Abstract
AIM: To investigate serum alanine aminotransferase (ALT) levels in relation to the clinical, biochemical, ultrasonographic and histological characteristics of patients with hepatitis C virus.
METHODS: Duration of disease, HCV-RNA, liver steatosis, and the hepatitis activity index (HAI) were correlated with serum ALT in 36 patients with HCV. ALT values were also investigated in 16 control subjects without any liver diseases.
RESULTS: In bivariate analyses, ALT levels correlated with duration of HCV infection (P < 0.01), HCV-RNA (P < 0.05), and the HAI (P < 0.01). Among the components of the HAI, ALT concentrations were significantly associated with periportal bridging/necrosis (P < 0.01) and fibrosis (P < 0.05). In multivariate analysis, periportal bridging/necrosis (β = 0.508; P < 0.01), duration of HCV infection (β = 0.413; P < 0.01), and HCV-RNA (β = 0.253; P < 0.05) were independently associated with ALT activity. The normal ALT activity for men and women was < 23 IU/L and < 22 IU/L, respectively.
CONCLUSION: In patients with HCV, alterations in the liver tissue as reflected by ALT elevation are mainly associated with periportal bridging/necrosis, viral load and duration of disease. A cut-off value < 23 IU/L distinguished with high diagnostic accuracy healthy controls from patients with HCV.
Keywords: Hepatitis C virus, Disease duration, Viral Load, Inflammation, Normal alanine aminotransferase
INTRODUCTION
Hepatitis C virus (HCV) is a major cause of chronic liver disease, frequently progressing to cirrhosis and increased risk of hepatocellular carcinoma[1-3]. Chronic hepatitis C is often silent, most of the times discovered only by routine serologic or biochemical testing[4-6]. Many attempts to identify the natural history and progression of hepatitis C infection have been made, but several aspects remain to be elucidated[7]. In individuals with chronic hepatitis C, viral load and elevated serum alanine aminotransferase (ALT) levels may have clinical relevance[8-10]. When parenchymal liver cells are damaged, aminotransferases leak from the liver into the blood, resulting in elevated levels of these enzymes in the bloodstream. The exact definition of the normal levels of serum ALT activity is crucial for screening and follow-up studies in hepatitis C infection[11,12]. It should be noted, however, that half of the untreated patients with chronic HCV infections display normal or minimally elevated serum ALT levels[13,14]. Accordingly, several studies have recently questioned whether previously established values to define normal ALT range are clinically accurate[11,12]. In this regard, it has been posited that the limits of normal for serum ALT should be revised accordingly[12].
In the present study, we sought to investigate serum ALT levels in relation to the clinical, biochemical, ultrasonographic and histological characteristics of patients with hepatitis C. We also aimed to study the normal level of ALT in Turkish healthy adults at low risk for chronic liver diseases. This information, in addition to daily clinical practice, may be clinically useful for research studies of hepatitis C and chronic liver diseases in Turkey.
MATERIALS AND METHODS
Study sample
A total of 36 patients (24 females, 12 males; mean age: 47.9 ± 13.2 years) with HCV infection were studied before the treatment with antiviral drugs. Patients with hemochromatosis, Wilson's disease, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, biliary obstruction, alpha-1 antitrypsin deficiency, or malignancies were excluded from the present study. None of the subjects was using any medications, including estrogens, amiodarone, steroids, tamoxifen, or herbal supplements. Furthermore we excluded patients with daily alcohol intake exceeding 20 g/d. For control purposes, 16 healthy age- and gender-matched volunteers (9 females, 7 males) were recruited. All controls were judged to be in good health and confirmed as having normal liver by ultrasound. Subjects with a consumption of alcohol > 20 g/d or who were taking any medication were not included in the control group. All subjects underwent physical examination, anthropometric measurements and biochemical screening.
A written informed consent was obtained from all participants. Our study was in accordance with the ethical standards for human experimentation and approved by the Ethics Committee of the Uludag University Medical School.
Laboratory and virology assessment
Blood samples for the evaluation of alanine aminotransferase (ALT), and biochemical parameters were obtained after overnight fasting. Routine biochemical tests were carried out using commercially available kits (Hitachi, Tokyo, Japan). The HCV-RNA was determined in the sera using an RT-PCR assay (Amplicor, Roche, Mannheim), with a sensitivity of 70 copies/mL.
Ultrasound assessment
Liver ultrasound (US) scanning was performed to assess the degree of steatosis. All US procedures were performed by the same operator. Liver steatosis was assessed semi-quantitatively on a scale of 0 to 3:0, absent; 1, mild; 2, moderate and 3, severe.
Histological analysis
Ultrasonography-guided liver biopsies were performed under conscious sedation using a 16-gauge Klatskin needle. The length of histological specimens was not smaller than 2.5 cm. All biopsy specimens were placed in formalin solution for fixation and embedded in paraffin blocks. Serial sections (sectioned at 4 μm intervals) were stained with hematoxylin-eosin and Masson's trichrome. The hepatitis activity index (HAI), designed by Knodell and Desmet[15,16], was used to grade the severity of the necroinflammatory process and fibrosis. HAI comprises four separate scores, including periportal necrosis with or without bridging necrosis (0-10), intralobular degeneration and focal necrosis (0-4), portal inflammation (0-4) and fibrosis (0-4).
Statistical analysis
Variables are presented as counts and percentages, mean ± SD. Correlations among the study variables were assessed by means of the Pearson's correlation coefficients. Multivariate stepwise regression models were used to assess the independent predictors of ALT levels in patients with HCV infection. Cut-off values for serum ALT values were determined by means of the ROC curve analysis with the use of the MedCalc statistical software (Mariakerke, Belgium). A P < 0.05 was considered statistically significant. All computations were made using SPSS 11.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Bivariate analysis of serum ALT levels in patients with HCV infection
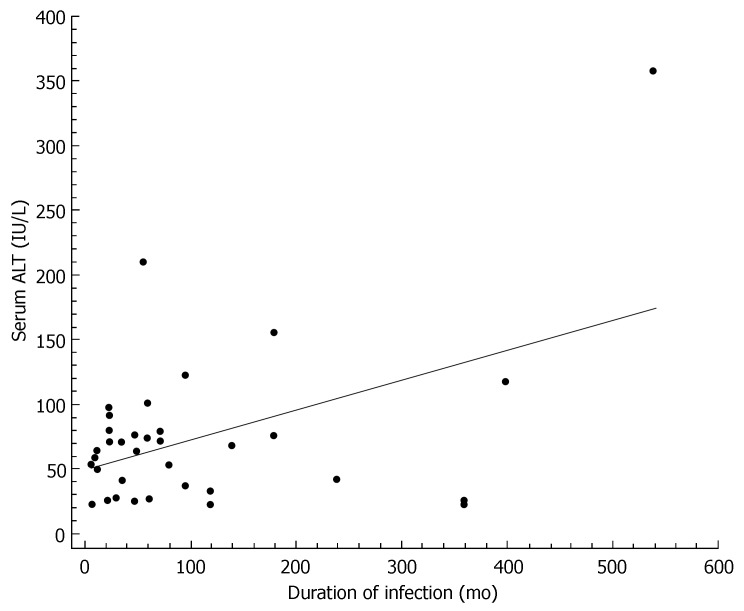
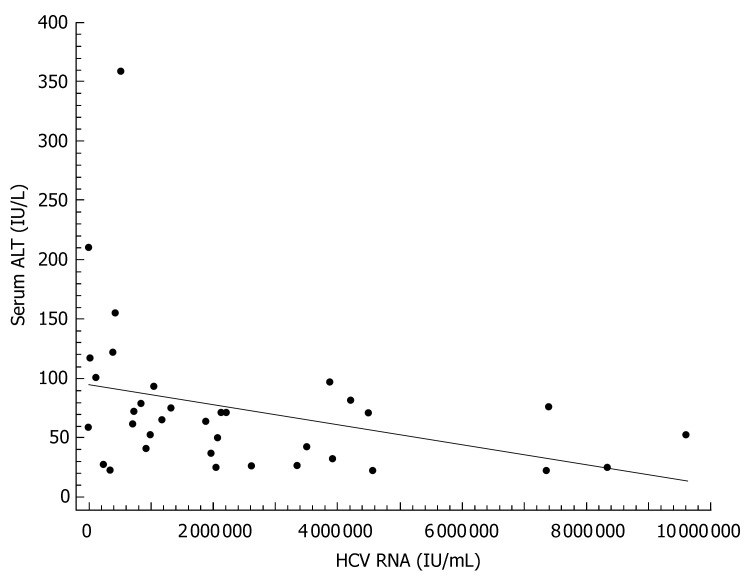
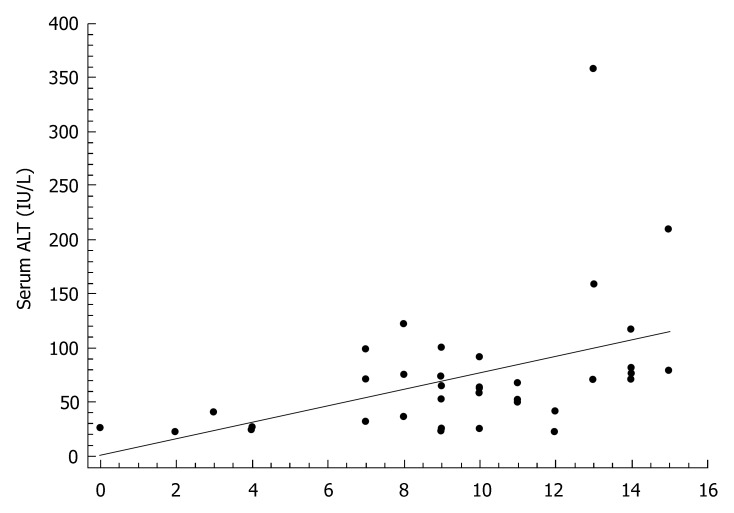
The characteristics of individuals with HCV infection are given in Table 1. The estimated median duration of HCV infection was 58 (interquartile range: 24-120) mo. Steatosis was present in 22 (61%) of the 36 HCV infected patients, of whom 8 (22%) had grade 1, 11 (30%) grade 2, and 3 (9%) grade 3. The histological findings of the study participants are shown in Table 2. In bivariate correlation analyses, ALT levels correlated with duration of HCV infection (r = 0.46, P < 0.01, Figure 1), HCV-RNA (r = -0.33, P < 0.05, Figure 2), and the HAI (r = 0.44, P < 0.01, Figure 3). Among the components of the HAI, ALT concentrations were significantly associated with periportal bridging/necrosis (r = 0.50, P < 0.01) and fibrosis (r = 0.37, P < 0.05).
Table 1.
General characteristics of the study patients with HCV infection
| Characteristic | Entire cohort (n = 36) |
| Female, n (%) | 24 (66.6%) |
| Age (yr) | 47.9 ± 13.2 |
| Estimated duration of HCV infection (mo) | 58 (24-120) |
| HCV RNA (IU/mL) | 2471075 ± 2490186 |
| Body mass index (kg/m2) | 28.5 ± 3.9 |
| Waist Circumference (cm) | 98 ± 11 |
| Fasting glucose (mg/dL) | 96 ± 10 |
| Haemoglobin (mg/dL) | 13.9 ± 1.5 |
| HOMA index | 3.1 ± 2.4 |
| Total cholesterol (mg/dL) | 161 ± 36 |
| HDL cholesterol (mg/dL) | 48 ± 11 |
| Triglycerides (mg/dL) | 98 ± 41 |
| Serum albumin (g/dL) | 4.4 ± 0.3 |
| Serum globulin (g/dL) | 3.1 ± 0.6 |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 |
| AST (IU/L) | 48 (32-67) |
| ALT (IU/L) | 64 (34-80) |
| ALP (IU/L) | 90 ± 33 |
| GGT (IU/L) | 45 (29-63) |
| LDH (IU/L) | 184 ± 32 |
| Total Bilirubin (mg/dL) | 0.7 ± 0.3 |
| Direct Bilirubin (mg/dL) | 0.3 (0.2-0.4) |
| Platelet count (103/mm3) | 202 ± 54 |
| Ferritin (ng/mL) | 45 (34-77) |
| Positive ANA, n (%) | 2 (5.5%) |
| Positive AMA, n | 0 |
| Positive ASMA, n | 0 |
| Positive LKM-1, n | 0 |
Data expressed as means ± SD, or median (interquartile range), as appropriate; HOMA: Homeostasis model assessment; HDL: High-density lipoprotein; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; GGT: Gamma glutam.yl transpeptidase; LDH: Lactate dehydrogenase; ANA: Antinuclear antibodies; AMA: Antimitochondrial antibodies; ASMA: Anti-smooth muscle antibody; LKM-1: Liver-kidney microsomal antigen.
Table 2.
Liver histology in the 36 study participants with HCV infection
| Variable | Score |
| Hepatitis activity index | 9.5 ± 3.7 |
| Periportal necrosis with or without bridging necrosis | 3.7 ± 2.2 |
| Intralobular degeneration and focal necrosis | 2.7 ± 1.1 |
| Portal inflammation | 3.0 ± 1.0 |
| Fibrosis | 1.9 ± 1.2 |
Figure 1.
Scatter diagram and regression line showing a significant positive relationship between duration of HCV infection and serum alanine aminotransferase (r = 0.46, P < 0.01).
Figure 2.
Scatter diagram and regression line showing a significant inverse relationship between viral load and serum alanine aminotransferase (r = -0.33, P < 0.05).
Figure 3.
Scatter diagram and regression line showing a significant positive relationship between the Histology Activity Index and serum alanine aminotransferase (r = 0.44, P < 0.01).
Multivariate analysis
Multivariate stepwise regression analysis was used to identify independent predictors of ALT levels in our patients with HCV infection. Serum ALT activity was considered as the dependent variable. All variables listed in Table 1 were entered into the multivariate model as independent variables. The results of this analysis showed that periportal bridging/necrosis (β = 0.508; P < 0.01), duration of HCV infection (β = 0.413; P < 0.01), and HCV-RNA (β = 0.253; P < 0.05) were, in the order they entered into the model, independently associated with ALT levels.
Identification of a cut-off value for serum ALT levels according to gender
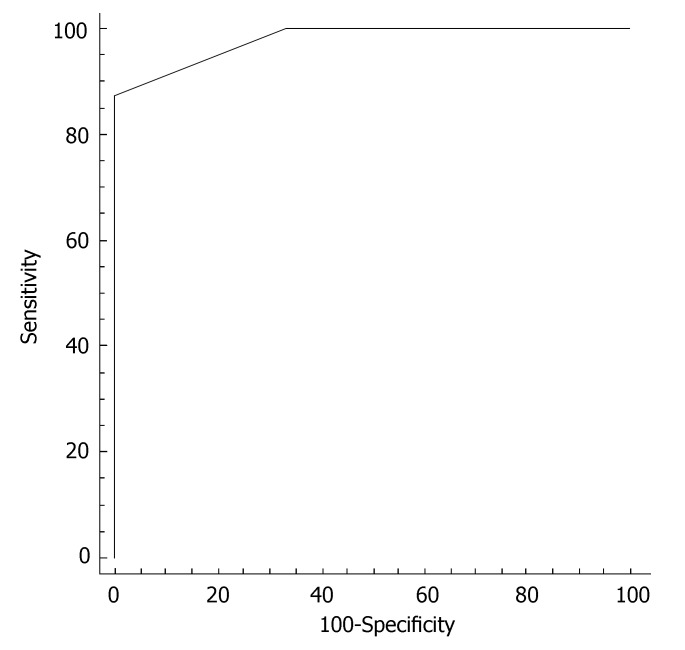
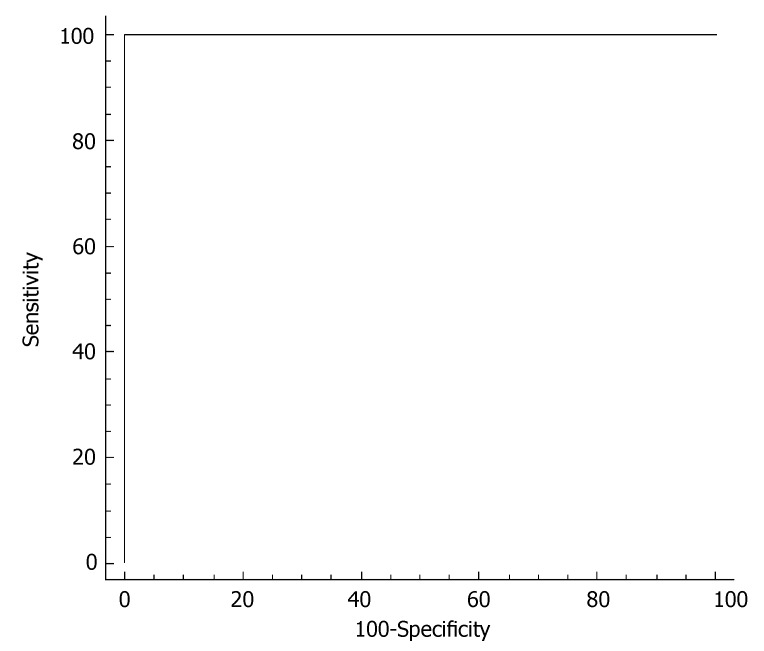
In order to establish a better cutoff value for ALT in hepatitis C screening in our Turkish population, the ALT levels were measured in 16 healthy age- and gender-matched volunteers. ALT levels in the control population were 18.2 ± 3.6 IU/L. The cutoff value was identified by construction of a ROC curve (receiver operating characteristic) in each gender. In females, the better cutoff value for ALT was at 22 IU/L, with sensitivity of 87.5% and specificity of 100% in identifying subjects without HCV infection (Figure 4). In males, the better cutoff value for ALT was at 22 IU/L, with sensitivity of 100% and specificity of 100% in identifying subjects without HCV infection (Figure 5).
Figure 4.
ROC curve of serum ALT for discriminating female HCV patients from women without liver disease. The better cutoff value for ALT was at 22 IU/L, with sensitivity of 87.5% and specificity of 100% in identifying subjects without HCV infection.
Figure 5.
ROC curve of serum ALT for discriminating male HCV patients from men without liver disease. The better cutoff value for ALT was at 23 IU/L, with sensitivity of 100% and specificity of 100% in identifying subjects without HCV infection.
DISCUSSION
This study provides insights into the correlates of ALT levels in the setting of patients with HCV infection. We found that, in our sample of Turkish patients, serum ALT levels were significantly and independently correlated with periportal bridging/necrosis, viral load and duration of HCV infection.
Serum ALT levels, as a measure of biochemical hepatitis activity, increased significantly with periportal bridging/necrosis, and this association was stronger than for other components of the HAI index. Our findings are in line with previous studies showing a statistically significant linear relationship between the degree of ALT elevation and the amount of liver injury based on the HAI score[17]. In our study, viral load was significantly and inversely correlated with mean ALT levels. This result is in keeping with the findings of Ito et al[18], who showed that mean viral load was significantly higher in chronic HCV patients with persistently normal ALT levels. In this regard, it has been hypothesized that immune response to HCV could play a role in rendering viral load smaller[18]. It should be noted, however, that some authors have reported higher ALT levels in patients with high viral load[19]. Another group has suggested that no significant difference in viral load exists between patients with abnormal ALT levels and those with normal ALT levels[20]. Although the reasons for conflicting data remain to be clarified, the discrepancies in the literature may be due at least in part to the presence of potential confounders such as ethnicity or different sample sizes. The duration of HCV infection may be of importance for the development of cirrhosis and patients with longer periods of infection may be more likely to have higher ALT levels[20]. In our study we found a positive association between ALT activity in the serum and duration of HCV infection. Some authors, however, have failed to demonstrate such a relationship[21]. In any case, it should be kept in mind that the onset of HCV infection may be difficult to establish in some patients, thereby rendering disease duration undefined.
Growing evidence has suggested that up to 25% of patients with chronic hepatitis C virus infection have persistently normal aminotransferase levels (10% to 40%, according to different studies)[14,22,23]. The normal range for ALT level was set in the 1950 s and has changed little since then. However, several recent studies have questioned whether previously established reference values to define normal ALT range are really accurate. Under these circumstances, it has been repeatedly suggested that the limits of normal ALT activity should be revised[24-26]. In most countries, the cutoff value for ALT is defined as twice the upper limit of the normal range of healthy individuals[27]. The normal ALT activity for men and women is < 23 IU/L and < 18 IU/L, respectively[27]. In order to gain more insights on the normal level of ALT in Turkish healthy adults at low risk for chronic liver diseases, we measured ALT activity in a control population from our country. By ROC curve analysis, we found that the optimal cutoff point in our population was 23 IU/L in males and 22 IU/L in females. Using our newly calculated cutoff value, we found that the sensitivity and specificity for detection of subjects without HCV infection were 100% and 100% in men and 87.5% and 100% in women, respectively. Hopefully, these new data obtained in the Turkish population should contribute to the ongoing discussion regarding new reference systems for the measurement of the catalytic activity of ALT in the clinical practice[28,29]. Given the small sample size, we believe that our findings may stimulate future studies on a larger number of patients.
In conclusion, we have provided evidence that the main correlates of ALT levels in HCV patients are periportal bridging/necrosis, viral load and duration of HCV infection. A cut-off value < 23 IU/L in males and 22 IU/L in females may distinguish with high diagnostic accuracy healthy control subjects from patients with HCV.
ACKNOWLEDGMENTS
The authors thank the assistance of the Research Fellows and the Scientific Staff at the Uludag University Medical School.
COMMENTS
Backgrounds
The exact definition of the normal levels of serum ALT activity is crucial for screening and follow-up studies in hepatitis C infection. However, half of the untreated patients with chronic HCV infections display normal or minimally elevated serum ALT levels. Accordingly, several studies have recently questioned whether previously established values to define normal ALT range are clinically accurate.
Research frontiers
Recent evidence has suggested that the limits of normal for serum ALT should be revised. In the present study, we sought to investigate serum ALT levels in relation to the clinical, biochemical, ultrasonographic and histological characteristics of patients with HCV.
Innovations and breakthroughs
We have provided evidence that the main correlates of ALT levels in HCV patients are periportal bridging/necrosis, viral load and duration of HCV infection.
Applications
A cut-off value < 23 IU/L in males and 22 IU/L in females may distinguish with high diagnostic accuracy healthy control subjects from patients with HCV.
Terminology
ALT elevation: aminotransferases leak from the liver into the blood when parenchymal liver cells are damaged.
Peer review
The study is of particular interest to the practical medicine. The results provide sufficient evidence that the activity of ALT correlates with histological changes, viral load and duration of infection in patients with HCV.
Footnotes
S- Editor Zhu LH L- Editor Alpini GD E- Editor Wang HF
References
- 1.Hughes CA, Shafran SD. Chronic hepatitis C virus management: 2000-2005 update. Ann Pharmacother. 2006;40:74–82. doi: 10.1345/aph.1G263. [DOI] [PubMed] [Google Scholar]
- 2.Chevaliez S, Pawlotsky JM. Hepatitis C virus: virology, diagnosis and management of antiviral therapy. World J Gastroenterol. 2007;13:2461–2466. doi: 10.3748/wjg.v13.i17.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevaliez S, Pawlotsky JM. Hepatitis C virus serologic and virologic tests and clinical diagnosis of HCV-related liver disease. Int J Med Sci. 2006;3:35–40. doi: 10.7150/ijms.3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sievert W. Management issues in chronic viral hepatitis: hepatitis C. J Gastroenterol Hepatol. 2002;17:415–422. doi: 10.1046/j.1440-1746.2002.02725.x. [DOI] [PubMed] [Google Scholar]
- 5.Teoh NC, Farrell GC. Management of chronic hepatitis C virus infection: a new era of disease control. Intern Med J. 2004;34:324–337. doi: 10.1111/j.1445-5994.2004.00615.x. [DOI] [PubMed] [Google Scholar]
- 6.Sherman M. Chronic hepatitis C and screening for hepatocellular carcinoma. Clin Liver Dis. 2006;10:735–752. doi: 10.1016/j.cld.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Liu C. Hepatitis C virus: virology and experimental systems. Clin Liver Dis. 2006;10:773–791. doi: 10.1016/j.cld.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Beld M, Penning M, McMorrow M, Gorgels J, van den Hoek A, Goudsmit J. Different hepatitis C virus (HCV) RNA load profiles following seroconversion among injecting drug users without correlation with HCV genotype and serum alanine aminotransferase levels. J Clin Microbiol. 1998;36:872–877. doi: 10.1128/jcm.36.4.872-877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mateescu RB, Rimbaş M, Stăniceanu F, Zurac S, Dragomir P, Voiosu MR, Tătaru M, Zota M, Bucurică S. Histological and nonhistological criteria in the evaluation of liver involvement in chronic hepatitis C. Rom J Intern Med. 2006;44:117–130. [PubMed] [Google Scholar]
- 10.Al-Quaiz MN, Madani TA, Karawi MA. The natural history of hepatitis C virus infection. Saudi Med J. 2003;24 Suppl 2:S67–S70. [PubMed] [Google Scholar]
- 11.Mohamadnejad M, Pourshams A, Malekzadeh R, Mohamadkhani A, Rajabiani A, Asgari AA, Alimohamadi SM, Razjooyan H, Mamar-Abadi M. Healthy ranges of serum alanine aminotransferase levels in Iranian blood donors. World J Gastroenterol. 2003;9:2322–2324. doi: 10.3748/wjg.v9.i10.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 13.Puoti C, Castellacci R, Montagnese F. Hepatitis C virus carriers with persistently normal aminotransferase levels: healthy people or true patients? Dig Liver Dis. 2000;32:634–643. doi: 10.1016/s1590-8658(00)80850-6. [DOI] [PubMed] [Google Scholar]
- 14.Shiffman ML, Diago M, Tran A, Pockros P, Reindollar R, Prati D, Rodríguez-Torres M, Lardelli P, Blotner S, Zeuzem S. Chronic hepatitis C in patients with persistently normal alanine transaminase levels. Clin Gastroenterol Hepatol. 2006;4:645–652. doi: 10.1016/j.cgh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 16.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 17.McCormick SE, Goodman ZD, Maydonovitch CL, Sjogren MH. Evaluation of liver histology, ALT elevation, and HCV RNA titer in patients with chronic hepatitis C. Am J Gastroenterol. 1996;91:1516–1522. [PubMed] [Google Scholar]
- 18.Ito H, Yoshioka K, Ukai K, Watanabe K, Yano M, Ishigami M, Mizutani T, Sasaki Y, Katano Y, Goto H. The fluctuations of viral load and serum alanine aminotransferase levels in chronic hepatitis C. Hepatol Res. 2004;30:11–17. doi: 10.1016/j.hepres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Hassan MI, Kassim SK, Ali HS, Sayed el-DA, Khalifa A. Evaluation of nitric oxide (NO) levels in hepatitis C virus (HCV) infection: relationship to schistosomiasis and liver cirrhosis among Egyptian patients. Dis Markers. 2002;18:137–142. doi: 10.1155/2002/647961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt AA, Tsevat J, Ahmad J, Shakil AO, Mrus JM. Biochemical and virologic parameters in patients co-infected with hepatitis C and HIV versus patients with hepatitis C mono-infection. Am J Med Sci. 2007;333:271–275. doi: 10.1097/MAJ.0b013e31805341f0. [DOI] [PubMed] [Google Scholar]
- 21.Haydon GH, Jarvis LM, Blair CS, Simmonds P, Harrison DJ, Simpson KJ, Hayes PC. Clinical significance of intrahepatic hepatitis C virus levels in patients with chronic HCV infection. Gut. 1998;42:570–575. doi: 10.1136/gut.42.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gholson CF, Morgan K, Catinis G, Favrot D, Taylor B, Gonzalez E, Balart L. Chronic hepatitis C with normal aminotransferase levels: a clinical histologic study. Am J Gastroenterol. 1997;92:1788–1792. [PubMed] [Google Scholar]
- 23.Persico M, Perrotta S, Persico E, Terracciano L, Folgori A, Ruggeri L, Nicosia A, Vecchione R, Mura VL, Masarone M, et al. Hepatitis C virus carriers with persistently normal ALT levels: biological peculiarities and update of the natural history of liver disease at 10 years. J Viral Hepat. 2006;13:290–296. doi: 10.1111/j.1365-2893.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- 24.Lozano M, Cid J, Bedini JL, Mazzara R, Gimenez N, Mas E, Ballesta A, Ordinas A. Study of serum alanine-aminotransferase levels in blood donors in Spain. Haematologica. 1998;83:237–239. [PubMed] [Google Scholar]
- 25.Khedmat H, Fallahian F, Abolghasemi H, Hajibeigi B, Attarchi Z, Alaeddini F, Holisaz MT, Pourali M, Sharifi S, Zarei N. Serum gamma-glutamyltransferase, alanine aminotransferase, and aspartate aminotransferase activity in Iranian healthy blood donor men. World J Gastroenterol. 2007;13:889–894. doi: 10.3748/wjg.v13.i6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, Sansonetti N, Opolon P. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC Group. Hepatology. 1998;27:1213–1219. doi: 10.1002/hep.510270505. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann T, Dreier J, Diekmann J, Götting C, Klauke R, Schumann G, Kleesiek K. Alanine aminotransferase cut-off values for blood donor screening using the new International Federation of Clinical Chemistry reference method at 37 degrees C. Vox Sang. 2003;85:159–164. doi: 10.1046/j.1423-0410.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- 28.Tsai JF, Jeng JE, Ho MS, Wang CS, Chang WY, Hsieh MY, Lin ZY, Tsai JH. Serum alanine aminotransferase level in relation to hepatitis B and C virus infections among blood donors. Liver. 1997;17:24–29. doi: 10.1111/j.1600-0676.1997.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 29.Kariv R, Leshno M, Beth-Or A, Strul H, Blendis L, Kokia E, Noff D, Zelber-Sagie S, Sheinberg B, Oren R, et al. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int. 2006;26:445–450. doi: 10.1111/j.1478-3231.2006.01197.x. [DOI] [PubMed] [Google Scholar]