Abstract
Purpose
In low–tumor burden follicular lymphoma (FL), maintenance rituximab (MR) has been shown to improve progression-free survival when compared with observation. It is not known whether MR provides superior long-term disease control compared with re-treatment rituximab (RR) administered on an as-needed basis. E4402 (RESORT) was a randomized clinical trial designed to compare MR against RR.
Patients and Methods
Eligible patients with previously untreated low–tumor burden FL received four doses of rituximab, and responding patients were randomly assigned to either RR or MR. Patients receiving RR were eligible for re-treatment at each disease progression until treatment failure. Patients assigned to MR received a single dose of rituximab every 3 months until treatment failure. The primary end point was time to treatment failure. Secondary end points included time to first cytotoxic therapy, toxicity, and health-related quality of life (HRQOL).
Results
A total of 289 patients were randomly assigned to RR or MR. With a median follow-up of 4.5 years, the estimated median time to treatment failure was 3.9 years for patients receiving RR and 4.3 years for those receiving MR (P = .54). Three-year freedom from cytotoxic therapy was 84% for those receiving RR and 95% for those receiving MR (P = .03). The median number of rituximab doses was four patients receiving RR and 18 for those receiving MR. There was no difference in HRQOL. Grade 3 to 4 toxicities were infrequent in both arms.
Conclusion
In low–tumor burden FL, a re-treatment strategy uses less rituximab while providing disease control comparable to that achieved with a maintenance strategy.
INTRODUCTION
Rituximab is effective therapy in follicular lymphoma (FL).1–3 The safety and adverse effect profiles make it an attractive alternative to cytotoxic chemotherapy. According to the National LymphoCare database, 15% to 20% of patients with FL receive single-agent rituximab as their initial therapy.4 How to dose rituximab for the optimal blend of efficacy, toxicity, and resource use is unclear. A strategy of maintenance rituximab (MR), after a rituximab induction, has been shown to prolong response duration.5–7 However, it is unclear if MR ultimately translates into better disease control, because patients under observation have the option of receiving re-treatment with rituximab at disease progression.8 One randomized phase II study previously examined the question of MR versus re-treatment rituximab (RR).9 This trial demonstrated that progression-free survival was improved by MR, but it found no difference in the duration of disease control (defined as time to chemotherapy). However, the trial was not definitive, because it was relatively small, with 45 patients per arm, and used a subjective primary end point.
For patients with asymptomatic low–tumor burden FL, it has long been considered reasonable to defer therapy until the development of symptoms or high tumor burden. This strategy of watch and wait (WW) was shown to produce survival equivalent to that with immediate therapy in three randomized clinical trials.10–12 Whether the WW strategy remains appropriate in the rituximab era is unknown. Those with low–tumor burden FL are an appealing patient population for clinical trials with nontoxic agents such as rituximab, the long-term efficacy of which has been established.13 In addition, rituximab therapy may delay the time to first cytotoxic chemotherapy, potentially affecting health-related quality of life (HRQOL).14–16
The Eastern Cooperative Oncology Group (ECOG) protocol E4402—RESORT (Rituximab Extended Schedule or Re-Treatment Trial)—was designed to definitively address the rituximab dosing question of MR versus RR in patients with previously untreated low–tumor burden FL. RESORT enrolled patients with both FL and non-FL indolent lymphoma, with stratification and planned analysis by histology (FL v other). Here we report the results obtained in the FL cohort.
PATIENTS AND METHODS
Eligibility
Patients were considered eligible if the following parameters were met: biopsy-proven grade 1 or 2 FL (small lymphocytic lymphoma, marginal zone nodal, marginal zone splenic, and mucosal-associated lymphoid tissue were eligible for trial but not included in this analysis), age ≥ 18 years, Ann Arbor stage III or IV, ECOG performance status 0 to 1, and low tumor burden by Groupe D'Etude des Lymphomes Folliculaires (GELF) criteria.10 Specifically, low tumor burden was defined as: no mass > 7 cm, < three masses > 3 cm, no systemic or B symptoms, no splenomegaly > 16 cm by computed tomography (CT) scan, no risk of vital organ compression, no leukemic phase > 5,000/μL circulating lymphocytes, and no cytopenias (defined as platelets < 100,000/μL, hemoglobin < 10 g/dL, or absolute neutrophil count < 1,500/μL. Patients were excluded if they had received prior lymphoma therapy, were HIV positive, were pregnant or breastfeeding, had active infections requiring antibiotics, or tested positive for the hepatitis B surface antigen.
Pathology Review
Diagnostic biopsies were to be centrally reviewed by expert pathologists of ECOG to confirm correct histology in accordance with WHO guidelines. Patients with both diffuse and follicular architectural elements were eligible if the histology was predominantly follicular, and there was no evidence of transformation to large-cell histology. If the interval since diagnosis was > 12 months, repeat biopsy was required to confirm the histology remained unchanged.
Baseline Studies
At study entry, a history was obtained for all patients; physical examination, baseline weight, and performance status were also obtained. Baseline laboratory tests were performed within 2 weeks of registration and included CBC, blood chemistries, β2-microglobulin, lactate dehydrogenase, serum pregnancy test (if applicable), quantitative immunoglobulin levels, and hepatitis B surface antigen test. Baseline imaging included CT scans of the neck, chest, abdomen, and pelvis within 6 weeks of registration. Baseline bone marrow biopsies were required unless the patient had had a marrow positive for lymphoma within the previous year.
Protocol Treatment
All patients received single-agent rituximab at a dose of 375 mg/m2 administered once per week for four doses (ie, induction rituximab), with restaging at week 13 (Fig 1). Response was assessed using the standard lymphoma response criteria applicable in 2004.17 Patients with stable or progressive disease (PD) at week 13 were taken off study. Patients demonstrating partial (PR) or complete response (CR) at week 13 were randomly assigned to either RR or MR, stratified by histology (FL v other), age (< 60 years v other), and time from diagnosis (< 1 v ≥ 1 year). Patients randomly assigned to RR were observed, without any additional treatment until PD. At PD, patients receiving RR were re-treated with four doses, once per week, of rituximab, which were repeated at each PD, until the development of treatment failure. Treatment failure was defined as: no response to RR, time to progression < 26 weeks, initiation of alternative therapy, or inability to complete planned therapy. Patients randomly assigned to MR received a single dose of rituximab every 13 weeks until treatment failure. The dosing interval was based on the known rituximab pharmacokinetics at the time of protocol design.18,19 By the definition of treatment failure described, any PD between scheduled rituximab doses constituted treatment failure. Randomly assigned patients were evaluated every 13 weeks with repeat CT scan imaging every 26 weeks.
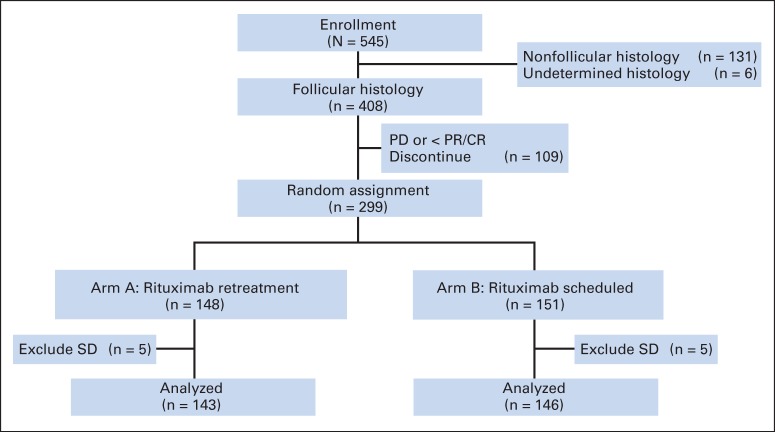
Fig 1.
CONSORT diagram for E4402 (RESORT [Rituximab Extended Schedule or Re-Treatment Trial]). CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Statistical Considerations
The primary end point of the study was time to treatment failure (TTF) in patients with FL. Patients with nonfollicular indolent lymphoma were enrolled as part of a prespecified exploratory analysis. Secondary end points included time to first cytotoxicity therapy (eg, chemotherapy, external-beam radiation, or radioimmunotherapy), toxicity, and HRQOL. The protocol did not include criteria for initiation of cytotoxic therapy, and such decisions were left to the discretion of the treating physician and the patient. On the basis of an estimated 166 events among 270 randomly assigned patients with FL enrolled over 45 months, the study was designed to detect a 36% reduction in the TTF rate, with 81% power at a one-sided .025 significance level. This corresponds to an improvement of median TTF from 21 months in the RR arm to 33 months in the MR arm. Interim analysis was planned for all semiannual data monitoring committee (DMC) meetings. At 78% information, the DMC concluded futility for the primary end point and released the results. Patients still receiving therapy were given the option of coming off study and choosing the best treatment strategy based on personal choice. Although inability to complete protocol therapy was considered an event for the primary end point of TTF, it was determined that treatment withdrawals after the DMC letter would not be considered failure events. Therefore, the data for the TTF analysis were locked as of November 1, 2011. There were no other censoring events except this administrative locking. For all other end points, the data were locked on September 20, 2013.
Additional analysis included overall survival (OS) and duration of response, which was defined as the time from documented response to documented progression. The log-rank test was used for all time-to-event comparisons, stratified on age (< 60 years v other) and time from diagnosis (< 1 v ≥ 1 year). The Kaplan-Meier method and Cox proportional regression models were used to estimate failure rate, hazard ratios, and 95% CIs. Fisher's exact and χ2 tests and t test were used to compare proportions and means, respectively.
RESULTS
Patient Characteristics
Between November 21, 2003, and September 12, 2008, 545 patients with indolent non-Hodgkin lymphoma were enrolled (Fig 2). Central pathology review was required per protocol, and tissue was submitted in 507 patient cases (93%). Review of local pathology reports was used in the remaining 38 patient cases. Follicular histology was noted in 408 patients (75%) and nonfollicular histology in 131 patients (not analyzed or reported here); for six patients, the correct histology could not be determined.
Fig 2.
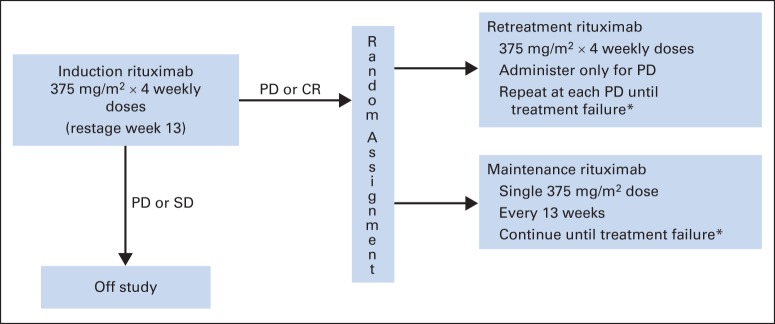
RESORT (Rituximab Extended Schedule or Re-Treatment Trial) treatment schema. CR, complete response; PD, progressive disease; SD, stable disease. (*) Treatment failure definition: no response to re-treatment or PD within 26 weeks of last rituximab dose, initiation of alternative treatment, or inability to complete planned rituximab treatment.
Baseline characteristics of the entire FL cohort and the 289 randomly assigned patients are listed in Table 1. The two treatment arms were well balanced for key baseline features such as age, sex, race, Ann Arbor stage, FL International Prognostic Index (FLIPI) score, and histologic grade. Of note, despite the fact that the population had low tumor burden by GELF criteria, 45% of the patients had intermediate-risk and 39% had high-risk FLIPI scores.
Table 1.
Demographic and Clinical Characteristics of Patients With FL Enrolled Onto and Randomly Assigned in E4402
| Characteristic | All Patients (N = 408) |
Randomly Assigned Patients |
||||
|---|---|---|---|---|---|---|
| RR (n = 143) |
MR (n = 146) |
|||||
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Median | 59.1 | 59.7 | 59.0 | |||
| Range | 25.2-86.4 | 26.0-86.2 | 25.2-86.1 | |||
| < 60 | 218 | 53.4 | 73 | 51.0 | 78 | 53.4 |
| Male sex | 195 | 48 | 67 | 47 | 66 | 45 |
| White race | 387 | 95 | 135 | 94 | 139 | 95 |
| PS 0 | 347 | 85 | 120 | 84 | 128 | 88 |
| BM involvement | 191 | 47 | 62 | 43 | 73 | 50 |
| Elevated β2-microglobulin | 171 | 42 | 65 | 46 | 55 | 38 |
| Elevated LDH | 52 | 13 | 18 | 13 | 22 | 15 |
| HgB < 12 g/dL | 25 | 6 | 12 | 8 | 7 | 5 |
| Stage | ||||||
| I-II | 4 | 1 | 1 | 1 | 2 | 1 |
| III | 201 | 49 | 79 | 55 | 67 | 46 |
| IV | 202 | 50 | 63 | 44 | 77 | 53 |
| FLIPI score | ||||||
| Low | 69 | 17 | 21 | 15 | 23 | 16 |
| Intermediate | 192 | 47 | 65 | 45 | 66 | 45 |
| High | 147 | 36 | 57 | 40 | 57 | 39 |
| FL histology | ||||||
| Grade 1 | 291 | 71 | 101 | 71 | 97 | 66 |
| Grade 2 | 92 | 23 | 33 | 23 | 42 | 29 |
| Grade 3a | 8 | 2 | 3 | 2 | 2 | 1 |
| Not classifiable | 17 | 4 | 6 | 4 | 5 | 3 |
| Time since diagnosis < 1 year | 376 | 92 | 132 | 92 | 132 | 90 |
Abbreviations: BM, bone marrow; FL, follicular lymphoma; FLIPI, Follicular Lymphoma International Prognostic Index; HgB, hemoglobin; LDH, lactate dehydrogenase; MR, maintenance rituximab; PS, performance status; RR, re-treatment rituximab.
Clinical Responses
Of the 408 patients with FL, 289 (70.8%; 95% CI, 67% to 76%) responded to induction rituximab and were randomly assigned to RR (n = 143) or MR (n = 146). The CR/unconfirmed CR (CRu) rate was 11.8%. This is likely lower than expected because of missing repeat bone marrow assessments. When comparing patients receiving RR versus those receiving MR, there was no difference in the proportion of patients achieving CR/CRu versus PR (data not shown). Response quality for patients in both treatment arms tended to improve over time. Of the 120 patients achieving PR assigned to RR, 49 (41%) later converted to CR/CRu, with either no further treatment (n = 29) or after re-treatment at progression (n = 20). Of the 113 patients achieving PR assigned to MR, 58 (51%) converted to CR/CRu while receiving maintenance therapy.
TTF and Time to First Cytotoxic Therapy
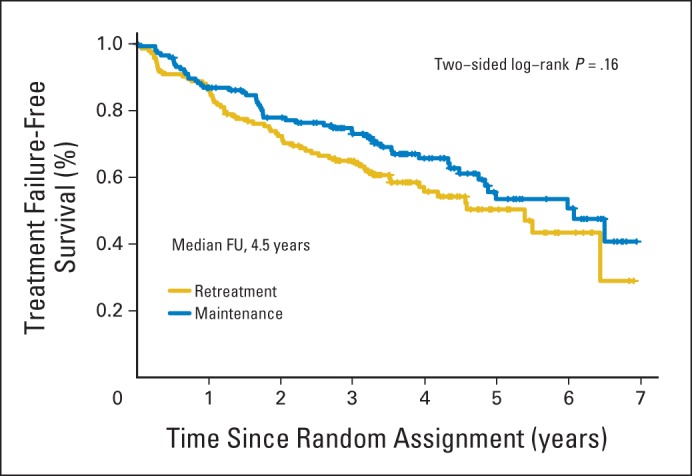
With a median follow-up of 4.5 years, the estimated median TTF was 3.9 years for patients receiving RR and 4.3 years for those receiving MR (P = .54; Fig 3A). The 3-year TTF was 61% for the RR arm and 64% for the MR arm (P = .33). There were 80 treatment failure events in patients assigned to RR and 78 in those assigned to MR (Appendix Table A1, online only). When comparing reasons for treatment failure, more patients receiving RR developed rituximab resistance (n = 37 v 29) or required initiation of alternative therapy (n = 15 v 2), whereas more patients receiving MR experienced an inability to complete planned therapy (n = 23 v 43). A more detailed analysis of the inability to complete therapy revealed more discontinuations because of toxicity, death, or other disease (n = 7 v 19), as well as more withdrawals for reasons other than toxicity (n = 16 v 24), in those receiving MR. Because some withdrawals were for social reasons (eg, patient tired of study commitment), which were potentially more common in the MR arm, a sensitivity analysis was performed, censoring patient withdrawals for nonmedical reasons. The estimated 3-year TTTF was 65% for the RR arm and 73% for the MR arm (P = .16; Appendix Fig A1, online only). For the secondary end point of time to first cytotoxic therapy, MR was superior to RR, with 95% of patients assigned to MR cytotoxic therapy free at 3 years versus 84% of those assigned to RR (P = .03; Fig 3B).
Fig 3.
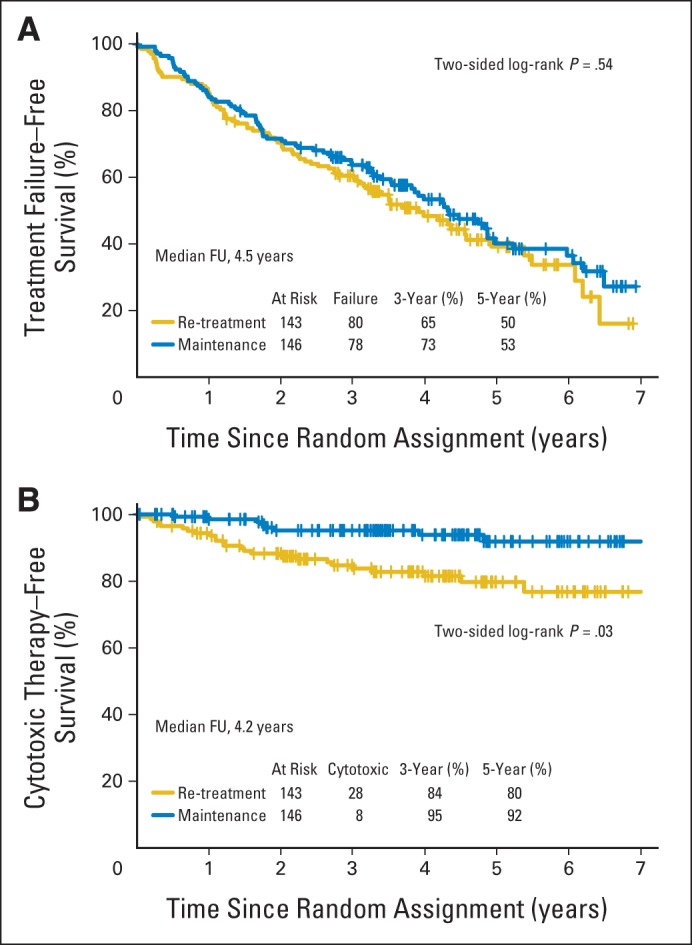
Time to (A) treatment failure and (B) first cytotoxic therapy in 289 patients with follicular lymphoma randomly assigned to re-treatment rituximab or maintenance rituximab. FU, follow-up.
Response Duration, OS, and Risk of Transformation
As anticipated, patients receiving MR were more likely to sustain their first remission than were patients receiving RR (78% v 50% at 3 years; Fig 4A). When tracking the 143 patients assigned to RR over time, the median response duration to induction treatment was 34.4 months; 56 patients received a first re-treatment, with 34 responses (61%) and median response duration of 18.5 months; 12 patients received a second re-treatment, with eight responses (67%) and median response duration of 12 months (Fig 4B); four patients received a third re-treatment, with no responses. There have been relatively few deaths (RR, 11; MR, 13), and there was no difference in OS between those assigned to RR and those assigned to MR (94% v 94% at 5 years). To date, eight patients assigned to RR and six to MR have experienced transformation to more aggressive histology.
Fig 4.
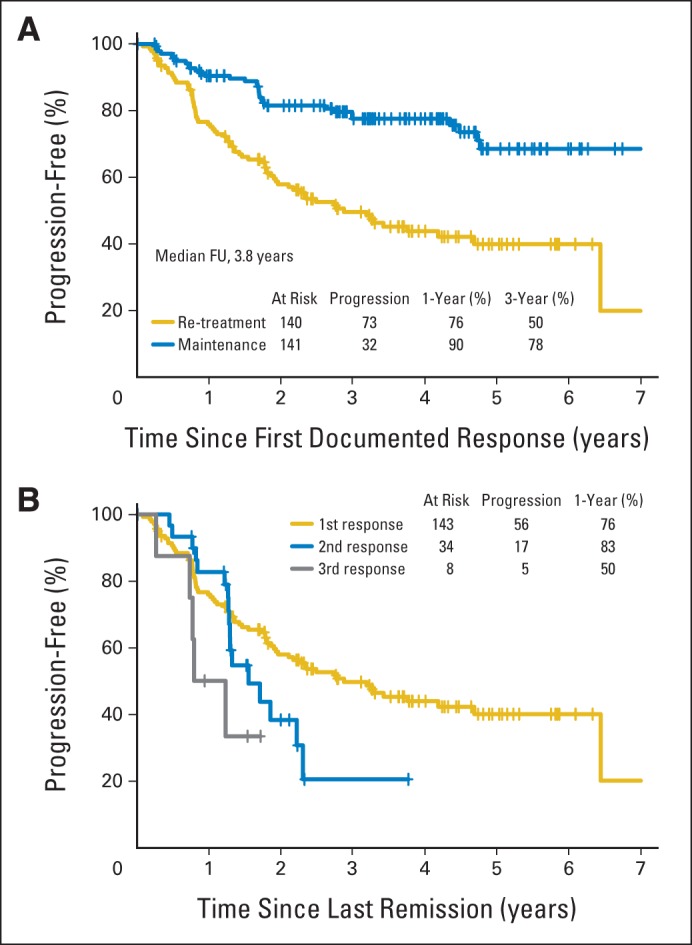
Response duration of patients with follicular lymphoma (A) assigned to re-treatment rituximab (RR; n = 143) or maintenance rituximab (n = 146) and (B) assigned to RR according to first rituximab treatment (n = 143), first rituximab re-treatment (n = 34), and second rituximab re-treatment (n = 8). Zero of four patients responded to third rituximab re-treatment. FU, follow-up.
Prognostic Factor Analysis
A Cox proportional hazards regression analysis was performed to identify features associated with TTF. By univariable and multivariable analyses, treatment arm (RR v MR), age (< 60 v ≥ 60 years), time from diagnosis (< 1 v ≥ 1 year), sex (male v female), and histologic grade (1 v 2) were not associated with risk for treatment failure (Table 2). FLIPI score was prognostic when comparing intermediate versus low, although only a trend was noted when comparing high versus low.
Table 2.
Prognostic Factor Analyses
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Treatment arm (RR v MR) | 1.17 | 0.85 to 1.60 | .33 | 1.07 | 0.77 to 1.49 | .68 |
| Age (< 60 v ≥ 60 years) | 0.86 | 0.63 to 1.17 | .34 | 0.89 | 0.61 to 1.30 | .54 |
| Time since diagnosis (< 1 v ≥ 1 year) | 0.83 | 0.48 to 1.41 | .48 | 0.99 | 0.55 to 1.79 | .97 |
| Sex (female v male) | 0.93 | 0.68 to 1.27 | .66 | 0.96 | 0.69 to 1.33 | .80 |
| Histologic grade (1 v 2) | 0.84 | 0.59 to 1.20 | .33 | 0.82 | 0.57 to 1.18 | .29 |
| FLIPI score (intermediate v low) | 1.76 | 1.06 to 2.92 | .03 | 1.71 | 1.01 to 2.87 | .04 |
| FLIPI score (high v low) | 1.57 | 0.94 to 2.61 | .09 | 1.45 | 0.81 to 2.58 | .21 |
Abbreviations: FLIPI, Follicular Lymphoma International Prognostic Index; HR, hazard ratio; MR, maintenance rituximab; RR, re-treatment rituximab.
Toxicity
Both treatment strategies were well tolerated, with infrequent grade 3 to 5 toxicities noted (Table 3). One patient developed progressive multifocal leukoencephalopathy (PML) after MR dose 15 and died. Second malignancies were reported in 16 patients receiving RR and 14 receiving MR, with no obvious trends toward specific cancers. Immunoglobulin (Ig) levels were measured yearly in patients receiving MR. From year 1 to 7, the median IgG level decreased 22%, from 856 to 668 mg/dL; the median IgA level decreased 45%, from 161 to 89 mg/dL; and the median IgM level decreased 48%, from 54 to 28 mg/dL (Appendix Table A2, online only).
Table 3.
Grade 3 to 5 Toxicities in Randomly Assigned Patients
| Toxicity | Grade |
|||||
|---|---|---|---|---|---|---|
| RR (n = 143) |
MR (n = 146) |
|||||
| 3 | 4 | 5 | 3 | 4 | 5 | |
| Thrombocytopenia | 1 | — | — | — | — | — |
| Neutropenia | — | 2 | — | — | — | — |
| Fever without neutropenia | — | — | — | 1 | — | — |
| Infection without neutropenia | — | — | — | 1 | — | — |
| Fatigue | 1 | — | — | 3 | — | — |
| Hypertension | 1 | — | — | 1 | — | — |
| Insomnia | — | — | — | 1 | — | — |
| Hearing loss | — | — | — | 1 | — | — |
| Generalized weakness | — | — | — | 1 | — | — |
| Encephalopathy | — | — | — | — | — | 1 |
| Syncope | 1 | — | — | — | — | — |
| Larynx pain | — | — | — | 1 | — | — |
| Worst degree | 4 | 2 | — | 10 | — | 1 |
Abbreviations: MR, maintenance rituximab; RR, re-treatment rituximab.
QOL and Resource Use
HRQOL and anxiety were measured throughout the study. Comparison of patients assigned to RR and those assigned to MR revealed no detectable difference in HRQOL or anxiety at any time point. A detailed analysis of HRQOL data from RESORT is reported separately.
Patients assigned to MR received more rituximab compared with those assigned to RR. Including the four induction doses, patients assigned to RR received a median of four doses (range, four to 16), whereas those assigned to MR received a median of 18 doses (range, five to 35).
DISCUSSION
Multiple trials have demonstrated that MR, after single-agent rituximab, chemotherapy, or rituximab plus chemotherapy, prolongs remissions in FL.5,6,20,21 It is not surprising that prolonged treatment with an effective agent generates more durable remissions. The larger question is whether MR can achieve something more meaningful, such as improvement in OS, time to next treatment, or HRQOL—and if it can, will MR continue to show that same benefit when compared with a re-treatment strategy? The goal of the RESORT trial was to definitively determine whether a maintenance dosing strategy provided superior disease control compared with a re-treatment strategy in low–tumor burden FL.
The data from RESORT indicate that in low–tumor burden FL, an RR strategy uses less rituximab and provides disease control comparable to that of an MR strategy. For the primary end point of TTF, there was no difference between RR and MR (median, 3.9 v 4.3 years, respectively; P = .54). For the secondary end point of time to first cytotoxic therapy, a statistically significant difference favoring MR was observed (cytotoxic therapy free at 3 years: RR, 84% v MR, 95%; P = .03). The improvement in time to cytotoxic therapy came at a cost. While on study, the typical patient assigned to RR received four doses of rituximab, whereas the typical patient assigned to MR received 18 doses. On the basis of current Centers for Medicare and Medicaid Services payments of $693.56 per 100 mg of rituximab, for a 1.9-m2 individual, the cost difference (not including infusion costs, nursing, pharmacy preparation, and so on) is approximately $69,000 per patient.22
It is notable that patients randomly assigned to the RR strategy fared extremely well. Approximately 50% of the randomly assigned patients remained in their first remission at 3 years. First and second re-treatment courses produced response rates of 61% and 67%, respectively, with median response durations of 18 and 12 months, respectively. The excellent outcome for patients assigned to RR was likely in part the result of a protocol-derived selection process. By design, only patients responding to induction rituximab were randomly assigned (71%), an important fact to bear in mind when comparing these results with results of other trials in similar patient populations. Nevertheless, for patients responding to rituximab induction, the RR strategy is highly likely to result in avoidance of chemotherapy or radiation therapy for many years.
A unique aspect of RESORT was the application of indefinite MR, permitting several novel observations. Our data suggest that MR does not accelerate the development of rituximab-resistant clones (Appendix Table A1, online only). IgG levels changed only modestly, with a 22% reduction over 7 years, and remained within the normal range for most patients. However, the changes in IgA and IgM levels were more pronounced, decreasing by 45% and 48%, respectively. Our trial did not collect data on grade 1 to 2 infections, but other trials have reported an increased number of infections in patients receiving MR.21,23 Grade 3 to 4 toxicities were infrequent in both the MR and RR arms (10 v six). Although both MR and RR were well tolerated, there was one death resulting from PML in the MR arm, and there were more treatment failures resulting from adverse events in patients receiving MR (nine v one).
RESORT does not specifically address the question of WW versus rituximab for low–tumor burden FL. That question was the subject of a large international trial, based in the United Kingdom and conducted in parallel with RESORT.24 In the UK study, 379 patients were randomly assigned to WW or rituximab induction plus MR for 2 years. The primary end points were time to initiation of new therapy and HRQOL, measured 6 months after the completion of rituximab induction. Rituximab-treated patients were far less likely to require new therapy at 3 years (88% v 46%), and they had significant improvement in HRQOL, with more patients feeling in control of their situation and experiencing less anxiety around their diagnosis. The authors concluded that “rituximab monotherapy should be regarded as a standard approach in the management of many patients with low tumor burden FL.”25(p434) The HRQOL tools in the UK study and the RESORT study were identical, by design. Of interest, an HRQOL advantage for MR was detected in the UK trial (MR v no treatment), whereas no HRQOL difference was found in RESORT (MR v RR), suggesting that intermittent treatment with rituximab overcomes the anxiety associated with a WW strategy.25
In summary, the RESORT trial demonstrates that single-agent rituximab, administered in an RR strategy, is as efficacious as MR in low–tumor burden FL. There was no advantage for MR in TTF or HRQOL. There was a small advantage for MR in time to first cytotoxic therapy, but significantly more rituximab was required to achieve this benefit. This trial has implications for both research and practice. With regard to research, it would be highly interesting to determine if an RR strategy could produce outcomes comparable to those of an MR strategy, when applied after rituximab plus chemotherapy in high–tumor burden FL.21 Given the widespread use of MR, the resource use implications are large. With regard to practice, RESORT indicates that if opting for single-agent rituximab as initial therapy for low–tumor burden FL, a strategy of re-treatment at disease progression is recommended over a strategy of continuous maintenance therapy.
Acknowledgment
Presented at the 53rd Annual Meeting of the American Society of Hematology, San Diego, December 10-13, 2011.
Appendix
Table A1.
Treatment Failure by Category
| Category | RR | MR |
|---|---|---|
| Rituximab resistance | 37 | 29 |
| No response to retreatment | 23 | 0 |
| TTP < 6 months | 14 | 29 |
| Alternative treatment | 15 | 2 |
| Inability to complete planned therapy because of: | 23 | 43 |
| AE* | 1 | 9 |
| Other disease state† | 6 | 10 |
| Patient withdrawal‡ | 16 | 24 |
| Other | 5 | 4 |
| Total | 80 | 78 |
Abbreviations: AE, adverse event; MR, maintenance rituximab; RR, re-treatment rituximab; TTP, time to progression.
AE deemed possibly treatment related.
Development of comorbid condition, deemed unlikely related to treatment (eg, stroke, second malignancy), which precluded continuation of treatment.
Patient withdrawal for nonmedical reason (eg, patient unwilling to continue with computed tomography scans twice per year).
Table A2.
Ig Levels Over 7 Years for Patients Assigned to MR
| Year | IgG (mg/dL) |
IgA (mg/dL) |
IgM (mg/dL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Samples | Minimum | Maximum | Median | No. of Samples | Minimum | Maximum | Median | No. of Samples | Minimum | Maximum | Median | |
| 1 | 103 | 435 | 1528 | 856 | 103 | 24 | 412 | 161 | 103 | 8 | 368 | 54 |
| 2 | 87 | 390 | 1530 | 800 | 86 | 28 | 441 | 154 | 84 | 5 | 371 | 49 |
| 3 | 81 | 343 | 1500 | 765 | 81 | 27 | 435 | 135 | 80 | 4 | 268 | 47 |
| 4 | 48 | 472 | 1550 | 731 | 49 | 40 | 416 | 115 | 46 | 7 | 93 | 37 |
| 5 | 39 | 431 | 1464 | 655 | 47 | 39 | 322 | 127 | 39 | 12 | 134 | 43 |
| 6 | 25 | 493 | 1011 | 710 | 25 | 40 | 218 | 131 | 25 | 13 | 128 | 44 |
| 7 | 13 | 482 | 1410 | 668 | 13 | 32 | 225 | 89 | 13 | 10 | 113 | 28 |
Abbreviations: Ig, immunoglobulin; MR, maintenance rituximab.
Fig A1.
Sensitivity analysis of time to treatment failure with censoring of treatment failures resulting from nonmedical reasons. FU, follow-up.
See accompanying editorial on page 3093; listen to the podcast by Dr Friedberg at www.jco.org/podcasts
Processed as a Rapid Communication manuscript.
Support information appears at the end of this article.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00075946.
Support
Supported in part by Public Health Service Grants No. CA21115, CA23318, CA66636, CA49957, CA21076, CA17145, and CA13650 from the National Cancer Institute, National Institutes of Health, and Department of Health and Human Services.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Brad S. Kahl, Michael E. Williams, Lynne I. Wagner, Sandra J. Horning
Provision of study materials or patients: Brad S. Kahl, Michael E. Williams, Randy D. Gascoyne, John C. Krauss, Thomas M. Habermann, Lode J. Swinnen, Stephen J. Schuster, Christopher G. Peterson, Mark D. Sborov, S. Eric Martin, Matthias Weiss, W. Christopher Ehmann, Sandra J. Horning
Collection and assembly of data: Brad S. Kahl, Fangxin Hong, Michael E. Williams, Randy D. Gascoyne
Data analysis and interpretation: Brad S. Kahl, Fangxin Hong, Michael E. Williams, Lynne I. Wagner, John C. Krauss, Thomas M. Habermann, Lode J. Swinnen, Stephen J. Schuster, Christopher G. Peterson, Mark D. Sborov, S. Eric Martin, Matthias Weiss, W. Christopher Ehmann, Sandra J. Horning
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Rituximab Extended Schedule or Re-Treatment Trial for Low–Tumor Burden Follicular Lymphoma: Eastern Cooperative Oncology Group Protocol E4402
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Brad S. Kahl
Consulting or Advisory Role: Genentech, Roche
Research Funding: Genentech, Roche
Fangxin Hong
No relationships to disclose
Michael E. Williams
Consulting or Advisory Role: Genentech, Roche
Research Funding: Genentech, Roche
Randy D. Gascoyne
Honoraria: Genentech, Roche
Consulting or Advisory Role: Genentech, Roche, Celgene, Seattle Genetics
Speakers' Bureau: Seattle Genetics
Research Funding: Genentech, Roche, Janssen (Inst)
Lynne I. Wagner
No relationships to disclose
John C. Krauss
Consulting or Advisory Role: Seattle Genetics
Thomas M. Habermann
No relationships to disclose
Lode J. Swinnen
Research Funding: AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: Use of allogeneic donor stem cells
Stephen J. Schuster
No relationships to disclose
Christopher G. Peterson
No relationships to disclose
Mark D. Sborov
No relationships to disclose
S. Eric Martin
No relationships to disclose
Matthias Weiss
No relationships to disclose
W. Christopher Ehmann
Research Funding: Pfizer, Novartis
Sandra J. Horning
Employment: Genentech, Roche
Stock or Other Ownership: Genentech, Roche
REFERENCES
- 1.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 2.Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: Clinical and molecular evaluation. Blood. 2001;97:101–106. doi: 10.1182/blood.v97.1.101. [DOI] [PubMed] [Google Scholar]
- 3.Witzig TE, Vukov AM, Habermann TM, et al. Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin's lymphoma: A phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005;23:1103–1108. doi: 10.1200/JCO.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: First report of the National LymphoCare Study. J Clin Oncol. 2009;27:1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hainsworth JD, Litchy S, Burris HA, 3rd, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:4261–4267. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 6.Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly × 4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 7.Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol. 2010;28:4480–4484. doi: 10.1200/JCO.2010.28.4786. [DOI] [PubMed] [Google Scholar]
- 8.Davis TA, Grillo-López AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: Safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 9.Hainsworth JD, Litchy S, Shaffer DW, et al. Maximizing therapeutic benefit of rituximab: Maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma—A randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23:1088–1095. doi: 10.1200/JCO.2005.12.191. [DOI] [PubMed] [Google Scholar]
- 10.Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: A randomized study from the Groupe d'Etude des Lymphomes Folliculaires—Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 1997;15:1110–1117. doi: 10.1200/JCO.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- 11.Young RC, Longo DL, Glatstein E, et al. The treatment of indolent lymphomas: Watchful waiting v aggressive combined modality treatment. Semin Hematol. 1988;25(suppl 2):11–16. [PubMed] [Google Scholar]
- 12.Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: A randomised controlled trial. Lancet. 2003;362:516–522. doi: 10.1016/s0140-6736(03)14110-4. [DOI] [PubMed] [Google Scholar]
- 13.Colombat P, Brousse N, Salles G, et al. Rituximab induction immunotherapy for first-line low-tumor-burden follicular lymphoma: Survival analyses with 7-year follow-up. Ann Oncol. 2012;23:2380–2385. doi: 10.1093/annonc/mds177. [DOI] [PubMed] [Google Scholar]
- 14.Webster K, Cella D. Quality of life in patients with low-grade non-Hodgkin's lymphoma. Oncology (Williston Park) 1998;12:697–714. discussion 714, 717, 721. [PubMed] [Google Scholar]
- 15.Stabler B, Berkowitz L, Schell M, et al. Quality of life in patients treated with rituximab as a single agent. J Clin Oncol. 2002;20(suppl):279a. abstr 2693. [Google Scholar]
- 16.Witzens-Harig M, Reiz M, Heiss C, et al. Quality of life during maintenance therapy with the anti-CD20 antibody rituximab in patients with B cell non-Hodgkin's lymphoma: Results of a prospective randomized controlled trial. Ann Hematol. 2009;88:51–57. doi: 10.1007/s00277-008-0560-2. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI-sponsored international working group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 18.Berinstein NL, Grillo-López AJ, White CA, et al. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 19.Gordan LN, Grow WB, Pusateri A, et al. Phase II trial of individualized rituximab dosing for patients with CD20-positive lymphoproliferative disorders. J Clin Oncol. 2005;23:1096–1102. doi: 10.1200/JCO.2005.12.171. [DOI] [PubMed] [Google Scholar]
- 20.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 study. J Clin Oncol. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare and Medicaid Services. Medicare Part B Drug Average Sales Price. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html?redirect=/McrPartBDrugAvgSalesPrice/
- 23.Vidal L, Gafter-Gvili A, Leibovici L, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: Systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2009;101:248–255. doi: 10.1093/jnci/djn478. [DOI] [PubMed] [Google Scholar]
- 24.Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: An open-label randomised phase 3 trial. Lancet Oncol. 2014;15:424–435. doi: 10.1016/S1470-2045(14)70027-0. [DOI] [PubMed] [Google Scholar]
- 25.Wagner LI, Zhao F, Williams ME, et al. Quality of life results from Eastern Cooperative Oncology Group protocol E4402 (RESORT): A randomized phase III study comparing two different rituximab dosing strategies for indolent non-Hodgkin's lymphoma. Blood. 2012:120. (abstr 235) [Google Scholar]