Abstract
Purpose
The goal in this study was to determine the incidence of subclinical neuropathy in treatment-naive patients with multiple myeloma (MM) with no history of peripheral neuropathy using quantitative sensory tests (QSTs) and its correlation with innervation density of the extremities using noninvasive laser reflectance confocal microscopy.
Patients and Methods
QST results were collected for 27 patients with a diagnosis of MM and compared with data collected from 30 age- and sex-matched healthy volunteers. Skin temperature, sensorimotor function (grooved pegboard test), and detection thresholds for temperature, sharpness, and low-threshold mechanical stimuli (von Frey monofilaments and bumps detection test) were measured. Meissner's corpuscle (MC) density in the fingertips was assessed using in vivo laser reflectance confocal microscopy.
Results
Patients showed a high incidence (> 80%) of ≥ one subclinical QST deficit. These included increased von Frey, bumps, and warmth detection thresholds as compared with healthy volunteers. Patients also showed increases in cold pain, sensorimotor deficits (grooved pegboard test), and higher overall neuropathy scores. MC density was significantly lower in patients than controls and showed significant inverse correlation with bumps detection threshold.
Conclusion
Patients with MM commonly present with sensory and sensorimotor deficits before undergoing treatment, and these deficits seem to result from disease-related decreases in peripheral innervation density.
INTRODUCTION
Patients with multiple myeloma (MM) typically seek care with signs of renal insufficiency, anemia, and bone lesions.1 Overt neurologic complications may also occur from tumor invasion into the vertebral space that compresses the spinal cord, cranial nerves, or nerve roots; intracranial invasion of tumor; and metabolic derangements.2,3 Clinically significant peripheral neuropathy without clear etiology before treatment has been reported in 5% to 10%2,4 and as high as 20% of patients.5,6 During treatment, peripheral neuropathy is the single most common cause of dose reduction or discontinuation of treatment, potentially affecting survival.7,8 The incidence of treatment-related chemotherapy-induced peripheral neuropathy (CIPN) affects approximately 70% of patients, depending on the therapeutic agent.4,5 The proteasome inhibitor bortezomib (Velcade; Millennium Pharmaceuticals, Cambridge, MA) is a common treatment for MM and is associated with high rates of peripheral neuropathy, which may become chronic and refractory to treatment.5,6
Given the potential profound impact of neurologic complications on disease treatment and quality of life, interest has centered on identifying means of avoiding the occurrence of treatment-related neuropathy in patients with MM. Quantitative sensory tests (QSTs) are a series of noninvasive measures capable of detecting deficiencies in sensory nerve fiber function. In one recently published study, the presence of subclinical sensory deficits in patients with MM was suggested as predictive of the development of CIPN.9 Although patients with pretreatment impairments in sharpness detection (test assessing Aδ fiber function) were at decreased risk for developing CIPN, baseline impairments in warmth detection (test assessing C fiber function) were associated with more severe pain and numbness after chemotherapy treatment.
Biopsies collected from glabrous skin sites in patients treated with chemotherapy show decreases in peripheral innervation, including loss of both intraepidemal nerve fibers and Meissner's corpuscles (MCs).6 MCs are cutaneous receptors consisting of primary afferent terminals interdigitated between stacks of flattened epithelial (laminar) cells that rapidly adapt to vibrations of 30 to 50 Hz and tactile stimuli. Noninvasive in vivo confocal microscopy is a novel imaging technique for visualizing the epidermis and superficial dermis that allows clear identification of MCs within dermal papillae. MC density assessed by in vivo confocal microscopy is well correlated with MC density assessed by confocal microscopy of skin biopsy.10 The goals of this study were to determine the prevalence of subclinical peripheral neuropathy in patients with MM and determine whether these deficits correlate with decreased peripheral innervation density as assessed using in vivo laser reflectance confocal microscopy.
PATIENTS AND METHODS
Patients and Volunteers
Twenty-seven patients with no previous symptoms or complaints of peripheral neuropathy or clear risk factors for neuropathy were recruited for this study through the Multiple Myeloma Clinic at the University of Texas MD Anderson Cancer Center. A group of 30 age- and sex-matched volunteers recruited from the staff of the institution provided comparative data. All participants provided informed consent to participate in the research protocols that had been reviewed and approved by the institutional review board of the MD Anderson Cancer Center.
Quantitative Sensory Analysis
Quantitative sensory analysis was performed as previously described.6,10,11 Three areas—the fingertip, thenar eminence, and volar surface of the forearm—were selected for sensory testing based on the distribution of sensory disturbances that have been documented in CIPN.6,11
Touch Detection Thresholds and Grooved Pegboard Test
Touch detection thresholds were determined using von Frey monofilaments (Semmes-Weinstein) in an up/down manner.6,11 Starting with a bending force of 0.02 g, each filament was applied to the skin for approximately 1 second in each of the three test sites. If the participant did not detect the stimulus, the next higher force was applied to the same location. When the participant detected the presence of the stimulus, the next lower force was administered. This procedure continued until the same filament was detected for three applications, and the associated force was considered the touch detection threshold.
A second, finer touch detection method based on detection of minute elevations (bumps detection) on a smooth surface was applied in 22 patients and 15 healthy volunteers.6,12 The bumps device consisted of three etched glass plates (11.5 × 15 cm), each of which contained 12 1.5- × 1.5-in squares. Within each square were five flat circles, each of a different color. Located on one of the circles within each square was a bump 550 μm in diameter. Bumps on plate one of the three-plate series varied from 2.5 to 8.0 μm in height; bumps on plate two varied from 8.5 to 14.0 μm in height; bumps on plate three ranged from 14.5 to 26 μm in height. Participants began each session using bumps that ranged from 8.5 to 14 μm. They were instructed to use the index finger of the dominant hand to explore the five circles within each square. Patients were unable to see the location of the bump and reported to the examiner which color they perceived the bump to be located on. If participants could correctly identify the location of bumps on plate two, they progressed to plate one (2.5 to 8 μm). Patients unable to detect the location of bumps on plate two were presented with plate two (14.5 to 26 μm). The bumps detection threshold was determined to be the smallest bump correctly identified in sequence to the next two higher bumps.12
Manual dexterity was assessed with the grooved pegboard test.13 Participants were instructed to fill a 5 × 5 slotted pegboard in an ordered fashion, either across rows or down columns. The time a participant took to complete the board was measured for both the dominant and nondominant hands. A faster time indicated greater dexterity.10
Sharpness Detection Threshold
The ability to detect sharpness was determined using weighted needle devices of 8, 10, 16, 20, 30, 32, 64, and 128 g.14 Each stimulus was applied for 1 second in ascending order. Participants were instructed to state whether the sensation produced by each stimulus was that of touch, pressure, sharpness, or pain. The sharpness detection threshold was defined as the mean force deemed sharp or painful from three trials that were separated by an average interval of 30 to 90 seconds.
Heat and Cold Detection Thresholds
Warmth detection and heat pain threshold was determined as previously described.6,11 Heat stimuli were applied using a 3.6- × 3.6-cm Peltier probe applied to the skin. The baseline temperature of the probe was set at 32°C, and the temperature increased at a rate of 0.30°C/sec. Participants signaled when the probe was first perceived as warm and then painful. The trial was immediately terminated, and the probe returned to baseline temperature. The final warmth detection and heat pain threshold for each site was defined as the mean of three trials that were separated by an average of 30 to 90 seconds. If a participant did not perceive warmth or heat pain, the cutoff temperature of 52°C was recorded as the default.
The threshold to detection of cooling (cool threshold) and then cold pain (cold threshold) was similarly determined, except the temperature was decreased at a rate of 0.50°C/sec. If a participant did not perceive cold pain, the cutoff of 3°C was recorded as the default value.
Skin Temperature
Skin temperature was measured using a radiometer, placed gently against the skin for approximately 2 seconds.
Imaging and Meissner Quantification
In vivo confocal imaging was performed on the skin of 12 patients and 10 healthy controls using Lucid Vivascope 1500 (Caliber I.D., Rochester, NY) as previously described.10 The microscope was centered on the tip of the fifth digit over a plastic ring and produced an image with a 2.0- × 2.0-mm field of view. Skin architecture was assessed using a stack of four images with a vertical resolution of 3 to 5 μm at different depths (z plane, 20 μm). MCs were quantified at the depth where they were most easily visualized by a research assistant blinded to the study group. Images that were 4.0 mm2 were divided into four quadrants, and MCs were quantified on one randomly chosen quadrant. MCs were identified as round light-colored structures 40 to 60 μm in diameter located in dermal papillae as previously described.10
Statistical Analysis
Comparisons of sensory and sensory-motor thresholds were performed between volunteers and patients by first evaluating for normality using the Shapiro-Wilk test and then using the nonparametric Wilcoxon signed-rank test. Results are reported as mean ± SEM. Correlations were performed with the nonparametric Spearman rank-order correlation. For every comparison, P < .05 was considered significant.
RESULTS
Study Group
Patient characteristics are listed in Table 1. None of the patients had a history of chemotherapy treatment, AIDS, diabetes, or radiation exposure that might have contributed to the development of neuropathy. All patient QSTs were collected before chemotherapy was initiated. Healthy volunteers had no exposure to equipment or testing procedures before undergoing QSTs. QSTs and scans were collected by a coordinator who did not participate in the data analysis.
Table 1.
Patient Demographic and Clinical Characteristics (n = 27)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Mean | 60.4 | |
| SD | 9.7 | |
| Sex | ||
| Male | 13 | 48.1 |
| Female | 14 | 51.9 |
| Race | ||
| White | 20 | 74.1 |
| Black | 5 | 18.5 |
| Hispanic | 2 | 7.4 |
| ISS stage | ||
| I | 11 | 40.7 |
| II | 10 | 37.0 |
| III | 6 | 22.3 |
| Amyloidosis | ||
| Yes | 3 | 11.1 |
| No | 24 | 88.9 |
Abbreviations: ISS, International Staging System; SD, standard deviation.
Touch Detection Thresholds and Grooved Pegboard Times
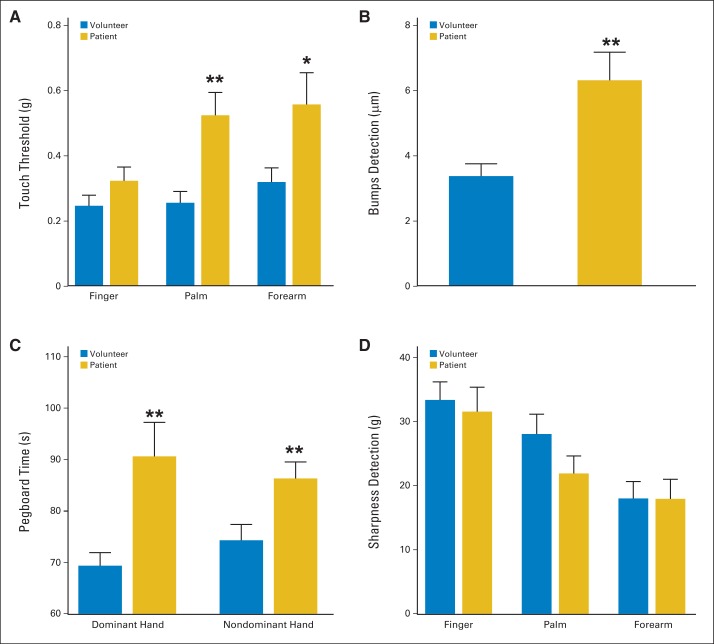
Touch detection thresholds—a gauge of Aβ fiber function15–17 obtained using von Frey monofilaments—were higher at the thenar eminence and volar forearm, but not the fingertip, in patients as compared with healthy volunteers (Fig 1A). Specifically, the touch detection thresholds in the palm and forearm were 0.26 ± 0.03 g and 0.32 ± 0.04 g in the volunteers, whereas the respective values for patients at these sites were 0.52 ± 0.07 g (P < .01) and 0.56 ± 0.10 g (P < .05). Importantly, patients exhibited significant impairment in bumps detection (Fig 1B). The mean bump detection threshold for patients was 6.30 ± 0.86 μm but only 3.37 ± 0.38 μm for the volunteers (P < .01). Thus, the bumps test was more sensitive than von Frey monofilaments in detecting impaired touch sensation at the fingertip.
Fig 1.
Touch detection, bumps detection, pegboard performance, and sharpness detection. Bar graphs show means (and SEs; horizontal bars) for measures of large myelinated fiber or Aδ function in patients with multiple myeloma (gold) versus healthy volunteers (blue). Results of (A) touch detection determined using von Frey monofilaments, (B) bumps detection threshold performed using index finger of dominant hand, (C) dominant and nondominant hands in completing slotted pegboard task, and (D) sharpness detection threshold. (*) P < .05; (**) P < .01.
Patients also showed a pronounced impairment in the sensory-motor slotted pegboard task (Fig 1C). The completion times for the dominant hand were 69.36 ± 2.56 seconds for volunteers and 90.63 ± 6.60 seconds for patients (P < .01); the respective values for the nondominant hand were 74.30 ± 3.08 seconds and 86.33 ± 3.20 seconds (P < .01). Combined, these findings indicate that patients with MM with no outward signs or symptoms of neuropathy have impaired Aβ fiber function before chemotherapy.
Sharpness Detection Thresholds
The results for the sharpness detection task are shown in Figure 1D. No significant deficits in sharpness detection were observed between the patient and volunteer groups.
Skin Temperature and Thermal Detection Thresholds
Baseline skin temperature was significantly different at the thenar eminence (patients, 34.56 ± 0.24°C v volunteers, 33.66 ± 0.35°C; P < .05) but not at the other test sites (Fig 2A). Figure 2 also shows differences between the groups in detection thresholds for heat and cold. The patient group showed significantly higher (P < .05) thresholds for warmth detection across all three test sites (patients, 39.45 ± 0.38; P < .01, 37.83 ± 0.29; P < .01, and 37.78 ± 0.34°C; P < .05 v volunteers, 37.90 ± 0.45, 36.72 ± 0.26, and 36.54 ± 0.40°C; Fig 2B). Heat pain threshold was similar between groups at all sites. Heat threshold at the fingertip occurred at the expected range of 45 to 47°C, whereas it was slightly lower at the thenar eminence and volar forearm (Fig 2B). The threshold to detect innocuous cool sensation was comparable between patients and volunteers. However, cold pain threshold was significantly elevated at the thenar eminence and volar forearm. Mean cold pain thresholds for patients versus volunteers were 14.67 ± 1.55 versus 7.33 ± 1.10°C at the thenar eminence (P < .001) and 13.31 ± 1.62 versus 7.46 ± 1.04°C at the volar forearm (P < .01; Fig 2C).
Fig 2.
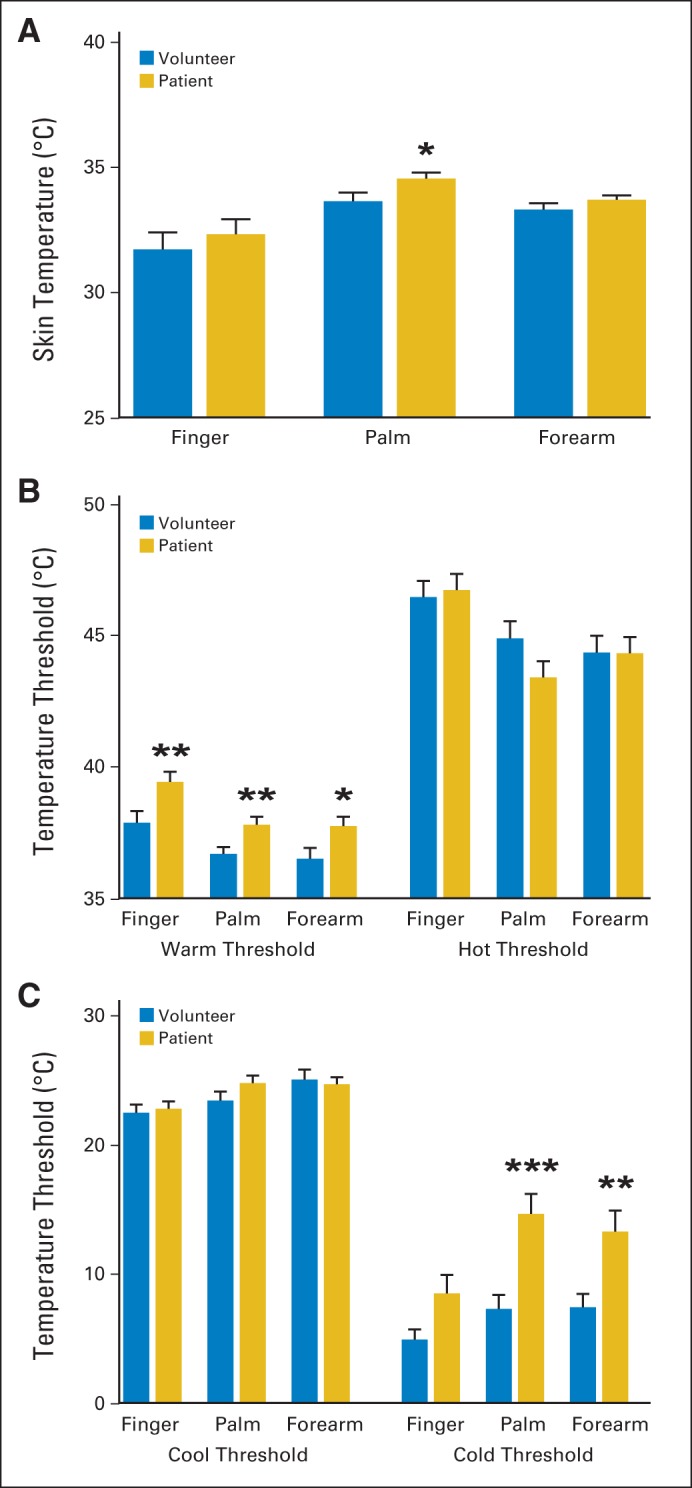
Skin temperature and thermal detection threshold. Bar graphs show means (and SEs; horizontal bars) for baseline skin temperature and thermal detection temperatures in patients with multiple myeloma (gold) versus healthy volunteers (blue). (A) Skin temperature measurements; (B) warmth detection (left) and heat pain threshold (right); (C) cool detection (left) and cold pain threshold (right). (*) P < .05; (**) P < .01; (***) P < .001.
General Neuropathy Score
An overall neuropathy score was generated for each patient and volunteer by summing the number of observations for each participant where any of the measures was > two standard deviations from the mean of the volunteer data set. In total, 22 (81.5%) of 27 patients versus 10 (33.3%) of 20 healthy volunteers had at least one out-of-range measure, defined as a measure two standard deviations above or below the volunteer mean. Patients had a mean 2.48 ± 0.34 observed out-of-range measures. In contrast, volunteers had a mean 0.60 ± 0.19 out-of-range measures (P < .001; Fig 3A).
Fig 3.
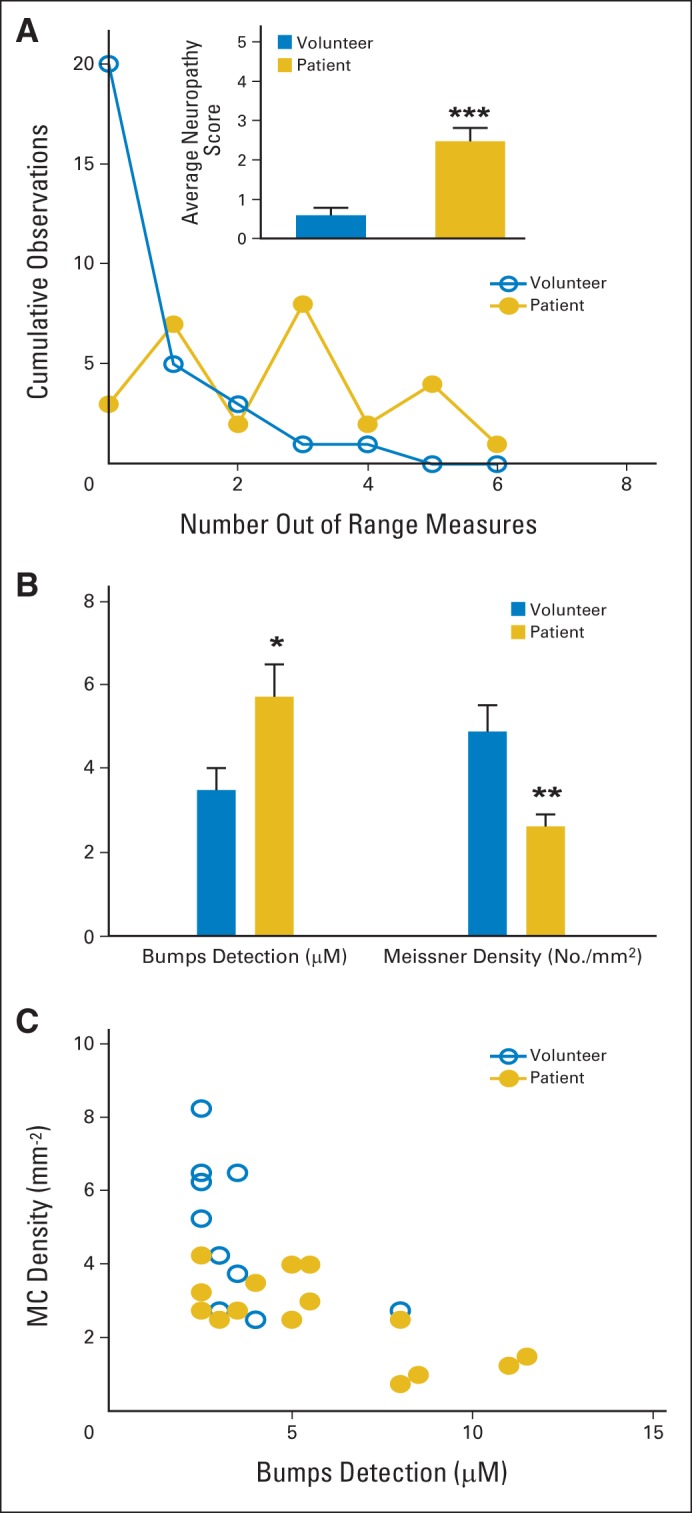
Neuropathy score and correlation of bumps to Meissner's corpuscle (MC) density. (A) Scatter and line plot shows percentage of cumulative observations for quantitative sensory test (QST) measures > two standard deviations from volunteer mean for each volunteer (open blue circles) and patient (solid gold circles). Inset bar graph shows mean out-of-range QST observations per volunteer (blue) versus patient (gold). (B) Means (and SEs; horizontal bars) for bumps detection score and MC density for subset of patients (gold) and healthy volunteers (blue). (C) Inverse correlation between bumps detection threshold and MC density in patients and volunteers; as bumps detection threshold increases, MC density decreases (overall, ρ = −0.69; P < .001). (*) P < .05; (**) P < .01; (***) P < .001.
Quantification of MCs
A subset of 13 patients with MM and 10 healthy volunteers underwent noninvasive confocal imaging of the fingertip of the fifth digit (Fig 4). Two patients underwent one repeat scan 3 months after the first scan, for a total of 15 quantified patient images. These patients had a significantly higher mean bumps score than healthy controls (5.73 ± 0.78 v 3.50 ± 0.53 μm; P < .05). Confocal images showed that patients had a decreased mean density of MCs as compared with controls (2.63 ± 0.28 v 4.88 ± 0.62 MCs/mm2; P < .01; Fig 3B). The Spearman rank-order correlation was used to assess the linear relationship between MC density and bump detection threshold. As bump detection threshold increased, MC density decreased (ρ = −0.69; P < .001; Fig 3C).
Fig 4.
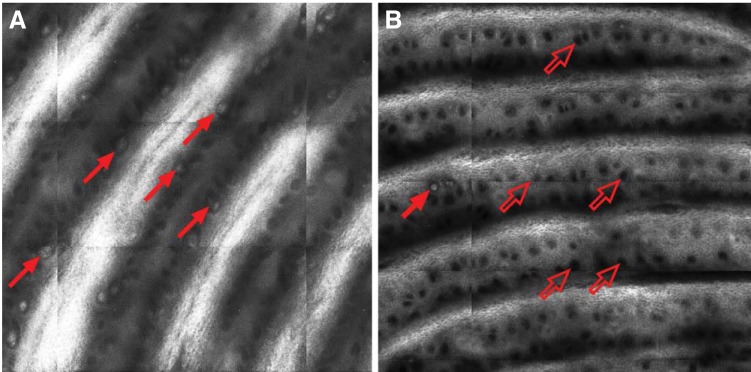
Representative Meissner's corpuscle (MC) scan images. Representative (A) healthy volunteer and (B) patient images of 1.0 × 1.0-mm in vivo laser reflectance confocal micrograph. In volunteer image, numerous MCs can be seen as white orb-shaped structures sitting at base of dermal papillae (dark bands). Several MCs are indicated by solid red arrows. One MC is visible in patient image, whereas numerous empty MC pits are visible, as indicated by open red arrows.
DISCUSSION
The results shown here indicate that subclinical sensory dysfunction consistent with early-onset neuropathy is highly prevalent in patients with MM before chemotherapy treatment. Impairments were observed in low-threshold mechanosensation, sensorimotor tasks, and thermal detection consistent with dysfunction in all three classes—Aβ, Aδ, and C—of primary afferent fibers.18–21 MC density on confocal scans was similar to MC density quantified in skin biopsies, which suggests that in vivo confocal microscopy is a noninvasive, quantitative method of assessing MC density. Patients showed decreased density of MCs by confocal imaging that was correlated with their ability to detect small bumps in the bumps detection test. These data suggest that a decrease in tactile sensitivity is well correlated with MC density as visualized by in vivo confocal imaging and is consistent with studies comparing bump threshold with MC density using quantified skin biopsy.18 Taken together, this suggests that nervous system complications are more prevalent in treatment-naive patients with MM than previously appreciated.
Neurologic complications in MM are multifaceted. The most common neurologic involvement is radicular pain resulting from spinal cord or nerve root compression after lytic bone lesions.21 Consistent with the findings reported here, electrophysiologic assessments before therapy revealed that roughly one third of patients with newly diagnosed MM had evidence of peripheral nerve involvement.21,22 The increased incidence of patients identified with subclinical neuropathy in our study is simply a result of the higher sensitivity of QSTs to reveal nerve fiber dysfunction compared with electrophysiologic methods. The important implication in this work is that drug-induced neuropathy occurs more frequently in patients who have existing neuropathy, and the severity of peripheral neuropathy is higher in those patients.8,9,23
Neuropathy before treatment in patients with MM implicates mechanisms based on individual and disease-related factors. In part, the patient cohort affected by MM is largely an elderly patient population diagnosed at age ≥ mid-50s. Advanced age is associated with a decline in innervation density (eg, density of MCs).24,25 This factor was accounted for with an age match of the nonpatient volunteers, indicating that a disease-related process is linked with a decrease in MCs in patients with MM. Despite their similar age, healthy volunteers had significantly more distal fingertip innervation than their patient counterparts, evidenced by higher MC density. All healthy volunteers included in this analysis were employed; future studies could include employed and unemployed volunteers for a more diverse control sample. MC density visualized on confocal scans was correlated with fine tactile discrimination. Of note, healthy volunteers with varying numbers of MCs were able to discern the smallest bump during the bumps test. Thus, individuals with the highest density of MCs may have been able to detect bumps smaller than 2.5 μm (ie, smallest bump used in QST), creating a floor effect in the data and weaker-than-expected correlation between MCs and tactile discrimination. Despite the presence of a floor effect, individuals who tended to perform worse on the tactile discrimination test had lower densities of MCs and more sensory abnormalities, consistent with sensory neuropathy. These data suggest that in vivo confocal imaging may be a novel and sensitive method for early detection of sensory deficits consistent with neuropathy. Although this technology is a potentially useful tool for quantifying peripheral innervation, several limitations of this technology warrant mention. MC innervation is composed of at least two types of C fibers and both unbranched and branched Aβ fibers. It is not clear how long the structure of MCs can persist in the absence of innervation by myelinated and unmyelinated sensory fibers or whether their structure depends on both or one type of innervation. Although the density of MCs is easily quantifiable with in vivo confocal microscopy, innervation of the structures cannot be assessed in these images.
Contributions of the disease process to the generation of neuropathy are well documented as overt clinical signs secondary to plasma cell dyscrasia (particularly in POEMS [polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes] syndrome) or as the result of compression of the nerve roots, cryoglobulinemia, or light chain deposits (amyloidosis).2,26 Given the more subtle deficits observed here, immune-mediated mechanisms seem more likely to be involved. Kelly et al27 suggested that neuropathy associated with disease in myeloma is a heterogeneous entity resembling carcinomatous neuropathy and that treatment of myeloma does not affect the course of neuropathy. Others have noted that a number of common disorders of the peripheral nervous system, termed paraproteinemic neuropathies, are closely connected with the presence of excessive amounts of an abnormal immunoglobulin in the blood.28 In at least some patients, these antibodies are directed at components of myelin or the axolemma, resulting in complement-mediated damage to Schwann cells and axons.28 However, baseline testing in patients with colorectal cancer with no clinical evidence or reported symptoms of neuropathy before chemotherapy revealed subclinical peripheral neuropathy is a surprisingly common occurrence (46 of 52 participants) in this type of cancer as well.29 This suggests that cancers in general engage biologic responses that impair nerve function. Given the strong connection between pre-existing neuropathy and its exacerbation by disease treatment, these findings underscore the need for careful screening and individualized treatment plans for patients at risk.
Acknowledgment
We thank Tina Peters and Tony Perez for their assistance in collecting quantitative sensory testing data.
Footnotes
Supported by National Institutes of Health Grant No. NS046606 and National Cancer Institute Grant No. CA124787.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Alyssa K. Kosturakis, Zijing He, Yan Li, Jessica A. Boyette-Davis, Sheeba K. Thomas, Haijun Zhang, Elisabeth G. Vichaya, Xin Shelley Wang, William R. Kennedy, Donald A. Simone, Charles S. Cleeland, Patrick M. Dougherty
Financial support: Patrick M. Dougherty
Administrative support: Patrick M. Dougherty
Collection and assembly of data: Alyssa K. Kosturakis, Yan Li, Jessica A. Boyette-Davis, Sheeba K. Thomas, Haijun Zhang, Elisabeth G. Vichaya, Xin Shelley Wang, William R. Kennedy, Donald A. Simone, Charles S. Cleeland, Patrick M. Dougherty
Data analysis and interpretation: Alyssa K. Kosturakis, Zijing He, Yan Li, Jessica A. Boyette-Davis, Nina Shah, Haijun Zhang, Elisabeth G. Vichaya, Xin Shelley Wang, Gwen Wendelschafer-Crabb, William R. Kennedy, Donald A. Simone, Charles S. Cleeland, Patrick M. Dougherty
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Talamo G, Farooq U, Zangari M, et al. Beyond the CRAB symptoms: A study of presenting clinical manifestations of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2010;10:464–468. doi: 10.3816/CLML.2010.n.080. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein A, Doniger DE. Neurologic complications of myelomatosis. Arch Neurol. 1963;9:534–544. doi: 10.1001/archneur.1963.00460110102011. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein A. Neurological complications of anticoagulation therapy: A neurologist's review. Arch Intern Med. 1979;139:217–220. [PubMed] [Google Scholar]
- 4.Plasmati R, Pastorelli F, Cavo M, et al. Neuropathy in multiple myeloma treated with thalidomide: A prospective study. Neurology. 2007;69:573–581. doi: 10.1212/01.wnl.0000267271.18475.fe. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Xie W, Mitsiades C, et al. Single-agent bortezomib in previously untreated multiple myeloma: Efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009;27:3518–3525. doi: 10.1200/JCO.2008.18.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyette-Davis JA, Cata JP, Zhang H, et al. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain. 2011;12:1017–1024. doi: 10.1016/j.jpain.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608. doi: 10.1038/leu.2011.346. [DOI] [PubMed] [Google Scholar]
- 8.Delforge M, Bladé J, Dimopoulos MA, et al. Treatment-related peripheral neuropathy in multiple myeloma: The challenge continues. Lancet Oncol. 2010;11:1086–1095. doi: 10.1016/S1470-2045(10)70068-1. [DOI] [PubMed] [Google Scholar]
- 9.Vichaya EG, Wang XS, Boyette-Davis JA, et al. Subclinical pretreatment sensory deficits appear to predict the development of pain and numbness in patients with multiple myeloma undergoing chemotherapy. Cancer Chemother Pharmacol. 2013;71:1531–1540. doi: 10.1007/s00280-013-2152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann DN, Boger JN, Jansen C, et al. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology. 2007;69:2121–2127. doi: 10.1212/01.wnl.0000282762.34274.94. [DOI] [PubMed] [Google Scholar]
- 11.Cata JP, Weng HR, Burton AW, et al. Quantitative sensory findings in patients with bortezomib-induced pain. J Pain. 2007;8:296–306. doi: 10.1016/j.jpain.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy WR, Selim MM, Brink TS, et al. A new device to quantify tactile sensation in neuropathy. Neurology. 2011;76:1642–1649. doi: 10.1212/WNL.0b013e318219fadd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: Normative values for the finger tapping and grooved pegboard tests. Percept Mot Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–149. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- 15.Cata JP, Cordella JV, Burton AW, et al. Spinal cord stimulation relieves chemotherapy-induced pain: A clinical case report. J Pain Symptom Manage. 2004;27:72–78. doi: 10.1016/j.jpainsymman.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty PM, Cata JP, Cordella JV, et al. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Voorhees PM, Dees EC, O'Neil B, et al. The proteasome as a target for cancer therapy. Clinical Cancer Res. 2003;9:6316–6325. [PubMed] [Google Scholar]
- 18.Fruhstorfer H, Zenz M, Nolte H, et al. Dissociated loss of cold and warm sensibility during regional anesthesia. Pflugers Arch. 1974;349:73–82. doi: 10.1007/BF00587918. [DOI] [PubMed] [Google Scholar]
- 19.Konietzny F, Hensel H. Warm fiber activity in human skin nerves. Pflugers Arch. 1975;359:265–267. doi: 10.1007/BF00587384. [DOI] [PubMed] [Google Scholar]
- 20.Torebjörk HE. Afferent C units responding to mechanical, thermal and chemical stimuli in human non-glabrous skin. Acta Physiol Scand. 1974;92:374–390. doi: 10.1111/j.1748-1716.1974.tb05755.x. [DOI] [PubMed] [Google Scholar]
- 21.Silberman J, Lonial S. Review of peripheral neuropathy in plasma cell disorders. Hematol Oncol. 2008;26:55–65. doi: 10.1002/hon.845. [DOI] [PubMed] [Google Scholar]
- 22.Richardson PG, Laubach JP, Schlossman RL, et al. Complications of multiple myeloma therapy, part 1: Risk reduction and management of peripheral neuropathy and asthenia. J Natl Compr Canc Netw. 2010;8(suppl 1):S4–S12. doi: 10.6004/jnccn.2010.0115. [DOI] [PubMed] [Google Scholar]
- 23.Sonneveld P, Jongen JL. Dealing with neuropathy in plasma-cell dyscrasias. Hematology Am Soc Hematol Educ Program. 2010;2010:423–430. doi: 10.1182/asheducation-2010.1.423. [DOI] [PubMed] [Google Scholar]
- 24.Bolton CF, Winkelmann RK, Dyck PJ. A quantitative study of Meissner's corpuscles in man. Neurology. 1966;16:1–9. doi: 10.1212/wnl.16.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Bolton CF, Winkelmann RK, Dyck PJ. A quantitative study of Meissner's corpuscles in man. Trans Am Neurol Assoc. 1964;89:190–192. [PubMed] [Google Scholar]
- 26.Denier C, Lozeron P, Adams D, et al. Multifocal neuropathy due to plasma cell infiltration of peripheral nerves in multiple myeloma. Neurology. 2006;66:917–918. doi: 10.1212/01.wnl.0000203345.29020.db. [DOI] [PubMed] [Google Scholar]
- 27.Kelly JJ, Jr, Kyle RA, Miles JM, et al. The spectrum of peripheral neuropathy in myeloma. Neurology. 1981;31:24–31. doi: 10.1212/wnl.31.1.24. [DOI] [PubMed] [Google Scholar]
- 28.Ropper AH, Gorson KC. Neuropathies associated with paraproteinemia. N Engl J Med. 1998;338:1601–1607. doi: 10.1056/NEJM199805283382207. [DOI] [PubMed] [Google Scholar]
- 29.Boyette-Davis JA, Eng C, Wang XS, et al. Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clin Cancer Res. 2012;18:3180–3187. doi: 10.1158/1078-0432.CCR-12-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]