Abstract
Hypersensitivity reactions (HSR) to oxaliplatin in patients with colorectal cancer include facial flushing, erythema, pruritis, fever, tachycardia, dyspnea, tongue swelling, rash/hives, headache, chills, weakness, vomiting, burning sensations, dizziness, and edema. We report a patient with fever as the sole manifestation of initial HSR, review the literature and discuss the management of HSR. A 57-year-old female with T3N2M0 rectal adenocarcinoma received modified FOLFOX-6. She tolerated the first 8 cycles without any toxicities except grade 1 peripheral neuropathy and nausea. During 9th and 10th infusions, she developed fever to a maximum of 38.3°C with stable hemodynamic status despite medications. During 11th infusion, she developed grade 3 HSR consisting of symptomatic bronchospasm, hypotension, nausea, vomiting, cough, and fever. On examination, she was pale, cyanotic, with a temperature of 38.8°C, BP dropped to 95/43 mm Hg, pulse of 116/min and O2 saturation of 88%-91%. She was hospitalized for management and recovered in 24 h. Fever alone is not a usual symptom of oxaliplatin HSR. It may be indicative that the patient may develop serious reactions subsequently, as did our patient who developed hypotension with the third challenge. Treatment and prevention consists of slowing the infusion rate, use of steroids and antagonists of Type 1 and 2 histamine receptor antagonists, whereas desensitization could help to provide the small number of patients who experience severe HSR with the ability to further receive an effective therapy for their colorectal cancer.
Keywords: Oxaliplatin, Hypersensitivity reaction, Fever, Colon cancer
INTRODUCTION
Oxaliplatin (C8H14N2O4) (Eloxatin; Sanofi-Aventis), is an organoplatinum in which the platinum atom is complexed with diaminocyclohexane and with an oxalate ligand. It is a third generation platinum which is indicated for the first-line treatment of metastatic colorectal cancer in combination with 5-fluorouracil (5-FU) and leucovorin (LV)[1] and for the adjuvant treatment of stage III colorectal cancer[2]. Oxaliplatin is safely administered in the outpatient setting but hypersensitivity reactions (HSR) can occur. Hypersensitivity is defined as an unexpected reaction that can not be explained by the known toxicity profile of the chemotherapeutic agent[3]. HSR are seen usually with the taxanes (paclitaxel), the platinum compounds, asparaginase, the epipodophyllotoxins and procarbazine[3]. Doxorubicin and 6-mercaptupurine have also been associated with these reactions. Acute onset and delayed reactions have been described which can cause flushing, pruritis, hypotension, dyspnea, nausea, back pain and rash[3,4].
We report a patient with fever as the sole manifestation of initial hypersensitivity reaction, who subsequently developed a serious HSR including hypotension with the third challenge.
CASE REPORT
The patient is a 57-year-old female diagnosed with T3N2M0 (Stage III/Dukes C) rectal adenocarcinoma who was treated with oxaliplatin-based regimen in the adjuvant setting. The tumor was 5.7 cm in size and six lymph nodes were positive for cancer. She was started on treatment with adjuvant chemotherapy with modified FOLFOX-6 (mFOLFOX-6; oxaliplatin 85 mg/m2 with concurrent LV 400 mg/m2 on d 1 followed by IV bolus 5-FU 400 mg/m2 followed by a single continuous infusion of 5-FU 2400 mg/m2 over 46 h every 2 wk). As per institutional standard premedication, she received dexamethasone 20 mg and ondansterone 16 mg intravenously. She tolerated 8 infusions without any complications. One and a half hour after starting the 9th infusion, she developed fever of 37.7°C. Other toxicities were grade 1 vomiting and grade 1 diarrhea. She was treated with promethazine (25 mg IV), hydrocortisone (100 mg IV), lorazepam (1 mg IV) and acetaminophen (650 mg PO). During this episode, her BP was 100/60 mm of Hg and pulse of 100/min. Despite the treatment, the fever continued to rise and reached a maximum of 38.8°C (Grade 2).She was discharged with the infusional 5-FU pump after observation and once fever came down to 37.8°C.
Two weeks later when she returned for the 10th infusion, she again developed fever of 37.7°C towards the end of the 2 h infusion. She also had emesis (grade 1) with nausea (grade 1) during oxaliplatin infusion. She was treated with promethazine and acetaminophen. Temperature rose to maximum of 38.4°C (Grade 2). The pulse and BP were preserved. Work-up for infectious etiology including line infection was done and ruled out. Fever defervesced and patient was discharged and returned home.
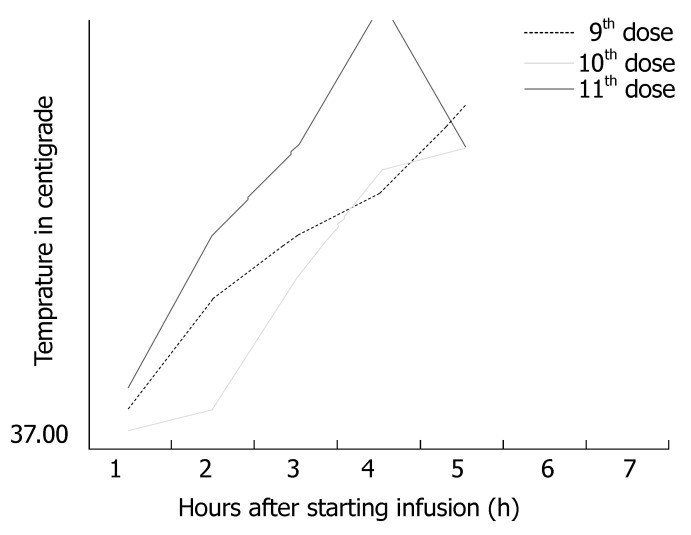
The patient was pre-medicated with promethazine before cycle 11th. She was planned to receive oxaliplatin over 2-h infusion. One and half hour into the infusion, she complained of itching for which diphenhydramine (25 mg) was given. After this she developed nausea (Grade 1) and vomiting (Grade 1) for which she received promethazine. One hour later she complained of cough and gagging sensation and on examination was found to be pale and cyanosis was visualized on the nail bed. Her temperature rose to 38.8°C (Figure 1) and soon her BP dropped to 95/43 mm Hg, pulse of 116/min and O2 saturation of 88%-91% (Grade 3). Two liters of oxygen were administered with nasal canula and the saturations improved to 91%. She was admitted to the hospital. Fever defervesced overnight and blood and urine cultures were negative. Hypotension responded to fluids and patient was discharged the next day. Desensitization was offered but patient refused further therapy with oxaliplatin.
Figure 1.
Fever in relation to infusion of oxaliplatin in the present case during 9th-11th cycles of modified FOLFOX-6 regimen in a patient with Stage III rectal cancer.
DISCUSSION
HSR have been reported to occur in a minority of patients (12%) who receive oxaliplatin, and as less than 0.55% of the patients develop grade 3 or 4 reactions[5-8] (Table 1). However, the incidence of HSR to oxaliplatin is rising recently as a result of increasing clinical use both in the adjuvant and metastatic treatment of colorectal cancer. The reactions usually occur after multiple infusions (mean 2-17) cycles of therapy with variable and unpredictable clinical features[9]. Patients can experience flushing, alterations in heart rate and blood pressure, bronchospasm, back pain, chest discomfort, fever, pruritis, erythema, nausea, and rash[3,5]. Usually the symptoms are mild but life-threatening anaphylactic reactions can occur. For this reason the FDA and Sanofi-Aventis has included a BOX warning for such reactions[10]. The incidence of HSR in pivotal phase III trials of oxaliplatin both in metastatic and adjuvant setting is show in Table 2[2,10,11].
Table 1.
Common terminology criteria for adverse events grading of hypersensitivity reactions (version 3.0)[26]
| Grade | Hypersensitivity reactions |
| 1 | Transient flushing or rash; drug fever < 38°C |
| 2 | Rash; flushing; urticaria; dyspnea; drug fever ≥ 38°C |
| 3 | Symptomatic bronchospasm, with or without urticaria; parenteral medication(s) indicated; allergy-related edema/angioedema; hypotension |
| 4 | Anaphylaxis |
Table 2.
Incidence of HSR in pivotal studies of oxaliplatin in metastatic and adjuvant treatment of colorectal cancer
| Study | Number of patients | Patients with HSR (%) | Patients with Grade 3/4 HSR (%) |
| Data from first-line[10] | 259 | 12 | 2 |
| Data from second-line[10] | 150 | 10 | < 1 |
| MOSAIC (adjuvant)[2] | 1123 | 10.3 | 2.9 |
| Siu et al[1] all patients | 180 | 15 | 2.2 |
| Siu et al[11] adjuvant/ first-line metastatic | 88 | 10.2 | |
| Siu et al[11] second-line metastatic | 92 | 19.6 |
HSR: Hypersensitivity reactions.
All platinum compounds are known to cause HSR[12-15]. Being a platinum derivative, it is not surprising that oxaliplatin can also develop HSR[5-9]. The differences in the reactions have been summarized in the Table 3.
Table 3.
Characteristics of HSR with different Platinum compounds
| Cisplatinum[12,13] | Carboplatinum[4,15] | Oxaliplatin[5-9] | |
| Incidence | 5%-20% ( Increased with radiation) | 16% | 12% (14), < 1% grade 3-4 |
| Initial onset | - | After 6 cycles( range 2-12) | After 7 cycles ( range 2-25) |
| Time of onset | Minutes | Minutes-days | Minutes-hours |
| Symptoms | Variable( fever, anxiety, pruritus, cough, dyspnea, diaphoresis, angioedema, vomiting, bronchospasm, rash and pruritus, and hypotension | Variable (itching ,rash, chest tightness, Blood pressure changes, facial swelling) | Variable (flushing, alterations in heart rate, dyspnea, back pain, fever, pruritis, erythema, nausea, rash) |
| Can it be re-introduced? | - | Yes, with slowing the infusion rate (6% discontinued) | Yes, with pre-medications, slowing the infusion rate, and/or desensitization |
The pathophysiology of HSR is not well understood. Development of this reaction after multiple infusions in most patients suggests the need for sensitization. Some investigators have described these reactions as Type I (IgE-mediated) allergic reactions[16]. Santini et al reported a case of an idiosyncratic reaction; the serum analysis of the patient showed elevated TNF-alpha and IL6. The investigator postulated it to be a T-cell mediated reaction[17]. It was also postulated that oxaliplatin acted as a super-antigen on mononuclear cells and resulted in the release of these cytokines. Other mechanisms that have been suggested include binding of the platinum salts to different peptides of major histocompatibility complex (MHC). In fact, HLA phenotype is a significant determinant of occupational sensitization to inhaled hapten of complex platinum salts and the strength of this association varies according to the intensity of exposure[18]. Furthermore, the relationship between hypersensitivity reactions and HLA-haplotype has been described for other drugs[19]. Additional factors are deemed to be necessary to the immune system for developing the reaction after several infusions.
Fever alone is not a usual symptom of oxaliplatin hypersensitivity. In another study among 39 patients who received a FOLFOX regimen in first line or beyond, the most common manifestations of an allergic reaction included: respiratory (50%), cutaneous (40%), and an anaphylactic shock (7.6%)[20]. Ulrich-Pur et al[21] reported a case of 74-year-old man who developed fever hours after receiving the third infusion of oxaliplatin (5-FU + LV d 1 and d 5 every 28 d + mitomycin C d 1). The temperature was recorded to 39°C and lasted for 3 d. Patient had similar episodes of fever with 4th to 6th infusion. A rise in serum levels of IL 6 levels with 5th and 6th infusion corresponded to the rise in temperature. Pre-medication with dexamethasone, clarithromycin and metamizol for 3 d prior to therapy did not prevent the febrile reaction. Because the reactions occurred 1.5 to 2 h after infusion, the authors did not consider it to be hypersensitivity reactions, and thought to be definitely some kind of acquired allergic reaction because the 1st and the 2nd infusions were well-tolerated. It is to be noted that mitomycin C can also cause fever as a reaction in some cases. Thomas et al[5] reported hypersensitivity reactions in 3 patients; one of them reported fever of 39.6°C 2 h after the infusion of the 9th cycle of oxaliplatin. Fever defervesced overnight and cultures were negative. The patient again developed fever of 39.0°C with rigors and chest tightness several hours after the next cycle. Before the next cycle, the patient was pre-medicated with dexamethasone 20 mg, 6 and 12 h. Thirty minutes before the infusion the patient received solumedrol 125 mg, diphenhydramine 50 mg and cimetidine 30 mg. Despite this, the patient still developed a single spike of 38.3°C without associated symptoms[5].
When a HSR occurs, the infusion of oxaliplatin should be immediately stopped and replaced by a saline infusion, an intravenous antihistaminic drug and a low-dose corticosteroids administration. In the case of more severe reactions (dyspnoea, sweating, bronchospasm, laryngospasm), immediately administer a high dose of steroid. The steroid dose range between 100 and 1000 mg of hydrocortisone[9]. After the reaction disappears, the oxaliplatin infusion should not be restarted and the decision to administer the other scheduled drugs must be taken evaluating the clinical status of the patient after the reaction, the risk of additional toxicity and the clinical utility of the chemotherapy. Mild hypersensitivity reactions to oxaliplatin and other platinum compounds can be ameliorated in some patients through the use of steroids and antihistamines before administration of subsequent cycles. However, premedication cannot prevent all hypersensitivity reactions, and mild reactions may escalate to severe reactions even when steroids and antihistamines are administered prior to oxaliplatin infusion[22].
Over the years many protocols have been devised to desensitize patients against these life-threatening reactions so that they can benefit from Oxaliplatin. The various approaches have been summarized in the Table 4. One of the approaches is to increase the duration of the infusion. This was described in a retrospective study by Brandi et al[6] In the review of 124 patients the author reported fewer hypersensitivity reactions as the infusion time was increased from standard 2 to 6 h. In this review, 17 patients out of 124 (13.7%) developed hypersensitivity. Out of the 17 only 2 developed reactions at the end of infusion while in the rest the reaction appeared 10-15 min from start of the infusion. Six of the 17 patients were successfully re-exposed after pre-medication with steroids and antihistaminic drugs. Five patients developed hypersensitivity symptoms again and only one had no further reactions.
Table 4.
Published cases of hypersensitivity associated with oxaliplatin
| Reference | Presenting Features | Premedication | Oxaliplatin dose and serial dilution if used | Duration of therapy |
| Lydia et al[23] | Diaphoresis, hypotension, nausea, abdominal cramping rash, coryza | D1-prednisone 20 mg Po Q6 h × 4 D2 45 min pre chemo ondansterone 8 mg + dexamethasone 20 mg iv 30 min prior diphenylhydramine 50 mg iv + cemetidine 300 mg iv | 140 mg on d 2 dilutions from 1:10000 to 1:1 | 8 h |
| Thomas et al[5] | Erythematic, flushing | Dexamethasone 20 mg 6 h and 12 h prior 30 min pre infusion: solumedrol 125 mg iv, diphenylhydramine 50 mg iv, cimetidine 50 mg iv | - | 2-4 h |
| Bhargava et al[22] | Palpitation, flushing, hypotension | Dexamethasone starting 24 h prior 30 min before infusion received dexamethasone, diphenylhydramine, hydrocortisone 100 mg iv | 1:10000, 1:1000, 1:100, 1:10 each bag infuse over one hour | 6-8 h |
| Meyer et al[7] | dexamethasone-famotidine and diphenylhydramine | 90% of total dose 1:1000, 1:100 and 1:10 dilution over 90 min | 6 h | |
| Lim et al[24] | Abdominal distension, heat, pruritis | D 1 diphenylhydramine 50 mg QID 30 min prior to infusion metoclopramide 9 mg, morazepam 2 mg, dexamethasone 5 mg iv | Fixed dose infusion over 24 h with dilute solution (0.15 mg/mL) | 24 h |
| Present case | Fever | - | Refused further oxaliplatin therapy | - |
Another approach is desensitization to the drug. Lydia et al[23] reported a 53-year-old female with metastatic colon cancer who was treated with CAPOX (capecitabine 1000 mg/m2 per day Monday to Friday with 2 weekly 85 mg/m2 oxaliplatin) and bevacizumab (Avastin) 10 mg/kg. With the cycle 5th she developed diaphoresis, hypotension, hypoxia, nausea, and abdominal cramps 12 min from the start of infusion. She was immediately resuscitated with fluid bolus, oxygen, diphenylhydramine (25 mg IV) and dexamethasone (10 mg IV). The patient received cycle 5th through 7th without oxaliplatin. Because she had partial response to combination chemotherapy with oxaliplatin and she refused to take irinotecan (camptosar; Pfizer) due to toxicity profile, it was decided to desensitize her. On d 1 of desensitization, the patient was given prednisone 20 mg every 6 h for 4 doses and was hospitalized the same day. On d 2, 45 min before infusion 8 mg of ondansterone and 20 mg of dexamethasone were administered. Then 30 min before infusion, diphenyhydramine 50 mg and cimetidine 300 mg was administered. Oxaliplatin was administered over 8 h in serial dilutions from 1:10000 to 1:1 and she received a total dose of 140 mg. Intravenous epinephrine (1:1000) and diphenyhydramine and methylprednisolone 125 mg were placed at bedside. The patient tolerated the therapy without complications. The next infusion was given as outpatient. Thus desensitization helped this patient to receive an additional three doses. In this report 24-h pre-medication schedule with serial dilution of oxaliplatin over longer period of time was used based on reports used for desensitization of carboplatinum. Lim et al[24] also published his case with successful desensitization.
With carboplatin, an intradermal skin test after 6th dose is a good predictor for occurrence of this reaction. However, no intradermal test is recommended before oxaliplatin administration. An intradermal skin test for hypersensitivity to oxaliplatin has been reported in small series to be 75%-80% accurate[25]. The investigators suggested that desensitization might be considered for patients with a mild to moderate skin reaction in whom oxaliplatin would be beneficial. The investigators also suggested that a rechallenge should not be attempted for patients with markedly positive skin test reactions. Garufi et al reported the skin test to be negative in 15 patients with no previous reactions to oxaliplatin. A positive skin-test reaction is helpful; however, negative results may be seen in some patients who experience hypersensitivity[25]. Two of 8 patients with prior hypersensitivity reactions to oxaliplatin were reported by Meyer et al[7] to have negative skin tests with oxaliplatin, as did 1 of 3 patients described by Thomas et al[5].
It is expected to see a rising incidence of HSR to oxaliplatin as has been observed as a result of increasing clinical use. Siu et al[11] recently reported epidemiological and clinical features of these reactions in his institution. Among 180 patients, 15% were labeled as allergic to oxaliplatin, the proportion being higher among those receiving oxaliplatin in palliative second-line or above settings (19.6%) than in adjuvant or palliative first-line settings (10.2%). Overall, 2.2% of them developed grade 3-4 reactions. Re-exposure to oxaliplatin in 14 patients resulted in 28.6% HSR with 14.3%reactions of grade 3-4 intensity.
Although the reported incidence of HSR is about 12% of the patients who receive oxaliplatin (1%-2% grade 3 or 4 in severity), the recent rising incidence of HSR to oxaliplatin observed is the result of increasing clinical use. It is also important to remember that fever can be the sole manifestation of initial HSR in few cases akin to ours; a harbinger that this patient can subsequently develop serious HSR with continued use of oxaliplatin. Therefore, proper recognition and management can prevent a serious HSR. Few patients can be managed with pre-medication with steroids and antihistamines, but the majority of patients require intensive desensitization. Due to the desensitization regimens many patients can successfully continue to receive this agent. Reintroductions have only been reported as single case studies or small cohorts. Large scale validation on desensitization strategies are still missing. Knowledge of this rare but real toxicity of oxaliplatin is paramount since the use of this drug is wide-spread both for metastatic and adjuvant settings in the treatment of colorectal cancer-the second leading cause of cancer mortality in USA.
Footnotes
S- Editor Zhu LH L- Editor Li M E- Editor Li JL
References
- 1.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts S. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 2.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd GM. Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol. 2003;24:253–262. doi: 10.1385/CRIAI:24:3:253. [DOI] [PubMed] [Google Scholar]
- 4.Robinson JB, Singh D, Bodurka-Bevers DC, Wharton JT, Gershenson DM, Wolf JK. Hypersensitivity reactions and the utility of oral and intravenous desensitization in patients with gynecologic malignancies. Gynecol Oncol. 2001;82:550–558. doi: 10.1006/gyno.2001.6331. [DOI] [PubMed] [Google Scholar]
- 5.Thomas RR, Quinn MG, Schuler B, Grem JL. Hypersensitivity and idiosyncratic reactions to oxaliplatin. Cancer. 2003;97:2301–2307. doi: 10.1002/cncr.11379. [DOI] [PubMed] [Google Scholar]
- 6.Brandi G, Pantaleo MA, Galli C, Falcone A, Antonuzzo A, Mordenti P, Di Marco MC, Biasco G. Hypersensitivity reactions related to oxaliplatin (OHP) Br J Cancer. 2003;89:477–481. doi: 10.1038/sj.bjc.6601155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer L, Zuberbier T, Worm M, Oettle H, Riess H. Hypersensitivity reactions to oxaliplatin: cross-reactivity to carboplatin and the introduction of a desensitization schedule. J Clin Oncol. 2002;20:1146–1147. doi: 10.1200/JCO.2002.20.4.1146. [DOI] [PubMed] [Google Scholar]
- 8.Wrzesinski SH, McGurk ML, Donovan CT, Ferencz TM, Saif MW. Successful desensitization to oxaliplatin with incorporation of calcium gluconate and magnesium sulfate. Anticancer Drugs. 2007;18:721–724. doi: 10.1097/CAD.0b013e32802ffbcb. [DOI] [PubMed] [Google Scholar]
- 9.Saif MW. Hypersensitivity reactions associated with oxaliplatin. Expert Opin Drug Saf. 2006;5:687–694. doi: 10.1517/14740338.5.5.687. [DOI] [PubMed] [Google Scholar]
- 10. Available from: http: //www.eloxatin.com/hcp/about_eloxatin/default.aspx(accessed on July 25, 2007).
- 11.Siu SW, Chan RT, Au GK. Hypersensitivity reactions to oxaliplatin: experience in a single institute. Ann Oncol. 2006;17:259–261. doi: 10.1093/annonc/mdj042. [DOI] [PubMed] [Google Scholar]
- 12.Koren C, Yerushalmi R, Katz A, Malik H, Sulkes A, Fenig E. Hypersensitivity reaction to cisplatin during chemoradiation therapy for gynecologic malignancy. Am J Clin Oncol. 2002;25:625–626. doi: 10.1097/00000421-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Basu R, Rajkumar A, Datta NR. Anaphylaxis to cisplatin following nine previous uncomplicated cycles. Int J Clin Oncol. 2002;7:365–367. doi: 10.1007/s101470200056. [DOI] [PubMed] [Google Scholar]
- 14.Polyzos A, Tsavaris N, Kosmas C, Arnaouti T, Kalahanis N, Tsigris C, Giannopoulos A, Karatzas G, Giannikos L, Sfikakis PP. Hypersensitivity reactions to carboplatin administration are common but not always severe: a 10-year experience. Oncology. 2001;61:129–133. doi: 10.1159/000055363. [DOI] [PubMed] [Google Scholar]
- 15.Zanotti KM, Rybicki LA, Kennedy AW, Belinson JL, Webster KD, Kulp B, Peterson G, Markman M. Carboplatin skin testing: a skin-testing protocol for predicting hypersensitivity to carboplatin chemotherapy. J Clin Oncol. 2001;19:3126–3129. doi: 10.1200/JCO.2001.19.12.3126. [DOI] [PubMed] [Google Scholar]
- 16.Stahl M, Köster W, Wilke H. Reaction after oxaliplatin--prevention with corticosteroids? Ann Oncol. 2001;12:874. doi: 10.1023/a:1011161126611. [DOI] [PubMed] [Google Scholar]
- 17.Santini D, Tonini G, Salerno A, Vincenzi B, Patti G, Battistoni F, Dicuonzo G, Labianca R. Idiosyncratic reaction after oxaliplatin infusion. Ann Oncol. 2001;12:132–133. doi: 10.1023/a:1008366223918. [DOI] [PubMed] [Google Scholar]
- 18.Newman Taylor AJ, Cullinan P, Lympany PA, Harris JM, Dowdeswell RJ, du Bois RM. Interaction of HLA phenotype and exposure intensity in sensitization to complex platinum salts. Am J Respir Crit Care Med. 1999;160:435–438. doi: 10.1164/ajrccm.160.2.9807065. [DOI] [PubMed] [Google Scholar]
- 19.Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, Lai E, Davies K, Handley A, Dow DJ, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–1122. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 20.Maindrault-Goebel F, André T, Tournigand C, Louvet C, Perez-Staub N, Zeghib N, De Gramont A. Allergic-type reactions to oxaliplatin: retrospective analysis of 42 patients. Eur J Cancer. 2005;41:2262–2267. doi: 10.1016/j.ejca.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich-Pur H, Penz M, Fiebiger WC, Schüll B, Kornek GV, Scheithauer W, Raderer M. Oxaliplatin-induced fever and release of IL-6. Oncology. 2000;59:187–189. doi: 10.1159/000012159. [DOI] [PubMed] [Google Scholar]
- 22.Bhargava P, Gammon D, McCormick MJ. Hypersensitivity and idiosyncratic reactions to oxaliplatin. Cancer. 2004;100:211–212. doi: 10.1002/cncr.11901. [DOI] [PubMed] [Google Scholar]
- 23.Mis L, Fernando NH, Hurwitz HI, Morse MA. Successful desensitization to oxaliplatin. Ann Pharmacother. 2005;39:966–969. doi: 10.1345/aph.1E532. [DOI] [PubMed] [Google Scholar]
- 24.Lim KH, Huang MJ, Lin HC, Su YW, Chang YF, Lin J, Chang MC, Hsieh RK. Hypersensitivity reactions to oxaliplatin: a case report and the success of a continuous infusional desensitization schedule. Anticancer Drugs. 2004;15:605–607. doi: 10.1097/01.cad.0000132235.38297.7c. [DOI] [PubMed] [Google Scholar]
- 25.Garufi C, Cristaudo A, Vanni B, Bria E, Aschelter AM, Santucci B, Terzoli E. Skin testing and hypersensitivity reactions to oxaliplatin. Ann Oncol. 2003;14:497–498. doi: 10.1093/annonc/mdg092. [DOI] [PubMed] [Google Scholar]
- 26.Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse events, version 3.0. Available from: http: //ctep.cancer.gov/forms/CTCAEv3.pdf.