Abstract
AIM: To investigate the poor prognosis of HCC with PVTT, we evaluated the efficacy by a new combination chemotherapy for advanced hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT).
METHODS: From 2002 to 2007, a total of 10 consecutive patients with Stage IVA HCC accompanied by PVTT were studied prospectively to examine the efficacy of treatment by intra-arterial infusion of a chemotherapeutic agents consisting of etoposide, carboplatin, epirubicin and pharmacokinetic modulating chemotherapy by 5-FU and enteric-coated tegafur/uracil.
RESULTS: The mean course of chemotherapy was 14.4 (range, 9-21) mo. One patient showed complete response (CR) with disappearance of HCC and PVTT after treatment, and the two patients showed partial response (PR), response rate (CR + PR/All cases 30%). The median survival time after the therapy was 457.2 d. The one-year survival rate was 70%. Adverse reactions were tolerable.
CONCLUSION: Although the prognosis of most patients with Stage IVA HCC by PVTT is poor, our combination chemotherapy may induces long-term survival and is an effective treatment and produced anti-tumor activity with tolerable adverse effects in patients for advanced Stage IVA HCC accompanied by PVTT.
Keywords: Hepatocellular carcinoma, Portal vein tumor thrombus, Intra-arterial regional chemotherapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide including Japan[1]. Although the development of imaging modalities has made the early diagnosis of HCC possible, surgically resectable cases are relatively uncommon because of a hepatic function reserve and/or an advanced stage at presentation. Several modalities, such as percutaneous ethanol injection (PEI), transcatheter arterial embolization (TAE), chemolipiodolization, microwave coagulation therapy (MCT), and radiofrequency ablation therapy (RFA) are reportedly useful in treating patients with unresectable disease[2,3]. However, unfortunately, many hepatocellular carcinoma patients have tumor recurrence. Furthermore, HCC has a predilection for portal vein invasion, which has been shown to be a poor prognostic factor. An effective therapy regimen is needed for advanced HCC with portal vein tumor thrombus (PVTT). Recent trials have been reported that combination therapy of intra-arterial 5-FU and systemic interferon for HCC with PVTT is effective[4,5]. However, portal venous invasion is a crucial factor that can worsen the prognosis of patients with HCC. It often leads to extensive spreading of the tumor throughout the liver, and can increase portal venous blood pressure, resulting in the fatal rupture of esophageal varices, and can decrease portal flow which causes ascites, jaundice, hepatic encephalopathy, and liver failure. Previous studies have reported that the median survival time of patients with portal venous invasion was 2.7-4 mo if left untreated[6,7].
As systemic therapy of HCC, we had previously achieved complete remission of multiple HCC associated with hepatitis C virus-related decompensated liver cirrhosis by oral administration of enteric-coated tegafur/uracil[8]. Furthermore, we reported that oral administration of enteric-coated tegafur/uracil induces long-term survival and is an effective treatment for Stage IV-A HCC[9]. However, this therapy by single agent is not effective HCC with PVTT. Therefore, there is an urgent need for new method and active drugs for PVTT from HCC.
The aim of this study is to evaluate the usefulness by intra-arterial infusion of a chemotherapeutic agents consisting of etoposide, carboplatin, epirubicin and pharmacokinetic modulating chemotherapy by 5-FU and enteric-coated tegafur/uracil for HCC with tumor thrombosis of the main trunk of the portal vein.
MATERIALS AND METHODS
Ethics
The study protocol was reviewed and approved by the Hospital Ethics Committee. Informed consent was obtained from each patient who entered a randomized controlled trial and from family member(s).
Patients
A group of 10 consecutive patients with HCC accompanied by portal vein tumor thrombus (PVTT) were enrolled in the therapeutic trial between April 2002 and April 2007. The diagnosis of HCC was made by histologically and/or imaging study.
All patients received intra-arterial regional chemotherapy carried out at the Department of Gastroenterology and Hepatology, Saiseikai Niigata Second Hospital and gave their informed consent according to our situational guidelines, and the study received ethical approval.
Eligibility criteria for patients in this study included the following: (1) diagnosed Stage IVA HCC with PVTT (2) unresectable carefully assessed by the individual experts; (3) no recent active treatments including surgery, radiotherapy, chemotherapy, transarterial embolization, percutaneous ethanol injection, or other regional treatment within six month; (4) HCC with PVTT diagnosed by total image systems such as computed tomography (CT) or magnetic resonance imaging (MRI). (5) the ability to manage the indwelling catheters and implanted injection ports; (6) adequate hematologic function (white blood cell count > 3000/L, platelet count > 80000/L, and hemoglobin level > 9.5 gm/dL); adequate renal function (serum creatinine < 1.5 mg/dL and a creatinine clearance > 60 mL/min); (7) adequate hepatic function, (8) a performance status less than 3 at pre-treatment, (9) portal tumor thrombi located the first portal branch, or the main portal trunk, and (10) informed consent.
Treatment schedule and follow up
For intra-arterial regional chemotherapy, catheters were introduced into the proper or common hepatic artery placed via the right femoral artery using the Seldinger method. The gastroduodenal artery and the right artery were occluded by a steel coil as indicated to prevent gastroduodenal injury from the anticancer agents. Intra-arterial regional chemotherapy was performed by puncturing a thin needle percutaneously into the port. Before every infusion, we confirmed that the catheters were patent, either by checking the blood back-flowing in the catheter or by injecting a contrast medium under fluoroscopy. Using an infusion pump (Syringe Pump, Terumo Co. Ltd., Osaka, Japan), 50 mg/body of etoposide (VePesid, Bristol-Myers Squibb Co. Ltd., Tokyo, Japan), 300 mg/body of carboplatin (Paraplatin, Bristol-Myers Squibb Co. Ltd., Tokyo, Japan) and 60 mg/body of epirubicin (Farmorubicin, Kyowa Hakko Kogyo Co. Ltd., Tokyo, Japan) were infused from each catheter over a 30-minute period. Subsequently, a continuous arterial infusion of 5-FU (500 mg/m2) (5-FU, Kyowa Hakko Kogyo Co. Ltd., Tokyo, Japan) was given for 24 h. Before the needle was removed from the port, both the port and connected catheter were filled with undiluted heparin (1000 U/mL). An antiemetic and an H2-receptor antagonist were given intravenously. This treatment was repeated once weekly for 3 consecutive weeks of every 4 wk or biweekly, mainly at our outpatient clinic, for as long as possible. All patients were given also enteric-coated tegafur/uracil (Taiho Pharmaceutical, Co. Ltd., Tokyo, Japan) at a dose rate of 200 mg/body twice daily. Treatment was continued for a minimum of 8 wk and given until patient withdrawal or death even after the progression of disease.
Studies during follow-up included a physical examination, complete blood count, platelet count, and determination of levels of AFP, PIVKA-II, amylase, liver transaminase, urea nitrogen, and creatinine. Either an ultrasonogram or a computed tomogram of the abdomen was obtained at least every two months, to determine the size of HCC and PVTT.
Evaluation of therapeutic response
The therapeutic clinical response of the liver was assessed in accordance with the World Health Organization Criteria. Clinical responses were graded as follows. Complete response (CR) was defined as disappearance of all measurable lesions in the liver, continuing for at least 4 weeks when assessed by both computed tomography and ultrasonography. Reduction of tumor size by more than 50%, continuing for at least 4 wk, was regarded as partial response (PR). No change (NC) was determined as tumors showing a decrease in size of less than 25%. Progressive disease (PD) was defined as tumors that had grown over 25%.
Statistical analysis
Survival curves were calculated by the Kaplan-Meier method and the difference between survival curves was evaluated using the log-rank test.
Statistical analyses were performed using Stat View-J4.11 software (Abacus Concepts; Berkeley, California) to assess the relative prognostic importance of variables in predicting the survival rate. Differences at P < 0.05 were considered significant.
RESULTS
Background clinical and laboratory data of patients
Patients' profiles before the combination chemotherapy are listed in Table 1. Seven men and three women, with a median age of 60.2 years, were treated. Positive HBsAg was found in 70% of patients, while anti-hepatitis C virus (HCV) serology was positive in 30% of patients.
Table 1.
Characteristics of the patients
| 10 patients |
| Age mean 60.20 y.o (range 35-70) |
| Sex: Male/Female 7/3 |
| Child-Pugh's stage: A,B/C 8/2 |
| "Virus: HBsAg/anti-HCV 8/2" |
Child-Pugh Grade A/B was 2/6, the remaining patients had Grade C. Only 2 patients received the full courses of chemotherapy. The median number of cycles received per patients was 15.3. Dose reduction was required in 80% of the patients, mainly due to profound bone marrow suppression from the previous cycle.
Cumulative survival rate of patients by Kaplan-Meier survival curves
Of the 10 patients in the treated group, a total of 3 (30.0%) were classed as CR or PR, and a total 7 (70.0%) were classed as NC or PD. At the time of the final analysis (April 2007) 2 patients (from the treatment group) were alive. A total of 153 treatment cycles were administered.
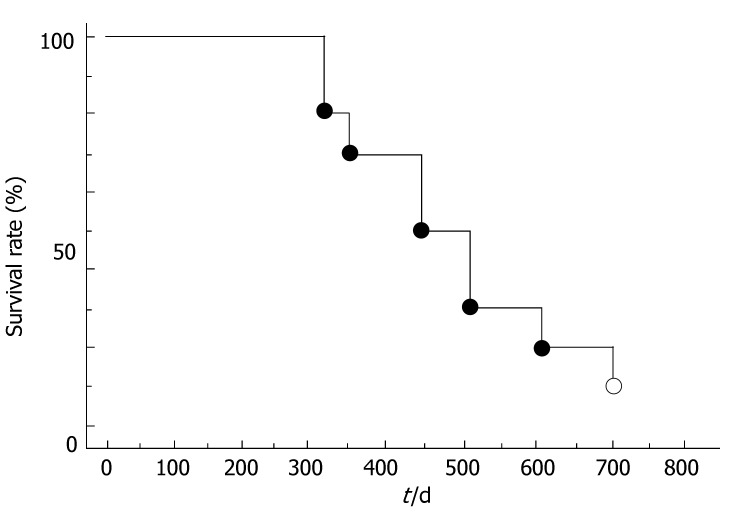
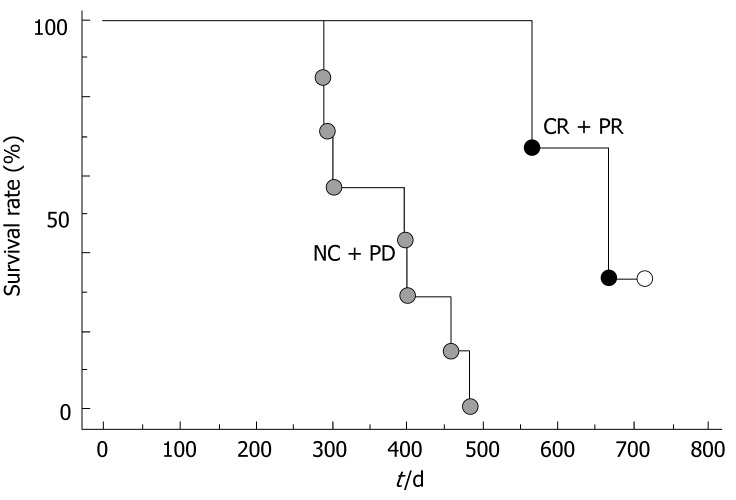
The overall survival curve for all 10 patients is shown in Figure 1. The median survival was 457.2 d. The survival rates at the end of 1-year and 2-year were 70.0% and 20.0%, respectively. Of the 10 patients studied, 8 had died by the time of this analysis. The survival curves for clinical responders (CR or PR) and the others (NC or PD) are shown in Figure 2. The 6-mo and one-year survival rates were 100% and 100.0%, respectively, in the responders and 100% and 57.1%, respectively, in the non-responder. There was a significant difference in survival between the two groups (P < 0.01).
Figure 1.
Cumulative survival of patients with hepatocellular carcinoma accompanied by PVTT who treated combination therapy.
Figure 2.
Survival of patients with hepatocellular carcinoma accompanied by PVTT according to the response and control (Log-rank P < 0.05). CR: Complete response, PR; Partial response, NC; No change, PD; Progressive disease.
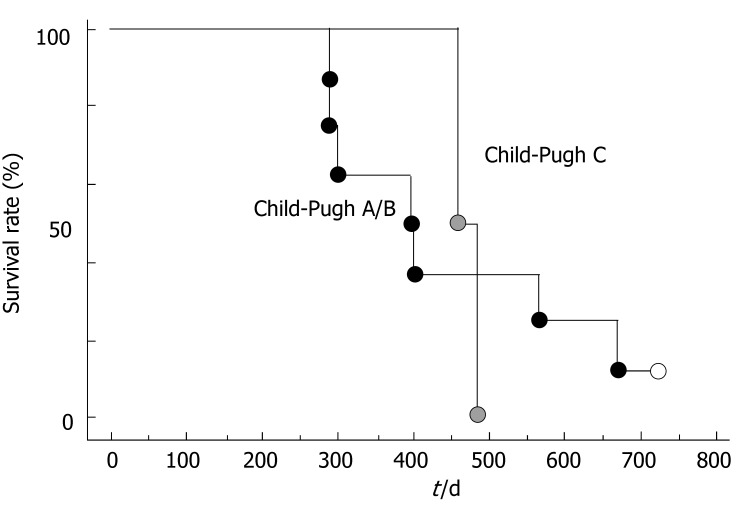
However, there was not a significant difference in survival rates between Child-Pugh A/B group and Child-Pugh C group (Figure 3).
Figure 3.
Survival of patients with hepatocellular carcinoma accompanied by PVTT according to Child-Pugh's stage (Log-rank P = 0.98).
Side effects and complications due to regional chemotherapy
The side-effects and complications encountered during therapy are summarized in Table 2. Local complications at the femoral artery entry sites did not occur in any patient. No serious complications that necessitated intensive care were encountered during therapy. The side-effects included oral dryness, diarrhea and liver dysfunction, and bone marrow suppression. Mild oral dryness was noted at the beginning of the treatment in 40.0% of patients, but this subsided as treatment continued. Mild diarrhea was noted at the beginning of the treatment in 30.0% of patients, but this also subsided with time. Such symptoms resolved spontaneously or after appropriate therapy. The complications experienced by patients treated with regional chemotherapy were well tolerated. No other serious complications, such as gastric ulcer, liver damage, renal damage, vascular complications, or cardiac toxicity were encountered. However, dose reduction was required in 80% of the patients, mainly due to profound bone marrow suppression from the previous cycle. There were no treatment-related deaths from administration of regional chemotherapy.
Table 2.
Main clinical side effects observed during the treatment (n represents the number of patients having experienced the effect in any of the courses)
|
Grade of toxicity |
||||
| 1 | 2 | 3 | 4 | |
| Oral dryness | 3 | 1 | 0 | 0 |
| Diarrhea | 1 | 2 | 0 | 0 |
| Vomiting | 1 | 0 | 0 | 0 |
| Liver dysfunction | 1 | 2 | 0 | 0 |
| Fever | 0 | 0 | 0 | 0 |
| Hair loss | 1 | 0 | 0 | 0 |
| BM suppression | 4 | 4 | 0 | 0 |
DISCUSSION
Patients with HCC are highly compromised by failing liver function. HCC is associated with a high risk of portal vein involvement. PVTT is one of important prognostic factors in patients with HCC. However, HCC with PVTT is refractory to treatment. The treatment of HCC with PVTT is still problematic and major challenge item for oncologists because of its high and dismal outcomes. None of the reported treatment regimens can be considered to be standard treatment for HCC with PVTT.
Meanwhile, regional hepatic arterial infusion chemotherapy is a reasonable drug delivery system for patients with advanced HCC because the tumors derive most of their blood supply from the hepatic artery, whereas the portal vein supplies the normal parenchyma[10]. Furthermore, chemotherapy combined with interferon is reported to be effective for HCC with PVTT.
Combined treatment with 5-FU and alpha-interferon for HCC patients was first reported by Patt et al[11] in 1993. The response rate was reportedly 22%. Urabe et al[12] treated 16 patients with HCC and PVTT in the main trunk or the major branches of the portal vein by intrahepatic infusion of methotrexate, 5-FU, and cisplatin, and administered alpha-interferon subcutaneously. The response rate and median survival time were 46.7% and 7 mo, respectively.
Moreover, combined intra-arterial 5-FU and subcutaneous alpha-interferon therapy for 8 patients with HCC accompanied by PVTT in the major portal vein was reported by Sakon et al[5]. The response rate was 63%. In another study by this group in 2005, 55 patients received this treatment, and 8 (14.5%) showed a complete response, 16 (29.1%) showed a partial response, 4 (7.3%) showed no response, and 27 (49.1%) showed progressive disease[13]. The median survival time and 5-year survival rate were 11.8 mo and 16.4%, respectively. Using this combination protocol, Obi et al[4] treated 116 patients with unresectable HCC accompanied by PVTT in the main trunk or the 1st branches of the portal vein. The survival rates at 1 and 2 years among overall patients were 34% and 18%, respectively, in contrast to 15% and 5% among the historical controls. Survival rates at 1 and 2 years were 81% and 59% among complete responders, respectively, and 43% and 18% among partial responders. The median survival time was prolonged to 11 mo in patients with an active response, although there appears to be no benefit in patients without an active response.
However, it is chaotic to determine whether the combination chemotherapy with interferon is effective or not for HCC accompanied by PVTT.
We previously reported[8,9] that administration of enteric-coated tegafur/uracil induces long-term survival and is an effective treatment for Stage IV-A HCC. However, single agent such as enteric-coated tegafur/uracil was not effective for HCC with PVTT. So, combination chemotherapy is needed for HCC with PVTT.
The choice of the anticancer agent is important in achieving favorable clinical results. We selected etoposide, carboplatin, epirubicin and pharmacokinetic modulating chemotherapy by 5-FU and enteric-coated tegafur/uracil.
Firstly, etoposide is agent which has shown significant antitumor against HCC[14-16]. It could be suggested as part of intensive multidrug regimens for HCC and high-risk HBV[17-19]. Though response rates to cisplatin and etoposide[20] given systemically as single agents are 5 and 15%, intra-arterial combination chemotherapy using cisplatin and etoposide produces a high rate of objective tumor remissions in patients with HCC[21].
However, cisplatin has been reported that it has a lot of nephrotoxic and emetic effects. To the contrary, carboplatin has demonstrated antitumor activity comparable to cisplatin and has been shown to have fewer nephrotoxic and emetic effects. In fact, carboplatin is thought to be a useful anticancer agent in patients with HCC treated with TACE. Furthermore, it is reported that carboplatin is effective for HCC[22-24].
So we selected carboplatin as combination with etoposide. In addition, a combination of epirubicin and etoposide appears to be an active and tolerable therapeutic option for HCC patients who are not candidates for surgical or locoregional procedures[19,25-27].
Recently, Kusunoki et al[28,29] reported that pharmacokinetic modulating chemotherapy, based on the concept that the benefit of a continuous venous 5-fluorouracil infusion can be potentiated by low-dose oral tegafur/uracil is useful for a variety of cancers.
In fact, it is reported that modified pharmacokinetic modulating chemotherapy had no severe side effect and was effective for advanced unresectable HCC[30].
Based on these facts, we tried combination chemotherapy for HCC with PVTT. In our series, the treatment resulted in an objective response rate of 30% and a median survival of 457.2 d. Only the three patients who had an objective response had a survival of long duration.
As our group was small, we did not perform a statistical analysis to determine a predictive factor for response. However, our results are comparable with those of most interferon combination chemotherapy.
In our study, the toxicity of this therapy was low despite the fact that all of the patients had cirrhosis. It is noteworthy that there were no patients showing overt liver toxicity. Moreover, there was no treatment-related death.
In this study, no hepatotoxicity due to this combination chemotherapy was observed. The side effects of this regimen were minimal and well tolerated.
In conclusion, this chemotherapeutic regimen ameliorated the survival of patients with advanced HCC without serious adverse effects.
We suggest that, in the near future, this chemotherapy method should be subjected to a prospective randomized controlled study for HCC with PVTT. Further prospective randomized clinical trials of chemotherapy for HCC with PVTT will be needed.
COMMENTS
Background
Portal venous tumor thrombus (PVTT) is a crucial factor that can worsen the prognosis of patients with hepatocellular carcinoma (HCC). It often leads to extensive spreading of the tumor throughout the liver, and can increase portal venous blood pressure, resulting in the fatal rupture of esophageal varices, and can decrease portal flow which causes ascites, jaundice, hepatic encephalopathy, and liver failure. However, there is not effective useful therapy for HCC combined with PVTT. Therefore, there is an urgent need for new and active drugs and combination chemotherapy of advanced HCC.
Research frontiers
HCC has a predilection for portal vein invasion, which has been shown to be a poor prognostic factor. An effective therapy regimen is needed for advanced HCC with PVTT. Combination chemotherapy is needed for HCC with PVTT urgently. The choice of the anticancer agent is important in achieving favorable clinical results. The authors selected etoposide, carboplatin, epirubicin and pharmacokinetic modulating chemotherapy by 5-FU and enteric-coated tegafur/uracil. Progress in implantable drug delivery systems has made possible the repeated arterial infusion of chemotherapeutic agents for patients with advanced HCC recently. Therefore, hepatic arterial infusion chemotherapy has been often selected as a therapeutic option for advanced HCC with PVTT.
Innovations and breakthroughs
The authors investigated the efficacy, the feasibility, usefulness, and complication rate of arterial combination therapies for HCC with PVTT.
Applications
Intra-arterial combination chemotherapy is useful and inducing long-term survival for advanced HCC accompanied by PVTT. Further prospective randomized clinical trials of chemotherapy for HCC with PVTT will be needed.
Terminology
PVTT: Portal vein tumor thrombus meaning tumor thrombus locating the first portal branch, or the main portal trunk.
Peer review
This is an interesting manuscript reporting the strategy for HCC with PVTT. This new information is certainly worthy of publication.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Wang HF
References
- 1.Okuda K. Hepatocellular carcinoma: recent progress. Hepatology. 1992;15:948–963. doi: 10.1002/hep.1840150532. [DOI] [PubMed] [Google Scholar]
- 2.Ichida T, Van Thiel DH, Hassanein T. The medical management of hepatocellular carcinoma (HCC) in Japan: a review with implications for HCC seen in the west. Hepatogastroenterology. 1996;43:1575–1583. [PubMed] [Google Scholar]
- 3.Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound. 2001;13:159–166. doi: 10.1016/s0929-8266(01)00128-8. [DOI] [PubMed] [Google Scholar]
- 4.Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990–1997. doi: 10.1002/cncr.21832. [DOI] [PubMed] [Google Scholar]
- 5.Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, Kawata S, Imai Y, Iijima S, Monden M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435–442. doi: 10.1002/cncr.10246. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 7.Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, De Santis M, Manenti F. Natural history of inoperable hepatocellular carcinoma: estrogen receptors' status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233–238. doi: 10.1053/jhep.2000.9603. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa T, Ichida T, Ishimoto Y, Yokoyama J, Nomoto M, Ebe Y, Usuda H, Naito M, Asakura H. Complete remission of multiple hepatocellular carcinomas associated with hepatitis C virus-related, decompensated liver cirrhosis by oral administration of enteric-coated tegafur/uracil. Am J Gastroenterol. 1999;94:1682–1685. doi: 10.1111/j.1572-0241.1999.01163.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa T, Ichida T, Sugitani S, Tsuboi Y, Genda T, Sugahara S, Uehara K, Inayoshi J, Yokoyama J, Ishimoto Y, et al. Improved survival with oral administration of enteric-coated tegafur/uracil for advanced stage IV-A hepatocellular carcinoma. J Gastroenterol Hepatol. 2001;16:452–459. doi: 10.1046/j.1440-1746.2001.02352.x. [DOI] [PubMed] [Google Scholar]
- 10.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 11.Patt YZ, Yoffe B, Charnsangavej C, Pazdur R, Fischer H, Cleary K, Roh M, Smith R, Noonan CA, Levin B. Low serum alpha-fetoprotein level in patients with hepatocellular carcinoma as a predictor of response to 5-FU and interferon-alpha-2b. Cancer. 1993;72:2574–2582. doi: 10.1002/1097-0142(19931101)72:9<2574::aid-cncr2820720911>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Urabe T, Kaneko S, Matsushita E, Unoura M, Kobayashi K. Clinical pilot study of intrahepatic arterial chemotherapy with methotrexate, 5-fluorouracil, cisplatin and subcutaneous interferon-alpha-2b for patients with locally advanced hepatocellular carcinoma. Oncology. 1998;55:39–47. doi: 10.1159/000011833. [DOI] [PubMed] [Google Scholar]
- 13.Ota H, Nagano H, Sakon M, Eguchi H, Kondo M, Yamamoto T, Nakamura M, Damdinsuren B, Wada H, Marubashi S, et al. Treatment of hepatocellular carcinoma with major portal vein thrombosis by combined therapy with subcutaneous interferon-alpha and intra-arterial 5-fluorouracil; role of type 1 interferon receptor expression. Br J Cancer. 2005;93:557–564. doi: 10.1038/sj.bjc.6602742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yodono H, Sasaki T, Tarusawa K, Midorikawa H, Saito Y, Takekawa SD. Arterial infusion chemotherapy for advanced hepatocellular carcinoma using EPF and EAP therapies. Cancer Chemother Pharmacol. 1992;31 Suppl:S89–S92. doi: 10.1007/BF00687114. [DOI] [PubMed] [Google Scholar]
- 15.Yodono H, Takekawa SD, Tarusawa K, Ikami I, Kanehira J, Saito Y, Takahashi S, Sasaki T, Nishi N, Kimura T. Combination therapy consisting of arterial infusion chemotherapy (EPF, EAP) and transcatheter arterial embolization (TAE) Cancer Chemother Pharmacol. 1994;33 Suppl:S79–S83. doi: 10.1007/BF00686673. [DOI] [PubMed] [Google Scholar]
- 16.Wierzbicki R, Ezzat A, Abdel-Warith A, Ayoub A, Kagevi I, Fadda M, Sieck J, Abdulkareem M, Amin T, Yazigi A. Phase II trial of chronic daily VP-16 administration in unresectable hepatocellular carcinoma (HCC) Ann Oncol. 1994;5:466–467. doi: 10.1093/oxfordjournals.annonc.a058882. [DOI] [PubMed] [Google Scholar]
- 17.Casanova M, Massimino M, Ferrari A, Spreafico F, Piva L, Coppa J, Luksch R, Cefalo G, Terenziani M, Polastri D, et al. Etoposide, cisplatin, epirubicin chemotherapy in the treatment of pediatric liver tumors. Pediatr Hematol Oncol. 2005;22:189–198. doi: 10.1080/08880010590921441. [DOI] [PubMed] [Google Scholar]
- 18.Aita P, Robieux I, Sorio R, Tumolo S, Corona G, Cannizzaro R, Colussi AM, Boiocchi M, Toffoli G. Pharmacokinetics of oral etoposide in patients with hepatocellular carcinoma. Cancer Chemother Pharmacol. 1999;43:287–294. doi: 10.1007/s002800050897. [DOI] [PubMed] [Google Scholar]
- 19.Bobbio-Pallavicini E, Porta C, Moroni M, Bertulezzi G, Civelli L, Pugliese P, Nastasi G. Epirubicin and etoposide combination chemotherapy to treat hepatocellular carcinoma patients: a phase II study. Eur J Cancer. 1997;33:1784–1788. doi: 10.1016/s0959-8049(97)00163-9. [DOI] [PubMed] [Google Scholar]
- 20.Melia WM, Johnson PJ, Williams R. Induction of remission in hepatocellular carcinoma. A comparison of VP 16 with adriamycin. Cancer. 1983;51:206–210. doi: 10.1002/1097-0142(19830115)51:2<206::aid-cncr2820510206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Sangro B, Rios R, Bilbao I, Beloqui O, Herrero JI, Quiroga J, Prieto J. Efficacy and toxicity of intra-arterial cisplatin and etoposide for advanced hepatocellular carcinoma. Oncology. 2002;62:293–298. doi: 10.1159/000065059. [DOI] [PubMed] [Google Scholar]
- 22.Akimoto M, Yoshikawa M, Ebara M, Sato T, Fukuda H, Kondo F, Saisho H. Relationship between therapeutic efficacy of arterial infusion chemotherapy and expression of P-glycoprotein and p53 protein in advanced hepatocellular carcinoma. World J Gastroenterol. 2006;12:868–873. doi: 10.3748/wjg.v12.i6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita F, Tanaka M, Andou E, Yutani S, Kato O, Tanikawa K. Carboplatin as an anticancer agent for transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Oncology. 1997;54:28–33. doi: 10.1159/000227657. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Seo HY, Choi JG, Sul HR, Sung HJ, Park KH, Choi IK, Oh SC, Yoon SY, Seo JH, et al. Phase II study with a combination of epirubicin, cisplatin, UFT, and leucovorin in advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2006;57:436–442. doi: 10.1007/s00280-005-0067-7. [DOI] [PubMed] [Google Scholar]
- 25.Boucher E, Corbinais S, Brissot P, Boudjema K, Raoul JL. Treatment of hepatocellular carcinoma (HCC) with systemic chemotherapy combining epirubicin, cisplatinum and infusional 5-fluorouracil (ECF regimen) Cancer Chemother Pharmacol. 2002;50:305–308. doi: 10.1007/s00280-002-0503-x. [DOI] [PubMed] [Google Scholar]
- 26.Pohl J, Zuna I, Stremmel W, Rudi J. Systemic chemotherapy with epirubicin for treatment of advanced or multifocal hepatocellular carcinoma. Chemotherapy. 2001;47:359–365. doi: 10.1159/000048544. [DOI] [PubMed] [Google Scholar]
- 27.Dobbs NA, Twelves CJ, Rizzi P, Warwick JD, Metivier EM, Williams R, Johnson PJ. Epirubicin in hepatocellular carcinoma: pharmacokinetics and clinical activity. Cancer Chemother Pharmacol. 1994;34:405–410. doi: 10.1007/BF00685565. [DOI] [PubMed] [Google Scholar]
- 28.Kusunoki M, Yanagi H, Kotera H, Noda M, Yamamura T. Effects of pharmacokinetic modulating chemotherapy using oral UFT and continuous venous 5FU infusion on the prognosis of irradiated rectal carcinomas with p53 overexpression. Int J Oncol. 1998;13:653–657. doi: 10.3892/ijo.13.4.653. [DOI] [PubMed] [Google Scholar]
- 29.Kusunoki M, Yanagi H, Noda M, Yoshikawa R, Yamamura T. Results of pharmacokinetic modulating chemotherapy in combination with hepatic arterial 5-fluorouracil infusion and oral UFT after resection of hepatic colorectal metastases. Cancer. 2000;89:1228–1235. doi: 10.1002/1097-0142(20000915)89:6<1228::aid-cncr6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Kamiyama T, Matsushita M, Kurauchi N, Nakagawa T, Kamachi H, Kondo M, Ito T, Ogata T, Nishikawa M, Todo S. Modified pharmacokinetic modulation chemotherapy (PMC) with medication of UFT and intraarterial infusion of 5-FU for advanced unresectable HCC. Gan To Kagaku Ryoho. 2002;29:2527–2531. [PubMed] [Google Scholar]