Abstract
Background
Despite significant cost differences, the comparative effect of combination treatments of disease modifying anti-rheumatic drugs (DMARDs) with and without biologic agents has rarely been examined. Thus we performed a network meta-analysis on the effect of combination therapies on progression of radiographic joint erosions in patients with rheumatoid arthritis (RA).
Methods and Findings
The following combination drug therapies compared versus single DMARD were investigated: Double DMARD: 2 DMARDs (methotrexate, sulfasalazine, leflunomide, injectable gold, cyclosporine, chloroquine, azathioprin, penicillamin) or 1 DMARD plus low dose glucocorticoid (LDGC); triple DMARD: 3 DMARDs or 2 DMARDs plus LDGC; biologic combination: 1 DMARD plus biologic agent (tumor necrosis factor α inhibitor (TNFi) or abatacept or tocilizumab or CD20 inhibitor (CD20i)). Randomized controlled trials were identified in a search of electronic archives of biomedical literature and included in a star-shaped network meta-analysis and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol. Effects are reported as standardized mean differences (SMD). The effects of data from 39 trials published in the period 1989–2012 were as follows: Double DMARD: −0.32 SMD (CI: −0.42, −0.22); triple DMARD: −0.46 SMD (CI: −0.60, −0.31); 1 DMARD plus TNFi: −0.30 SMD (CI: −0.36, −0.25); 1 DMARD plus abatacept: −0.20 SMD (CI: −0.33, −0.07); 1 DMARD plus tocilizumab: −0.34 SMD (CI: −0.48, −0.20); 1 DMARD plus CD20i: −0.32 SMD (CI: −0.40, −0.24). The indirect comparisons showed similar effects between combination treatments apart from triple DMARD being significantly better than abatacept plus methotrexate (−0.26 SMD (CI: −0.45, −0.07)) and TNFi plus methotrexate (−0.16 SMD (CI: −0.31, −0.01)).
Conclusion
Combination treatment of a biologic agent with 1 DMARD is not superior to 2–3 DMARDs including or excluding LDGC in preventing structural joint damage. Future randomized studies of biologic agents should be compared versus a combination of DMARDs.
Introduction
In a meta-analysis of 70 randomized controlled trials (RCTs) of rheumatoid arthritis (RA) patients investigating the effect of drug treatment on radiographic joint destruction (erosions), disease modifying anti rheumatic drugs (DMARDs), low-dose glucocorticoids (LDGC), biologic agents, and combinations of these significantly reduced radiographic progression with a relative effect of 48–84% compared with placebo treatment [1]. Although several biologic agents have been investigated as single therapy, biologic treatment is usually given in combination with a DMARD (typically methotrexate) in order to minimize the risk of developing neutralizing antibodies and to improve efficacy. A biologic agent plus methotrexate is superior to single methotrexate and superior to a single biologic agent [1]. Furthermore a combination of DMARDs is superior to a single DMARD [1]. Due to the lack of combination DMARD arms in the studies of biological drugs [1], [2], the comparative effect of combination treatments with and without biologic agents is unclear.
Hitherto only one randomized trial has directly compared the combination of a biologic agent plus methotrexate with a combination of DMARDs [3]. This study and its follow-up study [4] showed no difference between these two treatment principles. Very recently, additionally three studies have confirmed these observations [5]–[7]. Due to the shortage of direct comparisons, network (or mixed treatment comparison (MTC)) meta-analyses [8] have been performed to indirectly compare the effects of different biologic agents [9]–[10]. In contrast, the combination of conventional DMARDs versus biologic agents plus DMARDs have not been analysed in network meta-analyses, although such comparisons seem more interesting due to the cost differences between treatments with and without biologic agents. As our previous study [1] indicated that combination drug treatment was effective irrespective of the drugs involved in the combination, we intended to test the hypothesis that in patients with RA combination treatments of at least two DMARDs, or at least one DMARD plus LDGC or one DMARD plus a biologic agent do not differ significantly in their ability to reduce radiographic joint destruction (erosions) when compared with a single DMARD. Consequently we performed a network meta-analysis of the available direct and indirect evidence from RCTs comparing combination treatment versus single DMARD treatment.
Methods
The analysis is reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [11] and supplied with an analysis of consistency between indirect and direct evidence [12]. The first version of a protocol for the present study was performed on October 12, 2010 and was based on our previous meta-analysis [1].
Definition of network
Unlike a traditional meta-analysis, which summarizes the results of trials that have evaluated the same treatment/placebo combination (direct comparison), a network meta-analysis consist of a network of treatment effects for all possible pairwise comparisons from RCTs, whether or not they have been compared head to head (i.e. include both direct and indirect comparisons). The fundamental principle of the network is that the indirectly compared treatment effects have a common comparator on which they are anchored. In a simple network there is only one common comparator, whereas more complex networks may have several comparators, which are connected in the network.
The disadvantage of complex networks with many anchor treatments is that at least some of the many different treatment principles usually will be unbalanced and thus contribute to heterogeneity, which may complicate the interpretation of the outcome of the analysis. Furthermore, many of the treatments in a complex network often originates from a single study and thus do not benefit from the statistical power, which is the advantage of a conventional meta-analysis. Thus a complex network meta-analysis may result in numerous pairwise comparisons with low power and a high degree of undefined heterogeneity. Consequently, although the universality of the complex models is appealing, it is important to design a network with caution to avoid creating statistical results of limited clinical value.
For instance the total number of treatment principles in our first analysis [1] was 34. If all these principles should be compared in one network meta-analysis the result would be 561 comparisons, many of which would be clinically uninteresting and most of which would have low power. Inclusion of different doses of the same treatment would increase the problem.
In order to minimize the number of low power comparisons and the amount of heterogeneity we intended to create a simple network focussing on the interesting question and eliminating repetition of established evidence on the ability of drugs to reduce inflammation and joint destruction in RA.
First it is established in several conventional meta-analyses of direct comparisons that a single DMARD is better than placebo. Furthermore direct comparisons have shown that DMARDs generally have similar effects. Finally it has been established in direct comparisons that 2–3 DMARDs are better than one DMARD. In addition treatment principles, which are not fully investigated, should be avoided in the network. For instance the 10 known DMARDs can be combined in 45 different double combinations. However only 6 of these combinations have been tested, and therefore it is not possible to determine the most effective of the 45 combinations. Furthermore 4 of the combinations have only been tested in one study. Therefore statistical conclusions based on indirect comparisons of these combinations would be weak. In contrast, a comparison of a group of combination DMARD studies with other treatments would be powerful. The different biologic drugs combined with methotrexate have all been investigated in large studies, and therefore these combinations could all be included in powerful comparisons. Elimination of non-standard doses of biologics, which in direct comparisons have been shown to be inferior, would contribute to the reduction of heterogeneity.
The issue of interest does not only depend on the effect of the treatment, but also on the cost of the treatment. For instance a large difference between cheap DMARDs is interesting, whereas a small difference is not. Similarly a large difference between expensive biologics may be interesting, whereas a small difference is not. In contrast, it would be very interesting if there was only a small or no difference in effect between DMARDs and biologics.
We already know from previous conventional meta-analyses and network meta-analyses that the mutual effects of DMARDs and the mutual effects of biologics are similar, and that biologics as single treatment are better than single DMARD treatment. Furthermore we know the optimal standard dose of the biologics. Considering the 100 fold difference in cost, the remaining interesting question is whether a combination of a standard dose of a biologic plus methotrexate is better than a combination of cheap DMARDs. Consequently it was the intention to create a network to answer that question. Existing evidence was used to simplify the network in order to decrease heterogeneity and increase the power of the comparisons:
Placebo controlled single DMARD studies are eliminated, because the effects of single DMARDs are established
Single DMARD controlled single DMARD studies are eliminated, because the similar effects of single DMARDs are established
The combination DMARD studies are combined in one group and the comparison of different DMARD combinations are eliminated due to lack of investigations and power
To ensure the comparability with other network meta-analyses, the different biologic combinations are not combined but compared separately.
Only standard doses of biologics are investigated
IL1i treatment (anakinra) was excluded as IL1i has been shown to be inferior to other biologics in several network meta-analyses.
Eligibility criteria
Types of studies
Full-length studies published in peer-reviewed journals that were performed according to a RCT design and that scored joint radiographs as the primary or secondary outcome at 2 separate time points with a time interval of at least 3 months were included, irrespective of sample size and publication year.
Types of participants
Patients with RA diagnosed according to the 1958 or the 1987 criteria of the American College of Rheumatology (ACR; formerly, the American Rheumatism Association) were included. In studies performed before 1959, the stated study definitions of RA were accepted.
Type of outcome
The outcome was the difference between follow-up radiographic erosion score and baseline radiographic erosion score.
Types of intervention
As our previous meta-analysis [1] showed no statistically significant difference in radiographic progression between methotrexate (Mt), sulfasalazine (Su), cyclosporine (Cs), leflunomide (Lf) and injectable gold (Au, ij), we included combination DMARD studies, which had one of these effective DMARDs in the single DMARD arm, but excluded those that included the less effective DMARDs (chloroquine (Cl), D-penicillamine (Dp) and Dp analogue bucillamin (Bu), azathioprine (Az), cyclophosphamide (Cph) and peroral gold (Au, po)) in the single DMARD arm. Furthermore, we showed that LDGC, defined as maximally 7.5 mg prednisone or prednisolone per day, had an effect similar to the effective DMARDs [1], and therefore LDGC was included as a DMARD equivalent. Any DMARD was allowed in the combination arm. Finally, we included combination treatments of methotrexate plus TNF inhibitors (etanercept (Et), infliximab (In), adalimumab (Ad), certolizumab (Cz), and golimumab (Go)), methotrexate plus abatacept (Ab), methotrexate plus tocilizumab (Tz), and methotrexate plus CD20 inhibitors (rituximab (Rt), ocrelizumab (Oc)).
Information sources
Trials were identified by searching the electronic databases (PubMed, the Cochrane database, and ClinicalTrials.gov) and by scanning the lists of references from the identified randomized trials.
Search methods for identification of studies
The search was based on the following combination of search terms:
“rheumatoid arthritis and randomized and methotrexate OR rheumatoid arthritis and randomized and sulfasalazine OR rheumatoid arthritis and randomized and leflunomide OR rheumatoid arthritis and randomized and gold OR rheumatoid arthritis and randomized and cyclosporine OR rheumatoid arthritis and randomized and infliximab OR rheumatoid arthritis and randomized and etanercept OR rheumatoid arthritis and randomized and adalimumab OR rheumatoid arthritis and randomized and certolizumab OR rheumatoid arthritis and randomized and golimumab OR rheumatoid arthritis and randomized and tocilizumab OR rheumatoid arthritis and randomized and abatacept OR rheumatoid arthritis and randomized and rituximab OR rheumatoid arthritis and randomized and ocrelizumab OR rheumatoid arthritis and randomized and ofatumumab OR rheumatoid arthritis and randomized and glucocorticoid OR rheumatoid arthritis and randomised and methotrexate OR rheumatoid arthritis and randomised and sulfasalazine OR rheumatoid arthritis and randomised and leflunomide OR rheumatoid arthritis and randomised and gold OR rheumatoid arthritis and randomised and cyclosporine OR rheumatoid arthritis and randomised and infliximab OR rheumatoid arthritis and randomised and etanercept OR rheumatoid arthritis and randomised and adalimumab OR rheumatoid arthritis and randomised and certolizumab OR rheumatoid arthritis and randomised and golimumab OR rheumatoid arthritis and randomised and tocilizumab OR rheumatoid arthritis and randomised and abatacept OR rheumatoid arthritis and randomised and rituximab OR rheumatoid arthritis and randomised and ocrelizumab OR rheumatoid arthritis and randomised and ofatumumab OR rheumatoid arthritis and randomised and glucocorticoid.”
Data collection
Selection of trials
Titles were screened, abstracts read, and possible papers retrieved. Trials fulfilling eligibility criteria were included in the systematic review.
Data extraction
Eligibility assessment, data collection and risk of bias assessment were performed independently by two authors and disagreement resolved by consensus. All data were entered into standardized extraction forms.
Data items
Mean radiographic scores and standard deviations (SD) were assessed based on the change scores from baseline to follow-up for each treatment arm. In addition the following variables were recorded: Study identification, year of publication, scoring system, initial radiographic score, maximum radiographic score of scoring system, number of patients in each treatment arm, duration of RA at baseline, duration of study, DMARD inadequate response (i.e. whether included patients previously had had an inadequate response to a least one DMARD), strategy change (i.e. whether a change of treatment strategy was allowed during the course of the study) and mean daily glucocorticoid use in all treatment arms. We used the baseline radiographic score, the maximum radiographic score of scoring system and the duration of RA to calculate the percentage annual radiographic progression rate (PARPR) [1] in the period before baseline as a marker of disease activity at baseline.
Risk of bias in individual studies
Six different risk-of-bias domains defined by Cochrane [13] were assessed on the outcome level: sequence generation, allocation concealment, study blinding, outcome assessor blinding, incomplete outcome data and selective outcome reporting. In addition we included radiographic sequence blinding and company sponsoring as risk of bias domains.
Measures of treatment effect
For each randomized combination drug group and single DMARD group the difference between follow-up radiographic erosion score and baseline radiographic erosion score and the corresponding SDs were recorded. The difference between the mean effect in the combination drug group and the single DMARD group was the treatment effect.
Data analysis
Unit of analysis issues
If radiographic scoring was performed more than once during follow-up, the scoring with the most complete data was recorded. In trials with multi dose arms, only the defined standard dose arm was included. If the treatment arms of multi-armed trials consisted of different combination treatments (direct comparisons), these treatment arms were included in the network meta-analysis and additionally analyzed separately for the purposes of a consistency analysis of indirect comparisons versus direct comparisons. In this case the shared control group was split into a number of subgroups corresponding to the number of treatment arms to avoid multiple count of the control group.
Missing data
In articles where the median, but not the mean, was given, the median value was used in the calculations. If SD was not given, it could often be calculated from a 95% confidence interval, a standard error or a p-value [13]. An interquartile range (2 quartiles) was made equivalent to 1.35 x SD [13] and a full range was converted to an SD according to a conversion factor defined by Walther and Yao [14].
Heterogeneity
Heterogeneity between studies was tested statistically for all studies and each intervention by a χ2 (chi square) test, and quantified by means of the I2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error: I2, 0%–30%, unimportant heterogeneity; I2, 30%–60%, moderate heterogeneity; I2, >60%, substantial heterogeneity [13]. Both fixed and random effect models were used, but the existence of statistically significant heterogeneity would determine whether a fixed effect model (non-significant heterogeneity test) or a random effect model (significant heterogeneity test) would be used in the primary or secondary analysis [13]. Potential heterogeneity would be explored by means of subgroup analyses of extracted data.
Outcome data synthesis
A network meta-analysis consist of a network of treatment effects for all possible pairwise comparisons from RCTs, whether or not they have been compared head to head (i.e. include both direct and indirect comparisons) [7], [8]. We used a stepwise approach [15], [16], first performing multiple pairwise meta-analyses of the direct comparisons of each of the combination treatments versus single DMARD followed by an indirect comparison of the pooled results of each of these meta-analyses. As the outcome measure (radiographic progression) was estimated at different time points (6–24 months) and as the maximum score of the different scoring systems (Sharp, Larsen) differed, we standardized the outcome measure by dividing the outcome with the SD, thus converting the outcome unit to the unitless standardized mean difference (SMD) [13]. Consequently, we interpreted our analyses of the pairwise meta-analyses on the basis of the SMD, whereas the indirect comparisons were performed as weighted mean differences of the SMDs calculated in the pairwise meta-analyses.
Consistency analysis
Consistency analyses of the effects obtained in the trials directly comparing combination treatments versus the effects obtained by means of the exclusively indirect comparisons were performed to explore possible differences between the direct and the indirect comparisons [12].
Risk of bias across studies
Each of the above eight assessed risk of bias domains were evaluated in 3 groups: A: Low risk; B: Unclear risk; C: High risk [13]. In addition publication bias was evaluated visually by means of a funnel plot in which the effect of each trial was plotted by the inverse of its standard error [13].
Additional analyses
The outcome effect (radiographic progression) of combination DMARD treatments including LDGC was compared versus combination DMARD treatments not including LDGC.
Measures of bias domains and of other possible confounders were compared between the combination treatment groups with the purpose of performing sensitivity analyses for those, which differed. The outcome effect was compared between the grading (A, B, C) of the relevant bias domains and between the upper and lower 50% percentiles of possible confounders of continuous variables (PARPR (as a marker of disease activity at baseline), disease duration, differences in the mean use of glucocorticoids) and between groups of possible confounders of category variables (DMARD inadequate response and strategy change).
Data synthesis method
The combined effect measures of the direct comparisons of the individual combination treatments, the indirect comparisons of the combined effect measures of the individual combination treatments, the consistency analyses and the additional analyses were compared by means of the inverse variance method in Review Manager (RevMan) (Computer program), version 5.1. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2008 [13].
Results
Trial selection
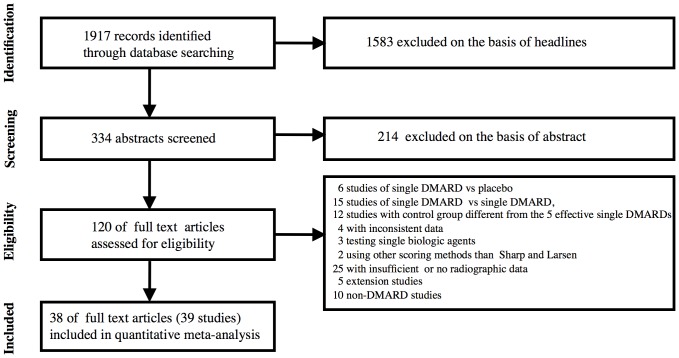
The search was repeated during the review period, by two authors by turns. The final search was performed July 5, 2012. A flow diagram of the literature search is shown in Figure 1.
Figure 1. Flow diagram of literature search.
The PubMed search revealed 1917 references. A search of ClinicalTrials.gov using the key-words “rheumatoid arthritis” and “radiographic progression” revealed 3 published studies with radiographic data, which also were identified during our primary search, 1 published study with no radiographic data and 2 finished but not published studies out of a total of 21 ongoing studies.
This search was supplied with a search in Cochrane Central Register of Controlled Trials using the terms “rheumatoid arthritis and radiographic progression” or “rheumatoid arthritis and joint destruction” resulting in 65 hits, none of which supplied the list of included studies.
After eliminating references which were considered irrelevant according to the headlines, 334 abstracts were read. On the basis of the abstracts 120 articles were retrieved in full length. From these a total of 38 references were identified (Figure 1). Until December 31 2009 the present search identified all 28 combination studies [3], [17]–[43] identified in our previous search [1] plus one additional study published in 2005 [44]. In addition the present search revealed three new references [45]–[47] (4 investigations) published in 2011 and 6 studies published in 2012 [48]–[53]. In total 38 “combination treatment” references (39 trials, 45 treatment groups) were included.
Characteristics of included studies
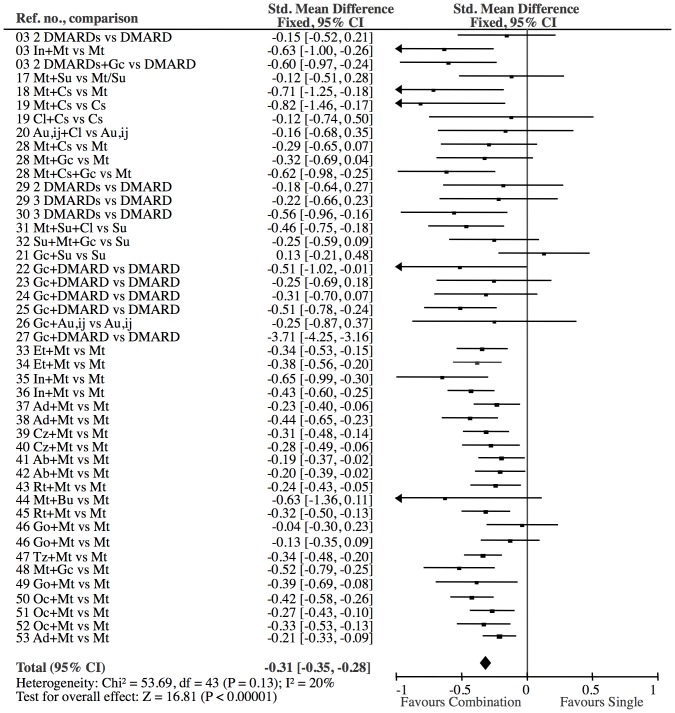
All were parallel studies. Individual study characteristics and risk of bias domains are shown in Table 1. A forest plot of the individual study results is shown in Figure 2. Heterogeneity seemed to be unimportant (I2 = 20%, p = 0.13).
Table 1. Study Characteristics and Risk of Bias Factors.
| Reference no. | Sequence generation | Concealed allocation | Study blinding | Outcome blinding | Radiographic sequence | Incomplete outcome | Sponsor | Test drug | Treatment group | N_(radiograph) | Duration RA, years | Scoring system | Initial radiographic score | Radiographic score, Max | Duration of study, months | PARPR | Mean Dose GC mg | Strategy change allowed | DMARD inadequate response |
| [3] | A | A | C | A | A | C | C | Mt | Single | 115 | 0,5 | Sharp | 2,0 | 280 | 12 | 1,4 | 0,03 | Yes | No |
| [3] | A | A | C | A | A | C | C | MtSu | Double | 110 | 0,5 | Sharp | 2,0 | 280 | 12 | 1,4 | 0,04 | Yes | No |
| [3] | A | A | C | A | A | C | C | MtSuGc | Triple | 125 | 0,5 | Sharp | 2,0 | 280 | 12 | 1,4 | 0,01 | Yes | No |
| [3] | A | A | C | A | A | C | C | InMt | TNFiMt | 119 | 0,5 | Sharp | 2,0 | 280 | 12 | 1,4 | 0,02 | Yes | No |
| [17] | B | B | A | A | C | C | C | Mt | Single | 49 | 1,5 | Sharp | 3,8 | 280 | 12 | 0,9 | 0,00 | No | No |
| [17] | B | B | A | A | C | C | C | Su | Single | 46 | 0,9 | Sharp | 2,8 | 280 | 12 | 1,1 | 0,00 | No | No |
| [17] | B | B | A | A | C | C | C | MtSu | Double | 49 | 0,9 | Sharp | 4,3 | 280 | 12 | 1,7 | 0,00 | No | No |
| [18] | B | B | C | A | C | C | A | Mt | Single | 30 | 0,9 | Sharp | 1,2 | 280 | 12 | 0,5 | 2,10 | Yes | No |
| [18] | B | B | C | A | C | C | A | MtCs | Double | 28 | 0,9 | Sharp | 1,1 | 280 | 12 | 0,4 | 2,10 | Yes | No |
| [19] | B | B | C | A | B | C | B | Cs | Single | 30 | 1,3 | Larsen | 14,5 | 200 | 12 | 5,6 | 2,20 | No | No |
| [19] | B | B | C | A | B | C | B | CsMt | Double | 30 | 1,3 | Larsen | 13,5 | 200 | 12 | 5,2 | 3,25 | No | No |
| [19] | B | B | C | A | B | C | B | CsCl | Double | 29 | 1,5 | Larsen | 13,7 | 200 | 12 | 4,6 | 2,15 | No | No |
| [20] | B | B | A | A | A | C | C | Au | Single | 32 | 2,0 | Larsen | 30,5 | 240 | 12 | 6,4 | 0,00 | No | No |
| [20] | B | B | A | A | A | C | C | AuCl | Double | 27 | 2,0 | Larsen | 31,2 | 240 | 12 | 6,5 | 0,00 | No | No |
| [21] | A | A | A | A | C | C | A | Su | Single | 66 | 1,0 | Sharp | 6,0 | 448 | 12 | 1,3 | 0,09 | No | No |
| [21] | A | A | A | A | C | C | A | SuGc | Double | 64 | 1,0 | Sharp | 8,0 | 448 | 12 | 1,8 | 0,09 | No | No |
| [22] | B | B | A | B | B | C | A | Mt | Single | 30 | 16,0 | Larsen | 37,0 | 240 | 12 | 1,0 | 0,09 | Yes | No |
| [22] | B | B | A | B | B | C | A | MtGc | Double | 32 | 13,0 | Larsen | 47,0 | 240 | 12 | 1,5 | 0,06 | Yes | No |
| [23] | A | B | C | A | A | C | A | Mt | Single | 39 | 8,5 | Larsen | 31,5 | 160 | 12 | 2,3 | 0,04 | Yes | No |
| [23] | A | B | C | A | A | C | A | MtGc | Double | 43 | 2,8 | Larsen | 28,5 | 160 | 12 | 6,4 | 0,05 | Yes | No |
| [24] | B | A | A | A | A | C | A | Su | Single | 49 | 1,3 | Larsen | 6,2 | 140 | 12 | 3,4 | 0,00 | No | No |
| [24] | B | A | A | A | A | C | A | SuGc | Double | 54 | 1,3 | Larsen | 2,7 | 140 | 12 | 1,5 | 0,00 | No | No |
| [25] | A | B | C | A | C | C | A | Mt | Single | 115 | 0,5 | Sharp | 1,9 | 280 | 12 | 1,4 | 0,62 | Yes | No |
| [25] | A | B | C | A | C | C | A | MtGc | Double | 99 | 0,5 | Sharp | 1,9 | 280 | 12 | 1,4 | 0,28 | Yes | No |
| [26] | B | B | A | B | B | A | A | Au | Single | 20 | 2,5 | Sharp | 17,0 | 280 | 10 | 2,4 | 0,04 | Yes | No |
| [26] | B | B | A | B | B | A | A | AuGc | Double | 20 | 1,8 | Sharp | 11,0 | 280 | 10 | 2,2 | 0,01 | Yes | No |
| [27] | A | B | A | A | C | C | C | Mt | Single | 72 | 0,8 | Sharp | 4,7 | 280 | 12 | 2,1 | 0,00 | No | No |
| [27] | A | B | A | A | C | C | C | MtGc | Double | 70 | 0,8 | Sharp | 6,0 | 280 | 12 | 2,7 | 0,00 | No | No |
| [28] | A | A | A | A | B | A | A | Mt | Single | 117 | 0,2 | Larsen | 7,0 | 240 | 24 | 14,6 | 0,82 | Yes | No |
| [28] | A | A | A | A | B | A | A | MtCs | Double | 119 | 0,3 | Larsen | 8,0 | 240 | 24 | 11,1 | 0,82 | Yes | No |
| [28] | A | A | A | A | B | A | A | MtGc | Double | 115 | 0,4 | Larsen | 5,0 | 240 | 24 | 5,2 | 0,82 | Yes | No |
| [28] | A | A | A | A | B | A | A | MtCsGc | Triple | 116 | 0,3 | Larsen | 5,0 | 240 | 24 | 6,9 | 0,82 | Yes | No |
| [29] | B | B | C | A | B | C | B | Mt | Single | 57 | 2,3 | Larsen | 32,8 | 200 | 24 | 7,1 | 1,50 | Yes | No |
| [29] | B | B | C | A | B | C | B | MtSu | Double | 56 | 2,5 | Larsen | 33,6 | 200 | 24 | 6,7 | 1,50 | Yes | No |
| [29] | B | B | C | A | B | C | B | MtSuCl | Triple | 58 | 2,2 | Larsen | 33,4 | 200 | 24 | 7,6 | 1,50 | Yes | No |
| [30] | A | A | C | A | C | C | A | Su | Single | 47 | 1,7 | Sharp | 32,0 | 280 | 18 | 6,7 | 1,15 | Yes | No |
| [30] | A | A | C | A | C | C | A | MtSuCl | Triple | 52 | 1,6 | Sharp | 28,0 | 280 | 18 | 6,3 | 4,08 | Yes | No |
| [31] | A | A | C | A | B | A | A | Mt | Single | 98 | 0,7 | Larsen | 2,0 | 240 | 24 | 1,2 | 3,59 | No | No |
| [31] | A | A | C | A | B | A | A | MtSuCl | Triple | 97 | 0,6 | Larsen | 2,0 | 240 | 24 | 1,4 | 5,14 | No | No |
| [32] | A | A | A | A | C | C | B | Su | Single | 65 | 0,3 | Sharp | 5,0 | 280 | 13 | 6,0 | 0,27 | No | No |
| [32] | A | A | A | A | C | C | B | SuMtGc | Triple | 70 | 0,3 | Sharp | 2,0 | 280 | 13 | 2,4 | 0,27 | No | No |
| [33] | B | A | A | A | A | C | C | Mt | Single | 212 | 6,8 | Sharp | 11,5 | 280 | 12 | 0,6 | 3,20 | No | No |
| [33] | B | A | A | A | A | C | C | EtMt | TNFiMt | 218 | 6,8 | Sharp | 9,5 | 280 | 12 | 0,5 | 3,10 | No | No |
| [34] | A | A | A | A | A | C | C | Mt | Single | 230 | 0,8 | Sharp | 5,0 | 448 | 12 | 1,4 | 2,50 | No | No |
| [34] | A | A | A | A | A | C | C | EtMt | TNFiMt | 246 | 0,7 | Sharp | 5,0 | 448 | 12 | 1,6 | 2,45 | No | No |
| [35] | B | B | B | A | A | C | C | Mt | Single | 64 | 11,0 | Sharp | 82,0 | 280 | 12 | 2,7 | 3,20 | No | Yes |
| [35] | B | B | B | A | A | C | C | InMt | TNFiMt | 71 | 10,0 | Sharp | 79,0 | 280 | 12 | 2,8 | 3,15 | No | Yes |
| [36] | A | A | A | A | A | C | C | Mt | Single | 226 | 0,9 | Sharp | 8,3 | 280 | 12 | 3,3 | 1,90 | Yes | No |
| [36] | A | A | A | A | A | C | C | InMt | TNFiMt | 306 | 0,8 | Sharp | 8,8 | 280 | 12 | 3,9 | 1,85 | Yes | No |
| [37] | B | B | A | A | A | A | C | Mt | Single | 251 | 0,8 | Sharp | 13,6 | 230 | 12 | 7,4 | 2,27 | No | No |
| [37] | B | B | A | A | A | A | C | AdMt | TNFiMt | 267 | 0,7 | Sharp | 11,0 | 230 | 12 | 6,8 | 2,40 | No | No |
| [38] | B | B | A | A | A | C | C | Mt | Single | 172 | 10,9 | Sharp | 37,2 | 230 | 12 | 1,5 | 2,71 | Yes | Yes |
| [38] | B | B | A | A | A | C | C | AdMt | TNFiMt | 183 | 11,0 | Sharp | 41,4 | 230 | 12 | 1,6 | 2,46 | Yes | Yes |
| [39] | B | B | A | A | A | A | C | Mt | Single | 199 | 6,2 | Sharp | 20,0 | 440 | 12 | 0,7 | 1,70 | No | Yes |
| [39] | B | B | A | A | A | A | C | CzMt | TNFiMt | 393 | 6,1 | Sharp | 20,0 | 440 | 12 | 0,7 | 1,70 | No | Yes |
| [40] | B | B | A | A | A | A | C | Mt | Single | 127 | 5,6 | Sharp | 23,1 | 280 | 6 | 1,5 | 2,85 | No | Yes |
| [40] | B | B | A | A | A | A | C | CzMt | TNFiMt | 246 | 6,1 | Sharp | 19,0 | 280 | 6 | 1,1 | 2,75 | No | Yes |
| [41] | A | A | B | A | A | A | C | Mt | Single | 195 | 8,9 | Sharp | 21,8 | 145 | 12 | 1,7 | 3,45 | Yes | Yes |
| [41] | A | A | B | A | A | A | C | AbMt | ABAMt | 391 | 8,5 | Sharp | 21,7 | 145 | 12 | 1,8 | 3,60 | Yes | Yes |
| [42] | B | B | A | A | B | C | C | Mt | Single | 223 | 0,5 | Sharp | 4,8 | 145 | 12 | 6,6 | 4,56 | Yes | No |
| [42] | B | B | A | A | B | C | C | AbMt | ABAMt | 218 | 0,5 | Sharp | 5,4 | 145 | 12 | 7,4 | 3,63 | Yes | No |
| [43] | B | B | C | A | A | C | C | Mt | Single | 184 | 11,6 | Sharp | 46,2 | 145 | 12 | 2,7 | 3,05 | No | Yes |
| [43] | B | B | C | A | A | C | C | RtMt | CD20iMt | 273 | 12,0 | Sharp | 46,2 | 145 | 12 | 2,7 | 3,25 | No | Yes |
| [44] | A | A | A | A | B | C | A | Mt | Single | 15 | 0,7 | Sharp | 24 | 448 | 22 | 7,7 | 1.6 | Yes | No |
| [44] | A | A | A | A | B | C | A | MtBu | Double | 15 | 0,8 | Sharp | 14 | 448 | 22 | 3,9 | 0.8 | Yes | No |
| [45] | A | A | A | A | A | A | C | Mt | Single | 232 | 0,90 | Sharp | 3,70 | 145 | 12 | 2,8 | 2,40 | No | No |
| [45] | A | A | A | A | A | A | C | RtMt | CD20iMt | 244 | 0,90 | Sharp | 3,50 | 145 | 12 | 2,7 | 2,20 | No | No |
| [46a] | B | A | A | B | B | A | C | Mt | Single | 160 | 2,9 | Sharp | 19,7 | 448 | 12 | 1,5 | 3,40 | Yes | No |
| [46a] | B | A | A | B | B | A | C | GoMt | TNFiMt | 159 | 3,5 | Sharp | 18,7 | 448 | 12 | 1,2 | 3,50 | Yes | No |
| [46b] | B | A | A | B | B | A | C | Mt | Single | 133 | 6,5 | Sharp | 36,7 | 448 | 12 | 1,3 | 4,80 | Yes | Yes |
| [46b] | B | A | A | B | B | A | C | GoMt | TNFiMt | 89 | 4,5 | Sharp | 29,7 | 448 | 12 | 1,5 | 5,60 | Yes | Yes |
| [47] | B | B | A | B | B | B | C | Mt | Single | 393 | 9 | Sharp | 28,8 | 145 | 12 | 2,2 | 3,45 | Yes | Yes |
| [47] | B | B | A | B | B | B | C | TzMt | TZMt | 398 | 9,3 | Sharp | 29,1 | 145 | 12 | 2,2 | 3,10 | Yes | Yes |
| [48] | A | A | A | A | C | C | A | Mt | Single | 110 | 0,6 | Sharp | 0 | 280 | 24 | 0,0 | 0 | Yes | No |
| [48] | A | A | A | A | C | C | A | MtGc | Double | 112 | 0,6 | Sharp | 0 | 280 | 24 | 0,0 | 0 | Yes | No |
| [49] | B | B | A | A | A | B | C | Mt | Single | 84 | 8,7 | Sharp | 30,8 | 280 | 6 | 1,3 | NA | Yes | Yes |
| [49] | B | B | A | A | A | B | C | GoMt | TNFiMt | 81 | 8,8 | Sharp | 32,1 | 280 | 6 | 1,3 | NA | Yes | Yes |
| [50] | B | A | A | A | B | C | C | Mt | Single | 285 | 7,2 | Sharp | NA | 448 | 11 | NA | 3,54 | No | Yes |
| [50] | B | A | A | A | B | C | C | OcMt | CD20iMt | 324 | 7,6 | Sharp | NA | 448 | 11 | NA | 3,83 | No | Yes |
| [51] | B | B | A | A | C | C | C | Mt | Single | 282 | 11,8 | Sharp | NA | 448 | 11 | NA | 4,07 | No | Yes |
| [51] | B | B | A | A | A | C | C | OcMt | CD20iMt | 277 | 12,3 | Sharp | NA | 448 | 11 | NA | 3,81 | No | Yes |
| [52] | B | A | A | A | B | C | C | Mt | Single | 196 | 1,23 | Sharp | 13,3 | 448 | 12 | 2,4 | 2 | No | No |
| [52] | B | A | A | A | B | C | C | OcMt | CD20iMt | 194 | 1,2 | Sharp | 12,4 | 448 | 12 | 2,3 | 2,11 | No | No |
| [53] | B | A | A | A | B | A | C | Mt | Single | 514 | 4,5 | Sharp | 5,1 | 280 | 6 | 0,4 | 2,3 | No | No |
| [53] | B | A | A | A | B | A | C | AdMt | TNFiMt | 508 | 4 | Sharp | 5,4 | 280 | 6 | 0,5 | 2,05 | No | No |
*Percentage of Annual Radiographic Progression Rate
Figure 2. Combination treatment versus single DMARD.
The effect on all studies is −0.33 SMD (CI: −0.36, −0.29). Test for overall effect: Z = 17.66 (P<0.00001). Heterogeneity: Chi2 = 201.54, df = 44 (P<0.00001); I2 = 78%. One study [27] contributed to heterogeneity due an extreme effect (−3.71 SMD). The elimination of this study resulted in a little more conservative estimate (−0.31 SMD (CI:−0.35, −0.28), Z = 16.81), but eliminated the significant heterogeneity (I2 = 20, p = 0.13). Consequently, reference [27] was excluded from all comparisons. N, combination: 6725; N, single: 5446.
Description of network
On the basis of the included treatment arms and doses, we defined 6 combination treatments versus single DMARD:
Two DMARDs/LDGC (Double);
Three DMARDs/LDGC (Triple);
Standard dose of TNFi (Infliximab: 3 mg/kg/8 weeks; etanercept: 50 mg/1 week; adalimumab: 40 mg/2 weeks; certolizumab: 200 mg/2 weeks; golimumab: 50 mg/4 weeks);
Standard dose of CD20 inhibitor treatment (rituximab 2 g/6 months; ocrelizumab 1 g/6 months);
Abatacept 10 mg/kg/4 weeks;
Tocilizumab 8 mg/kg/4 weeks.
The star shaped network is shown in Figure 3. As one study included a direct comparison between TNFi, double and triple [3] and additionally two studies included direct comparisons between double and triple [28], [29], the star includes loops to indicate the direct comparisons between TNFi, double and triple.
Figure 3. Star shaped network showing the 6 different combination treatments anchored on single treatment as the common comparator.
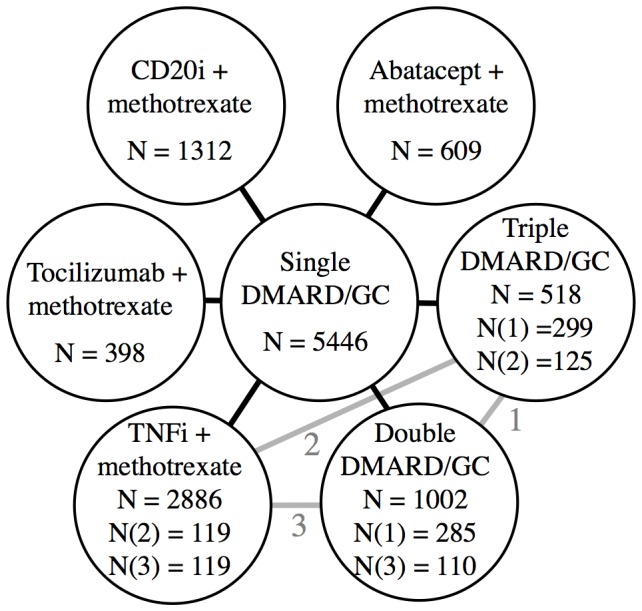
The loops (grey lines) with corresponding numbers (1, 2, 3) show the subgroups, which were directly compared in addition to being indirectly compared. N indicates the number of patients in the groups.
Synthesis of results
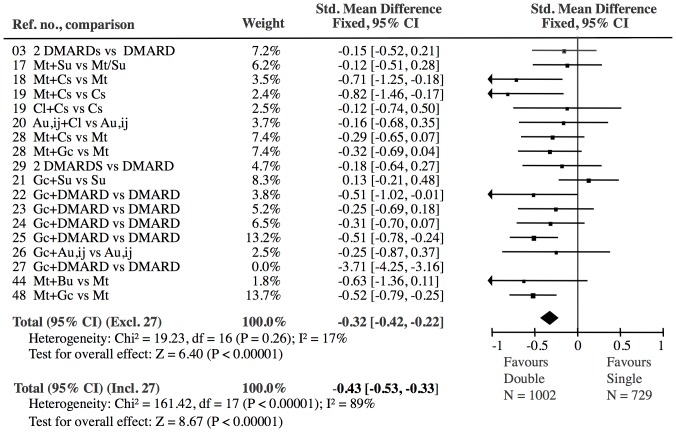
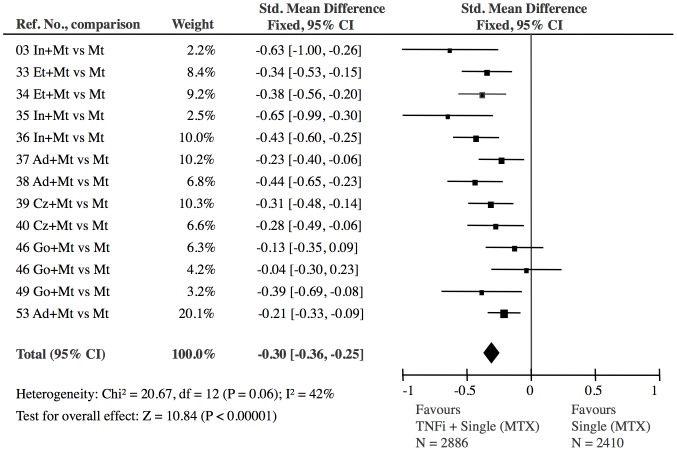
Only one study [27] contributed to heterogeneity in the analyses of all 45 treatment groups (I2 = 78%) (Figure 2) and in the analysis of double DMARD vs. single DMARD (I2 = 89%) (Figure 4). All other heterogeneity analyses were non-significant (I2 varying in the range 0–42%, Figures 5–9). Consequently we eliminated this study [27] from the statistical analyses (reducing I2 to 17–20%) and used a fixed effect model in the primary analyses and a random effect model in the secondary analyses. The results of the conventional meta-analyses of the 6 combination treatments are shown in Figures 4–9. The borderline heterogeneity in the TNFi analysis (I2 = 42%) (Figure 6) was due to two golimumab studies [46]. Elimination of these studies reduced heterogeneity (I2 = 27%) but did not change the overall result (SMD: −0.33 (CI: −0.39, −0.27)).
Figure 4. Double DMARD versus single DMARD: The effect of the Double DMARD treatment was highly significant (Z = 6.40).
All 18 Double studies showed heterogeneity (I2 = 89%). The exclusion of one reference [27], which had an extreme effect (−3.71 SMD), eliminated the significant heterogeneity (I2 = 17%).
Figure 5. Triple DMARD versus single DMARD: The effect of the Triple DMARD treatment was highly significant (Z = 6.13).
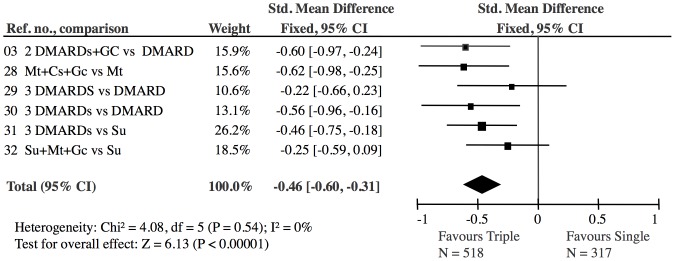
The 6 Triple studies showed no heterogeneity (I2 = 0).
Figure 9. Tocilizumab combined with methotrexate versus single DMARD (methotrexate): The effect of tocilizumab is significant (Z = 4.70).
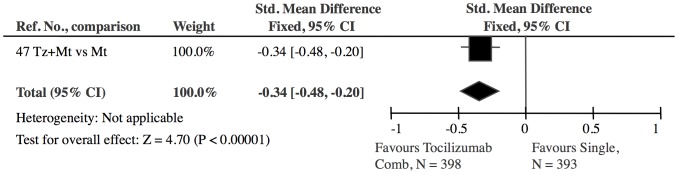
Figure 6. TNF inhibitor combined with methotrexate versus single DMARD (methotrexate): The effect of TNF inhibitor was highly significant (Z = 10.84).
The 13 TNF inhibitor studies showed no significant heterogeneity (I2 = 42%, p = 0.06). The borderline heterogeneity was due to two golimumab studies (GoBefore, GoForward) [46]. The exclusion of these, did, however, not change the overall result (−0.33 SMD (CI: −0.39, −0.27)).
Figure 7. Abatacept combined with methotrexate versus single DMARD (methotrexate): The effect of abatacept was significant (Z = 3.08).
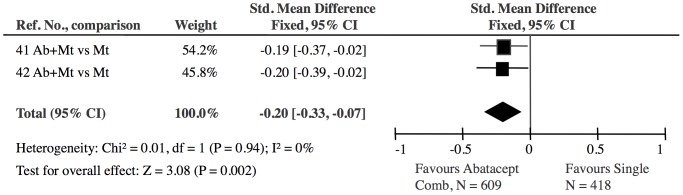
The 2 abatacept studies showed no heterogeneity (I2 = 0).
Figure 8. CD20 inhibitor treatment combined with single DMARD versus single DMARD: The effect of CD20 inhibitor treatment was highly significant (Z = 7.87).
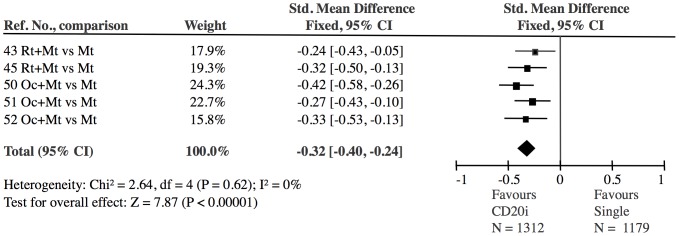
The 5 CD20 inhibitor studies showed no heterogeneity (I2 = 0).
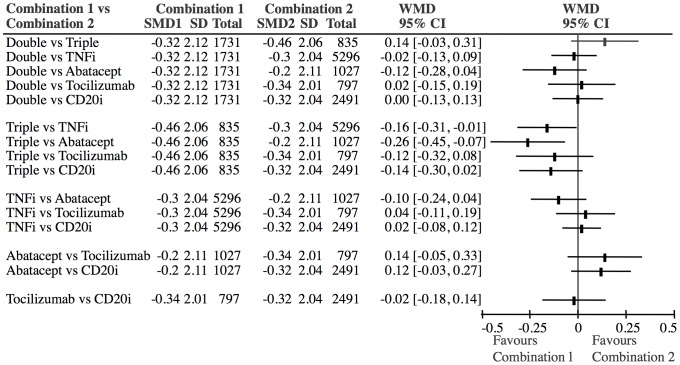
Because all interventions are connected in the network (i.e. each pair has a path from one to the other) indirect comparisons can be performed for each of the combination treatments in the star versus each other. Figure 10 shows the results of the indirect comparisons of the 6 combination treatments. The effects varied between −0.46 SMD (triple) and −0.20 SMD (abatacept). Statistically, triple treatment with DMARDs was a little better than abatacept plus methotrexate (−0.26 SMD (CI: −0.45, −0.07)) and TNFi plus methotrexate (−0.16 SMD (CI: −0.31, −0.01)), but no other significant differences between the different combination treatments were identified (Figure 10).
Figure 10. Indirect comparisons of different combination treatments.
There is a trend towards triple treatment being superior to abatacept and TNFi. All other differences between the combination treatments are non-significant. Abbreviations: SMD: Standardized mean difference. WMD: Weighted mean difference (SMD1-SMD2).
Risk of bias across studies
The cumulated grade (A, B, C) frequencies are shown in Table 2. Six of the eight bias domains are predominantly graded as being of low (A) or unclear (B) risk, whereas two domains (incomplete outcome reporting and study sponsoring) are predominantly classified as being of high risk. Concerning the 6 Cochrane bias domains, 28 of 39 trials contained at least one high risk (C) grade.
Table 2. Observed Frequencies of bias factors for treatment groups.
| Double | Triple | TNFi | ABA | CD20i | TZ | ?2 | p | |
| Sequence generation | ||||||||
| A | 9 | 5 | 3 | 1 | 1 | 0 | ||
| B | 9 | 1 | 10 | 1 | 4 | 1 | ||
| C | 0 | 0 | 0 | 0 | 0 | 0 | 8.3 | 0.14 |
| Allocation concealment | ||||||||
| A | 7 | 5 | 7 | 1 | 3 | 0 | ||
| B | 11 | 5 | 6 | 1 | 2 | 1 | ||
| C | 0 | 0 | 0 | 0 | 0 | 0 | 4.8 | 0.44 |
| Study blinding | ||||||||
| A | 11 | 2 | 11 | 1 | 4 | 1 | ||
| B | 0 | 0 | 1 | 1 | 0 | 0 | ||
| C | 7 | 4 | 1 | 0 | 1 | 0 | 19.7 | 0.03 |
| Outcome blinding | ||||||||
| A | 16 | 11 | 6 | 2 | 5 | 0 | ||
| B | 2 | 2 | 1 | 0 | 0 | 1 | ||
| C | 0 | 0 | 0 | 0 | 0 | 0 | 9.7 | 0.09 |
| Radiographic sequence | ||||||||
| A | 4 | 1 | 10 | 1 | 3 | 0 | ||
| B | 8 | 3 | 3 | 1 | 2 | 1 | ||
| C | 6 | 2 | 0 | 0 | 0 | 0 | 16.3 | 0.09 |
| Incomplete outcome data | ||||||||
| A | 3 | 2 | 6 | 1 | 1 | 0 | ||
| B | 0 | 0 | 1 | 0 | 0 | 1 | ||
| C | 15 | 4 | 6 | 1 | 4 | 0 | 27.7 | 0.002 |
| Selective outcome reporting | ||||||||
| A | 18 | 6 | 13 | 2 | 5 | 1 | ||
| B | 0 | 0 | 0 | 0 | 0 | 0 | ||
| C | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Sponsorship | ||||||||
| A | 11 | 3 | 0 | 0 | 0 | 0 | ||
| B | 3 | 2 | 0 | 0 | 0 | 0 | ||
| C | 4 | 1 | 13 | 2 | 5 | 1 | 30.1 | 0.001 |
A funnel plot indicates a minor degree of publication bias (Figure 11).
Figure 11. Funnel plot of all combination studies ([27] eliminated).
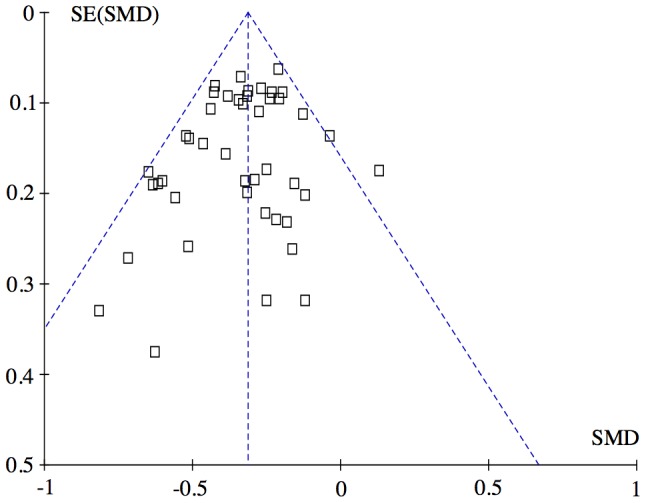
The left lower corner is empty compared with the right lower corner. This asymmetry may indicate that small studies with no effect was not published (publication bias). However, this asymmetry is quantitatively small, and probably does not affect the overall result. Exclusion of the three lower right studies [18], [19], [44] to eliminate the asymmetry did not change the overall result shown in Figure 2: −0.31 SMD (CI: −0.35, −0.27), test for overall effect: Z = 16.49 (P<0.00001). Heterogeneity: Chi2 = 48.41, df = 40 (P = 0.17); I2 = 17%. Abbreviations: SMD: Standardized mean difference.
Consistency analysis
Three trials [3], [28], [29] of the 39 trials contributed with treatment arms to three combination treatment groups (TNFi, Double and Triple). Pairwise consistency analyses of the SMD effects obtained in the trials directly comparing combination treatments versus the SMD effects obtained by means of the exclusively indirect comparisons were performed to explore possible differences between the direct and the indirect comparisons.
Triple versus Double: Direct comparison (n = 584) versus indirect comparison (n = 1616): Weighted mean difference = 0.20 SMD (CI: −0.08, 0.48).
Double versus TNFi plus methotrexate:
Direct comparison (BeSt study [3], 1. year data) (n = 229) versus indirect comparison (n = 6722): Weighted mean difference = 0.55 SMD (CI: 0.28, 0.82).
Supplementary analysis including the second year data from the BeSt study [4]: Direct comparison (n = 236) versus indirect comparison (n = 6722): Weighted mean difference = 0.05 SMD (CI: −0.32, 0.42).
Triple versus TNFi plus methotrexate: Direct comparison (n = 244) versus indirect comparison (n = 5810): Weighted mean difference = 0.23 SMD (CI: −0.07, 0.53).
Additional analyses
Using a random effect model instead of a fixed effect model eliminated the small significant difference between triple DMARD and TNFi (weighted mean difference: −0.14 SMD (CI: −0.30; 0.02)), but all other indirect comparisons as shown in Figure 10 were unchanged.
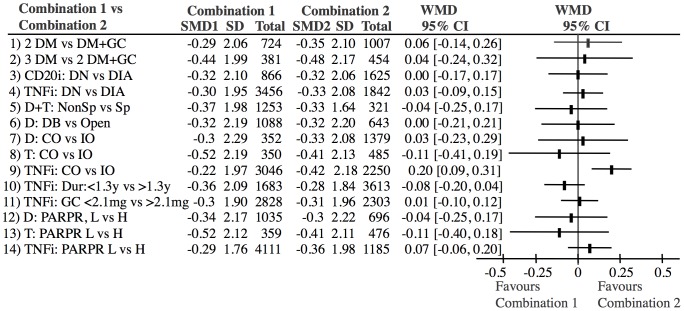
There was no difference between DMARD combination studies using LDGC as a DMARD equivalent and those using only DMARDs (Figure 12, lines 1–2). There was no difference between biologic studies performed in DMARD naïve (DN) patients and DMARD inadequate responders (DIA) (Figure 12, lines 3–4).
Figure 12. Analyses of bias factors and confounders, which differed significantly across treatment groups.
Only 1 bias factor (TNFi studies: Complete outcome versus incomplete outcome, line 9) had a significant influence on the outcome. Abbreviations: SMD: Standardized mean difference. WMD: Weighted mean difference (SMD1-SMD2); DM: DMARD; GC: Glucocorticoid; DN: DMARD naive; DIA: DMARD inadequate responder; D: double; T: Triple; Sp: Sponsoring; DB: double-blind; CO: Complete outcome; IO: Incomplete outcome; Dur: Disease duration at baseline; PARPR: Percentage of annual radiographic progression rate; L: low; H: High.
Table 3 shows other possible confounders across treatment groups. Sensitivity analyses were performed for the bias domains (Table 2) and possible confounding variables (Table 3), which differed across studies and the results are shown in Figure 12. The results of these analyses showed that these factors did not influence the results significantly (Figure 12, lines 5–14) with the exception TNFi studies with incomplete outcome reporting (high risk of bias), which had a significantly higher effect than those with complete outcome reporting (low risk of bias) (Figure 12, line 9).
Table 3. Other possible confounders across treatment groups.
| Double | Triple | TNFi | ABA | CD20i | TZ | p | |
| N | 18 | 6 | 13 | 2 | 5 | 1 | |
| Duration (years) of RA at baseline | |||||||
| Mean | 2.1 | 0.9 | 5.0 | 4.6 | 6.7 | 9.2 | |
| SD | 3.4 | 0.8 | 3.6 | 4.7 | 5.1 | 0.2 | 0.0001 |
| Percentage of annual radiographic progression rate at baseline | |||||||
| Mean | 3.5 | 5.2 | 1.9 | 4.4 | 2.6 | 2.2 | |
| SD | 3.3 | 3.9 | 1.7 | 3.1 | 0.2 | 0.04 | 0.03 |
| Glucocorticoid use during study | |||||||
| Mean | 0.6 | 1.6 | 2.6 | 3.8 | 3.0 | 3.3 | |
| SD | 0.9 | 1.7 | 1.2 | 0.5 | 0.8 | 0.2 | 0.0001 |
| Strategy change during study | |||||||
| No | 7 | 2 | 7 | 0 | 5 | 0 | |
| Yes | 11 | 4 | 6 | 2 | 0 | 1 | 0.09 |
Discussion
In contrast to our previous meta-analysis [1], which was a compilation of conventional meta-analyses, the present network meta-analysis indirectly compared the different treatment principles arranged in a network anchored on single DMARD therapy. The analysis is the first network meta-analysis to use the essential outcome (joint destruction) and to show that different biologic treatments combined with methotrexate may not be superior to treatments with 2–3 DMARDs or 1–2 DMARDs + LDGC (Figure 10). Furthermore the different biologic treatments did not differ from each other. The latter finding confirms the reliability of the analysis, as it is in agreement with previous network meta-analyses using ACR50 as an outcome [9]–[10], [54]–[59], which indicate that TNF inhibitors, tocilizumab and rituximab have similar effects, abatacept is borderline inferior and IL1i is clinically and statistically inferior. Most of these used a Bayesian framework, but one used a statistical method based on Bucher’s design, similar to ours [57]. The outcome of this analysis corresponded to the outcome of the others and ours. A limitation is that the outcomes of the present and previous network meta-analyses are based on indirect data. Therefore doubt can be raised that the treatment arms compared may not be as comparable as randomized treatment arms from one population. This doubt can never be completely eliminated and therefore some reservation concerning the outcomes should be acknowledged. Consequently, the present analysis cannot be considered to be definite evidence that two or more DMARDs prevent structural joint damage to the same degree as a biologic agent combined with methotrexate. The reverse conclusion is also not definite. Therefore confirmation of the present results in direct comparison studies and meta-analyses would be desirable. Recently, a few such studies did confirm that the effect of triple DMARD therapy was comparable with the effect of TNFi plus methotrexate [5]–[7]. These studies, which were published after the date of our final literature search, did not fulfill our inclusion criteria, as they did not use a single DMARD therapy treatment arm. Similar direct comparisons of the other biologic drugs (tocilizumab, abatacept and rituximab) with combination DMARD treatment have not been performed.
Our approach to reduce heterogeneity was successful, as there was no heterogeneity after exclusion of a single study, neither when the studies were analyzed in one group (Figure 2) nor when the treatments were analyzed separately (Figures 4–9). Most within study bias sources (Table 1) were equally distributed across the defined treatment groups (Table 2) and only one of the Cochrane defined bias domains (incomplete outcome data) was dominated by the high risk of bias grade C (26 of 39). Sensitivity analyses of the bias sources, which were unequally distributed in the combination treatment groups (Tables 2 and 3), did not change the results (Figure 12) with the exception TNFi studies with incomplete outcome data (Figure 12, line 9). This bias could inflate the effect of TNFi, but not change the main finding of the study. In general the results were robust. The amount of evidence in the network was significant (Figure 3), the heterogeneity analysis of the study effects was insignificant indicating similar results from study to study (Figure 2) and direct and indirect comparisons were consistent when comparing treatment balanced data. The main reason for the low degree of heterogeneity was probably that all comparisons were anchored on a similar comparator (single DMARD) and that the baseline differences between included populations were moderate. Finally, publication bias (Figure 11), or other possible confounders such as different disease duration , different disease activity at baseline (PARPR), different use of glucocorticoid or treatment strategy change during the treatment period (Table 3) could not explain the similar outcome effects (Figure 12).
A recent study indicated that patients included in newer studies have a lower baseline disease activity than in older studies [60]. This could in theory explain why the effect of the biologics did not exceed the effect of the DMARDs. This theory is in part confirmed by the fact that there was a difference in baseline disease activity between TNFi studies (PARPR = 1.9%) and triple DMARD studies (PARPR = 5.2%). However, the sensitivity analyses of studies with high baseline activity versus low baseline activity showed no differences (Figure 12, lines 12–14). Furthermore, the baseline activity of the double DMARD studies did not differ from the baseline activity of the other biologic studies (Table 3). Consequently the different time periods of the different studies could probably not explain the similar effects.
The chosen outcome (joint destruction) is the essential outcome of RA [61]–[62]. Furthermore, the ACR response criteria used in the meta-analyses of biologic studies [9]–[10], [54]–[59] are not available in older DMARD studies. We accepted two different scoring methods as our previous analysis showed concordant results for both methods [1]. This outcome and other outcome measures of RA are mutually dependent. Although joint inflammation and joint destruction are not always linked, several studies have shown that on the average there is a very high association between integrated measures of inflammatory variables (i.e. ESR, CRP, swollen joint count) and the radiographic score, as shown and reviewed previously [63]–[64]. Therefore, the radiographic score is a cumulative measure that not only shows the existing status of the patient, but also reflects the preceding disease course [63]–[64]. The assumption that the radiographic progression sufficiently reflects the outcome of RA is further verified by the fact that network-meta-analyses comparing biologic drugs using ACR response criteria as outcome measure also do not find differences between the different biologic drugs except that the IL1 inhibitor has an inferior effect [9]–[10], [54]–[59].
All approved treatment principles were investigated. The grouping of DMARDs and LDGC was based on the findings of our previous analyses, which showed that these drugs had similar effects [1]. The present study confirms that the effect of LDGC corresponds to the effect of a DMARD (Figure 12, line 1–2). Our assumption of equality between methotrexate, sulfasalazine and leflunomide has recently been verified in an independent review [65], which, however, did not investigate cyclosporine and gold.
In general, our results agree with those of an independent research group [66], which in an analysis of pairwise meta-analyses indicated that DMARD and TNFi/methotrexate combinations had equal efficacy on ACR response, withdrawals for inefficacy, disability and erosive progression.
Because of the high prices of biologics, their cost-effectiveness is a matter of debate [67]. This may be a reason why different official treatment recommendations are not completely concordant.
Our results are not consistent with the European League against Rheumatism (EULAR) recommendations [68], which suggest that in DMARD naive patients, irrespective of the addition of glucocorticoids, DMARD mono therapy rather than combination therapy of DMARDs may be applied followed by switching to another single DMARD or addition of a biologic agent. In contrast to the EULAR guidelines, the American College of Rheumatology (ACR) guidelines does recommend combination DMARD treatment [69]. However, ACR also recommends biologic treatment to subgroups of patients with poor prognostic factors, who have either received single DMARD therapy or never received DMARDs.
A recent analysis concluded that the continued use of placebo arms instead of active arms in the controlled trials of new biologic agents exposed patients in the control arms to possible deterioration [2]. In an accompanying editorial [70], the previous use of placebo was in part defended, but it was also acknowledged that new designs were necessary to reduce the risk of patients in the control arms. In our opinion there is now evidence that combination treatment with at least two DMARDs, one of which could be LDGC, may prevent structural joint damage to the same degree as a biologic agent combined with methotrexate. Therefore future study designers should not seek superiority of the new drug compared with placebo, but should design studies with sufficient power to demonstrate equality with a combination of conventional DMARDs. Biologic agents should, as originally intended, be reserved for patients that are insufficiently treated with a combination of at least two conventional DMARDs.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
This study was supported by The A.P. Møller Foundation for the Advancement of Medical Science. The Musculoskeletal Statistics Unit, the Parker Institute are supported by grants from the The Oak Foundation, Frederiksberg Hospital, and The Danish Medicines Agency. The A.P.M. Foundation, The Oak Foundation, and The Danish Medicines Agency are non-profit funding sources and had no role in the design and conduct of the study or in the collection, analysis, and interpretation of the data or in the preparation, review, and approval of the manuscript.
References
- 1. Graudal N, Jürgens G (2010) Similar effects of disease-modifying antirheumatic drugs, glucocorticoids and biologics on radiographic progression in rheumatoid arthritis: meta-analysis of 70 randomized placebo or drug controlled studies including 112 comparisons. Arthritis Rheum 62: 2852–2863. [DOI] [PubMed] [Google Scholar]
- 2. Estellat C, Ravaud P (2012) Lack of head-to-head trials and fair control arms: randomized controlled trials of biologic treatment for rheumatoid arthritis. Arch Intern Med 172: 237–44. [DOI] [PubMed] [Google Scholar]
- 3. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, et al. (2005) Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 52: 3381–90. [DOI] [PubMed] [Google Scholar]
- 4. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, et al. (2007) Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med 146: 406–15. [DOI] [PubMed] [Google Scholar]
- 5. Van Vollenhoven RF, Geborek P, Forslind K, Albertsson K, Ernestam S, et al. (2012) Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet 379: 1712–20. [DOI] [PubMed] [Google Scholar]
- 6. Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, et al. (2012) A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of early aggressive rheumatoid arthritis trial. Arthritis Rheum 64: 2824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, et al. (2013) Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 369: 307–18. [DOI] [PubMed] [Google Scholar]
- 8. Caldwell DM, Ades AE, Higgins JP (2005) Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nixon RM, Bansback N, Brennan A (2007) Using mixed treatment comparisons and meta-regression to perform indirect comparisons to estimate the efficacy of biologic treatments in rheumatoid arthritis. Stat Med 26: 1237–54. [DOI] [PubMed] [Google Scholar]
- 10. Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, et al. (2009) A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ 181: 787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, et al. (2009) Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 338: b1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Nordic Cochrane Centre, Higgins JP, Green S, editors (2009) Cochrane handbook for systematic reviews of interventions version 5.0.1, section 7.7.3. The Cochrane Collaboration. Available: www.cochrane-handbook.org.
- 14. Walter SD, Yao X (2009) Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol 60: 849–52. [DOI] [PubMed] [Google Scholar]
- 15. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, et al. (2011) Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 14: 417–28. [DOI] [PubMed] [Google Scholar]
- 16. Bucher HC, Guyatt GH, Griffith LE, Walter SD (1997) The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 50: 683–91. [DOI] [PubMed] [Google Scholar]
- 17. Dougados M, Combe B, Cantagrel A, Goupille P, Olive P, et al. (1999) Combination therapy in early rheumatoid arthritis: a randomised, controlled, double blind 52 week clinical trial of sulphasalazine and methotrexate compared with the single components. Ann Rheum Dis 58: 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marchesoni A, Battafarano N, Arreghini M, Panni B, Gallazzi M, et al. (2003) Radiographic progression in early rheumatoid arthritis: a 12-month randomized controlled study comparing the combination of cyclosporin and methotrexate with methotrexate alone. Rheumatology 42: 1545–49. [DOI] [PubMed] [Google Scholar]
- 19. Sarzi-Puttini P, D'Ingianna E, Fumagalli M, Scarpellini M, Fiorini T, et al. (2005) An open, randomized comparison study of cyclosporine A, cyclosporine A + methotrexate and cyclosporine A + hydroxychloroquine in the treatment of early severe rheumatoid arthritis. Rheumatol Int 25: 15–22. [DOI] [PubMed] [Google Scholar]
- 20. Scott DL, Dawes PT, Tunn E, Fowler PD, Shadforth MF, et al. (1989) Combination therapy with gold and hydroxychloroquine in rheumatoid arthritis: a prospective, randomized, placebo-controlled study. Br J Rheumatol 28: 128–33. [DOI] [PubMed] [Google Scholar]
- 21. Capell HA, Madhok R, Hunter JA, Porter D, Morrison E, et al. (2004) Lack of radiological and clinical benefit over two years of low dose prednisolone for rheumatoid arthritis: results of a randomised controlled trial. Ann Rheum Dis 63: 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choy EH, Kingsley GH, Khoshaba B, Pipitone N, Scott DL (2005) Intramuscular Methylprednisolone Study. A two year randomised controlled trial of intramuscular depot steroids in patients with established rheumatoid arthritis who have shown an incomplete response to disease modifying antirheumatic drugs. Ann Rheum Dis 64: 1288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansen M, Podenphant J, Florescu A, Stoltenberg M, Borch A, et al. (1999) A randomised trial of differentiated prednisolone treatment in active rheumatoid arthritis. Clinical benefits and skeletal side effects. Ann Rheum Dis 58: 713–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirwan JR (1995) The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study (1995) The effect of glucocorticoids on joint destruction in rheumatoid arthritis. N Engl J Med 333: 142–46. [DOI] [PubMed] [Google Scholar]
- 25. Svensson B, Boonen A, Albertsson K, van der Heijde D, Keller C, et al. (2005) Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum 52: 3360–3370. [DOI] [PubMed] [Google Scholar]
- 26. Van Gestel AM, Laan RF, Haagsma CJ, Van de Putte LB, van Riel PL (1995) Oral steroids as bridge therapy in rheumatoid arthritis patients starting with parenteral gold. A randomized double-blind placebo-controlled trial. Br J Rheumatol 34: 347–51. [DOI] [PubMed] [Google Scholar]
- 27. Wassenberg S, Rau R, Steinfeld P, Zeidler H (2005) Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 52: 3371–80. [DOI] [PubMed] [Google Scholar]
- 28. Choy EH, Smith CN, Farewell V, Walker D, Hassell A, et al. (2008) Factorial randomised controlled trial of glucocorticoids and combination disease modifying drugs in early rheumatoid arthritis. Ann Rheum Dis 67: 656–63. [DOI] [PubMed] [Google Scholar]
- 29. Calgüneri M, Pay S, Caliskaner Z, Apras S, Kiraz S, et al. (1999) Combination therapy versus monotherapy for the treatment of patients with rheumatoid arthritis. Clin Exp Rheumatol 17: 699–704. [PubMed] [Google Scholar]
- 30. Grigor C, Capell H, Stirling A, McMahon AD, Lock P, et al. (2004) Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 364: 263–69. [DOI] [PubMed] [Google Scholar]
- 31. Möttönen T, Hannonen P, Leirisalo-Repo M, Nissilä M, Kautiainen H, et al. (1999) Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet 353: 1568–73. [DOI] [PubMed] [Google Scholar]
- 32. Boers M, Verhoeven AC, Markusse HM, van de Laar MA, Westhovens R, et al. (1997) Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 350: 309–18. [DOI] [PubMed] [Google Scholar]
- 33. Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, et al. (2004) Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 363: 675–81. [DOI] [PubMed] [Google Scholar]
- 34. Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, et al. (2008) Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 372: 375–82. [DOI] [PubMed] [Google Scholar]
- 35. Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, et al. (2000) Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 343: 1594–602. [DOI] [PubMed] [Google Scholar]
- 36. St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, et al. (2004) Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 50: 3432–43. [DOI] [PubMed] [Google Scholar]
- 37. Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, et al. (2006) The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 54: 26–37. [DOI] [PubMed] [Google Scholar]
- 38. Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, et al. (2004) Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 50: 1400–1411. [DOI] [PubMed] [Google Scholar]
- 39. Keystone E, van der Heijde D, Mason D, Landewé R, Vollenhoven RV, et al. (2008) Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 58: 3319–29. [DOI] [PubMed] [Google Scholar]
- 40. Smolen JS, Landewé RB, Mease PJ, Brzezicki J, Mason D, et al. (2009) Efficacy and Safety of Certolizumab Pegol plus Methotrexate in Active Rheumatoid Arthritis: The RAPID 2 Study. Ann Rheum Dis 68: 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, et al. (2006) Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 144: 865–76. [DOI] [PubMed] [Google Scholar]
- 42. Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, et al. (2009) Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 68: 1870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keystone EC, Emery P, Peterfy CG, Tak PP, Cohen S, et al. (2009) Rituximab inhibits structural joint damage in rheumatoid arthritis patients with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis 68: 216–221. [DOI] [PubMed] [Google Scholar]
- 44. Ichikawa Y, Saito T, Yamanaka H, Akizuki M, Kondo H, et al. (2005) Therapeutic effects of the combination of methotrexate and bucillamine in early rheumatoid arthritis: a multicenter, double-blind, randomized controlled study. Mod Rheumatol 15: 323–8. [DOI] [PubMed] [Google Scholar]
- 45. Tak PP, Rigby WF, Rubbert-Roth A, Peterfy CG, van Vollenhoven RF, et al. (2011) Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis 70: 39–46. [DOI] [PubMed] [Google Scholar]
- 46. Emery P, Fleischmann R, van der Heijde D, Keystone EC, Genovese MC, et al. (2011) The Effects of Golimumab on Radiographic Progression in Rheumatoid Arthritis: Results of randomized controlled studies on Golimimab before methotrexate therapy and golimumab after methotrexate therapy. Arthritis Rheum 63: 1200–1210. [DOI] [PubMed] [Google Scholar]
- 47. Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, et al. (2011) Tocilizumab Inhibits Structural Joint Damage in Rheumatoid Arthritis Patients with an Inadequate Response to Methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 63: 609–621. [DOI] [PubMed] [Google Scholar]
- 48. Bakker MF, Jacobs JW, Welsing PM, Verstappen SM, Tekstra J, et al. (2012) Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med 156: 329–39. [DOI] [PubMed] [Google Scholar]
- 49. Tanaka Y, Harigai M, Takeuchi T, Yamanaka H, Ishiguro N, et al. (2012) Golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis: results of the GO-FORTH study. Ann Rheum Dis 71: 817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rigby W, Tony HP, Oelke K, Combe B, Laster A, et al. (2012) Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 64: 350–9. [DOI] [PubMed] [Google Scholar]
- 51. Tak PP, Mease PJ, Genovese MC, Kremer J, Haraoui B, et al. (2012) Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to at least one tumor necrosis factor inhibitor: results of a forty-eight–week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 64: 360–70. [DOI] [PubMed] [Google Scholar]
- 52. Stohl W, Gomez-Reino J, Olech E, Dudler J, Fleischmann RM, et al. (2012) Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial. Ann Rheum Dis 71: 1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kavanaugh A, Fleischmann RM, Emery P, Kupper H, Redden L, et al. (2013) Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 72: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bergman GJ, Hochberg MC, Boers M, Wintfeld N, Kielhorn A, et al. (2010) Indirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs. Semin Arthritis Rheum 39: 425–41. [DOI] [PubMed] [Google Scholar]
- 55. Devine EB, Alfonso-Cristancho R, Sullivan SD (2011) Effectiveness of biologic therapies for rheumatoid arthritis: an indirect comparisons approach. Pharmacotherapy 31: 39–51. [DOI] [PubMed] [Google Scholar]
- 56. Launois R, Avouac B, Berenbaum F, Blin O, Bru I, et al. (2011) Comparison of certolizumab pegol with other anticytokine agents for treatment of rheumatoid arthritis: a multiple-treatment Bayesian metaanalysis. J Rheumatol 38: 835–45. [DOI] [PubMed] [Google Scholar]
- 57. Salliot C, Finckh A, Katchamart W, Lu Y, Sun Y, et al. (2011) Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional diseasemodifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Ann Rheum Dis 70: 266–71. [DOI] [PubMed] [Google Scholar]
- 58. Schmitz S, Adams R, Walsh CD, Barry M, Fitzgerald O (2012) A mixed treatment comparison of the efficacy of anti-TNF agents in rheumatoid arthritis for methotrexate non-responders demonstrates differences between treatments: a Bayesian approach. Ann Rheum Dis 71: 225–30. [DOI] [PubMed] [Google Scholar]
- 59. Turkstra E, Ng SK, Scuffham PA (2011) A mixed treatment comparison of the short term efficacy of biologic disease modifying anti-rheumatic drugs in established rheumatoid arthritis. Curr Med Res Opin 27: 1885–97. [DOI] [PubMed] [Google Scholar]
- 60. Rahman MU, Buchanan J, Doyle MK, Hsia EC, Gathany T, et al. (2011) Changes in patient characteristics in anti-tumour necrosis factor clinical trials for rheumatoid arthritis: results of an analysis of the literature over the past 16 years. Ann Rheum Dis 70: 1631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brower AC (1990) Use of the radiograph to measure the course of rheumatoid arthritis. The gold standard versus fool's gold. Arthritis Rheum 33: 316–24. [DOI] [PubMed] [Google Scholar]
- 62. Graudal NA, Jurik AG, de Carvalho A, Graudal HK (1998) Radiographic progression in rheumatoid arthritis: a long-term prospective study of 109 patients. Arthritis Rheum 41: 1470–80. [DOI] [PubMed] [Google Scholar]
- 63. Graudal N, Tarp U, Jurik AG, Galloe AM, Garred P, et al. (2000) Inflammatory patterns in rheumatoid arthritis estimated by the number of swollen and tender joints, the erythrocyte sedimentationrate, and hemoglobin: long-term course and association to radiographic progression. J Rheumatol 27: 47–57. [PubMed] [Google Scholar]
- 64. Graudal N (2004) The natural history and prognosis of rheumatoid arthritis: association of radiographic outcome with process variables, joint motion and immune proteins. Scand J Rheumatol 33 Suppl 118 1–37. [DOI] [PubMed] [Google Scholar]
- 65.Donahue KE, Jonas DE, Hansen RA, Roubey R, Jonas B, et al.. (2012) Drug Therapy for Rheumatoid Arthritis in Adults: An Update [Internet]. Agency for Healthcare Research and Quality (US). Report No.: 12-EHC025-EF. AHRQ Comparative Effectiveness Reviews. [PubMed]
- 66. Ma MH, Kingsley GH, Scott DL (2010) A systematic comparison of combination DMARD therapy and tumour necrosis inhibitor therapy with methotrexate in patients with early rheumatoid arthritis. Rheumatology (Oxford). 49: 91–8. [DOI] [PubMed] [Google Scholar]
- 67. Scott DL (2012) Biologic based therapy for the treatment of rheumatoid arthritis. CPT 91: 30–43. [DOI] [PubMed] [Google Scholar]
- 68. Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, et al. (2010) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 69: 964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, et al. (2012) 2012 Update of the 2008 American College of Rheumatology Recommendations for the Use of Disease-Modifying Antirheumatic Drugs and Biologic Agents in the Treatment of Rheumatoid Arthritis. Arthritis Care Res 64: 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pearson SD (2012) Placebo-controlled trials, ethics, and the goals of comparative effectiveness research: comment on "lack of head-to-head trials and fair control arms". Arch Intern Med 172: 244–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)