Abstract
Purpose
The aim of this study was to investigate the association between two common single nucleotide polymorphisms (SNPs) in the vascular endothelial growth factor (VEGF) gene (−460C/T and +405G/C) and polycystic ovary syndrome (PCOS) risk in south Indian women.
Methods
This study involves clinically confirmed PCOS patients (n = 126) and non-PCOS controls (n = 130) of south Indian origin (Dravidian linguistic group). Genotyping of the VEGF gene −460C/T and +405G/C SNPs were performed by PCR and sequencing analysis. Haplotype frequencies for multiple loci and the standardized disequilibrium coefficient (D') for pairwise linkage disequilibrium (LD) were assessed by Haploview Software.
Results
The frequencies of +405G/G genotype (P = 0.03) and +405G alleles (P = 0.006) were significantly higher in patients compared to controls. Whereas the genotype and allele frequencies of −460C/T SNP were not significantly different between patients and controls. In addition, LD analysis revealed no significant difference between patients and controls.
Conclusion
Our findings suggest that the VEGF +405G/C polymorphism may constitute an inheritable risk factor for PCOS in south Indian women.
Keywords: PCOS, VEGF, SNP, Haplotype, South Indian women
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders affecting women of reproductive age with a prevalence of 6–10 % [1]. Important reproductive aspects associated with PCOS are polycystic ovaries, hyperandrogenism, hirsutism, acne, androgenic alopecia, anovulation (amenorrhea, oligomenorrhoea), and hyper secretion of LH. Metabolic disorders associated with PCOS include hyperinsulinaemia, insulin resistance, impaired pancreatic cell insulin secretion, type 2 diabetes [2]. In addition, disorders of general health such as preeclampsia and chronic thyroiditis [3, 4] are also shown in association with PCOS. The observation of familial aggregation indicates heritable tendency of the PCOS, but the etiology and pathogenesis remains uncertain.
A key pathophysiological feature of PCOS is the increased ovarian mass, supported by new blood vessel proliferation in stroma and theca [5]. Vascular endothelial growth factor (VEGF) or vascular permeability factor (VPF), a dimeric heparin-binding glycoprotein, is one of the most likely candidates for promoting angiogenesis and vascular permeability. Macrophages and granulosa cells are major sources for VEGF in female reproductive tract [6]. It mediates neovascularisation during corpus luteum formation, embryogenesis and tumorigenesis. In addition, it is also important for maintaining and regulation of perifollicular blood flow and intrafollicular oxygen levels [7].
Earlier studies have reported elevated serum VEGF levels in women with PCOS [8]. Increased secretion of VEGF in the serum of patients may induce an increased number of active granulosa lutein cells which have both increased secretory capacity as well as up-regulated gene expression level [8, 9]. Furthermore, increased VEGF levels have also been reported in the hyperthecotic stroma of PCOS which can induce connective stromal growth by promoting microvascular permeability [10]. High vascularisation may lead to the abnormal growth of the thecal interna, which is a major site for androgen steroidogenesis. Endothelial dysfunction in women with polycystic ovary syndrome has been demonstrated previously by colour Doppler blood flow imaging [11]. It has been suggested that increased intra-ovarian concentrations of VEGF are related to increased secretion of LH, an important pathophysiological feature of PCOS [12]. All these observations suggest that the development of ovarian follicle is strongly influenced by VEGF expression levels in PCOS patients.
The VEGF gene is located at 6p21.3 locus. Several number of single nucleotide polymorphisms (SNPs) have been identified within the VEGF gene, of which some are located in the promoter (eg: −460C/T) and in 5'-untranslated region (5'-UTR; eg: +405G/C) which are critical for transcriptional regulation. For example, the +405G/C SNP has a significant effect on VEGF expression [13]. Available literature indicates association between VEGF +405G/C, −460C/T SNPs and risk of various human diseases [14–17], but studies related to PCOS are very few. In the present study, we report for the first time association between the VEGF untranslated region SNPs (−460C/T and +405G/C) and PCOS risk in south Indian women.
Materials and methods
Study design
One hundred and twenty six women of Indian origin with PCOS were recruited at the Infertility Institute and Research Centre (IIRC), Secundrabad, India. They had no smoking and no caffeine habits. BMI was calculated as body weight (kg) divided by body height squared (m2). Patients were selected as per the Rotterdam consensus criteria to diagnose PCOS [18]. The characteristics of PCOS women and controls were summarized in Table 1. Polycystic appearing ovaries were defined sonographically as the presence of multiple (>10), small (2–9 mm in diameter) follicles in the periphery (in one plane) and increased stromal echogenicity. The presence of polycystic ovaries was confirmed by ultrasound scan, followed by laparoscopy to rule out any other reproductive disorders. In the study group, the indications for referral were menstrual cycle disturbances, infertility and symptoms of hyperandrogenism. Women with other infertility related disorders such as endometrial cysts on ovaries, adenomycosis, ovarian adhesions and presence of cysts on pelvic organs other than ovaries, even those showed symptoms of PCOS (like hyper androgenaemia and elevated hormone concentrations) but who had normal ovaries as revealed by ultra sound scan and laparoscopic examination were excluded from the present study. In addition, the study group women showed one or more of following clinical or biochemical disturbances: infertility, hirsutism, acne, irregular menstruation, laboratory tests revealing androgen excess (serum testosterone concentration >2.5 nmol/l or plasma testosterone >40 pmol/l), and an elevated LH/FSH ratio.
Table 1.
Clinical characteristics of PCOS and control group
| Variable | Total controls (n = 130) | Total PCOS (n = 126) | p-value a |
|---|---|---|---|
| Age (years) | 30.00 ± 5.28 | 26.46 ± 3.79 | <0.0001 |
| Weight (kg) | 57.32 ± 9.04 | 58.74 ± 11.75 | 0.2786 |
| BMI (kg/m2) | 23.85 ± 2.90 | 27.10 ± 5.16 | <0.0001 |
| FSH (mIU/ml) | 6.16 ± 1.74 | 6.01 ± 2.14 | 0.5383 |
| LH (mIU/ml) | 5.33 ± 1.64 | 7.90 ± 2.51 | <0.0001 |
| LH/FSH ratio | 0.88 ± 0.21 | 1.49 ± 0.99 | <0.0001 |
| Presence of overweight and obesity | 10 (6.41) | 58 (46.03) |
Data are given as mean ± S.D
p a values obtained by comparison of variables between controls and PCOS by Student’s t test
Controls
To compare the results obtained from the patient group, a total of 130 fertile women aged from 18 to 40 years (mean age: 26 years) of age were recruited as controls. They were selected on the basis of regular menstrual cycles and had a successful pregnancy record. The absence of polycystic ovaries in the controls was confirmed by ultrasound method.
Peripheral blood samples (5 ml) were collected from all the subjects in EDTA vacutainers and stored at −80 °C until further use. Written informed consent was obtained from all participants. The institutional review board of the Centre for Cellular and Molecular Biology (CCMB), Hyderabad, approved the study.
DNA extraction
Genomic DNA was extracted from 1 ml of EDTA anti-coagulated whole blood by the method described earlier [16]. Both cases and controls were genotyped in a randomized, blinded fashion.
Determination of VEGF genotype
Genotyping of VEGF polymorphisms (−460C/T and +405G/C) by PCR and sequencing analysis as per the protocols described earlier [17]. PCRs were carried out in a total volume of 25 μl, containing 50 ng genomic DNA, 2–6 pmole of each primer, 1X Taq polymerase buffer (1.5 mM MgCl2) and 0.25U of Amplitaq DNA polymerase (Perkin Elmer, Foster City, USA). The primers for -460C/T were 5' TGTGCGTGTGGGGTTGAGCG-3' (forward), and 5'-TACGTGCGGACAGGGCCTGA-3' (reverse), and 5'-ATTTATTTTTGCTTGCCATT-3' (forward) and 5'-GTCTGTCTGTCTGTCCGTCA-3' (reverse) for +405G/C. The primers were designed by using primer 3plus software (http://primer3plus.com/cgi-bin/dev/primer3plus.cgi). PCR amplification was performed in a programmable thermal cycler gradient PCR system (Eppendorf AG, Hamburg, Germany). The PCR amplification was carried out for 35 cycles (denaturation at 94 °C for 1 min, annealing for 1 min at 60 °C for −460 and 55 °C for +405, extension at 72 °C for 1 min and final extension for 10 min at 72 °C. PCR products of 175 bp (−460) and 304 bp (+405) were analysed by 1.5 % agarose gel stained with ethidium bromide and then sequenced with a Taq-Dye deoxy-terminator cycle sequencing kit (Applied BioSystems, USA) using an automated ABI 3770 DNA sequencer (Applied BioSystems, USA). Genotype calling was performed by using Chromas V.2 software (Technelysium Ltd., Australia).
Statistical analysis
Statistical analysis was performed using SPSS statistical package (V 11.0). The genotype distribution among subjects was tested for Hardy–Weinberg equilibrium (HWE) using Fisher’s exact test. The allele ratios and genotype distributions of cases and controls were analyzed using Fisher’s exact test. The odds ratio and 95 % confidence interval (CI) values were calculated using the online Vassar Stats Calculator (http://www.faculty.vassar.edu/lowry/VassarStats.html). Haplotype frequencies for multiple loci and the standardized disequilibrium coefficient (D') for pair-wise linkage disequilibrium (LD) were assessed by Haploview Software [19]. The data on haplotype and case–control were subjected to ANOVA and the four haplotypes were examined for their statistical significance applying Tukey’s test.
Results
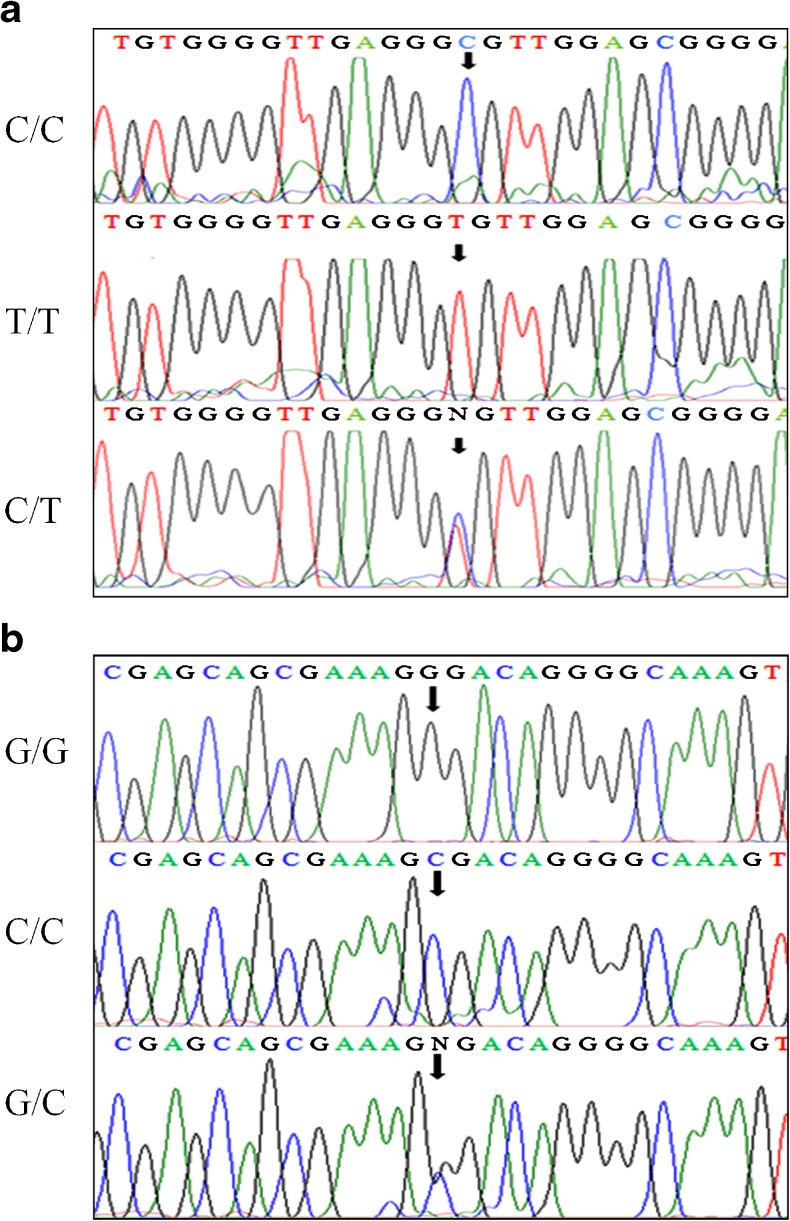
All subjects (n = 256) were successfully genotyped. Amongst both cases and controls, the genotype distributions of individual SNPs as well as VEGF allele system were all in Hardy–Weinberg equilibrium (P > 0.05). Sequencing analysis of 175 bp (−460C/T) and 304 bp (+405G/C) PCR products were shown in Fig. 1a and b. The TT, CC (−460) and GG, CC (+405) homozygotes manifested as a single peak, whereas heterozygotes CT (−460) and GC (+405) as double peaks.
Fig. 1.
a Genotyping of the VEGF gene -460C/T polymorphism by sequence analysis of the PCR-amplified product using a forward primer. b Genotyping of the VEGF gene +405G/C polymorphism by sequence analysis of the PCR-amplified product using a forward primer
+405G/C polymorphism
The genotype and allele distribution of the VEGF +405G/C SNP revealed significant differences between patients and controls (all P values <0.05). There was significant reduction of C/C genotype frequency and elevation of G/G genotype frequency in patients as compared to controls (Table 2). The allele frequency also showed similar trend indicating that ‘G’ allele might confer risk to PCOS and ‘C’ allele offer protection against the disease (P = 0.00694).
Table 2.
Genotype and allele frequencies of the VEGF -460C/T and +405G/C polymorphisms in PCOS patients and controls
| Genotypes/Alleles | PCOS (%) n = 126 | Controls (%) n = 130 | ‘P’-value | Odds ratio | 95 % CI |
|---|---|---|---|---|---|
| + 405 G/C Genotypes | |||||
| G/G | 70 (55.5) | 52 (40.0) | 0.03023a | ||
| G/C | 46 (36.5) | 59 (45.3) | |||
| C/C | 10 (7.9) | 19 (14.6) | |||
| Alleles | |||||
| G | 186 (0.74) | 163 (0.63) | 0.00694b | 1.6771 | 1.1505-2.4447 |
| C | 66 (0. 26) | 97 (0.37) | |||
| - 460 C/T Genotypes | |||||
| T/T | 40 (31.7) | 33 (25.3) | 0.37232a | ||
| T/C | 59 (46.8) | 72 (55.3) | |||
| C/C | 27 (21.4) | 25 (19.2) | |||
| Alleles | |||||
| T | 139 (0.55) | 138 (0.53) | 0.63676b | 1.0875 | 0.768 –1.5398 |
| C | 113 (0.45) | 122 (0. 47) | |||
CI, confidence interval
a Fisher’s exact test (3 × 2 table at 2 df); P < 0.05
b Fisher’s exact test (2 × 2 table at 1 df); P < 0.05
−460C/T polymorphism
The VEGF −460C/T SNP genotype distribution and allele frequencies amongst the cases and controls were shown in Table 2. The frequencies of genotypes (P = 0.372) and alleles (P = 0.636) were not significantly different between cases and controls.
Haplotype analysis
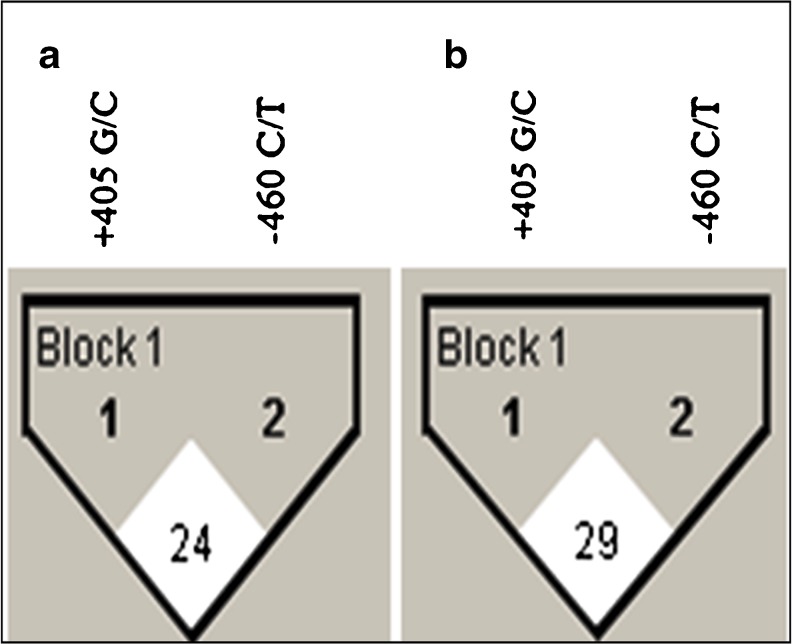
To analyze the combined effect of VEGF SNPs on PCOS development, the haplotype frequencies for multiple loci and the standardized disequilibrium coefficient (D') for pair-wise linkage disequilibrium (LD) were estimated (Table 3; Fig. 2). The LD between –460C/T and +405G/C loci was not much different between cases (D' = 24) and controls (D' = 29).
Table 3.
Haplotype frequencies of VEGF polymorphisms in PCOS patients and controls
| Haplotypes | Haplotype frequency | P valuea | Odds ratio | 95 % CI | ||
|---|---|---|---|---|---|---|
| −460 | +405 | Controls (%) | PCOS (%) | |||
| T | G | 98 (37.6) | 107 (42.4) | Reference | ||
| C | G | 66 (25.3) | 80 (31.7) | 0.63078 | 1.1102 | 0.7252 – 1.6996 |
| T | C | 40 (15.3) | 33 (13.0) | 0.30505 | 2.4271 | 1.5393 – 3.827 |
| C | C | 56 (21.5) | 32 (12.6) | 0.01285 | 1.472 | 0.9559 – 2.2668 |
CI, Confidence interval
a Fisher’s exact (2 × 2 table at I df); P < 0.05
Fig. 2.
LD analysis of cases and controls are shown separately. Haploview plots are presented along with the single nucleotide polymorphisms studied. The pair-wise linkage disequilibrium values (D’ = 0–100) of all single nucleotide polymorphisms are given in each diamond. A value of 100 represents maximum possible linkage disequilibrium. (a) LD analysis of cases. (b) LD analysis of controls
Our data suggests that the –460T/+405G is the most common haplotype in south Indian women. Hence, the relative risk of each haplotype was calculated by using this as reference (Table 3). The ANOVA results with Tukey’s correction showed a p value exceeding 0.05 indicating no statistically significant difference in the occurrence of the four haplotypes between cases and controls.
Discussion
Single nucleotide polymorphisms (SNPs) are common DNA sequence variations among individuals which play significant role in development of several human diseases, including cancer. SNPs particularly in gene promoters and protein encoding regions may modulate gene function and/or transcriptional efficiency. There are at least 80 SNPs places in this gene (NCBI Gene association no: NT 007592). Importantly, the –460C/T and +405G/C SNPs were extensively investigated by different scientific groups in various diseases with inconsistent results. Some of them have found an increased risk for the ‘C’ (−460) and/or ‘C’ (+405) allele carriers [20], while the others could not [21]. In regards to PCOS, only one case–control study have evaluated these SNPs [22]. Indeed these studies emphasize the importance of complex genetic traits for studying genetic variants in different ethnic and geographic populations.
The VEGF gene is located in the chromosome region 6p21.3 and consists of eight exons and seven introns exhibiting alternate splicing to form a family of proteins [23]. The regulatory region of the VEGF gene contains a number of transcription factor-binding sites and transcriptional regulation of this gene appears to be extremely complex, with levels of control at the transcription and translation [24]. The polymorphisms in the VEGF gene promoter region (−460) or 5'- untranslated region (+405) have been associated with different levels of VEGF expression [25]. It was reported that –460T [26] and +405G [13] alleles appear to correlate with increased VEGF expression. The differential VEGF expression could influence the etiology of a variety of pathological conditions. In addition, the –460C/+405G haplotype has been associated with higher promoter activity, than the –460T/+405C haplotype [26]. These observations suggest that the SNPs themselves have a regulatory function or, alternatively, there is an allelic linkage between these polymorphisms and functional polymorphisms elsewhere in the gene.
In the present study, for the first time we report significant association between VEGF +405G/C SNP and PCOS risk in south Indian women. The ‘G’ allele frequency was significantly higher in PCOS patients than unaffected controls drawn from the same population (Table 2). In addition, the G/G genotype frequency was also significantly higher in PCOS patients (P = 0.03). Hence, our results indicate VEGF as a candidate gene for PCOS. However, some of the previous investigations have reported no association between VEGF +405G/C SNP and PCOS risk which is not in agreement with the present result [22]. This discrepancy may be due to differences in ethnicity, demographic location, sample size, and/or allele frequencies among populations. Indeed drastic difference in ‘G’ allele distribution was observed in the two populations studied. The frequencies of ‘G’ allele in controls and cases were 85 and 82 % respectively compared to 63 and 74 % in the present study.
Our haplotype analysis showed that the –460C/+405C haplotype, which is associated with lower promoter activity, was significantly more common in controls than in cases (P = 0.0128). Interestingly, our data also showed higher frequency of –460T/+405G haplotype in the PCOS group compared to controls; however, it did not reach up to significant level (P>0.0125). This could be due to the smaller sample size. The –460T and +405G alleles are known to be associated with high production of VEGF [13]. Therefore, the –460T/+405G haplotype carriers may have a higher levels of VEGF which can lead to increased vascularity and accentuated stromal growth in the ovary with subsequent hyperplasia of the theca interna and excessive production of androgens. This hypothesis is supported by demonstration of a strong immunohistochemical staining of VEGF in the ovarian stroma of patients with PCOS [10]. VEGF can induce connective stromal growth by increasing microvascular permeability, which leads to extravasation of plasma proteins in the polycystic ovarian syndrome [27]. The extravascular matrix thus formed favours the growth of new blood vessels and fibroblasts, which in turn organize the avascular provisional fibrin matrix into a mature, vascularized connective tissue stroma [10].
The VEGF gene is reported to be regulated by estrogen, hypoxia, growth factors including epidermal growth factor, and cytokines including interleukin 6. [16, 28] The VEGF gene contains two ER-binding sites. One of these was in the 3'-untranslated region and worked as a conventional enhancer, and the other, located in exon 1 (+410 ERE). Previous studies reported that obese PCOS patients have elevated estrogen levels [29]. +405G/C polymorphism is located adjacent to the +410 ERE of VEGF gene and appeared to regulate transcription of the VEGF in the presence of estrogen [29]. Therefore, it is likely that carriage of these polymorphisms would alter the estrogen responsiveness of the promoter, and this would have important implications for regulation of VEGF expression. BRCA-1 affects the estrogen levels in granulosa cells of ovarian follicle by regulating the levels of aromatase, a rate limiting enzyme of estrogen biosynthesis [30]. However, BRCA-1 which is localized 1.2 kb upstream from the VEGF transcription start site also modulates VEGF transcription [31]. In conclusion we report that the +405G allele in the 5′-untranslated region of VEGF gene may influence the likelihood of a woman developing PCOS. However, further larger-scale population studies including other loci of the VEGF gene are necessary to confirm our findings.
Acknowledgments
Praveen Guruvaiah would like to thank University Grants Commission (UGC), India for awarding Junior Research Fellowship (JRF, NET).
Funding
This work was supported in part by grants from the SERB (DST), India (Lr No: SR/FT/LS-188/2009) to M.B.
Contributor Information
Praveen Guruvaiah, Email: renapraveen@gmail.com.
Suresh Govatati, Email: sureshgovatati@gmail.com.
Tumu Venkat Reddy, Email: venkatreddytm@gmail.com.
Dakshayani Lomada, Email: dlomada@yahoo.com.
Mamata Deenadayal, Email: iircoxegene@rediffmail.com.
Sisinthy Shivaji, Email: shivas@ccmb.res.in.
Manjula Bhanoori, Phone: 00-91-9989661469, Email: bhanoorim@yahoo.co.in.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endcrinol Met. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenisis and diagnosis. Nat Rev Endocrinol. 2011;7:219–31. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 3.De Vries MJ, Dekker GA, Schoemaker J. Higher risk of preeclampsia in the polycystic ovary syndrome. a case control study. Eur J Obstet Gynecol Reprod Biol. 1998;76:91–5. doi: 10.1016/S0301-2115(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 4.Garelli S, Masiero S, Plebani M, et al. High prevalence of chronic thyroiditis in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):248–51. doi: 10.1016/j.ejogrb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Pan HA, Wu MH, Cheng YC, Li CH, Chang FM. Quantification of Doppler signal in polycystic ovary syndrome using three-dimensional power Doppler ultrasonography: a possible new marker for diagnosis. Hum Reprod. 2002;17:201–6. doi: 10.1093/humrep/17.1.201. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal R, Tan SL, Wild S, Conway G. Serum vascular endothelial growth factor concentrations in in vitro fertilization cycles predict the risk of ovarian hyperstimulation syndrome. Fertil Steril. 1999;71:287–93. doi: 10.1016/S0015-0282(98)00447-6. [DOI] [PubMed] [Google Scholar]
- 7.Van Blerkom J, Antezak M, Schrader R. The development potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–55. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal R, Jacobs H, Payne N, Conway G. Concentration of vascular endothelial growth factor released by cultured human luteinized granulosa cells is higher in women with polycystic ovaries than in women with normal ovaries. Fertil Steril. 2002;78:1164–9. doi: 10.1016/S0015-0282(02)04242-5. [DOI] [PubMed] [Google Scholar]
- 9.Abd El Aal DE, Mohamed SA, Amine AF, Meki AR. Vascular endothelial growth factor and insulin-like growth factor1 in polycystic ovary syndrome and their relation to ovarian blood flow. Eur J Obstet Gynecol Reprod Biol. 2005;118(2):219–24. doi: 10.1016/j.ejogrb.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Frantz G, LeCounter J, Dillard-Telm L, Pham T, Draksharapu A, et al. Differential expression of the angiogenic factor genes vascular endothelial groeth factor (VEGF) and endocrine gland- derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162:1881–92. doi: 10.1016/S0002-9440(10)64322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowenstein L, Damti A, Pillar G, Shott S, Blumenfeld Z. Evalution of endothelial function in women with polycystic ovary syndrome. Eur J Obstet Gyncol Reprod Biol. 2007;134:208–12. doi: 10.1016/j.ejogrb.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Balen AH. Hyper secretion of luteinizing hormone in the polycystic ovary syndrome and a novel hormone ‘gonadotrophin surge attenuating factor’. J R Soc Med. 1995;88(6):339P–341P. [PMC free article] [PubMed] [Google Scholar]
- 13.Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine. 2000;12:1232–5. doi: 10.1006/cyto.2000.0692. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Liu L, Zhu ZM, Shao JH, Xin L. Five polymorphisms of vascular endothelial growth factor (VEGF) and risk of breast cancer. a meta-analysis involving 16,703 individuals. Cytokine. 2011;56:167–73. doi: 10.1016/j.cyto.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Bhanoori M, Babu KA, Reddy NGP, et al. The vascular endothelial growth factor (VEGF) + 405G>C 5’-untranslatedregion polymorphism and increased risk of endometriosis in South Indian women: a case control study. Hum Reprod. 2005;20:1844–9. doi: 10.1093/humrep/deh852. [DOI] [PubMed] [Google Scholar]
- 16.Tumu VR, Govatati S, Guruvaiah P, Deenadayal M, Shivaji S, Bhanoori M. An interleukin-6 gene promoter polymorphism is associated with polycystic ovary syndrome in South Indian women. J Assist Reprod Genet. 2013;30(12):1541–6. doi: 10.1007/s10815-013-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govatati S, Tangudu NK, Deenadayal M, Chakravarty B, Shivaji S, Bhanoori M. Association of E-cadherin single nucleotide polymorphisms with the increased risk of Endometriosis in Indian women. Mol Hum Reprod. 2012;18(5):280–87. doi: 10.1093/molehr/gar079. [DOI] [PubMed] [Google Scholar]
- 18.Rotterdam ESHRE/ASRM Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 19.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 20.Vural P, Baki M, Doğru-Abbasoğlu S, Özderya A, Karadağ B, Uysal M. Vascular endothelial growth factor polymorphisms increase the risk of developing graves’ disease. Int Immunopharmacol. 2012;14:133–7. doi: 10.1016/j.intimp.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Summers AM, Coupes BM, Brennan MF, Ralph SA, Short CD, Brenchley PE. VEGF −460 genotype plays an important role in progression to chronic kidney disease stage 5. Nephrol Dial Transplant. 2005;20:2427–32. doi: 10.1093/ndt/gfi029. [DOI] [PubMed] [Google Scholar]
- 22.Vural P, Küskü-Kiraz Z, Doğru-Abbasoğlu S, Cil E, Karadağ B, Akgül C, et al. Vascular endothelial growth factor −2578 A/C,-460 T/C and +405 G/C polymorphisms in polycystic ovay syndrome. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):57–60. doi: 10.1016/j.ejogrb.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Vincenti V, Cassano C, Rocchi M, Persico G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation. 1996;93:1493–5. doi: 10.1161/01.CIR.93.8.1493. [DOI] [PubMed] [Google Scholar]
- 24.Akiri G, Nahari D, Finkelstein Y, Le SY, Elroy-Stein O, Levi BZ. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene. 1998;17:227–36. doi: 10.1038/sj.onc.1202019. [DOI] [PubMed] [Google Scholar]
- 25.Shahbazi M, Fryer AA, Pravica V. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J Am Soc Nephrol. 2002;13:260–4. doi: 10.1681/ASN.V131260. [DOI] [PubMed] [Google Scholar]
- 26.Stevens A, Soden J, Brenchley PE, Ralph S, Ray DW. Haplotype analysis of the polymorphic vascular endothelial growth factor gene promoter. Cancer Res. 2003;63:812–6. [PubMed] [Google Scholar]
- 27.Aleem FA, Predanic M. Transvaginal color Doppler determination of the ovarian and uterine blood flow characteristics in polycystic ovary disease. Fertil Steril. 1996;65(3):510–6. doi: 10.1016/s0015-0282(16)58145-x. [DOI] [PubMed] [Google Scholar]
- 28.Solomon CG. The epidemiology of polycystic ovary syndrome: prevalence and associated disease risks. Endocrinol Metab Clin North Am. 1999;28:247–63. doi: 10.1016/S0889-8529(05)70069-4. [DOI] [PubMed] [Google Scholar]
- 29.Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res. 2000;60(12):3183–90. [PubMed] [Google Scholar]
- 30.Hu Y, Ghosh S, Amleh A, et al. Modulation of aromatase expression By BRCA1: a possible link to tissue-specific tumor suppression. Oncogene. 2005;24:8343–8. doi: 10.1038/sj.onc.1208985. [DOI] [PubMed] [Google Scholar]
- 31.Kawai H, Li H, Chun F, Avraham S, Avraham HK. Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene. 2002;21:7730–9. doi: 10.1038/sj.onc.1205971. [DOI] [PubMed] [Google Scholar]