Abstract
Background: Lymph node (LN) lymphatic sinuses transport lymph, cells, and antigens from the periphery through the LN. The lymphatic endothelium lining these sinuses appears to be an important contributor to the lymph node immune response. It has been challenging to obtain sufficient LN lymphatic endothelial cells for investigation of their functions, as they are minor constituents of LNs.
Methods and Results: A procedure was developed to purify lymphatic endothelial cells (LEC) from murine LNs, which yields large numbers of primary LN LEC. Two-dimensional in vitro cultures of dissociated LN stromal cells initially consist of multiple cell types, and then rapidly evolve to produce pure cultures of lymphatic endothelium within a few passages. One million LEC can be harvested after 4 weeks of culture, and much larger cell numbers can be obtained by continued culturing over long periods. The LEC cultures maintain endothelial morphology and expression of LEC markers, and preserve the same slow growth characteristics over at least 20 passages. The LEC cultures readily form tubes in Matrigel at early and at late passages, resembling those formed by LEC lines.
Conclusions: A simple and economical approach to obtain purified primary murine LN LEC was developed for in vitro studies of their function. The morphology, growth characteristics, and functional behavior of these cells in tube formation assays did not change between initial and long-term passages. Large numbers of these cells can be harvested after long-term passage, so that they can be studied in biochemical and biological assays.
Introduction
The lymphatic vasculature drains lymph, cells, and antigens from the periphery through lymph nodes (LN), to ultimately return to the blood circulation. Remodeling of LN architecture and extensive growth of lymphatic sinuses (lymphangiogenesis) is a feature of LNs activated by inflammation,1,2 as well as of tumor-reactive LNs.3,4 This LN lymphangiogenesis is thought to be important for lymph and antigen delivery to LNs,3,5 and it also can modify immune responses.6,7
The lymphatic endothelium normally is a minor component of LNs, so that it has been challenging to purify enough of these cells to investigate their function. Lymphatic endothelial cells (LEC) have been enriched from dermis8,9 by cell sorting and column purification techniques, which can be a costly approach. Cell lines derived from LN lymphatic endothelium have been obtained by SV40 transformation,10 limiting their utility for studies of normal lymphatic functions such as proliferation. However, a lymphatic endothelium cell line has successfully been cultured from normal rat lymphatic vessels,11 suggesting that transformation may not be required for primary LEC culture.
Recently a method for the isolation of murine LN stromal cells including LEC was published.12 Using this LN dissociation protocol, the cells obtained are a mixed population of all four LN stromal cell types: fibroblast reticular cells (FRC), 40%,blood endothelial cells (BEC) 30%, LEC 20%, and double negative (DN) stromal cells (10%). We cultured murine LNs to expand these stromal cells, and found that in two-dimensional culture a pure LEC population reproducibly emerges, which preserves functional characteristics of LN LECs in long-term culture.
Materials and Methods
Cell isolation and culture conditions
LN stromal cell populations were obtained by digesting pooled brachial, axillary, inguinal, and popliteal LNs from one wild-type C57BL/6J mouse in 1.5 mL RPMI with 0.2 mg/mL Collagenase P (Roche), and 0.8 mg/ml Dispase I (Worthington Biochemical) for 20 min, as described by Fletcher et al.12 The digest buffer was pipetted off with any free cells. Another 1.5 mL buffer with enzyme was added, and the clumps are digested further for 20 min. The cells from each digest step were then pooled, yielding all four LN stromal cell types.12 The cells isolated from the LNs of one mouse were plated in one well of a 48-well tissue culture treated plate in DMEM containing 10% FBS, 10 mM HEPES, 1% PenStrep, and 1/1000 β-mercaptoethanol. After 24 h, nonadherent cells were removed and the plate washed three times with 1X PBS, leaving behind the adherent LN stromal cells. Cells were passaged at a 1:5 dilution every 7–10 days, when they reached 80% confluency.
Immunofluorescent staining
Cells were harvested via trypsinization and plated at 40,000 cells/well in 12-well plates containing coverglass. The cells were allowed to recover from trypsin exposure for 1 day. The coverglass was washed 3 times with 1X PBS to remove media, and then cells were fixed with 4% paraformaldehyde. Primary antibodies: rat anti-mouse LYVE-1 (ALY7, eBiosciences), rat anti-mouse CD31 (MEC13.3, BD Biosciences), hamster anti-mouse podoplanin (8.1.1, Dr. A. Farr, University of Washington, Departments of Biological Structure and Immunology), rat anti-mouse ER-TR7 (Dr. A Farr), rat anti-mouse MECA-32 (ATCC), hamster anti-mouse 10.1.1 (Dr. A. Farr), rat anti-mouse smooth muscle α-actin Cy3 (1A4, Sigma), and rabbit anti-mouse Prox1 (Millipore). Secondary antibodies from Invitrogen: goat anti-hamster AlexaFluor 568, goat anti-hamster AlexaFluor 488, goat anti-rat AlexaFluor 568, and goat anti-rabbit AlexaFluor 488. Slides were mounted in Prolong Gold (Invitrogen) for imaging on a NikonE microscope using Nikon NIS Elements software.
Flow cytometry
Cells were harvested via trypsinization and stained with PE-Cy7 anti-mouse CD45 (30-F11, eBiosciences), PE anti-mouse CD31 (390, eBiosciences), and APC anti-mouse podoplanin (8.1.1, eBiosciences). Wild-type mouse LN was digested as described above as a positive control for the presence of the four LN stromal cell types. Cells were analyzed on a BD FACS-Canto II, and data processed using FlowJo software.13
Tube formation assay
Growth factor-reduced Matrigel (BD Biosciences) was allowed to solidify in a 96-well plate (50 μL/well) for 30 min at 37°C. SV-LEC14 or pure LEC P4 or P22 cultures were harvested via trypsinization and plated into each well at 30,000 cells/well (SV-LEC), and at 60,000 cells/well (P4, P8, or P22 LN LEC). Tubes were allowed to form for 4 h, and stained with Calcein AM viability dye (eBiosciences) in HBSS for 30 min to visualize tubes.
Results
Protease dissociation of LN stroma yields a mixture of LEC, BEC, FRC, and DN stromal cells, as detected by flow cytometry analysis of podoplanin and CD31 expression.12,15 The initial stromal cell yield per mouse is difficult to estimate, as most of the cells isolated are nonadherent lymphocytes that are removed after 24 h in culture. However, adherent stromal cells could be expanded in vitro from the initial well of a 48-well plate obtained from one mouse so that at passage 4 (P4) the cells could be plated into a T-25 flask, and by P5 approximately 106 cells were obtained. Six independent cell isolates were generated from six wild-type C57BL/6J mice with similar growth characteristics and morphology observed between the cultures. This finding demonstrates that primary LN stromal cells can expand for long periods in culture.
The cellular composition of the LN stromal cultures was examined by plating low passage number cells (P4) on coverglass and immunostaining them to examine expression of LEC, BEC, and FRC markers, for comparison with the cell types identified immediately after isolation of LN stroma.12 The LEC markers used were 10.1.1,3,13 Prox1,16 LYVE-1,17 podoplanin (8.1.1),18 and CD31.13,19 Smooth muscle α-actin (SMA1) and ER-TR7 were used to identify FRCs,20 while CD31 and MECA-32 distinguish BEC.21 Markers were chosen based on expression patterns defined by in situ immunostaining as summarized in Table 1. The cultured LN cells all expressed the LEC markers 10.1.1, Prox1, CD31, and podoplanin, suggesting that they are all LECs (Fig. 1 A-C, E). This marker expression pattern is similar to what has been published for the SV40 transformed LEC line SV-LEC.13,22,23 The LEC marker LYVE-1 was not expressed (Fig. 1G), likely due to downregulation of this lymphatic endothelial surface marker in culture.17 The BEC marker MECA-32 was not detected (Fig. 1D), perhaps because MECA-32 expression is rapidly lost in cultured cells.12,24 Cells expressing the FRC markers ER-TR7 and SMA1 were also not detected (Fig. 1F, H), even though FRC are the most abundant (70% of all cells) at the first passaging of the cells.12 This 10.1.1+ Prox1+ podoplanin+ CD31+ and MECA32- ER- TR7- SMA1- immunostaining pattern identifies an LEC phenotype16,18,19 for the cultured LN cells. These findings indicate that by P4, LECs proliferate and overgrow the LN culture, while other stromal cell types (FRC, DN, BEC) are lost. Similar data were obtained upon examination of higher passage (P5 to P8) cells (n=3 independent cell isolates for each P4, P5, and P8).
Table 1.
Cultured LN Stroma Exclusively Express LEC Markers
| P4 and P8 cultures | SV-LEC | LN LEC | LN FRC | LN BEC | |
|---|---|---|---|---|---|
| 10.1.1 | + | + | + | − | − |
| Prox1 | + | + | + | − | − |
| LYVE-1 | − | + | + | − | − |
| Podoplanin | + | + | + | + | − |
| CD31 | + | ND | + | − | + |
| SMA | − | ND | − | + | − |
| ER-TR7 | − | ND | − | + | − |
| MECA-32 | − | ND | − | − | + |
FIG. 1.
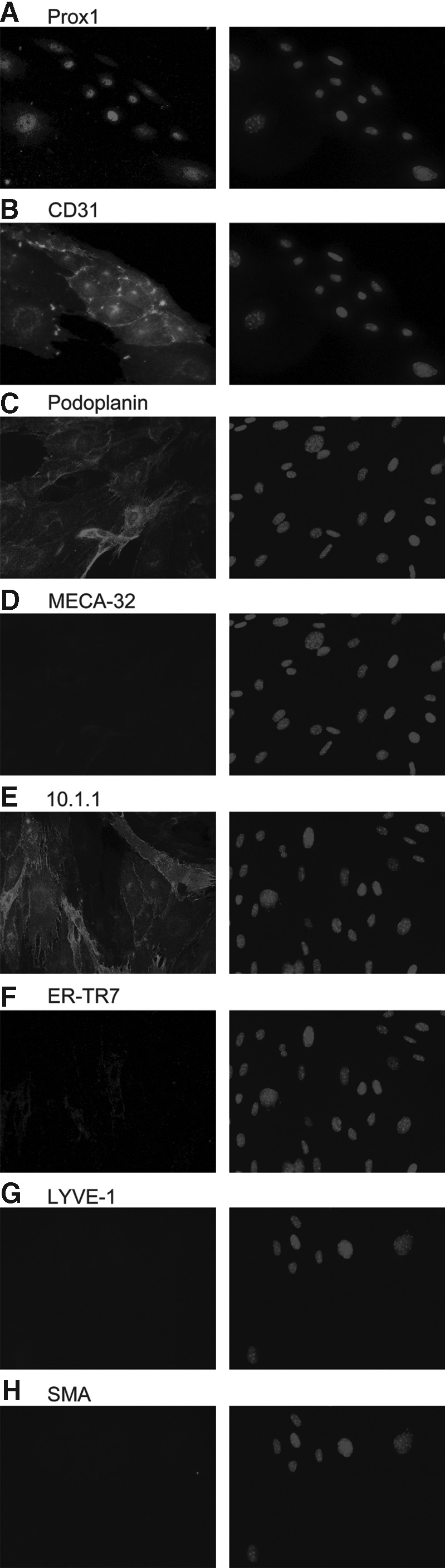
LN stromal cultures exclusively express LEC markers by the fourth passage. Immunofluorescent staining of cultured primary mouse LN cells at P4. Cells were plated in 12-well plates (40,000 cells/well), and immunostained for Prox1 (A), 10.1.1 (E), podoplanin (C), and CD31 (B), but not LYVE-1 (G), MECA-32 (D), ER-TR7 (F), or SMA (H), shown in the left panels. Nuclear DAPI staining is shown in the right panels. Adjacent images (A and B; C and D; E and F; and G and H) are of co-stains of the same field of cells. Staining was repeated on three independent cell isolates; representative images are shown.
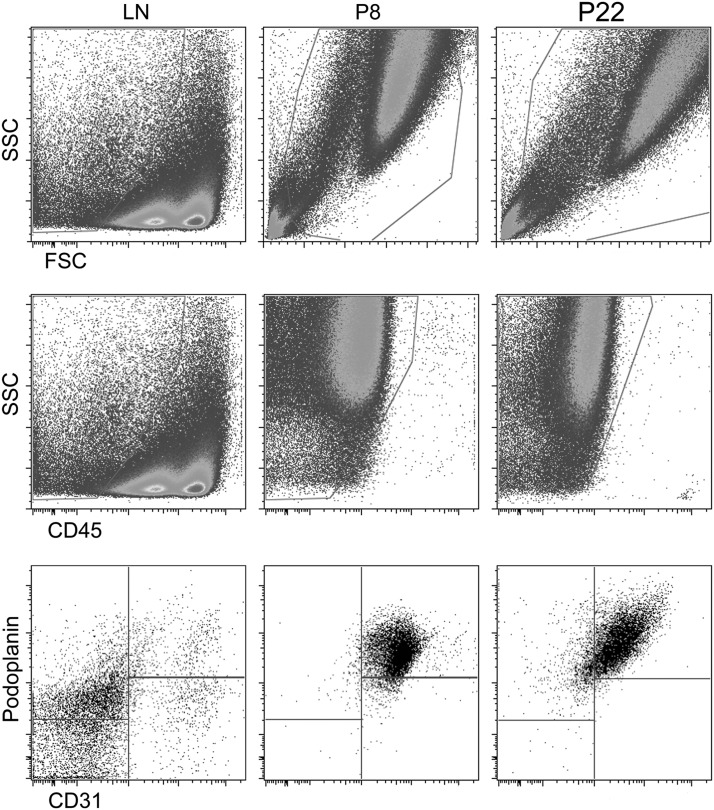
Flow cytometry using CD31 and podoplanin immunostaining can be used to distinguish LEC, BEC, FRC, and DN cells.12,15 To quantify the purity of the P8 population, cells were harvested and analyzed by flow cytometry. Freshly isolated mouse LN was used as a control to identify all four stromal cell populations. Forward scatter (FSC) and side scatter (SSC) demonstrate large cells in the culture (Fig. 2), as expected for endothelium. Staining with CD45 antibody confirms that the in vitro cultures are CD45-ve, so that the culture contains no leukocytes. CD31 and podoplanin immunostaining demonstrates that the cultures are CD31+ podoplanin+ LEC. Similar data were obtained upon analysis of later passages (P22, n=2 independent cell isolates). These findings confirm that simply passaging LN stromal isolates rapidly yields a pure population of LEC.
FIG. 2.
In vitro culture of LN stroma produces a pure LN LEC population. Flow cytometry analysis of freshly dissociated mouse LN stroma and of P8 and P22 cultures, stained with PE-Cy7-anti-CD45, APC-anti-podoplanin, and PE-anti-CD31 antibodies. Forward scatter (FSC) and side scatter (SSC) demonstrate large size of cells in P8 and P22 cultures. All P8 and P22 cells gated on this large population are CD45-ve CD31+ podoplanin+, relative to mixed composition of freshly dissociated LN stroma stained for podoplanin and CD31, which contain LEC, BEC, FRC, and DN stromal cells. Two independent cell isolates were analyzed.
The LN cultures can be continuously grown for more than 22 passages to generate much larger cell yields. At all stages of culture, the cells maintained monolayer (contact inhibited) growth and characteristic adherent endothelial morphology and extensive cytoplasm (Fig. 1), suggesting that the cells are not transformed.14,25 No alterations in morphology were observed, and the cells retained slow growth and long generation time with 7–10 days required for passaging through at least P22 (data not shown). This finding was obtained with six independent isolates for early passages, and two isolates were cultured up to P22, demonstrating that the cells consistently retain primary endothelial characteristics.
Endothelial cell function is often modeled in vitro using a tube formation assay, to measure the migration and adhesion of endothelial cells in Matrigel to form vessel-like tubes.26,27 The cultured LN LECs readily formed tube structures at early P4 (n=6 independent cell isolates) as well as late P22 passages (n=2 independent cell isolates), with examples shown in Figure 3. Early and late LEC cultures therefore both maintain the same ability to migrate and form vessel-like structures. The tubes formed resembled those formed by the SV-LEC cell line (Fig. 3), an SV40-transformed LEC line obtained from mesenteric adventitia.14 The primary LEC cultured from LNs thus show long-term ability to form tubes, suggesting that they will be useful to study various aspects of lymphatic endothelial biology.
FIG. 3.
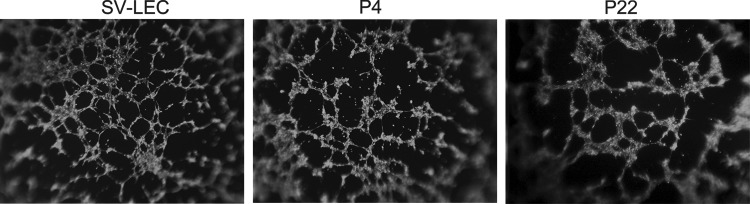
Cultured LN LECs retain tube formation potential. Calcein-stained early P4 and late P22 pure LN LEC cultures both readily form tubes in Matrigel. The morphology of these tubes is similar to that produced by SV-LEC cells. Tube formation assays were performed on six independent cell isolates at P4 and two independent isolates at P22.
Conclusions
A simple and economical method was developed which allows harvesting of large numbers of essentially pure murine LN LEC. These primary cells continuously exhibit the same endothelial morphology, slow cell division rate, and expression of lymphatic endothelial markers 10.1.1, Prox1, podoplanin, and CD31 for long periods in culture. These cells may be more widely useful for biological assays than LEC cell lines obtained by SV-40 transformation, which show altered cellular functions including increased proliferation. Normal primary LN LEC can be isolated by fluorescence activated cell sorting12,15 and/or by antibody affinity chromatography columns.8,9 However, these purification strategies are expensive and do not yield large numbers of cells per mouse. Our finding that primary LEC isolated by long-term culture retain tube formation activity suggests that they should be useful for investigations of LEC functions in vitro. For example, we have found that these cultured LEC proliferate in response to stimulation (data not shown). The ability to purify large numbers of nontransformed LN LEC should facilitate biological or biochemical assays or screening experiments requiring larger numbers of cells. Additionally, large numbers of LEC can be derived from individual mice, to facilitate genetic studies.
The LEC of mixed LN stromal cultures rapidly become the dominant cell type in these cultures in a reproducible manner, although the mechanism involved remains to be determined. The initial cellular composition of the LN stroma is approximately 30%–40% FRC, with 15%–25% LEC.12,15 At the first passage of the cells, after 5 days of culture, FRC dominate the culture comprising 70% of the cells, with LECs as a minor component.12 FRC can produce VEGF-A to support LEC growth,28 which could favor preferential establishment of the VEGFR2-expressing LEC in vitro.3 It remains to be determined why the FRC and DN stroma do not persist, or whether they are required for the initial establishment of LEC cultures. It is also possible that LEC are the only stromal cell types able to survive ex vivo, facilitating their purification by culturing.
Much of the characterization of primary LEC thus far has used dermal skin to isolate LEC by antibody affinity chromatography.8,9 Our LN LEC culture and purification strategy could potentially be useful as an economical approach to purify LEC from other lymphatic vessel sources such as the dermis. It should also now be possible to compare LN versus lymphatic vessel LEC types in vitro, to identify any organ-specific specialization of their functions.
Acknowledgments
The authors thank Andrew Farr and Sheila Ganti for their advice.
Author Disclosure Statement
No competing financial interests exist.
This research was supported by NIH NCI RO1 CA68328 (AR) and NIH Interdisciplinary Training Grant NIH T32 CA080416 (KJW).
References
- 1.Angeli V, Ginhoux F, Llodra J, et al. 2006. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 2006;24:203–215 [DOI] [PubMed] [Google Scholar]
- 2.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood 2007;110:3158–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruddell A, Mezquita P, Brandvold KA, Farr A, Iritani BM. B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis. Am J Pathol 2003;163:2233–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 22005;201:1089–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol 2007;170:774–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund AW, Swartz MA. Role of lymphatic vessels in tumor immunity: Passive conduits or active participants? J Mammary Gland Biol Neoplasia 2010;15:341–352 [DOI] [PubMed] [Google Scholar]
- 7.Tewalt EF, Cohen JN, Rouhani SJ, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 2012;120:4772–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clasper S, Royston D, Baban D, Cao Y, Ewers S, Butz S, Vestweber D, Jackson DG. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res 2008;68:7293–7303 [DOI] [PubMed] [Google Scholar]
- 9.Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci USA 2002;99:16069–16074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connell KA, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol 1990;144:521–525 [PubMed] [Google Scholar]
- 11.Hayes H, Kossmann E, Wilson E, Meininger C, Zawieja D. Development and characterization of endothelial cells from rat microlymphatics. Lymphat Res Biol 2003;1:101–119 [DOI] [PubMed] [Google Scholar]
- 12.Fletcher AL, Malhotra D, Acton SE, et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol 2011;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuya M, Kirschbaum SB, Paulovich A, et al. Lymphatic endothelial murine chloride channel calcium-activated 1 is a ligand for leukocyte LFA-1 and Mac-1. J Immunol 2010;185:5769–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ando T, Jordan P, Joh T, Wang Y, Jennings MH, Houghton J, Alexander JS. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat Res Biol 2005;3:105–115 [DOI] [PubMed] [Google Scholar]
- 15.Cohen JN, Guidi CJ, Tewalt EF, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med 2010;207:681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 2008;22:3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson LA, Prevo R, Clasper S, Jackson DG. Inflammation-induced uptake and degradation of the lymphatic endothelial hyaluronan receptor LYVE-1. J Biol Chem 2007;282:33671–33680 [DOI] [PubMed] [Google Scholar]
- 18.Farr AG, Berry ML, Kim A, Nelson AJ, Welch MP, Aruffo A. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J Exp Med 1992;176:1477–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruddell A, Mezquita P, Brandvold KA, Farr A, Iritani BM. B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis. Am J Pathol 2003;163:2233–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sixt M, Kanazawa N, Selg M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 2005;22:19–29 [DOI] [PubMed] [Google Scholar]
- 21.Leppink DM, Bishopp DK, Sedmak DD, et al. Inducible expression of an endothelial cell antigen on murine myocardial vasculature in association with interstitial cellular infiltration. Transplantation 1989;48:874–877 [DOI] [PubMed] [Google Scholar]
- 22.Ando T, Jordan P, Wang Y, Jennings MH, Houghton J, Alexander JS. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat Res Biol 2005;3:105–115 [DOI] [PubMed] [Google Scholar]
- 23.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Rüegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One 2009;4:e7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooley LS, Handsley MM, Zhou Z, et al. Reversible transdifferentiation of blood vascular endothelial cells to a lymphatic-like phenotype in vitro. J Cell Sci 2010;123:3808–3816 [DOI] [PubMed] [Google Scholar]
- 25.Arbiser JL, Moses MA, Fernandez CA, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA 1997;94:861–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnaoutova I, Kleinman HK. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 2010;5:628–635 [DOI] [PubMed] [Google Scholar]
- 27.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 1988;107:1589–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chyou S, Ekland EH, Carpenter AC, et al. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol 2008;181:3887–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]