Introduction
Because of scientific developments, life has been extended in recent decades, and among all diseases, cancer has gained a greater dimension and is recognized as a public health topic worldwide. There has been a major focus on breast cancer, which is recognized as the most common malignancy in women [1].
In 2012, there were an estimated 230,000 new cases of invasive breast cancer in the USA, mainly after menopause [2]. However, breast cancer at the reproductive age is considered a worst and invasive prognosis, for which chemotherapy using high doses of alkylating agents and radiotherapy with ionizing radiation should be applied. Such approach is known to reduce the primordial follicle reserve and cause premature ovarian failure, possibly with genetic damage to growing eggs [3].
Advances in reproductive techniques have provided many fertility preservation options [4]. However, initial trials on oocyte cryopreservation were limited by spindle misalignment and errors in the chromosomal arrangement due to ice formation inside the oocytes during the process [5]. In the last 15 years, a better understanding of the cryopreservation technique has resulted in improvements in the slow-freezing method and the introduction of the vitrification. Vitrification is the process of cryopreservation using ultra-rapid cooling at high concentrations of cryoprotectants, which avoids the formation of ice crystals [6]. The intracytoplasmic water is transformed into a glassy vitrified state, which thereby reduces cellular damage. This method has become recognized as a superior tool for oocyte cryopreservation [7].
In the present case report, we describe the outcome of a patient presenting breast cancer, who had her oocytes vitrified for 6 years and underwent in vitro fertilization (IVF) cycles that produced viable blastocysts and a successful healthy baby delivery.
Material and methods
In 2007, the patient SHC, 36 years old, married, nulliparous, had been recently submitted to a left quadrantectomy and axillary lymphadenectomy for breast cancer, which was recognized as being an IIB stage tumor (T2N1M0). Complementary evaluation classified as a Luminal A tumor that was estrogen and progesterone receptor positive, but the tumor displayed negative expression for the oncoprotein c-erbB-2 and p53, in addition to decreased cell proliferation (less than 14 %) as measured by Ki-67. No relatives had a previous breast cancer history. The patient was designated to receive complementary chemotherapy treatment, and the mastologist advised her about possible ovarian reserve damage due to the gonad-toxic effects of chemotherapy. She was referred to our center for fertility preservation.
At the initial appointment, she presented a normal clinical exam, adequate ovarian reserve evaluated by antral follicle count and basal levels of hormones: FSH: 6.0 UI/ml, E2: 42.5 ng/ml and P4: 0.3 ug/ml. Controlled ovarian stimulation (COH) for oocyte cryopreservation was indicated with oncologist acceptance.
Controlled ovarian stimulation was started on day 3 of the menstrual cycle with 225 IU of recombinant FSH (Puregon, Organon), which was adjusted during the cycle to 150 IU, for a total administered dose of 1,625 IU of recombinant FSH. Administration of a GnRH antagonist (Cetrotide, Merck Serono) was initiated on day 7, and final follicular maturation was triggered with 0.2 mg of GnRH agonist (Lupron, Abbott) on day 10 (Fig. 1). Oocyte aspiration was performed under sedation 35 h after triggering.
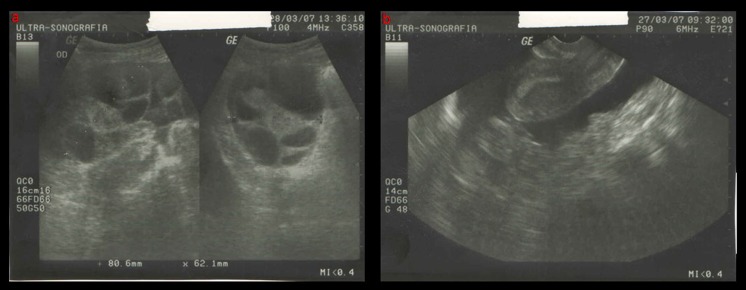
Fig. 1.
Vaginal ultrasonography, visualization of follicles in two ovaries (a) and the uterus (b) on the trigger day
A total of 35 oocytes were collected, and 28 were in the metaphase II (MII) stage. All MII oocytes were frozen, into four vials with seven oocytes each, using a Cryotip vitrification method (Irvine Scientific) following the manufacturer’s protocol.
Results
In 2013, 6 years after oocyte cryopreservation, the patient returned to our clinic, at 41 years of age, to use her cryopreserved oocytes for an IVF cycle. No signs of cancer recurrence were observed.
On the first attempt, one vial containing seven oocytes was warmed, resulting in five viable oocytes fertilized by ICSI. The embryos were cultured using G1 medium (Vitrolife), at 37 °C, 6 % CO2, and three were developed to eight-cell grade 1, on day 3, when they were transferred. Previously, endometrium preparation was performed with 4 mg of estradiol valerate through 13 days plus the introduction of micronized progesterone. No pregnancy was achieved.
A second cycle was proposed using other two vials of oocytes vitrified. A total of 12 viable oocytes were recovered after the warm process and submitted to ICSI, producing 11 embryos. Nine embryos reached day 3. Six of them were high quality (eight-cell grade 1), and three reached the blastocyst stage on day 5 of culture when they were transferred. The patient received the same endometrium preparation.
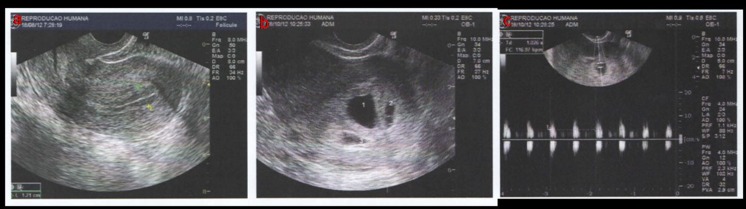
The hCG test was performed 9 days after the transfer (1,396 UI/mL), and two other tests indicated good progression (7,639 UI/mL and 53,715 UI/mL). A transvaginal ultrasound was performed on the sixth gestational week to confirm pregnancy. The presence of three gestational sacs (Fig. 2b) was confirmed, but only one had viable signs (Fig. 2c). The patient had a normal pregnancy, with no problems during the gestation, delivering by C-section a healthy baby, at term, with a weight of 2,970 g and no signs of malformation or congenital disease.
Fig. 2.
Transvaginal ultrasound: Endometrial tackiness before ET (a), presence of the three gestational sacs (b) and fetal heart (c)
Discussion
Breast cancer affects up to one in eight women in developed countries and is one of the most common malignancies during reproductive age. As the disease is usually more aggressive for young patients, chemotherapy with high doses of alkylating agents is usually proposed as a complementary therapy, which threatens patient ovarian reserve and fertility ability [8].
Until recently, oocyte cryopreservation was used as an experimental protocol for fertility preservation. Modifications in cryopreservation methods over the past few years are responsible for its routine use in IVF, especially for patients with cancer [9, 10]. Cryopreservation of mature oocytes using vitrification techniques to avoid ice crystal formation has become an effective method to store oocytes, which supports it usefulness for fertility preservation. The technique achieves pregnancy rates close to those of current IVF with fresh oocytes and no apparent increase in congenital abnormalities [11–15].
However, there are limited data regarding the effect of storage duration for oocyte cryopreservation survival in regards to achieving viable pregnancy. One study evaluated oocytes up to 48 months and did not find any differences of survival, fertilization, cleavage, embryo quality, implantation and live-birth rates compared with earlier thaws. However, oocyte vitrification was performed for non-medical purposes [16]. For women with cancer, at the reproductive age, oocyte cryopreservation is advisable. Gamete viability is essential, as there might be several years between the initiation of treatment until the absence of recurrences. The ability to maintain the cell efficiency for fertilization and development during this period at low temperatures is crucial. Only five cases have been reported and only one for breast cancer [17].
In the present case, the oocytes were cryopreserved for 6 years and then thawed after cancer remission, with a successful viable pregnancy achieved. Our results suggest that oocyte vitrification could offer a safe option for women at reproductive age with cancer for maintaining their ability to conceive in later years.
Nevertheless, a critical step in the technique is reinforcing the oocyte non-activity and protection among the process. As the patient had 28 recovered oocytes, which demonstrated her ability to conceive during the IVF attempts, her viable pregnancy was somewhat expected. On the other hand, given that only one out of three gestational sacs developed, it is possible that the cryopreservation technique did not fully protect the oocyte potential.
At the time of cryopreservation for the case described, it was routine to cryopreserve a high number of oocytes per Cryotip, as the rates of post-thaw survival were not well established, and seven oocytes per vial were loaded. Currently, the cryopreservation involves two or three oocytes per Cryotip, which is recognized to increase the survival rates [18]. In our case, we reported 81 % efficiency, which is low compared with the actual standards. Loading a lower number of oocytes per Cryotip most likely allows a better environment and protection against cryodamage, in addition to better management of the fertilization process.
Studies also have indicated that cryopreservation does not increase chromosomal abnormalities, birth defects or developmental deficits in the delivered children. Thus, given these successes in recent years, this technique should no longer be considered experimental [10]. However, this is a case report of a single cancer patient using her own oocytes cryopreserved for 6 years with a successful outcome. In spite other patients had cryopreserved their eggs in our clinic for fertility preservation, this is the unique case which patient requested to use her oocytes after cancer remission. We don’t know how many assisted reproduction physicians have tried similar methods applied here and were unsuccessful, as failed outcomes are not usually published in the literature. Hence, a series of similar cases would be useful to confirm the findings shown in our report. In conclusion, oocyte cryopreservation may be considered a good strategy for fertility preservation before cancer treatment.
Footnotes
Capsule This study reports a case of oocyte vitrification for fertility preservation before chemotherapy in a breast cancer patient. Twenty-eight mature oocytes were vitrified previous to chemotherapy and used for IVF treatment after six years with a healthy live birth baby delivered. Oocyte vitrification may be a safe option for women diagnosed with cancer that enables fertility preservation and pregnancy after treatment and curing.
References
- 1.Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36:237–48. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–9. doi: 10.1016/j.fertnstert.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Cobo A, Domingo J, Perez S, Crespo J, Remohi J, Pellicer A. Vitrification: an effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2008;10:268–73. doi: 10.1007/s12094-008-0196-7. [DOI] [PubMed] [Google Scholar]
- 5.Smith GD, Motta EE, Serafini P. Theoretical and experimental basis of oocyte vitrification. Reprod Biomed Online. 2011;23:298–306. doi: 10.1016/j.rbmo.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65:236–44. doi: 10.1016/j.theriogenology.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Burgos M, Herrero L, Megias D, Salvanes R, Montoya MC, Cobo AC, et al. Vitrification versus slow freezing of oocytes: effects on morphologic appearance, meiotic spindle configuration, and DNA damage. Fertil Steril. 2011;95:374–7. doi: 10.1016/j.fertnstert.2010.07.1089. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Wallberg KA, Oktay K. Fertility preservation in women with breast cancer. Clin Obstet Gynecol. 2010;53:753–62. doi: 10.1097/GRF.0b013e3181f96e00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ASRM PCo Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ubaldi F, Anniballo R, Romano S, Baroni E, Albricci L, Colamaria S, et al. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum Reprod. 2010;25:1199–205. doi: 10.1093/humrep/deq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Velasco JA, Domingo J, Cobo A, Martinez M, Carmona L, Pellicer A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril. 2013;99:1994–9. doi: 10.1016/j.fertnstert.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online. 2009;18:769–76. doi: 10.1016/S1472-6483(10)60025-9. [DOI] [PubMed] [Google Scholar]
- 15.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93:391–6. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 16.Parmegiani L, Garello C, Granella F, Guidetti D, Bernardi S, Cognigni GE, et al. Long-term cryostorage does not adversely affect the outcome of oocyte thawing cycles. Reprod Biomed Online. 2009;19:374–9. doi: 10.1016/S1472-6483(10)60171-X. [DOI] [PubMed] [Google Scholar]
- 17.Cobo A, Garcia-Velasco JA, Domingo J, Remohi J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil Steril. 2013;99:1485–95. doi: 10.1016/j.fertnstert.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 18.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]