Abstract
Background
Brain health may be affected by modifiable lifestyle factors; consuming fish and anti-oxidative omega-3 fatty acids may reduce brain structural abnormality risk.
Purpose
To determine whether dietary fish consumption is related to brain structural integrity among cognitively normal elders.
Methods
Data were analyzed from 260 cognitively normal individuals from the Cardiovascular Health Study with information on fish consumption from the National Cancer Institute Food Frequency Questionnaire and brain magnetic resonance imaging (MRI). The relationship between fish consumption data collected in 1989–1990 and brain structural MRI obtained in 1998–1999 was assessed using voxel-based morphometry in multiple regression analyses in 2012. Covariates were age, gender, race, education, white matter lesions, MRI-identified infarcts, waist/hip ratio, and physical activity as assessed by the number of city blocks walked in 1 week. Volumetric changes were further modeled with omega-3 fatty acid estimates to better understand the mechanistic link between fish consumption, brain health, and Alzheimer disease.
Results
Weekly consumption of baked or broiled fish was positively associated with gray matter volumes in the hippocampus, precuneus, posterior cingulate, and orbital frontal cortex even after adjusting for covariates. These results did not change when including omega-3 fatty acid estimates in the analysis.
Conclusions
Dietary consumption of baked or broiled fish is related to larger gray matter volumes independent of omega-3 fatty acid content. These findings suggest that a confluence of lifestyle factors influence brain health, adding to the growing body of evidence that prevention strategies for late-life brain health need to begin decades earlier.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia and is characterized by a progressive decline in multiple cognitive domains.1 There are approximately 24.3 million cases of dementia worldwide, and this number is projected to double every 20 years. This increase will result in over 80 million persons afflicted with dementia by 2040.2 Lifestyle factors are recognized as risk modifiers for the clinical expression of the disorder. We have previously shown and replicated in separate large cohorts the deleterious influence of obesity on brain structure.3–5 In addition, there is an identifiable benefit of physical activity on preserving brain structure and reducing the risk for dementia.6–8 Reducing physical inactivity, obesity, and smoking by 10%–25% may reduce the number of people afflicted by dementia by 1–3 million worldwide and by 184,000–492,000 per year in the U.S.9 Chronic diseases resulting from suboptimal lifestyle habits have been linked to an increased risk of dementia.10–12 Critical to the role of preventative medicine is that these modifiable lifestyle factors and their medical consequences have a cumulative effect over decades.
Other lifestyle factors such as diet are of increasing interest as another means to influence risk of dementia.13–15 For example, the consumption of fish may reduce risk for cognitive decline owing to the presence of anti-oxidative omega-3 fatty acids through their effects on the attenuation of cerebrovascular disease,16 improvement of mood-related disorders,17 and their support of neuronal health.18 However, although some studies have shown improved brain function and gray matter (GM) structure in elderly subjects using long-chain omega-3 fatty acids,19 others have not.16 Higher plasma eicosapentaenoic acid (EPA) levels are associated with a lower rate of atrophy over 4 years in the right mesial temporal lobe.20 Lower red blood cell docosahexaenoic acid (DHA) levels are associated with lower total brain volume and greater white matter lesion (WML) volumes21 that have also been reported in the oldest-old.15 A double-blind trial (26 weeks) of dietary supplementation found increases in grey matter volume and decreases in WMLs in cognitively normal people given fish oil daily.19
Understanding the effects of fish consumption on brain structure is critical for the determination of modifiable factors that can decrease the risk of cognitive deficits and dementia in the elderly population by intervention earlier in life.22 Greater dietary intake of omega-3 fatty acids23 and less intake of meat products24 are associated with increased total grey matter volume; local effects of DHA and EPA are seen in the amygdala and anterior cingulate gyrus.25
The purpose of this study was to investigate the relationship between dietary consumption of fish and brain structure among cognitively normal elderly subjects. The tested hypothesis is that frequency of fish consumption correlates with higher gray GM volumes in the brain areas responsible for memory and cognition in an elderly population. A second tested hypothesis predicts that the omega-3 fatty acid content of the consumed fish is itself related to specific increases in the volume of brain regions frequently targeted by AD in frontal, temporal, and parietal areas.
Methods
Subjects
The analysis modeled the relationship between dietary fish consumption data collected in 1989–1990 and brain structural magnetic resonance imaging (MRI) obtained in 1998–1999 using voxel-based morphometry in multiple regression analyses performed in 2012. The study sample was drawn from participants in the Cardiovascular Health Study Cognition Study (CHS-CS), which is nested within the larger Cardiovascular Health Study (CHS, www.chs-nhlbi.org). The CHS was initiated in 1989–1990 as a prospective, population-based, longitudinal study of risk factors for coronary heart disease and stroke in adults aged 65 years and older. Subjects were recruited if they were older than age 65, ambulatory, and non-institutionalized. The initial sample size was 5,888.
The CHS-CS, initiated in 1998–1999, was designed to identify subjects who had developed dementia or mild cognitive impairment (MCI) since 1992–1994.26, 27 The sample was limited to the 3,608 participants who had a brain MRI between 1991 and 1994. The classification in 1998–1999 of normal cognition, MCI, or dementia was determined by an adjudication committee upon review of clinical data. A cross-sectional analysis was conducted, which that analyzed MRI data from 260 CHS-CS participants from the Pittsburgh site who met the following inclusion criteria: (1) diagnosed as cognitively normal subjects in 1992 and 1998; (2) had a high-resolution MRI scan taken in 1998–1999 that met quality control standards; and (3) had data on dietary intake. People with AD and MCI were excluded from this analysis, as these conditions not only confound analysis of GM volume but also associated memory loss could bias self-report of fish consumption.
Assessment of Fish Consumption in the Cardiovascular Health Study
Dietary intake was assessed in 1989–1990 using a picture-sort version of the National Cancer Institute food frequency questionnaire (FFQ) (riskfactor.cancer.gov/diet/usualintakes/method.html). In this standardized questionnaire, participants were asked to indicate how often, on average, they had consumed various specific foods during the past year. This included tuna fish, other broiled or baked fish, and fried fish or fish sandwiches. This study specifically focused on consumption of broiled or baked fish because these preparations of fish have been previously related to a higher level of dietary omega-3 fatty acids in the CHS.28 These measures have been previously used to estimate total omega-3 fatty acid content at around study baseline in 1992–1993 that combines plasma phospholipid EPA (20:5n-3) and DHA (22:6n-3).29–31 The correlation between daily to weekly fish consumption and calculated quartile levels of plasma omega-3 fatty acid in this population is high (r =0.80, p<0.001).
Brain Imaging
Brain MRI scans were completed using a 1.5-tesla General Electric scanner, as detailed elsewhere.32 Briefly, a three-dimensional volumetric T1-weighted spoiled gradient recall (SPGR) sequence was also obtained (TE/TR=5/25, flip angle=40°, NEX=1, slice thickness=1.5 mm/0-mm interslice gap), with an in-plane acquisition matrix of 256 × 256 × 124 image elements, 250 × 250–mm field of view, and an in-plane voxel of 0.98 mm3. WMLs were rated using a 10-point standardized CHS visual grading system, ranging from 0 (normal) to 9 (mostly abnormal).33 WML ratings were based on the total extent of the subcortical and periventricular hyperintensities on either axial T2-weighted or spin-density images. MRI-identified infarcts were characterized as lesions >3 mm on proton density–weighted scans.
Voxel-Level Statistical Analysis
To summarize the analyses, fish intake assessed in 1989–1990 was correlated with MRI-derived voxel-level GM volume in 1998–1999. All MRI scans in this study were processed using previously described methods.34, 35 Whole brain voxel level–based statistical models used multiple regression to relate daily to weekly baked or broiled fish consumption to GM volume. This model consisted of fish consumption as an independent variable and GM volume as the dependent variable. Covariates in the analysis were total intracranial volume as a metric of head size, age, gender, race, education, WMLs, type two diabetes mellitus, MRI-identified infarcts, waist/hip ratio, and physical activity as assessed by the number of city blocks walked in 1 week. Fish consumption was defined with each participant classified as either eating baked/broiled fish one to four times per week, or not. The analysis also involved extracting the largest statistically significant clusters and eigenvariates for further analysis using the volume of interest tool in SPM5 (Wellcome Department of Imaging Neuroscience, University College, London) in an approach similar to previously described methods.35
Results
People who consumed baked or broiled fish at least one to four times per week had more years of education and higher scores on the Modified Mini-Mental State Examination (3MSE) than those who ate fish less frequently (Table 1). This relationship between fish consumption and 3MSE score was not statistically significant after controlling for level of education (rp =0.1, df=256, p=0.11). Fish consumption was unrelated to age (r= −0.02), gender (r=0.09), race (minority, r=0.02), infarcts (r= −0.03), WMLs (r= −0.06), physical activity (r =0.07), BMI (r= −0.06), or waist/hip ratio (r =0.02). Fish consumption was associated with level of education (r =0.21, p<0.001).
Table 1.
Subject demographics
| Variable of interest | Daily/weekly fish consumption (n=163) | Infrequent fish consumption (n=97) | t-test/chi-square | p-value |
|---|---|---|---|---|
| Age | 78.3 (3.54) | 78.4 (3.31) | 0.37 | 0.71 |
| Gender (percent (n) male) | 44 (71) | 34 (33) | 2.31 | 0.15 |
| Education (percent (n) >grade 12) | 71 (116) | 58 (56) | 4.9 | 0.03 |
| Race (percent (n) white) | 95 (155) | 96 (93) | 0.90 | 1.0 |
| APOE4 allelea | 76 (116) | 75 (70) | 0.04 | 0.88 |
| Hypertensiona | 53 (87) | 60 (58) | 1.22 | 0.30 |
| WMG 3+a | 65 (105) | 59 (57) | 0.83 | 0.43 |
| MRI infarctsa | 75 (122) | 72 (70) | 0.23 | 0.37 |
| 3MSE | 96.7 (3.67) | 95.5 (5.41) | −2.21 | 0.03 |
Percent (n) present.
3MSE, Modified Mini-Mental State examination; APOE4, apolipoprotein E4; MRI, magnetic resonance imaging; WMG, white matter grade
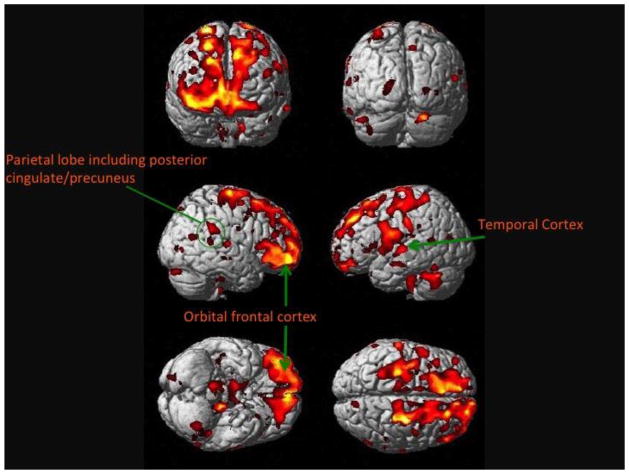
Weekly consumption of baked or broiled fish was positively associated with GM volumes in the hippocampus, precuneus, posterior cingulate, and orbital frontal cortex (Table 2). Figure 1 shows the main effects projected onto the standard single subject Montreal Neurological Institute (MNI) template.36 Brighter colors represent a larger positive effect size of weekly fish consumption on GM volume. As shown in the Figure 1, these neocortical regions include the frontal cortex bilaterally (right greater than left) extending from the inferior regions to the dorsolateral areas. All of the Voxel-Based Morphometry (VBM) analyses used a statistical correction for multiple comparisons of a false discovery rate (p=0.05).37
Table 2.
Standardized regression coefficients from analyses of brain region of interest volumes related to fish consumption
| Orbital frontal cortexa | Posterior cingulate/hippocampusb | |
|---|---|---|
| Gender (female) | −0.26* | −0.04 |
| Age | −0.10 | −0.09 |
| Education (<high school) | −0.13* | −0.11 |
| White matter grade (±3) | −0.24* | −0.16* |
| Blocks walked per week | 0.002 | 0.02 |
| BMI | −0.03 | −0.02 |
| Hypertension (present) | −0.10 | −0.13* |
| Fish consumption (weekly) | 0.29* | 0.21* |
| Plasma omega-3 | −0.08 | 0.05 |
Note: Boldface indicates statistical significance.
Cluster 1, F (7, 248)=14.8, p<0.001.
Cluster 2, F (7, 248)=5.41, p<0.001.
Figure 1. Main effect of weekly to daily baked or broiled fish consumption on gray matter.
This figure shows the main effect of daily to weekly fish consumption on brain structure in 260 cognitively normal individuals in the Cardiovascular Health Study. People who eat fish at least one to four times a week have larger gray matter volumes, as shown in the shaded regions, compared to those who do not eat fish on at least a weekly basis. Hotter colors denote a stronger main effect, seen here in the frontal lobes.
In Figure 2, the main effect of fish consumption is projected onto the Standard Single Subject MNI template, in radiologic convention. The gradient from green to yellow to red color illustrates the range of correlation coefficients (lower to higher) between fish consumption (at least weekly versus less than weekly) and GM volume. The specific region highlighted at the crosshairs is the right anterior hippocampus, which is critical for normal memory function and affected early in neurodegenerative processes such as AD.38–41
Figure 2. Main effect of weekly to daily consumption of baked or broiled fish on the hippocampus.
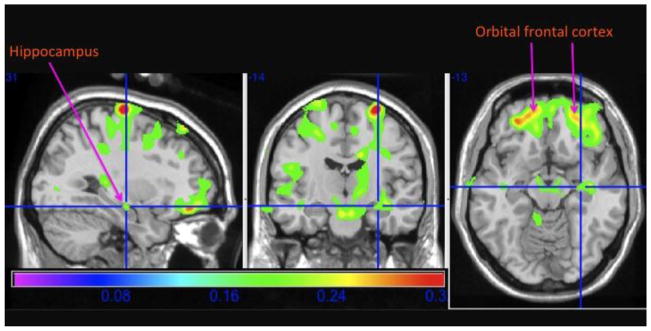
This figure shows the main effect of daily to weekly fish consumption on brain structure in cardinal sections projected onto the standard single subject Montreal Neurological Institute template brain. People who eat fish at least one to four times a week have larger gray matter volumes, as shown in the green, yellow, and red regions, compared to those who do not eat fish on at least a weekly basis. This includes the right hippocampus, as labeled and specified in the crosshairs.
There were two large, significant regions related to fish consumption: the first was the right frontal lobe including the right orbital frontal cortex with extension into the right anterior cingulate gyrus (Cluster 1). The second cluster included the right and left posterior cingulate gyrus and bilateral (right greater than left) hippocampus (Cluster 2). The analysis extracted eigenvariates for each of these clusters from SPM and then regressed these values on age, education, race, gender, hypertension, diabetes, WMLs, fish consumption, and plasma levels of omega-3s. The results of these analyses are shown in Table 2. The critical finding was that although these brain areas were affected by the amount of dietary fish intake, there was no significant association with plasma levels of omega-3 fatty acids. All statistically significant clusters are described in Table 3.
Table 3.
Statistically significant clusters from the main effect of regular fish consumption on gray matter volume
| Cluster locations/size | Montreal Neurological Institute coordinates/cluster size | t-score |
|---|---|---|
| Orbital frontal cortex | −27, 46, −14/164015 | 5.42 |
| Hippocampus/posterior cingulate gyrus | 7, −34, 16/3559 | 4.5 |
| Superior temporal cortex | −41, −14, −13/17611 | 4.4 |
Unstandardized regression coefficients were then utilized to estimate the effect of fish consumption on regional volumes at the mean of each of the covariates. Consumption of fish at least once per week resulted in a 4.3% increase in the volume of Cluster 1 (orbital frontal and anterior cingulate regions), and a 14% increase in the size of Cluster 2 (mesial temporal lobe).
Discussion
There are two main findings to this study. First, consuming baked or broiled fish at least weekly is related to larger GM volumes (4.3% and 14%, respectively) in areas of the brain responsible for memory and cognition in cognitively normal elderly individuals. Second, although the volumes in these brain regions were significantly related to fish consumption, they were not significantly associated with plasma omega-3 fatty acids. These findings suggest additional evidence that it is lifestyle factors—in this case, dietary intake of fish—and not necessarily the presumed biological factors that can affect the structural integrity of the brain.
These data and others42–44 are of particular relevance to clinicians who care for individuals in the 40–60-year age range and for the development of rational preventative interventions. The medical consequences of the so-called “Western” lifestyle, including hypertension and diabetes, are increasingly recognized as independent risk factors for cognitive impairment and dementia; interventions to modify this lifestyle have been proposed as disease-modifying approaches.9, 45 Previous work has shown how obesity (i.e., BMI >30) is related to deficits in brain structure in both cognitively normal older individuals3 and persons with MCI or AD.4 In fact, the hippocampus, a brain region critically important for normal memory function46 that is affected early in AD,47 is exquisitely sensitive to the deleterious effects of obesity.48 By contrast, physical activity can increase brain volumes and reduce AD risk.6 Thus, based on this range of data, a key implication is if these lifestyle factors can be modified during the middle-age years of life in the 40s and 50s (or prevented altogether), this will reduce burden on brain structure and function, increase brain and cognitive reserve,49, 50 and decrease incidence of clinical dementia.
This study showed that the effects of fish consumption on brain structure were independent of any effects of omega-3 fatty acids. This is consistent with previous studies that showed that omega-3 fatty acid supplementation has little effect on the prevention of dementia51 and does not improve cognition in AD patients.52 However, it may also be the case that other components contained in fish such as selenium, or in the diet of individuals who consume fish, can improve brain structure and decrease the risk for AD.53 One recent systematic review identified 11 observational studies of which nine demonstrated a relationship between a Mediterranean diet and improved cognition and lower risk of developing AD.54 It may also be the case that eating baked or broiled fish with some regularity is a marker of a healthier lifestyle, of which consuming fish is only one part. The fact that the CHS participants who consumed fish were more educated than those who were not may also indicate that this study could be measuring a general lifestyle effect, and not necessarily a diet-specific effect. Indeed, the Three City Study reported that fish consumption was not only associated with education, but also with higher income.55
This is not to suggest that omega-3 fatty acids are not relevant to brain health; in fact, a systematic review identified 13 animal studies that demonstrate a positive influence on brain structure and reduced AD pathology.56 That same review also identified 14 studies demonstrating the benefits of long-chain omega-3 fatty acids in slowing cognitive decline. Previous work also found a dissociation between blood omega-3 levels and brain DHA levels.57 Additionally, a postmortem study found that DHA levels decline significantly in the aging orbitofrontal cortex.58 By contrast, a diet high in saturated fats (which may also indicate a less healthy overall lifestyle) may increase AD risk.59 Also, the level of omega-3 fatty acids is inversely related to BMI.60
There are several mechanisms through which consumption of fish could benefit brain structure independent of other associated lifestyle factors. DHA and EPA can affect synaptic function and cognitive abilities by enhancing plasma membrane fluidity at the synapse.18 This has the effect of optimizing membrane permeability to cations and can potentiate the action of neurotrophic factors such as brain-derived neurotrophic factor (BDNF).61 BDNF in turn can promote neuronal growth and metabolism in such regions as the hippocampus.62 By contrast, animals fed diets deficient in omega-3 fatty acids have increased AD pathology caused by dysregulation of insulin-mediated amyloid clearance pathways.63 Fish consumption may also reduce dementia risk by reducing the burden of vascular risk factors, as manifested by infarcts and small vessel ischemic disease in the brain, as well as by reducing cellular inflammation.64
The main strengths of this study are its well-characterized population with extensive data on demographic, clinical, and lifestyle factors including dietary choices such as type and preparation of fish. This information, combined with quantitative analysis of volumetric MRI allows these findings to lend insight into how lifestyle choices may influence brain structure. The cross-sectional nature of this work, however, cannot establish causal relationships. Additionally, this study did not control for medications that may be associated with brain atrophy. These results should therefore stimulate a more extensive analysis of the genetic, epigenetic, behavioral, environmental, and dietary factors in the pathogenesis of cognitive decline. Future studies should examine, to the greatest extent possible, differences in diet among individuals as they change from a state of normal cognition to mild impairment and subsequently dementia.
Lifestyle factors affect a variety of body systems, and can have long-term effects on the health of the citizenry. The long-term impact of these factors on the brain, and on vascular systems that affect brain health, critically affects brain reserve capacity.65, 66 By reducing these risk factors, the long-term improvement in brain health will likely result in a delay in the clinical expression of any age-related neurodegenerative condition. The challenge is to implement prevention strategies decades prior to the peak time of dementia incidence when there are few, if any, signs of brain structural or functional abnormalities.
Acknowledgments
The research reported in this article was supported in part by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant no. HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through AG-023629, AG-15928, AG-20098, AG-027002, AG05133, and AG-027058 from the National Institute on Aging. A full list of principal Cardiovascular Health Study investigators and institutions can be found at chs-nhlbi.org/pi.htm. MR was supported by a grant from Fundación Caja Madrid (Spain).
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wade JP, Mirsen TR, Hachinski VC, Fisman M, Lau C, Merskey H. The clinical diagnosis of Alzheimer’s disease. Archives of neurology. 1987 Jan;44(1):24–9. doi: 10.1001/archneur.1987.00520130016010. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- 2.Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–45. doi: 10.2147/CLEP.S30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010 Mar;31(3):353–64. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging. 2010 Aug;31(8):1326–39. doi: 10.1016/j.neurobiolaging.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, et al. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci USA. 2010 May 4;107(18):8404–9. doi: 10.1073/pnas.0910878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2010 Jan 31; doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011 Feb 15;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res. 2012 Nov;43(8):615–21. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011 Sep;10(9):819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997 Feb 15;145(4):301–8. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 11.Forette F, Boller F. Hypertension and the risk of dementia in the elderly. Am J Med. 1991;90(3A):14S–9S. doi: 10.1016/0002-9343(91)90430-6. [DOI] [PubMed] [Google Scholar]
- 12.Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31(3):780–6. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- 13.Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Archives of neurology. 2007 Jan;64(1):86–92. doi: 10.1001/archneur.64.1.86. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 14.Panza F, Capurso C, D’Introno A, Colacicco AM, Del Parigi A, Gagliardi G, et al. Mediterranean diet, mild cognitive impairment, and Alzheimer’s disease. Exp Gerontol. 2007 Jan-Feb;42(1–2):6–7. doi: 10.1016/j.exger.2006.09.011. author reply 8–9. [DOI] [PubMed] [Google Scholar]
- 15.Bowman GL, Silbert LC, Howieson D, Dodge HH, Traber MG, Frei B, et al. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology. 2012 Jan 24;78(4):241–9. doi: 10.1212/WNL.0b013e3182436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virtanen JK, Siscovick DS, Lemaitre RN, Longstreth WT, Spiegelman D, Rimm EB, et al. Circulating omega-3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: the cardiovascular health study. J Am Heart Assoc. 2013 Oct;2(5):e000305. doi: 10.1161/JAHA.113.000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, et al. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. 2012 Jun;107(11):1682–93. doi: 10.1017/S0007114511004788. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008 Jul;9(7):568–78. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witte AV, Kerti L, Hermannstadter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, et al. Long-Chain Omega-3 Fatty Acids Improve Brain Function and Structure in Older Adults. Cereb Cortex. 2013 Jun 24; doi: 10.1093/cercor/bht163. [DOI] [PubMed] [Google Scholar]
- 20.Samieri C, Maillard P, Crivello F, Proust-Lima C, Peuchant E, Helmer C, et al. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology. 2012 Aug 14;79(7):642–50. doi: 10.1212/WNL.0b013e318264e394. [DOI] [PubMed] [Google Scholar]
- 21.Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, et al. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012 Feb 28;78(9):658–64. doi: 10.1212/WNL.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez OL, Becker JT, Kuller LH. Patterns of compensation and vulnerability in normal subjects at risk of Alzheimer’s disease. J Alzheimers Dis. 2013;33(1S):1:S427–S438. doi: 10.3233/JAD-2012-129015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titova OE, Sjogren P, Brooks SJ, Kullberg J, Ax E, Kilander L, et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordr) 2013 Aug;35(4):1495–505. doi: 10.1007/s11357-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Titova OE, Ax E, Brooks SJ, Sjogren P, Cederholm T, Kilander L, et al. Mediterranean diet habits in older individuals: Associations with cognitive functioning and brain volumes. Exp Gerontol. 2013 Dec;48(12):1443–8. doi: 10.1016/j.exger.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007 Jun 29;421(3):209–12. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick AL, Kuller LH, Ives D, Lopez OL, Jagust W, Breitner J, et al. Incidence and prevalence of dementia in the cardiovascular health study. J Am Geriatr Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 28.Virtanen JK, Siscovick DS, Longstreth WT, Jr, Kuller LH, Mozaffarian D. Fish consumption and risk of subclinical brain abnormalities on MRI in older adults. Neurology. 2008 Aug 5;71(6):439–46. doi: 10.1212/01.wnl.0000324414.12665.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the cardiovascular health study. Circulation. 2003 Mar 18;107(10):1372–7. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Longstreth WT, Jr, Lemaitre RN, Manolio TA, Kuller LH, Burke GL, et al. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005 Jan 24;165(2):200–6. doi: 10.1001/archinte.165.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, et al. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011 Aug 2;155(3):160–70. doi: 10.1059/0003-4819-155-3-201108020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryan RN, Manolio TA, Scertz LD, Jungreis C, Poirier VC, Elster AD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The cardiovascular health study. Am J Neuroradiol. 1994;15(9):1625–33. [PMC free article] [PubMed] [Google Scholar]
- 33.Manolio TA, Burke GL, O’Leary DH, Evans G, Beauchamp N, Knepper L, et al. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults: the cardiovascular health study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol. 1999 Feb;19(2):356–65. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 34.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009 Dec 1;73(22):1899–905. doi: 10.1212/WNL.0b013e3181c3f293. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010 Oct 19;75(16):1415–22. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998 Mar-Apr;22(2):324–33. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 37.Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 38.Olton DS, Becker JT, Handelman GE. Hippocampus, space, and memory. Behavioral and Brain Sciences. 1979;2:313–65. [Google Scholar]
- 39.Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cerebral cortex. 1996 Nov-Dec;6(6):823–9. doi: 10.1093/cercor/6.6.823. Clinical Trial Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- 40.Maguire EA, Frackowiak RS, Frith CD. Recalling routes around london: activation of the right hippocampus in taxi drivers. The Journal of neuroscience: the official journal of the Society for Neuroscience. [Research Support, Non-U.S. Gov’t] 1997 Sep 15;17(18):7103–10. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geula C. Abnormalities of neural circuitry in Alzheimer’s disease: hippocampus and cortical cholinergic innervation. Neurology. 1998;51(1S):S18–S29. doi: 10.1212/wnl.51.1_suppl_1.s18. [DOI] [PubMed] [Google Scholar]
- 42.Polidori MC, Nelles G, Pientka L. Prevention of dementia: focus on lifestyle. International journal of Alzheimer’s disease. 2010 doi: 10.4061/2010/393579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukaetova-Ladinska EB, Purshouse K, Andrade J, Krishnan M, Jagger C, Kalaria RN. Can healthy lifestyle modify risk factors for dementia? Findings from a pilot community-based survey in Chennai (India) and Newcastle (UK) Neuroepidemiology. 2012;39(3–4):163–70. doi: 10.1159/000338674. [DOI] [PubMed] [Google Scholar]
- 44.Norton MC, Dew J, Smith H, Fauth E, Piercy KW, Breitner JC, et al. Lifestyle behavior pattern is associated with different levels of risk for incident dementia and Alzheimer’s disease: the Cache County study. J Am Geriatr Soc. 2012 Mar;60(3):405–12. doi: 10.1111/j.1532-5415.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011 Sep;86(9):876–84. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8(3):198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. Research Support, Non-U.S. Gov’t Review. [DOI] [PubMed] [Google Scholar]
- 47.Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiology of aging. 2004 Mar;25(3):303–10. doi: 10.1016/S0197-4580(03)00084-8. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 48.Ho AJ, Raji CA, Saharan P, DeGiorgio A, Madsen SK, Hibar DP, et al. Hippocampal volume is related to body mass index in Alzheimer’s disease. Neuroreport. 2011 Jan 5;22(1):10–4. doi: 10.1097/wnr.0b013e3283412868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stern RA, Silva SG, Chaisson N, Evans DL. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Arch Neurol. 1996;53(2):148–53. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- 50.Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Annals of neurology. 2008 Jan;63(1):112–8. doi: 10.1002/ana.21212. Comparative Study Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- 51.Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012;6:CD005379. doi: 10.1002/14651858.CD005379.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, Baldauf-Wagner S, et al. A randomized placebo-controlled pilot trial of omega-3 Fatty acids and alpha lipoic Acid in Alzheimer’s disease. J Alzheimers Dis. 2014 Jan 1;38(1):111–20. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006 Jun;59(6):912–21. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013 Jul;24(4):479–89. doi: 10.1097/EDE.0b013e3182944410. [DOI] [PubMed] [Google Scholar]
- 55.Barberger-Gateau P, Jutand MA, Letenneur L, Larrieu S, Tavernier B, Berr C. Correlates of regular fish consumption in French elderly community dwellers: data from the three-city study. Eur J Clin Nutr. 2005 Jul;59(7):817–25. doi: 10.1038/sj.ejcn.1602145. [DOI] [PubMed] [Google Scholar]
- 56.Loef M, Walach H. The omega-6/omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J Nutr Gerontol Geriatr. 2013;32(1):1–23. doi: 10.1080/21551197.2012.752335. [DOI] [PubMed] [Google Scholar]
- 57.Cunnane SC, Chouinard-Watkins R, Castellano CA, Barberger-Gateau P. Docosahexaenoic acid homeostasis, brain aging and Alzheimer’s disease: Can we reconcile the evidence? Prostaglandins Leukot Essent Fatty Acids. 2012 Jan;88(1):61–70. doi: 10.1016/j.plefa.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 58.McNamara RK, Liu Y, Jandacek R, Rider T, Tso P. The aging human orbitofrontal cortex: decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot Essent Fatty Acids. 2008 Apr-May;78(4–5):293–304. doi: 10.1016/j.plefa.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Archives of neurology. 2003 Jul;60(7):940–6. doi: 10.1001/archneur.60.7.940. Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- 60.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005 Apr;40(4):343–7. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 61.Feher A, Juhasz A, Rimanoczy A, Kalman J, Janka Z. Association between BDNF Val66Met polymorphism and Alzheimer disease, dementia with Lewy bodies, and Pick disease. Alzheimer Dis Assoc Disord. 2009 Jul-Sep;23(3):224–8. doi: 10.1097/WAD.0b013e318199dd7d. [DOI] [PubMed] [Google Scholar]
- 62.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–61. doi: 10.1016/j.neuroscience.2005.03.026. Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S. [DOI] [PubMed] [Google Scholar]
- 63.Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012 May 1;590(Pt 10):2485–99. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orr SK, Bazinet RP. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs. 2008 Jul;9(7):735–43. [PubMed] [Google Scholar]
- 65.Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence of threshold theory. Neuropsychology. 1991;7(3):273–95. [Google Scholar]
- 66.Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32(3):371–5. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]