Abstract
Abusive head trauma (AHT) is a unique form of pediatric TBI with increased mortality and neurologic sequelae. Hemispheric hypodensity (HH) in association with subdural blood after AHT has been described. Though risk factors for HH are not understood, we hypothesized that risk factors could be identified. We retrospectively enrolled children under 5 years with TBI secondary to AHT (child advocacy diagnosis) who had undergone initial and interval brain imaging. Records were interrogated for prearrival and in-hospital physiologic and radiographic findings. HH was determined by a blinded observer. Twenty-four children were enrolled and 13 developed HH. HH was not significantly associated with age, initial Glascow Coma Scale, or mortality. Pediatric Intensity Level of Therapy (PILOT) scores (p=0.01) and daily maximal intracranial pressure (ICPmax; p=0.037) were higher in HH. Hypoxia, hypotension, cardiopulmonary arrest, need for blood transfusion, and daily blood glucoses tended to be greater in HH. Whereas all children with HH had acute subdural hematoma (SBH), many children without HH also had subdural blood; the presence of skull fracture was more likely in the children who did not develop HH (p=0.04), but no other intracranial radiographic pattern of injury was associated with HH. Surgical intervention did not appear to protect against development of HH. A variety of insults associated with ischemia, including intracranial hypertension, ICP-directed therapies, hypoxia, hypotension, and cardiac arrest, occurred in the children who developed HH. Given the morbidity and mortality of this condition, larger studies to identify mechanisms leading to the development of HH and mitigating clinical approaches are warranted.
Key words: : abusive head trauma, hemispheric hypodensity, intracranial hypertension, severe traumatic brain injury, subdural hematoma
Introduction
Injuries are the leading cause of death in children 1–19 years in the United States.1 Severe traumatic brain injury (sTBI), classified as a Glasgow Coma Scale (GCS)≤8, accounts for nearly half of these deaths and constitutes the leading cause of morbidity.2,3 Abusive head trauma (AHT) is a distinct subset of TBI, with an incidence of 29.7 per 100,000 person-years during the first year of life.4 Neurologic morbidity and mortality are worse in AHT, when compared to accidental TBI,5–8 with up to one third of infants dying and 40–90% experiencing long-term neurologic disability.4,9–15 Despite the high burden of disease, our understanding of the pathophysiology of TBI after AHT remains rudimentary.
Several previous investigations have described pathological patterns of injury in the very young with AHT. Decades ago, subdural hematoma (SDH) was recognized as nearly universal in infants with sTBI, leading to myriad neurologic deficits that range from life-long disability to persistent coma and death.16–18 More recently, investigators have described the so-called big black brain, referring to hypodensity of the cerebral cortex and underlying white matter on computed tomography (CT) involving the entirety of one or both cerebral hemispheres.19–21 This radiographic entity of hemispheric hypodensity (HH) was described in association with SDH and consists of a diffusely hypodense brain, spanning from the frontal to the occipital pole, and encompasses several cerebrovascular territories. The involved parenchyma can be ipsilateral to the subdural hemorrhage or show bilateral involvement. The brain itself demonstrates rapid, progressive atrophy and eventual diffuse brain destruction can be observed at initial presentation or develop over a period of days after the initial traumatic event.22 Mortality in children with HH approaches 70%,23 and survivors are at extremely high risk for poor neurological outcome.6,11,24 Though this entity can be observed in both accidental TBI and AHT, it was initially described and more commonly appreciated after AHT.19 The pathophysiological mechanisms responsible for the development of this condition remain elusive.21
Risk factors for HH in association with SDH are unknown. This phenomenon likely occurs after a particular mechanism of TBI as a response inherent to the developing, immature brain and may result from a particular type of secondary injury.21 We attempted to identify clinical risk factors for the development of HH after SDH in the AHT population.
Methods
With institutional review board approval, we conducted a retrospective review of the medical records of children with AHT admitted to the Pediatric Intensive Care Unit (PICU) of Children's Hospital of Pittsburgh (Pittsburgh, PA) from January 2008 to May 2011. Inclusion criteria for this study were 1) age <5 years, 2) diagnosis of TBI (including mild, moderate, and severe), 3) medical diagnosis of child abuse by the Child Advocacy Center of the University of Pittsburgh, and 4) the availability of serial imaging studies during their hospital stay. Importantly, children who underwent operative procedures were included in the analysis. All children received standard care based on the judgment of the clinical team, which included trauma surgeons, pediatric intensivists, neurosurgeons, and other specialists, as needed. Intracranial pressure (ICP) monitoring was performed in children with a GCS score≤8; a standardized monitoring and treatment protocol was utilized as previously described.25 Of note, the final decision to place ICP monitors was made by the attending neurosurgical staff in charge of the child's care.
Medical records were reviewed to determine a number of preinjury, injury, and hospital-based characteristics. Chart abstractions were performed by two of the authors (K.F. and M.R.) blinded to imaging results. If records were not available to assess a particular characteristic, it was assumed to be normal (i.e., if oxygen saturations during transport were not documented, it was recorded in this study as normal saturations). In the preinjury time period, demographics (age, sex, and race) and medical complications in the prehospital setting (documented hypoxia [saturations <94% for more than one reading or evidence of cyanosis], documented hypotension [systolic blood pressure (SBP) less than 5th percentile for age or evidence of decreased perfusion], and cardiac arrest [requirement for chest compressions to maintain cardiac output]) were collected. Based on the trauma survey, postresuscitation GCS score (using the modified GCS for infants, when appropriate) and pupil examination (stratified as bilaterally reactive, unilaterally nonreactive, or bilaterally nonreactive) performed by the neurosurgical personnel were collected. Other injury characteristics collected from records included presence of abdominal and retroperitoneal injuries (liver, splenic, gut, or renal injury or retroperitoneal hematoma), pulmonary insults (pneumothorax or pulmonary contusion), and orthopedic injuries (long bone and/or rib fractures). In-hospital data were also collected, including diagnosis of pneumonia, occurrence of one or more hypoxic events (as defined as bedside SpO2<94%, multiple consecutive readings), occurrence of cardiopulmonary arrest, hypotension (defined as mean arterial pressure [MAP]<5th percentile for age), use of vasopressor therapy, administration of blood product transfusion, and highest blood glucose (mg/dL). Retinal hemorrhage was recorded as documented on a formal retinal exam performed by a pediatric ophthalmologist. These hospital-based factors were collected for the first 5 days of the patient's hospital stay.
In this series, children who underwent ICP monitoring received an external ventricular drain (EVD; Integra Neurosciences, San Diego, CA) and intraparenchymal microsensor ICP monitor (Codman). Daily maximum ICP (ICPmax) was recorded for the first 5 days of monitoring. Daily PILOT (Pediatric Intensity Level of Therapy) scores were calculated for the first 5 days in children who underwent ICP monitoring.26 Whether or not the child received decompressive surgical intervention was noted.
All children were evaluated with a cranial 5-mm axial CT scan, obtained either at an outside, referring facility or immediately after resuscitation in the trauma bay at our institution. All postadmission imaging studies, including head CT and magnetic resonance imaging (MRI), were included. Of note, all imaging studies were reviewed by a blinded observer (E.C.T.K.) and compared to radiology staff final reports.
Statistical analysis
Statistics were performed using commercially available software (SigmaPlot 11.0; Systat Software, Inc., Chicago, IL). Patients were stratified into two groups based on those who developed HH and those that did not, as determined by an author blinded to all other data regarding the patients (E.C.T.K.). Univariate analyses between groups were performed using Mann-Whitney's rank-sum and equal variance tests, as appropriate. For variables measured over time, one-way analysis of variance (ANOVA) was performed. Statistical significance was defined as p value less than 0.05, and all data are presented as mean±standard error of the mean (SEM), unless otherwise defined.
Results
A total of 24 children were admitted to the Children's Hospital of Pittsburgh PICU over the 3-year period of this study who met the inclusion criteria. Thirteen (54%) of the children were male and age at admission ranged from 1 to 40 months. Of the overall population, 13 (54%) children were ultimately diagnosed with HH upon follow-up radiographic examination and 11 (46%) were not. Most children in the overall group had SDH on initial radiographic examination (n=22; 91.7%). The ages of children with and without HH were similar (15.4±3.3 months vs. 12.1±3.0 months, respectively).
Prehospital secondary injuries were documented in a number of children from both groups. A total of 9 (69%) children with HH had documented hypoxia, whereas 3 (27%) who did not develop HH had a similar episode described within the medical records. Similarly, 3 (23%) children with HH had hypotension documented, whereas no (0%) children without HH had this reported. Systemic injuries as a result of the initial trauma were generally less common in both groups, with 2 (15%) children with HH presenting with lung injury (pneumothorax and parenchymal injury), whereas no child without HH had such injuries. Long bone and/or rib fracture were found in both groups (8 [62%] with HH and 4 [36%] children without HH). All (100%) children with HH had documentation of retinal hemorrhage (RH) on formal ophthalmological examination, whereas 4 (36%) of those without HH were found to have RH.
Upon arrival to our institution, initial GCS scores obtained from the neurosurgical physician ranged from 3 to 15 for the entire cohort. Initial GCS scores did not differ between the HH and non-HH cohorts (median [range] 6.0±1.2 [3–7] vs. 6.0±0.4 [3–15], respectively). In the group of children who subsequently developed HH, only 4 (31%) had bilateral reactive pupils, whereas 6 (55%) without HH had bilateral reactive pupils (p=not significant). In the hospital, 3 (23%) children who went on to have HH had at least a single documented hypoxic event in the PICU, whereas only 1 (9%) child in the non-HH group had such an event. Vasopressor treatment was utilized in 10 (77%) patients with HH, whereas only 4 (36%) in the non-HH cohort needed blood pressure support. Of those who developed HH, 4 (31%) were the victim of cardiopulmonary arrest, whereas 1 (9%) of the non-HH patients had cardiopulmonary arrest. Blood products were given to 12 (92%) of the children in the HH group, whereas only 8 (73%) of those who did not develop HH received a transfusion. Other in-hospital results are summarized in Table 1.
Table 1.
In-Hospital Factors in the HH and Non-HH Cohorts
| Factor | HH | Non-HH | p value |
|---|---|---|---|
| Pneumonia | 6 (46%) | 2 (18%) | 0.29 |
| Hypoxic event | 3 (23%) | 1 (9%) | 0.69 |
| Cardiopulmonary arrest | 4 (31%) | 1 (9%) | 0.40 |
| Vasopressor use | 10 (77%) | 4 (36%) | 0.10 |
| Blood product transfusion | 12 (92%) | 8 (72%) | 0.43 |
| Average highest blood glucose (mg/dL) | 329±38.5 | 288±57.8 | 0.61 |
HH, hemispheric hypotensity.
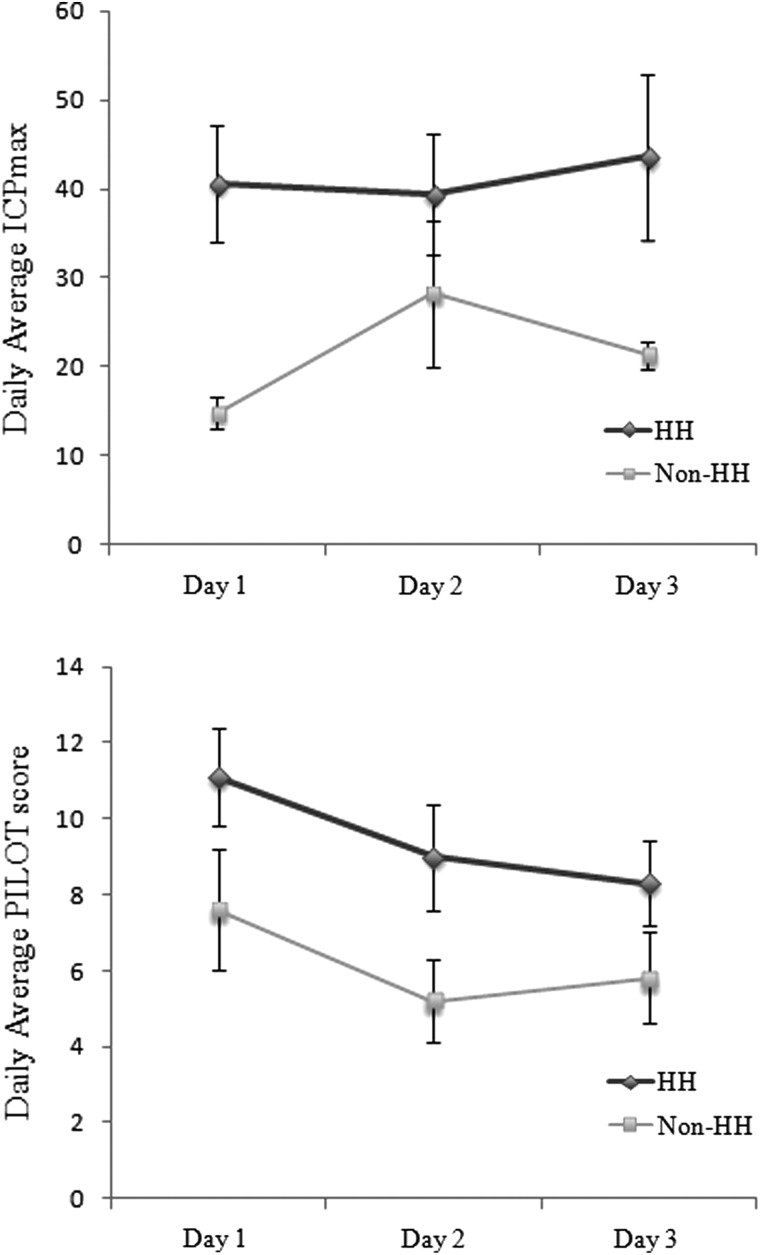
ICP monitoring was performed on 12 children in the cohort that developed HH and in 10 who did not develop HH, with the decision to place an ICP monitor made by neurosurgical staff (Table 2). Daily PILOT scores were found to be higher for the children who developed HH when compared to those who did not (p=0.01, ANOVA). Similarly, ICPmax recorded each day was greater in children who developed HH (p=0.037). Moreover, the overall average ICP on day 1 was greater in those that developed HH (40.6±6.5 mm Hg and 14.8±1.8 mm Hg; p=0.006). These results are depicted graphically in Figure 1.
Table 2.
Daily ICPmax and PILOT Scores for the HH and Non-HH Cohorts
| HH | Non-HH | p value | |
|---|---|---|---|
| ICPmax (mm Hg) | |||
| Day 1 | 40.6±6.5 | 14.8±1.8 | 0.037 |
| Day 2 | 39.4±6.8 | 28.2±8.1 | |
| Day 3 | 43.6±9.3 | 21.3±1.5 | |
| PILOT | |||
| Day 1 | 11.1±1.3 | 7.6±1.6 | 0.01 |
| Day 2 | 9.0±1.4 | 5.2±1.1 | |
| Day 3 | 8.3±1.1 | 5.8±1.2 | |
ICPmax, maximum intracranial pressure; PILOT, Pediatric Intensity Level of Change; HH, hemispheric hypotensity.
FIG. 1.
Graphic representation of average daily maximum ICP (ICPmax) for the HH and non-HH cohort on the first 3 days of admission; error bars represent standard error od the mean (SEM; top). Graphic representation of average daily PILOT scores for the HH and non-HH cohorts on the first 3 days of admission; error bars represent SEM (bottom). HH, hemispheric hypotensity; PILOT, Pediatric Intensity Level of Training.
Results of the radiographic findings in all children are summarized in Table 3. Every child with HH had SDH and most (82%) without HH also had SDH. Skull fractures were observed more often in the children who did not develop HH (3 [23%] vs. 8 [73%] for those with and without HH, respectively; p=0.04). No other radiographic criteria, including presence of epidural hematoma (EDH), traumatic subarachnoid hemorrhage (tSAH), intraparenchymal clot or contusion, cerebral edema, effacement of basal cisterns, or mid-line shift (MLS) correlated with development of HH. Representative imaging studies of 4 patients are presented in Figure 2.
Table 3.
Radiographic Findings in the HH and Non-HH Cohorts
| HH | Non-HH | p value | |
|---|---|---|---|
| SDH | 13 (100%) | 9 (82%) | 0.36 |
| >5 mm | 3 | 4 | |
| <5 mm | 10 | 5 | |
| EDH | 0 (0%) | 0 (0%) | 1.0 |
| tSAH | 2 (15%) | 3 (27%) | 0.81 |
| Intraparenchymal contusion/clot | 0 (0%) | 3 (27%) | 0.14 |
| Cerebral edema | 9 (69%) | 8 (73%) | 0.82 |
| Cisterns effaced | 7 (54%) | 7 (64%) | 0.92 |
| MLS | 5 (38%) | 4 (36%) | 0.75 |
| Skull fracture | 3 (23%) | 8 (73%) | 0.04 |
HH, hemispheric hypotensity; SDH, subdural hematoma; EDH, epidural hematoma; tSAH, traumatic subarachnoid hemorrhage; MLS, mid-line shift.
FIG. 2.
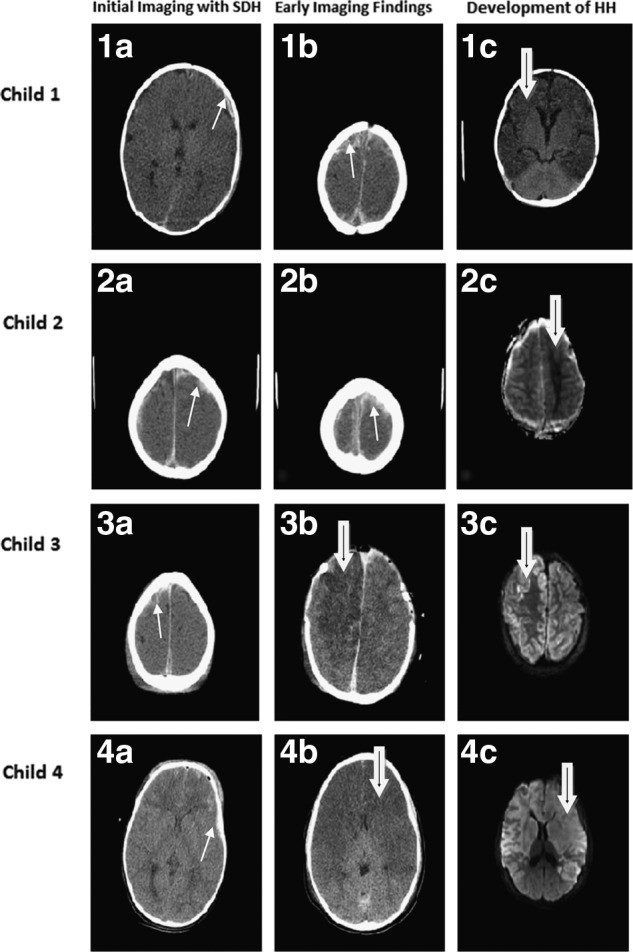
Representative cranial imaging of children at initial radiographic evaluation and after development of HH is shown. The first child suffered bilateral subdural hematomas (1a and 1b; white arrow) and showed development of bilateral HH (1c; black-in-white arrow). The second child also had bilateral injury (2a and 2b; white arrow) and showed predominately unilateral parenchymal insult, as shown on the apparent diffusion coefficient map (2c; black-in-white arrow). The third child had predominate right-sided subdural blood (3a; white arrow) and, despite emergent decompressive craniectomy, suffered HH as depicted on postoperative computed tomography and magnetic resonance imaging (diffusion-weighting imaging; DWI), as shown in 3b and 3c, respectively (black-in-white arrow). The fourth child showed bifrontal subdural blood with other bleeding patterns, particularly noted by traumatic subarachnoid hemorrhage and contusions (4a; white arrow) and rapidly developed HH (4b; black-in-white arrow), as corroborated on DWI (4c; black-in-white arrow). SDH, subdural hematoma; HH, hemispheric hypotensity.
Overall, 9 (37.5%) children underwent decompressive surgical intervention with craniotomy or craniectomy (5 and 4 children in the HH vs. non-HH cohorts, respectively). Mortality rate was 46% in children with HH, as compared to 18% in those who did not develop HH.
Discussion
HH in the pediatric population is a distinct radiographic pattern and associated with high neurologic morbidity and mortality. It is known that SDH is commonly observed in sTBI secondary to AHT,27–29 and therefore children suffering from AHT comprised a natural population for study. Herein, we found that neurologic exam and most radiographic hallmarks do not seem to predict HH. As expected, SDH was universally associated with HH, but whereas subdural blood was observed in all children with HH, many children without HH also had SDH. ICP was significantly elevated in children who developed this phenomenon. Mortality in this series approached 50% for children with HH, a rate slightly higher than other studies on sTBI secondary to AHT available in the literature.9,10,30
To date, experimental models and clinical evidence have failed to fully explain the development of HH in association with subdural blood. Animal studies designed to mimic SDH in the immature brain, such as the subdural murine model, in which blood is injected over the cerebral convexity through a burr hole31 or the cranial window infant piglet model,32 have failed to lead to HH. Moreover, studies to recapitulate the possible mechanisms of AHT using rat33 and piglet34 shaking models have not shown HH. Because of the inability to model HH, researchers are left with limited pathologic studies and isolated clinical reports to theorize on the pathophysiology of this disease.
Although clinical factors associated with HH are largely unknown, risk factors for morbidity and mortality in children with sTBI have been well established. Factors include intracranial bleeding,35–37 age,36,38,39 cerebral edema,36,40,41 low GCS score,35,40,42–45 and various physiologic disturbances.35,41,46–48 Shein and colleagues further identified risk factors associated with mortality in children with sTBI specifically secondary to AHT.15 Though the authors did not focus on children with HH, they did show that low initial GCS, RH, intraparenchymal hemorrhage, and cerebral edema independently correlated with mortality in AHT. In our small patient population, initial GCS and pupil exam in the trauma bay gave no indication as to whether or not a child had or would progress to have HH. All children in the HH cohort had documented RH; however, many children in the non-HH cohort also had RH, and this finding is not unexpected in children with AHT, irrespective of subsequent development of HH. This study reiterates the association of HH with the presence of subdural blood, but, HH does not appear to correlate with any other traumatic patterns of injury on intracranial imaging, including intraparenchymal hemorrhage or cerebral edema.
Brain oxygen (O2) supply-demand mismatch in children who manifest HH and systemic insults that impair O2 delivery to the brain may play a role in the development of HH. Neuropathologic examination of human brain tissue after AHT in infants has shown hypoxic/ischemic findings, with little or no evidence of diffuse axonal injury (DAI).49,50 Animal studies have shown decreased perfusion and increased brain metabolism in the region of SDH.51 The role of apnea and seizures, both of which stress brain metabolic needs and O2 demand, have been proposed as underlying the parenchymal changes in HH.21 In fact, apnea is known to occur in infants after a variety of insults,52 and very young children with SDH can present with apnea.53,54 Recent studies have shown that various physiologic aberrancies, including systemic hypotension and intracranial hypertension, independently correlate with adverse outcomes and death in children with sTBI.35,41,46–48 In this study, children who developed HH had increased ICPs, which were significantly higher, when compared to children who did not go on to display HH. Because this was an observational study, we could not determine whether raised ICP was a cause of HH or a manifestation of the process itself. Though failing to reach statistical significance, prearrival rates of hypoxia and systemic hypotension and in-house rates of hypoxia, pneumonia, hypotension, cardiac arrest, need for vasopressor therapy, and need for blood product transfusion were all higher in those children with HH. Taken together, these factors serve as a surrogate for diminished brain O2 supply; perhaps insults that hinder O2 delivery predispose children to development of HH.
Early surgical intervention for decompression and rapid evacuation of blood adjacent to the cerebral cortex did not seem to offer a protective effect to development of HH, as shown in this study and others.21,22,55 This finding supports the idea that inception of this phenomenon is likely early and may be a neurochemically mediated process. Cerebrospinal fluid (CSF) markers from ventriculostomy sampling in infants with TBI have shown higher levels of excitatory amino acids in inflicted infantile samples, when compared to older children with accidental mechanisms.56,57 Depletion of important CSF antioxidant reserves and evidence of free-radical–mediated lipid peroxidation have been shown in children with sTBI.58–60 In children with AHT who may suffer repeated offenses, the physiologic reserve may be even lower at the time of SDH; vulnerable, chronically insulted brain may be tipped over the edge by the presence of subdural blood.
Not all children with SDH develop HH, and therefore subdural blood alone appears insufficient to fully explain this entity. Given that some cases of HH involve only the hemisphere ipsilateral to the SDH, global physiologic aberrancy likely does not account entirely for development of big black brain or, presumably, both hemispheres would be equally affected. Of note, we observed children with unilateral SDH who developed HH either ipsilateral to the subdural blood or bilaterally, as well as children with bilateral SDH who developed either uni- or bilateral HH. No child in our small series developed exclusively contralateral HH from a unilateral SDH. Our study is vastly underpowered to analyze and compare each radiographic pattern separately. In the future, an animal model that would allow investigators to control for the pattern of SDH development and clinical studies powered sufficiently to look at each group individually would add to our understanding of the pathophysiologic origins of HH. The recently proposed “unified hypothesis” postulates that SDH in infants may occur after severe hypoxia, brain edema, and increased central venous pressures, which cause cortical veins to leak blood into the subdural compartment.61,62 Though this theory is widely debated,29,63–66 it nonetheless highlights our lack of understanding as to the causes and sequence of physiologic events leading to HH. Importantly, though this study focused on children with abuse-related mechanisms of injury, HH associated with SDH has been documented after accidental trauma.21 In children with accidental mechanisms, depletion of physiologic reserve cannot fully explain HH, given the presumed lack of chronic insult to the brain.
There are several limitations to this study. Data were collected from a small sample size at a single institution and reviewed retrospectively. Nevertheless, this manner of data collection permitted the comprehensive analysis of many preadmission and acute data points that may not be available in multi-center studies. The inclusion of only a single center necessarily eliminated any potential bias between centers and allowed standardization in obtaining the post-traumatic imaging necessary to stratify patients within the study. We were also limited to children who had the diagnosis of child abuse as established within the medical record and who were therefore subject to a mandatory imaging protocol. This may have altered the incidence of HH observed in our study and should be considered in future investigations. Although our child advocacy team performs comprehensive assessments to determine the diagnosis of abuse, it is likely that some children were not included in our analysis because this diagnosis could not be established with sufficient medical certainty. The selection of variables in our analysis may present another limitation within this study, and because of the limited sample size, controlling for such covariates was not possible. In addition, we chose to review variables that have been identified in association with sTBI and AHT in the extant literature and based on clinical experience, but other important variables were undoubtedly overlooked. We believe that our observations can form a basis for future studies of larger populations of children with AHT.
Given the retrospective nature of our data collection, we were limited by the information obtained for each patient, including the neurologic exams, adjunctive studies, and imaging modalities. The GCS accounts for only specific aspects of the neurologic exam and can have interobserver variability, which we attempted to minimize by utilizing the postresuscitation GCS in our analysis, as reported by the neurosurgical personnel (resident, fellow, or attending) on every patient. Moreover, preassessment factors, such as prearrival medications or seizures, can alter the GCS. Though a peripheral nerve stimulator (train-of-four) was used to assure pharmacologic paralysis was not altering the exam, sedatives, other medications, and the effect of seizures could have confounded the GCS score. The lack of distinction in GCS and other initial parameters at presentation among the two cohorts might be secondary to the fact that HH has not yet developed, and thus the children represent a homogenous group at this time, although the timing of HH inception remains to be discovered. Alternatively, we may have failed to identify and analyze other clinical indicators at presentation that could distinguish children with and without HH. Last, seizures (subclinical or clinical) have been hypothesized to play a critical role in the development of HH21; in our population, a very limited number of children underwent electroencephalography (EEG). Future studies and prospective analyses should include EEG analysis to assess the importance of seizure activity in the pathogenesis of HH.
There are inherent limitations to the imaging studies available for review in this analysis. Whereas our goal was to recognize clinical factors that may be associated with HH, we hypothesized, based on extrapolation, that, mechanistically, this could be related to oxygen delivery, which cannot be detected on standard CT and MRI. Importantly, noninvasive imaging modalities could help determine whether blood flow and oxygen delivery are playing a role in driving development of HH in the affected brain parenchyma, including positron emission tomography, dynamic susceptibility contrast perfusion MRI, and arterial spin labeling perfusion MRI.67–69 Though the availability and cost of such technologies may be prohibitive at some institutions, these modalities should be used in future studies for the targeted investigation of diminished regional blood flow. In our small series, there was not an association with radiographic patterns of injury on CT and MRI and the development of HH, but perhaps other types of imaging would delineate the HH and non-HH cohorts. Nonetheless, CT and MRI remain necessary in this patient population, because initial and interval radiographic studies make the diagnosis of HH possible, allow for the assessment of immediate and delayed postsurgical changes, can serve as a surrogate for the neurologic exam in sedated children (particularly those with raised ICP), and aid in providing prognosis for families.
In summary, our descriptive study demonstrates that a number of important clinical variables are associated with the development of HH, including intracranial hypertension and the intensity of ICP monitoring. In past studies of small patient populations, subdural blood was believed to be an important association, and perhaps mechanism, for the development of HH. The presence of this entity was so ubiquitous in our AHT population that it could not be linked exclusively to the development of HH. Given the morbidity and mortality associated with this process, understanding HH is essential for advancing the field of pediatric TBI. Larger studies are needed to determine factors that can be utilized for patient screening, identify mechanisms responsible for developing HH, and direct therapeutic approaches to prevent HH. Studies such as ours that attempt to understand HH more thoroughly are an important first step in this process.
Acknowledgment
This work received grant support from ADAPT (U01 NS081041; to M.J.B.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hamilton B.E., Hoyert D.L., Martin J.A., Strobino D.M., and Guyer B. (2013). Annual summary of vital statistics: 2010–2011. Pediatrics 131, 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keenan H.T., Runyan D.K., and Nocera M. (2006). Longitudinal follow-up of families and young children with traumatic brain injury. Pediatrics 117, 1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 4.Keenan H.T., Runyan D.K., Marshall S.W., Nocera M.A., Merten D.F., and Sinal S.H. (2003). A population-based study of inflicted traumatic brain injury in young children. JAMA 290, 621–626 [DOI] [PubMed] [Google Scholar]
- 5.Hymel K.P., Makoroff K.L., Laskey A.L., Conaway M.R., and Blackman J.A. (2007). Mechanisms, clinical presentations, injuries, and outcomes from inflicted versus noninflicted head trauma during infancy: results of a prospective, multicentered, comparative study. Pediatrics 119, 922–929 [DOI] [PubMed] [Google Scholar]
- 6.Ewing-Cobbs L., Kramer L., Prasad M., Canales D.N., Louis P.T., Fletcher J.M., Vollero H., Landry S.H., and Cheung K. (1998). Neuroimaging, physical, and developmental findings after inflicted and noninflicted traumatic brain injury in young children. Pediatrics 102, 300–307 [DOI] [PubMed] [Google Scholar]
- 7.Goldstein B., Kelly M.M., Bruton D., and Cox C. (1993). Inflicted versus accidental head injury in critically injured children. Crit. Care Med. 21, 1328–1332 [DOI] [PubMed] [Google Scholar]
- 8.Adamo M.A., Drazin D., Smith C., and Waldman J.B. (2009). Comparison of accidental and nonaccidental traumatic brain injuries in infants and toddlers: demographics, neurosurgical interventions, and outcomes. J. Neurosurg. Pediatr. 4, 414–419 [DOI] [PubMed] [Google Scholar]
- 9.King W.J., MacKay M., and Sirnick A. (2003). Shaken baby syndrome in Canada: clinical characteristics and outcomes of hospital cases. CMAJ 168, 155–159 [PMC free article] [PubMed] [Google Scholar]
- 10.Haviland J., and Russell R.I. (1997). Outcome after severe non-accidental head injury. Arch. Dis. Child. 77, 504–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duhaime A.C., Christian C., Moss E., and Seidl T. (1996). Long-term outcome in infants with the shaking-impact syndrome. Pediatr. Neurosurg. 24, 292–298 [DOI] [PubMed] [Google Scholar]
- 12.Duhaime A.C., Gennarelli T.A., Thibault L.E., Bruce D.A., Margulies S.S., and Wiser R. (1987). The shaken baby syndrome. A clinical, pathological, and biomechanical study. J. Neurosurg. 66, 409–415 [DOI] [PubMed] [Google Scholar]
- 13.Scavarda D., Gabaudan C., Ughetto F., Lamy F., Imada V., Lena G., and Paut O. (2010). Initial predictive factors of outcome in severe non-accidental head trauma in children. Childs Nerv. Syst. 26, 1555–1561 [DOI] [PubMed] [Google Scholar]
- 14.Barlow K.M., Thomson E., Johnson D., and Minns R.A. (2005). Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics 116, e174–e185 [DOI] [PubMed] [Google Scholar]
- 15.Shein S.L., Bell M.J., Kochanek P.M., Tyler-Kabara E.C., Wisniewski S.R., Feldman K., Makoroff K., Scribano P.V., and Berger R.P. (2012). Risk factors for mortality in children with abusive head trauma. J. Pediatr. 161, 716–722.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guthkelch A.N. (1971). Infantile subdural haematoma and its relationship to whiplash injuries. Br. Med. J. 2, 430–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caffey J. (1972). On the theory and practice of shaking infants. Its potential residual effects of permanent brain damage and mental retardation. Am. J. Dis. Child. 124, 161–169 [DOI] [PubMed] [Google Scholar]
- 18.Caffey J. (1974). The whiplash shaken infant syndrome: manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics 54, 396–403 [PubMed] [Google Scholar]
- 19.Duhaime A.C., Bilaniuk L., and Zimmerman R. (1993). The “big black brain”: radiographic changes after severe inflicted head injury in infancy. J. Neurotrauma 10, Suppl. 1, S59 [Google Scholar]
- 20.Cohen R.A., Kaufman R.A., Myers P.A., and Towbin R.B. (1986). Cranial computed tomography in the abused child with head injury. AJR Am. J. Roentgenol. 146, 97–102 [DOI] [PubMed] [Google Scholar]
- 21.Duhaime A.C., and Durham S. (2007). Traumatic brain injury in infants: the phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”). Prog. Brain Res. 161, 293–302 [DOI] [PubMed] [Google Scholar]
- 22.Dias M.S., Backstrom J., Falk M., and Li V. (1998). Serial radiography in the infant shaken impact syndrome. Pediatr. Neurosurg. 29, 77–85 [DOI] [PubMed] [Google Scholar]
- 23.Michaud L.J., Duhaime A.C., and Batshaw M.L. (1993). Traumatic brain injury in children. Pediatr. Clin. North Am. 40, 553–565 [DOI] [PubMed] [Google Scholar]
- 24.Gilles E.E., and Nelson M.D., Jr. (1998). Cerebral complications of nonaccidental head injury in childhood. Pediatr. Neurol. 19, 119–128 [DOI] [PubMed] [Google Scholar]
- 25.Shein S.L., Bell M.J., Su E., Kochanek P.M., and Exo J. (2012). Intracranial monitoring and continuous data collection. Crit. Care Med. 40, 3115–3116; author reply, 3116 [DOI] [PubMed] [Google Scholar]
- 26.Shore P.M., Hand L.L., Roy L., Trivedi P., Kochanek P.M., and Adelson P.D. (2006). Reliability and validity of the Pediatric Intensity Level of Therapy (PILOT) scale: a measure of the use of intracranial pressure-directed therapies. Crit. Care Med. 34, 1981–1987 [DOI] [PubMed] [Google Scholar]
- 27.Harding B., Risdon R.A., and Krous H.F. (2004). Shaken baby syndrome. BMJ 328, 720–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Case M.E., Graham M.A., Handy T.C., Jentzen J.M., and Monteleone J.A. (2001). Position paper on fatal abusive head injuries in infants and young children. Am. J. Forensic Med. Pathol. 22, 112–122 [DOI] [PubMed] [Google Scholar]
- 29.Matschke J., Voss J., Obi N., Gorndt J., Sperhake J.P., Puschel K., and Glatzel M. (2009). Nonaccidental head injury is the most common cause of subdural bleeding in infants <1 year of age. Pediatrics 124, 1587–1594 [DOI] [PubMed] [Google Scholar]
- 30.Jayawant S., Rawlinson A., Gibbon F., Price J., Schulte J., Sharples P., Sibert J.R., and Kemp A.M. (1998). Subdural haemorrhages in infants: population based study. BMJ 317, 1558–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller J.D., Bullock R., Graham D.I., Chen M.H., and Teasdale G.M. (1990). Ischemic brain damage in a model of acute subdural hematoma. Neurosurgery 27, 433–439 [DOI] [PubMed] [Google Scholar]
- 32.Shaver E.G., Duhaime A.C., Curtis M., Gennarelli L.M., and Barrett R. (1996). Experimental acute subdural hematoma in infant piglets. Pediatr. Neurosurg. 25, 123–129 [DOI] [PubMed] [Google Scholar]
- 33.Smith S.L., Andrus P.K., Gleason D.D., and Hall E.D. (1998). Infant rat model of the shaken baby syndrome: preliminary characterization and evidence for the role of free radicals in cortical hemorrhaging and progressive neuronal degeneration. J. Neurotrauma 15, 693–705 [DOI] [PubMed] [Google Scholar]
- 34.Raghupathi R., Mehr M.F., Helfaer M.A., and Margulies S.S. (2004). Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J. Neurotrauma 21, 307–316 [DOI] [PubMed] [Google Scholar]
- 35.Michaud L.J., Rivara F.P., Grady M.S., and Reay D.T. (1992). Predictors of survival and severity of disability after severe brain injury in children. Neurosurgery 31, 254–264 [DOI] [PubMed] [Google Scholar]
- 36.Levin H.S., Aldrich E.F., Saydjari C., Eisenberg H.M., Foulkes M.A., Bellefleur M., Luerssen T.G., Jane J.A., Marmarou A., Marshall L.F., and Young H.F. (1992). Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery 31, 435–443; discussion, 443–434 [DOI] [PubMed] [Google Scholar]
- 37.Levi L., Guilburd J.N., Linn S., and Feinsod M. (1991). The association between skull fracture, intracranial pathology and outcome in pediatric head injury. Br. J. Neurosurg. 5, 617–625 [DOI] [PubMed] [Google Scholar]
- 38.Campbell C.G., Kuehn S.M., Richards P.M., Ventureyra E., and Hutchison J.S. (2004). Medical and cognitive outcome in children with traumatic brain injury. Can. J. Neurol. Sci. 31, 213–219 [DOI] [PubMed] [Google Scholar]
- 39.Thakker J.C., Splaingard M., Zhu J., Babel K., Bresnahan J., and Havens P.L. (1997). Survival and functional outcome of children requiring endotracheal intubation during therapy for severe traumatic brain injury. Crit. Care Med. 25, 1396–1401 [DOI] [PubMed] [Google Scholar]
- 40.Feickert H.J., Drommer S., and Heyer R. (1999). Severe head injury in children: impact of risk factors on outcome. J. Trauma 47, 33–38 [DOI] [PubMed] [Google Scholar]
- 41.Ong L., Selladurai B.M., Dhillon M.K., Atan M., and Lye M.S. (1996). The prognostic value of the Glasgow Coma Scale, hypoxia and computerised tomography in outcome prediction of pediatric head injury. Pediatr. Neurosurg. 24, 285–291 [DOI] [PubMed] [Google Scholar]
- 42.Ducrocq S.C., Meyer P.G., Orliaguet G.A., Blanot S., Laurent-Vannier A., Renier D., and Carli P.A. (2006). Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr. Crit. Care Med. 7, 461–467 [DOI] [PubMed] [Google Scholar]
- 43.Hackbarth R.M., Rzeszutko K.M., Sturm G., Donders J., Kuldanek A.S., and Sanfilippo D.J. (2002). Survival and functional outcome in pediatric traumatic brain injury: a retrospective review and analysis of predictive factors. Crit. Care Med. 30, 1630–1635 [DOI] [PubMed] [Google Scholar]
- 44.White J.R., Farukhi Z., Bull C., Christensen J., Gordon T., Paidas C., and Nichols D.G. (2001). Predictors of outcome in severely head-injured children. Crit. Care Med. 29, 534–540 [DOI] [PubMed] [Google Scholar]
- 45.Lieh-Lai M.W., Theodorou A.A., Sarnaik A.P., Meert K.L., Moylan P.M., and Canady A.I. (1992). Limitations of the Glasgow Coma Scale in predicting outcome in children with traumatic brain injury. J. Pediatr. 120, 195–199 [DOI] [PubMed] [Google Scholar]
- 46.Chiaretti A., Piastra M., Pulitano S., Pietrini D., De Rosa G., Barbaro R., and Di Rocco C. (2002). Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv. Syst. 18, 129–136 [DOI] [PubMed] [Google Scholar]
- 47.Kokoska E.R., Smith G.S., Pittman T., and Weber T.R. (1998). Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. J. Pediatr. Surg. 33, 333–338 [DOI] [PubMed] [Google Scholar]
- 48.Jagannathan J., Okonkwo D.O., Yeoh H.K., Dumont A.S., Saulle D., Haizlip J., Barth J.T., Jane J.A., Sr., and Jane J.A., Jr. (2008). Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J. Neurosurg. Pediatr. 2, 240–249 [DOI] [PubMed] [Google Scholar]
- 49.Geddes J.F., Vowles G.H., Hackshaw A.K., Nickols C.D., Scott I.S., and Whitwell H.L. (2001). Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain 124, 1299–1306 [DOI] [PubMed] [Google Scholar]
- 50.Geddes J.F., Hackshaw A.K., Vowles G.H., Nickols C.D., and Whitwell H.L. (2001). Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain 124, 1290–1298 [DOI] [PubMed] [Google Scholar]
- 51.Kuroda Y., and Bullock R. (1992). Local cerebral blood flow mapping before and after removal of acute subdural hematoma in the rat. Neurosurgery 30, 687–691 [PubMed] [Google Scholar]
- 52.Johnston M.V., Trescher W.H., Ishida A., and Nakajima W. (2001). Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr. Res. 49, 735–741 [DOI] [PubMed] [Google Scholar]
- 53.Ichord R.N., Naim M., Pollock A.N., Nance M.L., Margulies S.S., and Christian C.W. (2007). Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion-weighted imaging. J. Neurotrauma 24, 106–118 [DOI] [PubMed] [Google Scholar]
- 54.Kemp A.M., Stoodley N., Cobley C., Coles L., and Kemp K.W. (2003). Apnoea and brain swelling in non-accidental head injury. Arch. Dis. Child. 88, 472–476; discussion, 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graupman P., and Winston K.R. (2006). Nonaccidental head trauma as a cause of childhood death. J. Neurosurg. 104, 245–250 [DOI] [PubMed] [Google Scholar]
- 56.Rose M.E., Huerbin M.B., Melick J., Marion D.W., Palmer A.M., Schiding J.K., Kochanek P.M., and Graham S.H. (2002). Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain Res. 943, 15–22 [DOI] [PubMed] [Google Scholar]
- 57.Han Y.Y., Carcillo J.A., Ruppel R.A., Adelson P.D., Wisniewski S.R., Bell M.J., Janesko K.L., Marion D.W., and Kochanek P.M. (2002). Cerebrospinal fluid procalcitonin and severe traumatic brain injury in children. Pediatr. Crit. Care Med. 3, 39–44 [DOI] [PubMed] [Google Scholar]
- 58.Ruppel R.A., Clark R.S., Bayir H., Satchell M.A., and Kochanek P.M. (2002). Critical mechanisms of secondary damage after inflicted head injury in infants and children. Neurosurg. Clin. N. Am. 13, 169–182, v [DOI] [PubMed] [Google Scholar]
- 59.Bayir H., Kagan V.E., Tyurina Y.Y., Tyurin V., Ruppel R.A., Adelson P.D., Graham S.H., Janesko K., Clark R.S., and Kochanek P.M. (2002). Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr. Res. 51, 571–578 [DOI] [PubMed] [Google Scholar]
- 60.Varma S., Janesko K.L., Wisniewski S.R., Bayir H., Adelson P.D., Thomas N.J., and Kochanek P.M. (2003). F2-isoprostane and neuron-specific enolase in cerebrospinal fluid after severe traumatic brain injury in infants and children. J. Neurotrauma 20, 781–786 [DOI] [PubMed] [Google Scholar]
- 61.Geddes J.F., and Plunkett J. (2004). The evidence base for shaken baby syndrome. BMJ 328, 719–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geddes J.F., Tasker R.C., Hackshaw A.K., Nickols C.D., Adams G.G., Whitwell H.L., and Scheimberg I. (2003). Dural haemorrhage in non-traumatic infant deaths: does it explain the bleeding in ‘shaken baby syndrome’? Neuropathol. Appl. Neurobiol. 29, 14–22 [DOI] [PubMed] [Google Scholar]
- 63.Punt J., Bonshek R.E., Jaspan T., McConachie N.S., Punt N., and Ratcliffe J.M. (2004). The ‘unified hypothesis’ of Geddes is not supported by the data. Pediatr. Rehabil. 7, 173–184 [DOI] [PubMed] [Google Scholar]
- 64.Miller M., Leestma J., Barnes P., Carlstrom T., Gardner H., Plunkett J., Stephenson J., Thibault K., Uscinski R., Niedermier J., and Galaznik J. (2004). A sojourn in the abyss: hypothesis, theory, and established truth in infant head injury. Pediatrics 114, 326. [DOI] [PubMed] [Google Scholar]
- 65.Punt J. (2006). Inflicted head injury in infants: issues arising from the Geddes hypothesis. Arch. Dis. Child. 91, 714–715 [PMC free article] [PubMed] [Google Scholar]
- 66.Smith C., Bell J.E., Keeling J.W., and Risden R.A. (2003). Dural haemorrhage in nontraumatic infant deaths: does it explain the bleeding in ‘shaken baby syndrome’? Geddes JE. A response. Neuropathol. Appl. Neurobiol. 29, 411–412; author reply, 412–413 [DOI] [PubMed] [Google Scholar]
- 67.Hattori N., Huang S.C., Wu H.M., Liao W., Glenn T.C., Vespa P.M., Phelps M.E., Hovda D.A., and Bergsneider M. (2004). Acute changes in regional cerebral (18)F-FDG kinetics in patients with traumatic brain injury. J. Nucl. Med. 45, 775–783 [PubMed] [Google Scholar]
- 68.Ragan D.K., McKinstry R., Benzinger T., Leonard J.R., and Pineda J.A. (2013). Alterations in cerebral oxygen metabolism after traumatic brain injury in children. J. Cereb. Blood Flow Metab. 33, 48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muir E.R., Watts L.T., Tiwari Y.V., Bresnen A., Shen Q., and Duong T.Q. (2014). Quantitative cerebral blood flow measurements using MRI. Methods Mol. Biol. 1135, 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]