Abstract
Purpose
Front-line treatment of peripheral T-cell lymphomas (PTCL) involves regimens such as cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) and results in a 5-year overall survival (OS) rate of less than 50%. This phase I open-label study evaluated the safety and activity of brentuximab vedotin administered sequentially with CHOP or in combination with CHP (CHOP without vincristine) as front-line treatment in patients with CD30+ PTCL.
Patients and Methods
Patients received sequential treatment (once every 3 weeks) with brentuximab vedotin 1.8 mg/kg (two cycles) followed by CHOP (six cycles) or brentuximab vedotin 1.8 mg/kg plus CHP (BV+CHP) for six cycles (once every 3 weeks). Responders received single-agent brentuximab vedotin for eight to 10 additional cycles (for a total of 16 cycles). The primary objective was assessment of safety; secondary end points included objective response rate, complete remission (CR) rate, progression-free survival rate (PFS), and OS. There were no prespecified comparisons of the two treatment approaches.
Results
After sequential treatment, 11 (85%) of 13 patients achieved an objective response (CR rate, 62%; estimated 1-year PFS rate, 77%). Grade 3/4 adverse events occurred in eight (62%) of 13 patients. At the end of combination treatment, all patients (n = 26) achieved an objective response (CR rate, 88%; estimated 1-year PFS rate, 71%). All seven patients without anaplastic large-cell lymphoma achieved CR. Grade 3/4 adverse events (≥ 10%) in the combination-treatment group were febrile neutropenia (31%), neutropenia (23%), anemia (15%), and pulmonary embolism (12%).
Conclusion
Brentuximab vedotin, administered sequentially with CHOP or in combination with CHP, had a manageable safety profile and exhibited substantial antitumor activity in newly diagnosed patients with CD30+ PTCL. A randomized phase III trial is under way, comparing BV+CHP with CHOP (clinical trial No. NCT01777152).
INTRODUCTION
Of all newly diagnosed non-Hodgkin lymphomas in the United States, 10% to 15% are aggressive T-cell lymphomas. The 2008 WHO Classification schema lists 18 subtypes of mature T- and natural-killer cell neoplasms, otherwise known as peripheral T-cell lymphomas (PTCLs).1 Many are known to express the cell-surface marker CD30—most notably, systemic anaplastic large-cell lymphomas (sALCL), in which CD30 expression is pathognomonic.2
PTCLs often present with advanced-stage, symptomatic disease.2,3 There has been little progress in the treatment of newly diagnosed PTCL patients with combination chemotherapy. These lymphomas are often grouped together in clinical trials based on their rarity and universally poor outcomes. Five-year overall survival (OS) rates in the large International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study were poor, ranging from 12% to 49%, depending on the patients' histologic subtypes.3
The majority of patients with these lymphomas do not obtain complete remissions (CRs) with multiagent chemotherapy regimens, such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).4 The estimated CR rate after CHOP was 39% in a recent prospective trial.5 In the same study, the median event-free survival was 12 months and the 2-year event-free survival rate was 41% to 45%, indicating that many events occurred in the first year after diagnosis. To improve long-term outcomes, novel treatment strategies are needed to increase CR rates and long-term survival.
Brentuximab vedotin is an antibody-drug conjugate (ADC) with demonstrated efficacy in the treatment of relapsed sALCL. It consists of the chimeric IgG1 antibody cAC10, specific for human CD30; the microtubule-disrupting agent monomethyl auristatin E (MMAE); and a protease-cleavable linker that covalently attaches MMAE to cAC10. Brentuximab vedotin 1.8 mg/kg administered once every 3 weeks was evaluated in a pivotal phase II study of patients with relapsed or refractory sALCL. The objective response rate (ORR) was 86% (CR rate, 57%).6 After a median observation time of 33.4 months, the median duration of objective response was 13.2 months overall and 26.3 months in patients who achieved CR.7 Brentuximab vedotin was generally well tolerated, with manageable adverse events. Peripheral neuropathy (PN), with an incidence of 53% (per Standardized Medical Dictionary for Regulatory Activities Query analysis), was the most clinically meaningful adverse event (AE).
Given the efficacy of brentuximab vedotin in relapsed sALCL patients, a phase I study was designed to evaluate brentuximab vedotin and multiagent chemotherapy in the front-line setting. Our trial explored the safety and activity of brentuximab vedotin, administered sequentially and in combination with multiagent chemotherapy, in patients with newly diagnosed CD30+ PTCL.
PATIENTS AND METHODS
Study Population
Treatment-naive adults with a diagnosis of CD30+ PTCL, including sALCL (anaplastic lymphoma kinase [ALK] -negative or ALK-positive with International Prognostic Index score ≥ 2), were eligible for this study. CD30+ disease for patients without anaplastic large-cell lymphoma (non-ALCL) was defined as ≥ 1% CD30 expression in malignant cells, confirmed by central pathology review. Other key eligibility requirements included fluorodeoxyglucose-avid disease by positron emission tomography (PET), measureable disease by computed tomography (CT; ≥ 1.5 cm), age ≥ 18 years, and an Eastern Cooperative Oncology Group performance status8 of no higher than 2. Patients could not be pregnant and must have met the following criteria: absolute neutrophil count ≥ 1,000/μL, platelet count ≥ 75,000/μL, serum bilirubin ≤ 1.5 × the upper limit of normal (ULN), serum creatinine ≤ 1.5 × ULN, and ALT and AST ≤ 3 × ULN.
Study Design and Treatment
This open-label, phase I study was conducted at 11 centers within the United States and Europe (ClinicalTrials.gov, trial No. NCT01309789). The latest assessment was approximately 6 months after the last patient's end-of-treatment visit. The study was approved by the institutional review board at each site, and written informed consent was obtained per the Declaration of Helsinki.
Patients could receive one of two treatment regimens: a sequential-treatment approach in which sALCL patients received 1.8 mg/kg brentuximab vedotin (two cycles, once every 3 weeks, intravenously [IV]) followed by standard-dose CHOP (six cycles, once every 3 weeks, IV) or a combination-treatment approach in which patients with PTCL, including those with sALCL, received brentuximab vedotin in combination with CHP (CHOP without vincristine; BV+CHP; six cycles, once every 3 weeks, IV). Vincristine was omitted from combination treatment with brentuximab vedotin to eliminate the potential for additional neurotoxicity. The combination-treatment approach included a six-patient sALCL cohort designed to determine the maximum-tolerated dose (MTD) of brentuximab vedotin in combination with CHP. The initial dose of brentuximab vedotin was 1.8 mg/kg and if one or fewer dose-limiting toxicities were observed, enrollment of PTCL patients would continue at that dose. Patients with an objective response at the end of multiagent therapy could receive single-agent brentuximab vedotin for eight to 10 additional cycles (for a total of 16; Appendix Fig A1 [online-only]).
Study Assessments
Baseline assessments included evaluation of disease-related signs and symptoms, physical examination, bone marrow biopsy, CT scans of the chest, neck, abdomen, and pelvis, and PET scan. Antitumor response assessments were per investigator according to the Revised Response Criteria for Malignant Lymphoma.9
For the sequential-treatment approach, responses were assessed by CT/PET scan after two cycles of single-agent brentuximab vedotin treatment and again after six cycles of CHOP. In the combination-treatment approach, responses were assessed by CT/PET scan after six combination treatment cycles. Scans were performed during subsequent single-agent brentuximab vedotin maintenance treatment (cycles 12 and 16). PET scans were not required once a negative scan was documented. Scans were not required following evidence of clinical progression. During follow-up, patients were assessed for survival and disease status every 3 months until death or study closure. For pharmacokinetic analyses, blood concentrations of brentuximab vedotin ADC, MMAE, and total antibody (TAb) were measured.
Safety assessments consisted of the recording of AEs, physical examination, and routine laboratory tests. AEs were summarized using the Medical Dictionary for Regulatory Activities, version 14.0, and were graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 3.0. Safety was monitored by a safety monitoring committee.
Statistical Analysis
Planned enrollment included approximately 20 patients in the sequential-treatment group and a minimum of approximately 26 patients in the combination-treatment group (at least six in the MTD and 20 in the expansion cohorts). Within each group, at least 12 patients provided a 72% chance of observing at least one occurrence (5%) of a clinically relevant AE with a true event rate of 10%.
The primary objective was to assess the safety of each treatment approach. Efficacy end points included response assessments (ORR and CR rates), progression-free survival (PFS), and OS. The ORR and CR rates were calculated, as were the two-sided 95% exact CIs. PFS and OS were estimated using Kaplan-Meier methodology. All statistical analyses were descriptive and formal inferences were limited to CIs.
RESULTS
Patients
Thirty-nine patients were enrolled onto the study (13 and 26 patients in the sequential- and combination-treatment groups, respectively; six patients enrolled onto the MTD cohort as MTD was not exceeded at 1.8 mg/kg of BV+CHP). Despite acceptable safety, the sponsor terminated enrollment in the sequential-treatment group after observing patients, who initially responded to brentuximab vedotin, experience disease progression while receiving CHOP.
The baseline characteristics of all patients are listed in Table 1. Data are summarized by disease subtype (sALCL and non-ALCL) and ALK status. Disease diagnoses included 32 patients with sALCL (ALK-positive, n = 6; ALK-negative, n = 26) and seven patients with other CD30+ PTCL (PTCL-not otherwise specified, n = 2; angioimmunoblastic T-cell lymphoma, n = 2; enteropathy-associated T-cell lymphoma, n = 1; adult T-cell leukemia/lymphoma, n = 2). CD30 expression in tumor cells in the non-ALCL patients assessed by immunohistochemistry ranged from 20% to 98% (Table 2). The median age of patients was 57 years (range, 21 to 82 years). ALK-negative patients were older than ALK-positive patients (median age, 60 v 35 years). Most patients had an Eastern Cooperative Oncology Group score of 0 or 1. At diagnosis, 19 (59%) of 32 sALCL patients and all seven patients with non-ALCL histologies had advanced-stage disease. Six (86%) of seven non-ALCL patients and all six ALK-positive patients had intermediate-risk disease (International Prognostic Index score, 2 to 3). Only one non-ALCL patient (14%) had baseline B symptoms compared with 17 (53%) of 32 ALCL patients. Baseline bone-marrow lymphoma involvement and malignant cutaneous lesions were reported in two and four patients, respectively.
Table 1.
Demographic and Baseline Disease Characteristics by Disease Diagnosis
| Characteristic | sALCL (n = 32) |
Non-ALCL (n = 7) |
Total (N = 39) |
|||||
|---|---|---|---|---|---|---|---|---|
| ALK Positive (n = 6) |
ALK Negative (n = 26) |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||||
| Median | 35 | 60 | 55 | 57 | ||||
| Range | 21-62 | 25-82 | 37-74 | 21-82 | ||||
| Sex | ||||||||
| Men | 3 | 50 | 16 | 62 | 1 | 14 | 20 | 51 |
| Women | 3 | 50 | 10 | 38 | 6 | 86 | 19 | 49 |
| Race | ||||||||
| American Indian or Alaska Native | 0 | 1 | 4 | 0 | 1 | 3 | ||
| Asian | 0 | 1 | 4 | 0 | 1 | 3 | ||
| Black or African American | 1 | 17 | 5 | 19 | 2 | 29 | 8 | 21 |
| White | 4 | 67 | 17 | 65 | 5 | 71 | 26 | 67 |
| Other | 1 | 17 | 2 | 8 | 0 | 3 | 8 | |
| ECOG performance status | ||||||||
| 0 | 0 | 11 | 42 | 2 | 29 | 13 | 33 | |
| 1 | 4 | 67 | 10 | 38 | 5 | 71 | 19 | 49 |
| 2 | 2 | 33 | 5 | 19 | 0 | 7 | 18 | |
| Disease diagnosis | ||||||||
| Adult T-cell leukemia/lymphoma | — | — | 2 | 29 | 2 | 5 | ||
| ALCL | 6 | 100 | 26 | 100 | — | 32 | 82 | |
| Angioimmunoblastic T-cell lymphoma | — | — | 2 | 29 | 2 | 5 | ||
| Enteropathy-associated T-cell lymphoma | — | — | 1 | 14 | 1 | 3 | ||
| Peripheral T-cell lymphoma NOS | — | — | 2 | 29 | 2 | 5 | ||
| Time from diagnosis to first dose, days | ||||||||
| Median | 24.0 | 29.5 | 39.0 | 29.0 | ||||
| Range | 9-36 | 7-79 | 21-109 | 7-109 | ||||
| ALK status | ||||||||
| Positive | 6 | 100 | — | — | 6 | 15 | ||
| Negative | — | 26 | 100 | — | 26 | 67 | ||
| Stage at diagnosis | ||||||||
| I | 0 | 4 | 15 | 0 | 4 | 10 | ||
| II | 1 | 17 | 8 | 31 | 0 | 9 | 23 | |
| III | 1 | 17 | 6 | 23 | 2 | 29 | 9 | 23 |
| IV | 4 | 67 | 8 | 31 | 5 | 71 | 17 | 44 |
| Baseline IPI score | ||||||||
| 0-1 | 0 | 13 | 50 | 0 | 0 | 13 | 33 | |
| 2-3 | 6 | 100 | 7 | 27 | 6 | 86 | 19 | 49 |
| 4-5 | 0 | 6 | 23 | 1 | 14 | 7 | 18 | |
| Baseline B symptoms | 3 | 50 | 14 | 54 | 1 | 14 | 18 | 46 |
| Baseline SPD, cm2* | ||||||||
| Median | 18.4 | 20.7 | 12.2 | 20.3 | ||||
| Range | 4-55 | 3-173 | 2-37 | 2-173 | ||||
| Baseline malignant cutaneous lesions | 2 | 33 | 2 | 8 | 0 | 4 | 10 | |
| Baseline bone marrow involvement | 0 | 2 | 8 | 0 | 2 | 5 | ||
Abbreviations: ALCL, anaplastic large-cell lymphoma; ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; NOS, not otherwise specified; sALCL, systemic anaplastic large-cell lymphoma; SPD, sum of the product diameters.
SPD of up to six of the largest dominant nodes or nodal masses.
Table 2.
CD30 Expression by IHC in Tumor Biopsies, Clinical Response, and Progression-Free Survival for Patients Without ALCL (n = 7)
| Diagnosis | CD30+ Cells (%)* | Tumor H-Score† | Stage at Diagnosis | IPI Score | Response‡ | PFS (months) |
|---|---|---|---|---|---|---|
| Adult T-cell leukemia/lymphoma | 25 | 60 | IV | 3 | CR | 7.1 |
| Adult T-cell leukemia/lymphoma | 98 | 291 | IV | 5 | CR | 22.8§ |
| Angioimmunoblastic T-cell lymphoma | 20 | 38 | IV | 2 | CR | 17.6§ |
| Angioimmunoblastic T-cell lymphoma | 25 | 50 | III | 2 | CR | 4.1§ |
| Enteropathy-associated T-cell lymphoma | 60 | 165 | IV | 2 | CR | 7.0 |
| Peripheral T-cell lymphoma NOS | 50 | 150 | IV | 2 | CR | 22.2§ |
| Peripheral T-cell lymphoma NOS | 80 | 200 | III | 3 | CR | 18.4§ |
Abbreviations: ALCL, anaplastic large-cell lymphoma; CR, complete remission; IHC, immunohistochemistry; IPI, International Prognostic Index; NOS, not otherwise specified; PFS, progression-free survival.
CD30 expression determined by IHC per central pathology review; at least 1% expression per local assessment required for enrollment.
Score that reflects CD30 staining intensity in conjunction with the percentage of CD30+ tumor cells.
Response assessment per investigator (Cheson9) at end of combination treatment, or at last available response assessment for patients who discontinued treatment before cycle 6.
Censored patients.
Safety
Sequential-treatment approach.
All 13 patients received the intended number of initial single-agent brentuximab vedotin treatment cycles (two cycles; median duration, 6 weeks). Eleven (85%) of 13 patients received the subsequent full CHOP-treatment regimen (median, six cycles; range, three to six cycles). One patient experienced progressive disease (PD) after three cycles of CHOP and another continued onto maintenance therapy after only four cycles of CHOP because of toxicity. Twelve of 13 patients went on to receive single-agent brentuximab vedotin maintenance treatment and eight (67%) of 12 patients received all eight cycles. The median absolute dose intensity of brentuximab vedotin was 0.6 mg/kg/week (the intended dose intensity), with a median relative dose intensity of 95.7% (range, 68% to 100%).
Treatment-emergent AEs (TEAEs) of any grade (incidence ≥ 30%) are listed in Table 3. TEAEs of at least grade 3 were experienced by 62% of patients (Table 4). In addition, six (46%) of 13 patients experienced serious adverse events (SAEs); febrile neutropenia was the only SAE that occurred in more than one patient (two patients; 15%). AEs led to treatment discontinuation in two of 13 patients, both times during single-agent brentuximab vedotin maintenance treatment.
Table 3.
Treatment-Emergent Adverse Events (incidence ≥ 30%) in Either Sequential or Combination Treatment
| Preferred Term* | Sequential Treatment (n = 13) |
Combination Treatment (n = 26) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Any event | 13 | 100 | 26 | 100 |
| Peripheral sensory neuropathy | 10 | 77 | 18 | 69 |
| Nausea | 10 | 77 | 17 | 65 |
| Fatigue | 8 | 62 | 15 | 58 |
| Diarrhea | 3 | 23 | 15 | 58 |
| Alopecia | 5 | 38 | 14 | 54 |
| Dyspnea | 6 | 46 | 12 | 46 |
| Constipation | 6 | 46 | 10 | 38 |
| Vomiting | 7 | 54 | 5 | 19 |
| Anemia | 4 | 31 | 8 | 31 |
| Pyrexia | 4 | 31 | 7 | 27 |
| Chills | 3 | 23 | 8 | 31 |
| Febrile neutropenia | 2 | 15 | 8 | 31 |
| Peripheral edema | 5 | 38 | 9 | 35 |
| Upper respiratory tract infection | 3 | 23 | 8 | 31 |
| Headache | 4 | 31 | 7 | 27 |
| Myalgia | 5 | 38 | 8 | 31 |
| Dizziness | 4 | 31 | 5 | 19 |
Medical Dictionary for Regulatory Activities, version 14.0.
Table 4.
Treatment-Emergent Adverse Events With Severity ≥ Grade 3 (occurring in more than one patient) in Either Sequential or Combination Treatment
| Preferred Term* | Sequential Treatment (n = 13) |
Combination Treatment (n = 26) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Any event | 8 | 62 | 19 | 73 |
| Febrile neutropenia | 2 | 15 | 8 | 31 |
| Neutropenia | 2 | 15 | 6 | 23 |
| Anemia | 2 | 15 | 4 | 15 |
| Peripheral sensory neuropathy | 2 | 15 | 2 | 8 |
| Leukopenia | 1 | 8 | 2 | 8 |
| Pulmonary embolism | 0 | 3 | 12 | |
| Septic shock | 1 | 8 | 2 | 8 |
| Syncope | 1 | 8 | 2 | 8 |
| Cardiac failure | 0 | 2 | 8 | |
| Constipation | 2 | 15 | 0 | |
| Fatigue | 2 | 15 | 0 | |
| Respiratory failure | 0 | 2 | 8 | |
Medical Dictionary for Regulatory Activities, Version 14.0.
Combination-treatment approach.
Twenty-three (88%) of 26 patients received the intended number of combination treatment cycles (six cycles; range, three to six cycles). Three patients discontinued treatment before completing six cycles of BV+CHP (two patients because of AEs; one because of investigator decision). Two additional patients discontinued treatment after completing all six cycles of BV+CHP (one patient, AE; one patient, patient decision). After achieving remission, all 21 remaining patients went on to receive single-agent brentuximab vedotin treatment. The median number of brentuximab vedotin cycles administered during the entire treatment period was 13 (range, three to 16 cycles). The median absolute dose intensity of brentuximab vedotin was 0.6 mg/kg/week, with a median relative dose intensity of 95.0% (range, 58% to 101%). The median relative dose intensities were 97.7% for cyclophosphamide and doxorubicin and 98.8% for prednisone. One dose-limiting toxicity was observed among six patients during combination treatment (grade 3 rash); this did not exceed the definition of the MTD, and enrollment continued at 1.8 mg/kg IV BV+CHP.
TEAEs of any grade (incidence ≥ 30%) included peripheral sensory neuropathy (PSN) (69%); nausea (65%); fatigue (58%); diarrhea (58%); alopecia (54%); dyspnea (46%); constipation (38%); peripheral edema (35%); and anemia, chills, febrile neutropenia, upper respiratory tract infection, and myalgia (31% each; Table 3 and Appendix Table A1). Dyspnea was observed in 12 patients, 11 of whom experienced dyspnea with a maximum severity of grade 1 or 2. In four (33%) of 12 patients, dyspnea was considered related to brentuximab vedotin. TEAEs of at least grade 3 were experienced by 73% of patients; febrile neutropenia (31%), neutropenia (23%), anemia (15%), and pulmonary embolism (12%) occurred in at least 10% of patients (Table 4 and Appendix Table A2). Two of the three instances of pulmonary embolism were not considered related to study treatment. SAEs were observed in 50% of patients, and the only SAEs observed in more than one patient were febrile neutropenia (eight patients, 31%), pyrexia (two patients, 8%), and cardiac failure (two patients, 8%). There was no treatment-related mortality.
Overall, six (23%) of 26 patients discontinued treatment as a result of an AE; half during combination treatment and half during single-agent maintenance. PSN was the only AE that led to discontinuation in more than one patient (three patients, 12%). Twenty-one (7%) of 309 brentuximab vedotin doses were delayed per protocol. AEs leading to dose delay and occurring in more than one patient were PSN (four patients) and febrile neutropenia (two patients). All four patients with dosing delays because of PSN experienced delays during single-agent brentuximab vedotin maintenance treatment, and one of these patients also experienced a dosing delay as a result of PSN during BV+CHP treatment. Thirty-nine (13%) of 309 brentuximab vedotin dose reductions occurred per protocol (reduction, 1.8 to 1.2 mg/kg). Nine (35%) of 26 patients experienced dose reductions per protocol, mostly during maintenance treatment. The only AE that led to dose reduction in more than one patient was PSN (n = 7; 27%). There were no unplanned brentuximab vedotin dose adjustments or infusion interruptions and no infusion-related reactions to brentuximab vedotin were reported.
Efficacy
Sequential-treatment approach.
At the end of single-agent brentuximab vedotin treatment, all 13 patients achieved an objective response (five CR; eight partial remissions [PR]). Following subsequent CHOP treatment, 11 of 13 patients maintained or improved their response rates (eight CR; three PR) and two experienced PD (Table 5). After a median observation time of 23.8 months, six (46%) of 13 patients experienced PD or death. Median PFS was 22.1 months (95% CI, 8.8 to not estimable) and the estimated 1-year PFS rate was 77% (95% CI, 44% to 92%; Appendix Fig A2). Median OS was not reached and the estimated 1-year OS rate was 85% (95% CI, 51% to 96%; Appendix Fig A3A).
Table 5.
Best Response After Sequential or Combination Treatment
| Response | Sequential ALCL (n = 13) |
Combination |
||||||
|---|---|---|---|---|---|---|---|---|
| ALCL (n = 19) |
Non-ALCL (n = 7) |
Total (n = 26) |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| Objective response | 11 | 85 | 19 | 100 | 7 | 100 | 26 | 100 |
| Complete remission | 8 | 62 | 16 | 84 | 7 | 100 | 23 | 88 |
| Partial remission | 3 | 23 | 3 | 16 | 0 | 3 | 12 | |
| Stable disease | 0 | 0 | 0 | 0 | ||||
| Progressive disease | 2 | 15 | 0 | 0 | 0 | |||
NOTE. Response assessment per investigator (Cheson9) at cycle 8 (sequential treatment), cycle 6 (combination treatment), or at last available response assessment for patients who discontinued treatment before these time points.
Abbreviation: ALCL, anaplastic large-cell lymphoma.
Combination-treatment approach.
At the end of combination therapy, all 26 patients had achieved an objective response (23 CR; three PR; CR rate, 88%; Table 5). All seven non-ALCL patients achieved CR (Table 2). Following a median observation time of 21.4 months, 11 (42%) patients experienced PD or death (two of seven non-ALCL patients and nine of 19 sALCL patients). Median PFS had not been reached (95% CI, 11.7 to not estimable) and the estimated 1-year PFS rate was 71% (95% CI, 49% to 85%; Fig 1). Of note, no patients went on to receive consolidative autologous or allogeneic stem-cell transplantation. Median OS was not reached and 21 of 26 patients remained alive at the time of analysis. The estimated 1-year OS rate was 88% (95% CI, 68% to 96%; Appendix Fig A3B). All patients in CR after combination therapy maintained their responses at restaging after cycles 12 and 16, and one patient with PR converted to CR during maintenance.
Fig 1.
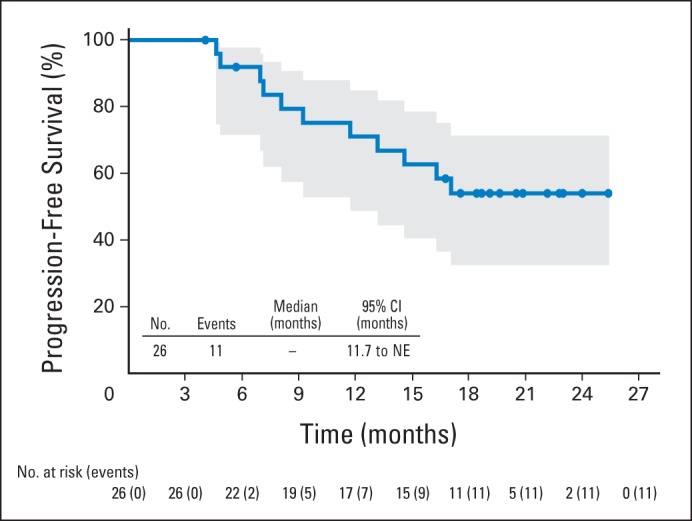
Progression-free survival by Kaplan-Meier analysis for patients receiving combination treatment. Eleven patients experienced progressive disease or death after a median observation time of 21.4 months. Median progression-free survival was not reached (95% CI, 11.7 to NE). Circles on the plot indicate censored patients. Shaded area indicates the 95% CIs. NE, not estimable.
Pharmacokinetics
Trough plasma concentrations of ADC, TAb, or MMAE collected at predose cycle 7 (combination treatment) likely represent steady-state levels with CHP. Plasma samples had geometric mean (% CV) concentrations of ADC, TAb, and MMAE of 1.1 μg/mL (71%), 3 μg/mL (36%), and 0.086 ng/mL (70%), respectively. On discontinuation of CHP, the geometric means of trough concentrations of ADC, TAb, and MMAE with single-agent brentuximab vedotin treatment (predose cycle 14) were comparable (Appendix Table A3).
DISCUSSION
The primary objective of this study was to assess the safety of brentuximab vedotin either in sequence with CHOP or in combination with CHP for the front-line treatment of PTCL. In combination with CHP, the MTD of brentuximab vedotin was not exceeded at 1.8 mg/kg, and the safety profile of BV+CHP was similar to the sequential approach with CHOP. Generally, toxicities with either treatment approach were manageable. Few patients discontinued treatment for treatment-emergent toxicities during the combination-treatment approach, and no treatment-related mortality was observed. The most common TEAEs experienced with either approach were PSN, nausea, fatigue, vomiting, diarrhea, alopecia, and dyspnea. The safety of these treatment approaches compares favorably with other combination anthracycline-based front-line PTCL regimens, such as the CD52 antibody alemtuzumab plus CHOP, which showed serious but manageable infection-related adverse events.10,11 Similarly, etoposide plus CHOP (CHOEP) was generally well tolerated. In one study, when CHOEP or CHOP every 2 weeks was consolidated by autologous stem-cell transplantation (ASCT), treatment-related mortality was 4%, with approximately half of deaths occurring during induction treatment (F. D'Amore, personal communication, March 2014).12
The higher rate of PN observed in this study compared with previous monotherapy experiences with brentuximab vedotin could be explained by sequential administration with vincristine, a microtubule-disrupting agent also associated with PN, and by greater cumulative exposure to brentuximab vedotin in the combination approach, in which the median number of cycles was 13, compared with a median of seven cycles in the pivotal phase II study.6 However, there was a low rate of grade 3 neuropathy events: two (18%) of 11 for sequential treatment and two (11%) of 19 for combination treatment. There were also few treatment discontinuations for neuropathy. Overall, PN was well managed with dose delays and reductions, with the majority of combination-therapy patients (14 of 19; 74%) achieving resolution or symptom improvement.
In the treatment of newly diagnosed PTCL patients, there are two distinct unmet needs: achieving high CR rates and translating these remissions into long-term survival. Several strategies have improved outcomes, including combining new agents with CHOP and upfront consolidation with ASCT.12,13–16 In several studies, achieving CR before ASCT was predictive of superior outcome.16 Historically, combining agents such as etoposide or alemtuzumab with CHOP or intensifying therapy have resulted in improved CR rates compared with CHOP alone (51% to 71% v 35% to 39% with CHOP alone) and came at the expense of increased toxicity.5,10–12,14 Adding agents such as these to CHOP may bias treatment toward younger, fit patients who are able to withstand additional toxicity. Other agents, including romidepsin and pralatrexate, which have shown activity in relapsed PTCL,17,18 are also being evaluated in phase III trials in combination or in sequence with CHOP.
Two strategies for incorporating brentuximab vedotin into front-line treatment were examined. Sequential treatment provided a window of opportunity to understand potential monotherapy activity in the front-line setting. This approach resulted in a high ORR after two cycles of brentuximab vedotin, whereas subsequent CHOP delivered CR in eight of 13 patients; however, two patients experienced PD. These data demonstrate the possibility of using brentuximab vedotin as a prephase treatment, resulting in reduced disease burden, which may be desirable for patients who initially are not candidates for multiagent chemotherapy because of significant comorbidities.
Combination treatment with BV+CHP was studied to provide safety and activity estimates to support future development efforts. The 88% CR rate and 100% ORR exceeded what would typically be expected for multiagent treatment in the PTCL front-line setting, despite the omission of vincristine. Notably, these results were achieved in patients of whom two thirds had advanced-stage or intermediate-high risk disease. Although direct comparisons cannot be made, the estimated 1-year PFS following BV+CHP is similar to that observed with consolidative ASCT, albeit in a slightly different patient population.12 Of note, none of the patients in the current study received post-treatment consolidative ASCT, which was permitted per investigator discretion. All non-ALCL PTCL patients achieved CR. Outcomes in these lymphomas are poor,2,3 and improved activity is particularly meaningful (Table 2).
Combining brentuximab vedotin with CHP provided an encouraging signal of activity, a manageable safety profile, and no treatment-related mortality. These data raise the possibility that BV+CHP may provide improved outcomes over standard CHOP in patients with CD30+ PTCL, and an ongoing double-blind, randomized phase III study of BV+CHP versus CHOP will formally test this hypothesis.
Acknowledgment
We thank J. Christian Hesketh for medical writing assistance and Emily Larsen for statistical support; they are employees of Seattle Genetics.
Appendix
Sequential-treatment approach. Five (38%) of 13 patients presented with preexisting peripheral neuropathy (PN; via Standardized Medical Dictionary for Regulatory Activities Query [SMQ] analysis). Treatment-emergent PN events of any grade were experienced by 11 (85%) of 13 patients. Treatment-emergent events are defined as not being present at baseline or having worsened after the first dose of study drug. Most of these events were grade 1 or 2 and sensory in nature. Grade 3 events occurred in two (18%) of 11 patients; no grade 4 events were observed. The median time to onset of PN was 10.9 weeks (range, 0 to 41). At the time of analysis, 10 patients (91%) had resolution of or symptom improvement of at least one grade. The median time to improvement was 3.8 months (range, 1 to 6 months).
Combination-treatment approach. Treatment-emergent PN events (via SMQ analysis) of any grade were experienced by 19 (73%) of 26 patients, the majority of which were grade 1 or 2 and sensory in nature. Two (11%) of 19 patients experienced grade 3 events; no grade 4 events were observed. The median time to onset of PN was 11 weeks (range, 0 to 34). At the time of analysis, 14 patients (74%) had resolution of or symptom improvement of at least one grade. The median time to improvement was 1.8 months (range, 0 to 6 months).
Table A1.
Treatment-Emergent Adverse Events (incidence ≥ 10%) in Combination Treatment
| Preferred Term* | Combination Treatment (n = 26) |
|
|---|---|---|
| No. | % | |
| Any event | 26 | 100 |
| Peripheral sensory neuropathy | 18 | 69 |
| Nausea | 17 | 65 |
| Diarrhea | 15 | 58 |
| Fatigue | 15 | 58 |
| Alopecia | 14 | 54 |
| Dyspnea | 12 | 46 |
| Constipation | 10 | 38 |
| Peripheral edema | 9 | 35 |
| Anemia | 8 | 31 |
| Chills | 8 | 31 |
| Febrile neutropenia | 8 | 31 |
| Myalgia | 8 | 31 |
| Upper respiratory tract infection | 8 | 31 |
| Anxiety | 7 | 27 |
| Back pain | 7 | 27 |
| Cough | 7 | 27 |
| Headache | 7 | 27 |
| Hypokalemia | 7 | 27 |
| Insomnia | 7 | 27 |
| Pyrexia | 7 | 27 |
| Decreased appetite | 6 | 23 |
| Hypomagnesemia | 6 | 23 |
| Neutropenia | 6 | 23 |
| Pain in extremity | 6 | 23 |
| Abdominal pain | 5 | 19 |
| Arthralgia | 5 | 19 |
| Dizziness | 5 | 19 |
| Dry skin | 5 | 19 |
| Rash | 5 | 19 |
| Vomiting | 5 | 19 |
| Weight decreased | 5 | 19 |
| Depression | 4 | 15 |
| Hot flush | 4 | 15 |
| Nasal congestion | 4 | 15 |
| Rash, maculopapular | 4 | 15 |
| Rash, pruritic | 4 | 15 |
| Urinary tract infection | 4 | 15 |
| Chest pain | 3 | 12 |
| Deep vein thrombosis | 3 | 12 |
| Dehydration | 3 | 12 |
| Dysuria | 3 | 12 |
| Hyperhidrosis | 3 | 12 |
| Muscular weakness | 3 | 12 |
| Musculoskeletal pain | 3 | 12 |
| Peripheral motor neuropathy | 3 | 12 |
| Pruritis | 3 | 12 |
| Pulmonary embolism | 3 | 12 |
| Vision blurred | 3 | 12 |
Medical Dictionary for Regulatory Activities, version 14.0.
Table A2.
Treatment-Emergent Adverse Events With Severity ≥ Grade 3 in Combination Treatment
| Preferred Term* | Combination Treatment (n = 26) |
|
|---|---|---|
| No. | % | |
| Any event | 19 | 73 |
| Febrile neutropenia | 8 | 31 |
| Neutropenia | 6 | 23 |
| Anemia | 4 | 15 |
| Pulmonary embolism | 3 | 12 |
| Cardiac failure | 2 | 8 |
| Leukopenia | 2 | 8 |
| Peripheral sensory neuropathy | 2 | 8 |
| Respiratory failure | 2 | 8 |
| Septic shock | 2 | 8 |
| Syncope | 2 | 8 |
| Abdominal pain | 1 | 4 |
| Alanine aminotransferase increased | 1 | 4 |
| Asthma | 1 | 4 |
| Atrial fibrillation | 1 | 4 |
| Candidiasis | 1 | 4 |
| Decreased appetite | 1 | 4 |
| Dehydration | 1 | 4 |
| Diarrhea | 1 | 4 |
| Diverticulitis | 1 | 4 |
| Dyspnea | 1 | 4 |
| Femoral neck fracture | 1 | 4 |
| Groin abscess | 1 | 4 |
| Hepatic failure | 1 | 4 |
| Hyperglycemia | 1 | 4 |
| Hyperuricemia | 1 | 4 |
| Hypocalcemia | 1 | 4 |
| Hypokalemia | 1 | 4 |
| Hyponatremia | 1 | 4 |
| Hypophosphatemia | 1 | 4 |
| Hypotension | 1 | 4 |
| Ligament rupture | 1 | 4 |
| Liver injury | 1 | 4 |
| Malnutrition | 1 | 4 |
| Meniscus lesion | 1 | 4 |
| Muscle spasms | 1 | 4 |
| Nausea | 1 | 4 |
| Neutropenic colitis | 1 | 4 |
| Pancytopenia | 1 | 4 |
| Paresthesia | 1 | 4 |
| Parametritis | 1 | 4 |
| Parotitis | 1 | 4 |
| Periodontitis | 1 | 4 |
| Peripheral edema | 1 | 4 |
| Peripheral motor neuropathy | 1 | 4 |
| Pneumocystis jiroveci pneumonia | 1 | 4 |
| Rash, maculopapular | 1 | 4 |
| Rash, pruritic | 1 | 4 |
| Rash, pustular | 1 | 4 |
| Renal impairment | 1 | 4 |
| Sepsis | 1 | 4 |
| Soft tissue infection | 1 | 4 |
| Strongyloidiasis | 1 | 4 |
| Subclavian vein thrombosis | 1 | 4 |
| Thrombocytopenia | 1 | 4 |
| Urinary tract infection | 1 | 4 |
| Vomiting | 1 | 4 |
| Weight decreased | 1 | 4 |
Medical Dictionary for Regulatory Activities, version 14.0.
Table A3.
Serum Concentrations of Brentuximab Vedotin Species at Steady-State BV+CHP (cycle 7 trough) and After CHP Washout (cycle 14 trough)
| Species, GM | Cycle 7 (n = 19) |
Cycle 14 (n = 12) |
||
|---|---|---|---|---|
| Serum Concentration (per mL) | % CV | Serum Concentration (per mL) | % CV | |
| ADC | 1.1 μg | 71 | 1.3 μg | 43 |
| TAb | 3.0 μg | 36 | 3.3 μg | 46 |
| MMAE | 0.086 ng | 70* | 0.11 ng | 69† |
Abbreviations: ADC, antibody-drug conjugate; BV+CHP, brentuximab vedotin plus cyclophosphamide, doxorubicin, prednisone; CHP, cyclophosphamide, doxorubicin, prednisone; CV, coefficient of variation; GM, geometric mean; MMAE, monomethyl auristatin E; TAb, total antibody.
Sixteen samples with measurable concentration numbers.
Eleven samples with measurable concentration numbers.
Fig A1.
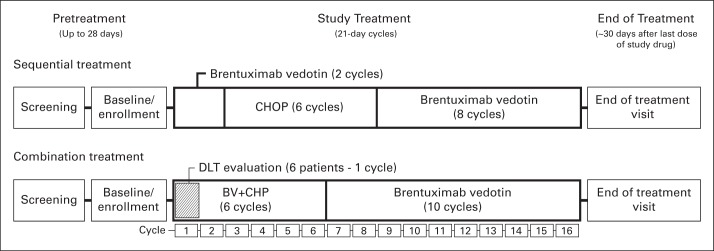
Study schema showing screening, study treatment, and end of treatment for the sequential and combination treatment approaches. Sequential treatment included brentuximab vedotin (BV; two cycles) followed by cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP; six cycles). The combination-treatment approach involved treatment with BV plus CHOP without vincristine (CHP; six cycles) and included a cohort to evaluate any dose-limiting toxicities (DLT; and the maximum-tolerated dose) during cycle 1. Responding patients were eligible to receive subsequent single-agent brentuximab vedotin for eight cycles (sequential treatment) or 10 cycles (combination treatment). During the follow-up period, after receiving the last dose of the study drug, patients were assessed for survival and disease status every 3 months until death or study closure.
Fig A2.
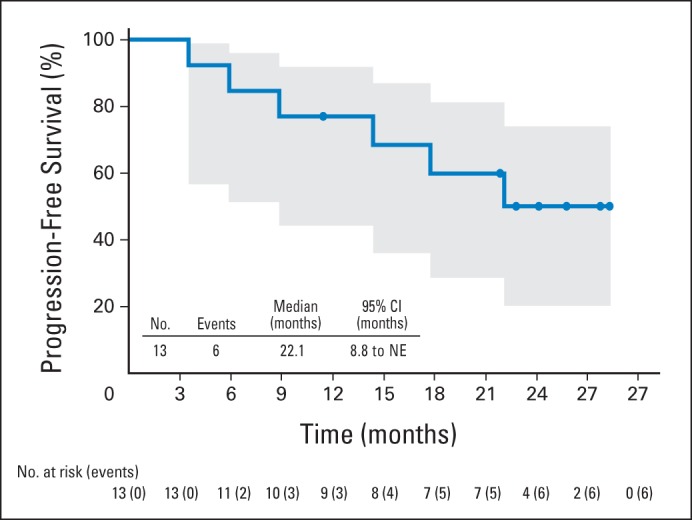
Progression-free survival among patients receiving sequential treatment by Kaplan-Meier analysis. Six patients experienced progressive disease or death after a median observation time of 23.8 months. Median progression-free survival was 22.1 months (95% CI, 8.8 to not estimable [NE]). Circles on the plot indicate censored patients. Shaded area indicates 95% CIs.
Fig A3.
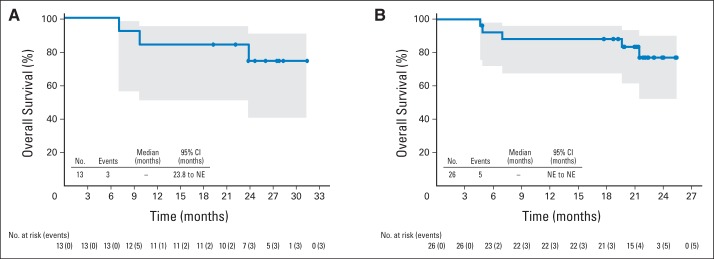
Overall survival among patients receiving (A) sequential treatment or (B) combination treatment by Kaplan-Meier analysis. Three patients receiving sequential treatment died after a median observation time of 23.8 months. Among patients receiving combination treatment, five patients died after a median observation time of 21.4 months. Median overall survival was not reached estimable (NE) for either treatment approach. Circles on the plots indicate censored patients. Shaded areas indicate 95% CIs.
Footnotes
Supported by Seattle Genetics and Takeda Pharmaceuticals International and by general support to R.W.C. by National Cancer Institute Grant No. K12CA001727.
Presented in part at the 54th American Society of Hematology Annual Meeting and Exposition, Atlanta, GA, December 8-11, 2012, and at the 55th American Society of Hematology Annual Meeting and Exposition, New Orleans, LA, December 7-10, 2013.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01309789.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Dirk Huebner, Takeda Pharmaceuticals (C); Dana A. Kennedy, Seattle Genetics (C) Consultant or Advisory Role: Michelle A. Fanale, Seattle Genetics (C); Steven M. Horwitz, Seattle Genetics (C), Takeda Pharmaceuticals (C); Nancy L. Bartlett, Seattle Genetics (C); Ranjana H. Advani, Seattle Genetics (U), Millennium Pharmaceuticals (C); Barbara Pro, Seattle Genetics (U); Robert W. Chen, Seattle Genetics (C); Andrew Davies, Seattle Genetics (C); Tim Illidge, Seattle Genetics (C), Takeda Pharmaceuticals (C); Andrei R. Shustov, Seattle Genetics (C) Stock Ownership: Dirk Huebner, Takeda Pharmaceuticals; Dana A. Kennedy, Seattle Genetics Honoraria: Michelle A. Fanale, Seattle Genetics; Andres Forero-Torres, Seattle Genetics; Robert W. Chen, Seattle Genetics; Andrew Davies, Seattle Genetics, Takeda Pharmaceuticals; Tim Illidge, Seattle Genetics; Andrei R. Shustov, Seattle Genetics Research Funding: Michelle A. Fanale, Seattle Genetics; Steven M. Horwitz, Seattle Genetics, Takeda Pharmaceuticals; Andres Forero-Torres, Seattle Genetics; Nancy L. Bartlett, Seattle Genetics; Ranjana H. Advani, Seattle Genetics, Millennium Pharmaceuticals; Barbara Pro, Seattle Genetics; Robert W. Chen, Seattle Genetics; Andrew Davies, Seattle Genetics; Tim Illidge, Seattle Genetics; Andrei R. Shustov, Seattle Genetics Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: Nancy L. Bartlett, Seattle Genetics; Barbara Pro, Seattle Genetics
AUTHOR CONTRIBUTIONS
Conception and design: Michelle A. Fanale, Steven M. Horwitz, Barbara Pro, Robert W. Chen, Dana A. Kennedy, Andrei R. Shustov
Provision of study materials or patients: Ranjana H. Advani, Andrei R. Shustov
Collection and assembly of data: Michelle A. Fanale, Steven M. Horwitz, Andres Forero-Torres, Nancy L. Bartlett, Barbara Pro, Robert W. Chen, Andrew Davies, Tim Illidge, Dana A. Kennedy, Andrei R. Shustov
Data analysis and interpretation: Michelle A. Fanale, Steven M. Horwitz, Andres Forero-Torres, Nancy L. Bartlett, Ranjana H. Advani, Robert W. Chen, Tim Illidge, Dirk Huebner, Dana A. Kennedy, Andrei R. Shustov
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Swerdlow SH, Campo E, Harris NL, et al. Chapter 11: Mature T- and NK-cell neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues (ed 4) Lyon, France: International Agency for Research on Cancer; 2008. pp. 269–319. [Google Scholar]
- 2.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 3.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ, Chhanabhai M, Gascoyne RD, et al. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- 5.Simon A, Peoch M, Casassus P, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma: Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151:159–166. doi: 10.1111/j.1365-2141.2010.08329.x. [DOI] [PubMed] [Google Scholar]
- 6.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large cell lymphoma: Results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 7.Pro B, Advani RH, Brice P, et al. Three-year survival results from an ongoing phase 2 study of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Presented at the Am Soc Hematol Annual Meeting; December 7-10, 2013; New Orleans, LA. [Google Scholar]
- 8.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 9.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 10.Gallamini A, Zaja F, Patti C, et al. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: Results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–2323. doi: 10.1182/blood-2007-02-074641. [DOI] [PubMed] [Google Scholar]
- 11.Kluin-Nelemans HC, van Marwijk Kooy M, Lugtenburg PJ, et al. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol. 2011;22:1595–1600. doi: 10.1093/annonc/mdq635. [DOI] [PubMed] [Google Scholar]
- 12.d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 13.Mercadal S, Briones J, Xicoy B, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19:958–963. doi: 10.1093/annonc/mdn022. [DOI] [PubMed] [Google Scholar]
- 14.Reimer P, Rüdiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: Results of a prospective multicenter study. J Clin Oncol. 2009;27:106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez J, Conde E, Gutiérrez A, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: A prospective study from The Gel-Tamo Study Group. Eur J Haematol. 2007;79:32–38. doi: 10.1111/j.1600-0609.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- 16.Corradini P, Tarella C, Zallio F, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–1538. doi: 10.1038/sj.leu.2404306. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: Results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]


