Abstract
Glucagon-like peptide 1 (GLP-1) has incretin effects that are well-documented, but the independent role of GLP-1 action in human satiety perception is debated. We hypothesized that blockade of GLP-1 receptors would suppress postprandial satiety and increase voluntary food intake. After an overnight fast, 8 normal weight participants (7 men, BMI 19–24.7 kg/m2, age 19–29 yr) were enrolled in a double-blind, placebo-controlled, randomized crossover study of the GLP-1 antagonist Exendin-[9–39] (Ex-9) to determine if the satiating effects of a meal are dependent on GLP-1 signaling in humans. Following a fasting blood draw, iv infusion of Ex-9 (600–750 pmol/kg/min) or saline began. Thirty minutes later, subjects consumed a standardized breakfast followed 90 minutes later (at the predicted time of maximal endogenous circulating GLP-1) by an ad libitum buffet meal to objectively measure satiety. Infusions ended once the buffet meal was complete. Visual analog scale ratings of hunger and fullness and serial assessments of plasma glucose, insulin, and GLP-1 concentrations were done throughout the experiment. Contrary to the hypothesis, during Ex-9 infusion subjects reported a greater decrease in hunger due to consumption of the breakfast (Ex-9 −62±5 Placebo −41±9; P=0.01) than during placebo. There were no differences in ad libitum caloric intake between Ex-9 and placebo. Ex-9 increased glucose, insulin, and endogenous GLP-1, which may have counteracted any effects of Ex-9 infusion to block satiety signaling. Blockade of GLP-1 receptors failed to suppress subjective satiety following a standardized meal or increase voluntary food intake in healthy, normal-weight subjects.
Keywords: GLP-1, satiety, insulin, visual analog scale
INTRODUCTION
Glucagon-like peptide 1 (GLP-1) is an incretin hormone released from the gut in response to food intake (D'Alessio and Vahl, 2004). Along with stimulating glucose-dependent insulin release (Drucker, 2006), GLP-1 reduces food intake in rodents (Williams et al., 2009). In humans, infusions of GLP-1 induce subjective satiety and reduce food intake (Flint et al., 1998) and synthetic GLP-1 agonists used to treat type 2 diabetes reduce both food intake and body weight (Edwards et al., 2001, DeFronzo et al., 2005). However, some studies suggest that GLP-1’s effects on satiety are dependent on synergism with other gut-derived satiety signals (Beglinger and Degen, 2006, De Silva et al., 2011) or are subject to physiologic condition such as elevated endogenous GLP-1, which occurs following gastric bypass surgery (Abegg et al., 2013).
Exendin-[9–39] (Ex-9) is a synthetic competitive antagonist to GLP-1 at the GLP-1 receptor. Ex-9 blocks the anorectic effect of both endogenous and exogenous GLP-1 in rodents (Chelikani et al., 2005, Williams et al., 2009). In humans, Ex-9 can suppress the insulinotropic effects of exogenous as well as endogenous GLP-1 stimulated by an oral glucose load (Schirra et al., 1998, Edwards et al., 1999, Salehi et al., 2008). However, the effects of Ex-9 on satiety have not been tested in humans.
In a double-blind, placebo-controlled, randomized crossover study, we used Ex-9 to investigate whether the satiating effects of a meal are dependent on endogenous GLP-1 signaling. While peak GLP-1 blood levels occur 30–60 minutes into the postprandial state (Flint et al., 1998, Flint et al., 2001, Verdich et al., 2001, Tomasik et al., 2002) and might play a role in satiation during meals, levels remain elevated for at least 180 minutes after meals (Verdich et al., 2001). It is unknown what role GLP-1 plays in enhancing satiety and suppressing further food intake in humans during the inter-meal interval, but animal studies demonstrate that Ex-9 has larger effects on food intake at times when baseline intake is low (Williams et al., 2009). We therefore presented subjects with an ad libitum buffet meal at a time point between meals when subjects would not normally be eating. We hypothesized that Ex-9 would suppress postprandial satiety and thereby increase hunger and voluntary food intake at a buffet meal.
SUBJECTS AND METHODS
Participants
Eight subjects (7 male, 1 female) were recruited by posted and print advertisements. Eligibility criteria were age 18–29 yr with a BMI 18.5–25 kg/m2 and habitual breakfast eater (4 or more times per week). Participants had mean BMI of 23.4±2.0 kg/m2 (range 19–24.7) and a mean age of 23.9±3.3 years (range 19–29). Exclusion criteria were: any chronic health conditions including diabetes; recent change in appetite; current dieting for weight loss; restrained eating; history of obesity, eating disorders, or weight loss surgery; random blood glucose >140 mg/dL; abnormal electrocardiogram, complete blood count, or kidney function; pregnancy; oral contraceptive use; smoking, drug or heavy alcohol use (>2 drinks a day); allergy or intolerance to study foods; vegetarian or vegan; extreme vigorous exercise (>21 hours per week); or medications known to alter appetite (e.g., atypical anti-psychotics). Study procedures were approved by the University of Washington (UW) Human Subjects Division and the U.S. Food and Drug Administration (IND #108604). All participants provided written informed consent.
Infusions
Exendin-[9–39] acetate (Clinalfa, Merck Biosciences, Germany) was diluted in 0.9% sodium chloride solution for administration via intravenous infusion. Prior use of Ex-9 in humans reveals that infusions of 300 pmol/kg/min reduce the insulinotropic action of GLP-1 (Schirra et al., 1998), whereas at higher doses of 500 (Edwards et al., 1999) and 600 pmol/kg/min (Salehi et al., 2008) the effect is completely blocked. Because doses sufficient to suppress satiety are unknown, the initial dose provided to 2 subjects was 600 pmol/kg/min. Thereafter, the dose was increased to 750 pmol/kg/min to achieve a maximal effect on satiety while ensuring that Ex-9 was safely tolerated. The placebo infusion was intravenous saline. Subjects were monitored throughout the study (EKG, symptoms) and no adverse events occurred.
Blinding and Randomization
Investigators and subjects were blinded to the treatment condition. Non-blinded pharmacists randomly assigned condition order in a blocked, counterbalanced design. Packaging and labeling of test and control infusions were identical.
Study Procedures
Subjects were asked to follow their usual eating habits and not to engage in strenuous activity prior to the study. Subjects fasted overnight beginning at 9:30 pm and arrived at the UW Clinical Research Center at 7:00 am the next morning. Following 2 antecubital or forearm IV placements, vital sign assessment, and a fasting blood draw, the randomly assigned infusion of either saline or Ex-9 was started at 8:00 am. Subjects then immediately drank a liquid solution of D-xylose consisting of 15g in 100mL of water (Letco Medical; Decauter, AL) (Medhus et al., 2000, Salehi et al., 2008). Administration of D-xylose with serial monitoring of blood levels was used as an indirect measure of nutrient entry into the duodenum (Pressman et al., 1987) and hence of gastric emptying (Drent et al., 1995, Frank et al., 1998, Salehi et al., 2008). After 30 minutes of infusion, subjects then had 15 minutes to consume a standardized breakfast meal (toast, orange juice, eggs; 35% fat, 20% protein, 45% carbohydrate). Meals were equal to 20%, 33%, or 40% of subjects’ estimated daily caloric needs (calculated by the Mifflin-St. Jeor equation and an activity factor). The size of the breakfast meal was varied as part of dose-response testing, however each subject received an identical meal during both Ex-9 and placebo infusions therefore group data were collapsed to examine our primary outcome of the effect of Ex-9 on satiety. Vital signs, blood draws and subjective satiety evaluations were repeated every 30 min. Ninety minutes after the start of the standardized breakfast subjects were presented with an ad libitum buffet meal at which they had 30 minutes to eat; however due to procedural delays the actual time between breakfast and the buffet varied, but was matched within subjects. This is reflected as hash marks in figures. Participants were not informed that the buffet was part of the study or that their food intake was being monitored until after all study procedures were complete. The 90-minute time point was chosen to represent a time at which subjects would not normally be eating as Ex-9 has larger effects on food intake at times when baseline intake is low (Williams et al., 2009). In humans, the effect of a preload on subsequent food intake diminishes with time (Rolls et al., 1991, Almiron-Roig et al., 2004). Moreover, after similar nutrient loads, subjective measures of satiety and GLP-1 levels remain elevated for 90 minutes, then return to baseline after approximately 150–180 minutes (Flint et al., 1998, Flint et al., 2001, Verdich et al., 2001, Vilsboll et al., 2003). Therefore, we sought to complete all ad libitum food intake well before the time at which GLP-1 would no longer be physiologically active. Infusions ended after the buffet meal.
Body weight and eating behavior
Height and weight were recorded to calculate BMI (weight(kg)/height(m2)). To identify individuals who may be less likely to eat according to their appetite, we administered the Revised Restraint Scale (Herman CP, 1980) as well as the Three-factor Eating Questionnaire (TFEQ).
Subjective appetite ratings
Serial visual analog scales (VAS) (Flint et al., 2000) were used to assess hunger and fullness at 30 minute intervals. Each scale was presented on a single sheet of paper with one the following statements on each side of a 100 mm line: “I am not hungry at all.” to “I have never been more hungry.” Or “I am not full at all.” to “I have never felt more full.” Subjects ratings were recorded by marking an “X” on a 100 mm scale.
Ad libitum buffet meal. The purpose of the buffet meal was to objectively measure satiety as well as food choice. The buffet included a wide range of foods suitable for a morning or midday meal. Foods varied in hedonic appeal, caloric and macronutrient content (e.g. bagels, turkey, fruit, pastries) and were provided in excess of estimated energy requirements (~5000 kcal). All uneaten food was weighed to determine total intake and macronutrients consumed (ProNutra, Viocare Technologies, Princeton, NJ).
Blood sampling
Blood samples were collected in EDTA tubes containing protease inhibitor, aprotinin and a dipeptidyl peptidase-4 inhibitor. Samples were cold centrifuged at −4°C and plasma was stored at −80°C. Glucose and D-xylose were measured by the hexokinase and colorimetric method, respectively (Eberts et al., 1979). Plasma insulin was measured by two-site immunoenzymometric assay using a Tosoh 2000 auto-analyzer (San Francisco, CA) with an assay sensitivity level of 0.5uU/mL. Total GLP-1 was measured by ELISA (ALPCO Diagnostics; Salem, NH) with an assay sensitivity level of 0.6 pmol/L and 100% specificity to GLP-1 (7–36) and GLP-1 (9–36). Intra- and inter-assay coefficients of variation for reference plasma for insulin and GLP-1 are between 2.0–2.8% and 3.7–9.5%, respectively.
Statistical analysis
Means and standard deviations are presented for descriptive statistics. Means and standard error of the mean are presented for all outcomes and graphs. Incremental area under the curve (AUC) was calculated using the trapezoidal method. Repeated m easures two-way ANOVA was used to evaluate: 1) treatment effect (matched) over multiple time points and 2) interactions between treatment and time points. Paired t-tests (2-tailed) were used to test group differences between Ex-9 and placebo. Three implausible glucose values were excluded (<70 mg/dL) and one subject was missing data for all GLP-1 timepoints. Statistical analyses were performed with GraphPad Prism (La Jolla, CA) and STATA (13.1; College Station, TX) and results were considered significant at P<0.05.
RESULTS
Participants
Eight participants (7 male, 1 female) self-reported fasting 8:00 AM hunger and fullness ratings that did not differ between treatment days (Figure 1A, Hunger: Ex-9 65±3.0 mm, Placebo 56±5.8 mm, P=0.11; Fullness: Ex-9 14±3.4 mm, Placebo 18±7.0 mm, P=0.42). Fasting plasma glucose, insulin, and GLP-1 concentrations also did not differ between treatment days (Glucose: Ex-9 92.9±8.9 mg/dL, Placebo 94.1±9.6 mg/dL, P=0.56; Insulin: Ex-9 4.88±1.7 uU/mL, Placebo 5.5±4.5 uU/mL, P=0.59; GLP-1: Ex-9 17.2±18.3 pmol/L, Placebo 16.0±17.6 pmol/L, P=0.36).
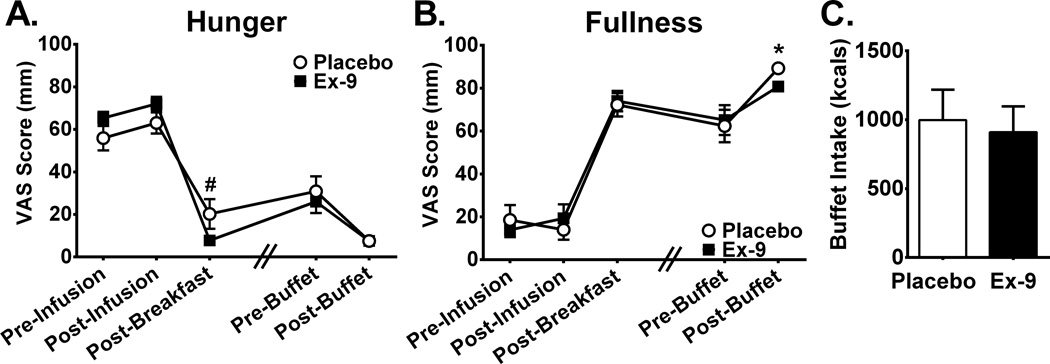
Figure 1. The effect of Ex-9 on appetite ratings and food intake.
Ex-9 had no overall effect on hunger (A) or fullness (B) ratings throughout the experimental protocol. Hunger ratings tended to be lower after the standardized breakfast whereas fullness ratings were significantly lower after the buffet meal during Ex-9 treatment. Buffet intake did not differ between treatment days (C). All time points are 30 minutes apart except for between Post-Breakfast and Pre-Buffet (indicated by hash marks) where actual time elapsed was ~60–90 min. and was matched within subjects. Ex-9, Exendin-[9–39], VAS, visual analog scale, *P<0.05, #P=0.05.
Effect of Ex-9 on subjective appetite and food intake
The standardized breakfast and buffet meal produced expected effects over time on VAS hunger and fullness ratings (Figure 1 A–B). We found no effect of treatment with Ex-9 on either hunger (F1,7=0.29, P=0.61) or fullness (F1,7=0.04, P=0.85). Contrary to the hypothesis, Ex-9 infusion decreased hunger ratings more due to consumption of the standardized breakfast (−62±6 mm) as compared to placebo infusion (−41±9 mm, P=0.01). Increases in fullness ratings following breakfast were comparable between treatments (Ex-9 54±8 mm, Placebo 58±8 mm, P=0.49). We tested if the effect of Ex-9 on appetite varied over time and found no evidence for interactions between treatment and time point for hunger (F3,19=2.14, P=0.13) or fullness (F3,19=1.21, P=0.33).
Total caloric intake at the buffet meal did not differ with Ex-9 treatment (Ex-9 910±187 kcal, Placebo 998±220 kcal, P=0.54, Figure 1C) nor did energy intake calculated as a percentage of total daily needs (Ex-9 29±6%, Placebo 32±7%, P=0.53). There was a tendency for subjects to consume less percentage of calories from fat on the placebo day (Ex-9 38.0±5%, Placebo 36.9±5%, P=0.05), but otherwise there were no differences in macronutrients consumed between conditions (Protein: Ex-9 12.6±2%, Placebo 13.6±2%, P=0.48; Carbohydrate: Ex-9 49.4±6%, Placebo 49.6±6%, P=0.90). Following the ad libitum buffet, subjects reported being less full during the Ex-9 treatment (Ex-9: 81±3 mm, Placebo 89±2 mm, P=0.02, Figure 1B). Of note, on the Ex-9 treatment day, subjects consumed, on average, 88 less total kilocalories at the buffet, a non-significant difference which may nonetheless have contributed to feeling less full. The change in VAS hunger and fullness scores resulting from the buffet meal did not differ during Ex-9 treatment compared to placebo (Hunger: Ex-9 −19±5 mm, Placebo −23±7 mm, P=0.61; Fullness: Ex-9 16±6 mm, Placebo 27±8 mm, P=0.22). It is possible a “floor effect” may have prevented subjects from expressing greater changes in subjective satiety, particularly for hunger, as most were scoring at the extreme of the 1–100 mm range after the buffet meal.
Effect of Ex-9 on plasma glucose, insulin, and GLP-1 concentrations
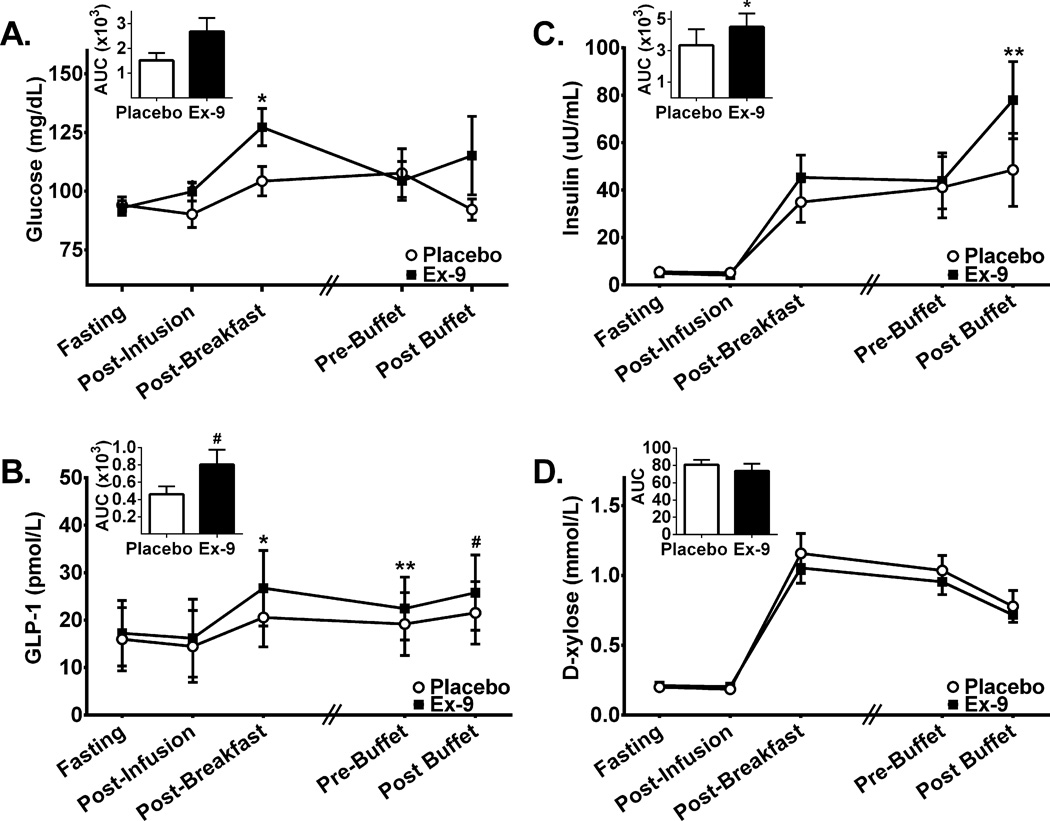
Treatment with Ex-9 significantly increased glucose (F1,7=8.40, P=0.02, Figure 2A) and GLP-1 concentrations (F1,6=15.43, P<0.01, Figure 2B) and tended to increase insulin (F1,7=5.24, P=0.06, Figure 2C). Time by treatment interactions were present for both glucose and insulin (Glucose: F3,16=3.40, P<0.05; Insulin: F3,19=6.01, P<0.01) As seen in Figures 2A and 2C, glucose concentrations were elevated with Ex-9 treatment compared to placebo most strongly before breakfast and after breakfast and insulin concentrations were elevated most strongly after the buffet. Ex-9 infusion increased endogenous GLP-1 concentrations most strongly following breakfast, prior to the buffet meal and following the buffet meal (Figure 2B).
Figure 2. Plasma glucose, insulin, GLP-1, and D-xylose concentrations during Ex-9 and placebo infusion.
Inset bar graphs represent incremental area under the curve (AUC) for the corresponding hormone throughout the protocol period. There was a main effect of Ex-9 for plasma glucose (A; F1,7=8.84; P=0.02) and GLP-1 (B; F1,6=17.94; P<0.01), and a trend was present for insulin as well (C; F1,7=5.10; P=0.06). Compared to placebo, Ex-9 treatment increased AUC values in insulin and GLP-1. Time point specific analyses revealed that Ex-9 infusion as compared to placebo resulted in elevations in levels of: A) glucose after infusion initiation and after breakfast, C) insulin after the buffet meal and B) GLP-1 after the breakfast meal and prior to the buffet. D-xylose concentrations were not significantly different between treatments (D; F1,7=4.35; P=0.08). All time points are 30 minutes apart except for between Post-Breakfast and Pre-Buffet (indicated by hash marks) where actual time elapsed was ~60–90 min. and was matched within subjects. A, C &D: N=8 for all points except for Post-Infusion where N=6. B: N=7 for all time points except for Post-Infusion where N=5. Ex-9, Exendin-[9–39], GLP-1, glucagon-like peptide-1, #P=0.06, *P<0.05, **P<0.01.
Effect of Ex-9 on gastric emptying
A trend suggested some effect of Ex-9 to lower D-xylose concentrations (F1,7=4.91; P=0.06, Figure 2D), although we cannot rule out that peak D-xylose concentrations during Ex-9 infusion preceded the 30-minute post-ingestion assessment.
DISCUSSION
Contrary to our hypothesis, we did not find that blockade of GLP-1 action was sufficient to reduce meal-induced satiety in humans, although effects of the antagonist were demonstrated on the incretin system. In fact, subjective hunger was suppressed to a greater degree by Ex-9 than by placebo infusion when subjects consumed a calorically matched standardized meal. There were no differences in ad libitum caloric intake. Although we saw no evidence that Ex-9 blocked satiety throughout the controlled intake period of the study, subjects did report slightly less fullness at the final time point assessed after the ad libitum buffet meal. Serial blood sampling demonstrated hyperglycemia, hyperinsulinemia, and elevated GLP-1 levels during Ex-9 infusion that together may have paradoxically promoted satiety. This is the first study to specifically focus on the effect of Ex-9 on subjective and behavioral measures of satiety in humans.
Elevated plasma glucose and insulin levels resulting from Ex-9 treatment (Calabria et al., 2012, Jorgensen et al., 2013) offer one explanation for the lack of effect on appetite of Ex-9. Hyperglycemia alone or in an insulin-dependent manner can suppress hunger in humans (Gielkens et al., 1998, Russell et al., 2001) and lower food intake in rodents (Davis et al., 1981, Kurata et al., 1986). Differences in the rate of nutrient emptying into the duodenum during Ex-9 infusion did not appear to explain the findings because we noted minimal effects of Ex-9 on gastric emptying. It is possible, however, that the tendency toward lower D-xylose levels we found reflects that peak D-xylose concentrations preceded the 30-minute post-ingestion assessment, potentially indicating enhanced gastric transit due to GLP-1 receptor blockade. The lack of an early post-ingestion time point limits interpretation of these data, but early nutrient delivery to the intestines cannot be entirely ruled out and may have stimulated endogenous GLP-1 release. In fact, endogenous GLP-1 levels were increased by Ex-9 infusion in the current study, as has been shown previously (Jorgensen et al., 2013). If receptor blockade was incomplete or increased sympathetic tone occurred (Perez-Tilve et al., 2010), then elevated endogenous GLP-1 concentrations could have counteracted the effect of Ex-9 to stimulate appetite. However, the significant differences we observed in glucose, insulin, and GLP-1 concentrations during Ex-9 treatment suggest that both doses provided achieved physiologically significant if not complete receptor blockade. One alternative interpretation of the elevated endogenous GLP-1 levels is the existence of a negative feedback loop in which activation of GLP-1 receptors on enteroendocrine cells are necessary to inhibit further GLP-1 secretion (Jorgensen et al., 2013). In sum, blockade of GLP-1 receptors was not sufficient to reduce meal-induced satiety in humans, but concurrent blockade of GLP-1’s incretin effects complicates interpretation of the results.
Several limitations of the study bear mentioning. First, we did not measure levels of either glucagon or peptide YY(3–36), which can also increase during Ex-9 infusion in humans (Edwards et al., 1999) and these hormones could have played a role in the paradoxical effect of Ex-9 to reduce self-reported hunger induced by a meal. Second, all but one of the participants were men. Third, the study was powered to detect increases in caloric intake at the buffet (300–400 kcal) due to Ex-9 and no such effects were found. Instead, a small reduction in caloric intake at the buffet meal and increased post-meal VAS hunger scores were observed during Ex-9 treatment. Much larger sample sizes would be required to evaluate whether these effects were due to chance alone. Fourth, gastric emptying was measured indirectly by the appearance of the absorbed nutrient D-xylose solution in the bloodstream, and a rapid gastric emptying of the solid food breakfast meal could have been missed. Fifth, we studied post-meal satiety and further study is required to test whether GLP-1 is necessary to reduce hunger during fasting or to enhance meal termination or satiation. Finally, there was variability in the size of the standardized meals between subjects and therefore average hormonal excursions must be interpreted with caution.
Given these caveats, these findings are nonetheless in line with prior studies showing that GLP-1 may not act independently, but rather in concert with other hormones and nutrients to promote satiety. Several studies do show effects of exogenous GLP-1(Flint et al., 1998, Gutzwiller et al., 1999, Gutzwiller et al., 1999, Degen et al., 2006) or GLP-1 agonists (Edwards et al., 2001) on appetite in humans. However, Ex-9 treatment alone in rats does not produce effects on food intake (Ruttimann et al., 2010), but when given in combination with leptin, it can attenuate the anorectic effect of leptin (Zhao et al., 2012). Also, infusion of GLP-1 was insufficient to significantly reduce food intake or modulate neural responses to visual food cues, whereas GLP-1 in combination with peptide YY(3–36) did suppress food intake and regional brain activation (De Silva et al., 2011). Moreover, w hen glucose utilization in the central nervous system is blocked, the anorectic effect of intraventricular GLP-1 is diminished (Sandoval et al., 2012). In sum, GLP-1 infusion or receptor stimulation appears to be sufficient to promote meal-related satiety in humans in a dose-dependent manner (Verdich et al., 2001). The current data do not support an independent physiologic role for GLP-1 in postprandial satiety in humans. In other words, intact GLP-1 signaling may not be necessary for relatively normal satiety processing, as evidenced by studies in GLP-1 receptor knockout mice (Ayala et al., 2010).
Because of the possibility that hyperglycemia and hyperinsulinemia counteracted any effect of Ex-9 on appetite, future studies could monitor satiety whilst controlling for the effects of GLP-1 receptor blockade on glucose and insulin through clamp methodologies. Alternatively, monitoring of peptide YY(3–36) and glucagon during Ex-9 could be informative as to the mechanism of paradoxical enhancement in satiety during Ex-9 infusion. In addition, recent data suggest that higher doses of Ex-9 than used in the current study can be given safely (Jorgensen et al., 2013), which might further enhance the near-complete receptor blockade assumed to be achieved with this and other studies (Edwards et al., 1999). Future studies could also target the genetic or physiologic mechanisms underlying documented individual variability of response to GLP-1 agonists (D'Alessio and Vahl, 2004, DeFronzo et al., 2005, Drucker, 2006) among healthy subjects and those with altered metabolic states, such as obesity and diabetes.
Following acceptance of this manuscript, Steinert et al (2014) published a similar study using Ex-9 in healthy men. Similar to the current study, they found no effect of intravenous infusion of Ex-9 on ad libitum food intake following a preload meal. They report a slight attenuation of meal-induced suppression of self-reported desire to eat with Ex-9 treatment, but only after a glucose preload, not a mixed-meal which would compare to the current study. Furthermore, they also report an elevation of GLP-1 with Ex-9 treatment and also a rise in total peptide YY and glucagon with both a glucose and mixed-meal preload. As discussed above and reported by Steinert and colleagues, the elevation of peptide YY(3–36) and glucagon by Ex-9 treatment could likely interfere with the hypothesized de-satiating effect of Ex-9 (Steinert et al., 2014). These published findings further support our conclusions that although GLP-1 is involved in satiation, it is not autonomous, and may not even play a strong role.
Highlights.
A double-blind, placebo-controlled crossover study of Exendin-[9–39] (Ex-9).
We examine the satiating effects of GLP-1 in normal weight humans.
Subjective appetite and objective food intake were similar between treatments.
Glucose, insulin and GLP-1 were elevated during treatment with Ex-9.
Antagonism of GLP-1 is not sufficient to suppress satiety or voluntary food intake.
Acknowledgments
Support: National Institutes of Health (NIH) DK083502 and DK070826 (Schur); University of Washington Diabetes and Endocrinology Research Center (P30DK017047); University of Washington Nutrition Obesity Research Center (Supported by the NIH P30DK035816); University of Washington Institute of Translational Health Sciences (Supported b y UL1TR000423, KL2TR000421, TL1TR000422 from the NIH National Center for Advancing Translational Sciences).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Susan J Melhorn, Email: smelhorn@u.washington.edu.
Vidhi Tyagi, Email: vtyagi@u.washington.edu.
Anne Smeraglio, Email: smeragli@stanford.edu.
Christian L Roth, Email: christian.roth@seattlechildrens.org.
Ellen A Schur, Email: ellschur@u.washington.edu.
REFERENCES
- Abegg K, Schiesser M, Lutz TA, Bueter M. Acute peripheral GLP-1 receptor agonism or antagonism does not alter energy expenditure in rats after Roux-en-Y gastric bypass. Physiol Behav. 2013;121:70–78. doi: 10.1016/j.physbeh.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almiron-Roig E, Flores SY, Drewnowski A. No difference in satiety or in subsequent energy intakes between a beverage and a solid food. Physiol Behav. 2004;82(4):671–677. doi: 10.1016/j.physbeh.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, James FD, Burmeister MA, Wasserman DH, Drucker DJ. Glucagon-like peptide-1 receptor knockout mice are protected from high-fat diet-induced insulin resistance. Endocrinology. 2010;151(10):4678–4687. doi: 10.1210/en.2010-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglinger C, Degen L. Gastrointestinal satiety signals in humans--physiologic roles for GLP-1 and PYY? Physiol Behav. 2006;89(4):460–464. doi: 10.1016/j.physbeh.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Calabria AC, Li C, Gallagher PR, Stanley CA, De Leon DD. GLP-1 receptor antagonist exendin-(9–39) elevates fasting blood glucose levels in congenital hyperinsulinism owing to inactivating mutations in the ATP-sensitive K+ channel. Diabetes. 2012;61(10):2585–2591. doi: 10.2337/db12-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1695–R1706. doi: 10.1152/ajpregu.00870.2004. [DOI] [PubMed] [Google Scholar]
- D'Alessio DA, Vahl TP. Glucagon-like peptide 1: evolution of an incretin into a treatment for diabetes. Am J Physiol Endocrinol Metab. 2004;286(6):E882–E890. doi: 10.1152/ajpendo.00014.2004. [DOI] [PubMed] [Google Scholar]
- Davis JD, Wirtshafter D, Asin KE, Brief D. Sustained intracerebroventricular infusion of brain fuels reduces body weight and food intake in rats. Science. 1981;212(4490):81–83. doi: 10.1126/science.7193909. [DOI] [PubMed] [Google Scholar]
- De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JD, Dhillo WS. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14(5):700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- Degen L, Oesch S, Matzinger D, Drewe J, Knupp M, Zimmerli F, Beglinger C. Effects of a preload on reduction of food intake by GLP-1 in healthy subjects. Digestion. 2006;74(2):78–84. doi: 10.1159/000097585. [DOI] [PubMed] [Google Scholar]
- Drent ML, Popp-Snijders C, Ader HJ, Jansen JB, van der Veen EA. Lipase inhibition and hormonal status, body composition and gastrointestinal processing of a liquid high-fat mixed meal in moderately obese subjects. Obes Res. 1995;3(6):573–581. doi: 10.1002/j.1550-8528.1995.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Eberts TJ, Sample RH, Glick MR, Ellis GH. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin Chem. 1979;25(8):1440–1443. [PubMed] [Google Scholar]
- Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281(1):E155–E161. doi: 10.1152/ajpendo.2001.281.1.E155. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes. 1999;48(1):86–93. doi: 10.2337/diabetes.48.1.86. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25(6):781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- Frank JW, Camilleri M, Thomforde GM, Dinneen SF, Rizza RA. Effects of glucagon on postprandial carbohydrate metabolism in nondiabetic humans. Metabolism. 1998;47(1):7–12. doi: 10.1016/s0026-0495(98)90185-8. [DOI] [PubMed] [Google Scholar]
- Gielkens HA, Verkijk M, Lam WF, Lamers CB, Masclee AA. Effects of hyperglycemia and hyperinsulinemia on satiety in humans. Metabolism. 1998;47(3):321–324. doi: 10.1016/s0026-0495(98)90264-5. [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. The American journal of physiology. 1999;276(5 Pt 2):R1541–R1544. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44(1):81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman CPPJ. Restrained eating. Philadelphia: W.B. Saunders; 1980. [Google Scholar]
- Jorgensen NB, Dirksen C, Bojsen-Moller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–3052. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Fujimoto K, Sakata T, Etou H, Fukagawa K. D-glucose suppression of eating after intra-third ventricle infusion in rat. Physiol Behav. 1986;37(4):615–620. doi: 10.1016/0031-9384(86)90295-7. [DOI] [PubMed] [Google Scholar]
- Medhus AW, Sandstad O, Bredesen J, Husebye E. Stimulation of the small intestine by nutrients in relation to phase of the migrating motor complex. Scand J Gastroenterol. 2000;35(5):494–500. doi: 10.1080/003655200750023750. [DOI] [PubMed] [Google Scholar]
- Perez-Tilve D, Gonzalez-Matias L, Aulinger BA, Alvarez-Crespo M, Gil-Lozano M, Alvarez E, Andrade-Olivie AM, Tschop MH, D'Alessio DA, Mallo F. Exendin-4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. Am J Physiol Endocrinol Metab. 2010;298(5):E1088–E1096. doi: 10.1152/ajpendo.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman JH, Hofmann AF, Witztum KF, Gertler SL, Steinbach JH, Stokes K, Kelts DG, Stone DM, Jones BR, Dharmsathaphorn K. Limitations of indirect methods of estimating small bowel transit in man. Dig Dis Sci. 1987;32(7):689–699. doi: 10.1007/BF01296133. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Kim S, McNelis AL, Fischman MW, Foltin RW, Moran TH. Time course of effects of preloads high in fat or carbohydrate on food intake and hunger ratings in humans. Am J Physiol. 1991;260(4 Pt 2):R756–R763. doi: 10.1152/ajpregu.1991.260.4.R756. [DOI] [PubMed] [Google Scholar]
- Russell AW, Horowitz M, Ritz M, MacIntosh C, Fraser R, Chapman IM. The effect of acute hyperglycaemia on appetite and food intake in Type 1 diabetes mellitus. Diabet Med. 2001;18(9):718–725. doi: 10.1046/j.1464-5491.2001.00545.x. [DOI] [PubMed] [Google Scholar]
- Ruttimann EB, Arnold M, Geary N, Langhans W. GLP-1 antagonism with exendin (9–39) fails to increase spontaneous meal size in rats. Physiol Behav. 2010;100(4):291–296. doi: 10.1016/j.physbeh.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Salehi M, Vahl TP, D'Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab. 2008;93(12):4909–4916. doi: 10.1210/jc.2008-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval D, Barrera JG, Stefater MA, Sisley S, Woods SC, D'Alessio DD, Seeley RJ. The anorectic effect of GLP-1 in rats is nutrient dependent. PLoS One. 2012;7(12):e51870. doi: 10.1371/journal.pone.0051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M. Exendin(9–39)amide is an antagonist of glucagon-like peptide-1(7–36)amide in humans. J Clin Invest. 1998;101(7):1421–1430. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert RE, Schirra J, Meyer-Gerspach AC, Kienle P, Fischer H, Schulte F, Goeke B, Beglinger C. Effect of glucagon-like peptide-1 receptor antagonism on appetite and food intake in healthy men. Am J Clin Nutr. 2014 doi: 10.3945/ajcn.114.083246. [DOI] [PubMed] [Google Scholar]
- Tomasik PJ, Sztefko K, Malek A. GLP-1 as a satiety factor in children with eating disorders. Horm Metab Res. 2002;34(2):77–80. doi: 10.1055/s-2002-20519. [DOI] [PubMed] [Google Scholar]
- Verdich C, Flint A, Gutzwiller JP, Naslund E, Beglinger C, Hellstrom PM, Long SJ, Morgan LM, Holst JJ, Astrup A. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. Journal of clinical endocrinology and metabolism. 2001;86(9):4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety--effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25(8):1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88(6):2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond) 2012;36(12):1522–1528. doi: 10.1038/ijo.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]