa model is amenable to dissection of biological processes associated with cancer cell motility through HA-rich connective tissues (Gurski et al., 2012).
Using adipic acid dihydrazide-crosslinked HA hydrogels, David et al. investigated the invasive behaviors of a total of thirteen different cancer cell lines (from primary tumor or metastases) (David et al., 2004). Similar to our observations, the invasiveness of the cancer cell is related to its production of hyaluronidase, the expression of HA binding receptors and the cellular secretion of HA. Moreover, optimal colonization occurred when cells produced hyaluronidase, but did not possess HA-binding sites and did not secrete HA. Separately, Pedron et al. revealed the pivotal roles of HA in regulating the invasion and malignancy of human glioblastoma cells in a 3D biomimetic environment using HA-integrated, radically polymerized gelatin hydrogels. Multicellular tumor-like structures were only observed in HA containing hydrogels, and a biphasic relationship between cellular malignancies and HA content in the hydrogels was discovered (Pedron et al., 2013). Overall, systematic manipulation of the local ECM microenvironment surrounding cancer cells enables cancer researchers to study the early growth of tumors.
3.2. Synthetic matrices
Synthetic hydrogels and scaffolds have been developed and extensively explored in tissue engineering applications to closely mimic the key features of the native ECM environment, owing to their flexible biochemical and biophysical characteristics (Drury and Mooney, 2003). These biomaterials are attractive alternatives to naturally derived matrices described above. Well-defined synthetic, yet biomimetic, matrices are attractive because they offer significantly improved batch-to-batch consistency with more controllable and reproducible characteristics, such as matrix mechanical properties, porosity and degradation profiles (Hutmacher et al., 2010).
Poly(ethylene glycol) (PEG)-based hydrogels, prepared by chemical crosslinking of the functionalized polymers, have been used widely as artificial matrices for 3D cell culture purposes (Lin and Anseth, 2009). While PEG is not a physiologically relevant molecule, biomimetic PEG hydrogels can be synthesized readily via the covalent incorporation of specific biological functionalities (e.g. functional peptides). Moreover, the mechanical and biological properties of PEG hydrogels can be tuned to profoundly influence the functions of the resident cells (Hutmacher et al., 2010). Taking advantage of the biocompatible nature of enzyme-catalyzed crosslinking reaction, Loessner et al synthesized PEG-based hydrogels using complementary peptide-functionalized multiarm-PEG as substrates for thrombin-activated factor XIII (FXIII) (Loessner et al., 2010). The design flexibility enabled the incorporation of arginine-glycine-aspartate (RGD) peptides for integrin-mediated cell adhesion, and a matrix metalloproteinase (MMP) sensitive peptide for protease-based matrix degradation. The authors demonstrated that both cell proliferation and spheroid formation from ovarian cancer cells in 3D depended on the integrin engagement and the ability of cells to proteolytically remodel their extracellular microenvironment.
In general, synthetic ECM can be designed to provide a biomimetic cell growth niche with precise control over both the physical and biological characteristics of the matrix for tumor tissue formation. In a recent study, researchers utilized a versatile PEG-peptide hydrogel system to investigate the influence of matrix properties and the inhibition of epidermal growth factor receptor (EGFR) on the growth of a pancreatic ductal adenocarcinoma cell line (PANC-1). The hydrogel was prepared via thiol-ene photoclick chemistry using 8-arm PEG-norbornene and MMP-sensitive peptide with terminal cysteines. These pathophysiologically-relevant hydrogels were designed to exhibit a wide range of stiffness (~1–18 kPa), with soft gels matching that of normal pancreatic tissue, and stiff gels reflecting the mechanical features of the tumor tissue. PANC-1 cells encapsulated in relatively soft hydrogels (G′ similar to 2 kPa) retained high initial viability and formed dusters after prolonged culture, whereas cells encapsulated in stiff hydrogels (G′ similar to 12 kPa) exhibited lower initial viability and reduced proliferation. Immobilization of an EGFR peptide inhibitor in soft hydrogels did not increase cell death, but the same peptide immobilized in stiff hydrogels induced significant cell apoptosis, owing to the reduced cellular expression of EGFR and reduced Akt activation in stiff hydrogels. These findings underscore the potential effects of matrix properties on the efficacy of antitumor drugs (Ki et al., 2013).
Porous scaffolds, widely used in tissue engineering applications, also have been adopted for tumor engineering purposes. Because scaffold preparation requires harsh conditions and organic solvents, cells must be introduced after the scaffold has been prepared. Mooney and colleagues reported the utility of polymeric scaffolds, prepared from poly(lactide-co-glycolide) (PLGA) via a gas foaming-particulate leaching process (Harris et al., 1998), for 3D culture of OSCC-3 cells (Fischbach et al., 2007). The angiogenic characteristics of the tumor cells were altered dramatically upon 3D culture in the porous scaffold, and corresponded much more closely to tumors formed in vivo. In another study (Kim et al., 2011), PLGA-based scaffolds, fabricated by a solvent casting and particulate leaching technique, were used for 3D culture of human glioma cells. Similar to other 3D culture studies, cells in this model were less sensitive to chemotherapy as evident by a lower caspase-3 activity, and a higher expression of anti-apoptotic proteins. Such a scaffold, although entirely synthetic, provides a useful 3D platform to study the effect of microenvironmental conditions on tumor progression in vitro.
To foster more intimate cell-cell and cell-matrix interactions, researchers resorted to electrospun, microfibrous meshes for 3D culture of cancer cells. In one example, porous electrospun poly(ε-caprolactone) (PCL) scaffolds were used for the culture of Ewing sarcoma cells in 3D (Fong et al., 2013). Cancer cells cultured in the 3D environment not only were more resistant to traditional cytotoxic drugs than were cells in 2D monolayer culture, but also exhibited remarkable differences in the expression pattern of the insulin-like growth factor-1 receptor/mammalian target of rapamycin pathway (Fong et al., 2013). Of note, since drug transport and interaction with the scaffold may have an impact on its efficacy, the decreased drug sensitivity found in 3D PCL scaffold may be partially attributed to the physical adsorption of the drug onto the scaffold. Overall, the reported PCL-based tumor model has the potential to provide a platform for the mechanistic studies of bone sarcomas and the evaluation of anticancer drug candidates for these malignancies (Fong et al., 2013). Modification of electrospun scaffolds with bioactive ECM-derived peptides also can be used to study pharmacokinetic properties of various drugs targeting cancer cells (Hartman et al., 2010). To gain mechanistic understandings of cancer metastasis, Sieh et al. cultured PCa cells on a tissue engineered bone constructs, fabricated by wrapping a human osteoblast cell sheet around a cell-seed, bone-mimetic composite scaffold (Sieh et al., 2010). Consistent with reported in vivo studies, the authors found that the intercellular and PCa cell-bone matrix interactions contributed to the observed increase in the expression level of various biomarkers associated with PCa bone metastasis.
Because of the defined amino acid composition and the structural and mechanical similarity to natural ECM, self-assembled peptide hydrogels have emerged as attractive 3D culture platforms (Loo et al., 2012). Gelation occurs via a process that involves concerted action of weak and non-covalent interactions, triggered by altering the composition of the aqueous media. The synthetic nature of the peptide building blocks allow for facile incorporation of specific biological relevant ligands to achieve desired biological functions (Ulijn and Smith, 2008; Zhang et al., 2005a). These physical gels contain entangled, amyloid-like fibers of ~ 10 nm thick and interstitial pores between 5 and 200 nm (Goldberg et al., 2007). Using RADA16-I peptide hydrogel, 3D models of ovarian cancer were created. Cells cultured in the peptide hydrogels not only exhibited strong invasion potentials, but also displayed significantly higher anticancer drug resistance than did cells cultured on the conventional 2D petri dish (Yang and Zhao, 2011). Hydrogels assembled from a rationally designed h9e peptide (Huang et al., 2011) containing sequences from an elastic segment of spider silk and a trans-membrane segment of human muscle L-type calcium channel, were used for 3D culture of human epithelial cancer cells (MCF-7). MCF-7 cells residing in the peptide hydrogel grew into 3D tumor-like clusters and responded to cisplatin treatment in a dose- and time-dependent manner (Huang et al., 2013). Gelation by supramolecular interactions through the entanglement of fibrillar structures is an attractive feature of self-assembled peptide hydrogels. Long term stability, however, needs to be improved.
4. 3D tumor models for drug testing
Although rapid advances in drug design methodology have led to dramatic increases in the discovery of both candidate drug compounds and their screenable drug targets (Dobson, 2004; Tan, 2005), a commensurate increase in the number of approved drugs has not been seen (LaBarbera et al., 2012). The high failure rate of drug candidates can be attributed in part to the usage of 2D monolayer cultures as the initial screening method that frequently produces inaccurate and unreliable results and does not predict chemoresistance (van de Waterbeemd and Gifford, 2003). As discussed above, the same drug treatment elicits distinctly different responses from cells in 3D and on 2D. The ability of 3D systems to recapitulate tumor-like microenvironment suggests the potential of engineered tumor models to provide more accurate and reproducible toxicity information for the early-stage drug discovery (Lee et al., 2008).
4.1. High-throughput screening of drug candidates
Adaptation of 3D tumor models to high-throughput drug screening systems are highly desirable because they can provide predictable results in an efficient manner, thereby enabling the prioritization of candidate compounds for further development in the in vivo studies (Friedrich et al., 2009). For use as in vitro drug testing platforms, the 3D models must recapitulate the pathophysiological features of the tumor microenvironment so that in vivo drug efficacy can be reliably predicted (Mueller-Klieser, 2000). For high-throughput purposes, the implementation of the 3D tumor models into drug evaluation routines requires an experimental design that guarantees tumor constructs of the same cell type cultured under the same external conditions are essentially identical in morphology, structure, microenvironment and cellular physiology (Friedrich et al., 2009). Routine high-throughput drug screening protocols based on various 3D systems (Kim, 2005) have been developed (Friedrich et al., 2009).
Using ultra-low attachment 96-well round-bottomed plates (Vinci et al., 2012), researchers demonstrated that tumor cells formed uniform, single and centrally located spheroids of reproducible size within each well. The resultant MCTS were subjected to automated multiparametric analyses, including measurements of spheroid diameter, perimeter and area, using a Celigo™ cytometer. This platform not only enabled reliable assessments of key hallmarks of cancer, i.e. cell motility, matrix invasion and tumor angiogenesis, but also facilitated evaluation of various types of cell growth inhibitors. Not surprisingly, 2D and 3D cultures exhibited differential sensitivities to targeted agents, and in general, tumor cells were less sensitive to soluble compounds in 3D than in 2D monolayer cultures. The utility of some agents in treating metastatic disease also has been identified. Other 3D culture techniques amendable to high throughput screening include liquid overlay (Li et al., 2011), hanging drop (Tung et al., 2011), microarray template (Hardelauf et al., 2011), microencapsulation (Zhang et al., 2005b) and magnetic cell levitation (Souza et al., 2010).
The differential drug sensitivity observed between 2D and 3D cultures can be attributed to decreased compound access or reduced drug sensitivity in response to hypoxic or more slowly cycling cells under 3D culture conditions (Fong et al., 2013; Minchinton and Tannock, 2006). It is also reported that cell-cell contact plays a role in drug resistance found in 3D. A negative correlation between cell-cell contact and drug sensitivity was observed in a PCL scaffold-based 3D tumor model (Fong et al., 2013). However, the exact signaling mechanism implicated in drug resistance in 3D is still unclear. Current work is focused on the study of the interplay among various factors (e.g. intracellular changes, paracrine signaling, modifications in the supporting matrix) that may contribute to the reduced drug sensitivity in 3D (Saraswathy and Gong, 2013).
Tumor models fabricated using matrix-assisted approaches have not yet been widely exploited for high throughput screening purposes because of the additional variables introduced by the matrix and challenges associated with manipulating and fabricating hydrogels or scaffolds in a high throughput fashion. If the matrix is simple, however, adaptation can be achieved. For example, using collagen or alginate gels, 3D tumor cell-culture array (Data Analysis Toxicology Assay Chip or DataChip) has been developed for the high-throughput toxicity evaluation of both the drug candidates and their cytochrome P450-generated metabolites (Lee et al., 2008). Specifically, cancer cells encapsulated within collagen or alginate gels with the volume as low as 20 nL, were spotted onto the surface of functionalized glass slides of the DataChip. One single DataChip contains 1,080 of this kind of individual microscale cell/gel tumor constructs. The system was used for spatially addressable screening against multiple anti-cancer compounds. In conjunction with the complementary human P450-containing microarray, the device provided the toxicity results for the metabolites of the compound from three human P450 isoforms (CYP3A4, CYP1A2, and CYP2D6), which were designed to mimic the function of human liver.
4.2. Evaluation on drug delivery systems
Over the past few decades, concerted efforts have been made for the development of nanotechnology-based cancer therapeutics, with the promise of potentially prolonging patients’ life expectancy, at the same time reducing treatment-related side effects (Byrne et al., 2008; Kumar et al., 2013; Peer et al., 2007). The engineered nano-formulations improve the pharmacological properties of drugs, and also potentially increase the drug efficacy through the enhanced permeability and retention (EPR) effect or the active targeting strategies (Byrne et al., 2008; Kang et al., 2010). The typical size of the developed nano-therapeutics for clinical applications ranges from 2 nm to 200 nm (Kamaly et al., 2012). For effective treatment of solid tumor, it is desirable that the nanomedicine can reach as many cells within the tumor parenchyma as possible (Kamaly et al., 2012). However, the penetration of the nano-therapeutics can be hindered by the elevated levels of interstitial fluid pressure (IFP), tortuosity and interstitial matrices of the tumor tissue (Wong et al., 2011). Moreover, the increased IFP limits the transport of nanomedicine to the poorly perfused regions of tumor tissues where access is dominated only by diffusion (Kim et al., 2010). The rate of diffusion and the penetration depth depend on the size of the drug carrier and the interactions between the carrier and the interstitial matrices (Kim et al., 2010).
Three-dimensional avascular tumor models have been used to assess the diffusion, distribution and drug efficacy of nano-therapeutics. Using a 3D MCTS model, Pun and coworkers reported that polystyrene nanoparticles (NPs) with an average diameter of 20 and 40 nm penetrated the MCTS, whereas larger NPs (100 or 200 nm) were restricted to the periphery of the spheroids (Goodman et al., 2007). When collagenase was immobilized on the particle surface, the penetration depth of the NPs was enhanced significantly. Therefore, incorporating ECM-modulating enzymes in drug carriers may improve NP penetration in solid tumors. In a separate investigation, researchers discovered that doxorubicin (Dox)-loaded nanoparticles (Dox-NPs) with an average diameter of 37 nm penetrated into the core of 400 μm human cervical tumor spheroids in 30 min. Compared to free Dox, Dox-NPs not only exhibited a greater tumor penetration capacity, but also suppressed tumor growth more efficiently in the MCTS model (Kim et al., 2010). Similar observations were found with regard to the transport property of a pseudo-peptide-based drug delivery system using a Hela MCTS model (Ho et al., 2011). Overall, NP penetration depended on both the carrier concentration and the incubation time.
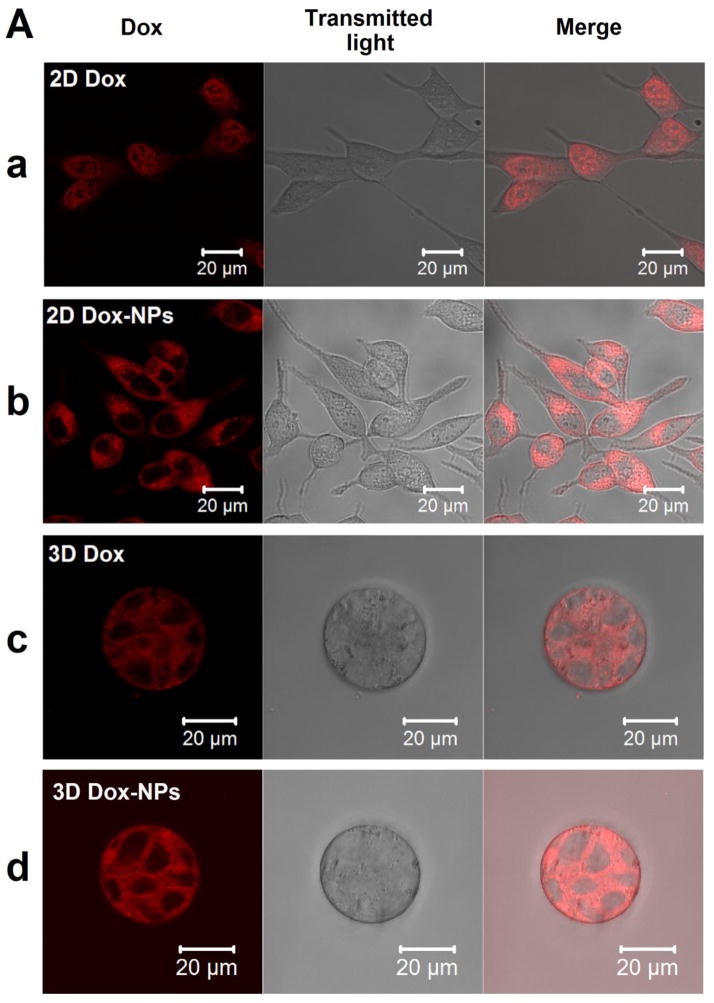
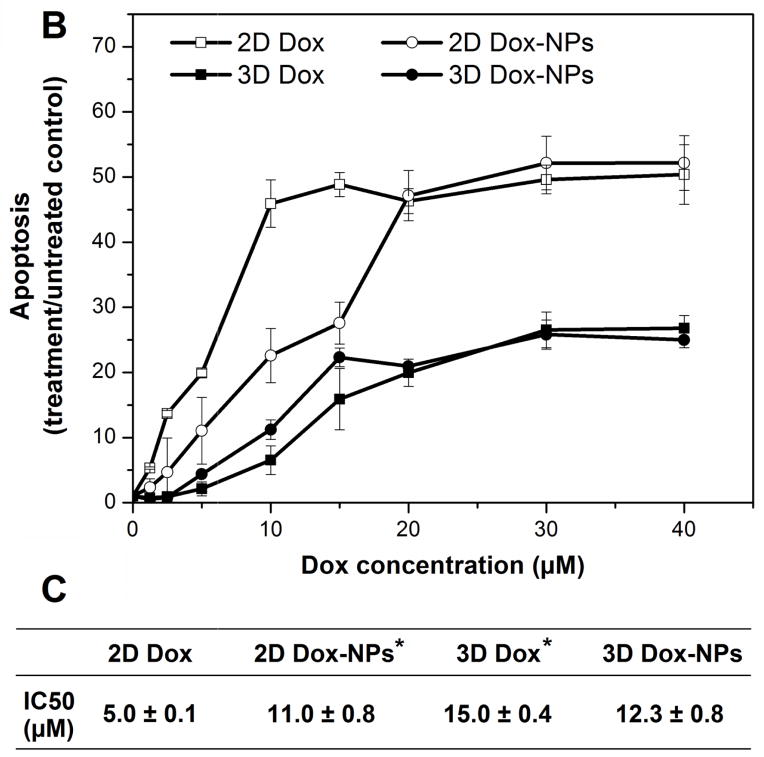
Three-dimensional tumor models assembled in hydrogel matrices also have been used for the evaluation of nanoformulations. The hydrogel network, with optimized stiffness and mesh size, was designed to simulate the ECM barrier of tumor tissues. Using a PCa tumor model created using an HA hydrogel, Xu et al discovered that Nile Red (NR)-loaded polymeric NPs with an average hydrodynamic size of 54 nm had a diffusion coefficient of 9.21 μm2/s and 4.04 μm2/s in water and the HA network, respectively. Dox-NPs introduced to the cell/gel constructs reached the embedded tumoroids within 2 hours, homogeneously distributed in the tumoroids and were internalized by tumor cells through caveolae-mediated endocytosis and macropinocytosis pathways. A comparison of intracellular drug localization revealed that on 2D cultures, free Dox was transported to the cell nuclei [Fig. 3A(a)] whereas Dox-NPs were excluded from the nuclei and distributed predominantly in the cytoplasm [Fig. 3A(b)]. Interestingly, under 3D culture conditions, both free Dox and Dox-NPs localized predominantly in the cytoplasm of individual cells in the tumoroids [Fig. 3A(c) and 3A(d)]. Finally, as indicated from the dose response curves and the IC50 values (Fig. 3B and 3C), tumor cells cultured as multicellular aggregates in HA hydrogel were more resistant to both free Dox and Dox-NP treatments compared to 2D cultures. The drug resistance profile observed in 3D has been attributed to the elevated expression of MDR proteins (multidrug resistance protein 1 and lung resistance-related protein) by LNCaP PCa cells cultured in HA hydrogels (Xu et al., 2014). It has been reported that primary cultures of PCa cells derived directly from the patients expressed significantly elevated levels of MDR proteins than PCa cell lines (i.e. LNCaP, PC3 and DU145 cell lines). As a result, patient-derived cells displayed lower drug sensitivity (Sanchez et al., 2009). The HA-based 3D tumor models closely mimic the in vivo tumor growth condition, and the increased expression of MDR proteins suggested a MDR cell phenotype. Therefore, the drug response from cells cultured in the 3D HA hydrogel should be more physiologically relevant than that from cells cultured on 2D.
Fig. 3.
Comparison of intracellular localization and cell-killing capacity of free Dox and Dox-NPs in 2D and 3D cultures of LNCaP prostate cancer cells. HA-based hydrogels were used for 3D cultures. (A) Confocal images show differential localizations of Dox and Dox-NPs in LNCaP cells after 2 h of drug exposure. Dox is inherently fluorescent and the internalized Dox or Dox-NPs were detected using a Zeiss510 NLO confocal microscope (excitation wavelength at 488 nm with a band pass filter of 565–615 nm). (B) Dose-dependent cell death induced by Dox or Dox-NPs on 2D and 3D cultures of LNCaP prostate cancer cells. Cell apoptosis analysis was performed using Cell Death Detection ELISAPLUS. (C) Summary of the IC50 values for various drug/culture combinations. (*, significant difference compared to the IC50 value of 2D Dox condition, p < 0.05). Reproduced with permission (Xu et al., 2014), Copyright 2014, Elsevier Ltd.
The Zaman and Grinstaff team created 3D models of osteosarcoma and breast adenocarcinoma by first allowing the cancer cells (U2OS and MDA-MB-231) to aggregate in an agarose well, then encapsulating the spheroids in collagen gels (Charoen et al., 2014). Using the engineered tumor models, the researchers characterized the chemotherapeutic responses from paclitaxel, delivered in bolus as free drug or controlled release using an expansile nanoparticle (eNP). The eNPs were designed to release the drug payload via a pH mediated particle expansion within the cell endosome (Zubris et al., 2013). Confocal microscopy imaging showed that the eNPs, with an average diameter of ~100 nm (Colby et al., 2013), dispersed throughout the spheroid after diffusing through the collagen and into the spheroid. Compared to the bolus delivery, the eNPs delivery provided a significantly more pronounced inhibition in cell growth, in accordance with the in vivo observations from a murine xenograft model. Contrarily, no difference in response between bolus and eNP delivered paclitaxel was detected in 2D monolayer (Liu et al., 2013). These findings highlight the importance of physiologically relevant tumor models for generating clinically predictable assessments on drug formulations.
5. Summary and outlook
Engineered 3D systems provide a realistic and controllable environment for the incorporation of specific cells, ECM molecules, growth factors and other biochemical cues to better simulate the native tumor microenvironment that favors tumor growth and progression. These models will likely be of significant use in delineating the biological mechanisms that govern the pathological abnormalities observed in cancer. They also will serve as more reliable platforms for generating predictive results on in vivo evaluation of chemotherapeutic agents and their delivery systems.
Despite substantial and continued success in creating biomimetic 3D tumor models, major challenges and limitations remain associated with existing models. First, many models are created using long term 2D culture adapted cancer cell lines that may no longer accurately reflect the pathology of the initial disease. The establishment of therapeutically relevant treatment predictions relies on the availability of patients’ cells, expanded in xenografts, with characteristic heterogeneity of the disease and different metastatic potentials. Successful culture of these cells ex vivo will provide access to a higher level of accuracy both in probing the biological underpinnings of metastasis, and in correctly identifying the susceptibility to pathway-targeted drugs (Fong et al., 2014). Equally important is the validation of the engineered tumor models, at least by the source xenografts, and ideally matched to each patient’s deidentified history of drug therapies to guide and refine the current model.
Secondly, current 3D systems remain relatively simplistic and do not faithfully represent the native tumor microenvironment. Many engineered models appear similar to native tumor tissues only morphologically. Phenotypic similarity and heterogeneity need to be recaptured. When synthetic matrices are employed, detailed signaling studies of cells in the engineered microenvironment is lacking. A future trend is to improve the physiological complexity, which includes cell types, matrix composition, as well as temporal and spatial presentation of soluble factors employing innovative materials chemistry and engineering designs. The recreation of the complex, tumor-associated vascular system in vitro is another challenge task but necessary to be addressed because these abnormal blood vessels not only influence cancer progression, but also greatly affect drug transport within the tumor tissues (Jain, 2012).
Finally, as the tumor models become more and more sophisticated, computational modeling (Shirinifard et al., 2009), system biology approaches (Deisboeck et al., 2011; Rajagopalan et al., 2013) and in situ real time imaging, detection and analysis modalities are sorely needed (Zaman, 2013). For example, crosslinking of hydrogel precursors in the presence of homogenously dispersed cancer cells results in the entrapment of cells in a porous network with mesh size in the micron to submicron scale (Nicodemus and Bryant, 2008; Xu et al., 2014). To form large tumor-like aggregates, cells initially trapped at a single cell state must proliferate within the matrix, at the same time migrate through the network, via amoeboid and/or mesenchymal movement (Wolf and Friedl, 2006). There is a need for quantitative understanding of the collective movement of aggregated cancer cells in the 3D environment. Overall, with the advances in 3D culture techniques (Hutmacher et al., 2010), cancer genomics (Chin et al., 2011) and mathematical modeling (Haeno et al., 2012), promising 3D engineered tumor models will serve as the bridge between 2D monolayer culture and cancer xenografts to accelerate the translation of novel therapeutics to the clinic.
Acknowledgments
Work in the authors’ laboratories has been funded by grants from the National Institutes of Health (P20 RR016458, X.J.; P01 CA098912, M.C.F.C.; R01 DE022969, M.C.F.C. and X.J.), Delaware Health Science Alliance (DHSA) and the University of Delaware (Graduate Fellowship to X.X.). The authors would like to thank Genzyme for generously providing hyaluronic acid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker JL, Souza GR. Using space-based investigations to inform cancer research on Earth. Nat Rev Cancer. 2013;13:315–27. doi: 10.1038/nrc3507. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med (N Y, NY, U S) 2011;17:320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LYW, Singleton PA, Zhu HB, Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (Macrophage-Colony stimulating factor) production and breast tumor progression. J Biol Chem. 2003;278:29420–34. doi: 10.1074/jbc.M301885200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LYW, Singleton PA, Zhu HB, Zhou B. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–12. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- Buchanan CF, Voigt EE, Szot CS, Freeman JW, Vlachos PP, Rylander MN. Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization. Tissue Eng. 2014;20:64–75. doi: 10.1089/ten.tec.2012.0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Delivery Rev. 2008;60:1615–26. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Chang TT, Hughes-Fulford M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng Part A. 2009;15:559–67. doi: 10.1089/ten.tea.2007.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoen KM, Fallica B, Colson YL, Zaman MH, Grinstaff MW. Embedded multicellular spheroids as a biomimetic 3D cancer model for evaluating drug and drug-device combinations. Biomaterials. 2014;35:2264–71. doi: 10.1016/j.biomaterials.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Delivery of molecular and nanoscale medicine to tumors: Transport barriers and strategies. Annu Rev Chem Biomol Eng. 2011;2:281–98. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- Chen L, Xiao ZF, Meng Y, Zhao YN, Han J, Su GN, et al. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials. 2012;33:1437–44. doi: 10.1016/j.biomaterials.2011.10.056. [DOI] [PubMed] [Google Scholar]
- Chin L, Andersen JN, Futreal PA. Cancer genomics: from discovery science to personalized medicine. Nat Med (N Y, NY, U S) 2011;17:297–303. doi: 10.1038/nm.2323. [DOI] [PubMed] [Google Scholar]
- Chung SW, Cooper CR, Farach-Carson MC, Ogunnaike BA. A control engineering approach to understanding the TGF-beta paradox in cancer. J R Soc Interface. 2012;9:1389–97. doi: 10.1098/rsif.2011.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby AH, Colson YL, Grinstaff MW. Microscopy and tunable resistive pulse sensing characterization of the swelling of pH-responsive, polymeric expansile nanoparticles. Nanoscale. 2013;5:3496–504. doi: 10.1039/c3nr00114h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Update. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L, Dulong V, Le Cerf D, Chauzy C, Norris V, Delpech B, et al. Reticulated hyaluronan hydrogels: a model for examining cancer cell invasion in 3D. Matrix Biol. 2004;23:183–93. doi: 10.1016/j.matbio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Deisboeck TS, Wang ZH, Macklin P, Cristini V. Multiscale Cancer Modeling. In: Yarmush ML, Duncan JS, Gray ML, editors. Annual Review of Biomedical Engineering. Vol. 13. Palo Alto: Annual Reviews; 2011. pp. 127–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelNero P, Song YH, Fischbach C. Microengineered tumor models: insights & opportunities from a physical sciences-oncology perspective. Biomed Microdevices. 2013;15:583–93. doi: 10.1007/s10544-013-9763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurjati R, Krishnan V, Shuman LA, Mastro AM, Vogler EA. Metastatic breast cancer cells colonize and degrade three-dimensional osteoblastic tissue in vitro. Clin Exp Metastasis. 2008;25:741–52. doi: 10.1007/s10585-008-9185-z. [DOI] [PubMed] [Google Scholar]
- Dhurjati R, Liu XM, Gay CV, Mastro AM, Vogler EA. Extended-term culture of bone cells in a compartmentalized bioreactor. Tissue Eng. 2006;12:3045–54. doi: 10.1089/ten.2006.12.3045. [DOI] [PubMed] [Google Scholar]
- Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2013;18:00615–6. doi: 10.1016/j.actbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM. Chemical space and biology. Nature. 2004;432:824–8. doi: 10.1038/nature03192. [DOI] [PubMed] [Google Scholar]
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Dutta RC, Dutta AK. Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv. 2009;27:334–9. doi: 10.1016/j.biotechadv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442:403–11. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeilsabzali H, Beischlag TV, Cox ME, Parameswaran AM, Park EJ. Detection and isolation of circulating tumor cells: Principles and methods. Biotechnol Adv. 2013;31:1063–84. doi: 10.1016/j.biotechadv.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Faute MAD, Laurent L, Ploton D, Poupon MF, Jardillier JC, Bobichon H. Distinctive alterations of invasiveness, drug resistance and cell-cell organization in 3D-cultures of MCF-7, a human breast cancer cell line, and its multidrug resistant variant. Clin Exp Metastasis. 2002;19:161–8. doi: 10.1023/a:1014594825502. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Steimberg N, Ponzoni M, Belloni D, Berenzi A, Girlanda S, et al. Ex-vivo dynamic 3-D culture of human tissues in the RCCS (TM) bioreactor allows the study of multiple myeloma biology and response to therapy. PLoS One. 2013:8. doi: 10.1371/journal.pone.0071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong EL, Martinez M, Yang J, Mikos AG, Navone NM, Harrington DA, et al. Hydrogel-based 3D model of patient-derived prostate xenograft tumors suitable for drug screening. Mol Pharm. 2014 doi: 10.1021/mp500085p. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong ELS, Lamhamedi-Cherradi S-E, Burdett E, Ramamoorthy V, Lazar AJ, Kasper FK, et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc Natl Acad Sci U S A. 2013;110:6500–5. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracasso G, Colombatti M. Effect of therapeutic macromolecules in spheroids. Crit Rev Oncol Hematol. 2000;36:159–78. doi: 10.1016/s1040-8428(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Franzmann EJ, Schroeder GL, Goodwin WJ, Weed DT, Fisher P, Lokeshwar VB. Expression of tumor markers hyaluronic acid and hyaluronidase (HYAL1) in head and neck tumors. Int J Cancer. 2003;106:438–45. doi: 10.1002/ijc.11252. [DOI] [PubMed] [Google Scholar]
- Fridman R, Benton G, Aranoutova I, Kleinman HK, Bonfil RD. Increased initiation and growth of tumor cell lines, cancer stem cells and biopsy material in mice using basement membrane matrix protein (Cultrex or Matrigel) co-injection. Nat Protoc. 2012;7:1138–44. doi: 10.1038/nprot.2012.053. [DOI] [PubMed] [Google Scholar]
- Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–24. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, et al. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. 2002;528:101–8. doi: 10.1016/s0014-5793(02)03262-3. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci-Polym Ed. 2007;18:241–68. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman TT, Ng CP, Pun SH. 3-D tissue culture systems for the evaluation and optimization of nanoparticle-based drug carriers. Bioconjugate Chem. 2008;19:1951–9. doi: 10.1021/bc800233a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellullar spheroids. Int J Nanomed. 2007;2:265–74. [PMC free article] [PubMed] [Google Scholar]
- Goodwin TJ, Jessup JM, Wolf DA. Morphologic differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating-wall vessels. In Vitro Cell Dev Biol. 1992;28A:47–60. doi: 10.1007/BF02631079. [DOI] [PubMed] [Google Scholar]
- Goodwin TJ, Prewett TL, Wolf DA, Spaulding GF. Reduced shear stress: a major component in the ability of mammalian tissues to form three-dimensional assemblies in simulated microgravity. J Cell Biochem. 1993;51:301–11. doi: 10.1002/jcb.240510309. [DOI] [PubMed] [Google Scholar]
- Grimes DR, Kelly C, Bloch K, Partridge M. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J R Soc Interface. 2014:11. doi: 10.1098/rsif.2013.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurski LA, Jha AK, Zhang C, Jia X, Farach-Carson MC. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials. 2009;30:6076–85. doi: 10.1016/j.biomaterials.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurski LA, Xu X, Labrada LN, Nguyen NT, Xiao L, van Golen KL, et al. Hyaluronan (HA) interacting proteins RHAMM and hyaluronidase impact prostate cancer cell behavior and invadopodia formation in 3D HA-based hydrogels. PLoS One. 2012;7:e50075. doi: 10.1371/journal.pone.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–75. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TG, Hammond JM. Optimized suspension culture: the rotating-wall vessel. Am J Physiol Renal Physiol. 2001;281:F12–F25. doi: 10.1152/ajprenal.2001.281.1.F12. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hardelauf H, Frimat JP, Stewart JD, Schormann W, Chiang YY, Lampen P, et al. Microarrays for the scalable production of metabolically relevant tumour spheroids: a tool for modulating chemosensitivity traits. Lab Chip. 2011;11:419–28. doi: 10.1039/c0lc00089b. [DOI] [PubMed] [Google Scholar]
- Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42:396–402. doi: 10.1002/(sici)1097-4636(19981205)42:3<396::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hartman O, Zhang C, Adams EL, Farach-Carson MC, Petrelli NJ, Chase BD, et al. Biofunctionalization of electrospun PCL-based scaffolds with perlecan domain IV peptide to create a 3-D pharmacokinetic cancer model. Biomaterials. 2010;31:5700–18. doi: 10.1016/j.biomaterials.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VHB, Slater NKH, Chen RJ. pH-responsive endosomolytic pseudo-peptides for drug delivery to multicellular spheroids tumour models. Biomaterials. 2011;32:2953–8. doi: 10.1016/j.biomaterials.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Hong JW, Quake SR. Integrated nanoliter systems. Nat Biotechnol. 2003;21:1179–83. doi: 10.1038/nbt871. [DOI] [PubMed] [Google Scholar]
- Hsiao AY, Torisawa YS, Tung YC, Sud S, Taichman RS, Pienta KJ, et al. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials. 2009;30:3020–7. doi: 10.1016/j.biomaterials.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HZ, Ding Y, Sun XZS, Nguyen TA. Peptide Hydrogelation and Cell Encapsulation for 3D Culture of MCF-7 Breast Cancer Cells. PLoS One. 2013;8:15. doi: 10.1371/journal.pone.0059482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HZ, Shi JS, Laskin J, Liu ZY, McVey DS, Sun XZS. Design of a shear-thinning recoverable peptide hydrogel from native sequences and application for influenza H1N1 vaccine adjuvant. Soft Matter. 2011;7:8905–12. [Google Scholar]
- Hutmacher DW. Biomaterials offer cancer research the third dimension. Nat Mater. 2010;9:90–3. doi: 10.1038/nmat2619. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW, Loessner D, Rizzi S, Kaplan DL, Mooney DJ, Clements JA. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 2010;28:125–33. doi: 10.1016/j.tibtech.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Lynch ME, Fischbach C. Engineered culture models for studies of tumor-microenvironment interactions. Annu Rev Biomed Eng. 2013;15:29–53. doi: 10.1146/annurev-bioeng-071811-150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18:356–64. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram M, Techy GB, Saroufeem R, Yazan O, Narayan KS, Goodwin TJ, et al. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell Dev Biol-Anim. 1997;33:459–66. doi: 10.1007/s11626-997-0064-8. [DOI] [PubMed] [Google Scholar]
- Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Delivery Rev. 2012;64:353–65. doi: 10.1016/j.addr.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Toita R, Katayama Y. Bio and nanotechnological strategies for tumor-targeted gene therapy. Biotechnol Adv. 2010;28:757–63. doi: 10.1016/j.biotechadv.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Kaur P, Ward B, Saha B, Young LL, Groshen S, Techy G, et al. Human breast cancer histoid: an in vitro 3-dimensional co-culture model that mimics breast cancer tissue. J Histochem Cytochem. 2011;59:1087–100. doi: 10.1369/0022155411423680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki CS, Shih H, Lin CC. Effect of 3D matrix compositions on the efficacy of EGFR inhibition in pancreatic ductal adenocarcinoma cells. Biomacromolecules. 2013;14:3017–26. doi: 10.1021/bm4004496. [DOI] [PubMed] [Google Scholar]
- Kim J, Taylor D, Agrawal N, Wang H, Kim H, Han A, et al. A programmable microfluidic cell array for combinatorial drug screening. Lab Chip. 2012;12:1813–22. doi: 10.1039/c2lc21202a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 2005;15:365–77. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Kim JW, Ho WJ, Wu BM. The Role of the 3D Environment in Hypoxia-induced Drug and Apoptosis Resistance. Anticancer Res. 2011;31:3237–45. [PubMed] [Google Scholar]
- Kim TH, Mount CW, Gombotz WR, Pun SH. The delivery of doxorubicin to 3-D multicellular spheroids and tumors in a murine xenograft model using tumor-penetrating triblock polymeric micelles. Biomaterials. 2010;31:7386–97. doi: 10.1016/j.biomaterials.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, et al. Basement-membrane complexes with biological-activity. Biochemistry. 1986;25:312–8. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4:66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 1999;59:1141–5. [PubMed] [Google Scholar]
- Krishnan V, Shuman LA, Sosnoski DM, Dhurjati R, Vogler EA, Mastro AM. Dynamic interaction between breast cancer cells and osteoblastic tissue: comparison of two- and three-dimensional cultures. J Cell Physiol. 2011;226:2150–8. doi: 10.1002/jcp.22550. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zhang X, Liang XJ. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol Adv. 2013;31:593–606. doi: 10.1016/j.biotechadv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Kundu B, Rajkhowa R, Kundu SC, Wang XG. Silk fibroin biomaterials for tissue regenerations. Adv Drug Delivery Rev. 2013;65:457–70. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- Kunz-Schughart LA, Kreutz M, Knuechel R. Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. Int J Exp Pathol. 1998;79:1–23. doi: 10.1046/j.1365-2613.1998.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarbera DV, Reid BG, Yoo BH. The multicellular tumor spheroid model for high-throughput cancer drug discovery. Expert Opin Drug Discov. 2012;7:819–30. doi: 10.1517/17460441.2012.708334. [DOI] [PubMed] [Google Scholar]
- Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. Bmc Cancer. 2011:11. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Kumar RA, Sukumaran SM, Hogg MG, Clark DS, Dordick JS. Three-dimensional cellular microarray for high-throughput toxicology assays. Proc Natl Acad Sci U S A. 2008;105:59–63. doi: 10.1073/pnas.0708756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- Li Q, Chen CY, Kapadia A, Zhou QO, Harper MK, Schaack J, et al. 3D models of epithelial-mesenchymal transition in breast cancer metastasis: high-throughput screening assay development, validation, and pilot screen. J Biomol Screen. 2011;16:141–54. doi: 10.1177/1087057110392995. [DOI] [PubMed] [Google Scholar]
- Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–43. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Gilmore DM, Zubris KAV, Xu XY, Catalano PJ, Padera RF, et al. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. Biomaterials. 2013;34:1810–9. doi: 10.1016/j.biomaterials.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TJ, Lin BC, Qin JH. Carcinoma-associated fibroblasts promoted tumor spheroid invasion on a microfluidic 3D co-culture device. Lab Chip. 2010;10:1671–7. doi: 10.1039/c000022a. [DOI] [PubMed] [Google Scholar]
- Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Obek C, Soloway MS, Block NL. Tumor-associated hyaluronic acid: A new sensitive and specific urine marker for bladder cancer. Cancer Res. 1997;57:773–7. [PubMed] [Google Scholar]
- Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, et al. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 2001;276:11922–32. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- Loo Y, Zhang SG, Hauser CAE. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol Adv. 2012;30:593–603. doi: 10.1016/j.biotechadv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Marrero B, Messina JL, Heller R. Generation of a tumor spheroid in a microgravity environment as a 3D model of melanoma. In Vitro Cell Dev Biol-Anim. 2009;45:523–34. doi: 10.1007/s11626-009-9217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–6. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 2012;164:192–204. doi: 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milane L, Duan Z, Amiji M. Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell Int. 2011:11. doi: 10.1186/1475-2867-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Moss NM, Liu YY, Johnson JJ, Debiase P, Jones J, Hudson LG, et al. Epidermal growth factor receptor-mediated membrane type 1 matrix metalloproteinase endocytosis regulates the transition between invasive versus expansive growth of ovarian carcinoma cells in three-dimensional collagen. Mol Cancer Res. 2009;7:809–20. doi: 10.1158/1541-7786.MCR-08-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Klieser W. Tumor biology and experimental therapeutics. Crit Rev Oncol Hematol. 2000;36:123–39. doi: 10.1016/s1040-8428(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Munson JM, Bellamkonda RV, Swartz MA. Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res. 2013;73:1536–46. doi: 10.1158/0008-5472.CAN-12-2838. [DOI] [PubMed] [Google Scholar]
- Murata T, Mizushima H, Chinen I, Moribe H, Yagi S, Hoffman RM, et al. HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells with cancer-associated fibroblasts to support progression of uterine cervical cancers. Cancer Res. 2011;71:6633–42. doi: 10.1158/0008-5472.CAN-11-0034. [DOI] [PubMed] [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–U10. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor D, Sionov RV, IshShalom D. CD44: Structure, function, and association with the malignant process. Advances in Cancer Research. 711997:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. 2008;14:149–65. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyga A, Cheema U, Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal. 2011;5:239–48. doi: 10.1007/s12079-011-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak CA, Mackay AA, Cowan DB. An improved method for the preparation of type I collagen from skin. J Vis Exp. 2014;21:51011. doi: 10.3791/51011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page H, Flood P, Reynaud EG. Three-dimensional tissue cultures: current trends and beyond. Cell Tissue Res. 2013;352:123–31. doi: 10.1007/s00441-012-1441-5. [DOI] [PubMed] [Google Scholar]
- Pedron S, Becka E, Harley BAC. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials. 2013;34:7408–17. doi: 10.1016/j.biomaterials.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, FaroKhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- Pekin D, Skhiri Y, Baret J-C, Le Corre D, Mazutis L, Ben Salem C, et al. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip. 2011;11:2156–66. doi: 10.1039/c1lc20128j. [DOI] [PubMed] [Google Scholar]
- Pluen A, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di Tomaso E, et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: Cranial vs. subcutaneous tumors. Proc Natl Acad Sci U S A. 2001;98:4628–33. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincloux R, Collin O, Lizarraga F, Romao M, Debray M, Piel M, et al. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc Natl Acad Sci U S A. 2011;108:1943–8. doi: 10.1073/pnas.1010396108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KJ, Tsykin A, Giles KM, Sladic RT, Epis MR, Ganss R, et al. Matrigel basement membrane matrix influences expression of microRNAs in cancer cell lines. Biochem Biophys Res Commun. 2012;427:343–8. doi: 10.1016/j.bbrc.2012.09.059. [DOI] [PubMed] [Google Scholar]
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan P, Kasif S, Murali TM. Systems biology characterization of engineered tissues. Annu Rev Biomed Eng. 2013;15:55–70. doi: 10.1146/annurev-bioeng-071811-150120. [DOI] [PubMed] [Google Scholar]
- Redden RA, Doolin EJ. Microgravity assay of neuroblastoma: in vitro aggregation kinetics and organoid morphology correlate with MYCN expression. In Vitro Cell Dev Biol-Anim. 2011;47:312–7. doi: 10.1007/s11626-011-9393-8. [DOI] [PubMed] [Google Scholar]
- Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–87. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell-adhesion: RGD and integrins. Science. 1987;238:491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Mendoza P, Contreras HR, Vergara J, McCubrey JA, Huidobro C, et al. Expression of multidrug resistance proteins in prostate cancer is related with cell sensitivity to chemotherapeutic drugs. Prostate. 2009;69:1448–59. doi: 10.1002/pros.20991. [DOI] [PubMed] [Google Scholar]
- Saraswathy M, Gong S. Different strategies to overcome multidrug resistance in cancer. Biotechnol Adv. 2013;31:1397–407. doi: 10.1016/j.biotechadv.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Sasser AK, Mundy BL, Smith KM, Studebaker AW, Axel AE, Haidet AM, et al. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett. 2007;254:255–64. doi: 10.1016/j.canlet.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Seo BR, DelNero P, Fischbach C. In vitro models of tumor vessels and matrix: Engineering approaches to investigate transport limitations and drug delivery in cancer. Adv Drug Deliv Rev. 2013;69–70:205–16. doi: 10.1016/j.addr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebriiskii I, Castello-Cros R, Lamb A, Golemis EA, Cukierman E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol. 2008;27:573–85. doi: 10.1016/j.matbio.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh AC, Swartz MA. Regulation of tumor invasion by interstitial fluid flow. Phys Biol. 2011;8:1478–3975. doi: 10.1088/1478-3975/8/1/015012. [DOI] [PubMed] [Google Scholar]
- Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–38. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Shirinifard A, Gens JS, Zaitlen BL, Poplawski NJ, Swat M, Glazier JA. 3D multi-cell simulation of tumor growth and angiogenesis. PLoS One. 2009;4:0007190. doi: 10.1371/journal.pone.0007190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoichet MS, Li RH, White ML, Winn SR. Stability of hydrogels used in cell encapsulation: An in vitro comparison of alginate and agarose. Biotechnol Bioeng. 1996;50:374–81. doi: 10.1002/(SICI)1097-0290(19960520)50:4<374::AID-BIT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA-Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Sieh S, Lubik AA, Clements JA, Nelson CC, Hutmacher DW. Interactions between human osteoblasts and prostate cancer cells in a novel 3D in vitro model. Organogenesis. 2010;6:181–8. doi: 10.4161/org.6.3.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardal A, Sarker SF, Crabbe A, Nickerson CA, Prestwich GD. The generation of 3-D tissue models based on hyaluronan hydrogel-coated microcarriers within a rotating wall vessel bioreactor. Biomaterials. 2010;31:8426–35. doi: 10.1016/j.biomaterials.2010.07.047. [DOI] [PubMed] [Google Scholar]
- Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5:291–6. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. Hyaluronan in cancer biology. Semin Cancer Biol. 2008a;18:237. doi: 10.1016/j.semcancer.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Stern R. Hyaluronidases in cancer biology. Semin Cancer Biol. 2008b;18:275–80. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Sutherland RM, Inch WR, McCredie JA, Kruuv J. Multi-component radiation survival curve using an in-vitro tumour model. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18:491–5. doi: 10.1080/09553007014551401. [DOI] [PubMed] [Google Scholar]
- Szot CS, Buchanan CF, Freeman JW, Rylander MN. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32:7905–12. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Kundu SC. A non-mulberry silk fibroin protein based 3D in vitro tumor model for evaluation of anticancer drug activity. Adv Funct Mater. 2012;22:4778–88. [Google Scholar]
- Tan DS. Diversity-oriented synthesis: exploring the intersections between chemistry and biology. Nat Chem Biol. 2005;1:74–84. doi: 10.1038/nchembio0705-74. [DOI] [PubMed] [Google Scholar]
- Tandon N, Marolt D, Cimetta E, Vunjak-Novakovic G. Bioreactor engineering of stem cell environments. Biotechnol Adv. 2013;31:1020–31. doi: 10.1016/j.biotechadv.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty TD, Coussens LM. Annual Review of Pathology-Mechanisms of Disease. Palo Alto: Annual Reviews; 2006. Tumor stroma and regulation of cancer development; pp. 119–50. [DOI] [PubMed] [Google Scholar]
- Tong Z, Duncan RL, Jia X. Modulating the behaviors of mesenchymal stem cells via the combination of high-frequency vibratory stimulations and fibrous scaffolds. Tissue Eng Part A. 2013;19:1862–78. doi: 10.1089/ten.tea.2012.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Zerdoum AB, Duncan RL, Jia X. Dynamic vibration cooperates with connective tissue growth factor to modulate stem cell behaviors. Tissue Eng Part A. 2014;23:23. doi: 10.1089/ten.tea.2013.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP, Hascall VC. Hyaluronan and tumor growth. Am J Pathol. 2002;161:745–7. doi: 10.1016/S0002-9440(10)64232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung YC, Hsiao AY, Allen SG, Torisawa YS, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–8. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulijn RV, Smith AM. Designing peptide based nanomaterials. Chem Soc Rev. 2008;37:664–75. doi: 10.1039/b609047h. [DOI] [PubMed] [Google Scholar]
- van de Waterbeemd H, Gifford E. ADMET in silico modelling: Towards prediction paradise? Nat Rev Drug Discovery. 2003;2:192–204. doi: 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]
- Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012:10. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, et al. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartenberg M, Frey C, Diedershagen H, Ritgen J, Hescheler J, Sauer H. Development of an intrinsic P-glycoprotein-mediated doxorubicin resistance in quiescent cell layers of large, multicellular prostate tumor spheroids. Int J Cancer. 1998;75:855–63. doi: 10.1002/(sici)1097-0215(19980316)75:6<855::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–7. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- Wei LY, Roepe PD. Low external pH and osmotic shock increase the expression of human MDR protein. Biochemistry. 1994;33:7229–38. doi: 10.1021/bi00189a027. [DOI] [PubMed] [Google Scholar]
- Wendt D, Riboldi SA, Cioffi M, Martin I. Potential and bottlenecks of bioreactors in 3D cell culture and tissue manufacturing. Adv Mater. 2009;21:3352–67. doi: 10.1002/adma.200802748. [DOI] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–73. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Wolf K, Friedl P. Molecular mechanisms of cancer cell invasion and plasticity. Br J Dermatol. 2006;1:11–5. doi: 10.1111/j.1365-2133.2006.07231.x. [DOI] [PubMed] [Google Scholar]
- Wong C, Stylianopoulos T, Cui JA, Martin J, Chauhan VP, Jiang W, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108:2426–31. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LY, Di Carlo D, Lee LP. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdevices. 2008;10:197–202. doi: 10.1007/s10544-007-9125-8. [DOI] [PubMed] [Google Scholar]
- Xu X, Gurski LA, Zhang C, Harrington DA, Farach-Carson MC, Jia X. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials. 2012a;33:9049–60. doi: 10.1016/j.biomaterials.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia X. Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter. 2012b;8:3280–94. doi: 10.1039/C2SM06463D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Sabanayagam CR, Harrington DA, Farach-Carson MC, Jia X. A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics. Biomaterials. 2014;35:3319–30. doi: 10.1016/j.biomaterials.2013.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XX, Liu C, Liu Y, Li N, Guo X, Wang SJ, et al. Encapsulated human hepatocellular carcinoma cells by alginate gel beads as an in vitro metastasis model. Exp Cell Res. 2013;319:2135–44. doi: 10.1016/j.yexcr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Yang ZH, Zhao XJ. A 3D model of ovarian cancer cell lines on peptide nanofiber scaffold to explore the cell-scaffold interaction and chemotherapeutic resistance of anticancer drugs. Int J Nanomed. 2011;6:303–10. doi: 10.2147/IJN.S15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates C, Shepard CR, Papworth G, Dash A, Beer Stolz D, Tannenbaum S, et al. Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Adv Cancer Res. 2007;97:225–46. doi: 10.1016/S0065-230X(06)97010-9. [DOI] [PubMed] [Google Scholar]
- Yip D, Cho CH. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem Biophys Res Commun. 2013;433:327–32. doi: 10.1016/j.bbrc.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Zaman MH. The role of engineering approaches in analysing cancer invasion and metastasis. Nat Rev Cancer. 2013;13:596–603. doi: 10.1038/nrc3564. [DOI] [PubMed] [Google Scholar]
- Zhang LR, Underhill CB, Chen LP. Hyaluronan of the surface of tumor-cells is correlated with metastatic behavior. Cancer Res. 1995;55:428–33. [PubMed] [Google Scholar]
- Zhang SG, Gelain F, Zhao XJ. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol. 2005a;15:413–20. doi: 10.1016/j.semcancer.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Wang W, Yu WT, Xie YB, Zhang XH, Zhang Y, et al. Development of an in vitro multicellular tumor spheroid model using microencapsulation and its application in anticancer drug screening and testing. Biotechnol Prog. 2005b;21:1289–96. doi: 10.1021/bp050003l. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nagrath S. Microfluidics and cancer: are we there yet? Biomed Microdevices. 2013;15:595–609. doi: 10.1007/s10544-012-9734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhau HE, Goodwin TJ, Chang SM, Baker TL, Chung LWK. Establishment of a three-dimensional human prostate organoid coculture under microgravity-simulated conditions: Evaluation of androgen-induced growth and PSA expression. In Vitro Cell Dev Biol-Anim. 1997;33:375–80. doi: 10.1007/s11626-997-0008-3. [DOI] [PubMed] [Google Scholar]
- Zhu H, Chen XP, Luo SF, Guan J, Zhang WG, Zhang BX. Involvement of hypoxia-inducible factor-1-alpha in multidrug resistance induced by hypoxia in HepG2 cells. J Exp Clin Cancer Res. 2005;24:565–74. [PubMed] [Google Scholar]
- Zhu H, Luo S-f, Wang J, Li X, Wang H, Pu W-y, et al. Effect of environmental factors on chemoresistance of HepG2 cells by regulating hypoxia-inducible factor-1 alpha. Chin Med J. 2012;125:1095–103. [PubMed] [Google Scholar]
- Zubris KAV, Liu R, Colby A, Schulz MD, Colson YL, Grinstaff MW. In vitro activity of paclitaxel-loaded polymeric expansile nanoparticles in breast cancer cells. Biomacromolecules. 2013;14:2074–82. doi: 10.1021/bm400434h. [DOI] [PMC free article] [PubMed] [Google Scholar]