Abstract
Infection by human immunodeficiency virus type 1 (HIV-1) causes acquired immunodeficiency syndrome (AIDS) after a long clinical latency. This disease is associated with a spectrum of cancers. Here we report that wild-type p53 is a potent suppressor of Tat, a major transactivator of HIV-1. Reciprocally, Tat inhibits the transcription of p53. Downregulation of p53 by upregulated tat may be important for the establishment of productive viral infection in a cell and also may be involved in the development of AIDS-related malignancies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Corallini A., Altavilla G., Pozzi L., Bignozzi F., Negrini M., Rimessi P., Gualandi F., Barbanti-Brodano G. Systemic expression of HIV-1 tat gene in transgenic mice induces endothelial proliferation and tumors of different histotypes. Cancer Res. 1993 Nov 15;53(22):5569–5575. [PubMed] [Google Scholar]
- Cullen B. R. Does HIV-1 Tat induce a change in viral initiation rights? Cell. 1993 May 7;73(3):417–420. doi: 10.1016/0092-8674(93)90126-b. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Gendelman R., Markham P., Fiorelli V., Colombini S., Raffeld M., Cafaro A., Chang H. K., Brady J. N., Gallo R. C. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature. 1994 Oct 20;371(6499):674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Gauss G. H., Lieber M. R. DEAE-dextran enhances electroporation of mammalian cells. Nucleic Acids Res. 1992 Dec 25;20(24):6739–6740. doi: 10.1093/nar/20.24.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P., Bos E., Braithwaite A. W. Wild-type mouse p53 down-regulates transcription from different virus enhancer/promoters. Oncogene. 1993 Mar;8(3):589–597. [PubMed] [Google Scholar]
- Kashanchi F., Piras G., Radonovich M. F., Duvall J. F., Fattaey A., Chiang C. M., Roeder R. G., Brady J. N. Direct interaction of human TFIID with the HIV-1 transactivator tat. Nature. 1994 Jan 20;367(6460):295–299. doi: 10.1038/367295a0. [DOI] [PubMed] [Google Scholar]
- Kastan M. B. P53: a determinant of the cell cycle response to DNA damage. Adv Exp Med Biol. 1993;339:291–296. doi: 10.1007/978-1-4615-2488-5_28. [DOI] [PubMed] [Google Scholar]
- Li C. J., Wang C., Pardee A. B. Camptothecin inhibits Tat-mediated transactivation of type 1 human immunodeficiency virus. J Biol Chem. 1994 Mar 11;269(10):7051–7054. [PubMed] [Google Scholar]
- Li C. J., Zhang L. J., Dezube B. J., Crumpacker C. S., Pardee A. B. Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1839–1842. doi: 10.1073/pnas.90.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Mack D. H., Vartikar J., Pipas J. M., Laimins L. A. Specific repression of TATA-mediated but not initiator-mediated transcription by wild-type p53. Nature. 1993 May 20;363(6426):281–283. doi: 10.1038/363281a0. [DOI] [PubMed] [Google Scholar]
- Nabel G. J., Rice S. A., Knipe D. M., Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988 Mar 11;239(4845):1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Campbell K., Haseltine W. A. Construction of recombinant murine retroviruses that express the human T-cell leukemia virus type II and human T-cell lymphotropic virus type III trans activator genes. J Virol. 1986 Jan;57(1):379–384. doi: 10.1128/jvi.57.1.379-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Huibregtse J. M., Vierstra R. D., Howley P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993 Nov 5;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Seto E., Usheva A., Zambetti G. P., Momand J., Horikoshi N., Weinmann R., Levine A. J., Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Subler M. A., Martin D. W., Deb S. Activation of the human immunodeficiency virus type 1 long terminal repeat by transforming mutants of human p53. J Virol. 1994 Jan;68(1):103–110. doi: 10.1128/jvi.68.1.103-110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck S. P., Crawford L. Characterization of the human p53 gene promoter. Mol Cell Biol. 1989 May;9(5):2163–2172. doi: 10.1128/mcb.9.5.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellutini C., Philippon V., Gambarelli D., Horschowski N., Nave K. A., Navarro J. M., Auphan M., Courcoul M. A., Filippi P. The maedi-visna virus Tat protein induces multiorgan lymphoid hyperplasia in transgenic mice. J Virol. 1994 Aug;68(8):4955–4962. doi: 10.1128/jvi.68.8.4955-4962.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wang X. W., Forrester K., Yeh H., Feitelson M. A., Gu J. R., Harris C. C. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
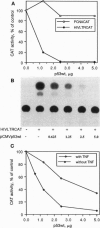
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]