Abstract
Examination of a drug’s abuse potential at multiple levels of analysis (molecular/cellular action, whole-organism behavior, epidemiological data) is an essential component to regulating controlled substances under the Controlled Substances Act (CSA). We reviewed studies that examined several central nervous system (CNS) stimulants, focusing on those with primarily dopaminergic actions, in drug self-administration, drug discrimination, and physical dependence. For drug self-administration and drug discrimination, we distinguished between experiments conducted with rats and nonhuman primates (NHP) to highlight the common and unique attributes of each model in the assessment of abuse potential. Our review of drug self-administration studies suggests that this procedure is important in predicting abuse potential of dopaminergic compounds, but there were many false positives. We recommended that tests to determine how reinforcing a drug is relative to a known drug of abuse may be more predictive of abuse potential than tests that yield a binary, yes-or-no classification. Several false positives also occurred with drug discrimination. With this procedure, we recommended that future research follow a standard decision-tree approach that may require examining the drug being tested for abuse potential as the training stimulus. This approach would also allow several known drugs of abuse to be tested for substitution, and this may reduce false positives. Finally, we reviewed evidence of physical dependence with stimulants and discussed the feasibility of modeling these phenomena in nonhuman animals in a rational and practical fashion.
Keywords: stimulant, animal model, abuse potential, self-administration, drug discrimination, physical dependence
1. Introduction
The Controlled Substances Act (CSA) was passed in the United States in 1970 and established five schedules of controlled substances (Title 21USC801; Title 21USC812). The scheduling of a controlled substance is based on its medical usefulness and potential for abuse where schedule I indicates no currently accepted medical use and high abuse potential (Title 21USC812). Schedule II through V drugs include those with currently accepted medical use and are categorized within this range (i.e., II–V) based on their abuse potential (Title 21USC812). The Food and Drug Administration (FDA) defines a drug as having abuse potential if it “… is used in nonmedical situations, repeatedly or even sporadically, for the positive psychoactive effects it produces” (FDA/Center for Drug Evaluation and Research; CDER, 2010, p. 4) or by O’Connor and colleagues (2011) as “…the potential for repeated taking of a drug for its reinforcing or subjective-effects, or the avoidance of associated negative effects” (p. 913).
Abuse-potential assessment predates the CSA and is an essential component to regulation of controlled substances (Balster and Bigelow, 2003). A complete assessment of abuse potential includes data collected at several levels, from cellular action to whole-organism behavior to collection of epidemiological data (Balster and Bigelow, 2003; FDA/CDER, 2010; Horton et al., 2013). The compounds being assessed may be putative therapeutics or emerging “street” drugs that are anecdotally abused but too new to have been characterized empirically. Readers are encouraged to refer to Calderon and Klein (Neuropharmacology, this issue), for a review of US regulatory procedures for evaluating abuse potential of central nervous system (CNS) stimulants. In this review, we focused on behavioral research with nonhuman animals in the characterization of abuse potential of CNS stimulants. The term “CNS stimulant” has been broadly defined as a centrally acting drug with actions on monoamine neurotransmitter systems that increases alertness, attention, energy, blood pressure, and heart and respiration rate (National Institute on Drug Abuse; NIDA, 2001). We focused on therapeutics including stimulant medications (i.e., those for attention-deficit/hyperactivity disorder (ADHD) and other dopamine uptake inhibitors) and illicit compounds such as methamphetamine, cocaine, and synthetic cathinones often referred to as “bath salts”. While dopamine agonists like those developed as potential therapies for stimulant abuse and Parkinson’s disease often lack some of the physiological characteristics associated with CNS stimulants, we reviewed these drugs because they are typically compared to illicit stimulants in assessment of their abuse potential.
1.1 Types of procedures reviewed
The FDA/CDER (2010) describes five types of procedures typically used in assessment of abuse potential in nonhuman subjects: drug self-administration, conditioned place preference, drug discrimination, psychomotor tests, and dependence potential. We reviewed drug self-administration and drug discrimination experiments because they are regarded as “gold-standard” procedures in abuse-potential testing, perhaps because they are good predictors of CSA scheduling status and abuse-potential measures obtained with humans (e.g., Horton et al., 2013; Kamien et al., 1993; Rush et al., 2001). With drug self-administration, we reviewed species differences and the importance of reinforcing effectiveness relative to known and well-characterized drugs of abuse. With drug discrimination, we reviewed the role of training stimulus in obtaining false positives. Finally, we discussed the nature of physical dependence with this drug class and whether it should be included in assessments of abuse potential of CNS stimulants.
1.2 Use of rodents and nonhuman primates (NHP)
The use of nonhuman animals in preclinical assessments of abuse potential offers distinct advantages compared with human participants, and there are ethical and safety reasons for understanding drug effects in nonhuman animals prior to their study in humans. Newly developed compounds can be characterized relatively early in the drug-development process, often as part of the safety/toxicology profile of the compound. A wider range of doses can be examined for a longer period of time. Studies with nonhuman animals can be conducted with greater experimental control than is feasible with humans because the investigator can control many of the environmental conditions such as drug history, enrichment, nutrition, and so on. In some cases with nonhuman primates (NHP), but particularly with rodents, each organism’s history is known and can be controlled by the experimenter. With rodents, subjects with similar genetic composition (e.g., inbred strains) or specific genetic modifications (e.g., knockout mice) can be selected and used depending on the experimental question. On the other hand, it is impossible to test drugs on naïve humans, and when conducting inpatient studies with drug abusers, extra-experimental events and genetic differences are extremely difficult, if not impossible, to control.
An important consideration in abuse-potential testing is the choice of animal model. Rodents and NHPs each have advantages and disadvantages (discussed below). In the current review, we distinguished between studies with rodents and NHPs to highlight the common and unique attributes of each model in characterizing abuse potential of CNS stimulants.
2. Drug self-administration
Drug self-administration is a procedure used to determine whether behavior can be maintained by the administration of a drug, a characteristic that defines a drug as a reinforcer. The drug is generally delivered intravenously (though other routes have been used, e.g., oral; Lemaire and Meisch, 1985; Meisch, 2001) contingent on a specific behavior, such as a lever press, or a pattern of lever presses (see Ator and Griffiths, 2003 for a methodological review). For a drug to be considered a reinforcer, it must maintain meaningfully higher levels of responding compared to a vehicle control.
2.1 Schedules of reinforcement in the characterization of drugs as reinforcers
The current approach to drug self-administration in abuse-potential assessment is often to use a simple fixed-ratio (FR) schedule to determine whether a drug functions as a reinforcer. With this schedule, drug delivery occurs after a specified number of lever presses. Particularly with rodents, it is common to use the simplest version of an FR, in which every lever press results in drug delivery (referred to as FR 1 or continuous reinforcement). Other simple schedules (fixed interval, variable interval, and variable ratio) and second-order schedules have been used less often. Under fixed-interval schedules, a specified period of time must pass before a single response results in drug delivery. Under variable-interval schedules, the specified period of time changes from delivery to delivery, yielding an average period of time. Variable-ratio schedules require an average number of responses per drug delivery, and the exact number or responses changes from delivery to delivery. All of these schedules have in common response rate and number of drug deliveries as primary dependent measures.
In the context of drug self-administration, many procedures have been used to determine whether a drug has abuse potential. However, it is common to use a “substitution” procedure, where a known drug reinforcer (or in some cases, the drug being tested for abuse potential) is used during acquisition and until responding is stable. Then, vehicle or different doses of drugs are substituted for the baseline drug for a fixed number of sessions (e.g., Caine and Koob, 1995; Gold and Balster, 1996; Motbey et al., 2013; Self and Stein, 1992) or for at least as many sessions as it took for responding to extinguish when vehicle was made available (e.g., Freeman et al., 2012; Sinnott et al., 1999; Woolverton et al., 1984).
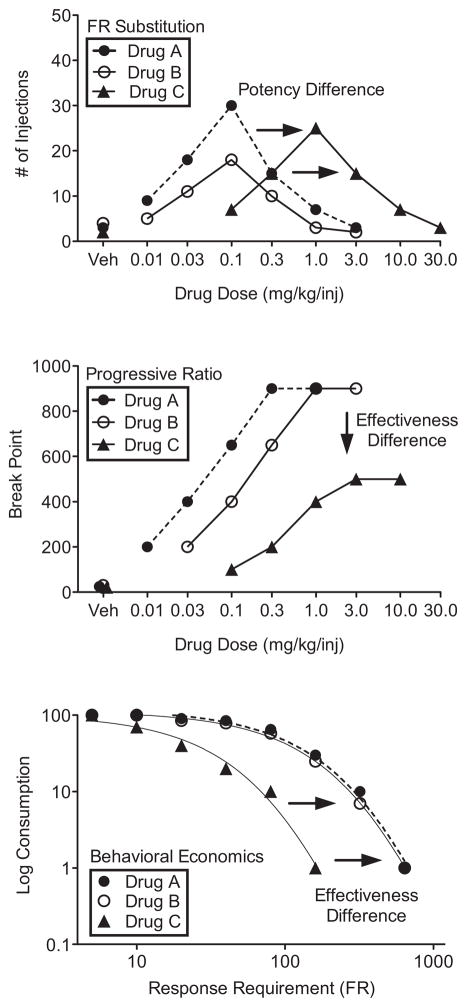
An important characteristic of self-administration of drugs under simple schedules is that the dose-response function is typically biphasic (higher response rates are maintained by smaller doses and lower response rates by larger doses; Pickens and Thompson 1968; Wilson et al. 1971). Figure 1 (top panel) shows biphasic functions for three hypothetical drugs (A, B, C) that might be obtained with a substitution procedure. All three drugs would be considered reinforcers because each shows behavior maintained above vehicle levels. Drugs A and B are similar in terms of potency as reinforcers because the ascending and descending portions of the curve occur at similar doses, while drug C would be considered less potent than A and B because the ascending and descending portions of the curve occur at larger doses.
Figure 1.
Hypothetical data for three drug self-administration procedures used in abuse-potential assessment. For all panels, closed circles are Drug A, open circles are Drug B, and closed triangles are Drug C. The top panel shows the number of injections as a function of drug dose for FR substitution. The middle panel shows PR breakpoints as a function of drug dose. The bottom panel shows consumption (i.e., normalized number of injections) as a function of response requirement for behavioral economics.
Determining drugs as reinforcers with outcomes like those depicted in the top panel of Figure 1 yields a binary, yes-or-no classification and does not provide quantitative information about how reinforcing a drug is. This is because response rate and number of injections can depend on several properties of the drug in addition to or other than its reinforcing properties (e.g., Balster and Bigelow, 2003; Richardson and Roberts, 1996; Wee et al., 2005). In Figure 1 (top panel), drugs A and C may have shorter durations of action, resulting in higher response rates and more injections per session while drug B may have a longer duration of action, resulting in lower response rates and fewer injections per session. In this example, we would not conclude that drugs A and C were more effective reinforcers than drug B because response rate may have been suppressed by direct or aversive effects of the drug or titrated to maintain a steady level of intake (see Lynch and Carroll, 2001 for a review). Similarly, we would not conclude that smaller doses of a drug that maintain higher response rates are more effective reinforcers than larger doses of the same drug that maintain lower response rates. Thus, response rates and number of injections are not appropriate measures of reinforcer effectiveness, and this has been recognized for some time (see Balster and Bigelow, 2003). In fact, response rates maintained by stimulant drugs are generally inversely or poorly related to choice (a measure of reinforcer effectiveness), and subjects choose larger doses over smaller ones (Johanson and Schuster, 1975; Woolverton and Johanson, 1984).
Additionally, response rates can depend on the schedule of reinforcement used (e.g., Carter and Griffiths, 2009; Hursh and Silberberg, 2008). Typically, higher response rates occur with ratio schedules and lower response rates with interval schedules, although response rates are also determined by schedule parameters like reinforcement frequency. It would not be appropriate to conclude that a drug is a less effective reinforcer under an interval schedule (with lower response rates) than under a ratio schedule (with higher response rates). Because response rate and number of drug deliveries can be influenced by factors other than reinforcing effectiveness, we suggest that the binary classification system for screening drugs for abuse potential may be in need of some revision. We, and others before us (e.g., Balster and Bigelow, 2003; Horton et al., 2013; O’Connor et al., 2011; Richardson and Roberts, 1996; Rowlett, 2000), suggest that it may be useful to examine drugs on a continuum of how reinforcing they are (relative reinforcing effectiveness). Two procedures suitable for this type of classification in both NHPs and rodents are progressive ratio (PR) and behavioral economics.
In PR procedures, the number of responses required for drug delivery systematically increases within or across sessions until responding ceases (see Richardson and Roberts, 1996; Rowlett, 2000; Stafford et al., 1998 for reviews). The response requirement in effect when responding stops is referred to as the “breakpoint” and reflects the maximal amount of responses maintained by delivery of a dose of a drug. Larger breakpoints are thought to reflect greater reinforcing effectiveness. Figure 1 (middle panel) shows breakpoints for three hypothetical drugs. Like the top panel, all three drugs would be considered reinforcers, but drugs A and B would be more effective reinforcers than drug C because the asymptotes for drugs A and B occur at larger breakpoints. Note that several doses are shown for each drug because this procedure is most useful when the maximal breakpoint of the test drug is compared to the maximal breakpoint of a known drug of abuse (i.e., the dose is increased until an asymptote is reached).
In behavioral-economic procedures, the number of responses required for each drug delivery systematically increases across several sessions until the number of drug injections reaches near-zero levels (e.g., Bickel et al., 1990; Hursh, 1991; Hursh and Silberberg, 2008). The number of injections is plotted as a function of response requirement, and the “elasticity” of the resulting function (termed demand curve) is calculated based on the slope obtained from nonlinear regression. Less elasticity is thought to reflect greater reinforcing effectiveness because it indicates that baseline drug intake will persist across increasingly larger response requirements. Figure 1 (bottom panel) shows demand curves plotted for three hypothetical drugs. The curves for drugs A and B are less elastic than the curve for drug C and thus, drugs A and B would be considered more effective reinforcers. A unique feature of the behavioral-economics approach is that the elasticity of the normalized demand function is thought to be independent of dose, allowing comparison between different types of drugs at single doses (Bickel et al., 1990; Hursh and Winger, 1995). Like PR procedures, behavioral economics is useful when elasticity is compared for the test drug and a known drug of abuse to determine relative reinforcing effectiveness.
2.2 Results of literature review
Table 1 shows several drugs with dopaminergic actions, including mixed and selective dopamine (DA) agonists, releasers, and uptake inhibitors and whether each of these compounds functioned as a reinforcer in rodents and NHPs. Among the drugs listed are ADHD medications (e.g., d-amphetamine and methylphenidate), potential therapies for stimulant abuse (e.g., bupropion, diethylpropion, bromocriptine), treatments for Parkinson’s disease and other disorders (e.g., pramipexole and ropinirole), cocaine analogs (e.g., RTI 177), and illicit compounds (e.g., methamphetamine and synthetic cathinones). Although most of the drugs in Table 1 also affect other monoamines, drugs with primary actions on or increased selectivity for serotonin and norepinephrine were not included in the review given our limited space and less consistent nature with which these drugs are self-administered by nonhuman animals (e.g., Lile and Nader, 2003; O’Connor et al., 2011; Roberts et al., 1999; Wee et al., 2005; Wee and Woolverton, 2004; Woolverton, 1987).
Table 1.
Drug Self-Administration
The first column shows a list of dopaminergic drugs. For rodents (second column) and nonhuman primates (NHP; fourth column), + symbols indicate that the drug was self administered, − symbols indicate that the drug was not self-administered, and −/+ indicates self-administration by approximately half of subjects tested. When choice, progressive ratio, or behavior economics procedures were used to compare the drug to cocaine or methamphetamine (* indicate when methamphetamine was the comparator, all other cases are cocaine), the symbol in parenthesis indicates whether the drug was less than (<), greater than (>) or equal (=) to cocaine or methamphetamine in terms of reinforcing effectiveness. References are shown in the third and fifth columns for each experiment. CSA scheduling status is shown in the final column; NS indicates that a drug is not scheduled.
Most of the drugs in Table 1 were self-administered by rodents and NHPs (indicated by plus signs), although there were some drugs that were not (indicated by minus signs). Despite several procedural differences, there was generally agreement within and between species. However, discrepancies occurred for rodents with SKF 82958 and SKF 77434. Caine and colleagues (1999) found that these compounds were not self-administered in rats whereas Self and Stein (1992) reported the opposite. There were procedural differences that may account for the discrepant results. Both trained animals to lever press with food before catheterization, but Caine et al. (1999) used a cocaine-substitution procedure and an FR 5 schedule. Self and Stein (1992) examined acquisition of SKF 82958 self-administration in drug-naïve rats responding under an FR 1 and later assessed different doses of SKF 82958 and SKF 77434 in rats that had acquired self-administration with SKF 82958 or cocaine. In Self and Stein (1992), drug delivery was paired with a tone. It is possible that different response requirements and stimuli paired with drug delivery were responsible for the differences between these experiments. In NHPs, SKF 82958 was self-administered and SKF 77434 was not (Grech et al., 1996; Weed and Woolverton, 1995; Weed et al., 1997).
For NHPs, there were no instances of a drug being self-administered in one study and not another. However, there were instances when a drug was a reinforcer in only a subset of subjects (indicated by a minus and plus symbol) for apomorphine, GBR-12909, mazindol, phentermine, and SKF 81297 (Bergman et al., 1989; Grech et al., 1996; Stafford et al., 2001; Woolverton et al., 1984). Three of these drugs (apomorphine, GBR-12909, and phentermine) were also self-administered in rodents. To our knowledge, self-administration of mazindol and SKF 81297 has not been examined in rodents.
There were few discrepancies between species for drugs examined in both rodents and NHPs, and this is consistent with a previous review suggesting high concordance between drug self-administration studies with rodents and NHPs (O’Connor et al., 2011). Modafinil is an exception. Two papers reported that modafinil was not self-administered by rodents (Deroche-Gamonet et al., 2002; Heal et al., 2013) while one paper reported that it was self-administered by NHPs (Gold and Balster, 1996). There are many procedural differences between these experiments, making it difficult to determine whether or which of those differences could have contributed to the discrepancy. Deroche-Gamonet et al. (2002) examined acquisition of modafinil self-administration under an FR 1 schedule in drug-naïve rats with no prior training. The FR 1 was in effect for the first 11 sessions and was gradually increased across the final eight sessions to an FR 10. Heal et al. (2013) examined self-administration of different doses of modafinil under an FR 2 in rats trained to self-administer cocaine. Each dose was in effect for at least three sessions and until responding was stable. Gold and Balster (1996) used a cocaine baseline and an FR 30 schedule. Different doses of modafinil were made available for four consecutive sessions followed by a return to the cocaine baseline. Therefore, it does not seem that drug-naïve versus drug-experienced or untrained versus trained animals resulted in the between-species discrepancy, which suggests that species is the determining issue in the discrepancy identified with modafinil. Notably, modafinil has been shown to induce pharmacological activity in brain dopamine systems in humans in a manner similar to amphetamine and cocaine (Volkow et al., 2009), suggesting that it may have abuse potential. However, studies with drug-experienced participants are inconsistent as to whether modafinil has abuse potential (e.g., Rush et al., 2002; Stoops et al., 2005; Vosburg et al., 2010). To our knowledge, there are no post-marketing surveillance reports of abuse of its commercial form (Provigil) in humans (Myrick et al., 2004).
A concern with the outcomes in Table 1 is that almost all of the drugs were self-administered, and there were numerous false positives in which a drug was self-administered by nonhuman animals but is not abused by humans or scheduled under the CSA (see last column of Table 1 for scheduling status). This is problematic for drug development because it impedes progress in development of medications with dopaminergic actions. In some cases, drugs in Table 1 may not be readily available to drug abusers (e.g., drugs that are synthesized in small quantities and limited to research use) and might reflect a true positive if they were more widely available. Other drugs in Table 1 are not scheduled or are schedule IV, which should indicate low abuse potential. For example, apomorphine, buproprion, diclofensine, diethylpropion, mazindol, modafinil, nomifensine, phentermine, piribedil, pramipexole, procaine, quinpirole, and ropinirole functioned as reinforcers in nonhumans but are not abused by humans. Many of these drugs (e.g., buproprion, modafinil, pramipexole, procaine, ropinirole) are used clinically and are therefore available for diversion. It is important to note that while drug self-administration yielded several false positives with dopaminergic drugs, it did not fail to detect true positives.
When a test of relative reinforcing effectiveness was conducted for a drug and the results compared to known stimulants of abuse, many of the drugs were less effective than cocaine or methamphetamine (7-OH-DPAT, d-amphetamine, diethylproprion, para- and meta-fluoroamphetamine, para- and meta-methylamphetamine, methylphenidate, modafinil, nomifensine, procaine, ropinirole, RTI-177, RTI-336, and SKF 81297). Only two of these 15 compounds (d-amphetamine and methylphenidate) that were less effective than cocaine or methamphetamine in at least one experiment are abused by humans and are schedule II drugs. Methylphenidate was equal in reinforcing effectiveness to cocaine in two other studies (Johanson and Schuster, 1975; Lile et al., 2003). Cathinone, mephedrone, GBR 12909, methamphetamine, methylphenidate, phentermine, and SKF 82958 were equal in their reinforcing effectiveness to cocaine, and four of these seven compounds (cathinone, mephedrone, methamphetamine, and methylphenidate) are abused by humans and are schedule I or II drugs (note that GBR 12909 and SKF 82958 are not used clinically). The synthetic cathinone MDPV was greater in reinforcing effectiveness compared to methamphetamine, and this compound is a schedule I drug (Aarde et al., 2013b).
2.3 Recommendations
Based on the studies reviewed, tests of relative reinforcing effectiveness could be used to more reliably predict abuse potential. This recommendation is supported by previous reviews from Horton and colleagues (2013), Richardson and Roberts (1996), and O’Connor and colleagues (2011). For example, Richardson and Roberts (1996) show that many stimulant drugs are reinforcers under an FR 1 but are less effective under a PR schedule compared to methamphetamine or cocaine. Horton et al. (2013) found that while drug self-administration was valuable in predicting CSA scheduling and human subjective effects, a PR schedule was associated with increased predictive value. Finally, O’Connor et al. (2011) note instances when a drug with low abuse potential maintained behavior under a simple schedule of reinforcement was identified as a weak reinforcer in a PR test relative to known drugs of abuse. Despite the consistent finding and previous arguments that tests of relative reinforcing effectiveness add predictive value to assessments of abuse potential, these procedures have not been adopted in drug development or by US regulatory agencies (Horton et al., 2013). Under current FDA guidelines (FDA/CDER, 2010), tests of relative reinforcing effectiveness are suitable but not required or recommended as a standard approach. Of the papers we reviewed, relatively few studies conducted such a test. Therefore, we suggest that such tests be conducted in situations when a drug has been shown to have reinforcing properties. If a drug is self-administered by rodents or NHPs under a simple FR schedule, it then could be examined in a test of relative reinforcing effectiveness, as part of a standard decision-tree approach. Indeed, the European Drug Abuse Testing Guidelines (EMEA, 2006) recommend using a PR schedule when possible.
It is important to emphasize that tests of reinforcing effectiveness must be conducted in a meaningful context. Some studies used a PR schedule, but only with the test drug and not compared to a known drug of abuse. In this scenario, the breakpoint for the test drug might be 100 responses. That number by itself does not provide much information, except that the test drug can maintain behavior. If the breakpoint for the test drug is 100 responses and for cocaine is 1,000 responses, it informs us that the test drug is much less effective than a known drug of abuse. In this scenario, it may be inappropriate to schedule the test drug with the same CSA status as cocaine. In other cases where a PR procedure was used, the test or comparator drug was compared at a single dose. As previously stated, reinforcing effectiveness of a drug is reflected in the asymptote of the dose-response function under a PR schedule of reinforcement. As such, testing a single dose of a drug in a PR procedure does not provide sufficient information for a conclusive determination of reinforcing effectiveness. For this reason, single-dose approaches should not be used for comparing drugs in terms of reinforcing effectiveness with a PR procedure.
We recommend tests of relative reinforcing effectiveness in an assessment of abuse potential, but this type of assessment brings with it several considerations. These assessments are longer, because they require more conditions (i.e., multiple drugs at multiple doses or response requirements). It is possible to do some of these tests in rodents, and rodents are advantageous for several reasons. They are more cost effective, easily cared for, and more readily available compared to NHPs, and while the FDA (FDA/CDER, 2010) does not specify a preference for rodents versus NHPs, the European Drug Abuse Testing Guidelines (EMEA, 2006) state a preference for rodent or non-primate subjects except when results with non-primates are unclear or conflict with clinical data. With rodents it is also easier to control each organism’s history and to obtain genetically homogeneous samples. However, limitations for rodents include shorter life span and shorter catheter life, and these limit the range of experimental designs that can be used. In rodents, tests of relative reinforcing effectiveness were only conducted in four studies in Table 1 relative to 22 instances for NHPs, some of which were within the same study. In three of these four cases with rodents, the experimental design was a mixed within- and between-subjects design, using drug type as a between-subject factor and drug dose as a within-subject factor (Aarde et al., 2013b; Motbey et al., 2013; Roberts, 1993). In one case, an entirely within-subjects design was used where each dose was examined for three consecutive sessions (e.g., Caine and Koob, 1995).
Longer lifespan and better long-term catheter patency in NHPs allow for more thorough characterization of self-administration of several drugs and several doses on a within-subjects basis and the ability to conduct each condition until responding is considered stable (i.e., steady state). Within-subjects design and steady-state behavior have several strengths, especially within the realm of abuse-potential assessment. This design reduces the impact of between-subject variability and differential drug sensitivities that are often obtained in behavioral studies. This is because each subject serves as its own control. Examination of behavior at steady state results in an added strength in that within-subject variability is also reduced compared to conditions that are conducted for a fixed or limited number of sessions. Additionally, fewer subjects are needed to obtain reliable results and draw meaningful conclusions, and subjects can be tested with multiple drugs and doses to allow relative effects of different compounds to be determined. Finally, many NHP researchers have adopted a strategy of beginning each new study with some experimentally naïve subjects and some experienced subjects. In this way, results obtained in those animals with and without a drug history can be compared. In our experience, naive and experienced animals typically do not differ. However, when differences do emerge, the effects of history can be thoroughly examined. Using some subjects with a history of drug self-administration is also useful because all subjects do not have to be trained prior to each experiment. Of the 22 instances in NHPs where relative reinforcing effectiveness of a drug was examined, the majority used a PR procedure, two used a drug-vs-drug choice procedure, and one used a behavioral-economic procedure.
3. Drug discrimination
Drug discrimination is a procedure used to determine whether the interoceptive nature of a drug will generalize to or substitute for other drugs. That is, comparisons can be made between centrally acting drugs to determine whether different drugs induce comparable interoceptive effects (see Ator and Griffiths, 2003 for a methodological review). These studies allow for behavioral and pharmacological characterization of drugs into different classes based on their discriminative-stimulus effects and can be useful for classifying new compounds (e.g., whether a compound is stimulant-like). Drug discrimination does not provide information about the reinforcing nature of a drug. If a drug has discriminative-stimulus properties similar to cocaine, it does not necessarily mean that it will function as a reinforcer in self-administration. Indeed, drugs that are not reinforcers can be trained on the basis of their interoceptive effects.
3.1 Procedures for characterizing discriminative-stimulus properties of drugs
There are several procedures used in drug-discrimination studies with nonhuman animals. Most commonly, a two-lever operant procedure is used, in which subjects are trained to press one lever following an experimenter-delivered injection of a dose of a drug and to press the other lever in a non-drug control condition, usually the drug’s vehicle or saline. Correct responses are reinforced, often with food or liquid, though shock avoidance has been used. With training, the interoceptive state associated with administration of a drug dose or vehicle functions as a discriminative stimulus that signals availability of a reinforcer contingent on correct responses.
Once subjects are trained to reliably respond to the correct lever, depending on whether the training dose or vehicle was administered, different doses of the training and test drugs are administered during test sessions to obtain dose-effect functions. Generally, doses of the training drug that are much smaller than the training dose engender responding on the vehicle-associated lever, and doses closer to and larger than the training dose engender responding on the drug-associated lever. A similar pattern of behavior occurs when drugs from the same class are administered. For instance, in subjects trained to discriminate cocaine from saline, d-amphetamine and methamphetamine administration result in dose-dependent increases in responding on the cocaine-associated lever. When drugs with mechanisms of action distinct from the training drug are administered, responding typically occurs on the vehicle-associated lever, even at doses that are behaviorally active. In the above example, pentobarbital engenders responding on the vehicle-associated lever in cocaine-trained subjects.
In abuse-potential assessment, novel compounds are directly compared to known drugs of abuse to determine to which drug class the novel compound belongs. Typically, a test drug is considered to fully substitute for a training drug (or said another way, a training drug is said to fully generalize to a test drug) and to share discriminative-stimulus properties when at least 80% of responding occurs on the drug-associated lever. Partial substitution is achieved when 21–79% of responding occurs on the drug-associated lever. Finally, a drug that engenders 20% or fewer responses on the drug-associated lever does not substitute for the training drug and is not considered to share discriminative-stimulus properties (note that these levels of drug-appropriate responding are the majority in the literature, and some investigators have used different criteria).
Several factors influence the nature of the discriminative-stimulus effects of drugs. One of those factors is the dose of the drug used for training (see Stolerman et al., 2011 for a recent review). For example, Schechter (1997) found that the dose-effect functions for cocaine, d-amphetamine, and methamphetamine were shifted to the left in rats trained to discriminate a low dose (2.0 mg/kg) relative to a high dose of cocaine (10.0 mg/kg) from saline. Additionally, which drug is used as a training stimulus and which is used as a testing stimulus could influence the outcome (full, partial, or no substitution) of drug-discrimination studies (D’Mello and Stolerman, 1977; Stolerman and D’Mello, 1981). Training with cocaine and testing novel compounds might yield different results than training with a novel compound and testing cocaine.
3.2 Results of the literature review
Table 2 shows a list of drug-discrimination studies conducted in rodents and NHPs using a number of CNS stimulants. Given the limited space, we reviewed studies where cocaine was used as a comparator drug, either as a training or test stimulus. Other stimulants like d-amphetamine and methamphetamine could be examined in a similar manner. A thorough examination of each of these drugs as comparators in characterizing discriminative-stimulus properties of potential drugs of abuse would be large enough in scope to constitute their own reviews. We focused on cocaine to establish a baseline of reasonable scope with which to make a critical analysis of the advantages and pitfalls of current applications of drug discrimination to assessment of abuse potential for stimulants. Table 2 shows several drugs with primary actions on dopamine. Those with primary actions on serotonin and norepinephrine were not included because these compounds generally do not fully substitute for cocaine (e.g., Baker et al., 1993; Callahan et al., 1997; Kleven et al., 1990). The first column indicates the drug that was used as the training stimulus and the second column indicates the drug tested for substitution.
Table 2.
Drug Discrimination
The first column lists the drug used as the training stimulus and the second column lists the drug tested for substitution. The training dose (in mg/kg unless otherwise noted) used for rodents is shown in the third column and for nonhuman primates (NHPs) in the sixth column. When multiple doses of the training drug were used for different subjects, doses are separated by a comma, and arrows indicate when the training dose was reduced for a group of subjects. In the fourth (rodents) and seventh columns (NHPs), “Full” indicates that the test drug substituted for the training drug, “Partial” indicates that the test drug partially substituted for the training drug, and “None” indicates that the test drug did not substitute for the training drug.
Most drugs in Table 2 fully substituted for the cocaine-training stimulus in at least one study regardless of species used. Exceptions are piribedil, SKF 82958, and terguride, and each of these were examined in only one study. Relatively few studies used NHPs, but when a drug was examined with both species, there was between-species consistency. When cocaine was the training stimulus, bupropion, d-amphetamine, diclofensine, GBR-12909, mazindol, methamphetamine, methcathinone, methylphenidate, nomifensine, and WIN 35,428 fully substituted for cocaine across all studies, and when examined in NHPs, fully substituted in both species. For all other drugs tested in more than one study with a cocaine-training stimulus, either full or partial substitution was obtained. These results raise two concerns. First, there are several false positives, or instances where a drug was cocaine-like but is not abused. Examples include 7-OH-DPAT, apomorphine, bromocriptine, bupropion, diclofensine, mazindol, modafinil, nomifensine, procaine, quinpirole, and others. Second, there is great discrepancy in the results obtained between studies, regardless of species used. In some instances, the discrepancies were related to the training dose of cocaine. With 7-OH-DPAT in rodents, full substitution occurred with larger cocaine-training doses (Acri et al., 1995; Garner and Baker, 1999; Ukai and Mitsunaga, 2005) and partial substitution occurred with smaller cocaine-training doses (Baker et al., 1998). The opposite occurred with 7-OH-DPAT in NHPs where larger cocaine-training doses yielded partial substitution (Lamas et al., 1996; Spealman, 1996) and smaller cocaine-training doses yielded full substitution (Sinnott et al., 1999). For procaine, a smaller cocaine-training dose fully generalized to procaine (Wilcox et al., 2001) while larger doses partially generalized (e.g., Graham and Balster, 1993; Middaugh et al., 1998). In other cases where there were discrepancies, training dose did not appear to correlate with different outcomes.
There are a few studies in Table 2 where the compound being tested for abuse potential was used as the training stimulus and cocaine was tested for substitution. These were conducted with mephedrone, 7-OH-DPAT, apomorphine, bupropion, cathinone, d-amphetamine, GBR-12909, methamphetamine, methcathinone, pramipexole, procaine, quinpirole, SKF 38393, or SKF 81297 as the training stimulus. When cocaine was tested for substitution in these instances, full substitution was obtained for six (bupropion, cathinone, d-amphetamine, GBR-12909, methamphetamine, methcathinone) of these 14 drugs. Partial substitution was obtained for four (mephedrone, 7-OH-DPAT, procaine, quinpirole) and no substitution was obtained for six (7-OH-DPAT, apomorphine, pramipexole, quinpirole, SKF 38393, SKF 81297) of these 14 drugs.
These results raise several issues. First, discriminative stimulus properties are not always symmetrical. If a drug substitutes for cocaine, it does not necessarily indicate that cocaine will substitute for that drug. This suggests a potential need to examine discriminative-stimulus properties in multiple situations, when a known drug of abuse is the training stimulus and when the drug being examined for abuse potential is the training stimulus. Unfortunately, there is a paucity of studies examining the latter situation. With the limited data shown in Table 2, it is not clear whether training with the drug being examined for abuse potential would result in more consistent findings within and across species than training with cocaine, and more research is needed on this topic. In the six cases where at least two different studies used the drug being assessed for abuse potential as the training stimulus, a discrepancy was obtained for 7-OH-DPAT and quinpirole.
The second issue concerns the large number of false positives obtained. When cocaine was used as the training stimulus, several drugs were found to be cocaine-like. Conversely, when the drug being examined for abuse potential was used as the training stimulus and cocaine was used as a test drug, about half of the drugs generalized fully to cocaine (i.e., cocaine substituted for six of 14 drugs versus all 14 drugs substituting for cocaine in at least one instance). Of those six compounds (bupropion, cathinone, d-amphetamine, GBR-12909, methamphetamine, methcathinone), only bupropion and GBR-12909 do not appear to be abused by humans, and GBR-12909 is not available clinically. Thus, in the limited cases available, training with the drug being examined for abuse potential resulted in fewer false positives without failing to detect true positives relative to those cases when cocaine was the training stimulus. Cocaine only substituted partially or did not substitute for eight of the 14 compounds examined as the training stimulus. Six of these eight compounds (7-OH-DPAT, apomorphine, pramipexole, quinpirole, SKF 38393, SKF 81297) are dopamine agonists, and one of these eight compounds (mephedrone) is abused by humans. In the experiment examining mephedrone as the training stimulus, Varner and colleagues (2013) found that cocaine and methamphetamine partially substituted for mephedrone, but one dose of methylenedioxymethamphetamine (MDMA) fully substituted. This highlights the third issue, a potential need to examine multiple drugs of abuse for their ability to substitute for novel compounds being examined for abuse potential.
3.3 Recommendations
Similar to results reported above with drug self-administration, drug-discrimination studies with cocaine as the training stimulus resulted in a large number of false positives (i.e., a drug is cocaine-like but is not abused) with the dopaminergic drugs included in our review. We have two recommendations and one consideration for future studies that focus on ways to potentially reduce the number of false positives obtained with dopaminergic drugs in this assay. Our recommendations would likely be feasible to examine in rodents. Relatively few studies were conducted with NHPs, but the limited data suggests that NHPs do not offer additional advantages relative to rodents. For our first recommendation, we suggest that the drug being examined for abuse potential be used as the training stimulus in cases when it has been shown to fully substitute for cocaine (or another known stimulant drug of abuse) as part of a standard decision-tree approach. Based on the few studies in Table 1, this situation resulted in fewer false positives with dopamine agonists (but not with uptake inhibitors, bupropion and GBR 12909) without failing to detect true positives (e.g., d-amphetamine, methamphetamine, cathinone).
Our second suggestion is to examine the drug being evaluated for abuse potential against multiple known drugs of abuse, also as part of a decision-tree approach. If, for example, a novel compound fully substitutes for cocaine, but not vice versa, the test compound could be used as the training stimulus and cocaine, methamphetamine, and MDMA could be tested for substitution. In one study in Table 2, an emerging drug of abuse, mephedrone, was used as the training stimulus and failed to fully generalize to cocaine (Varner et al., 2013). This might have reflected a case where a true positive was not detected. In that study, mephedrone was not considered to be cocaine- or methamphetamine-like, but it was similar to a different known drug of abuse, MDMA. Our second recommendation might prove most useful in cases of emerging “street” drugs. More research is necessary to determine whether our recommendations would be useful for reducing false positives while maintaining detection of true positives in this assay. Our recommendations could require additional time to fully assess drugs for abuse potential. However, we believe that adopting a standard decision-tree approach could maintain efficiency where possible while reducing the overall number of false positives.
Finally, we noted a considerable amount of variability in results obtained with drug discrimination. Coupled with the large number of false positives with many of the drugs in Table 2, we suggest that the field consider adopting more stringent criteria in determining whether a compound has abuse potential with this assay. Perhaps only those compounds that fully substitute for a known drug of abuse should be considered to have abuse potential while partial and no substitution should be considered to have no or low abuse potential. Note that this criterion, coupled with our decision-tree approach, would not result in false negatives with the list of drugs in Table 2. Synthetic cathinones (bath salts), cathinone, d-amphetamine, methamphetamine, methcathinone, and methylphenidate would be considered to have abuse potential with the criterion of full substitution coupled with our decision-tree approach. This approach would reduce the overall number of false positives and mostly those obtained with DA agonists. It should be noted that this approach would still result in DA uptake inhibitors (e.g., bupropion, GBR 12909, nomifensine) being considered to have abuse potential.
4. Physical dependence and withdrawal
The FDA broadly defines dependence potential as “…the propensity of a substance, as a consequence of its pharmacological effects on physiological or psychological functions, to give rise to a need for repeated doses of the substance” (FDA/CDER, 2010, p. 11). A state of physical dependence can be identified when administration of the drug is abruptly ceased (i.e., spontaneous withdrawal) or induced pharmacologically by an antagonist (i.e., precipitated withdrawal) following a protracted period of repeated drug administration, particularly with relatively high doses. In the case of opioids, a class of drug reinforcers for which physical dependence may be the most thoroughly characterized, overt and adverse signs of drug withdrawal are evident upon cessation of administration and are thought to be related to compensatory homeostatic adaptations that occur during chronic drug administration (Rehni et al., 2013). Drug withdrawal from opioids and other drugs with established physical dependence-inducing properties (e.g., ethanol, sedatives) can be alleviated by reestablishing administration of the drug, which is a likely contributor to continued drug-taking, or by administering another drug of comparable pharmacological effect (e.g., agonist substitution therapy; Kosten and O’Connor, 2003; Negus and Banks, 2013). During chronic drug administration, homeostatic adaptations also result in tolerance to the drug’s effects, which is manifest by a need to progressively increase the dose of the drug to maintain a stable level of effect. Thus, there is a clear need to characterize new drugs that fall within classes of known dependence-inducing compounds because of the possibility of adverse effects related to withdrawal and the potential impact that withdrawal and tolerance could have on continued drug-taking.
Historically, the case for physical dependence as a significant contributor to the abuse potential of stimulants has been more equivocal than for opioids and other compounds. In humans and animal models, the overt and often harmful physiological signs of withdrawal that are observed upon cessation of chronic administration of opioids, barbiturates, and ethanol do not occur with cessation of chronic stimulant administration (Barr and Markou, 2005; Carlson et al., 2012; Lago and Kosten, 1994; West and Gossop, 1994). This undoubtedly has contributed to a long-standing position that stimulant withdrawal, to whatever degree it exists, does not represent an acute medical danger to the user and may not be a significant contributor to the abuse potential of these compounds (Balster & Bigelow, 2003; Eddy et al., 1965).
The Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition (APA, 2013) includes under the category of Substance-Related and Addictive Disorders diagnostic criteria for stimulant dependence and a stimulant-withdrawal syndrome. The symptoms of the withdrawal syndrome include dysphoric mood, fatigue, unpleasant dreams, insomnia or hypersomnia, increased appetite and psychomotor retardation or agitation. Dysphoria is given special emphasis among the other symptoms in that it is the only one that is necessary (but not sufficient) for a diagnosis of stimulant-withdrawal syndrome. This emphasis on dysphoria is consistent with numerous claims that stimulant withdrawal is unique among other forms of drug withdrawal in that it is primarily a psychological experience (West and Gossop, 1994; Zorick et al., 2010). When the symptoms of stimulant withdrawal are compared and contrasted with a list of symptoms for the opioid-withdrawal syndrome (APA, 2013), which includes diarrhea, vomiting, fever, piloerection, and pupillary dilation, it becomes apparent that opioid withdrawal presents an overt array of physiological/somatic symptoms that are more easily observed, modeled, and measured in nonhuman animals than a mental state like dysphoria. A similar contrast is apparent when the symptoms of stimulant withdrawal are compared to those listed for other drugs that have established physical dependence-inducing properties (e.g., ethanol, sedatives; APA, 2013). Thus, modeling stimulant withdrawal in nonhuman animals, which includes an affective state as its linchpin, presents a unique challenge in terms of construct validity. Simply stated, how can we know through observation of physiology or behavior the mental state of an organism? However, regardless of the challenges of modeling, we should also question if the affective features of stimulant withdrawal represent a sufficient level of harm or risk to warrant added investment in the testing of an entire class of compounds in nonhuman subjects. If the harm or risk is low and/or the symptoms too difficult to model, it may be suitable to forgo such testing in nonhuman subjects and proceed to more valid determinations of affective processes related to the cessation of stimulant administration in humans under controlled laboratory conditions or in Phase II clinical trials.
Before considering these issues further, the discussion should be informed by a review of preclinical data relevant to the characterization of stimulant dependence. As stated above, a core feature of drug dependence is withdrawal, which for stimulants is manifest by a dysphoric affective state occurring when drug administration is abruptly ceased. It has been suggested that this dysphoric “crash” leads to subsequent drug taking through a negative reinforcement process, and thus represents harm by facilitating continued drug use. There is a growing body of evidence in nonhuman animals demonstrating that repeated administration of stimulants such as cocaine, amphetamine, and methamphetamine results in marked neuronal changes when drug administration is abruptly ceased (Barr and Markou, 2005; Calipari et al., 2013; Kitanaka et al., 2008; Larson et al., 2010; Perrine et al., 2008). However, for the current review we will focus on the affective and behavioral aspects of stimulant withdrawal because these factors are most likely more relevant to preclinical characterizations of abuse potential. Using a place conditioning procedure, Ettenberg et al. (1999) demonstrated that cocaine could condition either place preferences or aversions in rats, depending on the pretreatment interval. Specifically, shorter pretreatment intervals conditioned preferences while longer intervals conditioned aversions, demonstrating that cocaine administration can produce a presumably aversive state subsequent to its reinforcing effect that conditions aversions in the same manner that pain, emetics, and other classically “aversive” stimuli condition aversions (see also Frisch et al., 1995; Minami, 2009; Sufka, 1994). Linking stimulant withdrawal more specifically to dysphoria, Cryan et al. (2003) reported that rodents withdrawn from daily administrations of amphetamine demonstrated decreased mobility in forced-swim and tail-suspension tests, assays that have been used as nonhuman animal models of depression. Indeed, stimulant withdrawal has been proposed as a nonhuman animal model of depression (see Barr and Markou, 2005). Furthermore, abrupt cessation of stimulant administration increased thresholds for intracranial self-stimulation in rats, which has been interpreted as evidence that stimulant withdrawal causes a transient episode of anhedonia (Barr and Markou, 2005). Thus, despite the challenges of construct validity, current animal models do appear to capture some behavioral manifestations of the negative affective state associated with stimulant withdrawal.
In the context of abuse-potential testing, particular focus should be placed on determining if drug withdrawal facilitates subsequent drug use. If a state of withdrawal is aversive, it stands to reason that an organism will respond for stimuli that alleviate that state. However, whether in withdrawal or not, one cannot truly determine a nonhuman organism’s motive for drug-taking, which complicates the task of discerning the relative roles of positive and negative reinforcement processes in drug-taking during withdrawal. It may be possible to infer something about the nature of stimulant self-administration in a withdrawal state by comparing it to self-administration of another drug with a well-established withdrawal syndrome, such as an opioid. An excellent opportunity arises in a comparison of studies similar in design in which monkeys chose between drug injections and food. In the first study, the potency of heroin as a reinforcer was increased when monkeys were tested in a withdrawal state relative to a pre-dependence baseline (Negus, 2006). These results are consistent with other reports in nonhuman animals indicating that withdrawal from opioids enhances their reinforcing effects (Cooper et al., 2010; Hutcheson et al., 2001), which in turn could facilitate subsequent drug taking. However, in a follow-up study in which monkeys chose between cocaine and food, a period of extended daily access to cocaine that resulted in relatively high daily intake did not alter cocaine’s potency as a reinforcer relative to the pre extended-access baseline (Banks and Negus, 2010). More importantly, when monkeys were tested in a subsequent drug abstinence phase, cocaine’s potency as a reinforcer was comparable to the pre extended-access baseline and the extended-access period, evidence that withdrawal from cocaine does not alter its reinforcing effects. Similar results have been reported in monkeys (Czoty et al., 2006) and in rats (Li et al., 1994) self-administering cocaine before and after an abstinence period under a PR schedule.
4.1 Recommendations
Taken together, the studies cited above suggest that opioid withdrawal, which is qualitatively distinct from stimulant withdrawal, has a much more robust effect on the reinforcing effects of drugs within its class. Based on these empirical findings, we can infer a potential for harm though facilitation of drug abuse resulting from opioid withdrawal, which justifies current approaches for assessing drugs within the opioid class for physical dependence as part of the abuse potential testing process. Although we accept that an affect-oriented stimulant withdrawal exists and can be reasonably captured in animal models, current evidence does not, in our opinion, reveal a sufficient level of harm to justify the need to screen stimulants for physical dependence as a matter of practice in abuse-potential testing.
5. Conclusions
We have reviewed studies examining stimulant and other dopaminergic drugs in assessment of abuse potential with drug self-administration, drug discrimination, and physical dependence. We believe drug self-administration and drug discrimination are valuable in predicting abuse potential of these types of drugs, and rarely do these procedures fail to detect true positives. On the other hand, both procedures yielded many false positives, and we made recommendations for each procedure that may help reduce the number of false positives obtained with these drugs. Finally, screening stimulant drugs for dependence potential presents unique challenges relative to other drug classes, and stimulants may not present sufficient risk to necessitate assessment of physical dependence preclinically. Future reviews with other drug classes would be necessary to determine whether our findings and recommendations with stimulants and other dopaminergic drugs would generalize to drugs from different classes.
Highlights.
Drug self-administration and discrimination are used in abuse-potential testing
These procedures rarely fail to detect true positives but often yield false positives
Our recommendations may help reduce false positives with each procedure
Screening for dependence potential of stimulants preclinically may not be warranted
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Vandewater SA, Creehan KM, et al. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013a;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerosn TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxyprovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacol. 2013b;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Platt DM, Spealman RD. Antagonism of metabotropic glutamate 1 receptors attenuates behavioral effects of cocaine and methamphetamine in squirrel monkeys. J Pharmacol Exp Ther. 2012;343:214–224. doi: 10.1124/jpet.112.196295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acri JB, Carter SR, Alling K, Geter-Douglass B, Dijkstra D, Wikström H, et al. Assessment of cocaine-like discriminative stimulus effects of dopamine D3 receptor ligands. Eur J Pharmacol. 1995;281:R7–R9. doi: 10.1016/0014-2999(95)00411-d. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Appel JB, Weathersby RT, Cunningham KA, Callahan PM, Barrett RL. Transduction mechanisms of drug stimuli. Springer; Berlin Heidelberg: 1988. Stimulus properties of dopaminergic drugs: Comparisons involving selective agonists and antagonists; pp. 44–56. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Baker LE, Riddle EE, Saunders RB, Appel JB. The role of monoamine uptake in the discriminative stimulus effects of cocaine and related compounds. Behav Pharmacol. 1993;4:69–79. [PubMed] [Google Scholar]
- Baker LE, Svensson KA, Garner KJ, Goodwin AK. The dopamine D3 receptor antagonist PNU 99194A fails to block (+)-7-OH-DPAT substitution for D-amphetamine or cocaine. Eur J Pharmacol. 1998;358:101–109. doi: 10.1016/s0014-2999(98)00582-2. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Feeding condition and the relative contribution of different dopamine receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacol. 2013 doi: 10.1007/s00213-013-3271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend. 2003;70:S13–S40. doi: 10.1016/s0376-8716(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. A comparison of d-amphetamine, l-amphetamine, and methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1973;1:67–71. doi: 10.1016/0091-3057(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Effects of extended cocaine access and cocaine withdrawal on choice between cocaine and food in rhesus monkeys. Neuropsychopharmacol. 2010;35:496–504. doi: 10.1038/npp.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Markou A. Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev. 2005;29:675–706. doi: 10.1016/j.neubiorev.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Barrett RL, Appel JB. Effects of stimulation and blockade of dopamine receptor subtypes on the discriminative stimulus properties of cocaine. Psychopharmacol. 1989;99:13–16. doi: 10.1007/BF00634445. [DOI] [PubMed] [Google Scholar]
- Baxter BL, Gluckman MI, Stein L, Scerni RA. Self-injection of apomorphine in the rat: Positive reinforcement by a dopamine receptor stimulant. Pharmacol Biochem Behav. 1974;2:387–391. doi: 10.1016/0091-3057(74)90085-9. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci. 1990;47:1501–1510. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Becker RE. Characterization of the bupropion cue in the rat: lack of evidence for a dopaminergic mechanism. Psychopharmacol. 1985;85:173–177. doi: 10.1007/BF00428409. [DOI] [PubMed] [Google Scholar]
- Bondareva TS, Young R, Glennon RA. Central stimulants as discriminative stimuli: Asymmetric generalization between (−)ephedrine and S(+)methamphetamine. Pharmacol Biochem Behav. 2002;74:157–162. doi: 10.1016/s0091-3057(02)00963-2. [DOI] [PubMed] [Google Scholar]
- Botly LCP, Burton CL, Rizos Z, Fletcher PJ. Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacol. 2008;199:55–66. doi: 10.1007/s00213-008-1093-z. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Sci. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different scheduels in the rat. Behav Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D1-like and D2-like agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- Calderon SV, Klein M. A regulatory perspective on the abuse potential evaluation of novel stimulant drugs in the United States. Neuropharmacol. 2014 doi: 10.1016/j.neuropharm.2014.04.001. this issue. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Beveridge TJR, Jones SR, Porrino LJ. Withdrawal from extended-access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur J Neurosci. 2013 doi: 10.1111/ejn.12381. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan PM, Appel JB, Cunningham KA. Dopamine D1 and D2 mediation of the discriminative stimulus properties of d-amphetamine and cocaine. Psychopharmacol. 1991;103:50–55. doi: 10.1007/BF02244073. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. Discriminative stimulus properties of cocaine in relation to dopamine D2 receptor function in Rats. J Pharmacol Exp Ther. 1993;266:585–592. [PubMed] [Google Scholar]
- Callahan PM, de La Garza R, II, Cunningham KA. Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav. 1997;57:601–607. doi: 10.1016/s0091-3057(96)00434-0. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Kumar NN, Wong-Mckinstry E, Ayyagari S, Puri N, Jackson FK, Shashikumar S. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28:549–585. doi: 10.1016/j.ccc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105S:S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian AJ, Goodwin AK, Baker LE. Antagonism of the discriminative stimulus effects of (+)-7-OH-DPAT by remoxipride but not PNU-99194A. Pharmacol Biochem Behav. 2001;68:371–377. doi: 10.1016/s0091-3057(00)00470-6. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJ, Janssen PA. Discriminative stimulus properties of cocaine: Neuropharmacological characteristics as derived from stimulus generalization experiments. Pharmacol Biochem Behav. 1979;4:535–546. doi: 10.1016/0091-3057(79)90229-6. [DOI] [PubMed] [Google Scholar]
- Cook CD, Carroll FI, Beardsley PM. RTI 113, a 3-phenyltropane analog, produces long-lasting cocaine-like discriminative stimulus effects in rats and squirrel monkeys. Eur J Pharmacol. 2002;442:93–98. doi: 10.1016/s0014-2999(02)01501-7. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Shi YG, Woods JH. Reinforcer-dependent enhancement of operant responding in opioid-withdrawn rats. Psychopharmacol. 2010;212:369–378. doi: 10.1007/s00213-010-1966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Zuch CL, Fox RAV. Comparison of the stimulus properties of a pre- vs. a putative postsynaptic dose of Quinpirole. Pharmacol Biochem Behav. 1996;55:423–432. doi: 10.1016/s0091-3057(96)00113-x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiat. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM. Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacol. 1991;104:177–180. doi: 10.1007/BF02244175. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, Bergman J. Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacol. 2004;175:170–178. doi: 10.1007/s00213-004-1798-6. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Influence of abstinence and conditions of cocaine access on the reinforcing strength of cocaine in nonhuman primates. Drug Alcohol Depend. 2006;85:213–220. doi: 10.1016/j.drugalcdep.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Carroll FI, Nader MA. Lower reinforcing strength of the phenyltropane cocaine analogs RTI-336 and RTI-177 compared to cocaine in nonhuman primates. Pharmacol Biochem Behav. 2010;96:274–278. doi: 10.1016/j.pbb.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello GD, Stolerman IP. Comparison of the discriminative stimulus properties of cocaine and amphetamine in rats. Br J Pharmacol. 1977;61:415–422. doi: 10.1111/j.1476-5381.1977.tb08434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Garza R, Johanson CE. The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav. 1983;19:145–148. doi: 10.1016/0091-3057(83)90323-4. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudery M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naïve and cocaine-experienced rats. Psychopharmacol. 2002;161:387–389. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, Desai R, Bergman J. Monoaminergic psychomotor stimulants: Discriminative stimulus effects and dopamine efflux. J Pharmacol Exp Ther. 2010;333:834–843. doi: 10.1124/jpet.110.165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [3H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568:112–123. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Doty P, Johnson AR, Picker MJ. Discriminative stimulus properties of cocaine, alone and in combination with buprenorphine, morphine and naltrexone. Drug Alcohol Depend. 1992;30:227–234. doi: 10.1016/0376-8716(92)90056-i. [DOI] [PubMed] [Google Scholar]
- Eddy NB, Halbach H, Isbell H, Seevers MH. Drug dependence: Its significance and characteristics. B World Health Organ. 1965;32:721–733. [PMC free article] [PubMed] [Google Scholar]
- Engeln M, Ahmed SH, Vouillac C, Tison F, Bezard E, Fernagut PO. Reinforcing properties of pramipexole in normal and parkinsonian rats. Neurobiol Dis. 2013;49:79–86. doi: 10.1016/j.nbd.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency: Evaluation of Medicines for Human Use (EMEA) Guideline on the non-clinical investigation of the dependence potential of medicinal products 2006 [Google Scholar]
- Filip M, Przegaliński E. The role of dopamine receptor subtypes in the discriminative stimulus effects of amphetamine and cocaine in rats. Pol J Pharmacol. 1997;49:21–30. [PubMed] [Google Scholar]
- Ford RD, Balster RL. Reinforcing properties of intravenous procaine in rhesus monkeys. Pharmacol Biochem Behav. 1977;6:289–296. doi: 10.1016/0091-3057(77)90027-2. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Heal DJ, McCreary AC, Woolverton WL. Assessment of ropinirole as a reinforcer in rhesus monkeys. Drug Alcohol Depend. 2012;125:173–177. doi: 10.1016/j.drugalcdep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Frisch C, Hasenohrl RJ, Mattern CM, Hacker CM, Huston JP. Blockade of lithium chloride-induced conditioned place aversion as a test for antiemetic agents: comparison of metoclopramide with combined extracts of Zingiver officinale and Ginkgo biloga. Pharmacol Biochem Behav. 1995;52:321–327. doi: 10.1016/0091-3057(95)00073-6. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA)/Center for Drug Evaluation and Research (CDER) Draft guidance for industry: Assessment of abuse potential of drugs. Silver Spring, MD: US Department of Health and Human Service; 2010. Retrieved from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM198650.pdf on August 29, 2013. [Google Scholar]
- Garner KJ, Baker LE. Analysis of D2 and D3 receptor-selective ligands in rats trained to discriminate cocaine from saline. Pharmacol Biochem Behav. 1999;64:373–378. doi: 10.1016/s0091-3057(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Gasior M, Bergman J, Kallman MJ, Paronis CP. Evaluation of the reinforcing effects of monoamine reuptake inhibitors under a concurrent schedule of food and i.v. drug delivery in rhesus monkeys. Neuropsychopharmacol. 2005;30:758–764. doi: 10.1038/sj.npp.1300593. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R, Martin BR, Dal Cason TA. Methcathinone (“Cat”): An enantiomeric potency comparison. Pharmacol Biochem Behav. 1995;50:601–606. doi: 10.1016/0091-3057(94)00348-3. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacol. 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Yracheta JM, Bell SM, Lane KE. Intravenous self-administration of cathinone by rats. Behav Pharmacol. 1996;7:526–531. [PubMed] [Google Scholar]
- Götestam KG, Andersson BE. Self-administration of amphetamine analogues in rats. Pharmacol Biochem Behav. 1975;3:229–233. doi: 10.1016/0091-3057(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Graham JH, Balster RL. Cocaine-like discriminative stimulus effects of procaine, dimethocaine and lidocaine in rats. Psychopharmacol. 1993;110:287–294. doi: 10.1007/BF02251283. [DOI] [PubMed] [Google Scholar]
- Grech DM, Spealman RD, Bergman J. Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacol. 1996;125:97–104. doi: 10.1007/BF02249407. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Snell JD. Progressive-ratio performance maintained by drug infusions: Comparison of cocaine, diethylpropion, chlorphentermine, and fenfluramine. Psychopharmacol. 1978;56:5–13. doi: 10.1007/BF00571401. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Winger G, Brady JV, Snell JD. Comparison of behavior maintained by infusions of eight phenylethylamines in baboons. Psychopharmacol. 1976;50:251–258. doi: 10.1007/BF00426841. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Findley JD, Brady JV, Dolan-Gutcher K, Robinson WW. Comparison of progressive-ratio performance maintained by cocaine, methylphenidate and secobarbital. Psychopharmacologia. 1975;43:81–83. doi: 10.1007/BF00437619. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-methylmethacathinone (mephedrone): Neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerbeck DM, Mitchell CL. The reinforcing properties of procaine and d-amphetamine compared in rhesus monkeys. J Pharmacol Exp Ther. 1978;204:558–569. [PubMed] [Google Scholar]
- Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D. A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to d-amfetamine, methylphenidate and modafinil. Neuropharmacol. 2013;73:348–358. doi: 10.1016/j.neuropharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Differential interaction of GBR 12909, a dopamine uptake inhibitor, with cocaine and methamphetamine in rats discriminating cocaine. Psychopharmacol. 2001;155:180–186. doi: 10.1007/s002130100684. [DOI] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol. 2013;24:410–436. doi: 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. J Pharmacol Exp Ther. 1991;258:178–185. [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Kuhar MJ, Carrol FI. Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor RTI-113 in the squirrel monkey. J Pharmacol Exp Ther. 2000;292:521–529. [PubMed] [Google Scholar]
- Huang D, Wilson MC. Comparitive discriminative stimulus properties of dl-cathinone, d-amphetamine, and cocaine in rats. Pharmacol Biochem Behav. 1986;24:205–210. doi: 10.1016/0091-3057(86)90339-4. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56:377–393. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat. Neurosci. 2001;4:943–947. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Johanson CE. The reinforcing properties of procaine, chloroprocaine and proparacaine in rhesus monkeys. Psychopharmacol. 1980;67:189–194. doi: 10.1007/BF00431976. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: Cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975;193:676–688. [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A comparison of the behavioral effects of l- and dl- cathinone and d-amphetamine. J Pharmacol Exp Ther. 1981;219:355–362. [PubMed] [Google Scholar]
- Jones CN, Howard JL, McBennett ST. Stimulus properties of antidepressants in the rat. Pschopharmacol. 1980;67:111–118. doi: 10.1007/BF00431964. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Woolverton WL. A pharmacological analysis of the discriminative stimulus properties of d-amphetamine in rhesus monkeys. J Pharmacol Exp Ther. 1989;248:938–946. [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Hughes JR, Higgins ST, Smith BJ. Drug discrimination by humans compared to nonhumans: Current status and future directions. Psychopharmacol. 1993;111:259–270. doi: 10.1007/BF02244940. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Griffiths RR. Intravenous self-injection of methcathinone in the baboon. Pharmacol Biochem Behav. 1994;47:981–983. doi: 10.1016/0091-3057(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Sannerud CA, Griffiths RR. Intravenous self-injection of psychomotor stimulant-anorectics in the baboon. Exp Clin Psychopharmacol. 1996;4:141–150. [Google Scholar]
- Katz JL, Alling KL. Discriminative stimulus effects of putative D3 dopamine receptor agonists in rats. Behav Pharmacol. 2000;11:483–493. doi: 10.1097/00008877-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Terry P. Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacol. 2000;148:90–98. doi: 10.1007/s002130050029. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Takemura M. Neurochemical consequences of dysphoric state during amphetamine withdrawal in animal models: a review. Neurochem Res. 2008;33:204–219. doi: 10.1007/s11064-007-9409-7. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990;254:312–317. [PubMed] [Google Scholar]
- Koetzner L, Riley AL, Glowa JR. Discriminative stimulus effects of dopaminergic agents in rhesus monkeys. Pharmacol Biochem Behav. 1996;54:517–523. doi: 10.1016/0091-3057(95)02282-1. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Greedy B, Husbands SM, Grundt P, Newman AH, Woods JH. The discriminative stimulus effects of dopamine D2- and D3-preferring agonists in rats. Psychopharmacol. 2009;203:317–327. doi: 10.1007/s00213-008-1323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. New Engl J Med. 2003;348:1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- Lago JA, Kosten TR. Stimulant withdrawal. Addict. 1994;89:1477–1481. doi: 10.1111/j.1360-0443.1994.tb03746.x. [DOI] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Nader MA, Mello NK. Effects of the putative dopamine D3 receptor agonist 7-OH-DPAT in rhesus monkeys trained to discriminate cocaine from saline. Psychopharmacol. 1996;124:306–314. doi: 10.1007/BF02247435. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR. Self-administration in baboons and the discriminative stimulus effects in rats of bupropion, nomifensine, diclofensine and imipramine. Psychopharmacol. 1990;102:183–190. doi: 10.1007/BF02245920. [DOI] [PubMed] [Google Scholar]
- Larson EB, Akketli F, Edwards S, Graham DL, Simmons DL, Alibhai IN, Nestler EJ, Self DW. Striatal regulation of ΔFosB, FosB, and sFos during cocaine self-administration and withdrawal. J Neurochem. 2010;115:112–122. doi: 10.1111/j.1471-4159.2010.06907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire GA, Meisch RA. Oral drug self-administration in rhesus monkeys: Interactions between drug amount and fixed-ratio size. J Exp Anal Behav. 1985;44:377–389. doi: 10.1901/jeab.1985.44-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DH, Depoortere RY, Emmett-Oglesby MW. Tolerance to the reinforcing effects of cocaine in a progressive ratio paradigm. Psychopharmacol. 1994;116:326–332. doi: 10.1007/BF02245336. [DOI] [PubMed] [Google Scholar]
- Lile JA, Charnigo RJ, Nader MA. The relative reinforcing strength of methamphetamine and d-amphetamine in monkeys self-administering cocaine. Behav Pharmacol. 2013;24:482–485. doi: 10.1097/FBP.0b013e3283644d44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HML, Nader MA. The reinforcing efficacy of psychostimulants in rhesus monkeys: The role of pharmacokinetics and pharmacodynamic. J Pharmacol Exp Ther. 2003;307:356–366. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Lile JA, Nader MA. The abuse liability and therapeutic potential of drugs evaluated for cocaine addiction as predicted by animal models. Curr Neuropharmacol. 2003;1:21–46. [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Methylphenidate as a reinforcer for rats: Contingent delivery and intake escalation. Exp Clin Psychopharmacol. 2010;18:257–266. doi: 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna ML, Ho BT. The role of dopamine in the discriminative stimulus properties of cocaine. Neuropharmacol. 1980;19:297–303. doi: 10.1016/0028-3908(80)90153-7. [DOI] [PubMed] [Google Scholar]
- Meisch RA. Oral drug self-administration: An overview of laboratory animal studies. Alcohol. 2001;24:117–128. doi: 10.1016/s0741-8329(01)00149-5. [DOI] [PubMed] [Google Scholar]
- Melia KF, Spealman RD. Pharmacological characterization of the discriminative-stimulus effects of GBR 12909. J Pharmacol Exp Ther. 1991;258:626–632. [PubMed] [Google Scholar]
- Middaugh LD, McGroarty KK, Groseclose CH, Adinoff B. Cocaine discrimination: Relationship to local anesthetics and monoamine uptake inhibitors in C57BL/6 mice. Psychopharmacol. 1998;136:44–49. doi: 10.1007/s002130050537. [DOI] [PubMed] [Google Scholar]
- Minami M. Neuronal mechanisms for pain-induced aversion behavioral studies using a conditioned place aversion test. Int Rev Neurobiol. 2009;85:135–144. doi: 10.1016/S0074-7742(09)85010-1. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Clemens KJ, Apetz N, Winstock AR, Ramsey J, Li KM, et al. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: Neural consequences and comparison with methamphetamine. J Psychopharmacol. 2013;27:823–836. doi: 10.1177/0269881113490325. [DOI] [PubMed] [Google Scholar]
- Munzar P, Justinova Z, Kutkat SW, Goldberg SR. Differential involvement of 5-HT2A receptors in the discriminative-stimulus effects of cocaine and methamphetamine. Eur J Pharmacol. 2002;436:75–82. doi: 10.1016/s0014-2999(01)01598-9. [DOI] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance—a review of abuse liability issues. Ann Clin Psychiat. 2004;16:101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA) Prescription drugs: Abuse and addition. Research Report Series. 2001 Revised October 2011. Retrieved from http://www.drugabuse.gov/sites/default/files/rrprescription.pdf on September 3, 2013.
- Negus SS. Choice between heroin and food in non-dependent and heroin-dependent rhesus monkeys: Effects of naloxone, buprenorphine and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Negus SS, Banks ML. Medications development for opioid abuse. Cold Spring Hrb Perspect Med. 2013;1:a012104. doi: 10.1101/cshperspect.a012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: Studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Newman JL, Negus SS, Lozama A, Prisinzano TE, Mello NK. Behavioral evaluation of modafinil and the abuse-related effects of cocaine in rhesus monkeys. Exp Clin Psychopharmacol. 2010;18:395–408. doi: 10.1037/a0021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of rat self-administration model for abuse liability. Neurosci Biobehav Rev. 2011;35:912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Papasava M, Singer G, Papasava CL. Self-administration of phentermine by naïve rats: Effects of body weight and a food delivery schedule. Pharmacol Biochem Behav. 1985;22:1071–1073. doi: 10.1016/0091-3057(85)90318-1. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Fedolak A, Olivier B, Hanania T, Ghavami A, Caldarone B. Psychostimulant-like discriminative stimulus and locomotor sensitization properties of the wake-promoting agent modafinil in rodents. Pharmacol Biochem Behav. 2010;95:449–456. doi: 10.1016/j.pbb.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW. Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther. 1996;277:212–218. [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacol. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: Effects of reinforcement magnitude and fixed ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Piercy M, Koob GF. Characterization of the effects of the partial dopamine agonist terguride on cocaine self-administration in the rat. J Pharmacol Exp Ther. 1998;286:1231–1238. [PubMed] [Google Scholar]
- Ranaldi R, Wang Z, Woolverton WL. Reinforcing effects of D2 dopamine receptor agonists and partial agonists in rhesus monkeys. Drug Alcohol Depend. 2001;64:209–217. doi: 10.1016/s0376-8716(01)00124-7. [DOI] [PubMed] [Google Scholar]
- Rehni AK, Jaggi AS, Singh N. Opioid withdrawal syndrome: emerging concepts and novel therapeutic targets. CNS Neurol Disord-DR. 2013;12:112–125. doi: 10.2174/1871527311312010017. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DCS. Self-administration of GBR 12909 on a fixed ratio and progressive ratio schedule in rats. Psychopharmacol. 1993;111:202–206. doi: 10.1007/BF02245524. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. Self-administration of cocaine analogs by rats. Psychopharmacol. 1999;144:389–397. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Bergman J. Dopamine D1 receptor involvement in the discriminative-stimulus effects of SKF 81297 in squirrel monkeys. J Pharmacol Exp Ther. 1993;267:765–775. [PubMed] [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: Antecedents, methodologies, and perspectives. Psychopharmacol. 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Rush CR, Ator NA, Simpson CA, Bickel WK. Behavioral pharmacology of commonly abused drugs: Concordance between laboratory studies conducted with animals and humans. In: Carroll ME, Overmier JB, editors. Animal research and human health: Advancing human welfare through behavioral science. American Psychological Association; Washington, DC: 2001. pp. 101–114. [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13:105–115. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Glennon RA. Cathinone, cocaine and methamphetamine: Similarity of behavioral effects. Pharmacol Biochem Behav. 1985;22:913–916. doi: 10.1016/0091-3057(85)90295-3. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Discriminative characteristics of high and low cocaine administration: Effect of other psychostimulants. Pharmacol Biochem Behav. 1997;56:457–463. doi: 10.1016/s0091-3057(96)00301-2. [DOI] [PubMed] [Google Scholar]
- Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res. 1992;582:349–352. doi: 10.1016/0006-8993(92)90155-3. [DOI] [PubMed] [Google Scholar]
- Silverman PB, Schultz KA. Comparison of cocaine and procaine discriminative stimuli. Drug Dev Res. 1989;16:427–433. [Google Scholar]
- Sinnott RS, Mach RH, Mach Nader MA. Dopamine D2/D3 receptors modulate cocaine’s reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend. 1999;54:97–110. doi: 10.1016/s0376-8716(98)00162-8. [DOI] [PubMed] [Google Scholar]
- Sorensen G, Sager TN, Petersen JH, Brennum LT, Thogersen P, Bengsten CH, et al. Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacol. 2008;199:37–46. doi: 10.1007/s00213-008-1069-z. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Bergman J, Madras BK, Melia KF. Discriminative stimulus effects of cocaine in squirrel monkeys: Involvement of dopamine receptor subtypes. J Pharmacol Exp Ther. 1991;258:945–953. [PubMed] [Google Scholar]
- Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- Spealman RD. Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1996;278:1128–1137. [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC. Intravenous self-administration of nomifensine in rats: Implications for abuse potential in humans. Sci. 1981;212:1167–1168. doi: 10.1126/science.7195072. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progessive-ratio schedules of drug delivery in the analysis of drug self-administration: A review. Psychopharmacol. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Rice KC, Glowa JR. A comparison of cocaine, GBR 12909, and phentermine self-administration by rhesus monkeys on a progressive-ratio schedule. Drug Alcohol Depend. 2001;62:41–47. doi: 10.1016/s0376-8716(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Childs E, Ford MW, Grant KA. Role of training dose in drug discrimination: A review. Behav Pharmacol. 2011;22:415–429. doi: 10.1097/FBP.0b013e328349ab37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, D’Mello GD. Role of training conditions in discrimination of central nervous system stimulants by rats. Psychopharmacol. 1981;73:295–303. doi: 10.1007/BF00422421. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PEA, Rush CR. Reinforcing effects of modafinil: Influence of dose and behavioral demands following drug administration. Psychopharmacol. 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Sufka KJ. Conditioned place preference paradigm: A novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58:355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Fukuoka Y, Mori T, Miyatake M, Narita M. Behavioral sensitization to the discriminative stimulus effects of methamphetamine in rats. Eur J Pharmacol. 2004;498:157–161. doi: 10.1016/j.ejphar.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Tang AH, Franklin SR. Discriminative stimulus effects of a low dose of apomorphine in the rat. Psychopharmacol. 1987;91:61–66. doi: 10.1007/BF00690928. [DOI] [PubMed] [Google Scholar]
- Tella SR, Goldberg SR. Subtle differences in the discriminative stimulus effects of cocaine and GBR-12909. Prog Neuropsychopharmacol Biol Psychiat. 2001;25:639–656. doi: 10.1016/s0278-5846(00)00180-9. [DOI] [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Andrews AM, Goldberg SR, Cadet JL. Differential reinforcing effects of cocaine and GBR-12909: Biochemical evidence for divergent neuroadaptive changes in the mesolimbic dopaminergic system. J Neurosci. 1996;16:7416–7427. doi: 10.1523/JNEUROSCI.16-23-07416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Cadet JL. Differential regulation of dopamine transporter after chronic self-administration of bupropion and nomifensine. J Pharmacol Exp Ther. 1997;281:508–513. [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–1048. [PubMed] [Google Scholar]
- Terry P, Katz JL. Dopaminergic mediation of the discriminative stimulus effects of bupropion in rats. Psychopharmacol. 1997;134:201–212. doi: 10.1007/s002130050443. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: Agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther. 1998;285:1163–1174. [PubMed] [Google Scholar]
- Title 21 United States Code (USC) Controlled Substances Act, Section 801 (21USC801). Congressional findings and declarations: Controlled Substances. Retrieved from http://www.deadiversion.usdoj.gov/21cfr/21usc/801.htm on August 29, 2013.
- Title 21 United States Code (USC) Controlled Substances Act, Section 812 (21USC812). Schedules of controlled substances. Retrieved from http://www.deadiversion.usdoj.gov/21cfr/21usc/812.htm on August 29, 2013.
- Ukai M, Mitsunaga H. Involvement of dopamine D3 and D4 receptors in the discriminative stimulus properties of cocaine in the rat. Methods Find Exp Clin Pharmacol. 2005;27:645–649. doi: 10.1358/mf.2005.27.9.939341. [DOI] [PubMed] [Google Scholar]
- Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, Winsauer PJ. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacol. 2013;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Higgins GA. Investigations into the nature of a 7-OH-DPAT discriminative cue: Comparison with d-amphetamine. Eur J Pharmacol. 1997;339:101–107. doi: 10.1016/s0014-2999(97)01388-5. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alesoff D, Zhu W, Telang F, et al. Effects of modafinil in dopamine transporters in the male human brain: clinical implications. J Am Med Assoc. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010;106:233–236. doi: 10.1016/j.drugalcdep.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CR, et al. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts.” Addict. Res Ther. 2012;S9:1–8. doi: 10.4172/2155-6105.S9-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2013 doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Evaluation of the reinforcing effects of atomoxetine in monkeys: Comparison to methylphenidate and desipramine. Drug Alcohol Depend. 2004;75:271–276. doi: 10.1016/j.drugalcdep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Weed MR, Vanover KE, Woolverton WL. Reinforcing effect of the D1 dopamine agonist SKF 81297 in rhesus monkeys. Psychopharmacol. 1993;113:51–52. doi: 10.1007/BF02244333. [DOI] [PubMed] [Google Scholar]
- Weed MR, Paul IA, Dwoskin LP, Moore SE, Woolverton WL. The relationship between reinforcing effects and in vitro effects of D1 agonists in monkeys. J Pharmacol Exp Ther. 1997;283:29–38. [PubMed] [Google Scholar]
- Weed MR, Woolverton WL. The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1367–1374. [PubMed] [Google Scholar]
- West R, Gossop M. Overview: A comparison of withdrawal symptoms from different drug classes. Addict. 1994;89:1483–1489. doi: 10.1111/j.1360-0443.1994.tb03747.x. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Ordway GA, Woolverton WL. Role of the dopamine transporter and the sodium channel in the cocaine-like discriminative stimulus effects of local anesthetics in rats. Psychopharmacol. 2001;157:260–268. doi: 10.1007/s002130100796. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: Practical and theoretical concerns. Psychopharmacol. 2000;153:139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Hitomi M, Schuster CR. Psychomotor stimulant self-administration as a function of dosage per injection in the rhesus monkey. Psychopharmacologia. 1971;22:271–281. doi: 10.1007/BF00401789. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Schuster CR. Mazindol self-administration in the rhesus monkey. Pharmacol Biochem Behav. 1976;4:207–210. doi: 10.1016/0091-3057(76)90017-4. [DOI] [PubMed] [Google Scholar]
- Winger G, Woods JH. Comparison of fixed-ratio and progressive-ratio schedules of maintenance of stimulant drug-reinforced responding. Drug Alcohol Depend. 1985;15:123–130. doi: 10.1016/0376-8716(85)90036-5. [DOI] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing rats. Psychopharmacol. 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Nichols DE, Terry P, Katz JL. Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther. 1991;257:706–713. [PubMed] [Google Scholar]
- Wood DM, Emmett-Oglesby MW. Substitution and cross-tolerance profiles of anorectic drugs in rats trained to detect the discriminative stimulus properties of cocaine. Psychopharmacol. 1988;95:364–368. doi: 10.1007/BF00181948. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav. 1987;26:835–839. doi: 10.1016/0091-3057(87)90618-6. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Comparison of the reinforcing efficacy of cocaine and procaine in rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacol. 1995;120:296–302. doi: 10.1007/BF02311177. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther. 1984;230:678–683. [PubMed] [Google Scholar]
- Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. J Exp Anal Behav. 1984;41:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Kamien JB, Goldberg LI. Pharmacological analysis of the apomorphine discriminative stimulus in rhesus monkeys. J Pharmacol Exp Ther. 1987;241:213–217. [PubMed] [Google Scholar]
- Woolverton WL, Ranaldi R. Comparison of the reinforcing efficacy of two dopamine D2-like receptor agonists in rhesus monkeys using a progressive-ratio schedule of reinforcement. Pharmacol Biochem Behav. 2002;72:803–809. doi: 10.1016/s0091-3057(02)00749-9. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Pickens R. Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther. 1973;187:27–33. [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Amphetamine-type reinforcement by dopaminergic agonists in the rat. Psychopharmacol. 1978;58:289–296. doi: 10.1007/BF00427393. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Narita M, Mori T, Miyatake M, Isotani K, Tomiyasu S, et al. Role of dopamine D2 and D3 receptors in mediating the U-50,488H discriminative cue: Comparison with methamphetamine and cocaine. Addict Biol. 2012;17:949–955. doi: 10.1111/j.1369-1600.2010.00257.x. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. Cocaine-stimulus generalization to two new designer drugs: Methcathinone and 4-methylaminorex. Pharmacol Biochem Behav. 1993;45:229–231. doi: 10.1016/0091-3057(93)90110-f. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. Discriminative stimulus effects of S(−)-methcathinone (CAT): A potent stimulant drug of abuse. Psychopharmacol. 1998;140:250–256. doi: 10.1007/s002130050765. [DOI] [PubMed] [Google Scholar]
- Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addict. 2010;105:1809–1818. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]