Abstract
Purpose
We aimed to determine whether embryo culture induces markers of cellular senescence and whether these effects were dependent on culture conditions.
Methods
Murine blastocysts were derived in vitro and in vivo and assessed for 2 primary markers of senescence: senescence-associated β-galactosidase (SA-β-gal) and phosphorylated H2A.X (γ-H2A.X), the latter being a mark of DNA oxidative damage. Expression of senescence-associated genes p21, p16, and interleukin 6 (IL6) were also assessed.
Results
Compared with in vivo–derived blastocysts, in vitro embryos had high levels of SA-β-gal, nuclear γ-H2A.X, and p21 mRNA expression, indicating that a senescence-like phenotype is induced by in vitro culture. To determine the role of culture conditions, we studied the effect of oxygen (5 % vs 20 %) and protein supplementation on senescence markers. Blastocysts in reduced oxygen (5 %) had low levels of both SA-β-gal and γ-H2A.X compared with blastocysts cultured in ambient oxygen. Senescence markers also were reduced in the presence of protein, suggesting that antioxidant properties of protein reduce oxidative DNA damage in vitro.
Conclusion
Elevated SA-β-gal, γ-H2A.X, and p21 suggest that in vitro stress can induce a senescence-like phenotype. Reduced oxygen during embryo culture minimizes these effects, providing further evidence for potential adverse effects of culturing embryos at ambient oxygen concentrations.
Keywords: Cellular senescence, Embryo culture, In vitro stress, Oxidative stress
Introduction
Assisted reproduction via in vitro fertilization (IVF) inherently requires culturing human cells in a foreign environment at a critical stage of development. The preimplantation period begins with the oocyte and zygote, which have minimal homeostatic regulatory mechanisms, and ends with the blastocyst. This 4- to 5-day window of development encompasses a period of critical epigenetic reprogramming and can therefore represent a time when suboptimal culture conditions may have latent effects.
Embryos in culture are destined for 1 of 3 fates: growth arrest, cell death, or development into a blastocyst. The majority of human embryos arrest or degenerate, with typically 30 to 40 % developing to the blastocyst stage [1]. In addition, in vitro–derived human blastocysts typically implant at a frequency of 50 %, indicating that not all blastocysts are developmentally competent. Suboptimal in vitro culture conditions alter gene expression of preimplantation embryos [2, 3], with effects that may be responsible for embryonic arrest; however, the effect of in vitro stress on the fate of individual cells in the blastocyst is not clearly understood. Although programmed cell death (apoptosis) of cells exposed to stress in vitro has been studied in embryos [4, 5], less is known about other cell fates.
Replicative cellular senescence is a term used to describe cells that are unable to replicate yet remain viable and metabolically active [6]. Senescence can be induced prematurely by various stressors such as oncogenes, irradiation, or oxidation [7]. This stress-induced premature senescence is a phenomenon of both in vitro cell culture and the in vivo setting. Markers to detect cellular senescence include senescence-associated β-galactosidase (SA-β-galactosidase) [8], proteins associated with DNA damage repair such as phosphorylated histone H2A.X (γ-H2A.X) [9], and increased expression of genes involved in the stress response. Cells in senescence differ from apoptotic cells because senescent cells remain viable and metabolically active.
In somatic cells, reactive oxygen species (ROS) are implicated in the induction of cellular senescence [10] and in premature senescence with elevated oxidative stress during in vitro culture [11, 12]. These observations may apply to preimplantation embryos as well. Bovine embryos cultured with 20 % oxygen have significantly elevated levels of intracellular ROS and higher frequencies of permanent embryo arrest at the 2- to 4-cell stage compared with embryos cultured with 5 % oxygen [13]. Embryos that arrested at the 2- to 4-cell stage had γ-H2A.X foci, suggesting that the senescence pathway may be active in early embryos [14, 15]. Because culture to the blastocyst stage in ambient oxygen is a common practice in clinical IVF laboratories, stress-induced premature senescence may occur in human blastocysts, with downstream effects on development.
The objective of the current study was to determine whether markers of premature senescence appeared in mouse blastocysts in response to in vitro culture conditions. Our first aim was to compare the senescence markers SA-β-galactosidase, γ-H2A.X, and expression of p21, p16, and interleukin 6 (IL6) for blastocysts derived in vivo and in vitro. We then determined the impact of oxygen concentration, with or without protein supplementation, on SA-β-galactosidase and γ-H2A.X for blastocysts cultured in vitro.
Materials and methods
The manuscript does not contain clinical studies or patient data. All procedures involving animals were performed under an active Institutional Animal Care and Use Committee protocol.
Embryo collection and culture
Friend Virus B (FVB) mice (4–7 weeks old) were superovulated with intraperitoneal injections of 5 IU pregnant mare’s serum (National Hormone and Peptide Program) followed 48 h later with 5 IU of human chorionic gonadotropin (APP Pharmaceuticals). Female mice were caged individually with male CF-1 mice for breeding overnight. Female mice were checked for copulation by observing a vaginal plug of semen the next morning.
In vitro group
Pregnant mice with vaginal plugs were euthanized at 18 to 20 h after human chorionic gonadotropin administration. Oviducts were excised and flushed with human tubal fluid medium containing N-2-hydroxyethylpiperazine-N’-2-ethane-sulfonate (HEPES) and 5 mg/mL human serum albumin (Cooper Surgical). One-cell embryos were cultured in Global medium (IVFonline) under mineral oil (Fisher Scientific) with ambient air with 7.0 % CO2 at 37 °C. Embryos were cultured for 96 h after embryo collection and development was assessed every 24 h. Embryos at the expanded or hatching stage at 96 h were included.
In vivo group
Pregnant mice were euthanized 3.5 days after coitum to obtain in vivo control blastocysts, which provides embryos at a similar developmental stage to in vitro blastocysts at 96 h of culture [16]. Oviducts were excised and flushed with human tubal fluid medium to obtain blastocysts.
Experiment one
In vitro and in vivo blastocysts were obtained from 3 replicates with 30 blastocysts for each marker studied. Markers included SA-β-galactosidase, γ-H2A.X, and expression of p21, p16, and IL6.
Experiment two
Effects of oxygen and protein were studied using a 2 × 2 factorial design, with 2 levels of oxygen (5 % vs 20 %), with or without 10 % v/v protein (serum substitute supplement (SSS), Irvine Scientific). After 96 h of culture, blastocysts were obtained from a minimum of 3 replicates, with at least 30 blastocysts obtained for both SA-β-galactosidase and γ-H2A.X.
Blastocyst preparation
Blastocysts from each experimental group were washed 3 times in phosphate-buffered saline (PBS; pH 7.4; Invitrogen) containing 0.1 % polyvinyl alcohol (PVA; Sigma Aldrich) and were either immediately snap-frozen in liquid nitrogen for RNA isolation or processed for SA-β-galactosidase or γ-H2A.X and 4’,6-diamidino-2-phenylindole (DAPI) analysis.
Senescence-associated β-galactosidase assay
Blastocysts from each experimental group were fixed in 2 % paraformaldehyde (ElectronMicroscopy Sciences) and 0.2 % glutaraldehyde (Sigma Aldrich) in PBS-PVA under mineral oil for 10 min at room temperature. Blastocysts were washed 3 times in PBS-PVA and incubated for 16 h in a CO2-free incubator at 37 °C in SA-β-galactosidase assay solution. The SA-β-galactosidase assay solution consisted of 50 μL X-gal (20 mg/mL; American Bioanalytical), 200 μL 0.2 M citric acid (pH 6.0; Sigma Aldrich), 50 μL 100 mM potassium ferrocyanide (Sigma Aldrich), 50 μL 100 mM potassium ferricyanide (MP Biomedicals, Solon, OH), 30 μL 5 M sodium chloride (Boston BioProducts), 2 μL 1 M magnesium chloride (Boston BioProducts), and 620 μL molecular biology–grade water (Cellgro; Mediatech, Inc). A positive control for β-galactosidase activity was performed at pH 4.0. Assay solution was prepared fresh for each replicate.
Following incubation, blastocysts were washed extensively with PBS-PVA and assessed using a light microscope at 400× magnification (Nikon). Embryos were considered positive when blue staining was evident.
γ-H2A.X immunofluorescence staining
Each experimental group of embryos was fixed in 4 % paraformaldehyde in PBS under mineral oil for 1 h at 4 °C. Embryos were washed 3 times in PBS with 0.1 % Tween 20 (Bio-Rad) and permeabilized in PBS with 1.0 % Triton X-100 (Sigma Aldrich) for 1 h at 4 °C. Embryos were washed 3 times in PBS with 0.1 % Tween 20 and blocked in PBS containing 8 % bovine serum albumin (BSA) overnight at 4 °C. Embryos were stained with primary anti–γ-H2A.X antibody (rabbit anti-mouse monoclonal immunoglobulin [Ig] G; Life Technologies) overnight at 4 °C using a dilution of 1:300 in 1 % BSA/PBS. After this, embryos were washed 3 times in PBS with 0.1 % Tween 20 and incubated with a secondary fluorescein isothiocyanate–labeled antibody (anti-rabbit IgG; Life Technologies), diluted 1:500 in 1 % BSA/PBS, for 2 h at room temperature. The immunostained embryos were washed 3 times in PBS with 0.1 % Tween 20 and counterstained with 3 % DAPI (Abbott Molecular) in Vectashield mounting medium (Vector Laboratories). Confocal images were obtained with a LSM510 or LSM780 microscope (Zeiss). Blastocysts were scanned in 3 dimensions using the Z-stack function, with Z-stack images projected as overlays. Each nucleus of a blastomere with more than 5 foci of γ-H2A.X was considered positive [17]. Furthermore, we determined the percentage of nuclei within a blastocyst that was γ-H2A.X positive.
RNA extraction and amplification
For every biological replicate, total RNA was extracted from a pool of 10 blastocysts from each experimental group using the Prelude Direct Lysis Module (NuGen Technologies, Inc). The Ovation Pico WTA System (NuGen Technologies) was used to amplify the isolated total RNA (5 μL) according to the manufacturer’s instructions. After amplification, samples were purified using DNA Clean & Concentrator (Zymo Research Corp). Concentrations of cDNA samples were confirmed with NanoDrop 1000 spectrophotometer analysis (Thermo Fisher Scientific, Inc).
Relative real-time polymerase chain reaction
Gene expression was determined with real-time polymerase chain reaction (PCR) using the TaqMan Gene Expression Assay in a 7500 Fast Real-Time PCR System (Applied Biosystems). In brief, 10 μL of TaqMan Fast Advanced Master Mix were mixed with 5 μL of amplified cDNA from each biological replicate, 1 μL of the appropriate TaqMan primer (to detect p21, p16, or IL6), and 4 μL of water. A housekeeping gene encoding the TATA-box binding protein (TBP) was used as an internal standard [18]. The in vivo group was used as the reference group. Expression levels for each mRNA transcript were determined relative to the reference group and housekeeping gene. We used the comparative threshold cycle method [19] for calculation. Five biological replicates, each with 3 technical replicates, were performed for the in vitro and in vivo groups.
Statistical analysis
Results of the experiments were analyzed by 1-way analysis of variance (ANOVA) and significance was determined by the Student t test for the in vivo vs in vitro experiment. Results of experiment 2 were analyzed by ANOVA and means were compared with Tukey’s test. P values < .05 were considered statistically significant. Analysis was performed using JMP software (SAS Institute, Inc) and Prism (GraphPad Software, Inc).
Results
Experiment one: in vivo vs in vitro blastocysts
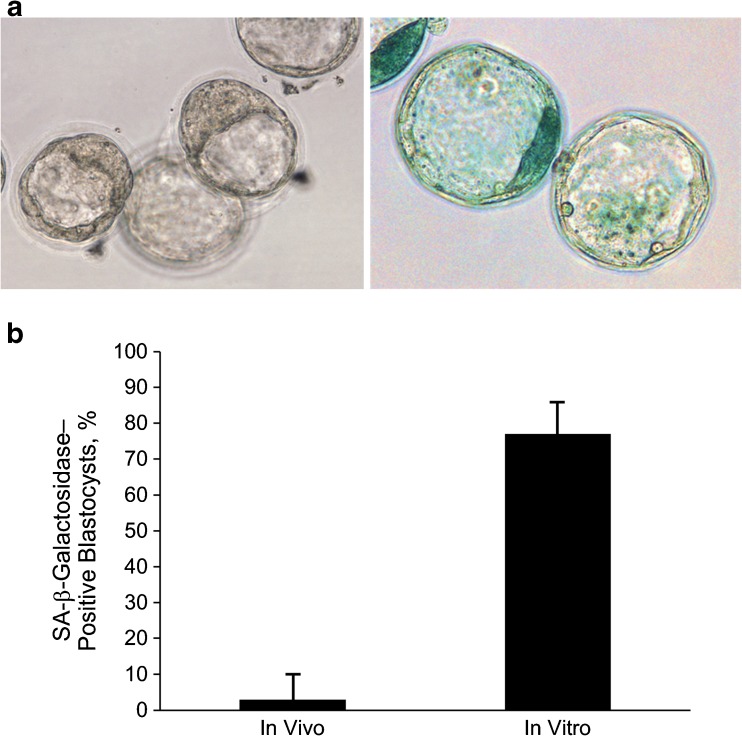
The classical senescence marker SA-β-galactosidase was readily apparent in in vitro–derived blastocysts (Fig. 1a); 76.7 % of blastocysts were positive for SA-β-galactosidase compared with 3.3 % of the in vivo group (P < .001; Fig. 1b). As an internal positive control, we performed the β-galactosidase assay at pH 4.0; under this condition, all in vivo and in vitro blastocysts were positive for β-galactosidase (data not shown).
Fig. 1.
A, Representative images of blastocysts (left: in vivo; right: in vitro) after staining for senescence-associated (SA-) β-galactosidase (blue color; original magnification, ×400). B, Percentage of blastocysts positive for SA-β-galactosidase activity (data shown are mean ± standard error; P < .001)
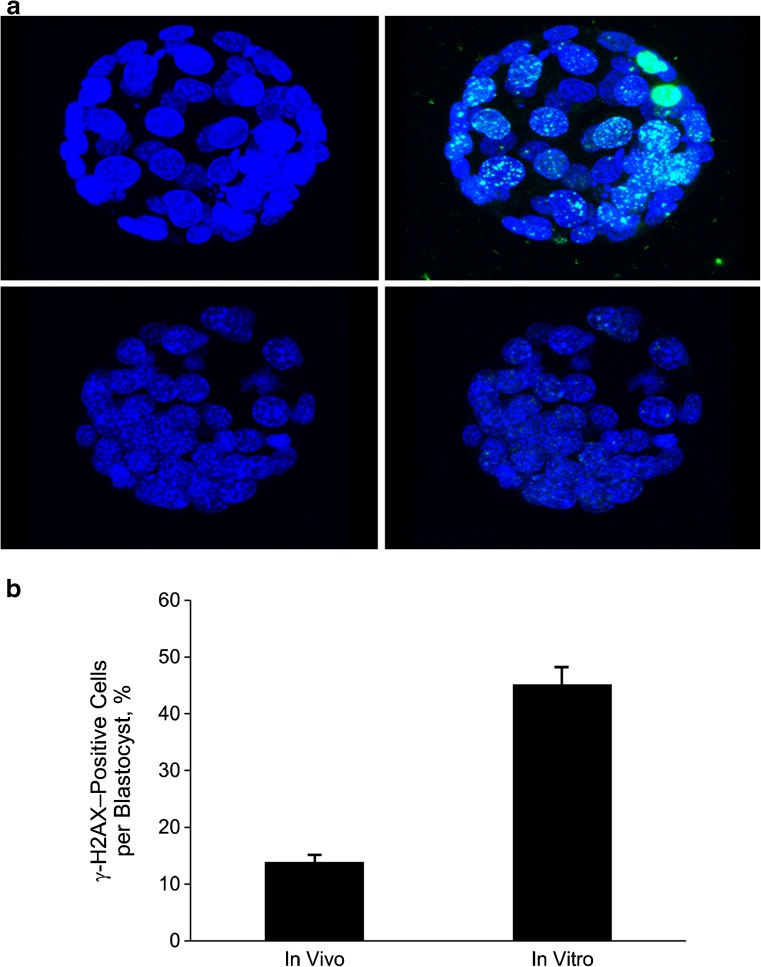
DNA damage response, as measured with phosphorylation of the histone variant H2A.X (γ-H2A.X), was increased in blastocysts from the in vitro group (Fig. 2a). In total, 45.2 % of nuclei from in vitro blastocysts had more than 5 γ-H2A.X foci, whereas only 14.2 % of the nuclei of in vivo blastocysts were γ-H2A.X positive (P < .001; Fig. 2b).
Fig. 2.
A, Upper left: In vitro blastocyst stained with DAPI (blue). Upper right: In vitro blastocyst stained with DAPI and overlaid with γ-H2A.X (green). Lower left; In vivo blastocyst stained with DAPI. Lower right: In vivo blastocyst stained with DAPI and overlaid with γ-H2A.X. B, Percentage of cells in each blastocyst positive for γ-H2A.X (>5 γ-H2A.X foci/nucleus) in the in vivo and in vitro groups (data shown are mean ± standard error; P < .001). DAPI denotes 4’, 6-diamidino-2-phenylindole; γ-H2A.X, phosphorylated histone H2A.X
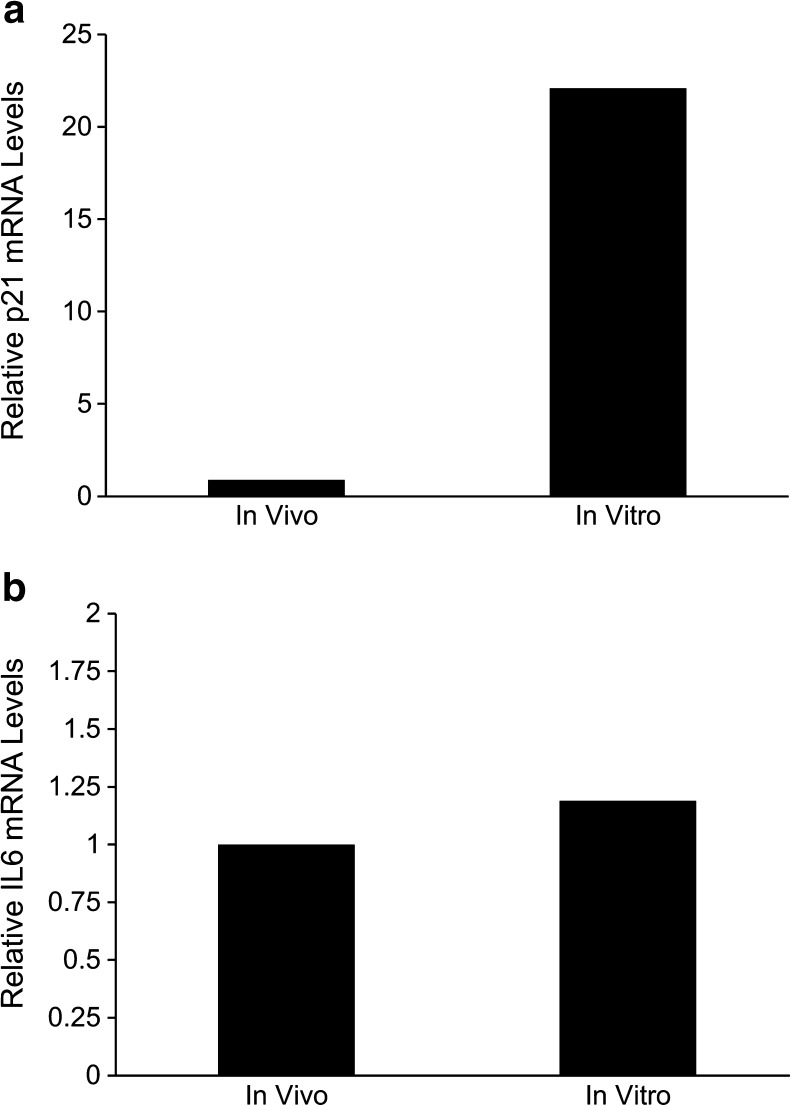
Another marker of cellular senescence is increased expression of specific cell cycle and inflammation genes. Expression of p21 was 22.1-fold higher in in vitro blastocysts compared with the in vivo group (P = .046; Fig. 3a). Expression of IL6 was similar between the 2 groups (P = .98; Fig. 3b). Expression of p16 was not detected in either group.
Fig. 3.
Relative expression of markers of senescence in blastocysts derived in vivo and in vitro. Data shown are mean ± standard error. A, Expression of p21 (P < .05). B, Expression of interleukin 6 (IL6) (P = .98)
Experiment two: effect of oxygen and protein on markers of cellular senescence
As described above, markers of senescence were present in blastocysts derived from in vitro culture without protein in 20 % oxygen. To determine whether these markers were dependent on culture conditions, we designed a 2 × 2 factorial study to compare the effects of 2 factors associated with oxidative stress, oxygen concentration and protein supplementation.
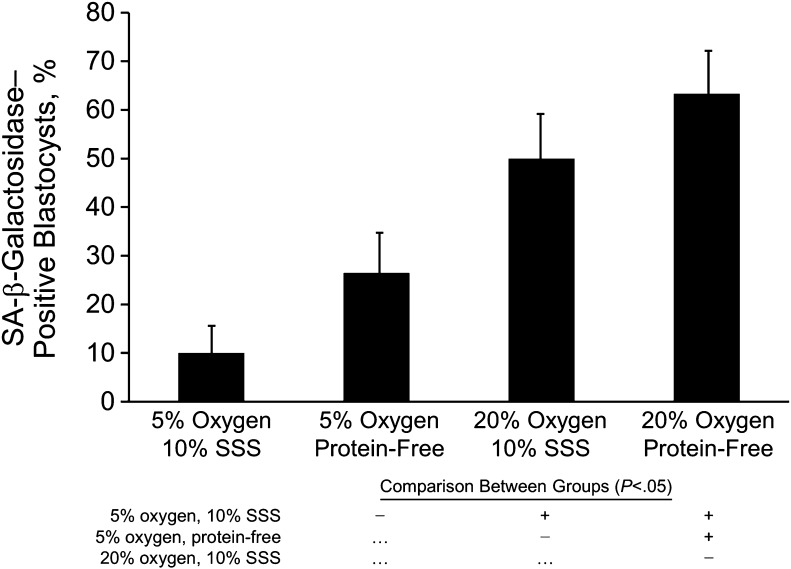
Blastocysts cultured in 20 % oxygen were positive for SA-β-galactosidase (Fig. 4); the proportion of positive blastocysts was similar for embryos cultured with or without protein supplementation (50.0 % vs 63.3 %, respectively). In contrast, for blastocysts cultured in 5 % oxygen, only 10.0 % stained positive for SA-β-galactosidase when protein was present but 26.7 % were positive when protein was absent (P < .05).
Fig. 4.
Percentage of blastocysts positive for senescence-associated (SA-) β-galactosidase activity, stratified by oxygen concentration and protein supplementation. Data shown are mean ± standard error. All culture conditions were compared individually in all possible combinations (each row shows comparison with the condition indicated for the histogram bar above); those with significant differences (P < .05) are indicated (+). SSS denotes serum substitute supplement
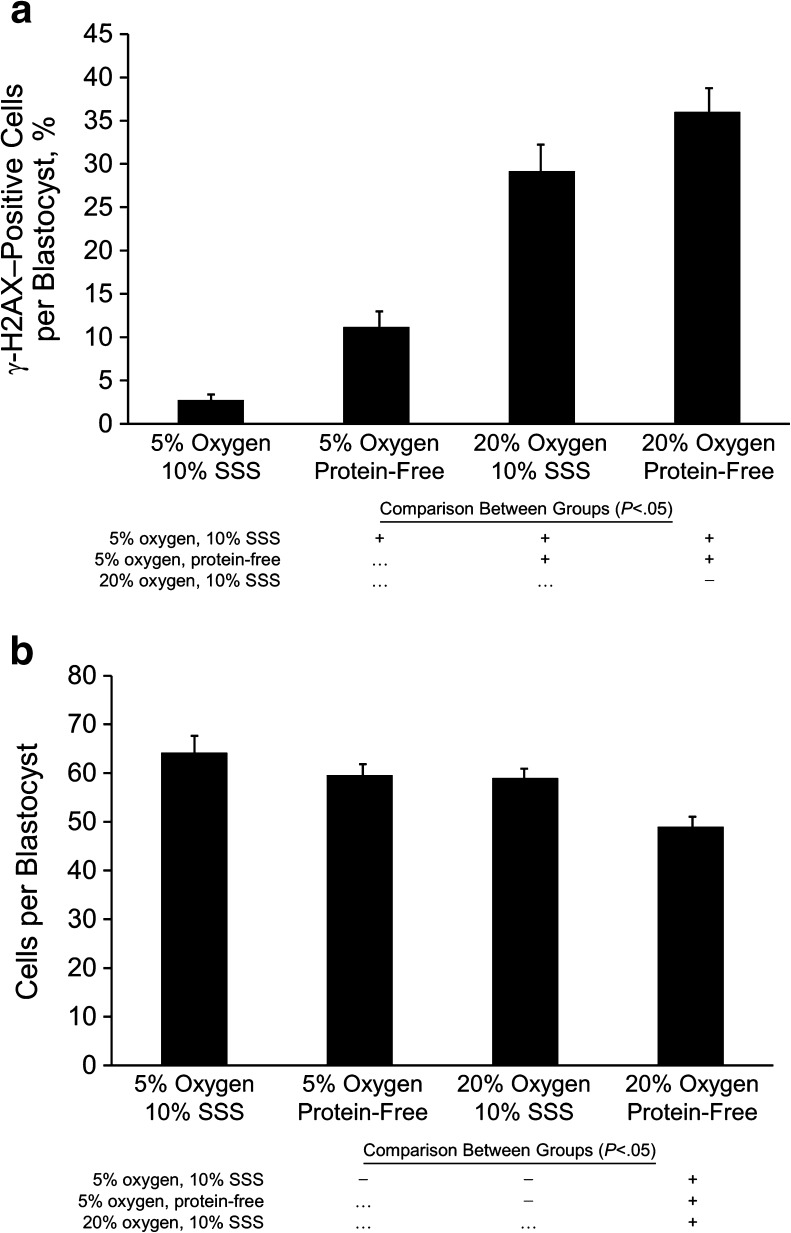
The DNA damage repair response marker, γ-H2A.X, was similarly affected by oxygen and by protein in the reduced oxygen group (Fig. 5a). Fewer blastomeres per blastocyst in the reduced-oxygen group, with or without protein supplementation, had at least 5 foci in the nucleus (2.8 vs 11.2 %, respectively) compared with blastocysts in the 20 % oxygen group (29.3 vs 36.0 %, respectively; P < .05). Although blastocyst development for all treatment groups exceeded 75 % (data not shown), total cell numbers were reduced in the group cultured in 20 % oxygen without protein supplementation (P < .01; Fig. 5b).
Fig. 5.
A, Percentage of cells in each blastocyst positive for γ-H2A.X (>5 γ-H2A.X foci/nucleus), stratified by oxygen concentration and protein supplementation. B, Number of cells per blastocyst for each oxygen and protein supplementation group. Data shown are mean ± standard error. All culture conditions were compared individually in all possible combinations (each row shows comparison with the condition indicated for the histogram bar above); those with significant differences (P < .05) are indicated (+). SSS denotes serum substitute supplement
Discussion
Most mammalian embryos undergo permanent growth arrest during the first 5 days of development in vitro or develop to the blastocyst stage but then fail to implant [1]. The reasons for arrest and the mechanisms that regulate this process are poorly understood. In our study of murine blastocysts, we investigated the presence and extent of markers of the senescence response in blastocysts developed in vitro and observed significantly higher expression of markers such as SA-β-galactosidase, γ-H2A.X, and p21 compared with in vivo–derived blastocysts. These findings indicate that components of the senescence response can be induced in embryos by stressors associated with in vitro culture. These energy-dependent cell responses to stress, particularly to DNA damage, may lead to cell cycle arrest and ultimately may affect viability or long-term development. Of potential importance for clinical assisted reproduction laboratories, we found that this senescence-like phenotype largely is due to culture in atmospheric oxygen, with 6- to 7-fold more γ-H2A.X–positive cells for blastocysts cultured in 20 % oxygen and similar levels of γ-H2A.X–positive cells when comparing blastocysts cultured in 5 % oxygen vs blastocysts cultured in vivo.
When actively growing cells accumulate a critical level of cellular damage, proliferation ceases and cells undergo programmed death (apoptosis or autophagy) or permanent cell cycle arrest (senescence). Although apoptosis has been reported in embryos [4, 5], it typically occurs in less than 10 % of blastomeres in blastocysts. Apoptosis represents an end point of cell fate: the culmination of cellular damage and the signature of a cell that is unable to overcome intrinsic defects or extrinsic insults. Cellular senescence is another programmed cell response to in vitro stress that, similar to apoptosis, is the culmination of damage and corresponding cellular responses. However, senescent cells, unlike apoptotic cells, remain viable and metabolically active, even though they do not divide [7]. Classical cellular senescence is linked to tumor suppression and aging in vivo [6] and is characterized by expression of cell cycle suppressors p16 and p21, expression of SA-β-galactosidase, persistent nuclear foci of γ-H2A.X, and dysfunctional telomeres [20].
Significant evidence indicates that most of the elements necessary to trigger a senescence phenotype exist during in vitro culture of embryos at ambient oxygen concentrations. High oxygen induces oxidative stress and ROS in embryos [13, 21–23] that lead to increased apoptosis [24, 25] and DNA damage relative to in vivo embryos [26] and embryos cultured in 5 % oxygen [21, 23]. Betts and King [14] proposed that permanent arrest of bovine embryos at the 2- to 4- cell stage is due to oxidative stress, which suggests that blastomeres of the preimplantation embryo are capable of responding with a senescence-like stress-signaling pathway. They demonstrated that arrested 2- to 4-cell bovine embryos have elevated levels of intracellular ROS [13], are still metabolically active, have high levels of γ-H2A.X foci [15], and have increased levels of the stress-sensing protein p66Shc [13]. Although embryos that arrest at the cleavage stage appear to have a senescent phenotype, senescence as a cell fate of blastomeres in embryos at later stages of development has not been reported.
The tumor suppressor p53 does not appear to have a role in early embryo senescence [13], but the p53 pathway is activated in blastocysts in response to genotoxic stress or in vitro culture [27–31]. DNA damage thus activates the DNA damage response, including the p53 pathway, which can lead to apoptosis or senescence. The role of apoptosis in blastocysts is not clear, however. Using a bovine model, Leidenfrost and colleagues [32] observed permanent cell cycle arrest and nonapoptotic cell death in blastocysts, the latter occurring mostly in the inner cell mass. Our findings suggest that although DNA damage due to ambient oxygen may not reach the threshold necessary to induce cell death, it nonetheless results in significant molecular responses that at a minimum require resources to repair; this response may ultimately lead to cell cycle arrest.
An early marker of cellular senescence is SA-β-galactosidase [8], an enzyme present in most senescent cells and induced by prolonged culture of somatic cells. Our study demonstrates for the first time that SA-β-galactosidase is present in in vitro–derived murine blastocysts. Classic acid β-galactosidase can be found in all eukaryotic cells in the lysosome and is distinguished from SA-β-galactosidase by its pKa, with the former active at a pH of 6.0. These enzymes are important for processing cellular waste components (eg, gangliosides, glycosaminoglycans, glycoproteins) by hydrolyzing β-linked terminal galactosyl residues from cell-derived (as well as artificial) substrates [33].
The importance of SA-β-galactosidase detection in embryos remains to be determined, but it could suggest an increase in lysosomal biogenesis, consistent with oxidative stress. Although SA-β-galactosidase is the first marker used to establish a senescent phenotype, the biological significance of this assay remains in question. SA-β-galactosidase activity at pH 6 increases in some nonsenescent cells [34] and may simply reflect an increase in lysosomal biogenesis [35]. Oxidative stress from mitochondrial ROS production during aging [10, 35] or exogenous ROS can lead to accumulation of oxidation products such as lipofuscin [36]; if lysosomal mass increases in an attempt to compensate for the high amount of oxidation products, then higher SA-β-galactosidase activity results [37].
γ-H2A.X is a highly sensitive marker of DNA damage [38] and is necessary for classification of the senescent phenotype [39]. γ-H2A.X forms foci at the site of double-strand breaks, causing recruitment of several DNA repair and cell cycle checkpoint proteins. Our results illustrate that embryos cultured in vitro at 20 % oxygen have a considerable number of cells with extensive DNA repair activity, likely due to oxidative stress. We used the threshold of at least 5 γ-H2A.X foci per nucleus [17] to classify cells as senescent. However, because the DNA damage response must be persistent to induce senescence [40], it is possible that these are transient foci that are repairable and, once restored, allow cells to continue proliferation. Although ours is the first report of γ-H2A.X in blastocysts and the fate of affected blastomeres is unknown, a prior report has shown that bovine embryos that arrest at the 2-cell stage are positive for γ-H2A.X and proliferating 2-cell embryos do not show expression of γ-H2A.X; taken together, γ-H2A.X may be a marker of senescence in preimplantation embryos [14, 15]. Similarly, genotoxic stress induces γ-H2A.X in 2-cell mouse embryos and results in cell cycle arrest [41].
The cellular response to DNA damage results in senescence via the p53-p21 or p16-pRB cell checkpoint pathways [7]. We demonstrated that the potent cell cycle inhibitor p21, but not p16, is upregulated during in vitro culture. p21 is a cyclin-dependent kinase inhibitor that is upregulated in dormant mouse blastocysts during delayed implantation [42] and causes cell cycle arrest in irradiated embryos [43]. DNA damage, specifically that associated with γ-H2A.X, initiates the p53-p21 signaling pathway at the onset of cellular senescence, whereas unphosphorylated H2A.X leads to degradation of p21 and cell death [7, 9]. The role of p16 (another cyclin-dependent kinase inhibitor) in the induction of senescence is less clear. Some studies support p16-driven senescence [44, 45], whereas others could not find any expression of p16 [46, 47]. We did not detect any p16 RNA, consistent with prior work suggesting that the pRB pathway does not develop until after implantation [48]. In contrast, p21 expression was higher in the in vitro group than the in vivo group, providing additional evidence that the senescence response may be active in blastocysts because p21 is a key target of p53-dependent senescence [49]. Another feature of senescent cells is a proinflammatory phenotype, mostly seen in chronic inflammation and commonly assessed by IL6 expression levels [20]. Because we did not observe a difference in IL6 gene expression between in vitro and in vivo embryos, the response to oxidative stress in in vitro blastocysts may be a premature, stress-induced, senescence-like phenotype, with the primary effect of upregulating p21.
The effect of in vitro culture on embryo health remains an important area of investigation because questions about potential adverse effects of culture continue to be raised [50, 51]. In the present study, embryos were cultured in 20 % oxygen, which likely contributed to the extent of oxidative stress because atmospheric oxygen levels adversely affect outcomes in most species studied [52, 53] and alter genetic imprinting [3]. Although reduced-oxygen culture is universally adopted in bovine embryo production and results in improved live birth rates for human IVF [54, 55], many clinical IVF laboratories continue to use 20 % oxygen [56]. Assessment of apoptosis in embryos shows greater apoptosis in in vitro–derived bovine embryos compared with in vivo embryos [25], suggesting the potentially deleterious effects of a suboptimal culture environment [57]. The detection of both apoptotic and arrested cells in bovine blastocysts [32] indicates coexistence of these 2 cell fates in a single blastocyst, resulting in fewer total cells. Cell stress might therefore lead to embryo lethality because of the loss of a critical number of cells.
In summary, markers of cellular senescence, including SA-β-galactosidase, γ-H2A.X, and p21, are expressed in murine blastocysts cultured in vitro and indicate that a senescence-like state may be induced through oxidative stress and suboptimal culture conditions. Culture in 5 % oxygen reduced markers to levels similar to that seen with in vivo embryos, illustrating another mechanism of damage induced by culture at ambient oxygen. The fate of these DNA-damaged cells in blastocysts is unknown, but the possibilities include repair with transient cell cycle arrest, senescence with complete cell cycle arrest, or apoptotic cell death. Further studies of DNA damage, the cellular senescence pathway in embryos, and the role of oxidative stress and other embryo culture conditions are warranted.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- ANOVA
Analysis of variance
- BSA
Bovine serum albumin
- DAPI
4’,6-diamidino-2-phenylindole
- FVB
Friend Virus B
- γ-H2A.X
phosphorylated histone H2A.X
- HEPES
N-2-hydroxyethylpiperazine-N’-2-ethane-sulfonate
- Ig
Immunoglobulin
- IL6
Interleukin 6
- IVF
In vitro fertilization
- PBS
Phosphate-buffered saline
- PCR
Polymerase chain reaction
- PVA
Polyvinyl alcohol
- ROS
Reactive oxygen species
- SA-β-galactosidase
Senescence-associated β-galactosidase
Footnotes
Capsule In vitro culture of murine embryos induces cellular and molecular changes that are similar to changes observed in cellular senescence.
Alexandra Meuter and Lisa-Marlen Rogmann contributed equally to the manuscript.
References
- 1.Thomas MR, Sparks AE, Ryan GL, Van Voorhis BJ. Clinical predictors of human blastocyst formation and pregnancy after extended embryo culture and transfer. Fertil Steril. 2010;94(2):543–8. doi: 10.1016/j.fertnstert.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 2.Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62(6):1526–35. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 3.Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction. 2004;128(3):301–11. doi: 10.1530/rep.1.00297. [DOI] [PubMed] [Google Scholar]
- 4.Hardy K. Cell death in the mammalian blastocyst. Mol Hum Reprod. 1997;3(10):919–25. doi: 10.1093/molehr/3.10.919. [DOI] [PubMed] [Google Scholar]
- 5.Jurisicova A, Acton BM. Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction. 2004;128(3):281–91. doi: 10.1530/rep.1.00241. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40. [DOI] [PubMed]
- 8.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fragkos M, Jurvansuu J, Beard P. H2AX is required for cell cycle arrest via the p53/p21 pathway. Mol Cell Biol. 2009;29(10):2828–40. doi: 10.1128/MCB.01830-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passos JF, von Zglinicki T, Kirkwood TB. Mitochondria and ageing: winning and losing in the numbers game. Bioessays. 2007;29(9):908–17. doi: 10.1002/bies.20634. [DOI] [PubMed] [Google Scholar]
- 11.Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276(4):2531–7. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- 12.Passos JF, Saretzki G, von Zglinicki T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection. Nucleic Acids Res. 2007;35(22):7505–13. doi: 10.1093/nar/gkm893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favetta LA, St John EJ, King WA, Betts DH. High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radic Biol Med. 2007;42(8):1201–10. doi: 10.1016/j.freeradbiomed.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Betts DH, King WA. Genetic regulation of embryo death and senescence. Theriogenology. 2001;55(1):171–91. doi: 10.1016/S0093-691X(00)00453-2. [DOI] [PubMed] [Google Scholar]
- 15.Betts DH, Madan P. Permanent embryo arrest: molecular and cellular concepts. Mol Hum Reprod. 2008;14(8):445–53. doi: 10.1093/molehr/gan035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaren A, Bowman P. Genetic effects on the timing of early development in the mouse. J Embryol Exp Morphol. 1973;30(2):491–8. [PubMed] [Google Scholar]
- 17.Lawless C, Wang C, Jurk D, Merz A, Zglinicki TV, Passos JF. Quantitative assessment of markers for cell senescence. Exp Gerontol. 2010;45(10):772–8. doi: 10.1016/j.exger.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Willems E, Mateizel I, Kemp C, Cauffman G, Sermon K, Leyns L. Selection of reference genes in mouse embryos and in differentiating human and mouse ES cells. Int J Dev Biol. 2006;50(7):627–35. doi: 10.1387/ijdb.052130ew. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–56. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi M, Keicho K, Takahashi H, Ogawa H, Schultz RM, Okano A. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Theriogenology. 2000;54(1):137–45. doi: 10.1016/S0093-691X(00)00332-0. [DOI] [PubMed] [Google Scholar]
- 22.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7(2):175–89. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62(7):1186–97. doi: 10.1016/j.theriogenology.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Van Soom A, Yuan YQ, Peelman LJ, de Matos DG, Dewulf J, Laevens H, et al. Prevalence of apoptosis and inner cell allocation in bovine embryos cultured under different oxygen tensions with or without cysteine addition. Theriogenology. 2002;57(5):1453–65. doi: 10.1016/S0093-691X(01)00726-9. [DOI] [PubMed] [Google Scholar]
- 25.Pomar FJ, Teerds KJ, Kidson A, Colenbrander B, Tharasanit T, Aguilar B, et al. Differences in the incidence of apoptosis between in vivo and in vitro produced blastocysts of farm animal species: a comparative study. Theriogenology. 2005;63(8):2254–68. doi: 10.1016/j.theriogenology.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Saka N, Takahashi H, Kanai Y, Schultz RM, Okano A. Assessment of DNA damage in individual hamster embryos by comet assay. Mol Reprod Dev. 1999;54(1):1–7. doi: 10.1002/(SICI)1098-2795(199909)54:1<1::AID-MRD1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Jurisicova A, Latham KE, Casper RF, Casper RF, Varmuza SL. Expression and regulation of genes associated with cell death during murine preimplantation embryo development. Mol Reprod Dev. 1998;51(3):243–53. doi: 10.1002/(SICI)1098-2795(199811)51:3<243::AID-MRD3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Lichnovsky V, Kolar Z, Murray P, Hlobilkova A, Cernochova D, Pospisilova E, et al. Differences in p53 and Bcl-2 expression in relation to cell proliferation during the development of human embryos. Mol Pathol. 1998;51(3):131–7. doi: 10.1136/mp.51.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frenkel J, Sherman D, Fein A, Schwartz D, Almog N, Kapon A, et al. Accentuated apoptosis in normally developing p53 knockout mouse embryos following genotoxic stress. Oncogene. 1999;18(18):2901–7. doi: 10.1038/sj.onc.1202518. [DOI] [PubMed] [Google Scholar]
- 30.Chandrakanthan V, Chami O, Stojanov T, O’Neill C. Variable expressivity of the tumour suppressor protein TRP53 in cryopreserved human blastocysts. Reprod Biol Endocrinol. 2007;5:39. doi: 10.1186/1477-7827-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganeshan L, Li A, O’Neill C. Transformation-related protein 53 expression in the early mouse embryo compromises preimplantation embryonic development by preventing the formation of a proliferating inner cell mass. Biol Reprod. 2010;83(6):958–64. doi: 10.1095/biolreprod.109.083162. [DOI] [PubMed] [Google Scholar]
- 32.Leidenfrost S, Boelhauve M, Reichenbach M, Gungor T, Reichenbach HD, Sinowatz F, et al. Cell arrest and cell death in mammalian preimplantation development: lessons from the bovine model. PLoS ONE. 2011;6(7):e22121. doi: 10.1371/journal.pone.0022121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishii N, Oohira T, Oshima A, Sakuraba H, Endo F, Matsuda I, et al. Clinical and molecular analysis of a Japanese boy with Morquio B disease. Clin Genet. 1995;48(2):103–8. doi: 10.1111/j.1399-0004.1995.tb04065.x. [DOI] [PubMed] [Google Scholar]
- 34.Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257(1):162–71. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 35.Zwerschke W, Mazurek S, Stockl P, Hutter E, Eigenbrodt E, Jansen-Durr P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem J. 2003;376(Pt 2):403–11. doi: 10.1042/BJ20030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sitte N, Merker K, Grune T, von Zglinicki T. Lipofuscin accumulation in proliferating fibroblasts in vitro: an indicator of oxidative stress. Exp Gerontol. 2001;36(3):475–86. doi: 10.1016/S0531-5565(00)00253-9. [DOI] [PubMed] [Google Scholar]
- 37.Liton PB, Lin Y, Gonzalez P, Epstein DL. Potential role of lysosomal dysfunction in the pathogenesis of primary open angle glaucoma. Autophagy. 2009;5(1):122–4. doi: 10.4161/auto.5.1.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24(4):679–86. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 39.von Zglinicki T, Saretzki G, Ladhoff J, d’Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126(1):111–7. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 40.d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8(7):512–22. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 41.Mu XF, Jin XL, Farnham MM, Li Y, O’Neill C. DNA damage-sensing kinases mediate the mouse 2-cell embryo’s response to genotoxic stress. Biol Reprod. 2011;85(3):524–35. doi: 10.1095/biolreprod.110.089334. [DOI] [PubMed] [Google Scholar]
- 42.Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6(1):117–31. doi: 10.1016/S1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- 43.Adiga SK, Toyoshima M, Shimura T, Takeda J, Uematsu N, Niwa O. Delayed and stage specific phosphorylation of H2AX during preimplantation development of gamma-irradiated mouse embryos. Reproduction. 2007;133(2):415–22. doi: 10.1530/REP-06-0048. [DOI] [PubMed] [Google Scholar]
- 44.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22(16):4212–22. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sviderskaya EV, Gray-Schopfer VC, Hill SP, Smit NP, Evans-Whipp TJ, Bond J, et al. p16/cyclin-dependent kinase inhibitor 2A deficiency in human melanocyte senescence, apoptosis, and immortalization: possible implications for melanoma progression. J Natl Cancer Inst. 2003;95(10):723–32. doi: 10.1093/jnci/95.10.723. [DOI] [PubMed] [Google Scholar]
- 46.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14(4):501–13. doi: 10.1016/S1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 47.Freedman DA, Folkman J. CDK2 translational down-regulation during endothelial senescence. Exp Cell Res. 2005;307(1):118–30. doi: 10.1016/j.yexcr.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 48.Egashira A, Kano K, Naito K. Preimplantation-embryo-specific cell-cycle regulation is attributable to a low expression of retinoblastoma protein rather than its phosphorylation. J Reprod Dev. 2011;57(4):492–9. doi: 10.1262/jrd.10-170O. [DOI] [PubMed] [Google Scholar]
- 49.Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277(5327):831–4. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 50.Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod. 2010;25(3):605–12. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- 51.Kallen B, Finnstrom O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Blastocyst versus cleavage stage transfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril. 2010;94(5):1680–3. doi: 10.1016/j.fertnstert.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 52.Catt JW, Henman M. Toxic effects of oxygen on human embryo development. Hum Reprod. 2000;15(Suppl 2):199–206. doi: 10.1093/humrep/15.suppl_2.199. [DOI] [PubMed] [Google Scholar]
- 53.Orsi NM, Leese HJ. Protection against reactive oxygen species during mouse preimplantation embryo development: role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Mol Reprod Dev. 2001;59(1):44–53. doi: 10.1002/mrd.1006. [DOI] [PubMed] [Google Scholar]
- 54.Meintjes M, Chantilis SJ, Douglas JD, Rodriguez AJ, Guerami AR, Bookout DM, et al. A controlled randomized trial evaluating the effect of lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program. Hum Reprod. 2009;24(2):300–7. doi: 10.1093/humrep/den368. [DOI] [PubMed] [Google Scholar]
- 55.Kovacic B, Sajko MC, Vlaisavljevic V. A prospective, randomized trial on the effect of atmospheric versus reduced oxygen concentration on the outcome of intracytoplasmic sperm injection cycles. Fertil Steril. 2010;94(2):511–9. doi: 10.1016/j.fertnstert.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 56.Bontekoe S, Mantikou E, van Wely M, Seshadri S, Repping S, Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database Syst Rev. 2012;7:CD008950. doi: 10.1002/14651858.CD008950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knijn HM, Gjorret JO, Vos PL, Hendriksen PJ, van der Weijden BC, Maddox-Hyttel P, et al. Consequences of in vivo development and subsequent culture on apoptosis, cell number, and blastocyst formation in bovine embryos. Biol Reprod. 2003;69(4):1371–8. doi: 10.1095/biolreprod.103.017251. [DOI] [PubMed] [Google Scholar]