Abstract
AIM: The purpose of this study is to find a better operative technique by comparing interrupted stitches with continuous stitches for the outer layer of the pancreaticojejunostomy, i.e., the stitches between the stump parenchyma of the pancreas and the jejunal seromuscular layer, and other risk factors for the incidence of pancreatic leakage.
METHODS: During the period January 1997 to October 2004, 133 patients have undergone the end-to-side and duct-to-mucosa pancreaticojejunostomy reconstruction after pancreaticoduodenectomy with interrupted suture for outer layer of the pancreaticojejunostomy and 170 patients with a continuous suture at our institution by one surgeon.
RESULTS: There were no significant differences between the two groups in the diagnosis, texture of the pancreas, use of octreotide and pathologic stage. Pancreatic fistula occurred in 14 patients (11%) among the interrupted suture cases and in 10 (6%) among the continuous suture cases (P = 0.102). Major pancreatic leakage developed in three interrupted suture patients (2%) and zero continuous suture patients (P = 0.026). In multivariate analysis, soft pancreatic consistency (odds ratio, 5.5; 95% confidence interval 2.3-13.1) and common bile duct cancer (odds ratio, 3.7; 95% CI 1.6-8.5) were predictive of pancreatic leakage.
CONCLUSION: Pancreatic texture and pathology are the most important factors in determining the fate of pancreaticojejunal anastomosis and our continuous suture method was performed with significantly decreased occurrence of major pancreatic fistula. In conclusion, the continuous suture method is more feasible and safer in performing duct-to-mucosa pancreaticojejunostomy.
Keywords: Pancreaticoduodenectomy, Pancreati-cojejunostomy, Pancreatic fistula
INTRODUCTION
Despite improvement of the operative technique, materials and instruments, pancreatic fistula after pancreaticoduodenctomy is the most common and serious complication. Recent large series have reported that the failure rate of the pancreaticoenteric anastomosis is 9%-18%[1-6], a complication rate not far improved from Dr. Whipple's report of a 19.5% fistula rate more than 50 years ago[7]. A number of methods for reducing the incidence of pancreatic fistula have been proposed and tested. Many of these involve technical aspects of the anastomosis, including the site of reconstruction (pancreaticogastrostomy versus pancreaticojejunosto-my)[8,9], the anastomotic technique (duct-to-mucosa anastomosis versus stump invagination)[10,11], the use of biologic adhesive[12,13] and the use of intraoperative transanastomotic stents[14]. In addition, in order to determine how to prevent pancreatic fistula, the risk factors for pancreatic fistula have been extensively studied. They include the patient's comorbid illness[8], age[15], texture of the pancreas[4], pancreatic duct size[16], intraoperative blood loss[15] and the surgeon's experience[15].
The present study tested the hypothesis that using continuous stitches for the outer layer of the panceaticojejunostomy (i.e., the stitches between the stump parenchyma of the pancreas and the jejunal seromuscular layer) is a better operative technique than using interrupted stitches in terms of safety and efficiency.
MATERIALS AND METHODS
During the period from January 1997 to October 2004, 133 patients underwent duct-to-mucosa pancreaticojejunostomy reconstruction after pancreaticoduodenectomy with the interrupted suture method for the outer layer of the pancreaticojejunostomy and 170 patients underwent the procedure with the continuous suture method at our institution. From 1997 to 2000, the interrupted suture method was performed, and from 2001 to 2004, the continuous suture method was performed. The operations were performed by one surgeon who had experienced more than 500 cases of pancreaticoduodenectomy before this study.
We retrospectively reviewed the medical records of the patients who underwent duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy noting parameters such as the existence of pancreatic fistula, age, sex, preoperative symptoms, preoperative laboratory tests results, amount of intraoperative bleeding, and postoperative octreotide usage. Postoperative octreotide was given subcutaneously (dose 100 mg every 8 hours) for the patients considered high risk for pancreatic fistula based on gland texture and duct size.
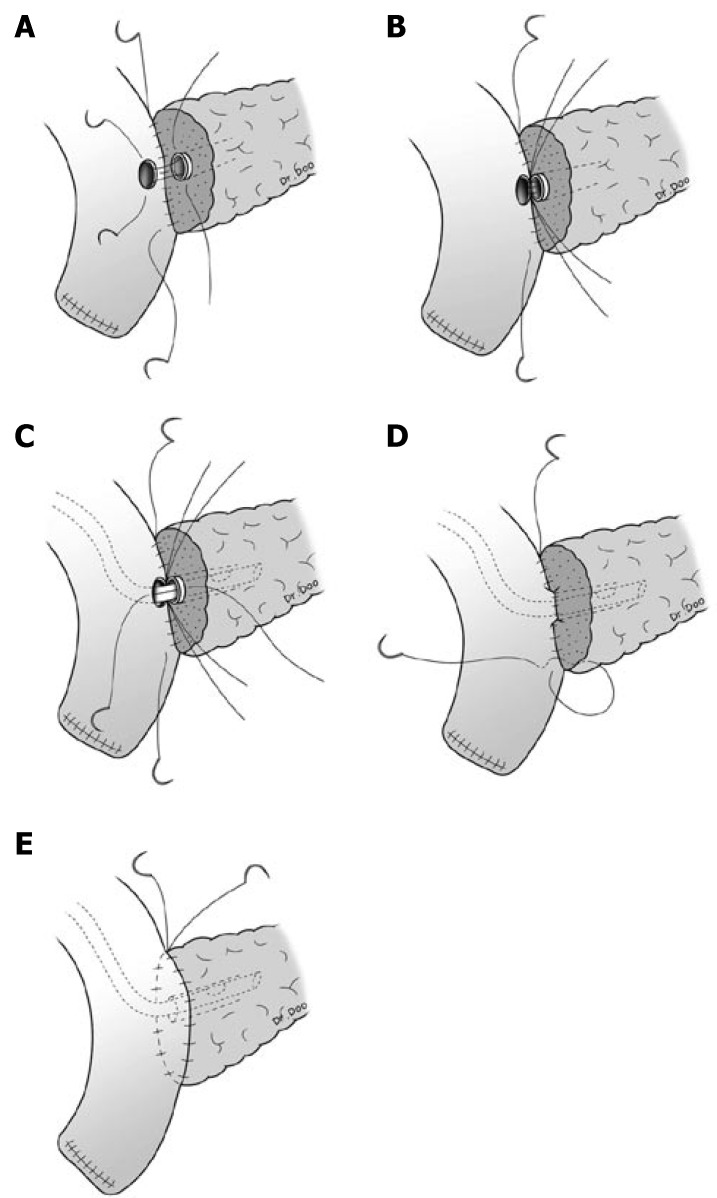
Pancreaticoduodenectomy was performed with conventional pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy (PPPD). Anastomosis for the remnant pancreas was performed between the pancreas and jejunum by a two layer pancreaticojejunostomy. The outer layer consisted of the remnant pancreatic parenchyma and the seromuscular layer of jejunum and interrupted suture or continuous suture between these two was performed with 5-0 polypropylene (Prolene*, Ethicon, Somerville, NJ). The inner layer consisted of the pancreatic duct and mucosa of the jejunum, and interrupted suture for duct-to-mucosa was performed with 5-0 polydioxanone (PDSTM II, Ethicon, Somerville, NJ). A silastic polyethylene tube was inserted into the pancreatic duct as a stent for all patients, and external drainage was done (Figure 1).
Figure 1.
Continuous suture method for the outer layer of pancreaticojejunostomy. A: The posterior outer layer consisted of the remnant pancreatic parenchyma and the seromuscular layer of jejunum and continuous suture between these two was performed with 5-0 polypropylene (Prolene*, Ethicon, Somerville, NJ); B: The posterior inner layer consisted of the pancreatic duct and mucosa of the jejunum, and interrupted suture for duct-to-mucosa was performed with 5-0 polydioxanone (PDSTMII, Ethicon, Somerville, NJ); C: A silastic polyethylene tube was inserted into the pancreatic duct and external drainage was done; D: For anterior inner layer consisted of the pancreatic duct and mucosa of the jejunum, interrupted suture was performed; E: Continuous suture for anterior outer layer was performed.
Two or three drains were routinely placed anterior and posterior to the pancreatico-jejunal anastomosis and exteriorized through the lateral abdominal wall.
A pancreatic fistula was defined as the drainage of more than 30 mL of fluid with an amylase level higher than 600 U/dL on or after postoperative week 1[17]. Also, three grades of fistula severity (A, B, C) were classified according to the International Study Group for Pancreatic Fistulas (ISGPF) clinical criteria[18] as follows: Grade A fistulas are transient, asymptomatic fistulas, with only elevated drain amylase levels and treatments or deviation in clinical management are not required. Grade B fistulas are symptomatic, clinically apparent fistulas that require diagnostic evaluation and therapeutic management. Grade C fistulas are severe, clinically significant fistulas that require major deviations in clinical management and aggressive therapeutic interventions are unquestionably warranted. Major pancreatic leakage was defined as the drainage of more than 200 mL of fluid or the development of an intra-abdominal abscess.
Pancreatic fistulas, according to the operative methods and clinicopathologic factors causing pancreatic fistula, were analyzed.
Data comparisons between the two groups were made using the χ2 test for qualitative parameters, a Student's t-test for quantitative parameters and logistic regression n for determining the effect of multiple risk factors on pancreatic leakage. A value of P < 0.05 was accepted as significant.
RESULTS
There were no significant differences in mean age, sex, prophylactic use of octreotide, pancreas texture, stage, and indication for pancreaticoduodenectomy between the two groups (Table 1).
Table 1.
Comparison of clinical characteristics between the interrupted suture group and the continuous suture group n (%)
| Interrupted suture group (n = 133) | Continuous suture group (n = 170) | P value | |
| Age (yr) | 58.2 ± 12.4 | 60.4 ± 10.9 | 0.072 |
| Male:Female | 1.8:1 | 1.5:1 | 0.484 |
| Pancreas texture | 0.893 | ||
| Hard | 35 (26) | 52 (31) | |
| Firm | 86 (65) | 76 (45) | |
| Soft | 12 (9) | 42 (24) | |
| Use of prophylactic octreotide | 83 (62) | 122 (72) | 0.072 |
| Jaundice | 59 (44) | 69 (41) | 0.643 |
| Diabetes mellitus | 21 (16) | 33 (19) | 0.544 |
| Diagnosis | 0.433 | ||
| Ampulla of vater cancer | 35 (26) | 45 (26) | |
| Common bile duct cancer | 38 (29) | 46 (27) | |
| Pancreatic cancer | 34 (25) | 57 (34) | |
| Duodenal cancer | 6 (5) | 6 (4) | |
| Etc. | 20 (15) | 16 (9) |
Etc.: Ampulla of Vater adenoma, choledochal cyst, chronic pancreatitis, duodenal GIST, gallbladder cancer, intraductal papillary mucinous tumor, islet cell tumor of pancreas, peripancreatic neurilemmoma, pseudocyst, serous cystic adenoma.
Although there was no significant difference in the mean total operation time between the interrupted suture and continuous suture groups, there was a statistically significant difference in the mean operation time of the outer layer anastomosis of pancreaticojejunostomy. The mean operation time of the interrupted suture method for the outer layer anastomosis of pancreaticojejunostomy was 35.4 ± 4.8 minutes, and the mean operation time of the continuous suture method was 29.1 ± 3.9 min (P < 0.001).
Pancreatic fistula occurred in 14 patients (11%) of the 133 patients who underwent interrupted suture for the outer layer and in 10 patients (6%) of the 170 patients who underwent continuous suture (P = 0.102). There were 5 grade A fistulas, 6 grade B fistulas, and 3 grade C fistulas in the interrupted suture group. There were 4 grade A fistulas, 5 grade B fistulas, and no grade C fistula in the continuous suture group. There was no significant difference between the two groups (P = 0.085). Major pancreatic leakage occurred in three patients (2%) in the interrupted suture group and zero patients in the continuous suture group (P = 0.026). For one patient of the interrupted suture group who experienced pancreaticojejunal anastomotic rupture, externalization of the pancreatic duct was performed at the 10th postoperative day. For the other two patients of the interrupted suture group with major pancreatic leakage, percutaneous drainage was added.
Two patients of the continuous suture group with distal common bile duct (CBD) cancer developed pseudoaneurysm with preceding pancreatic fistula. They were successfully managed by radiologic intervention. There was no postoperative hospital mortality in the two groups (Table 2).
Table 2.
Comparison of postoperative complications and mortality between the interrupted suture group and the continuous suture group n (%)
|
Number of patients |
P value | ||
| Interrupted suture group (n = 133) | Continuous suture group (n = 170) | ||
| Pancreatic fistula | 14 (10.5) | 10 (5.9) | 0.102 |
| ISGPF grade | 0.085 | ||
| Grade A | 5 (3.8) | 4 (2.4) | |
| Grade B | 6 (4.5) | 6 (3.5) | |
| Grade C | 3 (2.3) | 0 | |
| Major pancreatic fistula | 3 (2.3) | 0 | 0.026 |
| Disruption | 1 (0.8) | 0 | |
| Daily drainage > 200 cc | 0 | 0 | |
| Intra-abdominal abscess | 2 (1.5) | 0 | |
| Pseudoaneurysm | 0 | 2 | 0.128 |
| Reoperation for pancreatic fistula | 1 (0.8) | 0 | 0.199 |
| Hospital mortality | 0 | 0 | |
Of the total of 303 patients, 24 patients (8%) developed postoperative pancreatic fistula. There were no significant differences in age, sex, preoperative bilirubin and albumin levels, operation methods, amount of intraoperative bleeding, total operation time, postoperative prophylactic octreotide usage, and stage between the pancreatic fistula group and the non-pancreatic fistula group. There was a significant difference in pathologic features between the pancreatic fistula group and the non-pancreatic fistula group (P = 0.039). When the pathologic features were divided into CBD cancer and non-CBD cancer, there was a significant difference (P = 0.004). The consistency of the remnant pancreas correlates strongly with subsequent postoperative fistula rates. In the non-pancreatic fistula group, 43 (15%) were classified as soft and 85 as hard. In the pancreatic fistula group, 11 (46%) were classified as soft and 2 as hard (P < 0.001) (Table 3). In multivariate analysis, soft pancreatic consistency (odds ratio, 5.5; 95% confidence interval 2.3-13.1) and CBD cancer (odds ratio, 3.7; 95% confidence interval 1.6-8.5) were predictive of pancreatic leakage.
Table 3.
Perioperative risk factors for pancreatic fistula
| Pancreatic fistula (+) (n = 24) | P value | |
| Age (yr) | 62.3 ± 10.2 | 0.565 |
| Male:Female | 2:1 | 0.582 |
| Preoperative disease | ||
| Diabetes mellitus | 4 (7%, 4/54) | 0.959 |
| Laboratory findings | ||
| Hypoalbuminemia (3 < g/dL) | 3 (9%, 3/33) | 0.729 |
| Hyperbilirubinemia (> 2 mg/dL) | 14 (7%, 14/195) | 0.665 |
| Pathologic feature | 0.039 | |
| Ampulla of Vater cancer | 4 (5%, 4/80) | |
| Common bile duct cancer | 13 (16%, 13/84) | |
| Pancreatic cancer | 4 (4%, 4/91) | |
| Duodenal cancer | 0 (0%, 0/12) | |
| Others | 3 (8%, 3/36) | |
| Type of resection | 0.097 | |
| PPPD | 22 (10%, 22/218) | |
| Whipple's op. | 2 (2%, 2/85) | |
| Outer layer suture method | 0.102 | |
| Interrupted | 14 (11%, 14/133) | |
| Continuous | 10 (6%, 10/170) | |
| Pancreas consistency | < 0.001 | |
| Hard | 2 (2%, 2/87) | |
| Firm | 11 (7%, 11/162) | |
| Soft | 11 (20%, 11/54) | |
| Total operative time (min) | 383 ± 52 | 0.515 |
| Estimated blood loss (mL) | 564 ± 220 | 0.831 |
| Use of prophylactic Octreotide | 14 (7%, 14/205) | 0.317 |
| Lymph node metastasis | 0.351 | |
| Yes | 17 (9%, 17/197) | |
| No | 7 (7%, 7/106) | |
| Positive resection margin | 0.105 | |
| Yes | 1 (4%, 1/26) | |
| No | 23 (8%, 23/277) |
DISCUSSION
Recently, pancreaticoduodenectomy has become popularized as the standard treatment for various benign and malignant diseases of the periampullary region, including the pancreas head. Although the mortality rate has markedly decreased, the incidence of pancreatic fistula, the most catastrophic complication, remains high after pancreaticoduodenectomy. After the first successful pancreaticoduodenectomy was performed by the German surgeon Kausch in 1912[19], the risk factors for pancreatic anastomotic leakage and methods aimed at prevention of pancreatic anastomotic leakage have been extensively studied.
Several factors related to pancreatic anastomotic leakage have been described in the literature. They can be conveniently divided into disease factors (pancreatic texture[4], pancreatic pathology[8], pancreatic duct size[16], pancreatic juice output[8]), procedure-related factors (intraoperative blood loss[15], operative techniques[15]) and patient factors (age[15], sex[8], comorbid illness[8], jaundice[8]). Still, it is very difficult to predict the relationship of the risk factors and pancreatic fistula, and many studies have revealed heterogeneous results. In our study, two risk factors, common bile duct cancer and soft pancreatic texture were correlated with increased incidence of pancreatic fistula. The relative proportion of bile duct and ampulla of Vater cancers is much larger in Korea than in Western countries. The reason is not clear. The high incidence of hepatolithiasis, choledocholithiasis, choledochal cyst, and clonorchiasis in Korea is a possible explanation, at least in a proportion of cases. Unlike in the Johns Hopkins series[8], the leakage rate was lower in cases of ampulla of Vater cancer (5%, 4/80) than in others (9%, 20/223). Patients with ampulla of Vater cancer are diagnosed early, and their general condition is relatively good. More importantly, most of the patients have a dilated pancreatic duct and a firm pancreas, which facilitates the anastomosis and its healing. On the other hand, most of the patients with common bile duct cancer have a non-dilated pancreatic duct and a soft pancreas. The increased fistula rates with soft pancreatic texture may be interpreted by three explanations. First, most soft pancreases have no pancreatic duct dilatation, which makes secure duct-to-mucosa anastomosis difficult[20,21]. Second, a soft pancreas is more easily injured directly or via ischemia by stitches placed between the pancreas parenchyma and the seromuscular layer of the jejunum[20]. Third, a soft pancreas has a good exocrine function, secreting more pancreatic juices rich in proteolytic enzymes[20-22].
Prophylactic use of octreotide is expected theoretically to reduce the incidence rate of the pancreatic fistula through decreasing pancreatic juice secretion. However, randomized trials from Europe and United states showed opposite results[23-26]. In our study, there was no significant difference in the incidence rate of pancreatic fistula between postoperative prophylactic octreotide usage group and no usage group (P = 0.347). To prove the effect of octreotide in reducing the amount of postoperative pancreatic fistula, more organized randomized controlled studies are needed.
Various techniques for managing the pancreatic remnant have been studied with the aim of reducing the anastomotic leakage rate, including varying the site of the jejunum for pancreaticojejunostomy (end vs side), varying the type of anastomosis (duct-to-mucosa vs invagination)[10,11], comparing the difference between pancreaticogastrostomy and pancreaticojejunostomy[8,9], use of fibrin glue[12,13] and pancreatic duct stenting[14]. Unfortunately, randomized trials on these technical measures are scarce[11]. As a result, there is no universal agreement on which operative technique is safer and less prone to pancreatic leakage[11].
Because our study is not a prospective randomized controlled study, there is potential for a beta error. However, it could be thought as a periodic randomized study. The operations were performed by one surgeon who had experienced more than 500 cases of pancreaticoduodenectomy before this study, and the improved results are unlikely associated with a learning curve by doing more procedures.
Although there was no significant difference between the interrupted suture and continuous suture methods for preventing pancreatic fistula in our study, the incidence of major pancreatic fistula decreased significantly in the continuous suture group (P = 0.026). Theoretically, the continuous suture method has many advantages over the interrupted suture method[27-32]. First of all, a more even distribution of tension is possible in the continuous suture between the pancreatic parenchyma and jejunum[27]. Due to the coiled spring effect, the continuous suture method provides a reduction in the likelihood of focal tissue ischemia[27], an increase in tensile strength[27], and a reduction of the risk of pancreaticojejunal rupture. A continuous suture provides an enhanced air and watertight seal[28,29]. As our study shows, a continuous suture reduces anastomosis time[30,31]. A continuous suture is technically easier[30,31] and costs less[31,32]. For continuous sutures, we use one polypropylene (Prolene*, Ethicon, Somerville, NJ) thread, and the thread costs 5.7 dollars. However, for interrupted sutures, we use 16 polypropylene sutures and it costs 86 dollars more than continuous suture.
The study of two methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer in duct-to-mucosa pancreaticojejunostomy revealed that the incidence of major pancreatic fistula decreased significantly and operation time is reduced significantly in the continuous suture group. In conclusion, the continuous suture method is more feasible and safe to use in performing pancreaticojejunostomy.
COMMENTS
Background
Leakage of the pancreaticojejunal anastomosis has been a major complication after pancreaticoduodenectomy. Over the past decades, various measures directed towards prevention of pancreatic leakage have been studied.
Research frontiers
The purpose of this study is to find better operative technique as comparing the interrupted stitches with the continuous stitches for the outer layer of the panceaticojejunostomy, ie. the stitches between the stump parenchyma of the pancreas and the jejunal seromuscular layer, and other risk factors for the incidence of pancreatic leakage.
Innovations and breakthroughs
Soft pancreatic consistency (odds ratio, 5.5; 95% confidence interval 2.3-13.1) and CBD cancer (odds ratio, 3.7; 95% confidence interval 1.6-8.5) were predictive of pancreatic leakage and our continuous suture method performed with significantly decreasing major pancreatic fistula.
Applications
The study of two methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer in duct-to-mucosa pancreaticojejunostomy revealed that the incidence of major pancreatic fistula decreased significantly and operation time is reduced significantly in the continuous suture group. In conclusion, the continuous suture method is more feasible and safe to use in performing pancreaticojejunostomy.
Terminology
A pancreatic fistula was defined as the drainage of more than 30 mL of fluid with an amylase level higher than 600 U/dL on or after postoperative week 1. Also, three grades of fistula severity (A, B, C) were classified according to ISGPF clinical criteria as follows: Grade A fistulas are transient, asymptomatic fistulas, with only elevated drain amylase levels and treatments or deviations in clinical management are not required. Grade B fistulas are symptomatic, clinically apparent fistulas that require diagnostic evaluation and therapeutic management. Grade C fistulas are severe, clinically significant fistulas that require major deviations in clinical management and aggressive therapeutic interventions are unquestionably warranted. Major pancreatic leakage was defined as the drainage of more than 200 mL of fluid or the development of an intra-abdominal abscess.
Peer review
As this was a retrospective, non-randomized study in which the continuous suture was used after experience with 133 patients in whom an interrupted suture technique was used, the "better" results with the continuous suturing may simply be a learning curve.
Footnotes
Supported by grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea, No.0520320
S- Editor Liu Y L- Editor Kremer M E- Editor Li HY
References
- 1.Balcom JH, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 2.Bassi C, Falconi M, Salvia R, Mascetta G, Molinari E, Pederzoli P. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. 2001;18:453–457; discussion 458. doi: 10.1159/000050193. [DOI] [PubMed] [Google Scholar]
- 3.Marcus SG, Cohen H, Ranson JH. Optimal management of the pancreatic remnant after pancreaticoduodenectomy. Ann Surg. 1995;221:635–645; discussion 645-648. doi: 10.1097/00000658-199506000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg. 1997;185:18–24. doi: 10.1016/s1072-7515(97)00007-0. [DOI] [PubMed] [Google Scholar]
- 5.Yeh TS, Jan YY, Jeng LB, Hwang TL, Wang CS, Chen SC, Chao TC, Chen MF. Pancreaticojejunal anastomotic leak after pancreaticoduodenectomy--multivariate analysis of perioperative risk factors. J Surg Res. 1997;67:119–125. doi: 10.1006/jsre.1996.4974. [DOI] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257; discussion 257-260. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whipple AO. The rationale of radical surgery for cancer of the pancreas and ampullary region. Ann Surg. 1941;114:612–615. doi: 10.1097/00000658-194111440-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, Lillemoe KD, Pitt HA. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588; discussion 588-592. doi: 10.1097/00000658-199510000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aranha GV, Hodul P, Golts E, Oh D, Pickleman J, Creech S. A comparison of pancreaticogastrostomy and pancreaticojejunostomy following pancreaticoduodenectomy. J Gastrointest Surg. 2003;7:672–682. doi: 10.1016/s1091-255x(02)00432-8. [DOI] [PubMed] [Google Scholar]
- 10.Greene BS, Loubeau JM, Peoples JB, Elliott DW. Are pancreatoenteric anastomoses improved by duct-to-mucosa sutures? Am J Surg. 1991;161:45–49; discussion 49-50. doi: 10.1016/0002-9610(91)90359-l. [DOI] [PubMed] [Google Scholar]
- 11.Poon RT, Lo SH, Fong D, Fan ST, Wong J. Prevention of pancreatic anastomotic leakage after pancreaticoduodenectomy. Am J Surg. 2002;183:42–52. doi: 10.1016/s0002-9610(01)00829-7. [DOI] [PubMed] [Google Scholar]
- 12.D'Andrea AA, Costantino V, Sperti C, Pedrazzoli S. Human fibrin sealant in pancreatic surgery: it is useful in preventing fistulas? A prospective randomized study. Ital J Gastroenterol. 1994;26:283–286. [PubMed] [Google Scholar]
- 13.Suc B, Msika S, Fingerhut A, Fourtanier G, Hay JM, Holmières F, Sastre B, Fagniez PL. Temporary fibrin glue occlusion of the main pancreatic duct in the prevention of intra-abdominal complications after pancreatic resection: prospective randomized trial. Ann Surg. 2003;237:57–65. doi: 10.1097/00000658-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohwada S, Tanahashi Y, Ogawa T, Kawate S, Hamada K, Tago KI, Yamada T, Morishita Y. In situ vs ex situ pancreatic duct stents of duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy with billroth I-type reconstruction. Arch Surg. 2002;137:1289–1293. doi: 10.1001/archsurg.137.11.1289. [DOI] [PubMed] [Google Scholar]
- 15.Lerut JP, Gianello PR, Otte JB, Kestens PJ. Pancreaticoduodenal resection. Surgical experience and evaluation of risk factors in 103 patients. Ann Surg. 1984;199:432–437. doi: 10.1097/00000658-198404000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miedema BW, Sarr MG, van Heerden JA, Nagorney DM, McIlrath DC, Ilstrup D. Complications following pancreaticoduodenectomy. Current management. Arch Surg. 1992;127:945–949; discussion 949-950. doi: 10.1001/archsurg.1992.01420080079012. [DOI] [PubMed] [Google Scholar]
- 17.Kim SW, Youk EG, Park YH. Comparison of pancreatogastrostomy and pancreatojejunostomy after pancreatoduodenectomy performed by one surgeon. World J Surg. 1997;21:640–643. doi: 10.1007/s002689900286. [DOI] [PubMed] [Google Scholar]
- 18.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Kausch W. Das Carcinoma der Papilla duodeni und serine radikale Entfernung. Beitrag Z Klin Chir. 1912;78:439–486. [Google Scholar]
- 20.Suzuki Y, Fujino Y, Tanioka Y, Hiraoka K, Takada M, Ajiki T, Takeyama Y, Ku Y, Kuroda Y. Selection of pancreaticojejunostomy techniques according to pancreatic texture and duct size. Arch Surg. 2002;137:1044–1047; discussion 1048. doi: 10.1001/archsurg.137.9.1044. [DOI] [PubMed] [Google Scholar]
- 21.Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–959. doi: 10.1016/j.gassur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Sato N, Yamaguchi K, Chijiiwa K, Tanaka M. Risk analysis of pancreatic fistula after pancreatic head resection. Arch Surg. 1998;133:1094–1098. doi: 10.1001/archsurg.133.10.1094. [DOI] [PubMed] [Google Scholar]
- 23.Montorsi M, Zago M, Mosca F, Capussotti L, Zotti E, Ribotta G, Fegiz G, Fissi S, Roviaro G, Peracchia A. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery. 1995;117:26–31. doi: 10.1016/s0039-6060(05)80225-9. [DOI] [PubMed] [Google Scholar]
- 24.Friess H, Beger HG, Sulkowski U, Becker H, Hofbauer B, Dennler HJ, Büchler MW. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. Br J Surg. 1995;82:1270–1273. doi: 10.1002/bjs.1800820938. [DOI] [PubMed] [Google Scholar]
- 25.Lowy AM, Lee JE, Pisters PW, Davidson BS, Fenoglio CJ, Stanford P, Jinnah R, Evans DB. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg. 1997;226:632–641. doi: 10.1097/00000658-199711000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo CJ, Cameron JL, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, Campbell KA, Choti MA. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000;232:419–429. doi: 10.1097/00000658-200009000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behrend M, Kluge E, Schüttler W, Klempnauer J. A comparison of interrupted and continuous sutures for tracheal anastomoses in sheep. Eur J Surg. 2002;168:101–106. doi: 10.1080/11024150252884322. [DOI] [PubMed] [Google Scholar]
- 28.Friedman E, Perez-Atayde AR, Silvera M, Jonas RA. Growth of tracheal anastomoses in lambs. Comparison of PDS and Vicryl suture material and interrupted and continuous techniques. J Thorac Cardiovasc Surg. 1990;100:188–193. [PubMed] [Google Scholar]
- 29.Max E, Sweeney WB, Bailey HR, Oommen SC, Butts DR, Smith KW, Zamora LF, Skakun GB. Results of 1,000 single-layer continuous polypropylene intestinal anastomoses. Am J Surg. 1991;162:461–467. doi: 10.1016/0002-9610(91)90262-c. [DOI] [PubMed] [Google Scholar]
- 30.Chen YX, Chen LE, Seaber AV, Urbaniak JR. Comparison of continuous and interrupted suture techniques in microvascular anastomosis. J Hand Surg Am. 2001;26:530–539. doi: 10.1053/jhsu.2001.22933. [DOI] [PubMed] [Google Scholar]
- 31.Bardini R, Bonavina L, Asolati M, Ruol A, Castoro C, Tiso E. Single-layered cervical esophageal anastomoses: a prospective study of two suturing techniques. Ann Thorac Surg. 1994;58:1087–1089; discussion 1089-1090. doi: 10.1016/0003-4975(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 32.Burch JM, Franciose RJ, Moore EE, Biffl WL, Offner PJ. Single-layer continuous versus two-layer interrupted intestinal anastomosis: a prospective randomized trial. Ann Surg. 2000;231:832–837. doi: 10.1097/00000658-200006000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]