Abstract
The concentrations of major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil derived neurotoxin (EDN) and eosinophil peroxidase (EPO) have been associated with eosinophilic disease severity. Whereas a variety of techniques have been used to measure individual eosinophil granule protein concentration, none of these methods efficiently measures MBP, ECP, EDN and EPO simultaneously. A multiplex suspension array system was developed to simultaneously measure the concentration of MBP, ECP, EDN and EPO in serum. The assay showed excellent inter- and intra-assay reliability, and serum levels of MBP, ECP and EDN from eosinophilic subjects analyzed by ELISA and multiplex were highly correlated (r = 0.8579; P < 0.0001, r = 0.6356; P = 0.0006 and r = 0.8600; P < 0.0001, respectively, Spearman rank correlation). Moreover, the multiplex assay required 500-fold less serum than a single ELISA to achieve comparable sensitivity. Absolute eosinophil count and eosinophil surface expression of the activation marker, CD69, were significantly correlated with concentrations of MBP, EDN and EPO, but not ECP, in serum from eosinophilic subjects. Furthermore, subjects with eosinophilic gastrointestinal disorder and normal peripheral absolute eosinophil counts (< 0.5 × 109/L) had significantly increased concentrations of MBP (P < 0.0001), ECP (P < 0.0001), EDN (P = 0.0001) and EPO (P < 0.0001) compared to normal donors. In summary, the eosinophil granule protein multiplex assay provides a rapid and reliable way to measure eosinophil granule protein levels and should prove useful in assessing patterns of degranulation in patients with eosinophilic disorders.
Keywords: Multiplex, ELISA, Eosinophil granule proteins, Eosinophil, Degranulation
1. Introduction
The eosinophil granule proteins, major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil derived neurotoxin (EDN) and eosinophil peroxidase (EPO), are eosinophil effector molecules implicated in host defense (Malik and Batra, 2012), immunoregulatory responses (Yang et al., 2008) and allergic and inflammatory reactions (Sedgwick et al., 2004; Kato et al., 2012; Lee et al., 2013). These highly cationic proteins are stored preformed within the secondary granules of eosinophils, and can be released in intact membrane-bound granules during cell lysis or through a complex system of vesiculotubular structures during “piecemeal necrosis” (reviewed in (Muniz et al., 2012)). Although differential secretion of preformed cytokines (Spencer et al., 2009) and eosinophil granule proteins (Tomassini et al., 1991) has been shown to occur in vitro in response to specific stimuli, little is known about preferential release of individual granule proteins in vivo.
Because levels of eosinophil granule proteins have been associated with disease severity (Kita, 2011), assays have been developed to measure their levels in various biological fluids including sputum (Virchow et al., 1992), urine (Reimert et al., 1991a), feces (Peterson et al., 2002), plasma (Morioka et al., 2000), bronchoalveolar lavage (Modig et al., 1986), nasopharyngeal secretions (Colocho Zelaya et al., 1994) and cerebrospinal fluid (Hallgren et al., 1983). Although levels of MBP, ECP, EDN and EPO in serum, other body fluids, and cell supernatants can be measured by radioimmunoassay (RIA) (Wassom et al., 1981; Peterson et al., 1991; Robinson et al., 1995) and enzyme-linked immunosorbent assay (ELISA) (Reimert et al., 1991b; Yamamoto et al., 1994; Morioka et al., 2000; Ochkur et al., 2012), both of these methods are time-consuming and measure each granule protein individually. Suspension array technology has the potential to circumvent both of these problems and has been used for the sensitive and simultaneous measurement of multiple proteins in a wide variety of clinical samples, including serum, plasma, cerebrospinal fluid (Moller, 2012), sputum (Bafadhel et al., 2012) and urine (Campbell et al., 2013).
The present study describes the development of a suspension array assay in multiplex (hereafter referred to as multiplex) to simultaneously measure the eosinophil granule proteins, MBP, ECP, EDN and EPO. The results obtained with this method are compared to those using established ELISAs for MBP, ECP and EDN, and demonstrate similar sensitivity and maintenance of precision despite a decrease in total assay time. The use of eosinophil granule protein concentrations as a predictor of tissue eosinophilia in the setting of normal peripheral absolute eosinophil counts in subjects with eosinophilic gastrointestinal disease (EGID) and longitudinal variations of eosinophil granule protein release in subjects with episodic angioedema associated with eosinophilia (EAE) demonstrate the relevance of the eosinophil granule protein multiplex assay in a clinical setting.
2. Methods
2.1 Coupling of mouse anti-human MBP, ECP, EDN and EPO to microspheres
Mouse monoclonal antibodies (D4 anti-MBP, 1C8.5/D9 anti-ECP, 6D1.5/A5 anti-EDN and E5 anti-EPO) were raised in mice immunized with purified MBP, ECP, EDN and EPO proteins. Hybridomas were made with SP/2 cells using standard techniques. Specificity was ensured by reactivity to each specific granule protein and no reactivity to other granule proteins on Western blot. Ascites was made in nude mice and antibodies were purified by Protein A. The mouse monoclonal antibodies against MBP, ECP, EDN and EPO were coupled to carboxylated magnetic microspheres in MagPlex microsphere regions 12, 13, 14 and 15, respectively, via two-step carbodiimide reaction protocol provided in the xMAP Antibody Coupling Kit (Luminex Corporation, Austin, TX). Following titration, 0.5 µg anti-MBP, EDN and EPO and 10 µg anti-ECP were coupled per 106 microspheres. The final concentration of the coupled microspheres was determined by counting in a hemocytometer. Coupling was confirmed by detection with biotin goat-anti-mouse IgG, Fcγ fragment specific (2.6 µg /ml; Jackson ImmunoResearch Laboratories, West Grove, PA) and PE-streptavidin (2 µg /ml; Jackson ImmunoResearch Laboratories). Conjugated microspheres were stored in light safe vials at 4°C. Coupling of mouse anti-human MBP, ECP, EDN and EPO to microspheres was performed on two separate instances to verify reproducibility of the reaction (Supplemental Figure 1).
2.2 Serum samples
Serum from eosinophilic subjects (n = 55) and healthy volunteers (n = 19) and plasma from eosinophilic subjects (n = 12) were collected under Institutional Review Board (IRB)-approved clinical protocols to study eosinophilic disorders (NCT00001406), eosinophilic gastrointestinal disease (NCT01212016) and to obtain controls for in vitro research (NCT00090662) (Table 1). All participants gave written informed consent. Serum and plasma were spun within 30 minutes of venipuncture, frozen and stored at −80°C until use.
Table 1. Characteristics of the study population.
Absolute eosinophil counts are significantly different between each eosinophilic group and the normal donor group (P < 0.0001). AEC is also significantly different between the FIP1L1-PDGFRA/JAK2 group and the PARA (P = 0.0279) group as well as between the FIP1L1-PDGFRA/JAK2 group and the HEUS group (P = 0.0076).
| Diagnosis | Median Age (range) |
Sex (M / F) | GMa Eosinophils × 109/L (range) |
|---|---|---|---|
| HES/LHES (n = 13) | 58 (17 – 82) | 9 / 4 | 5.183 (1.514 – 60.920) |
| FIP1L1-PDGFRA/JAK2 (n = 9) | 35 (17 – 78) | 8 / 1 | 5.970 (2.425 – 11.821) |
| PARA (n = 10) | 28.5 (21 – 71) | 5 / 5 | 2.948 (1.071 – 10.400) |
| HEUS (n = 10) | 54.5 (18 – 74) | 7 / 3 | 2.864 (1.340 – 6.390) |
| EAE (n = 2) | 27 (21 – 33) | 2 / 0 | Not Applicable |
| EGID (n = 11) | 34 (22 – 54) | 9 / 2 | 0.173 (0.070 – 0.470) |
| ND (n = 19) | 34 (26 – 81) | 12 / 7 | 0.133 (0.039 – 0.390)b |
GM indicates geometric mean
Absolute eosinophil counts were available for 14 of the 19 ND.
2.3 Reduction and alkylation of serum samples
50 µl of subject serum or undiluted serum standard were incubated for 1 hour at room temperature with 130 µl Tris EDTA (0.33 M Tris pH 8.0, 0.12 M NaCl and 0.01 M EDTA pH 8.0) and 20 µl 0.075 M DTT followed by 20 minutes at room temperature in the dark with 20 µl 1.5 M iodoacetamide. Samples were further diluted in assay buffer (1× PBS, 1% BSA, 0.05% Tween-20) for a final dilution of 1:250. The purified eosinophil granule protein standards were acid-solubilized prior to purification, rendering reduction and alkylation unnecessary (Ackerman et al., 1985).
2.4 Multiplex assay
The concentrations of eosinophil granule proteins in serum and plasma were measured simultaneously by a suspension array assay in multiplex. The assay was performed in 96 well flat bottom non-binding µClear® bottom plates (Grenier Bio-One, Monroe, NC), which facilitate microsphere retention during washing. Microspheres were resuspended and dispersed by vortex and sonication for 20 seconds each. Several different buffer and reagent conditions were tested to optimize the assay: 1) 100, 500, 1000, 1250, 2500 (recommended by manufacturer) and 5000 beads per well, 2) serum dilutions of 1:5, 1:10, 1:20, 1:25 1:40, 1:50, 1:80, 1:100, 1:160, 1:250, 1:320, 1: 640, 1:1280, 1:2560, 1:5120 and 1:10240, and 3) wash buffer containing 1% BSA (recommended by manufacturer), 0.05% Tween-20, 4% AB serum, and 25% serum matrix (Millipore).
Five thousand microspheres of each specificity were plated in 50 µl of assay buffer per well. The microspheres were then washed with wash buffer (1× PBS, 0.05% Tween-20) using a HydroFlex microplate washer with magnetic plate carrier (Tecan Group, Männedorf, Switzerland). At least two wells in the plate were reserved for background determination. For these wells, only assay buffer was added. Purified MBP, ECP, EDN and EPO protein standards (Hamann et al., 1990) were diluted in assay buffer (1× PBS, 1% BSA, 0.05% Tween-20) to initial concentrations of 1 µg/ml and 500 ng/ml and then each serial diluted 1:10. The serum standard was diluted 1:50 in assay buffer and then serially diluted 1:2. Test samples were diluted 1:250 in assay buffer. The plate was protected from light exposure by wrapping with aluminum foil. Standards and test samples were incubated in the plate with microspheres at room temperature, 500 rpm orbital shaking for 1 hour. After incubation, the plate was washed with wash buffer.
Rabbit antisera were raised to purified MBP, ECP, EDN and EPO proteins by three 80 µg injections in Complete Freund’s Adjuvant (CFA) followed by Incomplete Freund’s Adjuvant (IFA). Rabbit antibodies were purified by ammonium sulfate precipitation followed by Protein A purification. Polyclonal rabbit anti-human MBP, ECP, EDN and EPO IgG detecting antibodies were diluted 1:500 in assay buffer and 100 µl was added to all wells. The plate was incubated at room temperature, 500 rpm shaking for 30 minutes. After incubation, the plate was washed with wash buffer. Subsequently, biotinylated goat anti-rabbit IgG, Fc fragment specific (Jackson ImmunoResearch Laboratories) conjugate antibody was diluted to 2.4 µg /ml and 100 µl was added to all wells in the plate. PE-streptavidin (Jackson ImmunoResearch Laboratories) substrate was diluted to 2 µg /ml in assay buffer and 50 µl was added to all wells on the plate, which was then incubated at room temperature, 500 rpm shaking for 15 minutes. After incubation, the plate was washed with wash buffer. Finally, microspheres were resuspended in 80 µl sheath fluid (Luminex Corporation) and incubated at room temperature, 500 rpm shaking for 5 minutes.
The microspheres were analyzed using a Bio-Plex 200 (Bio-Rad Laboratories, Hercules, CA) instrument. The instrument read a 50 µl sample of microspheres in regions 12, 13, 14 and 15 corresponding to MBP, ECP, EDN and EPO, respectively. A minimum of 100 microspheres was read per sample, and the mean fluorescence intensity (MFI) was determined for each region in each sample. The MFI was corrected for each sample by subtracting the average of the corresponding MFI from the background samples. All assays were performed in duplicate, with the final MFI determined by the average from duplicate samples. Concentrations were calculated based on a standard curve using SoftMax Pro Software (Molecular Devices, Sunnyvale, CA).
2.5 Standard curve accuracy
The difference between the expected value of the standard dilution and the measured value was assessed to evaluate the accuracy of the serum standard. The MFI output for each dilution of the standard was back calculated to give a measured concentration. The percentage of error was then determined for each dilution.
2.6 ELISA
To validate the eosinophil granule protein multiplex assay, the concentrations of EDN, ECP and MBP were compared with those from the corresponding enzyme-linked immunosorbent assay (ELISA) using methods previously described (Klion et al., 2004). OD was measured at 405 nm and 650 nm using a VERSA max microplate reader (Molecular Devices), and the reduced values were used for comparison of assay range and sensitivity.
2.7 Inter-assay and intra-assay precision
To determine inter-assay reproducibility, the serum standard control was tested 19 times over eight independent multiplex assays. Serum samples from test subjects were assessed in four independent multiplex assays under identical conditions. Coefficients of variation (CV) and the ratio of measured concentration to mean concentration were calculated for each sample. The working range for each eosinophil granule protein was determined as the concentrations for which CV was <10% and the measured concentration to mean concentration ratio deviated <20%. Precision of the multiplex assay within a plate was demonstrated by testing 24 replicates of a single eosinophilic subject sample and calculating the CV.
2.8 Statistical analysis
Standard curves were fitted by nonlinear regression and the equation of the curve was determined by one-site binding (hyperbola) or when possible by sigmoidal four-parameter interpolation. To determine the relationship between nonparametric values, Spearman rank correlation was used. Nonparametric comparisons of group means were made using Mann Whitney U test, and parametric comparisons of group means were made using Student’s t test for paired samples. A P value of less than 0.05 was considered statistically significant for all tests.
3. Results
3.1 Development and optimization of a suspension array assay in multiplex for MBP, ECP, EDN and EPO
After coupling of the antibodies to the microspheres was confirmed, different buffers and reagent concentrations were tested to optimize the assay. Greater sensitivity was achieved, especially for MBP and ECP, when the assay buffer contained 0.05% Tween-20 (Supplemental Figure 2). The addition of 5,000 microspheres per well rather than 2,500 microspheres per well (as recommended by the manufacturer) resulted in faster detection by the suspension array reader and consistent detection of at least 100 microspheres for each region (data not shown).
Once the assays were optimized, serial dilutions of purified MBP, ECP, EDN and EPO were assayed individually and in multiplex. The standard curves generated for each of the purified granule proteins measured in the multiplex format were identical to those obtained when the assays were done individually (Supplemental Figure 3). Finally, in order to circumvent the need for purified granule proteins for the standard curve, a panel of 32 serum samples from eosinophilic subjects was screened in the multiplex assay. One serum sample with high levels of all four granule proteins was identified. Serial dilutions were prepared for use as a serum standard control, and the concentrations of MBP, ECP, EDN and EPO for each dilution were determined by interpolation from the purified eosinophil granule protein standard curves. The purified eosinophil granule protein standard curves were limited by a marked prozone effect (data not shown), so the data points shown for the serum standard curve (Fig. 1) reflect the dilutions for which concentrations could be calculated.
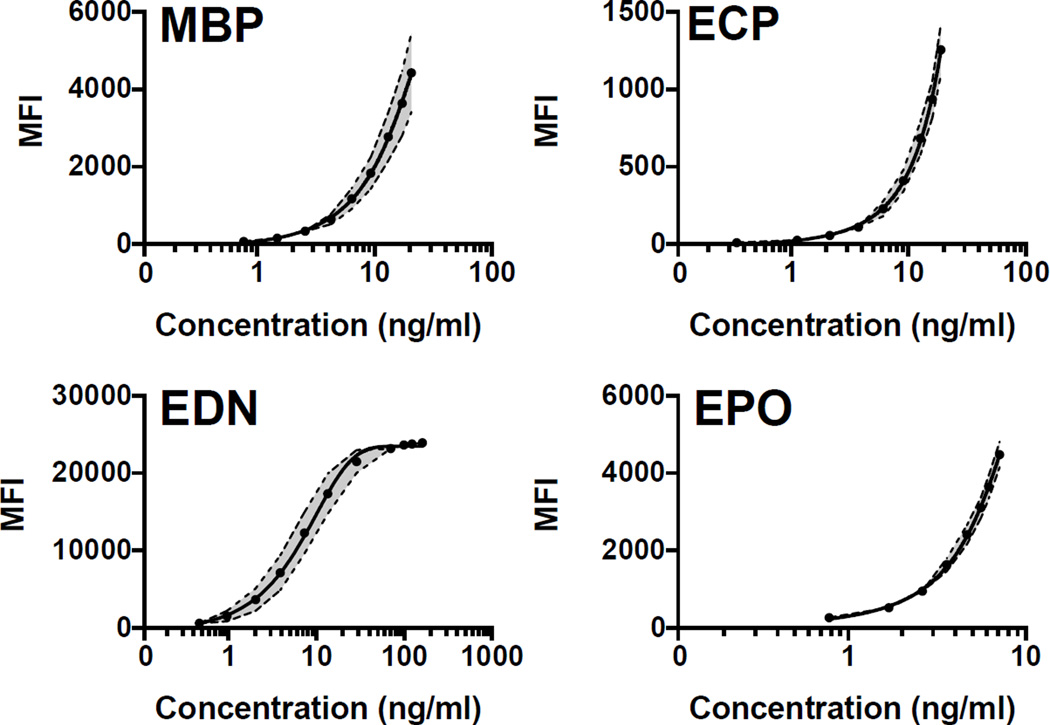
Fig. 1.
Reproducibility of serum standard control: 19 parallel measurements of the serum standard control. The solid line represents the mean of all standard curve measurements. The dotted lines represent the upper and lower limits of the standard deviation, with the space between the limits shaded in gray.
3.2 Reliability and accuracy of the serum standard control
Reliability of the serum standard control was determined by 19 parallel measurements (Fig. 1). The greatest variability in the serum standard control was seen at the highest concentrations of MBP (> 2.572 ng/ml; MFI SD > 100) and in the mid-range concentrations of EDN (0.958 – 28.599 ng/ml; MFI SD > 500). ECP and EPO standard curves displayed negligible variability.
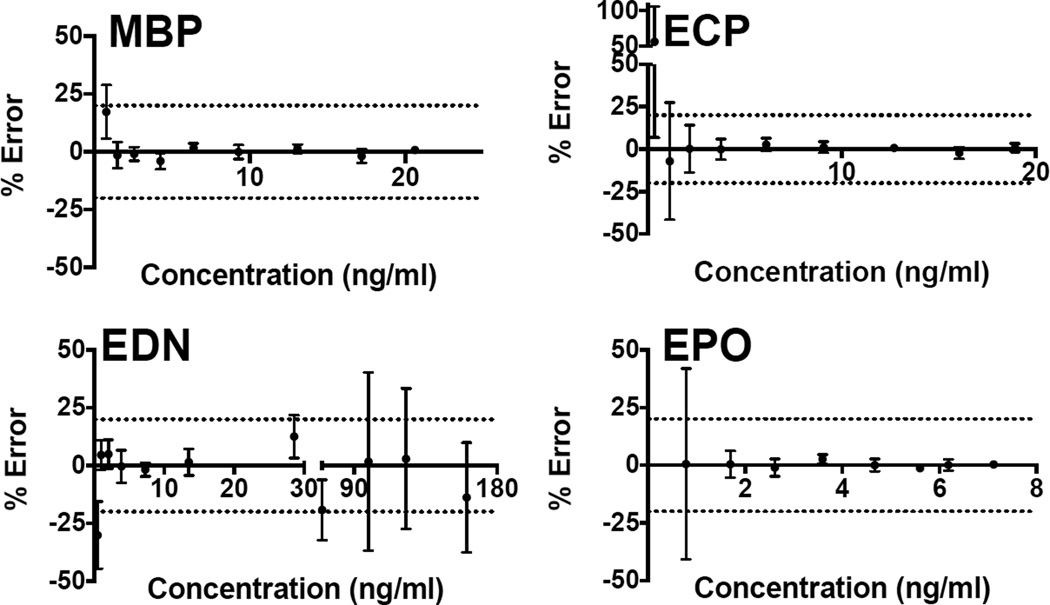
Accuracy of the serum standard control was assessed using 21 parallel measurements (Fig. 2). After determining the measured concentration of each standard dilution by back calculation and comparison to the expected concentration, the percentage error was ascertained. A percent error less than 20% was considered acceptable. Therefore, the serum standard curves were accurate at concentrations greater than 1.485 ng/ml, 2.149 ng/ml and 1.69 ng/ml for MBP, ECP and EPO, respectively. The serum standard curve for EDN was accurate within the range of 2.025 ng/ml and 28.599 ng/ml.
Fig. 2.
Accuracy of the serum standard curves: Serum standard curves (n = 21) were assayed by multiplex. The MFI output was back calculated to determine the observed concentration and then compared to the expected concentration to determine % error. An acceptable % error of +/− 20% is denoted by the dotted line.
3.3 Correlation of eosinophil granule protein concentrations in serum and plasma measured in multiplex
Because clotting of blood for serum collection can cause degranulation of eosinophil granule proteins (EGP) over time, all serum samples were spun within 30 minutes of collection. To verify results of the multiplex assay in serum, the concentrations of eosinophil granule proteins in serum were compared with those from concurrently obtained plasma samples from 12 subjects (Supplemental Figure 4). While plasma samples had consistently lower concentrations of all granule proteins, the levels of MBP (r = 0.8364; P = 0.0022), ECP (r = 0.6294; P = 0.0323), EDN (r = 0.7381; P = 0.0458) and EPO (r = 0.7727; P = 0.0074) were significantly correlated between serum and plasma.
3.4 Comparison of standard curves and correlation of results from ELISA and Multiplex
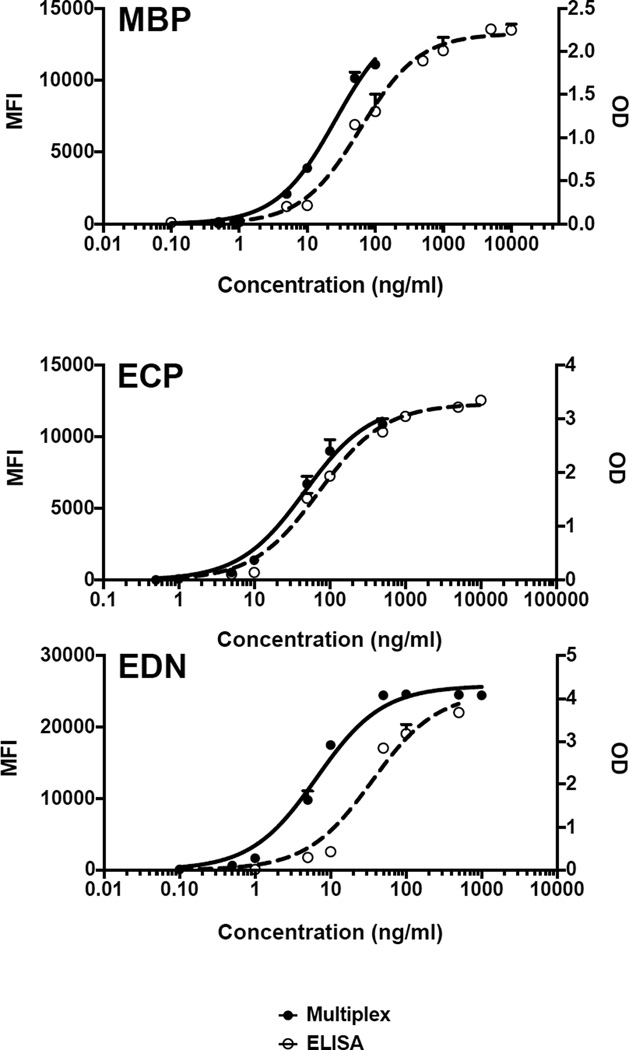
To verify consistency between the ELISA and multiplex assays, the MBP, ECP and EDN purified granule protein standard curves were compared between the two methods (Fig. 3). Whereas the ELISAs gave a broader range of detection, the multiplex assay was more sensitive for ECP and EDN and demonstrated comparable sensitivity for detection of MBP. By ELISA, eosinophil granule protein concentrations in the range of 0.1 – 10,000 ng/ml, 5 – 10,000 ng/ml and 5 – 500 ng/ml would be detectable for MBP, ECP and EDN, respectively. In contrast, the multiplex assay would allow detection in the range of 0.1 – 100 ng/ml for MBP, 0.5 – 500 ng/ml for ECP and 0.1 – 100 ng/ml for EDN. Despite the differences in range and sensitivity, the overall shape of the standard curves remained the same for all three eosinophil granule proteins, regardless of the assay.
Fig. 3.
Comparison of signals from ELISA (OD) and multiplex (MFI) for purified MBP, ECP and EDN standard curves: Closed circles indicate eosinophil granule protein standard curves assayed by multiplex, whereas open circles indicate those assayed by ELISA. Values that were out of range (above or below the curve) as determined by the program software are not represented on the graph.
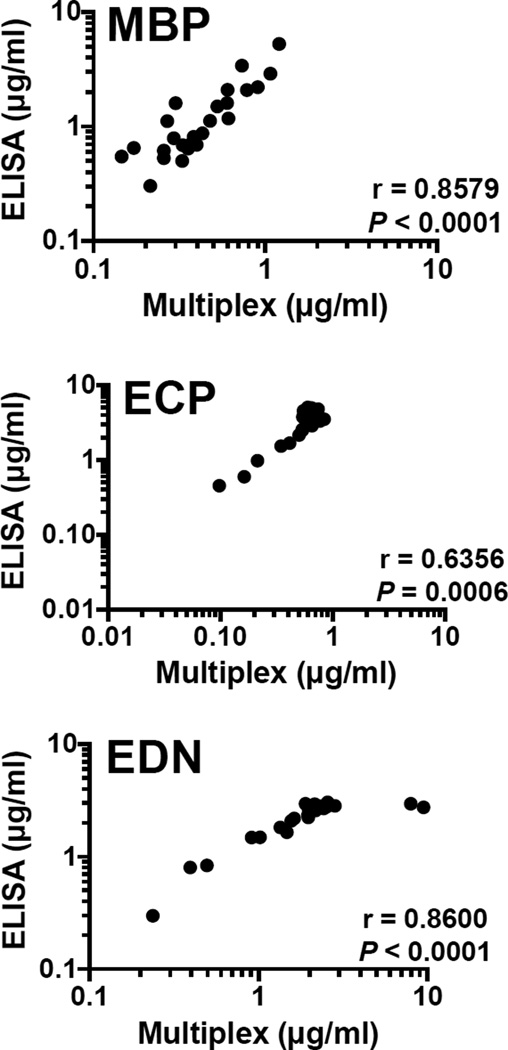
Once the reproducibility of the standard curves between assays was established, subject serum samples were analyzed by both ELISA and multiplex (Fig. 4). Despite systematically lower calculated values for MBP and ECP by ELISA as compared to multiplex, values were significantly correlated between the two methods for MBP, ECP and EDN (r = 0.8579, P < 0.0001; r = 0.6356, P = 0.0006 and r = 0.8600; P < 0.0001, respectively, Spearman rank correlation).
Fig. 4.
Correlation between concentration of MBP, ECP and EDN from ELISA and Multiplex for eosinophilic subject sera (n = 25)
3.5 Inter-assay and Intra-assay Precision of EGP Multiplex Assay
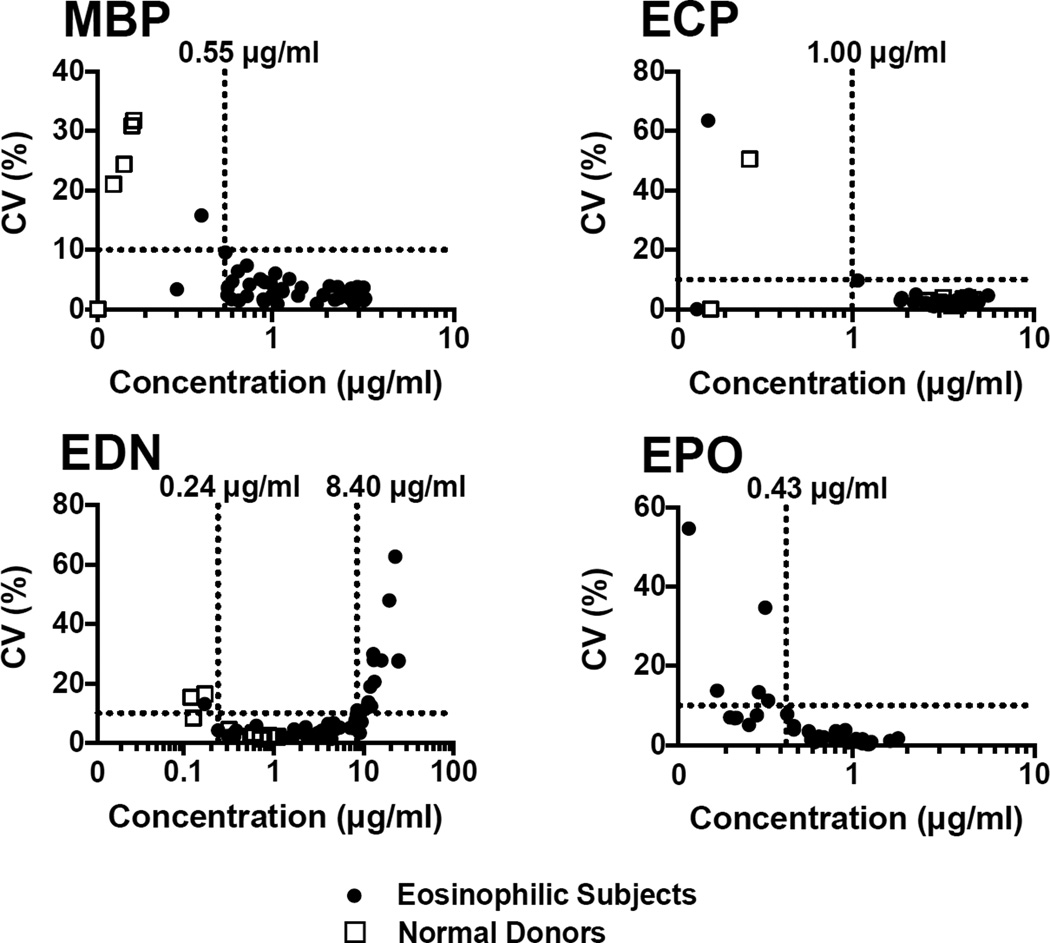
Serum samples from 42 eosinophilic subjects and 19 normal donors were analyzed by multiplex assay for MBP, ECP, EDN and EPO concentration. After analyzing each serum sample in four separate independent multiplex assays under identical conditions, the working range for each eosinophil granule protein was determined based on the evaluation of the coefficient of variation (Fig. 5) and the ratio of single concentration measurement of a sample to the mean of all concentration measurements of a sample (measured/mean ratio) (Supplemental Fig. 5). CV was acceptable (< 10%) at a concentration greater than 0.55 µg/ml for MBP, greater than 1.00 µg/ml for ECP, within the range of 0.24 µg/ml and 8.40 µg/ml for EDN and greater than 0.43 µg/ml for EPO. For MBP, ECP and EDN the measured/mean ratio was acceptable (< 20%) at the same concentrations as those found when measuring the acceptable CV. The range of acceptable measured/mean ratio for EDN was between 0.24 µg/ml and 11 µg/ml. To determine the working range for EDN, the most stringent range was chosen when comparing acceptable CV and acceptable measured/mean ratio, giving a working range for EDN of between 0.24 µg/ml and 8.40 µg/ml.
Fig. 5.
Precision of the eosinophil granule protein multiplex assay: Serum from 42 eosinophilic subjects (closed circles) and 19 normal donors (open squares) were assayed by multiplex (n = 4). The mean concentration of each granule protein was compared with % CV. An acceptable CV of less than 10% established the working range of the assay for each granule protein
Serum from a single eosinophilic subject was analyzed by multiplex in 24 wells of a single plate to determine intra-assay precision. The CVs for MBP, ECP, EDN and EPO were 3.30%, 3.52%, 10.33% and 2.91%, respectively. Only EDN was slightly outside of the acceptable range (< 10%) of CV.
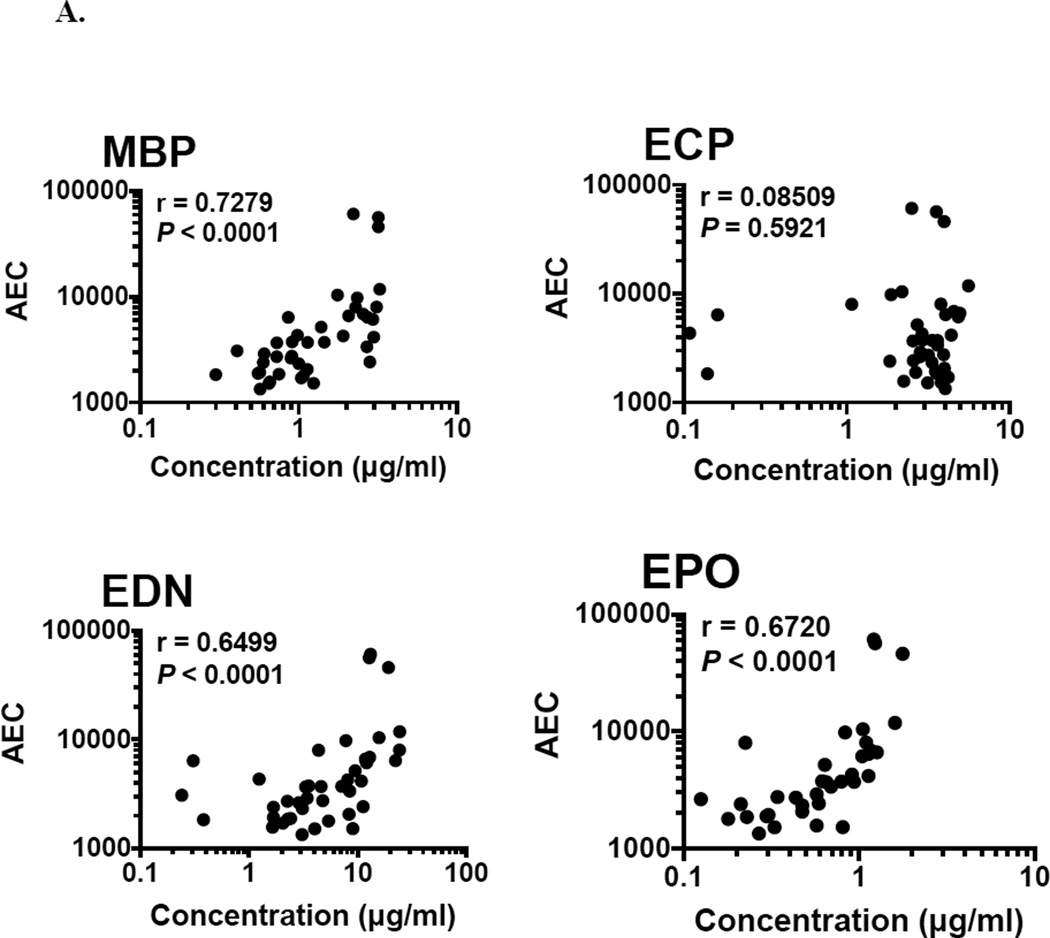
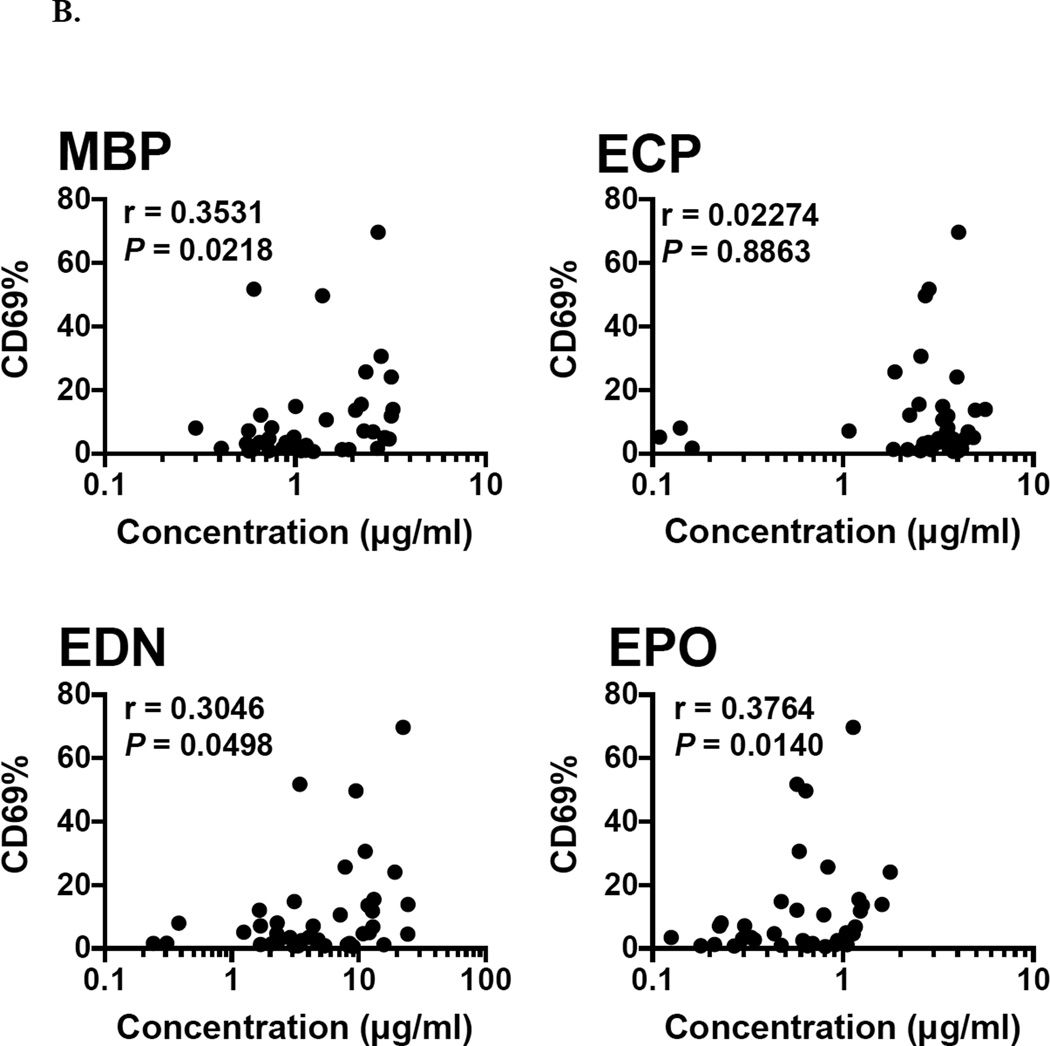
3.6 Correlation of EGP concentration with absolute eosinophil count and activation status
Absolute eosinophil count was significantly correlated with the concentration of MBP, EDN and EPO (r = 0.7279, 0.6499, and 0.6720 respectively; P < 0.0001), but not with the concentration of ECP in serum from eosinophilic subjects (n = 42; Fig. 6A). Expression of the activation marker, CD69, on the surface of eosinophils was assessed by flow cytometry. The percentage of eosinophils expressing CD69 was also correlated with serum levels of MBP (r = 0.3531; P = 0.0218), EDN (r = 0.3046; P = 0.0498) and EPO (r = 0.3764; P = 0.0140), but not ECP (n = 42; Fig. 6B).
Fig. 6.
Correlation between signals from the multiplex assay with (A) Absolute Eosinophil Count (AEC) and (B) CD69% in serum from eosinophilic subjects (n = 42).
3.7 Effect of reduction and alkylation of serum on activity of MBP, ECP, EDN and EPO
Since reduction and alkylation have been reported to increase serum MBP levels measured by ELISA by releasing MBP from complexes in serum (Furuta et al., 2012), the serum standard curve and serum from 42 subjects and 19 normal donors were reduced and alkylated and compared to corresponding untreated samples to determine if reduction and alkylation affect ECP, EDN and EPO levels in the multiplex assay. Levels of MBP (P = 0.0033, mean of differences = 2.947, SD of differences = 2.137), ECP (P < 0.0001, mean of differences = 3.602, SD of differences = 1.220), EDN (P < 0.0001, mean of differences = 9.045, SD of differences = 2.691) and EPO (P = 0.0001, mean of differences = 0.5067, SD of differences = 0.2971) in reduced and alkylated serum were consistently higher than those measured in untreated serum (Supplemental Figure 6A). Whereas ECP (r = 0.7692; P = 0.0049), EDN (r = 0.8182; P = 0.0019) and EPO (r = 0.7483; P = 0.0070) concentrations were highly correlated between reduced and alkylated serum and untreated serum, MBP (r = 0.2500; P = 0.5206) concentrations in reduced and alkylated samples were almost uniformly increased and not correlated with levels in untreated serum (Supplemental Figure 6B).
3.8 Patterns of EGP Expression in Eosinophilic Subjects
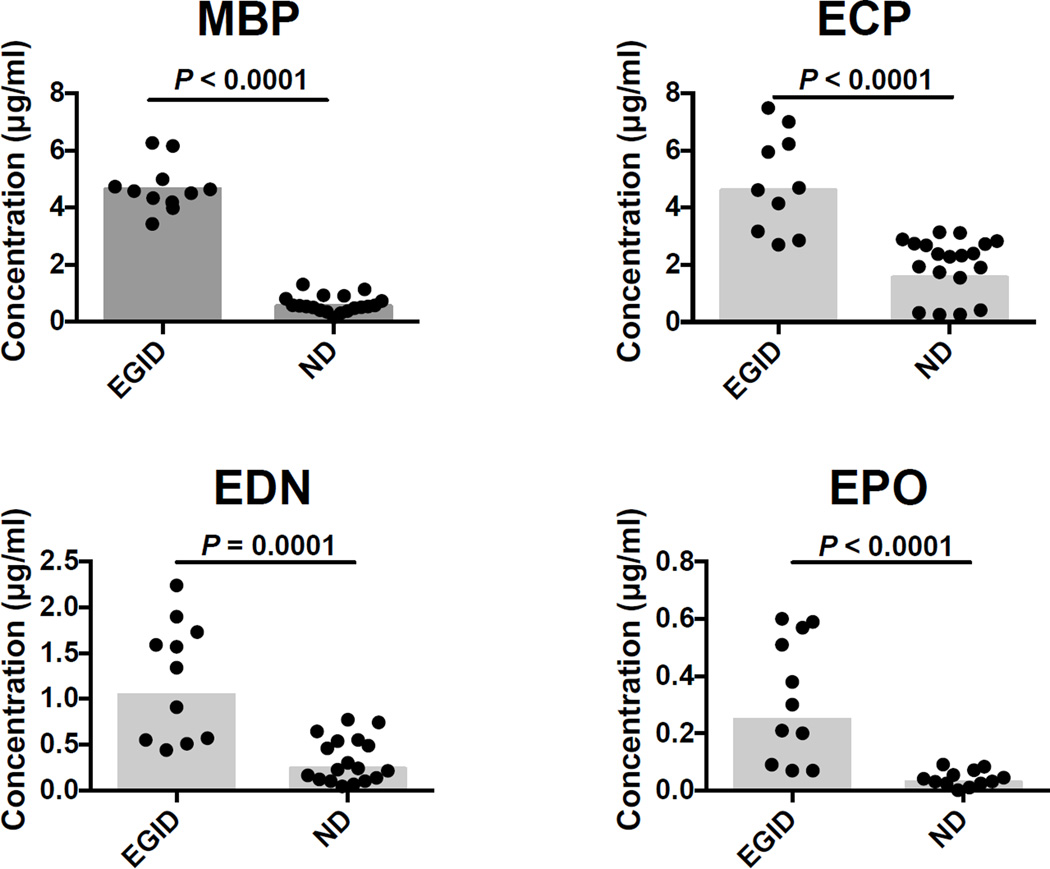
To begin to assess the utility of the EGP multiplex assay in identifying MBP, ECP, EDN and EPO as a biomarkers of tissue eosinophilia in the absence of peripheral blood eosinophilia, EGP levels in reduced and alkylated serum from subjects with eosinophilic gastrointestinal disease (EGID), who had eosinophilic infiltration on tissue biopsy and normal peripheral absolute eosinophil counts (< 0.5 × 109/L), were compared to samples from normal donors (Fig. 7). Whereas the absolute eosinophil count was not significantly different between EGID subjects and normal donors (P = 0.2976), concentrations of MBP (P < 0.0001, EGID geometric mean (GM) = 4.642 and SD = 0.8506, ND GM = 0.5520 and SD = 0.2925), ECP (P < 0.0001, EGID GM = 4.605 and SD = 1.721, ND GM = 1.555 and SD = 0.9928), EDN (P = 0.0001, EGID GM = 1.042 and SD = 0.6416, ND GM = 0.2392 and SD = 0.2424), and EPO (P < 0.0001, EGID GM = 0.2479 and SD = 0.2140, ND GM = 0.02909 and SD = 0.02794), were significantly higher in EGID subjects. In fact, using the GM MBP level + 3 SD of the normal subjects as the cutoff, serum MBP levels were able to identify EGID subjects with 100% sensitivity and specificity.
Fig. 7.
Comparison of eosinophil granule protein concentrations in reduced and alkylated serum between subjects with EGID without peripheral eosinophilia (n = 11) and normal donors (n = 19). The absolute eosinophil count was not significantly different between groups (Mann-Whitney Test)
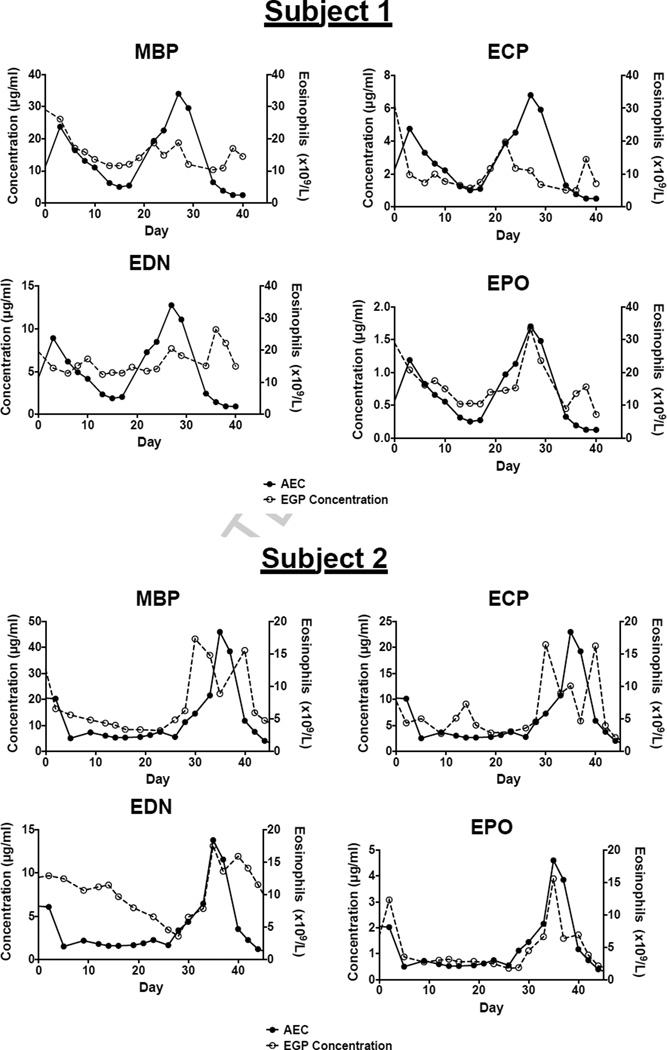
Subjects with episodic angioedema associated with eosinophilia (EAE) experience cyclic elevation of the absolute eosinophil count associated with recurrent symptoms, such as weight gain, fever, angioedema and urticaria, and resolution of symptoms and eosinophil count between episodes (Gleich et al., 1984). Longitudinal changes in the concentrations of ECP, MBP, EDN and EPO were assessed in 2 subjects with EAE using reduced and alkylated serum samples collected every 3 – 4 days during a single cycle (Fig. 8). Serum levels of all four EGP showed similar patterns in the 2 subjects. Serum levels of MBP and ECP peaked just prior to the peak AEC and again as the AEC approached a nadir. In contrast, peak serum levels of EPO appeared to coincide with the peak AEC with a smaller second rise as the eosinophilia resolved. Although serum EDN levels peaked after the peak AEC in both subjects, a second elevation at the time of the peak AEC was not consistently present.
Fig. 8.
Longitudinal variations of eosinophil granule protein (EGP) concentrations (open circles) in reduced and alkylated serum from two subjects with EAE in reference to temporal changes in absolute eosinophil count (closed circles).
4. Discussion
The suspension array assay in multiplex for MBP, ECP, EDN and EPO offers a number of technical advantages compared to conventional methods of EGP detection, such as ELISA and RIA. Despite comparable specificity to standard ELISAs using the same capture and detection antibodies, the multiplex assay uses less sample (0.4 µl in a single well vs. 50 µl in each of four wells), takes less time (1 day vs. 2 days) and requires significantly less antibody/well than the corresponding ELISAs (2.5 ng/5000 beads/well vs. 50 ng coating antibody/well, respectively). Coupling of multiple antibodies to the beads is straightforward and highly reproducible. Although some variability in antibody binding can occur, as demonstrated by the slight shift in the MBP standard curve between the two coupling experiments (Supplemental Figure 1), this limitation can easily be overcome by verification of the standard curves after each coupling. Consequently, this technology should be rapidly adaptable to other EGP ELISAs. Furthermore, since the magnetic beads are able to accommodate up to 80 different antibodies, assays for additional eosinophil granule proteins, such as MBP2 (Plager et al., 2006) and Charcot-Leyden crystal protein (Furuta et al., 2013), could be easily incorporated, allowing direct comparison between various proposed biomarkers of eosinophilic disease activity.
As demonstrated in prior studies (Wassom et al., 1981; Furuta et al., 2012), dissociation of complexes in serum by reduction and alkylation was necessary for accurate measurement of MBP levels. Although the effect of this process on measured levels of the other granule proteins was a theoretical concern in the multiplex assay, concentrations of ECP, EDN and EPO in treated serum were uniformly increased and highly correlated between treated and untreated samples (Supplemental Figure 6). The EGP multiplex assay was also able to reliably measure levels of MBP, ECP, EDN and EPO in plasma (Supplemental Figure 4), as well as in urine and supernatants from cultured eosinophils (data not shown).
Although peripheral blood eosinophil counts often correlate with disease activity in patients with eosinophilic disorders, eosinophilic tissue infiltration and end organ manifestations can occur in the absence of peripheral eosinophilia. This is particularly common in organ-restricted eosinophilic conditions, such as eosinophilic esophagitis (Konikoff et al., 2006) and asthma, but has also been observed following administration of eosinophil-targeted therapies that dramatically lower blood eosinophil counts but have variable effects on tissue eosinophilia (Flood-Page et al., 2003; Laviolette et al., 2013). In these settings, measurement of eosinophil granule proteins in serum, sputum, and/or other body fluids, has been reported to serve as a useful biomarker of disease activity (Pucci et al., 2000; Lonnkvist et al., 2001; Furuta et al., 2013; Nair et al., 2013), although most studies have not examined the relative performance of the different EGPs. In the present study, we confirmed that despite their having blood eosinophil counts in the normal range, subjects with active EGID have elevated serum levels of all four EGP as compared to normal controls (Figure 7) and demonstrate the relative superiority of serum MBP levels in distinguishing between subjects with EGID and normal controls. Comparisons between the individual EGP levels, alone or in combination, as biomarkers of disease activity in EGID will require additional longitudinal studies.
Whereas ultrastructural studies have clearly demonstrated differential localization of individual EGP in human eosinophils (Peters et al., 1986; Torpier et al., 1988; Karawajczyk et al., 2013) and preferential detection of individual EGP in the serum or plasma has been reported in a wide variety of cross-sectional studies (Ott et al., 1994; Karawajczyk et al., 1995; Koller et al., 1999), there are little data addressing the variations of serum/plasma levels of the different EGP in vivo over time. In one study, MBP and EDN levels were measured serially in the plasma of subjects with onchocerciasis before and after diethylcarbamazine treatment (Ackerman et al., 1990). Whereas the absolute eosinophil count and MBP level were maximal at 12 hours posttreatment, plasma levels of EDN peaked at 6–8 hours post treatment, the point at which the absolute eosinophil count is at its lowest, presumably reflecting transit of eosinophils out of the blood and into the tissues. In the present study, we examined the longitudinal differences of ECP, MBP, EDN and EPO concentrations in the setting of episodic angioedema associated with eosinophilia (EAE), a disorder in which the peripheral eosinophil count and clinical symptoms demonstrate a characteristic cyclic variation (Gleich et al., 1984). Consistent with the onchocerciasis study, serum levels of MBP and EDN peaked at different times in relation to the absolute eosinophil count and showed similar patterns in the two subjects studied (Figure 8). Although these findings suggest differential release of EGP over time, the contribution of EGP from other cell types, including neutrophils and basophils (Abu-Ghazaleh et al., 1992), cannot be excluded and extension of these studies to larger well-defined patient cohorts and varied clinical settings is needed.
In summary, a suspension array assay in multiplex has been developed to simultaneously measure the concentrations of MBP, ECP, EDN and EPO in clinical and research samples. The sensitivity and specificity of this new eosinophil granule protein detection method has been demonstrated by comparison with established ELISAs for MBP, ECP and EDN. Preliminary results using human sera from well-defined cohorts confirm detection of EGP in the setting of active eosinophilic tissue involvement without peripheral eosinophilia and differing patterns of individual EGP levels over time in subjects with cyclic eosinophilia. These findings suggest that the multiplex EGP assay is not only a useful tool for the comparison of potential biomarkers of disease activity in eosinophilic disorders, but that the ability to easily and reliably measure multiple EGP in clinical and research specimens may provide insights into the biology of eosinophil degranulation.
Supplementary Material
Highlights.
Four different eosinophil granule proteins can be measured simultaneously.
The multiplex assay requires 500-fold less serum than necessary for standard ELISA.
Serum levels of MBP, ECP and EDN measured by ELISA and multiplex were correlated.
AEC and CD69 expression were correlated with MBP, EDN and EPO concentration.
EGID subjects with normal AEC had increased levels of MBP, ECP, EDN and EPO compared to ND.
Acknowledgments
We would like to thank the Laboratory of Parasitic Diseases and Laboratory of Allergic diseases Clinical Center staff and the research subjects without whom the studies described would not have occurred. We would also like to thank Drs. Steven Ackerman and Gerald Gleich for their helpful suggestions and technical assistance with reduction and alkylation. This research was supported by the Intramural Research Program of the NIH, NIAID.
Abbreviations
- AEC
Absolute Eosinophil Count
- CFA
Complete Freund’s Adjuvant
- CV
Coefficients of variation
- EAE
Episodic Angioedema associated with Eosinophilia
- ECP
Eosinophil Cationic Protein
- EDN
Eosinophil Derived Neurotoxin
- EGID
Eosinophilic Gastrointestinal Disorder
- EGP
Eosinophil Granule Proteins (MBP, ECP, EDN and EPO)
- ELISA
Enzyme-linked immunosorbent assay
- EPO
Eosinophil Peroxidase
- FIP1L1-PDGFRA
Fip1-like1/platelet-derived growth factor α
- GM
Geometric Mean
- HES
Hypereosinophilic Syndrome
- HEUS
Hypereosinophilia of unknown significance
- IFA
Incomplete Freund’s Adjuvant
- JAK2
Janus Kinase 2
- LHES
Lymphocytic variant Hypereosinophilic Syndrome
- MBP
Major Basic Protein
- MFI
Mean fluorescence intensity
- ND
Normal Donor
- OD
Optical density
- SD
Standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Ghazaleh RI, Dunnette SL, Loegering DA, Checkel JL, Kita H, Thomas LL, Gleich GJ. Eosinophil granule proteins in peripheral blood granulocytes. Journal of leukocyte biology. 1992;52:611–618. doi: 10.1002/jlb.52.6.611. [DOI] [PubMed] [Google Scholar]
- Ackerman SJ, Gleich GJ, Loegering DA, Richardson BA, Butterworth AE. Comparative toxicity of purified human eosinophil granule cationic proteins for schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1985;34:735–745. doi: 10.4269/ajtmh.1985.34.735. [DOI] [PubMed] [Google Scholar]
- Ackerman SJ, Kephart GM, Francis H, Awadzi K, Gleich GJ, Ottesen EA. Eosinophil degranulation. An immunologic determinant in the pathogenesis of the Mazzotti reaction in human onchocerciasis. J Immunol. 1990;144:3961–3969. [PubMed] [Google Scholar]
- Bafadhel M, McCormick M, Saha S, McKenna S, Shelley M, Hargadon B, Mistry V, Reid C, Parker D, Dodson P, Jenkins M, Lloyd A, Rugman P, Newbold P, Brightling CE. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration; international review of thoracic diseases. 2012;83:36–44. doi: 10.1159/000330667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Cobbold M, Wang Y, Goodall M, Bonney SL, Chamba A, Birtwistle J, Plant T, Afzal Z, Jefferis R, Drayson MT. Development of a highly-sensitive multi-plex assay using monoclonal antibodies for the simultaneous measurement of kappa and lambda immunoglobulin free light chains in serum and urine. Journal of immunological methods. 2013;391:1–13. doi: 10.1016/j.jim.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Colocho Zelaya EA, Orvell C, Strannegard O. Eosinophil cationic protein in nasopharyngeal secretions and serum of infants infected with respiratory syncytial virus. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 1994;5:100–106. doi: 10.1111/j.1399-3038.1994.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. American journal of respiratory and critical care medicine. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, Masterson JC, Ochkur S, Protheroe C, Moore W, Pan Z, Amsden K, Robinson Z, Capocelli K, Mukkada V, Atkins D, Fleischer D, Hosford L, Kwatia MA, Schroeder S, Kelly C, Lovell M, Melin-Aldana H, Ackerman SJ. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2012 doi: 10.1136/gutjnl-2012-303171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, Masterson JC, Ochkur S, Protheroe C, Moore W, Pan Z, Amsden K, Robinson Z, Capocelli K, Mukkada V, Atkins D, Fleischer D, Hosford L, Kwatia MA, Schroeder S, Kelly C, Lovell M, Melin-Aldana H, Ackerman SJ. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62:1395–1405. doi: 10.1136/gutjnl-2012-303171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich GJ, Schroeter AL, Marcoux JP, Sachs MI, O'Connell EJ, Kohler PF. Episodic angioedema associated with eosinophilia. N Engl J Med. 1984;310:1621–1626. doi: 10.1056/NEJM198406213102501. [DOI] [PubMed] [Google Scholar]
- Gopinath R, Hanna LE, Kumaraswami V, Perumal V, Kavitha V, Vijayasekaran V, Nutman TB. Perturbations in eosinophil homeostasis following treatment of lymphatic filariasis. Infect Immun. 2000;68:93–99. doi: 10.1128/iai.68.1.93-99.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren R, Terent A, Venge P. Eosinophil cationic protein (ECP) in the cerebrospinal fluid. Journal of the neurological sciences. 1983;58:57–71. doi: 10.1016/0022-510x(83)90110-7. [DOI] [PubMed] [Google Scholar]
- Hamann KJ, Gleich GJ, Checkel JL, Loegering DA, McCall JW, Barker RL. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol. 1990;144:3166–3173. [PubMed] [Google Scholar]
- Karawajczyk M, Pauksen K, Peterson CG, Eklund E, Venge P. The differential release of eosinophil granule proteins. Studies on patients with acute bacterial and viral infections. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1995;25:713–719. doi: 10.1111/j.1365-2222.1995.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Karawajczyk M, Peterson CG, Venge P, Garcia RC. An extragranular compartment of blood eosinophils contains eosinophil protein X/eosinophil-derived neurotoxin (EPX/EDN) Inflammation. 2013;36:320–329. doi: 10.1007/s10753-012-9549-z. [DOI] [PubMed] [Google Scholar]
- Kato M, Ishioka T, Kita H, Kozawa K, Hayashi Y, Kimura H. Eosinophil granular proteins damage bronchial epithelial cells infected with respiratory syncytial virus. International archives of allergy and immunology. 2012;158(Suppl 1):11–18. doi: 10.1159/000337752. [DOI] [PubMed] [Google Scholar]
- Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunological reviews. 2011;242:161–177. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klion AD, Law MA, Riemenschneider W, McMaster ML, Brown MR, Horne M, Karp B, Robinson M, Sachdev V, Tucker E, Turner M, Nutman TB. Familial eosinophilia: a benign disorder? Blood. 2004;103:4050–4055. doi: 10.1182/blood-2003-11-3850. [DOI] [PubMed] [Google Scholar]
- Koller DY, Halmerbauer G, Muller J, Frischer T, Schierl M. Major basic protein, but not eosinophil cationic protein or eosinophil protein X, is related to atopy in cystic fibrosis. Allergy. 1999;54:1094–1099. doi: 10.1034/j.1398-9995.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- Konikoff MR, Blanchard C, Kirby C, Buckmeier BK, Cohen MB, Heubi JE, Putnam PE, Rothenberg ME. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:1328–1336. doi: 10.1016/j.cgh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, Busse WW, Wenzel S, Wu Y, Datta V, Kolbeck R, Molfino NA. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. The Journal of allergy and clinical immunology. 2013;132:1086–1096. e5. doi: 10.1016/j.jaci.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Lee HH, Choi BS, Jee HM, Kim KW, Sohn MH, Kim KE. Association between Eosinophilic Airway Inflammation and Persistent Airflow Limitation. The Journal of asthma : official journal of the Association for the Care of Asthma. 2013 doi: 10.3109/02770903.2013.776074. [DOI] [PubMed] [Google Scholar]
- Lonnkvist K, Hellman C, Lundahl J, Hallden G, Hedlin G. Eosinophil markers in blood, serum, and urine for monitoring the clinical course in childhood asthma: impact of budesonide treatment and withdrawal. The Journal of allergy and clinical immunology. 2001;107:812–817. doi: 10.1067/mai.2001.114246. [DOI] [PubMed] [Google Scholar]
- Malik A, Batra JK. Antimicrobial activity of human eosinophil granule proteins: involvement in host defence against pathogens. Critical reviews in microbiology. 2012;38:168–181. doi: 10.3109/1040841X.2011.645519. [DOI] [PubMed] [Google Scholar]
- Modig J, Samuelsson T, Hallgren R. The predictive and discriminative value of biologically active products of eosinophils, neutrophils and complement in bronchoalveolar lavage and blood in patients with adult respiratory distress syndrome. Resuscitation. 1986;14:121–134. doi: 10.1016/0300-9572(86)90116-4. [DOI] [PubMed] [Google Scholar]
- Moller JK. Detection of Neisseria meningitidis in cerebrospinal fluid using a multiplex PCR and the Luminex detection technology. Methods Mol Biol. 2012;799:37–53. doi: 10.1007/978-1-61779-346-2_3. [DOI] [PubMed] [Google Scholar]
- Morioka J, Kurosawa M, Inamura H, Nakagami R, Mizushima Y, Chihara J, Yokoseki T, Kitamura S, Omura Y, Shibata M. Development of a novel enzyme-linked immunosorbent assay for blood and urinary eosinophil-derived neurotoxin: a preliminary study in patients with bronchial asthma. International archives of allergy and immunology. 2000;122:49–57. doi: 10.1159/000024358. [DOI] [PubMed] [Google Scholar]
- Muniz VS, Weller PF, Neves JS. Eosinophil crystalloid granules: structure, function, and beyond. Journal of leukocyte biology. 2012;92:281–288. doi: 10.1189/jlb.0212067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P, Ochkur SI, Protheroe C, Radford K, Efthimiadis A, Lee NA, Lee JJ. Eosinophil peroxidase in sputum represents a unique biomarker of airway eosinophilia. Allergy. 2013;68:1177–1184. doi: 10.1111/all.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochkur SI, Kim JD, Protheroe CA, Colbert D, Condjella RM, Bersoux S, Helmers RA, Moqbel R, Lacy P, Kelly EA, Jarjour NN, Kern R, Peters A, Schleimer RP, Furuta GT, Nair P, Lee JJ, Lee NA. A sensitive high throughput ELISA for human eosinophil peroxidase: a specific assay to quantify eosinophil degranulation from patient-derived sources. Journal of immunological methods. 2012;384:10–20. doi: 10.1016/j.jim.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott NL, Gleich GJ, Peterson EA, Fujisawa T, Sur S, Leiferman KM. Assessment of eosinophil and neutrophil participation in atopic dermatitis: comparison with the IgE-mediated late-phase reaction. The Journal of allergy and clinical immunology. 1994;94:120–128. doi: 10.1016/0091-6749(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Peters MS, Rodriguez M, Gleich GJ. Localization of human eosinophil granule major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin by immunoelectron microscopy. Laboratory investigation; a journal of technical methods and pathology. 1986;54:656–662. [PubMed] [Google Scholar]
- Peterson CG, Eklund E, Taha Y, Raab Y, Carlson M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. The American journal of gastroenterology. 2002;97:1755–1762. doi: 10.1111/j.1572-0241.2002.05837.x. [DOI] [PubMed] [Google Scholar]
- Peterson CG, Enander I, Nystrand J, Anderson AS, Nilsson L, Venge P. Radioimmunoassay of human eosinophil cationic protein (ECP) by an improved method. Establishment of normal levels in serum and turnover in vivo. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1991;21:561–567. doi: 10.1111/j.1365-2222.1991.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Plager DA, Loegering DA, Checkel JL, Tang J, Kephart GM, Caffes PL, Adolphson CR, Ohnuki LE, Gleich GJ. Major basic protein homolog (MBP2): a specific human eosinophil marker. J Immunol. 2006;177:7340–7345. doi: 10.4049/jimmunol.177.10.7340. [DOI] [PubMed] [Google Scholar]
- Pucci N, Lombardi E, Novembre E, Farina S, Bernardini R, Rossi E, Favilli T, Vierucci A. Urinary eosinophil protein X and serum eosinophil cationic protein in infants and young children with atopic dermatitis: correlation with disease activity. The Journal of allergy and clinical immunology. 2000;105:353–357. doi: 10.1016/s0091-6749(00)90087-3. [DOI] [PubMed] [Google Scholar]
- Reimert CM, Minuva U, Kharazmi A, Bendtzen K. Eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN). Detection by enzyme-linked immunosorbent assay and purification from normal human urine. Journal of immunological methods. 1991a;141:97–104. doi: 10.1016/0022-1759(91)90214-z. [DOI] [PubMed] [Google Scholar]
- Reimert CM, Venge P, Kharazmi A, Bendtzen K. Detection of eosinophil cationic protein (ECP) by an enzyme-linked immunosorbent assay. Journal of immunological methods. 1991b;138:285–290. doi: 10.1016/0022-1759(91)90177-h. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Assoufi B, Durham SR, Kay AB. Eosinophil cationic protein (ECP) and eosinophil protein X (EPX) concentrations in serum and bronchial lavage fluid in asthma. Effect of prednisolone treatment. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1995;25:1118–1127. doi: 10.1111/j.1365-2222.1995.tb03259.x. [DOI] [PubMed] [Google Scholar]
- Sedgwick JB, Vrtis RF, Jansen KJ, Kita H, Bartemes K, Busse WW. Peripheral blood eosinophils from patients with allergic asthma contain increased intracellular eosinophil-derived neurotoxin. The Journal of allergy and clinical immunology. 2004;114:568–574. doi: 10.1016/j.jaci.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. Journal of leukocyte biology. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini M, Tsicopoulos A, Tai PC, Gruart V, Tonnel AB, Prin L, Capron A, Capron M. Release of granule proteins by eosinophils from allergic and nonallergic patients with eosinophilia on immunoglobulin-dependent activation. The Journal of allergy and clinical immunology. 1991;88:365–375. doi: 10.1016/0091-6749(91)90099-a. [DOI] [PubMed] [Google Scholar]
- Torpier G, Colombel JF, Mathieu-Chandelier C, Capron M, Dessaint JP, Cortot A, Paris JC, Capron A. Eosinophilic gastroenteritis: ultrastructural evidence for a selective release of eosinophil major basic protein. Clinical and experimental immunology. 1988;74:404–408. [PMC free article] [PubMed] [Google Scholar]
- Virchow JC, Jr., Holscher U, Virchow C., Sr. Sputum ECP levels correlate with parameters of airflow obstruction. Am Rev Respir Dis. 1992;146:604–606. doi: 10.1164/ajrccm/146.3.604. [DOI] [PubMed] [Google Scholar]
- Wassom DL, Loegering DA, Solley GO, Moore SB, Schooley RT, Fauci AS, Gleich GJ. Elevated serum levels of the eosinophil granule major basic protein in patients with eosinophilia. The Journal of clinical investigation. 1981;67:651–661. doi: 10.1172/JCI110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Ninomiya H, Yoshimatsu K, Uchiyama Y, Shibasaki M, Enokihara H, Tachibana S, Abe T. Serum levels of major basic protein in patients with or without eosinophilia: measurement by enzyme-linked immunosorbent assay. British journal of haematology. 1994;86:490–495. doi: 10.1111/j.1365-2141.1994.tb04778.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. The Journal of experimental medicine. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.