Abstract
Atrophy of the hippocampus and surrounding temporal regions occurs in Alzheimer’s disease (AD). APOE ε4, the major genetic risk factor for late-onset AD, has been associated with smaller volume in these regions before amyloidosis can be detected by AD biomarkers. To examine APOE ε4 effects in relation to aging, we performed a longitudinal MRI study involving cognitively normal adults (25 APOE ε4 carriers and 31 ε3 homozygotes), initially aged 51–75 years. We used growth curve analyses, which can provide information about APOE ε4-related differences initially and later in life. Hippocampal volume was the primary outcome; nearby medial temporal regions were secondary outcomes. BDNF val66met was a secondary covariate. APOE ε4 carriers had significantly smaller initial hippocampal volumes than ε3 homozygotes. Rate of hippocampal atrophy was not greater in the APOE ε4 group, even though age-related atrophy was detected in the overall sample. The findings add to the growing evidence that effects of APOE ε4 on hippocampal size begin early in life, underscoring the importance of early interventions to increase reserve.
Keywords: Aging, Apolipoprotein E4, Alzheimer Disease, Brain-Derived Neurotrophic Factor, Hippocampus, Longitudinal Studies, Magnetic Resonance Imaging
1. Introduction
After age, the APOE ε4 genetic variant is the major risk factor for late onset Alzheimer’s disease (LOAD) (Bertram et al., 2010). Furthermore, APOE ε4 is associated with an earlier age of onset (Corder et al., 1993; Khachaturian et al., 2004; Kwon et al., 2010); but the mechanisms by which APOE ε4 influences risk and age of onset of LOAD are unclear. Reports that APOE ε4 carriers frequently have greater accumulation of cerebral amyloid and smaller hippocampal volumes are particularly interesting in light of AD mouse models that suggest roles of APOE ε4 in prolonging clearance of beta amyloid (Aβ) (Castellano et al., 2011) and impairing hippocampal neurogenesis (Li et al., 2009).
In humans, APOE ε4 has been frequently associated with earlier accumulation of Aβ, as assessed by PET imaging of fibrillar amyloid or by cerebrospinal fluid (CSF) assay of Aβ42. The earliest indication of Aβ positivity may begin around age 55 among APOE ε4 carriers, 20 years earlier than in noncarriers (Fleisher et al., 2013). APOE’s influence on brain development is also increasingly recognized, particularly in regions affected early by AD. In an MRI study involving neonates, APOE ε4 carrier status was associated with reduced temporal lobe gray matter (GM), as analyzed by tensor-based morphometry (Knickmeyer et al., 2013). Similarly, a longitudinal MRI study of adolescents reported that APOE ε4 status was associated with thinner entorhinal cortex (ERC) (Shaw et al., 2007). Shaw et al. (2007) also noted that the APOE genotype differences seemed “fixed and non-progressive” from age 11 to 20 years, with no evidence of accelerated cortical loss. These indications of localized gray matter reduction are suggestive of lower tissue reserve in AD-vulnerable brain regions, and may also help explain why APOE ε4 carriers are at increased risk of AD. In later adult life, less age-related atrophy would need to occur before a critical anatomical threshold associated with cognitive impairment is reached.
In addition to influences of APOE ε4 on early brain morphology, influences of APOE ε4 on brain function have been seen. As revealed by FDG PET imaging, young adult APOE ε4 carriers showed signs of cortical hypometabolism that resembles an AD pattern (Reiman et al., 2004). As assessed by resting-state functional MRI, young (Filippini et al., 2009) and older adult APOE ε4 carriers, including those who did not show evidence of amyloidosis (Sheline et al., 2010), showed functional connectivity differences. How should we interpret these APOE ε4 differences in brain structure and function? Do these APOE ε4 effects reflect “preclinical AD” (i.e., the presence of AD pathology in apparently normal individuals) (Sperling et al., 2011) or do they reflect early developmental effects?
Given the considerable evidence suggesting that APOE ε4 affects both early brain development and Aβ-mediated risk of AD, we performed a longitudinal MRI study involving subjects of a wide age range. We examined effects of APOE ε4 on hippocampal volume and nearby medial temporal gray matter, using a growth curve approach, which can provide information on ε4-related differences initially and later in life. We expected that APOE ε4 carriers in our sample of 51- to 78-year-olds would have smaller initial volumes than noncarriers, in agreement with results on young subjects (Knickmeyer et al., 2013; Shaw et al., 2007). Second, APOE ε4 carriers might show progressive differences later in life, which would be consistent with reports of greater atrophy rates over time in older nondemented ε4 carriers (Lu et al., 2011; Risacher et al., 2010) and with the view that greater rates of MRI atrophy occur after Aβ deposition (Jack et al., 2010). The effect of the val66met variation in brain-derived neurotrophic factor (BDNF) was also included in the growth-curve analyses given its relevance to human brain development (Knickmeyer et al., 2013), hippocampal volume (Hajek et al., 2012), and hippocampal aging (von Bohlen und Halbach, 2010). In summary, our overall approach is relevant to assessing brain reserve in adult life and the age when a faster rate of atrophy may typically begin among APOE ε4 carriers.
2. Method
2.1. Participants
Participants were 56 volunteers (45 men and 11 women) who completed 1 to 3 MRI scans. Participants were selectively recruited into the MRI study with the aim of achieving an enriched proportion of APOE ε4 carriers. Twenty-five participants were APOE ε4 carriers (23 APOE ε3/ε4 heterozygotes; 2 APOE ε4/ε4 homozygotes); the remaining 31 were APOE ε3/ε3 homozygotes. These participants had previously been genotyped as part of the ongoing Stanford/VA Aviation Study, a longitudinal study of recreational and commercial pilots. The MRI study recruited Aviation Study participants who were at least 50 years old, were still actively flying (as assessed by FAA currency rules), and had a current medical certificate. Data for eight other MRI study participants were not included in the present analysis because their BDNF genotype was either unknown (n = 5) or it was the rare met/met genotype (n = 3). All participants agreed to have genotyping results withheld from them. Informed consent, approved by Stanford University and VA Palo Alto Health Care System Institutional Review Boards, was obtained from all participants.
2.2. Genotyping
APOE genotyping was performed on genomic DNA extracted from samples of frozen whole blood, buccal mucosa, or saliva using restriction isotyping (Hixson and Vernier, 1990) as previously described (Murphy et al., 1997). For blood samples, we used the Gentra PureGene kit (Gentra Systems, Minneapolis, MN); for buccal mucosa samples we used the protocol of Richards et al. (1993); for saliva, we extracted DNA from epithelial cells using the Oragene kit (DNA Genotek, Ottowa, ON).
2.3. Image acquisition
All MRI data were acquired on a 1.5 Tesla MRI scanner (General Electric Medical Systems, Milwaukee, WI) at the Veterans Affairs Palo Alto Health Care System. Participants were positioned on the scanner bed so that head movements were restricted. The following structural MR sequences were done on all participants using a standard head coil: a) a spin-echo, sagittal localizer 2D sequence of 5 mm thick slices (acquisition time = 1 min 44 s); b) a proton density and T2-weighted spin-echo MRI, TR/TE1/TE2 = 5000/30/80 ms, 51 oblique axial 3 mm slices covering the entire brain and angulated parallel to the long axis of the hippocampal formation (1.00 × 1.00 mm2 in plane resolution, acquisition time = 17 min); c) a 3D fast spoiled gradient recall acquisition, TR/TE = 9/2 ms, 15° flip angle, perpendicular to the long axis of the hippocampi (1 × 1 mm2 in plane resolution, 1.5 mm coronal slices covering the entire brain, no skip, acquisition time = 7 min 58 s).
2.4. Hippocampal voluming
Hippocampal volumetry was carried out using a commercially available high dimensional brain mapping tool (Medtronic Surgical Navigation Technologies [SNT], Louisville, CO), that has previously been validated and compared to manual tracing of the hippocampus (Hsu et al., 2002). This semi-automated technique involves manual placement of 22 landmarks by a trained technician. Next, fluid image transformation is used to match the individual brains to a template brain (Christensen et al., 1997). The pixels corresponding to the hippocampus are then labeled and counted to obtain volumes. The SNT method has been validated and compared to manual tracing (left: r = .92; right: r = .91; n=60) (Hsu et al., 2002). Intra- and inter-rater reliability coefficients are .90 or better (Hsu et al., 2002; Schuff et al., 2009b). The SNT method has been implemented by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (Schuff et al., 2009b), and SNT has been used in our prior work showing hippocampal volume correlates with memory performance (r = .47) (Adamson et al., 2010). SNT total hippocampal volume, which includes hippocampal gray matter only and no nearby regions such as the parahippocampal gyrus (PHG), was chosen as the a priori primary ROI.
2.5. Secondary ROIs
Because APOE ε4 has been found to preferentially influence MTL gray matter more negatively than parietal or frontal regions in both healthy young samples (Knickmeyer et al., 2013; Shaw et al., 2007) and AD samples (e.g., Pievani et al., 2009; Wolk et al., 2010), two MTL regions near the hippocampus were examined using FreeSurfer (FS 4.5) image analysis suite: the ERC and the posterior PHG (labeled by FreeSurfer as “EC” and “PHG” respectively). Details about FS 4.5 are described at http://surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalChangeLog (Reuter et al., 2012).
2.6. Statistical analyses
To adjust for differences in head size, each ROI was normalized by dividing the subject’s ROI volume by that subject’s Total Intracranial Volume (TIV). To convey results that are more meaningful than proportions, the ROI/TIV values were multiplied by the median TIV of the sample. For the longitudinal data analysis of each normalized ROI, we used a mixed-effects growth model (Singer and Willett, 2003), which can provide information about a developmental trajectory in terms of the estimated initial starting point (intercept “Ii”) and rate of change (slope “S”). We modeled effects of APOE ε4 and BDNF met status on initial volume “Ii” and on rate of change over age “S.” We assumed a linear age trend; inclusion of an age*age term did not significantly improve the fit of the growth model (results not reported). Thus, there were three independent variables—age (at time of MRI), APOE ε4, and BDNF met status. Age was centered at 61.25 yrs, the median age of this sample at the first scan. APOE ε4 status (ε3/ε3 vs. ε4 carrier) and BDNF met status (met/val vs. val/val) were centered around zero as −.5 and +.5 (Kraemer and Blasey, 2004). In the results below, the βI1 term is an estimate of how much APOE ε4 carriers and ε3 homozygotes differ in initial volume “Ii”. The βS1 term is an estimate of how much APOE ε4 carriers and ε3 homozygotes differ in rate of change over age “S” (i.e., the interaction of APOE with age). Analogously, the βI2 and βS2 terms indicate how much BDNF met carriers and noncarriers differ in initial volume and in change over age. To examine sex effects, the model parameters were initially allowed to reflect the influence of sex. There were no significant effects of sex or interactions of sex with age (Fs < 1; data not shown), so sex was dropped as a covariate in the final models of hippocampal, ERC and posterior PHG volume. Growth models were fit using the PROC MIXED procedure in SAS software, version 9.3 (SAS Institute, Cary, NC).
3. Results
3.1. Sample Characteristics
Table 1 summarizes baseline characteristics of the participants, separated by APOE ε4 carrier status. There were no significant differences between APOE ε4 carriers and ε3 homozygotes in memory performance (p = .29), as measured by the Rey Auditory Verbal Learning Test delayed recall score (Rey, 1958). No participant performed > 1.5 SDs below the mean of normative data (Geffen, 1995; Ivnik et al., 1992) (range of raw scores = 4 to 15 words recalled; percentile range = 14.5 to 99th percentile). A greater proportion of women were represented in the ε3/ε3 group than the ε4 carrier group, p = .049. Otherwise, the groups were comparable in terms of age, family history of dementia, years of education, and health-related variables; 96.8 % of all participants reported their health to be “good” or “excellent.”
Table 1.
Baseline demographic and health characteristics of the 56 participants, grouped according to APOE status.
| Characteristic |
APOE
ε3/ε3 homozygotes n = 31 |
APOE ε4
carriers n = 25 |
|---|---|---|
| Age, M ± SD | 62.2 ± 6.3 | 59.4 ± 5.7 |
| (range in years) | (51 – 75) | (51 – 70) |
| Rey AVLT delayed recall, M ± SD | 10.2 ± 2.8 | 9.4 ± 2.9 |
| % Family history of dementia: “yes/no/not sure” | 29/61/10% | 32/64/4% |
| Years of education, M ± SD | 16.9 ± 1.6 | 17.6 ± 2.0 |
| Women, n (%) | 9 (29%) | 2 (8%) |
| % Self-rated health “excellent/good/fair” | 42/55/3% | 44/52/4% |
| % Cholesterol-lowering medications | 23% | 32% |
| % Anti-hypertensive medications | 29% | 28% |
| BDNF val/val, n (%) | 18 (58%) | 12 (48%) |
| n (%) with multiple scans | 19 (61%) | 20 (80%) |
| Duration of follow-up (years)‡ | 3.2 ± 1.0 | 3.3 ± 1.1 |
Rey Auditory Verbal Learning Test (Rey, 1958), delayed recall score (max = 15 words).
SD: standard deviation
Subjects in this category had more than one scan.
Thirty-nine (69.6%) participants had multiple MRIs; the average interval from the first to the last scan was 3.3 ± 1.0 years (total 116 scans). Participants with follow-up memory assessments continued to perform within 1.5 SD of the recall score expected for their age (range of raw scores = 2 to 15; percentile range = 8th to 99th percentile), with ε3 homozygotes (M = 10.6 ± 2.8) and APOE ε4 carriers (M = 9.2 ± 3.7) performing comparably at the last assessment, p = .20. APOE groups did not differ in the percentage that had longitudinal data, p = .13 nor did they differ in the mean length of follow-up, p = .18.
3.2. Hippocampal Volume
Table 2 lists the parameter estimates of the mixed-effects growth model. The parameter βI1 is a test of the effect of APOE ε4 on ‘Initial Volume (Ii).’ The APOE ε4 group had significantly smaller initial hippocampal volumes than the ε3 homozygotes (βI1 = −0.25 cc; p = .042; estimated standardized effect size “d” = −.56). As illustrated in Figure 1, the mean trend line of the APOE ε4 group lies below that of the ε3/ε3 group. There was significant age-related atrophy in the sample as a whole (mean Change over Age ηS = −0.051 cc per year; p <0.0001, equivalent to 1% volume loss per year). The rate of atrophy did not differ significantly between ε4 carriers and ε3 homozygotes (βS1 = 0.006 cc; p =.74; d = .09). As can be seen in Figure 1, the mean trend lines for APOE ε4 carriers and ε3 homozygotes are nearly parallel (n.s. interaction of APOE with age). BDNF met status did not predict initial hippocampal volume or rate of atrophy, as indicated by the nonsignificant βI2 and βS2 terms in Table 2.
Table 2.
Growth Curve Analysis of Hippocampal Volume in Relation to APOE, BDNF and Age.a
| Parameter Estimate (SE) |
p | |
|---|---|---|
| Initial Volumeb (Ii) | ||
| Intercept (mean, ηI) | 5.04 (0.06) | <0.0001 |
| APOE (βI1) | −0.25 (0.12) | 0.042 |
| BDNF (βI2) | −0.01 (0.12) | 0.93 |
| Change over Agec (S) | ||
| Intercept (mean, ηS) | −0.051 (0.01) | <0.0001 |
| APOE (βS1) | 0.006 (0.02) | 0.74 |
| BDNF (βS2) | 0.002 (0.02) | 0.92 |
The model for the outcome at a given age was: Yit = Ii + S*(age centered) + eit, where the outcome Y for individual i at age t is a function of random initial level Ii . The residual eit is assumed to be normally distributed.
Fig. 1.
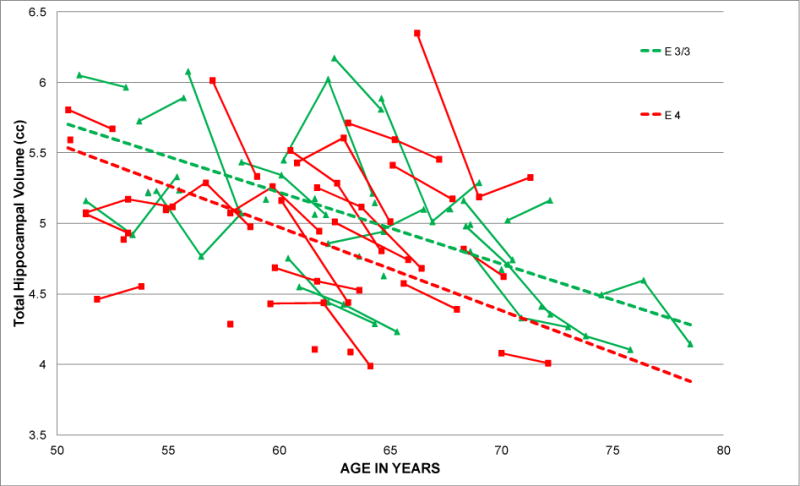
Observed individual trajectories and model-estimated mean trajectories for APOE ε4 carriers and ε3/ε3 homozygotes
The parameter estimates of the model can be used to predict total hippocampal volume for a particular combination of age and APOE ε4 values (.5, if APOE ε4 or −.5 if non ε4). The predicted hippocampal volume at the midpoint age of 61 years is 5.04 cc (ηI). At age 61, APOE ε4 carriers have an expected mean volume of 4.92 cc, and noncarriers have an expected volume of 5.17 cc. (Initial Level (I) = ηI + βI1; where ηI = 5.04 cc and βI1 = −0.25*(+.5. if APOE ε4 or −.5 if non ε4). This APOE ε4-related difference of 0.25 cc in hippocampal volume is roughly equivalent being five years older (ηS * 5 years, or −0.05 cc/year * 5 years = −0.25 cc).
3.3. Secondary ROIs
There were no significant APOE-related decreases in initial volumes of the ERC or the posterior PHG (p’s > .30); nor were there APOE ε4 effects on rates of atrophy (p’s > .30). The BDNF val/val group had larger initial posterior PHG volumes on average than the val/met group (βI2 = 0.52 cc; SE = 0.16, p = .0025) relative to the midpoint of 5.01 cc. Val/val status did not slow the rate of age-related atrophy in either ROI, p’s > .9, although, in the sample as a whole, significant age-related atrophy was detected (p’s <0.004). For the ERC, the average yearly loss was 0.028 cc (0.66%); for the posterior PHG, average loss was 0.038 cc/yr (0.75%).
4. Discussion
APOE ε4 carriers had hippocampal volumes that were 5% smaller than ε3 homozygotes on average, with no evidence of a significantly greater rate of atrophy with increasing age. This pattern of APOE ε4 differences in MTL gray matter is analogous to that observed in a longitudinal study of adolescents, in which the ERC was 4% thinner in APOE ε4 carriers, with no evidence of accelerated thinning across ages 11 to 20 years (Shaw et al., 2007). Less MTL gray matter in association with APOE ε4 has also been reported in cross-sectional studies of neonates (Knickmeyer et al., 2013), young adults (O’Dwyer et al., 2012), and healthy middle-aged to older adults (den Heijer et al., 2002; Potkin et al., 2009; Wishart et al., 2006). Often, MTL gray matter differences related to the ε4 allele have been subtle in that reductions were reported only for the right hippocampus (Lind et al., 2006; Tohgi et al., 1997), only among ε4 homozygotes (Lemaitre et al., 2005), or only in some MTL subregions, specifically the dentate gyrus, CA3 (Mueller et al., 2008), ERC and subiculum (Burggren et al., 2008). The subtleness is also reflected by many nonsignificant trends (e.g., Chen et al., 2012; Richter-Schmidinger et al., 2011; Schmidt et al., 1996; Tupler et al., 2007). In the present study, longitudinal MRI data helped model the effects of APOE ε4 (and aging) with greater power.
One mechanism of APOE ε4 that could account for reduced volume at any early age is decreased hippocampal neurogenesis. As demonstrated in a series of experiments examining GABAergic interneuron function in apoE4 Knock-In adult mice, neurons in the dentate gyrus did not mature normally or completely (Li et al., 2009). The SNT method we used for hippocampal voluming includes the dentate gyrus, CA1-3 and subiculum. The dentate gyrus, CA3, subiculum and ERC regions appear to be differentially affected in APOE ε4 carriers (Burggren et al., 2008; Mueller et al., 2008). In contrast to SNT, FreeSurfer parcellates the entire brain and is less specific to the hippocampal formation; it generates a larger hippocampal volume that includes not only the dentate gyrus, CA1-3 and subiculum, but also additional white and gray matter (fimbria, alveus, infralimbic gyrus, and parts of the amygdala and parahippocampal gyrus) (Schuff et al., 2009a). SNT is well suited to detect subtle effects of APOE ε4 on hippocampal volume.
Even though APOE ε4 carriers had significantly smaller initial hippocampal volumes than ε3 homozygotes, the rates of hippocampal atrophy did not differ significantly in this study. In the current model of AD biomarkers (Jack et al., 2010), initial deposition of Aβ is crucial for the rate of MRI atrophy to increase. Thus, Aβ deposition alone defines “Stage 1- Preclinical AD” and abnormal MRI atrophy is “Stage 2 – Preclinical AD” per recent guidelines for research on preclinical AD (Sperling et al., 2011). Based on these guidelines and estimates of the age-specific prevalence of Aβ positivity (Aβ+) among APOE ε4 carriers and noncarriers, the majority of our participants were too young to have preclinical AD at the time of their MRIs. Recent amyloid imaging data suggests that Aβ+ is rare among cognitively normal noncarriers younger than 75 (Fleisher et al., 2013). Among cognitively normal APOE ε4 carriers, the prevalence of Aβ+ may be 10 to 15% among 50–59 year-olds (Fleisher et al., 2013; Morris et al., 2010); rising to around 50% among 60–79 year-old APOE ε4 carriers (Fleisher et al., 2013; Morris et al., 2010; Reiman et al., 2009; Rowe et al., 2010). By applying these prevalence rates to the age distribution of the ε4 carriers in our study cohort, we could estimate that two-thirds of the ε4 carriers would not have had preclinical AD at the time of their MRIs. By this reasoning and the current rubric for biomarkers of preclinical AD, the non-significant effect of APOE ε4 on the rate of MRI atrophy is not surprising. Two important limitations of the present study are the modest sample size, which limits statistical power to detect an APOE ε4 difference in the slope assessing MRI atrophy rate, and the lack of amyloid imaging to assess preclinical AD.
The likelihood of detecting an effect of APOE ε4 on rate of hippocampal atrophy would be expected to be higher in older-age samples, in line with the increasing prevalence of preclinical AD. Indeed, there are several reports on older nondemented subjects in which hippocampal change was greater in APOE ε4 carriers (mean age 76) (Chiang et al., 2011; Jak et al., 2007; Morra et al., 2009; Risacher et al., 2010). The literature with regard to older-age samples is not entirely consistent, as one study detected faster atrophy only in APOE ε4 homozygotes (Crivello et al., 2010) and three studies did not detect atrophy-rate differences between ε4 carriers and noncarriers (Du et al., 2006; Jack et al., 1998; Schuff et al., 2009b). Also, some studies of late middle-aged subjects have reported significantly greater atrophy in APOE ε4 carriers (e.g., Cohen et al., 2001). It is worth noting however, that these studies may have had greater sensitivity to detect differences, in that APOE ε2/ε3 subjects were selected as the non-carrier group (Lu et al., 2011), or the ε4 carrier and noncarrier groups were enriched with APOE ε4/ε4 and APOE ε2/ε3 subjects, respectively (Moffat et al., 2000). Collectively, the MRI literature is reasonably consistent with the notion that hippocampal atrophy rate is age- and genotype-dependent in tandem with risk for preclinical AD.
Early interventions that increase tissue reserve in regions such as the hippocampus could potentially delay the onset of AD symptoms. Interventions that have potential to increase hippocampal volume include aerobic exercise and cognitive stimulation (Fotuhi et al., 2012). Two controlled trials of exercise interventions have demonstrated increases in hippocampal volume (Erickson et al., 2011; Pajonk et al., 2010). Because the BDNF val66met polymorphism is relevant to tissue reserve, it was included as a covariate in the mixed-effects growth model. No effect of BDNF val66met on hippocampal volume was detected. A recent meta-analysis reported an effect size of 0.41 for the effect of BDNF on hippocampal volume (Hajek et al., 2012), suggesting that the present study was not powered to detect a significant effect of BDNF on hippocampal volume. The BDNF val/val group had more MTL volume near the hippocampus, consistent with studies of young individuals (e.g., Knickmeyer et al., 2013). Even though the effect size of BDNF val66met is small (Hajek et al., 2012), it would be worthwhile to include it as a covariate in future studies because BDNF val66met genotype may interact with physical activity level (Kim et al., 2011).
In conclusion, it is remarkable the same regions that are smaller in APOE ε4 carriers early in life are the regions affected in early AD. Thus, it seems that the biological effects of apoE4 are in play throughout life. Indeed, the mechanisms that lead to less gray matter density early in life may overlap with the mechanisms that lead to earlier onset of preclinical AD. One possible lifelong mechanism could be disruption of synaptic input in the presence of apoE4 (Ji et al., 2003; Koffie et al., 2012; Li et al., 2009). It has been suggested that apoE4 impairs synaptic function via its role as a co-factor that stabilizes oligomeric Aβ and directs it to synapses, leading to toxic accumulation of Aβ and synapse shrinkage (Koffie et al., 2012). Blocking oligomeric Aβ-apoE4 interactions has been offered as a therapeutic strategy for preventing this AD-related process (Koffie et al., 2012).
Acknowledgments
We express appreciation to the participants for their tremendous interest and expenditure of time and we thank Jerome A. Yesavage MD for referring them. This research was supported by the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (VA Sierra-Pacific MIRECC), War Related Illness and Injury Study Center, and the Medical Research Service; and by NIA grants R37 AG12713, P41 RR023953and R01 AG021632 (with a Diversity Supplement to Dr. Adamson). Dr. Scanlon was supported by the Office of Academic Affiliations, VA Advanced Fellowship Program in Mental Illness Research and Treatment. These sponsors solely provided financial support or facilities to conduct the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIA or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement. None of the authors have conflicts of interest related to the topic of this paper. Dr. Weiner has received honoria from NeuroVigil, Inc. and Insitut Catala de Neurociencies Aplicades (2010), PMDA /Japanese Ministry of Health, Labour, & Welfare and Tohoku University (2011), and the Alzheimer’s Drug Discovery Foundation (2012). He holds stock options in Synarc and Elan and has received commercial funding from Merck and Avid. Dr. Weiner has current or recent consulting relationships with Astra Zeneca, Araclon, Bayer Healthcare, Ipsen, Medivation/Pfizer, TauRx Therapeutics LTD, Biogen Idec, Exonhit Therapeutics-SA, Servier, Synarc, Janssen, Harvard University, and KLJ Associates. Dr. Murphy is a consultant for Brain Resource Ltd. and is listed as an inventor on a patent application regarding CRHR2 and NR3C1 polymorphisms and the risk for depression. The remaining authors have no potential financial or personal conflicts of interest including relationships with other people or organizations within 3 years of beginning the work submitted that could inappropriately influence their work. The study was approved by the Stanford University Institutional Review Board. Written informed consent was obtained from all study participants.
References
- Adamson MM, Landy KM, Duong S, Fox-Bosetti S, Ashford JW, Murphy GM, Weiner M, Taylor JL. Apolipoprotein E epsilon4 influences on episodic recall and brain structures in aging pilots. Neurobiol Aging. 2010;31:1059–63. doi: 10.1016/j.neurobiolaging.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–81. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. NeuroImage. 2008;41:1177–83. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Ayutyanont N, Langbaum JB, Fleisher AS, Reschke C, Lee W, Liu X, Alexander GE, Bandy D, Caselli RJ, Reiman EM. Correlations between FDG PET glucose uptake-MRI gray matter volume scores and apolipoprotein E epsilon4 gene dose in cognitively normal adults: a cross-validation study using voxel-based multi-modal partial least squares. Neuroimage. 2012;60:2316–22. doi: 10.1016/j.neuroimage.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang GC, Insel PS, Tosun D, Schuff N, Truran-Sacrey D, Raptentsetsang ST, Thompson PM, Reiman EM, Jack CR, Jr, Fox NC, Jagust WJ, Harvey DJ, Beckett LA, Gamst A, Aisen PS, Petersen RC, Weiner MW. Impact of apolipoprotein varepsilon4-cerebrospinal fluid beta-amyloid interaction on hippocampal volume loss over 1 year in mild cognitive impairment. Alzheimers Dement. 2011;7:514–20. doi: 10.1016/j.jalz.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen GE, Joshi SC, Miller MI. Volumetric transformation of brain anatomy. IEEE Trans Med Imaging. 1997;16:864–77. doi: 10.1109/42.650882. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57:2223–8. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crivello F, Lemaitre H, Dufouil C, Grassiot B, Delcroix N, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Effects of ApoE-epsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage. 2010;53:1064–9. doi: 10.1016/j.neuroimage.2009.12.116. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–8. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27:733–40. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. PNAS. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Liu X, Ayutyanont N, Roontiva A, Thiyyagura P, Protas H, Joshi AD, Sabbagh M, Sadowsky CH, Sperling RA, Clark CM, Mintun MA, Pontecorvo MJ, Coleman RE, Doraiswamy PM, Johnson KA, Carpenter AP, Skovronsky DM, Reiman EM. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging. 2013;34:1–12. doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- Geffen G. A compendium of neuropsychological tests: Administration, norms and commentary. 2. Oxford University Press; New York: 1995. Normative data for the Auditory Verbal Learning Test. Personal communication, cited in Spreen and Strauss. [Google Scholar]
- Hajek T, Kopecek M, Hoschl C. Reduced hippocampal volumes in healthy carriers of brain-derived neurotrophic factor Val66Met polymorphism: meta-analysis. World J Biol Psychiatry. 2012;13:178–87. doi: 10.3109/15622975.2011.580005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of Lipid Res. 1990;31:545–8. [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–10. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s older Americans normative studies: Update AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(Supplement):83–104. [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51:993–9. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dement Geriatr Cogn Disord. 2007;23:382–9. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–15. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Khachaturian AS, Corcoran CD, Mayer LS, Zandi PP, Breitner JC. Apolipoprotein E epsilon4 count affects age at onset of Alzheimer disease, but not lifetime susceptibility: The Cache County Study. Arch Gen Psychiatry. 2004;61:518–24. doi: 10.1001/archpsyc.61.5.518. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Bae KY, Kim SW, Yang SJ, Park KH, Shin IS, Yoon JS. Role of BDNF val66met polymorphism on the association between physical activity and incident dementia. Neurobiol Aging. 2011;32:551 e5–12. doi: 10.1016/j.neurobiolaging.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Wang J, Zhu H, Geng X, Woolson S, Hamer RM, Konneker T, Lin W, Styner M, Gilmore JH. Common Variants in Psychiatric Risk Genes Predict Brain Structure at Birth. Cereb Cortex. 2013 Jan 2; doi: 10.1093/cercor/bhs401. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Hashimoto T, Tai HC, Kay KR, Serrano-Pozo A, Joyner D, Hou S, Kopeikina KJ, Frosch MP, Lee VM, Holtzman DM, Hyman BT, Spires-Jones TL. Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain. 2012;135:2155–68. doi: 10.1093/brain/aws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Blasey CM. Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13:141–51. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OD, Khaleeq A, Chan W, Pavlik VN, Doody RS. Apolipoprotein E polymorphism and age at onset of Alzheimer’s disease in a quadriethnic sample. Dement Geriatr Cogn Disord. 2010;30:486–91. doi: 10.1159/000322368. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Dufouil C, Grassiot B, Tzourio C, Alperovitch A, Mazoyer B. No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. Neuroimage. 2005;24:1205–13. doi: 10.1016/j.neuroimage.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5:634–45. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson LG, Backman L, Adolfsson R, Cruts M, Sleegers K, Van Broeckhoven C, Nyberg L. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neurosci Lett. 2006;396:23–7. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- Lu PH, Thompson PM, Leow A, Lee GJ, Lee A, Yanovsky I, Parikshak N, Khoo T, Wu S, Geschwind D, Bartzokis G. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. J Alzheimers Dis. 2011;23:433–42. doi: 10.3233/JAD-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology. 2000;55:134–6. doi: 10.1212/wnl.55.1.134. [DOI] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Toga AW, Jack CR, Jr, Schuff N, Weiner MW, Thompson PM. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. Neuroimage. 2009;45:S3–15. doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of neurology. 2010;67:122–31. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer’s disease using high resolution MRI at 4 T. Neuroimage. 2008;42:42–8. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GM, Taylor J, Kraemer HC, Yesavage J, Tinklenberg JR. No association between apolipoprotein E epsilon 4 allele and rate of decline in Alzheimer’s disease. Am J Psychiatry. 1997;154:603–8. doi: 10.1176/ajp.154.5.603. [DOI] [PubMed] [Google Scholar]
- O’Dwyer L, Lamberton F, Matura S, Tanner C, Scheibe M, Miller J, Rujescu D, Prvulovic D, Hampel H. Reduced Hippocampal Volume in Healthy Young ApoE4 Carriers: An MRI Study. PLoS One. 2012;7:e48895. doi: 10.1371/journal.pone.0048895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Muller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–43. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Pievani M, Rasser PE, Galluzzi S, Benussi L, Ghidoni R, Sabattoli F, Bonetti M, Binetti G, Thompson PM, Frisoni GB. Mapping the effect of APOE epsilon4 on gray matter loss in Alzheimer’s disease in vivo. NeuroImage. 2009;45:1090–8. doi: 10.1016/j.neuroimage.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Guffanti G, Lakatos A, Turner JA, Kruggel F, Fallon JH, Saykin AJ, Orro A, Lupoli S, Salvi E, Weiner M, Macciardi F. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PLoS One. 2009;4:e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. PNAS. 2004;101:284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:6820–5. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–18. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen clinique en psychologie. Presse Universitaire de France; Paris: 1958. [Google Scholar]
- Richards B, Skoletsky J, Shuber AP, Balfour R, Stern RC, Dorkin HL, Parad RB, Witt D, Klinger KW. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum Mol Genet. 1993;2:159–63. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, Lewczuk P, Sidiropoulos C, Kneib T, Perneczky R, Doerfler A, Kornhuber J. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. 2011;118:249–57. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, Harvey DJ, Jack CR, Jr, Weiner MW, Saykin AJ. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2010;31:1401–18. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–83. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Schmidt R, Fazekas F, Semmler J, Kapeller P, Reinhart B, Kostner GM. Apolipoprotein E e4 allele in the normal elderly: neuropsychologic and brain MRI correlates. Clin Genet. 1996;50:293–9. doi: 10.1111/j.1399-0004.1996.tb02377.x. [DOI] [PubMed] [Google Scholar]
- Schuff N, Truran D, Raptentsetsang S, Buckley S, Tosun D, Insel P, Fischl B, Weiner M. ADNI hippocampal measurements, brain atrophy and cognitive decline. 2009a Retrieved from http://www.adni-info.org/Scientists/Meetings/ADNIDataPresentationsSpring2009.aspx.
- Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR, Jr, Weiner MW. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009b;132:1067–77. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, Mintun MA. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30:17035–40. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurence. Oxford University Press; New York: 2003. [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgi H, Takahashi S, Kato E, Homma A, Niina R, Sasaki K, Yonezawa H, Sasaki M. Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E epsilon4 allele. Neurosci Lett. 1997;236:21–4. doi: 10.1016/s0304-3940(97)00743-x. [DOI] [PubMed] [Google Scholar]
- Tupler LA, Krishnan KR, Greenberg DL, Marcovina SM, Payne ME, MacFall JR, Charles HC, Doraiswamy PM. Predicting memory decline in normal elderly: genetics, MRI, and cognitive reserve. Neurobiol Aging. 2007;28:1644–56. doi: 10.1016/j.neurobiolaging.2006.07.001. [DOI] [PubMed] [Google Scholar]
- von Bohlen, Halbach O. Involvement of BDNF in age-dependent alterations in the hippocampus. Front Ag Neurosci. 2010;2:36. doi: 10.3389/fnagi.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart Ha, Saykin aJ, McAllister TW, Rabin La, McDonald BC, Flashman La, Roth RM, Mamourian aC, Tsongalis GJ, Rhodes CH. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67:1221–4. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC, the Alzheimer’s Disease Neuroimaging Initiative Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional – executive network function in Alzheimer’s disease. PNAS. 2010;107:10256–61. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]


