Abstract
Small molecule kinase inhibitors have proven enormously successful at delivering impressive responses in patients with cancers as diverse as chronic myeloid-leukemia, melanoma, breast cancer and small cell lung cancer. Despite this, resistance is commonplace and most patients ultimately fail therapy. One emerging observation is the rapid rewiring of signaling that occurs across multiple cancer types when driver oncogene function is inhibited. These adaptive signaling changes, seem critical in delivering some of the earliest survival signals that allow small numbers of cells to evade therapy. In this commentary we review the mechanisms that contribute to the robustness of signaling networks within cancer cells and suggest new therapeutic strategies to limit treatment failure.
Keywords: melanoma, BRAF, NRAS, signaling phosphoproteomics
Introduction
The normal physiological behavior of a cell is dependent upon its ability to sense and react to multiple signals from its internal milieu, the local and distant microenvironments and neighboring cells. The summation of all of this information is channeled through a discrete number of signaling pathways that dictate whether a cell should grow, move or alter its metabolic state. Regulation of signals through these pathways occurs through changes in phosphorylation, ubiquitination, and other post-translational modifications. Among the pathways known to regulate the behavior of normal cells, the mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase (PI3K)/AKT pathways have consistently emerged as being some of the most critical for cell behavior and the development of cancer [1, 2]. The recent years have seen considerable efforts directed towards the targeting of these pathways through small molecule inhibitors. At this time a number of highly specific signal transduction inhibitors directed against components of the MAPK and PI3K/AKT signaling pathways are undergoing clinical evaluation in multiple cancers [3-7]. Although these drugs frequently give encouraging results, the duration of effect is usually limited and most patients fail therapy [4, 8-12]. It is now clear that oncogene-driven signaling networks are highly plastic and adapt rapidly to reactivate the target pathway. The development of strategies that limit these adaptive signaling process are expected to prove critical in delivering more durable responses to patients on targeted cancer therapy. In this commentary we will review our current understanding of how signaling networks adapt to perturbations and will outline how this knowledge can be exploited therapeutically.
Physiological regulation of signaling networks
The prototypic signaling pathways for the majority of human cells are those driven by receptor tyrosine kinases (RTKS). RTKs are cell surface receptors that bind to exogenous ligands derived from the target, neighboring or distant cells and their activity drives many cellular processes including proliferation, growth, differentiation and survival [13] (Figure 1). The human genome contains fifty-eight RTKs that are organized by amino acid sequence and structure into 20 families [14]. Although diverse in their affinity for different ligands and the structure of their extracellular domains, most RTKs share common molecular features, particularly with regards to their highly conserved intracellular domains. RTKs become activated following ligand binding which leads to receptor dimerization and the recruitment of adaptor proteins that facilitate the activation of downstream signaling pathways [14]. Some RTKs do not require any physical interaction between each other for their activation and are instead activated through binding to their ligand. Despite the existence of common activation mechanisms for most RTKs, exceptions do occur. There is evidence that some RTKs, such as the Discoidin Domain Receptors (DDR1 and DDR2), are instead stimulated through binding to components of the extracellular matrix [15].
Figure 1. Physiological receptor tyrosine kinase signaling.
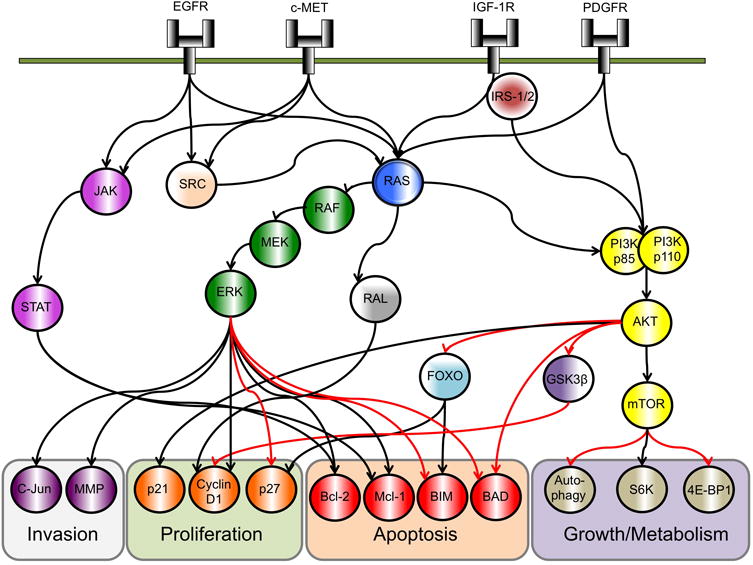
Figure shows the common signaling cascades initiated from upstream RTK activity, resulting in the activation of MAPK, PI3K/AKT/mTOR, JAK/STAT and SRC activity along with the regulation of diverse cellular processes.
Activation of RTKs leads to the recruitment and initiation of downstream signaling pathways including those mediated by the small GTPase RAS (Figure 1). In normal cells, activation of RAS occurs following the RTK autophosphorylation and the recruitment of GRB2 adaptor molecules to the intracellular regions via SH2 domains [16]. This in turn facilitates the recruitment of SOS (son of sevenless) to the plasma membrane via two SH3 domains and the exchange of GDP for GTP which switches RAS from its inactive (GDP- bound) state to an active (GTP-bound) state [17]. Activation of RAS through GDP to GTP exchange is mediated by a family of guanine nucleotide exchange factors (GEFs), that include RASGRF, SOS1 and SOS2. GEFs accelerate GDP release by RAS, allowing more robust GTP binding and activation [18]. The stimulatory effects of GEFs are counter balanced by GTPase-activating proteins (GAPs), which promote rapid GTP hydrolysis, returning RAS to its inactive state [18, 19]. Activated RAS then recruits and activates a number of intracellular signaling cascades including the MAPK pathway (RAF/MEK/ERK) the PI3K/AKT pathway and other signaling cascades including Ral-GDS, phospholipase C, and other small G-proteins [19, 20] (Figure 1).
The MAPK pathway is a key regulator of cell growth and survival and is constitutively activated following the hyperactivation of RTKs, activating BRAF mutations, RAS mutations, MEK mutations and loss of function mutations in negative pathway regulators such as sprouty (SPRY) [21-24] (Figure 2). In the absence of activating mutations RAF becomes activated following its dimerization and binding to RAS. The process of dimerization leads to RAF stimulation through its phosphorylation at Ser338. The activation of RAF leads in turn to the phosphorylation of MEK1 and MEK2 on two adjacent sites in the activation segment (Ser218 and Ser222) [25]. Other kinases have also been discovered capable of phosphorylating/activating MEK, including PAK1 and COT [26, 27]. The activation of MEK1/MEK2 leads to the subsequent phosphorylation of ERK1 and ERK2 on Thr202 and Tyr204. MEK1 and MEK2 are tyrosine and serine/threonine dual-specificity kinases and although no other known targets beyond ERK proteins have been characterized, activation of ERK regulates the downstream activity of more than 600 nuclear and cytoplasmic targets (Figure 2). In melanoma, constitutive MAPK pathway signals drive cell growth through increasing levels of cyclin D1 and by reducing expression of the cyclin dependent kinase inhibitor p27KIP1 [28]. It can also enhance cell survival through the phosphorylation and down regulation of the pro-apoptotic protein BIM and plays key roles in increasing cell motility via regulation of the actin cytoskeleton [29-31].
Figure 2. MAPK pathway signaling plasticity.
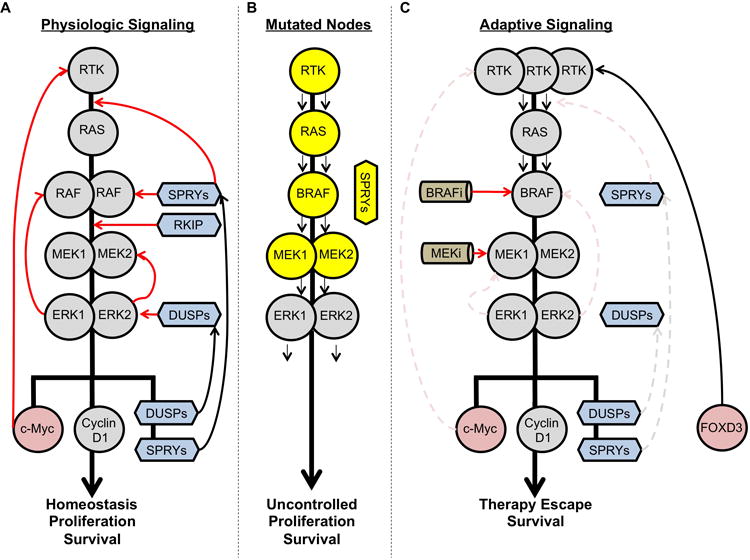
A. Upon upstream activation, RAS becomes activated, resulting in signal transduction through RAF, MEK and ERK. The MAPK pathway can turn itself off via negative feedback loops at multiple nodes to prevent hyperactivity. Inhibitory function shown as red arrows; activating functions are shown as black arrows. B. Characterized oncogenic mutations in the MAPK pathway are shown in yellow. C. A simplified schema of adaptive MAPK signaling following pathway inhibition.
The PI3K/AKT pathway is an important regulator of cell survival, motility, and cell metabolism [32]. Its activation in cancer can be secondary to acquisition of RAS mutations, constitutive RTK signaling mutations in pathway components such as PI3K and AKT, and through loss of negative pathway regulators such as phosphatase and tensin homolog (PTEN) and neurofibromatosis (NF)-1 [20, 33-35] (Figure 3). PI3K exists as multiple isoforms and consists of a heterodimer comprising a p85 regulatory and a p110 catalytic subunit [32]. Activation of PI3K occurs following RTK stimulation via adaptor proteins that bind to the SH2 domain of the p85 subunit. The p110 domain can also be recruited following the activation of RAS. PI3K then localizes to the inner leaflet of the cell membrane and phosphorylates the phosphatidylinositol-4,5,bisphosphate ring (PIP2) at the 3′ position, converting PIP2 to PIP3 [32]. The downstream serine-threonine kinases PDK1 and AKT are then stimulated through the actions of PIP3. AKT has a critical role in cancer development and regulates apoptosis by phosphorylating BAD; it also affects many other pathways, including ribosomal S-6-kinase (S6K), forkhead (FOXO) and glycogen synthase kinase-3 (GSK3β) [1, 36] (Figure 3).
Figure 3. PI3K/AKT/mTOR pathway signaling plasticity.
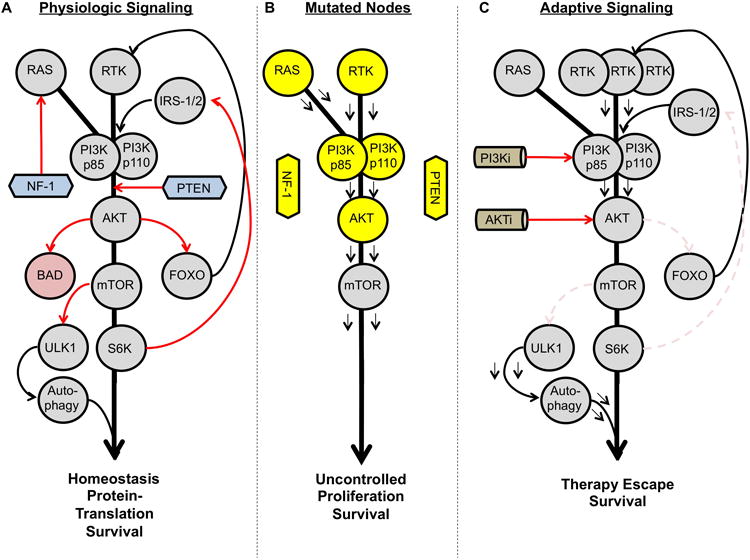
A. PI3K can become active, under physiologic conditions, via upstream RTK signaling and/or RAS. Activation of PI3K leads to downstream signal amplification through AKT and mTOR, resulting in increased protein translation, proliferation and survival. The PI3K/AKT/mTOR signaling axis can regulate its own activity through the negative feedback loops shown, as well as from negative pathway regulators PTEN and NF-1. B. Known nodes within the pathway mutated/hyperactivated in cancer are shown. C. Adaptive signaling in response to PI3K/AKT/mTOR inhibition is shown, resulting in therapy evasion.
The mTOR signaling module is an important part of the PI3K/AKT pathway that regulates cell metabolism by integrating information about nutrient availability, growth factors and the cells' energy state [37]. The function of mTOR is complicated, and involves the formation of two separate complexes mTORC1 and mTORC2 that both function upstream and downstream of AKT activation, respectively. The mTORC1 complex comprises mTOR, RAPTOR, GBL/mLST8 and PRAS40 whereas mTORC2 consists of mTOR, Rictor, Protor, mSIN1 and MLST8/GBL [38]. The two mTOR complexes subserve different functions, with mTORC1 being primarily involved in the regulation of protein translation via S6K and 4E-BP1. In contrast, mTORC2 complex directly regulates AKT by phosphorylating it at S473.
Maintaining homeostasis in signaling pathways through feedback inhibition and pathway inactivation
In normal cells, both MAPK and PI3K/AKT pathway activation engages homeostatic mechanisms that eventually downregulate signaling throughput. These mechanisms of regulation are critical for the maintenance of ordered signaling and are evolutionary conserved from yeast. The central feature of the structures of both these pathways is feedback inhibition; where the output of signals from multiple levels of the pathway inactivates upstream signaling [39]. This network design conveys stability and robustness, allowing the cell to respond rapidly as well as maintaining a stable “off” state [40]. It is perhaps not surprising that malignant transformation, which often relies on unrestricted high levels of signaling, is frequently associated with loss of feedback control [39].
In normal cells, induction of MAPK activity leads to its inactivation. One such inhibitory mechanism, operating at the level of RAF is RAF kinase inhibitory protein (RKIP) [41] (Figure 2). This protein, which was identified through a yeast-two hybrid screen, acts in a competitive manner to prevent MEK phosphorylation by disrupting RAF/MEK interaction [41]. Another level of control involves the spatial organization of the MAPK pathway modules, which require close physical proximity for their activation; an effect mediated through scaffolding proteins such as KSR1 (kinase suppressor of Ras) [42, 43]. Dual specificity MAPK phosphatases (MKPs) are a family of discrete phosphatases (DUSPs) that inactivate MAPK signaling, with DUSP6 (otherwise known as MKP3) and DUSP1 known to bind to inactivate both ERK1 and ERK2 [44] (Figure 2). The SPRYs are another family of proteins that negatively regulate signaling through multiple RTKs including EGFR, and c-MET [23]. From a mechanistic standpoint, the SPRYs deactivate MAPK signaling through an interaction with the catalytic domain of RAF, preventing MEK activation and by sequestering the adaptor protein GRB2, which prevents signals being transduced from RTKs to RAS [45]. Many of the negative modulators of the MAPK pathway, including the DUSPs and the SPRYs are transcriptionally regulated by ERK, so that increased signaling output ultimately deactivates the pathways [39, 40]. Cancer cells with a dependency upon MAPK signaling often show impaired feedback inhibition. In BRAF mutant melanoma feedback inhibition is disabled in part because the SPRY proteins are unable to bind to the conformation of mutated BRAF [46, 47]. Interestingly, DUSP activity in BRAF mutant melanoma cells remains functional, ensuring that phospho-ERK levels are not significantly increased despite the output of the pathway being high [39].
Feedback inhibition also exists within the PI3K/AKT/mTOR signaling pathway. In non-transformed cells, activation of the PI3K pathway through insulin like growth factor (IGF)-1 can be limited through decreased expression of the adaptor proteins IRS-1 and IRS-2, which link IGF1R to PI3K [48] (Figure 3). This downregulation occurs as a result of PI3K activating AKT, leading to enhanced mTOR and S6K kinase activity. Stimulation of S6K leads to phosphorylation of IRS-1, and its degradation, leading to a disruption of signaling between IGFR1 and PI3K [49, 50]. Further downstream, AKT signaling also participates in feedback inhibition through the regulation of RTK expression [51, 52]. In this instance, AKT modulates the transcription of RTKs through the phosphorylation and inactivation of FOXO-family transcription factors [51] (Figure 3).
Adaptive signaling in the PI3K/AKT/mTOR pathway
The vast majority of cancers are initiated and sustained through the activity of oncogenes - many of which drive signaling through the MAPK and the PI3K/AKT signaling pathways. In some cases, tumors become dependent upon the activity of one oncogene for their growth and survival, a state termed oncogene addiction. Examples of this are numerous and include the Bcr-Abl fusion protein in chronic myeloid leukemia (CML), c-KIT signaling in gastrointestinal stromal tumors (GIST), mutant KRAS in pancreatic cancer, EML4-ALK fusions and EGFR in non-small cell lung cancer (NSCLC) and mutant BRAF in melanoma and hairy cell leukemia [10, 22, 53-57]. The reliance of cancers upon one oncogene offers a molecular weakness that can be targeted through small molecule inhibitors and is the basis for targeted therapy.
Oncogene transformed cells exhibit high levels of feedback inhibition that is relieved following treatment with targeted therapeutics or shRNA knockdown. This phenomenon has been well described for inhibitors of the PI3K/AKT/mTOR pathway, with the abrogation of feedback inhibition constituting a major mediator of resistance and therapeutic escape. One of the first PI3K/AKT/mTOR pathway inhibitors to be developed was rapamycin, a macrolide mTORC1 inhibitor derived from an Easter Island streptomycete. As a single agent, the anti tumor activity of rapamycin was limited, partly through the relief of feedback inhibition in the pathway leading to increased AKT signaling. Functional studies showed that mTOR inhibition suppressed both IRS-1 and S6K activity, leading in turn to the reciprocal activation of AKT, a finding confirmed both in vitro and in clinical studies of glioblastoma [52, 58, 59] (Figure 3). The mechanism underlying this abrogation of feedback inhibition was revealed by two independent proteomic studies that identified the adaptor protein GRB10 as an mTORC1 target that negatively regulated PI3K signaling [60, 61]. In this context, the rapamycin-mediated inhibition of mTORC1 led in turn to a decrease in GRB10 stability, relieving its inhibition of PI3K, causing an increase in AKT signaling [60, 61]. Inhibition of one pathway can also stimulate reciprocal signaling in other parallel pathways. An analysis of pre and post-treatment biopsies from patients receiving treatment with the mTOR inhibitor RAD001 showed drug treatment to stimulate the MAPK pathway through the relief of feedback inhibition at the level of IRS-1 and an enhancement of RAS activity [58]. There is also evidence that increased MAPK activity can inhibit PI3K/AKT signaling through a mechanism involving the EphA2 receptor mediated inhibition of RAS activity [62].
The finding that mTORC1 inhibitors such as rapamycin paradoxically activated AKT signaling through mTORC2 activation led to the development of combined mTORC1/2 inhibitors. Although it was expected that combined mTORC1/2 inhibition would limit the rebound AKT signaling it was instead observed that although phosphorylation of AKT was decreased in a sustained manner at Ser473 it was only transiently inhibited at Thr308 [63]. A more in-depth analysis showed mTORC inhibition to initially block AKT signaling followed by the relief of feedback inhibition at the RTK level, the recruitment of PI3K and the reactivation of AKT [63]. Multiple RTKs were involved in the recovery of AKT activity including HER kinases (HER2, 3 and 4 and EGFR), IGFR1-R, and multiple FGFR receptors. The reactivation of PI3K/AKT signaling was blocked by treatment with either class I PI3K inhibitors or the HER family kinase inhibitor lapatinib [63]. The in vivo relevance of PI3K/AKT reactivation was confirmed in animal xenograft studies with tumor regression being seen following treatment with the combination of an mTOR kinase inhibitor with the HER kinase inhibitor lapatinib [63].
The reliance of many cancer cells upon the PI3K/AKT pathway has also led to the development of small molecule AKT inhibitors including MK-2206, GSK690693, GDC0068 and AZ5363. Like mTOR inhibitors, AKT inhibitors are also associated with adaptive signaling and the relief of feedback inhibition, albeit in a subtly different manner. In many cell lines, AKT inhibition led to decreased activity through the pathway followed by reactivation some hours later [51]. Experimental studies showed the recovery of signaling to be associated with the induction of a restricted set of RTKs that included HER3, IGF1R and the insulin receptor [51]. From a signaling standpoint, AKT inhibition led to the increased association of HER3 with HER2, an increase in HER3 phosphorylation and the recruitment and activation of PI3K The increase in RTK expression was dependent upon AKT inhibition and could be recapitulated by the siRNA knockdown of each AKT isoform. Inhibition of AKT directly modulated RTK expression through regulation of FOXO transcription factors, with chromatin immunoprecipitation (CHIP) assays showing AKT inhibitors to directly increase the level of FOXO3 binding to the RTK promoters. The effects of AKT inhibitors upon RTK upregulation could be reversed following siRNA knockdown of FOXO3a [51]. The role of adaptive HER kinase expression in the escape from AKT inhibitor therapy was further demonstrated in vivo, where the combination of an AKT inhibitor with either lapatinib or gefitinib led to significant tumor regression [51]. Previous studies have shown that both TORC1 inhibitors and some PI3K inhibitors can also increase HER3 signaling suggesting that TORC1 inhibition may underlie part of the observed effects of AKT inhibition. However in this instance it was noted that rapamycin was much weaker at upregulating HER3 than AKT inhibition and did not induce the same cohort of RTKs [64, 65].
RAS mutations are some of the most common driver events in cancer, and occur in up to 20% of all tumors [20]. The frequency of these mutations, coupled with the multiplicity of signaling pathways activated through RAS has made this a highly attractive therapeutic target [21]. Although attempts to target RAS through the inhibition of its post-translational processing were initially unsuccessful there has been renewed interest in developing novel RAS inhibitors that target different binding domains [66]. One approach has been to utilize binding to the cysteine in KRAS G12 as a mechanism to achieve selectivity for mutant KRAS over the wild-type [67]. Another strategy depends upon the direct targeting of the interaction between KRAS and the prenyl binding protein PDEδ (which maintains KRAS signaling by localizing it to the endomembrane) [68]. Despite RAS being one of the most potentially appealing therapeutic targets in cancer, it is also subject to feedback inhibition. It is already known from studies in colorectal carcinoma that the shRNA mediated downregulation of KRAS leads to both the inhibition of MAPK signaling and a paradoxical increase in phospho-AKT [69]. In this case, KRAS knockdown increased the association between PI3K and IGFR1, and increased AKT signaling. The importance of RTK signaling for the PI3K-mediated escape from KRAS inhibition was demonstrated by the observation that combined treatment with inhibitors of IGF1R and MEK inhibition enhanced levels of cytotoxicity in vivo compared to either agent alone [69].
Negative feedback and adaptive resistance in the MAPK pathway
The discovery that 8% of all cancers harbor activating BRAF mutations has raised interest in targeting the MAPK signaling pathway. It is now known that 50% of cutaneous melanomas, 100% of hairy cell leukemias as well as lower percentages ovarian cancer, multiple myeloma, thyroid carcinoma, colorectal carcinoma and lung cancer all harbor BRAF mutations [22, 53, 70-73]. In preclinical studies, BRAF mutant melanoma cell lines and xenografts were highly sensitive to BRAF inhibitors such as vemurafenib and dabrafenib, with cell cycle arrest, apoptosis induction and ER-stress induction being observed [74-76]. In clinical trials, BRAF inhibitor therapy was associated with impressive levels of tumor shrinkage and significant improvements in PFS compared to patients treated with the then standard-of-care dacarbazine [4]. Unfortunately, the majority of patients on vemurafenib and dabrafenib therapy relapsed after relatively short time periods (median PFS 5.1 and 5.3 months respectively). Pharmacodynamic analysis of post-treatment biopsies from patients on vemurafenib therapy showed that >90% pathway inhibition was required for clinical benefit, suggesting that low-level pathway recovery facilitated therapeutic escape [77]. Further studies showed reactivation of MAPK signaling to be prevalent in the majority (>70%) of post-relapse specimens, identifying this as the major mechanism of treatment failure [78].
One surprise early preclinical finding was the rapidity of MAPK pathway reactivation following BRAF inhibitor treatment, with some level of pERK signaling recovering following 24-48 hr of drug treatment [79]. An in-depth mechanistic analysis of BRAF mutant melanoma cells showed RAS signaling to be very low when the BRAF inhibitor was absent and that this occurred in conjunction with a reduced sensitivity to exogenous growth factors [80]. In the absence of drug, the cells exhibited a high level of output from the MAPK pathway, and showed strong feedback inhibition at the level of RAS, mediated through SPRY2 and DUSP6 [80]. With applied BRAF or MEK inhibitor treatment, the feedback inhibition upon RAS was relieved through the downregulation of SPRY2 and DUSP6 expression (Figure 2). At this point the cells regained sensitivity to signals mediated through growth factors such as EGF, which induced the reactivation of the Ras/Raf/MEK/ERK signaling pathway. Interestingly knockdown of SPRY2 was found to both increase the activity of RAS and RAF and decrease the sensitivity of the cells to BRAF inhibition [80]. The effects of BRAF inhibition on adaptive RTK signaling are known to be manifold. In addition to relieving the feedback inhibition upon RTK signals, BRAF inhibition was also found to increase RTK expression. Some of the earliest studies on BRAF inhibitor resistance proposed elevated RTK signaling (particularly IGF1R and PDGFR) to be mechanisms of acquired BRAF inhibitor resistance [81, 82]. It now seems likely that RTK signaling may be critical at the earliest stages of drug resistance and allows minor populations of cells to survive drug treatment, remaining in a semi-dormant state until secondary driver mutations are acquired.
Like adaptive signaling in the PI3K/AKT pathway, BRAF inhibitor treatment is also dependent upon members of the forkhead box transcription factor family. In BRAF mutant melanoma, the stemness factor FOXD3 was identified as a potential regulator of adaptive RTK expression following BRAF inhibition [83, 84] (Figure 2). Use of a combined microarray/CHIP-seq analysis showed FOXD3 to be upregulated following BRAF/MEK inhibition and that this increased the transcription of the RTK ERBB3/HER3. In this context the HER3 dimerized with HER2 and showed an increased responsiveness to neuregulin that mediated resistance to vemurafenib through the PI3K/AKT pathway [84]. Interestingly, the increased HER3 signaling seen following vemurafenib treatment was dependent upon continual drug selection pressure, and could be reversed upon drug withdrawal leading to an impaired neuregulin response [84]. Recent work from our lab showed adaptive RTK signaling to also occur when NRAS mutant melanoma cell lines were treated with MEK inhibitors. In this instance PDGFR-B emerged as the critical receptor involved in therapeutic escape with co-treatment with the MEK inhibitor AZD6244 and the PDGFR inhibitor crenolanib leading to an increased apoptotic response compared to either drug alone [85].
The role of adaptive RTK signaling in the escape from MEK inhibition has also been comprehensively studied in triple-negative breast cancer. A quantitative chemical proteomics approach was developed in which multiplexed kinase inhibitor beads and mass spectrometry was used to interrogate the activity of the kinome following treatment with the MEK inhibitors U0126 and AZD6244 [86]. It was found that inhibition of MEK was associated with a rapid rewiring of kinase signaling, with alterations seen in virtually every branch of the tyrosine and serine/threonine kinase family. One of the major mechanisms of resistance identified from these studies was the recovery of MAPK signaling associated with increased expression and activity of multiple RTKs including PDGFR, VEGFR2, HER3, DDR1, RON and AXL [86]. The increase in RTK expression was mirrored by enhanced expression of growth factors including EGF, GAS6, PDGF-B and PDGF-D, suggesting that multiple autocrine signaling loops were being established. At the transcriptional level, MEK inhibition led to decreased c-Myc stability and expression and a destabilization of Myc-Max complexes leading to increased RTK expression [86]. Co-treatment of triple negative breast cancer cell lines and a C3-Tag mouse model of breast cancer with the combination of a MEK inhibitor and the pan-RTK inhibitor sorafenib showed synergistic anti-tumor activity.
Increased RTK signaling has also been identified as a mechanism of intrinsic resistance in some tumors that harbor BRAF mutations. In colon carcinoma BRAF mutations often co-occur with high levels of EGFR expression [73, 87]. Upon treatment with BRAF inhibitors, adaptive signaling through EGFR and RAS abrogates the response to these drugs and it has been suggested that better therapeutic responses could be achieved through co-targeting of BRAF and EGFR [73, 87]. Similar findings have also been noted in BRAF mutant papillary thyroid carcinomas, where vemurafenib treatment is associated with increased expression and signaling through HER3, limiting the efficacy of the BRAF inhibitor [88]. Other studies have shown that growth factors emanating from the host microenvironment, such as those derived from endothelial cells and fibroblasts can also drive the adaptive signaling to kinase inhibition. There is already evidence that resistance to the ALK inhibitor crizotinib can be mediated by host-derived EGF and HGF and that the escape of melanoma cells from vemurafenib is driven through HGF released from stromal fibroblasts [89, 90].
Future perspectives: limiting adaptive signaling responses
Small molecule kinase inhibitors have proven highly effective at delivering impressive but short-lived responses to patients with a diverse range of advanced cancers. Adaptive signaling seems critical for both the intrinsic resistance (such as the lack of response to BRAF inhibitors in colon cancer) and the earliest stages of therapeutic adaptation (to BRAF, MEK, AKT and mTOR inhibitors). The data thus far has identified multiple mechanisms of escape including relief of feedback inhibition, cross-talk between parallel pathways and increased RTK signaling. The development of strategies to limit this escape is likely to prove critical in improving the long-term durability and responsiveness to these drugs.
The observation that ERK signaling becomes reactivated following BRAF inhibition led to an interest in targeting the MAPK pathway at two (or more) points – a strategy termed vertical pathway inhibition. Initial preclinical studies demonstrated that the reactivation of ERK signaling could be abrogated through the co-targeting of BRAF and MEK and showed that this limited therapeutic escape by enhancing the level of apoptosis and preventing reentry into the cell cycle [79, 80]. In a phase II clinical trial, the use of dabrafenib (a BRAF inhibitor) in combination with trametinib (a MEK inhibitor) was associated with an increased PFS compared to dabrafenib alone [91]. The BRAF/MEK inhibitor combination received accelerated FDA-approval in early 2014 on the basis of these data. Despite the increase in PFS seen to the combination, therapeutic escape still occurred and early indications suggest this is mediated through resistance mechanisms similar to those seen to single-agent BRAF inhibitor therapy (e.g. MEK mutations, BRAF splice-mutants etc) [92]. Other vertical pathway combinations, including the co-targeting of BRAF and ERK and MEK and ERK, are being explored preclinically at this time.
Many of the proteins known to be involved in adaptive signaling; including RTKs and components of the MAPK and PI3K pathways have been identified as clients of heat shock protein 90 and rely upon its chaperone function for proper protein folding and stability. At this time >200 proteins have been identified as clients as HSP90, making these very broadly targeted drugs. Although HSP90 inhibitors have had a checkered clinical history, promising activity has been seen when HSP90 inhibitors are used as drug combination partners. In patients with HER2-positive breast cancer who progressed on trastuzumab therapy, the combination of trastuzumab with the HSP90 inhibitor tanespimycin showed clinical responses by RECIST criteria [93]. There is also evidence that HSP90 inhibitors can overcome resistance to proteasome inhibitors in multiple myeloma [94].
HSP90 inhibitors are expected to enhance the efficacy of multiple targeted therapies by limiting adaptive responses. In lung cancer, the combination of an EGFR inhibitor with an HSP90 inhibitor is effective against resistance mediated through the EGFR T790M mutation and MET amplification [95, 96]. In melanoma, virtually every protein implicated thus far in both acquired and intrinsic BRAF inhibitor resistance is known to be an HSP client. Work from our lab demonstrated the HSP90 inhibitor XL888 to be highly effective at abrogating BRAF inhibitor resistance mediated through multiple mechanisms including PDGFR overexpression, NRAS mutation, COT amplification and PTEN loss [97]. These findings were confirmed by two other preclinical studies that demonstrated HSP90 inhibition to be effective at reversing acquired BRAF inhibitor resistance [98, 99]. In animal models, the HSP90 inhibitor ganetespib led to the regression of vemurafenib resistant xenografts following combination with a MEK inhibitor [99]. A phase I clinical trial evaluating the frontline combination of vemurafenib + XL888 (NCT01657591) in unresectable BRAF mutant melanoma is close to achieving its accrual goals, with early evidence of clinical response.
Another therapeutic strategy being actively explored is the design of MAPK targeted drugs that limit feedback inhibition. In BRAF mutant tumors, such as melanoma, BRAF and MEK inhibitors have good efficacy, an effect due in part to the fact that feedback inhibition is disabled at the level of RAS but remains effective at the level of MEK. RAS mutant tumors are less sensitive to MEK inhibitors because feedback inhibition remains intact and the inhibition of MEK is associated with a rapid induction of MEK phosphorylation. A systematic investigation of the mechanism of MEK reactivation through means of an siRNA screen showed CRAF to be critical for MEK stimulation and that CRAF/MEK complexes showed a reduced sensitivity to MEK inhibitors [100]. Allosteric MEK inhibitors such as CH5126766 inhibit both RAF and MEK function and prevent the reactivation of MEK associated with relief of feedback inhibition [101]. In KRAS mutant HCT116 colon carcinoma xenografts, CH5126766 prevented the reactivation of MEK signaling and induced a greater level of tumor regression than the MEK inhibitor PD0325901 [101].
Combination strategies to prevent adaptive RTK signaling following PI3K/AKT/mTOR signaling are focusing upon the targeting of HER family RTKs and IGF1R. At this stage it is not clear which level of the pathway will need to be targeted to achieve the greatest therapeutic index (e.g. mTOR, AKT etc) and whether the patterns of adaptive RTK signaling are specific to individual tumors or defined tumor histologies (e.g. do breast tumors show different patterns of RTK signaling than lung cancers). There is however a strong preclinical rationale for the co-targeting of PI3K with HER kinases and a number of clinical trials in lung cancer are currently recruiting to investigate this (Table 1).
Table 1.
Active or recently completed phase I/II clinical trails with combinatorial RTK targeted strategies.
| RTK Inhibitor | Second Agent | Clinical Trial Number | Status |
|---|---|---|---|
| Dasatinib(c-Kit/ephrins) | Erlotinib (EGFR) | NCT00444015 | Completed |
| Afatinib (Her2/EGFR) | Dasatinib (c-Kit, ephrins) | NCT01999985 | Recruiting |
| Erlotinib (EGFR) | Docetaxol (chemo) | NCT00054275 | Completed |
| PF-02341066 (c-MET) | PF-00299804 (pan-HER) | NCT01121575 | Completed |
| Imatinib (PDGFR) | LBH589 (HDAC inhibitor) | NCT01175109 | Active, not recruiting |
| Gefitinib (EGFR) | Cetuximab (EGFR) | NCT00820417 | Completed |
| Lapatinib (Her2/EGFR) | MK-2206 (AKT) | NCT01281163 | Active, not recruiting |
| Gefitinib (EGFR) | Docetaxol (chemo) | NCT01746277 | Recruiting |
| Erlotinib (EGFR) | Pemetrexed/Carboplatin(chemo) | NCT02066038 | Active, not recruiting |
| Erlotinib (EGFR) | Bevacizumab (VEGF-1) | NCT01532089 | Recruiting |
| Vatalanib (VEGFR) | Gemcitabine (chemo) | NCT00185588 | Completed |
| Enzalutamide (Androgen-R) | Gemcitabine/Paclitaxel(chemo) | NCT02138383 | Recruiting |
| Sorafenib (VEGFR2/PDGFRβ) | Cetuximab (EGFR) | NCT00326495 | Recruiting |
| Vandetanib (VEGFR/EGFR/RET) | AZD6244 (MEK) | NCT01586624 | Recruiting |
| Dasatinib (c-Kit/ephrins) | Fulvestrant (Estrogen-R agonist) | NCT00754325 | Completed |
| Erlotinib (EGFR) | Tivantinib (c-MET) | NCT01244191 | Active, not recruiting |
The development of targeted cancer therapies that deliver durable responses to patients is likely to depend upon an in-depth knowledge of how cancer cells adapt to kinase inhibition through the abrogation of feedback inhibition. Newer generations of MEK inhibitors that prevent RAF/MEK binding, combined mTOR/PI3K inhibitors and vertical pathway inhibition are strategies that may limit the recovery of signaling in some of the key pathways required for oncogene-mediated tumor progression. Further strategies will likely depend upon the co-targeting of MEK or AKT in conjunction with multiple RTKs. These approaches are likely to require further personalization and tools to interrogate adaptations in tumors undergoing therapy. It is our hope that the development of these and similar approaches will allow us to achieve the ultimate goal of reducing cancer to the level of a chronic disease.
Acknowledgments
Grant support: Work in the Smalley lab is supported by SPORE grant 1P50CA168536-01A1 and R01 CA161107-01 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Madhunapantula SV, Robertson GP. The PTEN-AKT3 signaling cascade as a therapeutic target in melanoma. Pigment Cell Melanoma Res. 2009;22:400–19. doi: 10.1111/j.1755-148X.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan RJ, Flaherty K. MAP kinase signaling and inhibition in melanoma. Oncogene. 2013;32:2373–9. doi: 10.1038/onc.2012.345. [DOI] [PubMed] [Google Scholar]
- 3.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine. 2012;367:107–14. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 6.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Molecular Cancer Therapeutics. 2010;9:1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Z, Sarbassov dos D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–12. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Zhang G, Haura EB. Targeting epidermal growth factor receptor: central signaling kinase in lung cancer. Biochemical Pharmacology. 2010;80:613–23. doi: 10.1016/j.bcp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7535–40. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duensing S, Duensing A. Targeted therapies of gastrointestinal stromal tumors (GIST)--the next frontiers. Biochem Pharmacol. 2010;80:575–83. doi: 10.1016/j.bcp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocrine-related cancer. 2001;8:161–73. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]
- 15.Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. The Journal of clinical investigation. 2001;107:727–35. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowy DR, Willumsen BM. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–91. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 17.Rojas JM, Oliva JL, Santos E. Mammalian son of sevenless Guanine nucleotide exchange factors: old concepts and new perspectives. Genes Cancer. 2011;2:298–305. doi: 10.1177/1947601911408078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cully M, Downward J. SnapShot: Ras Signaling. Cell. 2008;133:1292–e1. doi: 10.1016/j.cell.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 20.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nature reviews Cancer. 2003;3:459–65. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 21.Downward J. Targeting RAS signalling pathways in cancer therapy. Nature reviews Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 22.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nature reviews Molecular cell biology. 2004;5:441–50. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- 24.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3085–96. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 26.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010 doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong CC, Jubb AM, Jakubiak D, Zhou W, Rudolph J, Haverty PM, et al. P21-activated kinase 1 (PAK1) is a therapeutic target in BRAF wild-type melanoma. Journal of the National Cancer Institute. 2013 doi: 10.1093/jnci/djt054. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt KV, Spofford LS, Aram G, McMullen M, Pumiglia K, Aplin AE. Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling. Oncogene. 2005;24:3459–71. doi: 10.1038/sj.onc.1208544. [DOI] [PubMed] [Google Scholar]
- 29.Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–44. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein RM, Aplin AE. Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions. Cancer Res. 2009;69:2224–33. doi: 10.1158/0008-5472.CAN-08-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 33.Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 34.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–14. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 36.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–83. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, et al. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A. 2009;106:4519–24. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sturm OE, Orton R, Grindlay J, Birtwistle M, Vyshemirsky V, Gilbert D, et al. The mammalian MAPK/ERK pathway exhibits properties of a negative feedback amplifier. Science signaling. 2010;3:ra90. doi: 10.1126/scisignal.2001212. [DOI] [PubMed] [Google Scholar]
- 41.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–7. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 42.Therrien M, Chang HC, Solomon NM, Karim FD, Wassarman DA, Rubin GM. KSR, a novel protein kinase required for RAS signal transduction. Cell. 1995;83:879–88. doi: 10.1016/0092-8674(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 43.Roy F, Therrien M. MAP kinase module: the Ksr connection. Current biology : CB. 2002;12:R325–7. doi: 10.1016/s0960-9822(02)00831-x. [DOI] [PubMed] [Google Scholar]
- 44.Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. The FEBS journal. 2013;280:489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross I, Bassit B, Benezra M, Licht JD. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. The Journal of biological chemistry. 2001;276:46460–8. doi: 10.1074/jbc.M108234200. [DOI] [PubMed] [Google Scholar]
- 46.Brady SC, Coleman ML, Munro J, Feller SM, Morrice NA, Olson MF. Sprouty2 association with B-Raf is regulated by phosphorylation and kinase conformation. Cancer Research. 2009;69:6773–81. doi: 10.1158/0008-5472.CAN-08-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsavachidou D, Coleman ML, Athanasiadis G, Li S, Licht JD, Olson MF, et al. SPRY2 is an inhibitor of the ras/extracellular signal-regulated kinase pathway in melanocytes and melanoma cells with wild-type BRAF but not with the V599E mutant. Cancer Res. 2004;64:5556–9. doi: 10.1158/0008-5472.CAN-04-1669. [DOI] [PubMed] [Google Scholar]
- 48.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Molecular endocrinology. 2000;14:783–94. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 49.White MF, Yenush L. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Current topics in microbiology and immunology. 1998;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 50.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- 51.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Research. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF Mutations in Hairy-Cell Leukemia. New Engl J Med. 2011;364:2305–15. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567–72. doi: 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20:1692–703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 56.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 57.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 58.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Molecular Cancer Therapeutics. 2005;4:1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 60.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–22. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–6. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menges CW, McCance DJ. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2008;27:2934–40. doi: 10.1038/sj.onc.1210957. [DOI] [PubMed] [Google Scholar]
- 63.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–59. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amin DN, Sergina N, Ahuja D, McMahon M, Blair JA, Wang D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Science translational medicine. 2010;2:16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6:541–55. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 67.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–51. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimmermann G, Papke B, Ismail S, Vartak N, Chandra A, Hoffmann M, et al. Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature. 2013;497:638–42. doi: 10.1038/nature12205. [DOI] [PubMed] [Google Scholar]
- 69.Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, et al. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. Journal of Clinical Investigation. 2011;121:4311–21. doi: 10.1172/JCI57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 71.Andrulis M, Lehners N, Capper D, Penzel R, Heining C, Huellein J, et al. Targeting the BRAF V600E Mutation in Multiple Myeloma. Cancer Discov. 2013;3:862–9. doi: 10.1158/2159-8290.CD-13-0014. [DOI] [PubMed] [Google Scholar]
- 72.Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. The Journal of clinical endocrinology and metabolism. 2003;88:4393–7. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 73.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 74.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY, et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res. 2006;66:11100–5. doi: 10.1158/0008-5472.CAN-06-2554. [DOI] [PubMed] [Google Scholar]
- 76.Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. The Journal of clinical investigation. 2014;124:1406–17. doi: 10.1172/JCI70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–30. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, et al. Relief of Profound Feedback Inhibition of Mitogenic Signaling by RAF Inhibitors Attenuates Their Activity in BRAFV600E Melanomas. Cancer Cell. 2012;22:668–82. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basile KJ, Abel EV, Aplin AE. Adaptive upregulation of FOXD3 and resistance to PLX4032/4720-induced cell death in mutant B-RAF melanoma cells. Oncogene. 2011 doi: 10.1038/onc.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abel EV, Basile KJ, Kugel CH, 3rd, Witkiewicz AK, Le K, Amaravadi RK, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. The Journal of clinical investigation. 2013;123:2155–68. doi: 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rebecca VW, Wood ER, Fedorenko IV, Paraiso KH, Haarberg HE, Chen Y, et al. Evaluating Melanoma Drug Response and Therapeutic Escape with Quantitative Proteomics. Molecular & cellular proteomics : MCP. 2014 doi: 10.1074/mcp.M113.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149:307–21. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mao M, Tian F, Mariadason JM, Tsao CC, Lemos R, Jr, Dayyani F, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:657–67. doi: 10.1158/1078-0432.CCR-11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3:520–33. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du JY, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–U118. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamada T, Takeuchi S, Nakade J, Kita K, Nakagawa T, Nanjo S, et al. Paracrine receptor activation by microenvironment triggers bypass survival signals and ALK inhibitor resistance in EML4-ALK lung cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:3592–602. doi: 10.1158/1078-0432.CCR-11-2972. [DOI] [PubMed] [Google Scholar]
- 91.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wagle N, Van Allen EM, Treacy DJ, Frederick DT, Cooper ZA, Taylor-Weiner A, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014;4:61–8. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, et al. HSP90 Inhibition Is Effective in Breast Cancer: A Phase II Trial of Tanespimycin (17-AAG) Plus Trastuzumab in Patients with HER2-Positive Metastatic Breast Cancer Progressing on Trastuzumab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5132–9. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 94.Richardson PG, Chanan-Khan AA, Lonial S, Krishnan AY, Carroll MP, Alsina M, et al. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. British journal of haematology. 2011;153:729–40. doi: 10.1111/j.1365-2141.2011.08664.x. [DOI] [PubMed] [Google Scholar]
- 95.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Science translational medicine. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shimamura T, Li D, Ji H, Haringsma HJ, Liniker E, Borgman CL, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Research. 2008;68:5827–38. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paraiso KHT, Haarberg HE, Wood E, Rebecca VW, Chen YA, Xiang Y, et al. The HSP90 Inhibitor XL888 Overcomes BRAF Inhibitor Resistance Mediated through Diverse Mechanisms. Clinical Cancer Research. 2012;18:2502–14. doi: 10.1158/1078-0432.CCR-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu X, Marmarelis ME, Hodi FS. Activity of the heat shock protein 90 inhibitor ganetespib in melanoma. PLoS ONE. 2013;8:e56134. doi: 10.1371/journal.pone.0056134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Acquaviva J, Smith DL, Jimenez JP, Zhang C, Sequeira M, He S, et al. Overcoming acquired BRAF inhibitor resistance in melanoma via targeted inhibition of Hsp90 with ganetespib. Molecular Cancer Therapeutics. 2014;13:353–63. doi: 10.1158/1535-7163.MCT-13-0481. [DOI] [PubMed] [Google Scholar]
- 100.Lito P, Saborowski A, Yue J, Solomon M, Joseph E, Gadal S, et al. Disruption of CRAF-Mediated MEK Activation Is Required for Effective MEK Inhibition in KRAS Mutant Tumors. Cancer Cell. 2014 doi: 10.1016/j.ccr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ishii N, Harada N, Joseph EW, Ohara K, Miura T, Sakamoto H, et al. Enhanced inhibition of ERK signaling by a novel allosteric MEK inhibitor, CH5126766, that suppresses feedback reactivation of RAF activity. Cancer Research. 2013;73:4050–60. doi: 10.1158/0008-5472.CAN-12-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]


