Abstract
Hepatitis C virus (HCV) infection affects 180 million people worldwide with the predominant prevalence being infection with genotype 1, followed by genotypes 2 and 3. Standard anti-HCV therapy currently aims to enhance natural immune responses to the virus, whereas new therapeutic concepts directly target HCV RNA and viral enzymes or influence host-virus interactions. Novel treatment options now in development are focused on inhibitors of HCV-specific enzymes, NS3 protease and NS5B polymerase. These agents acting in concert represent the concept of specifically targeted antiviral therapy for HCV (STAT-C). STAT-C is an attractive strategy in which the main goal is to increase the effectiveness of antiviral responses across all genotypes, with shorter treatment duration and better tolerability. However, the emergence of resistant mutations that limit the use of these compounds in monotherapy complicates the regimens. Thus, a predictable scenario for HCV treatment in the future will be combinations of drugs with distinct mechanisms of action. For now, it seems that interferon will remain a fundamental component of any new anti-HCV therapeutic regimens in the near future; therefore, there is pressure to develop forms of interferon that are more effective, less toxic, and more convenient than pegylated interferon.
Keywords: Hepatitis C virus, Chronic hepatitis C, Polymerase inhibitors, Protease inhibitors, Cyclophilin B inhibitors
INTRODUCTION
Hepatitis C virus (HCV) infection affects 180 million people worldwide, about 3% of the world’s population. Globally, 3-4 million persons are newly infected each year, with the predominant prevalence being infection with genotype 1, followed by genotypes 2 and 3. The other genotypes, 4, 5, and 6, have specific geographical distributions. The acute phase of infection is asymptomatic in the majority of cases, but leads to chronic infection in about 70%-80% of affected individuals[1,2]. This results in progressive liver disease and eventually liver cirrhosis, with increased risk of hepatocellular carcinoma[3].
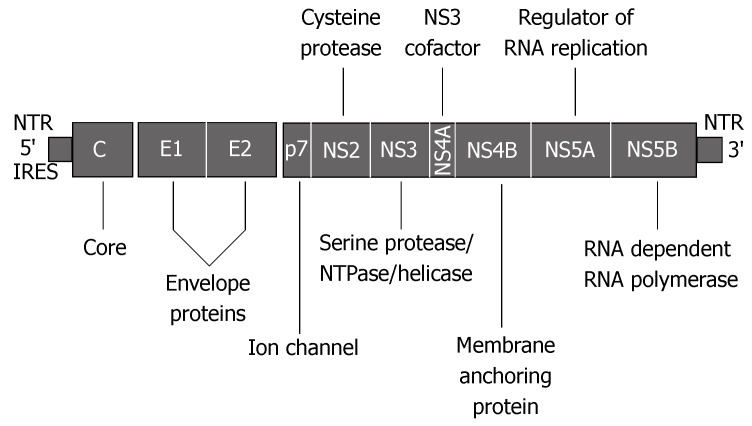
HCV is an enveloped virus belonging to the Flaviviridae family. The virion consists of an outer envelope composed of E1 and E2 proteins covering the nucleocapsid, with a single-stranded RNA genome (Figure 1). The HCV genome encodes structural proteins that form the capsid and envelope, as well as non-structural proteins required for virus replication. The latter are potential targets for new drugs aimed at directly affecting HCV. The non-structural components include proteases and helicase (NS2, NS3 and NS4A), a protein responsible for anchoring the replication complex to intracytoplasmic membranes (NS4B), the RNA-binding protein (NS5A), and RNA-dependent RNA polymerase (NS5B). After entering the cell, HCV RNA is released into the cytoplasm. The genome is directly translated by host enzymes. The HCV polyprotein is subsequently processed by the host and viral proteases into structural and non-structural proteins, including NS5B, which is crucial for HCV replication. There are two viral proteases, NS2-3 and NS3. NS2-3 is responsible for cleavage at the NS2/3 site. NS3 catalyses cleavage at four sites: NS3/4A, NS4A/B, NS4B/5A and NS5A/B. The proteolytic activity of NS3 protease is significantly enhanced by heterodimerization with its cofactor NS4B[4].
Figure 1.
Organization of the HCV genome (adapted from Appel et al[4]).
CURRENT TREATMENT OPTIONS
The therapy currently regarded as the standard consists of pegylated interferon injections administered once weekly, along with daily oral ribavirin. This combination exerts synergistic antiviral effects, although it is efficient in only about 50% of treated individuals. The most important predicting factor appears to be the HCV genotype. Sustained viral response (SVR) expressed as an absence of detectable serum HCV RNA 24 wk after completion of therapy is achieved in only 33%-42% of patients with genotype 1, but in about 90% of those with genotypes 2 and 3[5]. There are patients who do not achieve an SVR due to unresponsiveness or relapse after treatment, as well as those who lack tolerance to adverse events that occur during treatment. Therefore, there is a need for new therapeutic strategies with higher efficacy, shorter treatment duration, convenient routes of administration and favorable side-effect profiles.
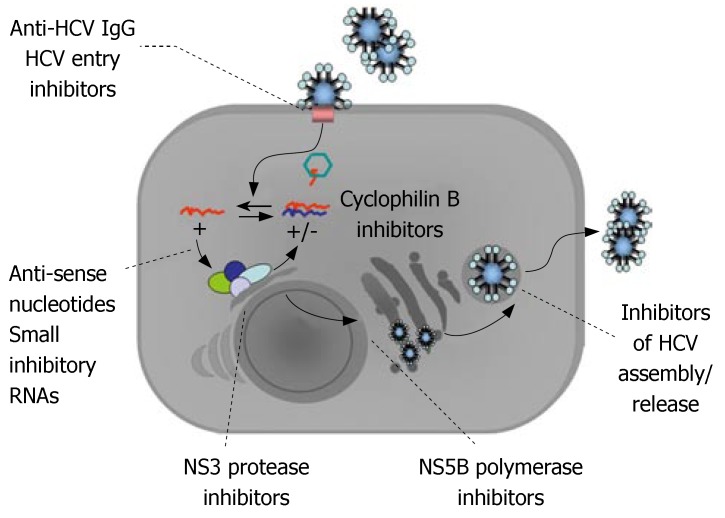
The standard anti-HCV therapy currently aims to enhance natural immune responses to the virus; whereas, new therapeutic concepts directly target HCV RNA (anti-sense nucleotides, small inhibitory RNA) and viral enzymes (protease and polymerase inhibitors), or influence host-virus interactions (cyclophilin inhibitors, bavituximab, inhibitors of viral entry) (Figure 2)[6-9]. These findings essentially came from attempts to create an experimental model for studies of the molecular details of the virus and HCV infection of cells. The development of the replicon system and pseudoviruses has greatly contributed to the current knowledge of HCV replication. However, a highlight in recent studies on HCV has been the establishment of a complete cell culture replication system that enables further research into the HCV lifecycle and novel antivirals.
Figure 2.
Potential targets for new anti-HCV agents.
NOVEL ANTIVIRAL AGENTS
Novel treatment options now under intensive development are focused on the inhibitors of HCV-specific enzymes, NS3 protease and NS5B polymerase. The actual protease inhibitors under clinical development are telaprevir and SCH 503034. Among the polymerase inhibitors, several drugs have reached the clinical stage. These include valopicitabine, R1479, HCV-796 and BILB1941. Such agents acting in concert represent the concept of specifically targeted antiviral therapy for HCV (STAT-C). The principle of this new therapeutic strategy is the achievement of higher rates of efficacy, shortened treatment duration, improved tolerability and adherence, oral administration and the possibility for use in special populations (e.g., those with contraindications to interferon). However, the emergence of resistance mutations that limit the use of these compounds in monotherapy complicates the regimens. Thus, a predictable scenario for treatment of HCV in the future will be combinations of drugs with distinct mechanisms of action. Interferon, however, appears pivotal for any new therapeutic regimens.
INTERFERONS IN DEVELOPMENT
Pegylated interferon underpins the current standard of care therapy for HCV. Its immunomodulatory activities with positive pharmacokinetics significantly improve the efficacy of anti-HCV regiments, although the effectiveness of antiviral treatment remains unsatisfactory, especially as regards genotype-1 infections. Another limitation is the frequent occurrence of adverse events, which greatly influence adherence to the treatment regimen. Therefore, there is pressure to develop forms of interferon that are more effective, less toxic, and more convenient than pegylated interferon. Phase 1 clinical trials are in progress with oral forms of interferon (Belerofon; oral interferon alpha). Another goal in the field of interferon development is to obtain forms with a longer half life, which will allow a reduction in the number of injections required and lengthen the period between injections. These agents [IL-29 (PEG-interferon lambda), Belerofon Interferon (long-acting), BLX-883 (Locteron), and Medusa Interferon (Multiferon)] are mostly in the first or second phase of their clinical development. Omega interferon, which is in phase II development, is used in an implantable infusion pump that releases a steady amount of interferon for about 1 mo[10]. Albinterferon (IFN-α2b genetically fused to human albumin) and interferon β-1a (REBIF) have reached phase III development. Albinterferon is a protein that combines the antiviral properties of IFN-α2b with the prolonged half-life of human serum albumin. Clinical trials have shown that albinterferon administered every 2 wk exerts an antiviral efficacy comparable to that of Peg-IFN-α2a, with a similar frequency of occurrence of adverse events and laboratory abnormalities[11]. Consensus interferon (Infergen) administered daily is currently in phase IV clinical trials, and research evaluating its usefulness in treatment of prior non-responders is ongoing, with promising interim results[12,13].
PROTEASE INHIBITORS
Telaprevir (VX-950), an investigational oral selective inhibitor of HCV NS3/4A protease[14], appears to be the one of most advanced therapeutic agents that specifically target the HCV lifecycle. Clinical phase IIb studies utilizing VX-950 are ongoing. These include the PROVE 1 and PROVE 2 trials that have enrolled 260 and 320 treatment-naïve HCV genotype 1 patients, respectively, and the PROVE 3 study in patients with HCV genotype 1, previously treated by standard of care therapy (at least one prior course of peginterferon with ribavirin).
The phase Ib studies showed that VX-950 had potent anti-HCV activity in monotherapy, which led to a rapid decline in HCV RNA of about 3 log within 3 d in all dosage groups (450 mg q8h, 750 mg q8h and 1250 mg q12h), both in genotype 1 treatment-naïve patients and non-responders to prior treatment. In the optimal dose group, 750mg q8h, the highest plasma concentration was achieved with a 4.4 log reduction in HCV RNA load at d 14. When sub-optimal doses were applied, maximal HCV RNA reduction was seen after 3-7 d treatment, with any following increase in viral load being related to the selection of HCV variants less sensitive to VX-950[15,16]. Moreover, other studies have reported that the abovementioned HCV RNA fluctuations, as well as alanine aminotransferase (ALT) levels during treatment correlated with the concentration of neopterin - a monocyte/macrophage-derived factor that can be considered an indicator of inflammatory activity during the course of chronic C hepatitis[17,18]. Furthermore, the ultrasound evaluation of perihepatic lymph nodes (PLNs) in HCV-infected individuals undergoing treatment with telaprevir alone showed a significant reduction in PLN volume after 14 d therapy. This suggests that VX-950 may have the ability to reduce inflammatory activity in liver tissue; however, this research is limited by a lack of objective parameters for inflammatory activity, such as histopathological evaluation[19].
The HCV NS3 protease diversity determined before administration of VX-950 appears to have no predictive impact for the effectiveness of antiviral responses[20]. Whereas, identification of HCV NS3 protease variants after 14 d treatment demonstrates the emergence of mutations that implicate varying decreases in sensitivity to VX-950, low-level resistance characterized by V36A/M, T54A, R155K/T, and A156S mutations, and high-level resistance determined by the emergence of A156V/T, V36A/M-R155K/T, and V36A/M-A156V/T variants. Each dosage group has a distinct mutation pattern that results from the different levels of drug pressure on the variants selected. It has been suggested that these drug-resistant variants pre-exist at a minimal frequency before the introduction of treatment, because of the low fidelity of HCV polymerase and the high rate of HCV replication. Resistant variants appear to be less fit and have lower replication rates compared to wild type; therefore, they may not be detected prior to treatment[21,22].
Further studies of VX-950 administered with peginterferon, with or without ribavirin, have confirmed the advantageous effect of combination therapy on virus selection. Data presented at the American Association for the Study of Liver Diseases (AASLD) Meeting in 2006 demonstrated a 5.5 log HCV RNA drop when VX-950 was combined with peginterferon α2a vs a 1 log drop in those receiving peginterferon alone, and a 4 log decrease in monotherapy with VX-950 at d 14 of the study[23]. With regard to selection of the resistant variant, this study provides evidence of the suppressing effect of peginterferon when it is included in a combination therapy regimen, or is applied as a follow-on after discontinuation of VX-950, thus indicating that VX-950-resistant variants remain sensitive to the standard care therapy. This observation is consistent with in vitro research confirming the decreased replication capacity of resistant variants while the sensitivity to interferon is fully retained[22]. Interestingly, in a few patients receiving VX-950 alone, the higher level resistant variant A156V/T emerged, but was subsequently suppressed by therapy with VX-950 followed by peginterferon and ribavirin. In this study, all patients receiving peginterferon and ribavirin subsequent to 14 d treatment with VX-950 had undetectable HCV RNA at the end of wk 24. However, the discontinuation of therapy at that point in individuals with undetectable HCV RNA at wk 12, resulted in relapses in two of the four patients from the VX-950 monotherapy group, and one of six from the VX-950 with peginterferon group, which showed the advantages of combination therapy over monotherapy[23,24]. The interim results after 12 wk of the PROVE 1 study, the first major phase II clinical trial to evaluate VX-950, are now available. Analysis shows a definitely higher incidence of undetectable HCV RNA [limit of detection (LOD) 10 IU/mL] at wk 12 in patients receiving VX-950 in combination with peginterferon and ribavirin, in contrast to those receiving standard therapy (88% vs 52%). The frequency of adverse events was comparable in the telaprevir-treated and control groups. However, discontinuation due to adverse events was higher in the telaprevir than in the control groups, 9% vs 3%. The adverse events most frequently reported in the telaprevir group included rashes (3%), gastrointestinal disorders and anemia. The incidence of serious adverse events in the telaprevir groups was about 3% compared to 1% in the control[25]. Further research on telaprevir that aimed to assess its activity against the NS3/4A proteases of HCV genotypes 2, 3 and 4 was presented at the European Association for the Study of the Liver (EASL) Meeting in 2007. In vitro studies have demonstrated that the VX-950 activity against genotypes 2a, 2b, 3a and 4a is similar to that for genotypes 1a or 1b. Moreover, NS3/4A protease heterogeneity seems to have an unremarkable impact on VX-950 suppressive activity. Hence, it has been suggested that the majority of genotype-specific polymorphic sequences are located peripherally to the active sites of HCV protease, and do not affect binding of the agent molecule[26]. This investigation confirms the necessity for further research in this subject area. By contrast to the above are observations of telaprevir activity in a liver biopsy model of HCV infection. Cell cultures from liver biopsies of patients with genotype 1 and non-genotype 1 HCV were exposed to VX-950, which resulted in a significant decrease in HCV genotype 1 RNA, but only a minimal effect in non-genotype 1 HCV[27]. Thus further studies are required.
Another HCV protease inhibitor, SCH 503034, orally bioavailable with satisfactory pharmacokinetics and a good safety profile, is being tested in a phase II clinical trial[28]. In vitro studies have determined its anti-viral activity on its own, and an additive effect in combination with interferon α-2b[29,30]. Monotherapy with SCH 503034, at a dose of 400 mg q8h, in HCV-genotype-1-infected non-responders to prior standard therapy resulted in a 2.06 log reduction of the mean maximum viral load during the 14 d period of observation. Moreover, the HCV RNA decline correlated with ALT activity, and both of these parameters appeared to be dose-dependent. It is important to mention that the frequency of adverse events was comparable to the control group receiving a placebo[31]. Similar to telaprevir, further research into combination therapy with SCH 503034 and peginterferon has shown advantages over monotherapy with SCH 503034 or peginterferon. The mean maximum HCV RNA decline was dose-dependent, 2.4 log and 2.9 log for 200 mg q8h and 400 mg q8h, respectively, of SCH 503034 with peginterferon[32]. Another study designed to evaluate the antiviral effects of a combination of an NS3 protease inhibitor with a polymerase inhibitor strongly supports the concept of STAT-C. In this research, genotype 1b HCV replicon cells, wild-type, as well as replicons with reduced susceptibility to protease inhibitors or polymerase inhibitors, were exposed to a combination of SCH 503034 with the polymerase inhibitor HCV-796. The results were encouraging; the antiviral effect of these two agents was additive, with a favorable cross-resistance profile. Furthermore, the emergence of resistant viral variants was less frequent compared to that for monotherapy[33].
ITMN-191, a novel agent with a high genetic barrier to emergence of resistance mutations, is in preclinical development and has been shown to retain significant potency against variants with decreased sensitivity to other protease inhibitors[34]. Other in vitro studies have shown that ITMN-191 displays synergistic antiviral activity in combination with interferon[35,36], as well as HCV polymerase inhibitor R1479[37].
Recently, the clinical trials for BILB2061, another specific and potent inhibitor of HCV NS3 protease, were halted due to drug-induced cardiotoxicity[38].
ACH-806 is a novel anti-HCV agent, currently in phase I/II clinical studies, which has a distinct mechanism of action. Preclinical data have demonstrated that ACH-806 inhibits HCV genotype 1 replication via binding to NS4A and consequently blocks the formation of HCV replication complexes[39]. Resistance induction studies in replicon cells have shown a lack of cross resistance between ACH-806 and protease or polymerase inhibitors; thus confirming its different mode of action. Interestingly, viral quasispecies resistant to protease or polymerase inhibitors remain sensitive to ACH-806 and vice versa[40]. In vitro studies of a combination of ACH-806 with telaprevir or valopicitabine or interferon have demonstrated potent antiviral effects, which are synergistic when cells are exposed to ACH-806 with telaprevir or ACH-806 with valopicitabine. Hence the authors have concluded that ACH-806 could in the future become a potent component of STAT-C. Clinical studies preformed on genotype 1 HCV-infected subjects have shown an ~ 1 log reduction in HCV RNA at d 5. Further research on ACH-806 has been limited because of reversible nephrotoxicity reported in a clinical trial[41].
POLYMERASE INHIBITORS
The inhibitors of HCV NS5B polymerase influence different steps in RNA synthesis, from initiation to elongation through binding to the enzyme's active sites. Valopicitabine (NM283) appears the most advanced orally bioavailable pro-drug of a ribonucleoside analogue that displays an ability to sufficiently inhibit HCV NS5B polymerase. It is currently in phase II clinical development. It has been shown that NM283 given as monotherapy exhibits significant, dose-related antiviral activity in both treatment-naïve and non-responders to prior interferon therapy. This effect was marked and synergistic, with a mean HCV RNA reduction of 2.7 log at d 28 when NM283 was administered in combination with peginterferon[42]. Studies in genotype 1 HCV-infected treatment-naïve patients showed an intense HCV RNA decline of about 4 log at wk 24 when NM283 was included in the regimen[43]. However evaluation of the side effects, mainly gastrointestinal and hematological disorders at higher doses, resulted in revision of the dosages. Interestingly, at higher doses, NM283 developed superior rapid response rates, although after 12, 24 and 36 wk of treatment the results were similar in all the different dose groups. Hence, low-dose NM283 in combination with peginterferon seems to display marked antiviral effects with fewer adverse events[43,44]. Moreover, there are promising results of in vitro research into the efficacy of NM283 against HCV variants resistant to protease inhibitors. All mutations tested conferring resistance to protease inhibitors were sensitive to NM283, thus supporting the advantages of combination therapy. As well, only a single mutation (S282T), described in the highly conserved B domain of NS5B, has so far been identified as determining moderate resistance to NM283[45]. In a separate study, a combination of two agents with distinct mechanisms of action, NM283 and SCH 503034, revealed enhanced anti-replicon activity, without observed cross-resistance[46]. These data support the initiative to develop combination regimens for treatment of HCV infections.
Other polymerase inhibitors currently in clinical development are nucleoside analogue R1479 (prodrug R1626) and non-nucleoside analogues HCV-796 and BILB1941. In phase I trials, R1626 has been shown to induce the greatest viral load reduction in genotype 1 HCV-infected patients, so far reported among this class of antiviral agents. It was a 3.7 log mean HCV RNA decline for 4500 mg q12h at d 14, and a 2.6 log decline for the lower dose of 3000 mg q12h, but with better tolerability[47]. In vitro analysis of the antiviral effects exerted by combinations of R1479 with interferon or ribavirin have demonstrated synergistic effects, whereas with other direct antivirals the effects were additive[48]. Another compound, HCV-796, when utilized in monotherapy exhibited dose-related anti-HCV activity across multiple genotypes, and with a good tolerability profile. Although, a selection of variants with reduced susceptibility was observed, they retained sensitivity to interferon[49]. Associated studies of combined HCV-796 and peginterferon have confirmed the increased antiviral response to therapy, which was more intense in non-genotype 1 HCV[50]. In a clinical evaluation, polymerase inhibitor BILB 1941 showed anti-HCV activity; however, a high discontinuation rate due to adverse events within the group with appropriate pharmacokinetics was observed[51].
Several novel compounds are now being tested in preclinical trials; these include PSI-6130, GSK 625433, A-848837, A-837093 and AG-021541[52-56]. Additional studies should determine whether these polymerase inhibitors reach a clinical development stage in the future.
CYCLOPHILIN INHIBITORS
The favorable complementary elements of various prospective therapies seem to be the cyclophilin inhibitors, which exhibit peculiar mechanisms of action based on targeting of virus-host interactions. Cyclophilin B (Cyp B) has been shown to serve as a cellular cofactor of the NS5B RNA-dependent RNA polymerase that promotes HCV replication[57,58]. Thus the blocking of Cyp B appears to be an attractive target for the development of new anti-HCV agents[59-61]. The first compound shown to exhibit inhibitory properties with reference to cyclophilins was cyclosporin A (CsA)[60-62]. It was shown that its antiviral activity against HCV was distinct from its immunosuppressive function, which was dependent on anti-calcineurin activity[60-63]. Clinical studies on a regimen based on a combination of CsA with interferon-α demonstrated a high antiviral potency compared to that with interferon alone[63]. However, the immunosuppressive function of CsA limits its usefulness in anti-HCV therapy. Moreover, there are various opinions regarding the benefits of administering CsA vs tacrolimus in post-liver transplantation management of individuals with HCV infection[64,65]. The available data are limited, and the results are equivocal[66,67]. Thus, further studies are required in this area.
The first synthesized CsA derivate found to be devoid of immunosuppressive activity was NIM811. It has been investigated in regard to its anti-HIV inhibitory features[68,69]. Recent studies have confirmed its anti-HCV activity in vitro. The reduction of HCV RNA in replicon cells was more potent, even at low concentrations, compared to CsA. Moreover, NIM811 shows activity against HCV in replicons with high resistance to protease or polymerase inhibitors. Also, combinations of NIM811 with other groups of HCV inhibitors display at least additive effects[71]. Analogous results, with potentially synergistic effects, have been obtained when HCV replicon cells were exposed to a combination of NIM811 with interferon-α[72]. The anti-HCV activity of NIM-811 exerted at low drug concentrations infers reduced potential side effects from the therapy. Hence, in vivo evaluations of the safety profile, pharmacokinetics and anti-HCV activity need to be undertaken.
The first oral non-immunosuppressive cyclophilin B inhibitor, Debio 025, to enter into clinical trials displayed potent antiviral effects in chimeric mice, and the effect was enhanced in combination with pegylated interferon[72]. In vitro studies have demonstrated that Debio 025 has a unique potency for clearing HCV replicon cells of virus when used alone or in combination with other antivirals. The combination of Debio 025 with interferon α-2a results in additive to slightly synergistic antiviral effects. Resistant replicon cells, selected by passage in increasing concentrations of Debio 025, are sensitive to protease or polymerase inhibitors and interferon[73]. Moreover, Debio 025 exerts antiviral activity against wild-type HCV, as well as HCV replicons resistant to protease or polymerase inhibitors. The emergence of resistant variants to protease inhibitors (VX-950, BILB 2061) is restrained by the presence of Debio 025[74]. Clinical research shows that Debio 025 exerts both anti-HIV and anti-HCV activity in HIV/HCV co-infected subjects. The anti-HIV effect at d 15 was a 1 log decrease in viral load when the dose was 1200 mg q12h. HCV viremia decline was more profound, reaching 3.6 log at d 15. However, at this dose, transitional hyperbilirubinemia led to the discontinuation of treatment in some patients[6]. Debio 025 has been shown to exert anti-HCV activity in both genotype 1 and non-genotype 1 infections. Interestingly, no signals of emerging mutation appeared during the period of observation, which suggests a possible ability for this agent to suppress selection of resistant variants[75].
Recently the novel non-immunosuppressive CsA derivate, SCY-635, has been evaluated in preclinical studies that have demonstrated its favorable properties as an anti-HCV agent. Compared to CsA, this compound has good affinity with cyclophilin, and exerts potent antiviral activity with suitable pharmacokinetics and lower cytotoxicity. The combination of SCY-635 with interferon-α has antiviral effects on HCV replicon cells that range form additive to synergistic; thus supporting the need for more evaluations of this drug in anti-HCV treatment options[7].
CONCLUSION
Specifically targeted antiviral therapy, through interference in the replication cycle, leads to the selection of resistant variants[76-78]. This observation has been confirmed in several studies focusing on STAT-C agents as used in monotherapy. Combination therapy displays higher antiviral efficacy and has a fundamental impact on the selection of HCV strains with concomitantly reduced sensitivity and decreased viral fitness. It has also been shown that protease and polymerase inhibitors demonstrate advantageous cross-resistance profiles with improved anti-HCV activity, which supports the STAT-C development initiatives. Interestingly, the introduction of interferon to regimens based on novel compounds not only augments antiviral effects, but has a strong suppressive activity against the emergence of resistant mutations. Considering this, it is seems unlikely that interferon will be eliminated from antiviral regimens in the near future.
STAT-C presents an attractive strategy, one in which the main goal is to increase the effectiveness of antiviral responses across all genotypes, with shorter treatment duration and better tolerability. Recent studies have shown that these aims are within reach. Novel therapeutic agents are already in advanced clinical development; listed here in Table 1. Although there are many limitations pertaining to the STAT-C strategy; including variable bioavailability, and different effects exerted against various HCV genotypes and even quasispecies. A disadvantage of direct interference in the virus replication cycle is the increased rate of resistant mutations, but this could be ameliorated by the use of multiply agents with different mechanisms of actions. In combination with interferon, these drugs could augment antiviral effects and reduce some of the toxic side effects of this cytokine. On the other hand, the toxicity of new agents needs to be carefully evaluated, along with their pharmacokinetics, safety and effective doses. New regimens will also need to be worked out. Currently, it appears that interferon will remain as an element of anti-HCV therapy in the near future; indeed its effectiveness could be elevated by the introduction of new drugs.
Table 1.
Anti-HCV agents currently under clinical development
| Compound | Clinical advancement nt | Viral efficacy | Resistance (HCV resistant variants) | Remarks | |
| Protease inhibitors | Telaprevir | II | (dose-750 mg q8h; genotype-1) | Low level: V36A/M, T54A, R155K/T, A156S | Rash, GI and hematological adverse events (AE) |
| (VX-950) | PROVE- 1, 2, 3 | VLR = 3 log d 3 | High level: A156V/T, V36A/M-R155K/T, V36A/M-A156V/T; | ||
| VLR = 4.4 log: d 14 | After 14 d of treatment | ||||
| VLR = 5.5 log: d 14 when combined with PEG-IFNa2a | |||||
| SCH 503034 | II | (dose 400 mg q8h; genotype-1) | Low to moderate levels: | Frequency of AE comparable to control group receiving placebo | |
| VLR = 2.06 log d 14 | V170A | ||||
| VLR = 2.9 log: when combined with PEG-IFN | T54A | ||||
| A156S | |||||
| High level: A156T | |||||
| Inhibitor of protease cofactor NS4A | ACH-806 | I/II | VLR = 1 log: d 5 | Single mutation at N-terminus of NS3; lack of cross resistance to any of the polymerase inhibitors or protease inhibitors now under development | Reversible nephrotoxicity |
| Polymerase inhibitors | Valopicitabine (NM283) | II | VLR = 0.8 log: d 28 | S282T | GI and hematological AE |
| VLR = 2.7 log: d 28, when combined with PEG-IFN | |||||
| VLR = 4.24 log: wk 24, when combined with PEG-IFN | |||||
| R1479 (R1626) | II | VLR = 3.7 log for 4500 mg q12h at d 14, VLR = 2.6 log for 3000 mg q12h | no data | Mild to moderate hematological AE with increasing doses | |
| HCV-796 | II | VLR = 1.4-1.5 log: d 4 | C316Y | Mild to moderate headache-the most frequent AE; no treatment-emergent serious AE | |
| VLR = 3.3-3.5 log: d 14, when combined with PEG-IFN | |||||
| BILB 1941 | I | Data incomplete due to high discontinuation rate | No data | GI AE | |
| Cyclophilin inhibitors | DEBIO 025 | I | VLR = 3.6 log: d 14 of monotherapy | No breakthrough during the treatment | Transitional hyperbilirubinemia |
Footnotes
S- Editor Ma N L- Editor Kerr C E- Editor Liu Y
References
- 1.Hepatitis C--global prevalence (update) Wkly Epidemiol Rec. 2000;75:18–19. [PubMed] [Google Scholar]
- 2.Barrera JM, Bruguera M, Ercilla MG, Gil C, Celis R, Gil MP, del Valle Onorato M, Rodés J, Ordinas A. Persistent hepatitis C viremia after acute self-limiting posttransfusion hepatitis C. Hepatology. 1995;21:639–644. [PubMed] [Google Scholar]
- 3.Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol. 2005;43:550–552. doi: 10.1016/j.jhep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Appel N, Schaller T, Penin F, Bartenschlager R. From structure to function: new insights into hepatitis C virus RNA replication. J Biol Chem. 2006;281:9833–9836. doi: 10.1074/jbc.R500026200. [DOI] [PubMed] [Google Scholar]
- 5.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 6.Flisiak R, Horban A, Kierkus J, Stanczak J, Cielniak I, Stanczak GP, Wiercinska-Drapalo A, Siwak E, Higersberger J, Aeschlimann C, et al. The cyclophilin inhibitor DEBIO-025 has a potent dual anti-HCV activity in treatment-naive HIV/HCV co-infected subjects. Hepatology. 2006;44 Suppl 1:S609. [Google Scholar]
- 7.Houck DR. Preclinical evaluation of SCY-635, a cyclophilin inhibitor with potent anti-HCV activity. Hepatology. 2006;44 Suppl 1:S534. [Google Scholar]
- 8.Godofsky EW, Shan JS. Phase I single-dose study of bavituximab, a chimeric anti-phosphatidyl serine monoclonal antibody, in subjects with chronic hepatitis C. Hepatology. 2006;44 Suppl 1:S236. [Google Scholar]
- 9.Matsumura T, Kato T, Hu Z, Juteau JM, Vaillant A, Liang JT. A novel class of amphipathic DNA polymers inhibits hepatitis C virus infection by blocking viral entry. Hepatology. 2006;44 Suppl 1:S693. doi: 10.1053/j.gastro.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novozhenov V, Zakharova N, Vinogradova E, Nikiton I, Gorbakov V, Yakovlev A, Pak S, Rafalski V, Bogomolov P, Alessi T, et al. Phase 2 study of omega interferon alone or in combination with ribavirin in subjects with chronic hepatitis C genotype-1 infection. J Hepatol. 2007;46 Suppl 1:S8. [Google Scholar]
- 11.Zeuzem S, Benhamou Y, Bain V, Shouval D, Pianko S, Flisiak R, Grigorescu M, Rehak V, Yoshida E, Kaita K, et al. Antiviral response at week 12 following completion of treatment with albinterferon α-2b plus ribavirin in genotype-1, IFN-naive, chronic hepatitis C patients. J Hepatol. 2007;46 Suppl 1:S293. [Google Scholar]
- 12.Chen K, Seraphin P, Murphy L, Shah N. Consensus interferon and ribavirin in patients with chronic hepatitis C who were nonresponders to prior therapy with either interferon alpha and ribavirin or pegylated interferon and ribavirin. J Hepatol. 2005;42 Suppl 1:S670. [Google Scholar]
- 13.Ghalib R, Levine C, Mouti M, Weinstein J, Schwartz A, Cheng S. Early viral response to consensus interferon plus ribavirin therapy in patients who are nonresponders or relapsers to prior PEG IFN plus ribavirin therapy. Hepatology. 2005;42 Suppl 1:S686. [Google Scholar]
- 14.Perni RB, Almquist SJ, Byrn RA, Chandorkar G, Chaturvedi PR, Courtney LF, Decker CJ, Dinehart K, Gates CA, Harbeson SL, et al. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob Agents Chemother. 2006;50:899–909. doi: 10.1128/AAC.50.3.899-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reesink HW, Zeuzem S, Weegink CJ, Forestier N, Vliet A, Wetering de Rooij J, McNair L, Purdy S, H M. C, Jansen PL. Final result of a phase 1b, multiple-dose study of VX-950, a hepatitis C virus protease inhibitor. Hepatology. 2005;42 Suppl 1:S234. [Google Scholar]
- 16.Reesink HW, Zeuzem S, Weegink CJ, Forestier N, van Vliet A, van de Wetering de Rooij J, McNair L, Purdy S, Kauffman R, Alam J, et al. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology. 2006;131:997–1002. doi: 10.1053/j.gastro.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Gelderblom HC, Zeuzem S, Weegink CJ, Forestier N, McNair L, Purdy S, Jansen PL, Reesink H. Decline in serum neopterin concentration correlated with HCV RNA decline during administration of VX-950, a hepatitis C virus protease inhibitor. Hepatology. 2005;42 Suppl 1:S533. [Google Scholar]
- 18.Gelderblom HC, Zeuzem S, Weegink CJ, Forestier N, McNair L, Purdy S, Jansen PL, Reesink H. Neopterin and ALT as markers of inflammation in chronic hepatitis C patients during administration of the HCV NS3-4A protease inhibitor telaprevir (VX-950) in combination with peginterferon alpha 2a. J Hepatol. 2007;46 Suppl 1:S225. [Google Scholar]
- 19.Friedrich-Rust M, Forestier N, Sarrazin C, Reesink H, Herrmann E, Zeuzem S. Ultrasound evaluation of perihepatic lymph nodes during antiviral therapy with the protease inhibitor telaprevir (VX-950) in patients with chronic hepatitis C infection. J Hepatol. 2007;46 Suppl 1:S205. doi: 10.1016/j.ultrasmedbio.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Kieffer T, Sarrazin C, Bartels D, Muh U, Welker M, Lin C, Grossman T, Weegink CJ, Purdy S, Reesink H, et al. Genetic heterogeneity in the HCV NS3 protease of untreated genotype 1 patients has little effect on the sensitivity to VX-950. Hepatology. 2005;42 Suppl 1:S537. [Google Scholar]
- 21.Sarrazin C, Kieffer T, Bartels D, Hanzelka B, Muh U, Welker M, Wincheringer D, Lin C, Grossman T, Purdy S, et al. Characterization of viral variants in the HCV NS3 protease domain of genotype 1 in patients that are selected during 14 days of dosing with VX-950. Hepatology. 2005;42 Suppl 1:S751. [Google Scholar]
- 22.Chu HM, Zhou Y, Bartels D, Khunvichai A, Rao BG, Kwong AD, Lin C. Telaprevir (VX-950)-resistant variants exhibit reduced replication capacity compared to wild-type HCV in vivo and in vitro. J Hepatol. 2007;46 Suppl 1:S230. [Google Scholar]
- 23.Kieffer T, Sarrazin C, Miller J, Traver S, Zhou Y, Bartels D, Hanzelka B, Muh U, Lin C, Reesink H, et al. Combination of telaprevir (VX-950) and Peg-INF alpha suppresses both wild type virus and resistance variants in HCV genotype 1-infected patients in a 14-day phase 1b study. Hepatology. 2006;44 Suppl 1:S222. [Google Scholar]
- 24.Forestier N, Weegink CJ, Purdy S, McNair L, Jansen PL, Zeuzem S, Reesink H. Current status of subjects receiving peginterferon-alfa-2a (Peg-IFN) and ribavirin (RBV) after a 14-day study of the hepatitis C protease inhibitor telaprevir (VX-950), with Peg-IFN. Hepatology. 2006;44 Suppl 1:S614. [Google Scholar]
- 25.McHutchison JG, Everson GT, Gordon S, Jacobson I, Kauffman R, McNair L, Muir A. Results of an interim analysis of a phase 2 study of telaprevir (VX-950) with peginterferon alfa-2a and ribavirin in previously untreated subjects with hepatitis C. J Hepatol. 2007;46 Suppl 1:S296. [Google Scholar]
- 26.Lin C, Hanzelka B, Muh U, Kovari L, Bartels D, Tigges AM, Miller J, Rao BG, Kwong AD. Telaprevir (VX-950) is a potent inhibitor of HCV NS3 proteases derived from genotype non-1 HCV-infected patients. J Hepatol. 2007;46 Suppl 1:S8. [Google Scholar]
- 27.Hibbert L, Foster GR. Effects of telaprevir (VX950) in a liver biopsy model of HCV infection: genotype-1 virions are inhibited in vitro but other genotypes are less responsive. J Hepatol. 2007;46 Suppl 1:S227. [Google Scholar]
- 28.Preston RA, Alonso AB, Feely W, Gupta S, Deckman D, Marchisin D, Kantesaria B, Hughes E. SCH 503034, an oral HCV protease inhibitor, is well-tolerated in patients with varying degrees of hepatic impairment. J Hepatol. 2007;46 Suppl 1:S207. [Google Scholar]
- 29.Malcolm BA, Arasappan A, Bennett F, Bogen SL, Chase R, Chen K, Chen T, Ingravallo P, Jao E, Kong J, et al. SCH 503034, a mechanism-based inhibitor of hepatitis C virus (HCV) NS3 protease suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon-a-2b (INF) Hepatology. 2005;42 Suppl 1:S535. [Google Scholar]
- 30.Malcolm BA, Liu R, Lahser F, Agrawal S, Belanger B, Butkiewicz N, Chase R, Gheyas F, Hart A, Hesk D, et al. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob Agents Chemother. 2006;50:1013–1020. doi: 10.1128/AAC.50.3.1013-1020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeuzem S, Sarrazin C, Rouzier R, Tarral A, Brion N, Forestier N, Gupta S, Deckman D, Fellows K, Hussain M, et al. Anti-viral activity of SCH 503034, a HCV protease inhibitor, administered as monotherapy in hepatitis C genotype-1 (HCV-1) patients refractory to pegylated interferon (Peg-IFN-alpha) Hepatology. 2005;42 Suppl 1:233A. [Google Scholar]
- 32.Zeuzem S, Sarrazin C, Wagner F, Rouzier R, Forestier N, Gupta S, Hussain M, Shah A, Cutler D, Zhang J. Combination therapy with the HCV protease inhibitor, SCH 503034, plus Peg-Intron in hepatitis C genotype-1 Peg-Intron non-responders: phase 1b results. Hepatology. 2005;42 Suppl 1:276A. [Google Scholar]
- 33.Howe AY, Ralston R, Chase R, Tong X, Skelton A, Flint M, Mullen S, Broom C, Emini EA. Favourable cross-resistance profile of two novel hepatitis C virus inhibitors, SCH-503034 and HCV-796, and enhanced anti-replicon activity mediated by the combined use of both compounds. J Hepatol. 2007;46 Suppl 1:S165. [Google Scholar]
- 34.Seiwert SD, Hong J, Lim SR, Wang T, Tan H, Blatt LM. Sequence variation of NS3/4A in HCV replicons exposed to ITMN-191 concentrations encompassing those likely to be achieved following clinical dosing. J Hepatol. 2007;46 Suppl 1:S244. [Google Scholar]
- 35.Tan H, Seiwert SD, Blatt LM. In vitro synergistic antiviral activity of ITMN-191, an orally active inhibitor of the hepatitis C virus (HCV) NS3/4A protease, in combination with Peg-Interferon alfa-2a. Hepatology. 2006;44 Suppl 1:534A. [Google Scholar]
- 36.Blatt LM, Tan H, Seiwert SD. ITMN-191 concentrations achieved in the liver of animals promote HCV replicon clearance in vitro and this effect is enhanced by Peg-IFN alpha-2a. J Hepatol. 2007;46 Suppl 1:S219. [Google Scholar]
- 37.Seiwert SD, Tan H, Blatt LM. Additive to synergistic antiviral effects of an NS3/4A protease inhibitor (ITMN-191) and an NS5B RNA-dependent RNA polymerase inhibitor (R1479) in an HCV replicon system. J Hepatol. 2007;46 Suppl 1:S167. [Google Scholar]
- 38.Hinrichsen H, Benhamou Y, Wedemeyer H, Reiser M, Sentjens RE, Calleja JL, Forns X, Erhardt A, Cronlein J, Chaves RL, et al. Short-term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology. 2004;127:1347–1355. doi: 10.1053/j.gastro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Huang M, Sun Y, Yang W, Hou H, Fabrycki J, Nie X, Sanchez A, Zhao Y, Phadke A. ACH-806: a potent inhibitor of HCV replication with a novel mechanism of action. J Hepatol. 2007;46 Suppl 1:S221. [Google Scholar]
- 40.Yang W, Zhao Y, Fabrycki J, Hou X, Nie X, Sun Y, Phadke A, Agarwal A, Deshpande M, Huang M. Induction of resistance by a novel class of compounds active against hepatitis C virus with no cross-resistance to NS3 protease and NS5B polymerase inhibitors. Hepatology. 2006;44 Suppl 1:S536. [Google Scholar]
- 41.Pottage JCJ, Lawitz E, Mazur D, Wyles D, Vargas H, Ghalib R, Gugliotti R, Donohue M, Robison H. Short-term antiviral activity and safety of ACH-806 (GS-9132), an NS4A antagonist, in HCV genotype 1 infected individuals. J Hepatol. 2007;46 Suppl 1:S294. [Google Scholar]
- 42.O'Brien C, Godofsky EW, Rodriguez-Torres M, Afdhal N, Pockros P, Lawitz E, Bzowej N, Rustgi V, Sulkowski M, Sherman K, et al. Randomized trial of valopicitabine (NM283) alone or with peg-interferon, vs. retreatment with peg-interferon plus ribavirin (PEGIFN/RBV) in hepatitis C patients with previous non-response to PEGIFN/RBV: first interim results. Hepatology. 2005;42 Suppl 1:S234. [Google Scholar]
- 43.Lawitz E, Nguyen TT, Younes Z, Santoro J, Gitlin N, McEniry D, Chasen R, Goff J, Dieterich D, Knox S, et al. Clearance of HCV RNA with valopicitabine (NM283) plus peg-interferon in treatment-naive patients with HCV-1 infection: results at 24 and 48 weeks. J Hepatol. 2007;46 Suppl 1:S9. [Google Scholar]
- 44.Lawitz E, Nguyen TT, Younes Z, Santoro J, Gitlin N, McEniry D, Chasen R, Goff J, Knox S, Kleber K, et al. Valopicitabine (NM283) plus peg-interferon in treatment-naive hepatitis C treatment-naive hepatitis C patients with HCV genotype-1 infection: HCV RNA clearance during 24 weeks of treatment. Hepatology. 2006;44 Suppl 1:S223. [Google Scholar]
- 45.Bichko V, Lallos L, Soubasakos M, LaColla M, Tausek MM, Gillum J, Standring DN. Valopicitabine (NM283) is fully active against known HCV protease resistance mutations in vitro. J Hepatol. 2007;46 Suppl 1:S163. [Google Scholar]
- 46.Ralston R, Bichko V, Chase R, LaColla M, Lallos L, Skelton A, Soubasakos M, Tausek M, Tong X, Standring D. Combination of two hepatitis C virus inhibitors, SCH 503034 and NM107, provides enhanced anti-replicon activity and suppresses emergence of resistant replicons. J Hepatol. 2007;46 Suppl 1:S298. [Google Scholar]
- 47.Roberts S, Cooksley G, Dore G, Robson R, Shaw D, Berns H, Brandl M, Fettner S, Hill G, Ipe D, et al. Results of a phase 1b, multiple dose study of R1626, a novel nucleoside analog targeting HCV polymerase in chronic HCV genotype 1 patients. Hepatology. 2006;44 Suppl 1:S692. [Google Scholar]
- 48.Jiang WR, Chiu S, Ali S, Chapman M, Daniel C, Kretz T, Cammack N, Symons J, Klumpp K. In vitro antiviral interactions of a novel HCV inhibitor R1479 with interferon alpha-2-a, ribavirin and other HCV inhibitors. Hepatology. 2006;44 Suppl 1:S533. [Google Scholar]
- 49.Villano S, Howe A, Raible D, Harper D, Speth J, Bichier G. Analysis of HCV NS5B genetic variants following monotherapy with HCV-796, a non-nucleoside polymerase inhibitor, in treatment-naive HCV-infected patients. Hepatology. 2006;44 Suppl 1:S607. [Google Scholar]
- 50.Villano S, Raible D, Harper D, Speth J, Chandra P, Shaw P, Bichier G. Antiviral activity of the non-nucleoside polymerase inhibitor, HCV-796, in combination with pegylated interferon alfa-2b in treatment-naive patients with chronic HCV. J Hepatol. 2007;46 Suppl 1:S24. [Google Scholar]
- 51.Erhardt A, Wedemeyer H, Benhamou Y, Moellekens C, Forns X, Pol S, Calleja JL, Ross S, Spangenberg HC, Garcia-Samaniego J, et al. Safety, pharmacokinetics and antiviral effect of BILB1941, a novel HCV RNA polymerase inhibitor, after 5 days oral treatment in patients with chronic hepatitis C. J Hepatol. 2007;46 Suppl 1:S222. [Google Scholar]
- 52.Wagner R, Larson D, Bosse T, Kati W, Mo HM, Koev G, Masse S, Jiang W, Liu Y, Montgomery D, et al. Discovery, characterization and anti-HCV activity of a novel NS5B inhibitor. Hepatology. 2006;44 Suppl 1:S236. [Google Scholar]
- 53.Furman PA, Murakami E, Bao H, Symons J, Otto MJ. Inhibition of HCV replication by PSI-6130: mechanism of biochemical activation and inhibition. J Hepatol. 2007;46 Suppl 1:S224. [Google Scholar]
- 54.Gray F, Amphlett E, Bright H, Chambers L, Cheasty A, Fenwick R, Haigh D, Hartley D, Howes P, Jarvest R, et al. GSK625433; a novel and highly potent inhibitor of the HCV NS5B polymerase. J Hepatol. 2007;46 Suppl 1:S225. [Google Scholar]
- 55.Molla A, Wagner R, Lu L, He D, Chen CM, Koev G, Masse S, Cai Y, Klein C, Beno D, et al. Characterization of pharmacokinetic/pharmacodynamic parameters for the novel HCV polymerase inhibitor A-848837. J Hepatol. 2007;46 Suppl 1:S234. [Google Scholar]
- 56.Shi ST, Herlihy KJ, Gonzalez J, Patick AK, Duggal R. In vitro resistance studies of AG-021541, a novel nonnucleoside inhibitor of the hepatitis C virus RNA- dependent RNA polymerase. Hepatology. 2006;44 Suppl 1:S534. doi: 10.1128/AAC.00834-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watashi K, Ishii N, Hijikata M, Inoue D, Murata T, Miyanari Y, Shimotohno K. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol Cell. 2005;19:111–122. doi: 10.1016/j.molcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Heitman J, Cullen BR. Cyclophilin B escorts the hepatitis C virus RNA polymerase: a viral achilles heel? Mol Cell. 2005;19:145–146. doi: 10.1016/j.molcel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Gallay P, Flisiak R, Horban A, Aeschlimann C, Dumont JM, Porchet H, Scalfaro P, Crabbe R. The reduction of both cyclophilin B and HCV-RNA by the cyclophilin inhibitor Debio-025 confirms the importance of cyclophilin B for HCV replication in man. J Hepatol. 2007;46 Suppl 1:S296. [Google Scholar]
- 60.Goto K, Watashi K, Murata T, Hishiki T, Hijikata M, Shimotohno K. Evaluation of the anti-hepatitis C virus effects of cyclophilin inhibitors, cyclosporin A, and NIM811. Biochem Biophys Res Commun. 2006;343:879–884. doi: 10.1016/j.bbrc.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 61.Watashi K, Shimotohno K. Chemical genetics approach to hepatitis C virus replication: cyclophilin as a target for anti-hepatitis C virus strategy. Rev Med Virol. 2007;17:245–252. doi: 10.1002/rmv.534. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawa M, Sakamoto N, Enomoto N, Tanabe Y, Kanazawa N, Koyama T, Kurosaki M, Maekawa S, Yamashiro T, Chen CH, et al. Specific inhibition of hepatitis C virus replication by cyclosporin A. Biochem Biophys Res Commun. 2004;313:42–47. doi: 10.1016/j.bbrc.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 63.Inoue K, Sekiyama K, Yamada M, Watanabe T, Yasuda H, Yoshiba M. Combined interferon alpha2b and cyclosporin A in the treatment of chronic hepatitis C: controlled trial. J Gastroenterol. 2003;38:567–572. doi: 10.1007/s00535-002-1104-5. [DOI] [PubMed] [Google Scholar]
- 64.Mueller AR, Platz KP, Blumhardt G, Bechstein WO, Steinmüller T, Christe W, Hopf U, Lobeck H, Neuhaus P. The optimal immunosuppressant after liver transplantation according to diagnosis: cyclosporine A or FK506? Clin Transplant. 1995;9:176–184. [PubMed] [Google Scholar]
- 65.Villamil F, Levy G, Grazi GL, Mies S, Samuel D, Sanjuan F, Rossi M, Lake J, Munn S, Mühlbacher F, et al. Long-term outcomes in liver transplant patients with hepatic C infection receiving tacrolimus or cyclosporine. Transplant Proc. 2006;38:2964–2967. doi: 10.1016/j.transproceed.2006.08.131. [DOI] [PubMed] [Google Scholar]
- 66.Hilgard P, Kahraman A, Lehmann N, Seltmann C, Beckebaum S, Ross RS, Baba HA, Malago M, Broelsch CE, Gerken G. Cyclosporine versus tacrolimus in patients with HCV infection after liver transplantation: effects on virus replication and recurrent hepatitis. World J Gastroenterol. 2006;12:697–702. doi: 10.3748/wjg.v12.i5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berenguer M, Aguilera V, Prieto M, San Juan F, Rayón JM, Benlloch S, Berenguer J. Effect of calcineurin inhibitors on survival and histologic disease severity in HCV-infected liver transplant recipients. Liver Transpl. 2006;12:762–767. doi: 10.1002/lt.20655. [DOI] [PubMed] [Google Scholar]
- 68.Billich A, Hammerschmid F, Peichl P, Wenger R, Zenke G, Quesniaux V, Rosenwirth B. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. J Virol. 1995;69:2451–2461. doi: 10.1128/jvi.69.4.2451-2461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenwirth B, Billich A, Datema R, Donatsch P, Hammerschmid F, Harrison R, Hiestand P, Jaksche H, Mayer P, Peichl P. Inhibition of human immunodeficiency virus type 1 replication by SDZ NIM 811, a nonimmunosuppressive cyclosporine analog. Antimicrob Agents Chemother. 1994;38:1763–1772. doi: 10.1128/aac.38.8.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin K, Ma S, Boerner JE, Huang MM, Ryder NS, Weidmann B, Cooreman MP. NIM811 exhibits potent anti-HCV activity in vitro and represents a novel approach for viral hepatitis C therapy. Hepatology. 2005;42 Suppl 1:S536. [Google Scholar]
- 71.Ma S, Boerner JE, TiongYip C, Weidmann B, Ryder NS, Cooreman MP, Lin K. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with alpha interferon. Antimicrob Agents Chemother. 2006;50:2976–2982. doi: 10.1128/AAC.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue K, Umehara T, Watanabe T, Durmont JM, Scalfaro P, Yoshiba M, Kohara M. Non immunosupressive cyclosporin Debio-025 with interferon shows synergistic anti-HCV effect in chimeric mouse. Hepatology. 2006;44 Suppl 1:S238. [Google Scholar]
- 73.Paeshuyse J, Coelmont L, Kaul A, Vliegen I, de Clercq E, Rosenwirth B, Dumont JM, Scalfaro P, Bartenschlager R, Neyts J. The Cyclophilin inhibitor Debio-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology. 2006;44 Suppl 1:S346. doi: 10.1002/hep.21102. [DOI] [PubMed] [Google Scholar]
- 74.Coelmont L, Paeshuyse J, Kaptein S, Vliegen I, Kaul A, De Clercq E, Rosenwirth B, Scalfaro P, Crabbe R, Bartenschlager R, et al. The Cyclophilin Inhibitor Debio-025 is a Potent Inhibitor of Hepatitis C Virus Replication in vitro With a Unique Resistance Profile. Antivir Res. 2007;74:S139. [Google Scholar]
- 75.Herrmann E, Flisiak R, Horban A, Crabbe R, Porchet H, Nicolas V, Scalfaro P, Zeuzem S. Viral kinetics during 14-day treatment with Debio-025 demostrate efficient blocking of viral replication in different HCV genotypes without signs of emerging viral resistance. J Hepatol. 2007;46 Suppl 1:S40. [Google Scholar]
- 76.Lin C, Gates CA, Rao BG, Brennan DL, Fulghum JR, Luong YP, Frantz JD, Lin K, Ma S, Wei YY, et al. In vitro studies of cross-resistance mutations against two hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061. J Biol Chem. 2005;280:36784–36791. doi: 10.1074/jbc.M506462200. [DOI] [PubMed] [Google Scholar]
- 77.Lin C, Lin K, Luong YP, Rao BG, Wei YY, Brennan DL, Fulghum JR, Hsiao HM, Ma S, Maxwell JP, et al. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: structural analysis indicates different resistance mechanisms. J Biol Chem. 2004;279:17508–17514. doi: 10.1074/jbc.M313020200. [DOI] [PubMed] [Google Scholar]
- 78.Tong X, Chase R, Skelton A, Chen T, Wright-Minogue J, Malcolm BA. Identification and analysis of fitness of resistance mutations against the HCV protease inhibitor SCH 503034. Antiviral Res. 2006;70:28–38. doi: 10.1016/j.antiviral.2005.12.003. [DOI] [PubMed] [Google Scholar]