Abstract
Background
Public concerns regarding the safety of transfused blood have prompted reconsideration of the indications for the transfusion of allogeneic red cells (blood from an unrelated donor), and a range of techniques designed to minimise transfusion requirements.
Objectives
To examine the evidence for the efficacy of pre‐operative autologous blood donation (PAD) in reducing the need for perioperative allogeneic red blood cell (RBC) transfusion.
Search methods
Articles were identified by searches of the electronic databases; MEDLINE (January 1950 to July 2009), EMBASE (January 1980 to Week 31, 2009), ISI Web of Science (inception to August 2009), The Cochrane Library 2009, Issue 3, and The Cochrane Injuries Group Specialised Register (searched August 7 2009). Reference lists in relevant publications were checked and authors were contacted to identify additional studies. The searches were updated in August 2009.
Selection criteria
Randomised controlled trials with a concurrent control group in which adult patients, scheduled for non‐urgent surgery, were randomised to PAD, or to a control group who did not receive the intervention.
Data collection and analysis
Data were independently extracted and the risk of bias was assessed. Relative risks (RR) and mean differences (MD) with 95% confidence intervals (CIs) were calculated. Data were pooled using a random‐effects model. The principal outcomes were the proportion of patients exposed to allogeneic red blood cells (RBCs) and the amount of blood transfused. Other clinical outcomes are detailed in the review.
Main results
Fourteen trials were included. Overall PAD reduced the risk of receiving an allogeneic blood transfusion by a relative 68% (RR 0.32; 95% CI 0.22 to 0.47). The absolute reduction in risk of allogeneic transfusion was 44% (risk difference (RD) ‐0.44; 95% CI ‐0.68 to ‐0.21). In contrast, the results show that the risk of receiving any blood transfusion (allogeneic and/or autologous) is increased by PAD (RR 1.24; 95% CI 1.02 to 1.51). There was evidence of significant heterogeneity for both of these outcomes.
Authors' conclusions
Although the trials of PAD showed a reduction in the need for allogeneic blood, the methodological quality of the trials was poor and the overall transfusion rates (allogeneic and/or autologous) in these trials were high, and were increased by recruitment into the PAD arms of the trials. This raises questions about the true benefit of PAD. In the absence of large, high quality trials using clinical endpoints, it is not possible to say whether the benefits of PAD outweigh the harms.
Plain language summary
Not certain that people are better off giving their own blood before surgery in case they need transfusion, when there is a safe blood bank
Although in developed countries the safety of blood supplies is high, there is still concern about contracting illness from transfusion. People often give their own blood before surgery for use if transfusion is needed (autologous donation). However, the review of trials found that it is not certain that people benefit. While pre‐operative donation may reduce the chances of needing someone else's blood, it increases the chances of transfusion overall. It may be that donation causes some anaemia (low red blood cells), or surgeons are more likely to transfuse if autologous blood is available. Over‐transfusion has risks, especially for older people.
Background
Public concern regarding the safety of transfused blood has prompted a reconsideration of the role of allogeneic red blood cell transfusion (whole blood or packed red cells from an unrelated donor). The risks associated with receiving transfusion of allogeneic blood that has been screened by a competent blood transfusion program are considered minimal, with very low risks of transmission of HIV, and hepatitis C (Whyte 1997). However, this only applies where there is a safe, plentiful, well‐regulated supply. The majority of the world's population does not have access to such a system, and the risks of transfusion in developing countries may be much higher (McFarland 1997). Concerns of patients and clinicians regarding blood safety have generated enthusiasm for the use of technologies intended to reduce the need for allogeneic blood (Bryson 1998; Forgie 1998; Huet 1999; Laupacis 1997; Laupacis 1998). Some of the alternatives to allogeneic blood have their own risks, and are expensive (Coyle 1999; Fergusson 1999a).
Generally, such interventions fall into two groups: (1) techniques for reinfusing a patient's own blood (pre‐operative autologous donation, acute normovolemic haemodilution, cell salvage), and (2) the administration of agents to diminish blood loss (aprotinin, tranexamic acid, epsilon aminocaproic acid, fibrin sealant) or to promote red blood cell production (erythropoietin). Erythropoietin (EPO) may be used in association with autologous blood donation.
Pre‐operative autologous donation (PAD) is applicable to patients scheduled for elective surgery who are judged likely to have blood losses that require red blood cell replacement. PAD involves the patient donating one or more units of his/her own blood pre‐operatively; this blood is held within the blood bank where it is available to the anaesthetist to administer during or after surgery.
This review examines the clinical evidence of the efficacy of pre‐operative autologous blood donation (PAD) in reducing the need for allogeneic blood transfusion in elective surgery, and whether there is a greater reduction in allogeneic blood transfusion requirements in identifiable patient sub‐groups. The review uses methods developed by the Cochrane Collaboration, and the International Study of Perioperative Transfusion (ISPOT) study group (a ten‐country study of evidence, attitudes and practices relating to the use of alternatives to perioperative allogeneic blood transfusion) (Fergusson 1999b). More detailed consideration of the benefits and harms of allogeneic red cell transfusion and the advantages of avoiding transfusion is provided in an accompanying review of the efficacy of anti‐fibrinolytic drugs (Henry 2001).
Objectives
To examine the evidence for the efficacy of PAD in reducing allogeneic blood transfusion and the evidence for any effect on clinical outcomes, such as mortality and infection rates.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials with a concurrent control group.
Types of participants
The study participants were adults (over 18 years). Trials were included if participants aged less than 18 years were enrolled, but the type of surgery was predominantly carried out in adult patients. The surgery performed was elective or non‐urgent.
Types of interventions
The intervention considered was pre‐operative autologous blood donation (PAD).
Types of outcome measures
Primary outcomes
the proportion of patients who were transfused with allogeneic blood or any blood transfusion (allogeneic or autologous), and the amounts of allogeneic and autologous blood transfused.
Secondary outcomes
adverse transfusion reactions; pre‐operative morbidity; pre‐operative haemoglobin levels, reported postoperative complications (thrombosis, infection), and mortality.
Search methods for identification of studies
The searches were last updated in July and August 2009.
Electronic searches
This review drew on a literature search that was constructed as part of the International Study of Perioperative Transfusion (ISPOT) (Laupacis 1997) which identified all articles containing any of the following terms: aprotinin, ddAVP, desmopressin acetate, I‐desamino‐8‐o‐arginine vasopressin, tranexamic acid, e‐aminocaproic acid, or 6‐aminocaproic acid. We have subsequently searched the following electronic databases;
Cochrane Injuries Group Specialised Register ‐ searched 7th August 2009.
Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2009, Issue 3.
MEDLINE (Ovid SP) ‐ January 1950 to July (week 5) 2009.
EMBASE (Ovid SP) ‐ January 1980 to week 31, 2009.
Current Contents ‐ inception to January 2004.
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) ‐1970 to August 2009.
ISI Web of Science: Conference Proceedings Citation Index‐ Science (CPCI‐S) ‐ 1990 to August 2009.
using the search strategies reported in Appendix 1
The websites of international health technology assessment agencies were searched to January 2004, through the International Network of Agencies of Health Technology Assessment (INAHTA), and the International Society of Technology Assessment in Health Care (ISTAHC).
Searching other resources
We contacted experts in the field to identify reports, or projects in progress, relevant to the review and checked the reference lists of published reviews and articles for relevant trials. In addition, we checked reference lists in the identified trials and contacted authors, where possible, to identify any additional published or unpublished data.
Data collection and analysis
Selection of studies
The titles and abstracts identified in the electronic searches were screened independently by two authors (by one author for the 2009 update). To be eligible for inclusion, studies had to include adult patients, scheduled for elective non‐urgent surgery, who were randomised to PAD or to a control group who did not receive PAD. To be eligible study reports had to have collected data on the proportion of patients transfused with red blood cells or the volumes of blood transfused.
Data extraction and management
Two authors (one author for the 2009 update) independently selected trials that met the defined inclusion criteria, with disagreements resolved by consensus.
One author performed data extraction. The following information was recorded on the data extraction form: the number of patients exposed to allogeneic blood; the amount of allogeneic blood transfused; the number of patients receiving any transfusion (allogeneic blood and/or autologous blood); the number of patients experiencing postoperative complications (thrombosis, infection) and mortality. Data were collected on pre‐operative haemoglobin levels when this was reported. Information regarding demographic characteristics (age, sex), the type of surgery, and the presence or absence of a transfusion protocol was also recorded. Data were extracted for allogeneic blood transfusion if it was expressed as whole blood or packed red cells. Information regarding the use of fresh frozen plasma (FFP) and/or platelets was also recorded.
Data were entered into the Review Manager database by one author. Articles identified as duplicate publications were combined to obtain one set of data. The report with the greatest number of patients for that study was then represented in the analysis. Data on the numbers of patients exposed to allogeneic blood, and the numbers of patients in each treatment group, were entered into Review Manager. The relative risks (RR) for allogeneic blood transfusion in the intervention group as compared with the control group, and the corresponding 95% confidence intervals, were calculated for each trial.
Assessment of risk of bias in included studies
This was assessed by one author using the Cochrane Collaboration's tool for assessing risk of bias presented in Higgins 2008. The following domains were assessed for each study;
sequence generation,
allocation concealment,
blinding,
intention‐to‐treat analysis.
We completed a risk of bias table for each study, incorporating a description of the study's performance against each of the above domains and our overall judgment of the risk of bias for each entry as follows; 'Yes' indicates low risk of bias 'Unclear' indicates unclear or unknown risk of bias 'No' indicates high risk of bias.
Measures of treatment effect
The treatment effects (relative risks/mean differences) were pooled across trials using the random‐effects model. The risk difference and number needed to treat were also calculated for exposure to allogeneic blood transfusion.
Assessment of heterogeneity
Statistical heterogeneity was examined by both the I2 statistic and Chi2 test. The I2 statistic describes the percentage of total variation across studies due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity; substantial heterogeneity is considered to exist when I2 > 50% (Higgins 2008). For the Chi2 statistic, a P value of < 0.10 was used to indicate the presence of statistically significant heterogeneity.
Subgroup analysis and investigation of heterogeneity
Analysis of subgroups of trials was performed to determine whether effect sizes varied according to the type of surgery and use of transfusion protocols. In practice these subgroup analyses were constrained by the small number of trials.
Results
Description of studies
Fourteen eligible studies were identified and included in the analysis. The included trials were conducted in Canada (n = 1), Germany (n = 3), Greece (n = 2), Japan (n = 1), Sweden (n = 3), The Netherlands (n = 2), and the USA (n = 2). Six trials involved orthopaedic surgery, four involved curative surgery for colorectal cancer, two involved liver surgery, one involved maxillofacial surgery and one involved cardiac surgery.
Autologous donation range
The volume of blood donated by each participant varied. Seven trials used predonation of two units of autologous blood and one trial evaluated predonation of three units (450 mL per unit) of autologous blood. The remaining trials studied a range of predonation volumes. In the trial conducted by Kajikawa 1994, 2‐6 units (200 mL per unit) were withdrawn from each patient prior to surgery. In the trial conducted by Elawad 1991, three study groups of 15 patients each donated on average between 2.8 to 3.0 units each prior to surgery. In the trial conducted by Ekback 1995 study participants predonated 2‐3 units of autologous blood within six weeks prior to surgery. In Bezwada 2003 two units were predonated for participants undergoing bilateral or revision arthroplasty and one unit for those undergoing primary unilateral arthroplasty. Participants in Billote 2002 predonated two units, although two patients only donated one unit. In Christopoulou 2001 24 patients predonated two units while four others undergoing bimaxillary osteotomies, donated two units. In Bouchard 2008 two units of 350 mL were predonated.
Transfusion 'triggers'/thresholds
Of the 10 trials that reported the use of transfusion protocols, all included a transfusion 'trigger'. The transfusion threshold or 'trigger' was either the haemoglobin or erythrocyte volume fraction (EVF) value at which point a transfusion of autologous and/or allogeneic blood was considered appropriate. There was significant variation between trials in the transfusion threshold value used. Lorentz 1991 and Kostopanagiotou 2007 commenced blood transfusion if the haemoglobin of the patient fell to less than 9.0 g/dL in the operating room, or less than 10.0 g/dL in the intensive care unit postoperatively. Heiss 1997 commenced blood transfusion if the haemoglobin of the patient fell to less than 9.0 g/dL. Hoynck 1992 commenced blood transfusion when the haemoglobin concentration repeatedly fell below 10.5 g/dL. Busch 1993 initiated blood transfusion if blood loss exceeded 500 mL or when the haemoglobin concentration fell below 10.5 g/dL. In the trial conducted by Heiss 1993 blood transfusion was recommended when the haemoglobin level fell below 10.0 g/dL. In the trial conducted by Ekback 1995 study participants were transfused to correct the EVF to greater than 27%. Bezwada 2003 initiated blood transfusion if the haemoglobin level reached 80 g/L or less and/or there was persistent tachycardia or hypotension requiring large volumes of crystalloid. In Billote 2002 a need for red blood cell transfusion was indicated when haemoglobin concentration reached less than 70 g/L in healthy patients or less than 80 g/L in patients with co‐morbidities (e.g. heart disease or peripheral vascular disease), or less than 100 g/L in the postoperative period. Bouchard 2008 commenced transfusion when the perioperative haemoglobin fell below 60 g/L or the postoperative level fell below 80 g/L.
Predonation time frames
Details are presented in Table 1.
1. Pre‐donation time frames.
| Trial | Units donated | Donation regimen |
| Bezwada 2003 | 1‐2 units | No information reported. |
| Billote 2002 | 2 units | Last unit donated no later than 2 weeks prior to surgery. |
| Bouchard 2008 | 2 units | One unit was donated weekly. Units were stored for a maximum of 35 days. |
| Busch 1993 | 2 units | A 72‐hour minimum between donations and the second donation occurred no later than 5 days before surgery. |
| Christopoulou 2001 | 1‐2 units | One week between donations. Surgery performed at least one week after the last donation. |
| Ekback 1995 | No information reported. | No information reported. |
| Elawad 1991 | 2‐3 units | Donated 31‐40 days before surgery with 9‐16 days between each phlebotomy. |
| Hedstrom 1996 | 2 units | Donated 4 weeks and 2 weeks before surgery. |
| Heiss 1993 | 2 units | Donated on 7th and 10th days prior to surgery. |
| Heiss 1997 | 2 units | Donated on 7th and 10th days prior to surgery. |
| Hoynck 1992 | 2 units | Donated 7‐14 days before surgery with a 3‐day minimum between donations. |
| Kajikawa 1994 | 1‐3 units | Donated 2‐3 weeks before surgery. |
| Kostopanagiotou 2007 | 2 units | No information reported. |
| Lorentz 1991 | 3 units | First donation 5 weeks before surgery. |
In the trial by Lorentz 1991 surgery was performed in the fifth week after the first donation of autologous blood. In two trials (Heiss 1993; Heiss 1997), study participants donated two units on the 7th and 10th days prior to surgery. In the trial conducted by Hedstrom 1996 study participants donated four weeks and two weeks prior to surgery. Study participants in the Elawad 1991 trial donated 31 to 40 days before operation, with nine to 16 days between each phlebotomy. In the trial conducted by Hoynck 1992 participants donated two units, seven to 14 days before the operation, with at least a three‐day interval between donations. In the trial conducted by Busch 1993 patients assigned to the autologous‐transfusion group were required to donate blood twice. The minimum interval between the two donations was 72 hours, and the second donation had to occur no later than five days before surgery. In the trial conducted by Kajikawa 1994 blood was collected once or twice weekly (400 to 1200 mL, total) from each participant, two to three weeks before surgery. Ekback 1995 did not describe predonation procedures. In Billote 2002, whole blood was donated once a week with the final unit collected no later than two weeks prior to surgery. Participants in the trial by Christopoulou 2001 donated at least one week before the operation and the time between donations was one week. Bouchard 2008 reports that patients donated blood weekly and that blood was stored for a maximum of 35 days. There was no information presented regarding predonation time frames in the reports for Bezwada 2003 or Kostopanagiotou 2007.
Iron supplementation
Details are presented in Table 2.
2. Summary of iron supplementation.
| Trial | Dose (mg) | Route/frequency | Commenced/ceased |
| Bezwada 2003 | 325 mg† | Oral, three times daily | No information reported. |
| Billote 2002 | 325 mg† | Oral, twice daily | Started after the first donation (PAD group). |
| 325 mg† | Oral, twice daily | 10 days prior to surgery (Control group). | |
| Bouchard 2008 | 300 mg† | Oral, three times daily | For the period of recruitment and surgery. |
| Busch 1993 | No information reported. | No information reported. | Received iron supplementation immediately after randomisation. |
| Christopoulou 2001 | 150 mg† | Oral, once daily | Ceased one week post‐operation. |
| Elawad 1991 | 100 mg† | Oral, three times daily | Started after the first phlebotomy. |
| 100 mg + 5 mg folate | Oral, three times daily | Started after the first phlebotomy. | |
| Hedstrom 1996 | 100 mg | Oral, twice daily | Commenced after the first donation until the day of the operation. |
| Heiss 1993 | 100 mg† | Oral, twice daily | No information reported. |
| Hoynck 1992 | 200 mg‡ | Oral, three times daily | Started before the first donation. |
| Kajikawa 1994 | 80 mg† | IV | Started on day of blood collection. |
†Ferrous sulphate
‡Ferrous fumarate
Ten trials reported giving iron supplementation to study participants in conjunction with pre‐operative autologous blood donation. All patients in the trial conducted by Hedstrom 1996, received iron supplementation, consisting of 100mg of iron given orally, twice a day, after the first donation until the day of the operation. The trial conducted by Elawad 1991 studied four groups of participants, two of which were exposed to iron supplementation. One of these groups received iron supplementation as ferrous sulfate 100 mg three times daily, starting after the first phlebotomy, and the other received the iron supplementation plus folate supplementation as folate 5 mg three times daily, starting after the first phlebotomy. In the trial conducted by Hoynck 1992 patients allocated to the autologous blood donation program were commenced on iron supplementation (200 mg of ferrous fumarate three times daily), before the first donation. In the trial conducted by Heiss 1993 patients in the autologous blood transfusion group received iron supplementation in the form of oral ferrous sulphate, 100 mg twice daily. Busch 1993 treated patients randomly allocated to the autologous‐transfusion group, to oral iron supplementation, which they received immediately after randomisation. The dose and frequency were not detailed. Kajikawa 1994 treated those participants who donated autologous blood with 80 mg of iron sulphate and 500 mL of Ringer's lactate solution intravenously on the day of blood collection, followed by 100 mg of iron sulphate given orally once daily until the operation. In Billote 2002 all patients received 325 mg of oral ferrous sulphate; twice daily after the first donation in the PAD group and 10 days prior to surgery in the control group. In Bezwada 2003 the PAD group received oral supplementation of 325 mg iron sulphate three times a day. In Christopoulou 2001 all patients received 150 mg ferrous sulphate orally each day until one week post‐operation. Bouchard 2008 gave patients in the PAD group 300 mg ferrous sulfate orally, three times daily throughout the period between recruitment and surgery.
Risk of bias in included studies
For further details regarding the performance of the studies against each domain, please see the 'Risk of bias' tables. A summary of the information in the tables is given below. Additionally, a visual summary of judgements about each methodological quality item for each included trial is shown in Figure 1.
1.
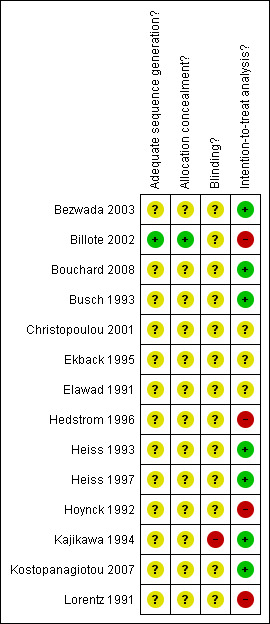
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Adequate sequence generation
The risk of bias for this item was judged to be low for one trial (Billote 2002) which used computer randomisation to allocate patients. The remaining 13 trials presented no information regarding the method of sequence generation and were rated as unclear.
Allocation concealment
The risk of bias for this item was judged to be low for one trial (Billote 2002) which used sequentially sealed envelopes. The remaining 13 trials presented no information regarding the allocation concealment and were rated as unclear.
Blinding
None of the trials were judged to be at low risk of bias for blinding, 13 were rated as being unclear and one (Kajikawa 1994) was rated as being at high risk of bias for this item.
Intention‐to‐treat
Seven trials were judged to have collected outcome data on an intention‐to‐treat basis and to be at low risk of bias, three were rated as being unclear and four were judged to be at high risk of bias.
Effects of interventions
Data from 13 of the 14 included trials were suitable for the pooled analysis.
Exposure to allogeneic blood transfusion
Thirteen trials reported data on the number of participants exposed to allogeneic blood transfusion. These trials included a total of 1506 participants, of whom 760 were randomised to pre‐operative autologous donation (PAD). Of the 760 patients randomised to PAD, the majority (n = 486) donated their blood prior to cancer surgery. Overall, PAD reduced the risk of allogeneic blood transfusion by a relative 68% (RR 0.32; 95% CI 0.22 to 0.47). Heterogeneity between these trials was statistically significant (Chi2 = 36.43, df = 11, P = 0.0001; I2 = 70%). The absolute reduction in risk of allogeneic transfusion exposure was 44% (RD ‐0.44; 95% CI ‐0.68 to ‐0.21). On average 2.3 patients would need to undergo PAD so that one would avoid allogeneic transfusion (NNT 2.27; 95% CI 1.47 to 4.76).
Exposure to any blood transfusion (allogeneic and/or autologous)
When all transfusions were considered, that is the proportion of patients exposed to allogeneic and/or autologous transfusion, the risk of receiving any transfusion (allogeneic and/or autologous) in those randomised to PAD compared to control was 1.24 (95% CI 1.02 to 1.51). Overall, 78% of patients randomised to PAD received a blood transfusion. Heterogeneity between these trials was statistically significant (Chi2 = 87.53, df = 10, P < 0.00001; I2 = 89%).
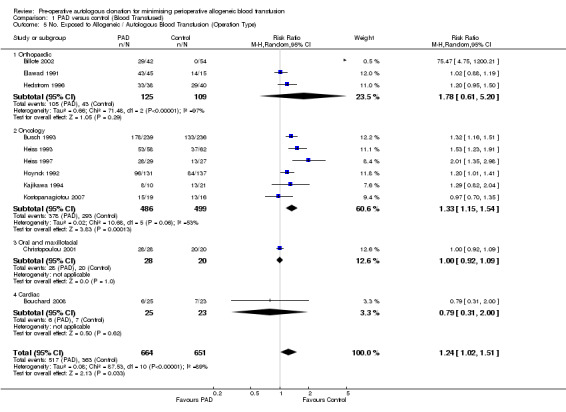
Type of surgery
Six trials of PAD involved surgery for cancer. These trials included a total of 985 participants, of whom 486 were randomised to PAD. The pooled relative risk of exposure to allogeneic blood transfusion in those patients randomised to PAD was 0.48 (95% CI 0.35 to 0.66) compared to control. Heterogeneity between these trials was statistically significant (Chi2 = 11.12, df = 5, P = 0.05; I2 = 55%).
Five trials of PAD involved patients preparing for orthopaedic surgery. These trials included a total of 425 participants of whom 221 were randomised to receive PAD. The pooled relative risk of exposure to allogeneic blood transfusion for those randomised to PAD was 0.21 (95% CI 0.11 to 0.43). Heterogeneity between these trials was statistically significant (Chi2 = 6.97, df = 3, P = 0.07; I2 = 57%).
One trial of PAD involved 48 patients undergoing maxillofacial surgery, 28 of which were randomised to PAD. The relative risk of exposure to allogeneic blood transfusion was 0.02 (95% CI 0.00 to 0.28).
One trial of PAD involved 48 patients undergoing cardiac surgery, 25 of which were randomised to PAD. The relative risk of exposure to allogeneic blood transfusion was 0.06 (95% CI 0.00 to 1.02).
Effect of transfusion protocols
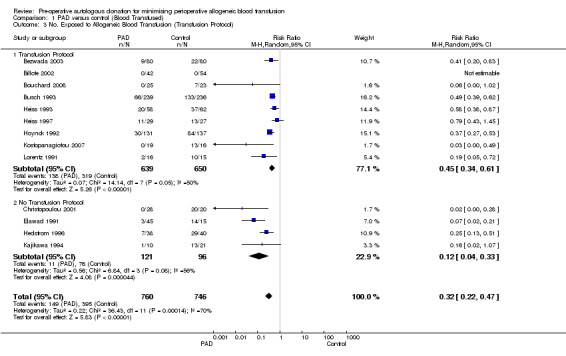
Of the 13 trials that reported data on the number of patients exposed to allogeneic blood transfusion, nine trials reported the use of transfusion protocols. These nine trials included a total of 1289 participants, of whom 639 were randomised to PAD. The pooled relative risk of exposure to allogeneic blood for those randomised to PAD was 0.45 (95% CI 0.34 to 0.61). Heterogeneity between these trials was statistically significant (Chi2 = 14.14, df = 7, P = 0.05; I2 = 50%). For the four trials that did not report the use of a transfusion protocol, the pooled relative risk of exposure to allogeneic blood for those randomised to PAD was 0.12 (95% CI 0.04 to 0.33). Heterogeneity between these trials was statistically significant (Chi2 = 6.84, df = 3, P = 0.08; I2 = 56%).
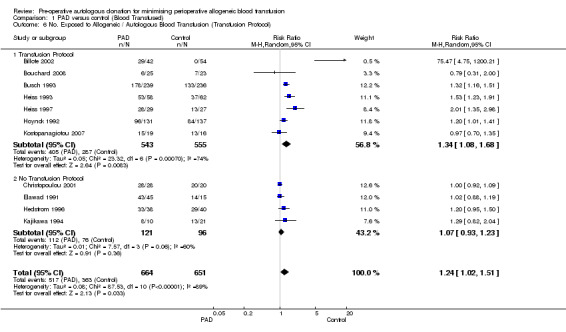
Seven trials reported data on the number of participants exposed to any transfusion (allogeneic and/or autologous) where a transfusion protocol was used. These trials included a total of 1098 participants of whom 543 were randomised to PAD. The pooled relative risk of exposure to any transfusion for those who were randomised to PAD was 1.34 (95% CI 1.08 to 1.68). Heterogeneity between these trials was statistically significant (Chi2 = 23.32, df = 6, P = 0.0007; I2 = 74%). For the four trials that did not report the use of a transfusion protocol, the pooled relative risk of exposure to any transfusion for those patients who were randomised to PAD was 1.07 (95% CI 0.93 to 1.23). Heterogeneity between these trials was statistically significant (Chi2 = 7.57, df = 3, P = 0.06; I2 = 60%).
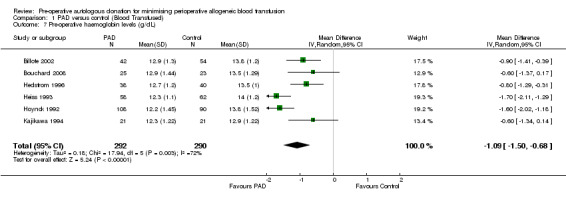
Pre‐operative haemoglobin
Six trials reported pre‐operative haemoglobin data. These trials included a total of 582 patients, of whom 292 were randomised to PAD. The MD in pre‐operative haemoglobin in patients randomised to PAD compared to control was statistically significant (MD ‐1.09 g/dL; 95% CI ‐1.50 to ‐0.68 g/dL). Heterogeneity between these trials was statistically significant (Chi2 = 17.94, df = 5, P = 0.003; I2 = 72%).
Infection
Three trials reported data on infection. These trials included a total of 621 participants of whom 309 were randomised to PAD. The relative risk of acquiring any infection was unaffected by PAD (RR 0.70; 95% CI 0.34 to 1.43). Heterogeneity between these trials was statistically significant (Chi2 = 5.25, df = 2, P = 0.07; I2 = 62%).
Any thrombosis
Three trials reported data on the incidence of any thrombosis. These studies included a total of 250 participants, of whom 140 were randomised to receive PAD. The pooled relative risk of developing any thrombosis for those patients randomised to PAD was 0.82 (95% CI 0.21 to 3.13). Heterogeneity between these trials was not statistically significant (Chi2 = 0.39, df = 1, P = 0.53; I2 = 0%).
Discussion
Evidence of benefit
The results of the meta‐analysis indicate that pre‐operative donation of autologous blood reduces exposure to allogeneic blood transfusion by 68%. However, for those patients who donated autologous blood the risk of receiving any transfusion (allogeneic and/or autologous) was increased by 24%. The increased rate of exposure to any transfusion may be attributed to two factors: (1) patients who donate autologous blood in general have lower preoperative haemoglobin levels than those patients who do not predonate autologous blood, and therefore have an increased probability of requiring an intra‐operative and/or postoperative blood transfusion; (2) the availability of predonated autologous blood engenders a more liberal transfusion policy (Faught 1998). An analysis we performed of 35 non‐randomised studies of PAD (Carless 2004) showed that the overall transfusion rate (allogeneic and/or autologous) was 67% in patients allocated to PAD. This result is similar to what was seen in this meta‐analysis of randomised controlled trials, which showed an overall transfusion rate (allogeneic and/or autologous) of 78% in those patients randomised to PAD.
On the basis of the current evidence, PAD appears effective in reducing exposure to allogeneic blood. However, pre‐operative autologous donation exposes patients to other potential risks associated with blood donation and blood transfusion. As reported by McVay 1990 the incidence of reactions occurring at the time of donation is similar for allogeneic and autologous donors (between 2% and 5%), with most reactions being mild and of a vasovagal origin. Autologous blood can become contaminated with bacteria, and can cause circulatory overload, particularly in elderly patients if used in a liberal fashion without a transfusion protocol. As with any transfusion there is the ever present risk of transfusing the wrong blood due to clerical, laboratory, or ward error (Faught 1998).
The overall benefits of PAD probably outweigh the harms for some groups, for instance those who have been alloimmunized through repeated transfusion and are contemplating elective surgery. However, a full assessment of the balance of benefit and harm requires a better understanding of the clinical value of legitimate indications for red cell transfusion. The trend in the last decade has been to promote lower transfusion thresholds (i.e. the haemoglobin or haematocrit levels below which transfusion is indicated) (Carson 1998; Hebert 1995; Hebert 1999). This is the best way of reducing exposure to allogeneic blood and conserving it for patients who really need it. The same rule should be applied in centres where donated blood is inadequately screened for viral contamination. However, it can be argued that there may be a stronger case for PAD where there are doubts about the safety of the blood supply.
Adverse effects
In individual studies the numbers of adverse events were small. Although we found a small decrease in the rate of infection in those patients randomised to PAD, the treatment effect did not reach statistical significance. There were insufficient data to draw any conclusions about the effect of PAD on mortality, stroke, deep venous thrombosis, and pulmonary embolus. PAD predisposes patients to pre‐operative anaemia, which increases a patient's risk of requiring a perioperative blood transfusion. The results of the meta‐analysis showed that on average the pre‐operative haemoglobin levels of those patients randomised to PAD was 1.09 g/dL (95% CI 0.68 to 1.50 g/dL) less than in those patients who did not donate their blood.
Sources of bias
In our review we found a number of small trials. Reliance on small trials raises concerns about the effects of publication bias. The degree to which publication bias may have impacted on the overall results of this review is difficult to determine as the small number of trials hampered any formal evaluation. However, the presence of publication bias cannot be discounted as the asymmetry of the generated funnel plot for allogeneic blood transfusion suggests a 'missing population' of small negative trials (Figure 2).
2.
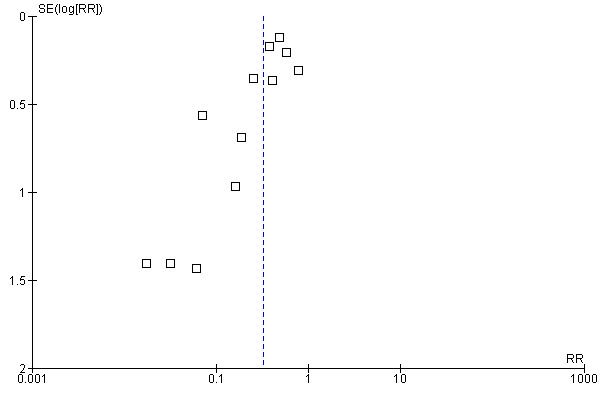
Funnel plot of comparison: 1 PAD versus control (Blood Transfused), outcome: 1.1 No. Exposed to Allogeneic Blood Transfusion.
The methodological quality of the assessed trials was generally poor. Only one trial was rated as being at low risk of bias for sequence generation and allocation concealment, with the remainder being rated as unclear. Additionally, none of the trials were judged to have been adequately blinded. The lack of allocation concealment and adequate blinding indicates that most of the trials assessed may have been open to bias, which may have been in favour of the intervention. The inadequate reporting of the methods used to generate allocation sequences also raises questions as to whether selection bias may have been introduced. In light of the poor methodological quality of the trials reviewed, the results may need to be viewed with some degree of caution.
As the decision to transfuse (the primary outcome in the meta‐analysis) is a practice variable (rather than a measured clinical variable), it therefore involves a degree of subjectivity. This raises concerns about blood transfusion exposure as an outcome variable in unblinded trials, particularly in the absence of transfusion protocols. There is little consensus on appropriate transfusion thresholds, and even in centres where transfusion triggers were promulgated, there was probably a large degree of subjectivity in the decision. To some degree the decision to transfuse will be based on the type of procedure, the anticipated degree of blood loss and the particular susceptibility of the patient to hypovolemia or anaemia. As the haemoglobin level used as the transfusion threshold was rather high (> 10.0 g/dL) for most of the trials assessed, it suggests perioperative blood transfusion was used quite liberally, and in unblinded studies this may have inflated the differences in the frequency of transfusion between groups. If a more conservative transfusion threshold (< 8.0‐9.0 g/dL) had been adopted then the frequency of allogeneic blood transfusion observed in the control group might have been significantly reduced. Therefore, it is conceivable that the choice of the primary outcome (the decision to transfuse with allogeneic red cells) has introduced bias.
Sources of heterogeneity
Marked heterogeneity in trial outcomes was observed in some outcomes. Overall the variation was in terms of the size, not the direction, of effect. We considered a number of factors that might explain variation in the size of the treatment effect. These included the concomitant use of clinical transfusion thresholds (or transfusion triggers), the type of operation, the clinical setting, and the type of clinical outcome studied. Most of the observed heterogeneity seemed to be explained by the type of surgery and the use of a transfusion protocol. As transfusion requirements (allogeneic and/or autologous) were assessed over the intra‐operative and postoperative periods (from surgery to hospital discharge), for all of the included trials, variation in the length of outcome assessment does not appear to be a source of heterogeneity.
Clinical significance of the results
The true value of avoiding allogeneic red cell transfusion is debatable. Conceivably, patients who are concerned about the risks of contracting illness through transfusion will be more interested in avoiding it completely, rather than reducing the volume of transfused blood. However, the importance of avoiding transfusion depends on the probability of avoiding disease transmission, or other adverse effects that have been attributed to blood transfusion (for instance immunosuppression) (Blumberg 1997). The rate of transmission of HIV or viral hepatitis in most developed countries is very low, because of the quality of screening of donated blood (Coyle 1999; Whyte 1997). As noted earlier in this review, this assumption does not apply equally in developing countries. Allogeneic red cell transfusion is administered frequently in developing countries, blood may be inadequately screened, and the prevalence of viral pathogens amongst donors is high (Kimball 1995; McFarland 1997). In these settings there may be greater clinical value in pre‐operative blood donation by individuals who are seronegative.
Conclusions
Although the trials of PAD showed a reduction in the need for allogeneic blood, they were unblinded, and transfusion practices may have been influenced by knowledge of the trial status of individual patients. Furthermore, the overall transfusion rates in these trials were very high, and were increased by recruitment into the PAD arms of the trials. This raises questions about the true benefit of PAD in the field. Over‐transfusion could lead to volume overload in elderly patients and those with left ventricular and renal impairment. Additional concerns about the use of PAD include handling errors, and the infection of the blood (Goldman 1997). Any benefit from avoidance of short‐term immunosuppression and reduction in the risk of viral diseases, have to be set against the risks associated with the technique itself.
Authors' conclusions
Implications for practice.
Whilst the results of the meta‐analysis showed that PAD apparently reduced exposure to allogeneic blood, the poor methodological quality of the trials may have impacted on the magnitude of the beneficial effect of PAD. The lack of adequate allocation concealment and blinding is of concern, as the primary outcome, the decision to transfuse (a practice variable rather than a measured clinical variable), requires a degree of subjectivity, which may have been influenced by prior knowledge of the patient's treatment status. The subjective nature of blood transfusion raises concerns about the use of blood transfusion exposure as an outcome variable, particularly in unblinded trials.
Although the use of PAD provides the patient with a sense of wellbeing, knowing they will receive their own blood if needed, the process is not without its own risks. The use of PAD would appear to be most justified in some patients who have developed immune responses to repeated transfusions, and in situations where there is doubt about the safety of the blood supply. However, where there is a safe, plentiful, and well‐regulated blood supply, the value of PAD is uncertain.
Implications for research.
Further trials of PAD, of the type reviewed here, are not warranted. Further research should attempt to establish the true clinical benefits of avoidance of allogeneic blood transfusion.
What's new
| Date | Event | Description |
|---|---|---|
| 1 December 2009 | New search has been performed | The searches were updated to August 2009, two new trials have been included and the Results amended accordingly. The overall conclusions of the review remain unchanged. As part of this update the assessment of methodological quality used in earlier versions of this review has been replaced with an assessment of the risk of bias. This amendment is in response to a change in the Cochrane Collaboration's methodological guidance. The list of review authors has also been updated. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 20 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Melissa Forgie and Philip Wells for their contributions as co‐authors of the original version of the review (published in 2002) and the update conducted in 2004.
We also thank Karen Blackhall who updated the electronic searches for the 2009 update.
Appendices
Appendix 1. Search strategies July 2009
Cochrane Injuries Group Specialised Register (searched August 7 2009) (autologous and transfus*) or (autologous and donat*) or (autologous and blood) or (blood and pre‐donat*) or (autologous and pre‐donat*) CENTRAL (The Cochrane Library 2009, Issue 3) #1MeSH descriptor Blood Transfusion, Autologous explode all trees #2pre‐operative autologous donat* #3autologous blood donat* #4autologous blood transfus* #5(autologous predonat*) or (autologous pre‐donat*) #6(#1 OR #2 OR #3 OR #4 OR #5) #7MeSH descriptor Blood Transfusion explode all trees #8MeSH descriptor Hemorrhage explode all trees #9MeSH descriptor Anesthesia explode all trees #10transfusion* #11bleed* #12(blood‐loss* or bloodloss*) #13hemorrhag* or haemorrhag* #14(#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15(#6 AND #14) #16 #15 (2004‐2009)
MEDLINE (Ovid SP) 1950 to July (week 5) 2009 1.exp Blood Transfusion, Autologous/ 2.pre‐operative autologous donat*.mp. 3.autologous blood donat*.mp. 4.autologous blood transfus*.mp. 5.(autologous predonat* or autologous pre‐donat*).mp. 6.or/1‐5 7.exp Blood Transfusion/ 8.exp Hemorrhage/ 9.exp Anesthesia/ 10.transfusion*.mp. 11.bleed*.mp. 12.(blood‐loss* or bloodloss*).mp. 13.(hemorrhag* or haemorrhag*).mp. 14.7 or 8 or 9 or 10 or 11 or 12 or 13 15.6 and 14 16.randomi?ed.ab,ti. 17.randomized controlled trial.pt. 18.controlled clinical trial.pt. 19.placebo.ab. 20.clinical trials as topic.sh. 21.randomly.ab. 22.trial.ti. 23.16 or 17 or 18 or 19 or 20 or 21 or 22 24.(animals not (humans and animals)).sh. 25.23 not 24 26.15 and 25 27.(2004* or 2005* or 2006* or 2007* or 2008* or 2009*).em. 28.26 and 27
EMBASE (Ovid SP) 1980 to week 31 2009 1.exp blood autotransfusion/ 2.pre‐operative autologous donat*.mp. 3.autologous blood donat*.mp. 4.autologous blood transfus*.mp. 5.(autologous predonat* or autologous pre‐donat*).mp. 6.or/1‐5 7.exp Blood Transfusion/ 8.exp Bleeding/ 9.exp Anesthesia/ 10.transfusion*.mp. 11.bleed*.mp. 12.(blood‐loss* or bloodloss*).mp. 13.(hemorrhag* or haemorrhag*).mp. 14.7 or 8 or 9 or 10 or 11 or 12 or 13 15.6 and 14 16.exp Randomized Controlled Trial/ 17.exp controlled clinical trial/ 18.randomi?ed.ab,ti. 19.placebo.ab. 20.*Clinical Trial/ 21.randomly.ab. 22.trial.ti. 23.16 or 17 or 18 or 19 or 20 or 21 or 22 24.exp animal/ not (exp human/ and exp animal/) 25.23 not 24 26.25 and 15 27.(2004* or 2005* or 2006* or 2007* or 2008* or 2009*).em. 28.27 and 26 ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) to August 2009 ISI Web of Science: Conference Proceedings Citation Index‐ Science (CPCI‐S) to August 2009 #1Title=(Auto‐transfusion or Autotransfusion or autologous or Blood or Bleed* or transfusion*or blood‐loss* or bloodloss* or hemorrhag* or haemorrhag*) AND Topic=(predonat* or pre‐donat* or preoper* or pre‐oper*) #2Topic=((clinical OR control* OR placebo OR random OR randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random) SAME (trial* or group* or study or studies or placebo or controlled)) #3#1 and #2
Data and analyses
Comparison 1. PAD versus control (Blood Transfused).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. Exposed to Allogeneic Blood Transfusion | 13 | 1506 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.22, 0.47] |
| 2 No. Exposed to Allogeneic Blood Transfusion (Operation Type) | 13 | 1506 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.22, 0.47] |
| 2.1 Orthopaedic | 5 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.11, 0.43] |
| 2.2 Oncology | 6 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.35, 0.66] |
| 2.3 Oral and maxillofacial | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.02 [0.00, 0.28] |
| 2.4 Cardiac | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.02] |
| 3 No. Exposed to Allogeneic Blood Transfusion (Transfusion Protocol) | 13 | 1506 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.22, 0.47] |
| 3.1 Transfusion Protocol | 9 | 1289 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.34, 0.61] |
| 3.2 No Transfusion Protocol | 4 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.04, 0.33] |
| 4 No. Exposed to Allogeneic / Autologous Blood Transfusion | 11 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.02, 1.51] |
| 5 No. Exposed to Allogeneic / Autologous Blood Transfusion (Operation Type) | 11 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.02, 1.51] |
| 5.1 Orthopaedic | 3 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.61, 5.20] |
| 5.2 Oncology | 6 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.15, 1.54] |
| 5.3 Oral and maxillofacial | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.09] |
| 5.4 Cardiac | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.31, 2.00] |
| 6 No. Exposed to Allogeneic / Autologous Blood Transfusion (Transfusion Protocol) | 11 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.02, 1.51] |
| 6.1 Transfusion Protocol | 7 | 1098 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.08, 1.68] |
| 6.2 No Transfusion Protocol | 4 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.23] |
| 7 Pre‐operative haemoglobin levels (g/dL) | 6 | 582 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.50, ‐0.68] |
1.1. Analysis.
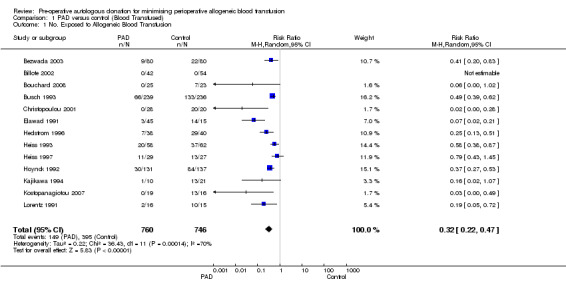
Comparison 1 PAD versus control (Blood Transfused), Outcome 1 No. Exposed to Allogeneic Blood Transfusion.
1.2. Analysis.
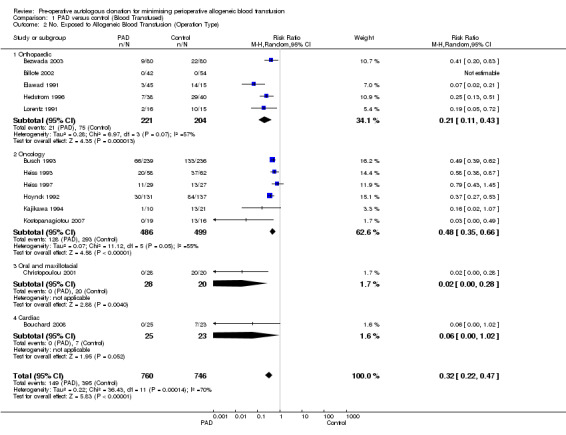
Comparison 1 PAD versus control (Blood Transfused), Outcome 2 No. Exposed to Allogeneic Blood Transfusion (Operation Type).
1.3. Analysis.
Comparison 1 PAD versus control (Blood Transfused), Outcome 3 No. Exposed to Allogeneic Blood Transfusion (Transfusion Protocol).
1.4. Analysis.

Comparison 1 PAD versus control (Blood Transfused), Outcome 4 No. Exposed to Allogeneic / Autologous Blood Transfusion.
1.5. Analysis.
Comparison 1 PAD versus control (Blood Transfused), Outcome 5 No. Exposed to Allogeneic / Autologous Blood Transfusion (Operation Type).
1.6. Analysis.
Comparison 1 PAD versus control (Blood Transfused), Outcome 6 No. Exposed to Allogeneic / Autologous Blood Transfusion (Transfusion Protocol).
1.7. Analysis.
Comparison 1 PAD versus control (Blood Transfused), Outcome 7 Pre‐operative haemoglobin levels (g/dL).
Comparison 2. Adverse events and other outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any Thrombosis | 3 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.21, 3.13] |
| 2 Any Infection | 3 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.34, 1.43] |
2.1. Analysis.

Comparison 2 Adverse events and other outcomes, Outcome 1 Any Thrombosis.
2.2. Analysis.
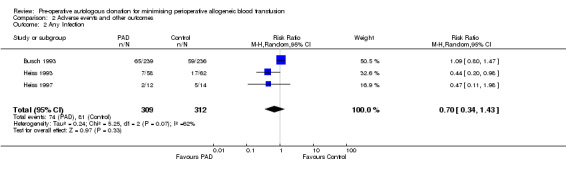
Comparison 2 Adverse events and other outcomes, Outcome 2 Any Infection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bezwada 2003.
| Methods | Design: Prospective, randomised, open‐label, parallel‐group trial. Setting: Single centre, Pennsylvania Hospital, Pennsylvania, USA. | |
| Participants | N = 240, patients undergoing total joint arthroplasty, 59% revision or bilateral.
Baseline risk factors: co‐morbidities = cardiovascular C: 36/80, I: 25/80; renal C: 5/80, I: 4/80; pulmonary C: 12/80, I: 7/80. Inclusion criteria: > 21 years of age; scheduled to undergo total joint arthroplasty; initial haemoglobin ≤ 140 g/L; willingness to participate in PAD programme; women had to be postmenopausal, sterile or taking oral contraceptives. Exclusion criteria: pregnancy; clinically relevant uncontrolled systemic disease or abnormal laboratory values; primary haematological disease; seizure disease; uncontrolled hypertension; recent gastrointestinal or intracranial haemorrhage; iron deficiency. |
|
| Interventions |
Timing of autologous blood collection/retransfusion: retransfusion performed intra‐operatively Volume of autologous blood collected/retransfusion: 2 units for bilateral or revision arthroplasty and one unit for primary unilateral arthroplasty. Iron supplementation: PAD group received oral supplementation of 325 mg iron sulphate 3 x daily. Control group: 600 IU/kg subcutaneously in a four‐dose regimen, 21, 14, 7 and 1 day prior to surgery; single dose 100 mg intravenous iron dextran with initial dose of EPO followed by oral supplementation with 325 mg iron sulphate 2 x daily. Use of transfusion threshold: indications for perioperative blood transfusion were Hb level of 80 g/L or less and/or persistent tachycardia or hypotension requiring administration of large volumes of crystalloid; clinical symptoms was an additional criterion for allogeneic blood transfusion in the postoperative period. Other active interventions given to both arms: all patients received warfarin for prophylaxis; all patients who underwent revision total hip arthroplasty had intra‐operative CS (14/80 in control group and 14/80 in PAD group); intra‐operative and immediate postoperative CS performed for all patients who underwent revision total knee arthroplasty (14/80 in control group and 10/80 in PAD group); and bilateral total knee and total hip arthroplasty (23/80 control group and 23/80 PAD group); Length of surgery: no details |
|
| Outcomes | Outcomes reported: number of patients transfused with allogeneic blood, volume of allogeneic blood transfused (no SDs), volume of autologous blood transfused (no SDs), pre‐operative Hb (range not SD), volume of autologous blood wasted (no SD), (wound haematomas, pulmonary embolus, mortality, stroke, DVT ‐ no groups stated). | |
| Notes | Period of study : not stated Length of study: not reported A priori sample size: unclear Baseline comparability: adequate except for Hb levels were significantly lower in group A (EPO) compared to group B (PAD+EPO). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | Participant blinding: no, open‐label. Assessor blinding: unclear but states only operating surgeon was blinded regarding whether patient had received EPO. |
| Intention‐to‐treat analysis? | Low risk | Data were analysed on an intention‐to‐treat basis. |
Billote 2002.
| Methods | Design: Randomised controlled trial. Setting: Single centre, Northwestern Memorial Hospital, Northwestern University Medical School, Illinois, USA. | |
| Participants | N = 112, patients undergoing elective total hip replacement (96 patients completed the trial).
Baseline risk factors: no significant medical co‐morbidities. Inclusion criteria: baseline Hb at least 120 g/L (finger‐prick). Exclusion criteria: severe or unstable cardiac disease; uncontrolled hypertension; symptomatic carotid or vertebral artery stenosis; a bleeding diathesis or bacteraemia. |
|
| Interventions |
Timing of autologous blood collection/retransfusion: whole blood donated once a week with last unit no later than 2 weeks prior to surgery, retransfused post‐operatively (1 patient had retransfusion intra‐operatively) (PAD group only) Volume of autologous blood collected/retransfused: 2 units, maximum 1 unit (approximately 500 g) at each time; 2 patients in PAD group only pre‐donated 1 unit each. Iron supplementation: Ferrous sulfate tablets (325 mg twice daily) were prescribed to all patients. PAD group started supplementation after their first autologous blood donation, control group started it 10 days before surgery. Use of transfusion threshold: need for intra‐operative blood transfusion decided by Anaesthetist (not a co‐investigator in the study) and identical thresholds for autologous and allogeneic, autologous blood transfused before allogeneic if available; standardised protocol indicated need for red blood cell transfusion when: acute blood loss of more than 25% estimated blood volume with or without hypovolaemic shock; tachycardia attributed to haemoglobin‐responsive hypoxia and unresponsive to intravenous fluid administration; haemoglobin concentration less than 70 g/L in healthy patients regardless of age; haemoglobin concentration of less than 80 g/L in patients with co‐morbid diseases such as cerebrovascular accident, heart disease, peripheral vascular disease or chronic obstructive pulmonary disease. Postoperative decision to transfuse made by orthopaedic surgeon (one of which was an author of the study), indication for autologous transfusion included haemoglobin level of less than 100 g/L in postoperative period. Other active interventions given to both arms: all patients received thromboprophylaxis on first postoperative day continued for one month. Length of surgery: no details. |
|
| Outcomes | Outcomes reported: number of patients transfused with allogeneic blood, number of patients transfused with autologous blood, volume of autologous blood transfused (no SD), pre‐operative Hb, blood loss (mLs), autologous blood wastage (no SD), length of hospital stay (no SD). | |
| Notes | Period of study: not stated Length of study: 6 weeks A priori sample size: Yes Baseline comparability: No significant difference between the two groups in regard to age, gender, BMI, blood volume or baseline medical condition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation scheme (Analysis Tool Pack, Microsoft Excel). |
| Allocation concealment? | Low risk | Sequential sealed envelope by an independent research nurse. |
| Blinding? All outcomes | Unclear risk | Participant blinding: unclear. Assessor blinding: unclear. |
| Intention‐to‐treat analysis? | High risk | Data were not analysed on an intention‐to‐treat basis. |
Bouchard 2008.
| Methods | Design: Randomised controlled trial. Setting: Montreal Heart Institute, Canada. | |
| Participants | N = 48, patients undergoing elective cardiopulmonary bypass surgery.
Inclusion criteria: Patients aged 18‐80 years who were in the pre‐operative phase of an elective cardiac surgery. Exclusion criteria: Patients with aortic stenosis, coronary disease in left main trunk or unstable angina, those who lived outside the Montreal area and were unable to attend pre‐operative blood donation appointments, used EPO pre‐operatively, had a haemoglobin level < 110 g/L and required emergency surgery. |
|
| Interventions |
Timing of autologous blood collection/transfusion: Donated weekly, blood stored for a maximum of 35 days. Volume of blood collected: Donated 2 units, 350 mL each. Iron supplementation: PAD group received 300 mg ferrous sulphate orally 3 times daily during the period before recruitment and surgery. Transfusion threshold: Both groups received postoperative mediastinal blood reinfusion during the first 6 hours after surgery when postoperative bleeding > 100 mL/h. Blood administered when haemoglobin level < 60 g/L during operation and < 80 g/L postoperatively. Length of surgery: 174.7 (44.9) and 177.6 (62.3) mean (SD) minutes in the PAD and control groups respectively. |
|
| Outcomes | Outcomes reported : Number of participants exposed to allogeneic blood; amount of allogeneic blood transfused; haemoglobin level; complications. | |
| Notes | Period of study: January 2001 to October 2002. Length of study: Not reported. A priori sample size: No information presented. Baseline comparability: There were no statistically significant differences between the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information reported. |
| Allocation concealment? | Unclear risk | No information reported. |
| Blinding? All outcomes | Unclear risk | Patient and surgeon were not blinded to group assignment. ICU intensivists, nurses and residents were blinded. |
| Intention‐to‐treat analysis? | Low risk | Data were analysed on an intention‐to‐treat basis. |
Busch 1993.
| Methods | Design: Randomised controlled trial. Setting: 14 hospitals in The Netherlands and one hospital in the UK. | |
| Participants | N = 475 patients scheduled for potentially curative resection of cancer of the colon or rectum.
Inclusion/exclusion criteria: Absence of severe cardiovascular and respiratory disease, no history of epilepsy after infancy, and a haemoglobin level > 11.3 g/dL, no evidence of metastatic disease, no evidence of ulcerative colitis, familial polyposis or fixed rectal carcinoma requiring pre‐operative radiotherapy, no history of blood transfusion during the 3 months before randomisation. |
|
| Interventions |
Timing of autologous blood collection/transfusion: PAD group were required to donate blood twice. The minimal interval between two donations was 72 hours, and the second donation had to occur no later than five days before surgery. Volume of blood collected: At each donation, 450 mL of blood was obtained by standard procedures. The patients were treated with oral iron supplementation immediately after randomisation. The collected blood was separated into packed red cells and fresh frozen plasma, except at one hospital, where autologous blood was given in transfusion as whole blood. Iron supplementation: The PAD group were treated with oral iron supplementation immediately after randomisation. Transfusion threshold: PAD group ‐ if the Hb concentration (> 10.5 g/dL) was not achieved after two autologous transfusions, additional allogeneic transfusions were made. In both groups fresh‐frozen plasma was given when indicated. Control group received RBC only if the loss of blood exceeded 500ml or if the Hb concentration dropped below 10.5 g/dL (6.5 mmol/liter). Length of surgery: Not reported. |
|
| Outcomes | Outcomes reported : Number of patients exposed to allogeneic blood (n), allogeneic blood transfused (units), blood loss (mL), Hb levels, mortality (n), postoperative infection (n), disease recurrence (n). | |
| Notes | Period of study: August 1986 to November 1991 Length of study: Patients were evaluated every 3 months during the first two years after surgery and every six months thereafter. A priori sample size: Not reported. Baseline comparability: Report states that none of the characteristics differed significantly between the groups. Other: All randomised patients were evaluated according to the intention‐to‐treat principle. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | Low risk | Data were analysed on an intention‐to‐treat basis. |
Christopoulou 2001.
| Methods | Design: Randomised controlled trial. Setting: Single centre, Evangelismos Hospital, University of Athens, Greece. | |
| Participants | N = 48 patients undergoing maxillofacial operations.
Baseline risk factors: haematocrit and red blood count levels of control group were significantly higher than of the intervention group. Inclusion criteria: orthognathic surgery, reconstruction after trauma or removal of tumours, removal of benign tumours or malformations; anticipated need for blood not to exceed 4 units; completion of donor form; haematological testing donor‐recipient to be carried out. Exclusion criteria: Hb greater than 11 g/dL; haematocrit less than 11 g/dL or 34%; anaemia; less than 10 years of age and greater than 65 years of age; active malignant tumour; coronary disease; recent MI; arterial hypertension (systolic blood pressure higher than 180 mmHg, diastolic blood pressure higher than 100 mmHg); pregnancy; AIDS; diabetes; active infection for which treatment being given. |
|
| Interventions |
[*not included in the review's analysis] Timing of autologous blood collection/retransfusion: blood donated at least one week before operation, time between donations was one week, retransfused intra‐operatively (PAD group only). Volume of autologous blood collected/retransfused: 24 PAD patients pre‐donated 1 unit each and 4 patients predonated 2 units (bimaxillary osteotomies). Iron supplementation: all patients received 150 mg ferrous sulphate daily by mouth pre‐operatively until 1 week postoperatively. Use of transfusion threshold: no details Length of surgery: no details |
|
| Outcomes | Outcomes reported: number of patients exposed to allogeneic blood, number of patients exposed to autologous blood, pre‐operative Hb. | |
| Notes | Period of study: 1990‐1995 Length of study: 4 weeks. A priori sample size: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Report states that 'consecutive patients randomly assigned'. No further information given. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | Unclear risk | Insufficient information presented to judge whether data were analysed on an intention‐to‐treat basis. |
Ekback 1995.
| Methods | Design: Randomised controlled trial. Setting: Sweden. | |
| Participants | N = 45 patients with severe hip arthrosis undergoing total hip arthroplasty. NB: No demographic data provided | |
| Interventions |
[* Not used as the control group for the meta‐analysis] Timing of autologous blood collection/retransfusion: Predonated blood was withdrawn in 2‐3 sessions within 6 weeks of the operation. In both PAD and control groups, blood loss was replaced with 3% dextran and by autotransfusion of washed and haemo‐concentrated blood salvaged by intraoperative suction and from wound drains up to 4 hours postoperatively. Volume of autologous blood collected/retransfused: 2‐3 units of SAGM‐ERC was withdrawn. Use of transfusion threshold: Blood transfused to maintain EVF > 27%. If necessary, heterologous SAGM‐ERC was used in PAD group if transfusion of all predonated autologous blood failed to maintain EVF > 27%. Length of surgery: no details |
|
| Outcomes | Outcomes reported: Amount of allogeneic units transfused, complications (n), autologous units transfused. | |
| Notes | Period of study: Not reported. Length of study: 10 days. A priori sample size: Not reported. Baseline comparability: The trial report states that there "was no significant difference in demographic data like age and sex between groups". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | Unclear risk | No information. |
Elawad 1991.
| Methods | Design: Randomised controlled trial. Setting: Malmo General Hospital, Sweden. | |
| Participants | N = 60 patients undergoing elective primary total hip replacement.
[*data for these groups were combined for the review's analysis to create one PAD group] |
|
| Interventions |
Timing of autologous blood collection/retransfusion: Donated 31 to 40 days before operation, with nine to 16 days between each phlebotomy. Volume of autologous blood collected/retransfused: Each donated on average between 2.8 and 3.0 units. Iron supplementation: Two of the four groups were exposed to iron supplementation. One of these received iron supplementation as ferrous sulfate 100 mg three times daily, starting after the first phlebotomy, and the other received the iron supplementation plus folate supplementation as folate 5 mg three times daily, starting after the first phlebotomy. Use of transfusion threshold: Not mentioned. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood (n), allogeneic blood transfused (units), blood loss (mL), deep vein thrombosis (n), pulmonary embolus (n), biochemical parameters. | |
| Notes | Period of study: January 1988 to February 1989 Length of study: Not reported. A priori sample size: Not reported. Baseline comparability: Age and gender of the patients were similar in all groups. Operation time (mean ± SD) was longer in the no‐PAD control group (125 ± 18) than the PAD groups (116 ± 19; 116 ± 26; 109 ± 20). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | Patients were allocated by means of sealed envelopes |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | Unclear risk | No information. |
Hedstrom 1996.
| Methods | Design: Randomised controlled trial. Setting: Sweden. | |
| Participants | N = 80 patients undergoing total hip replacement for primary coxarthrosis (78 patients included in the analysis).
Inclusion/Exclusion criteria: Patients with hepatitis, severe coronary artery disease or heart failure, haematologic diseases, Hb concentration < 110 g/L or body weight < 50 kg were not eligible. |
|
| Interventions |
Timing of autologous blood collection/retransfusion: one 4 weeks before surgery and the other 2 weeks before the scheduled operation. The blood was stored at +4.0 degrees celsius for not more than 6 weeks and was handled according to existing routines and regulations for allogeneic blood. All autologous blood, as well as the allogeneic blood, was retransfused as packed red blood cells (leukocyte depleted blood) if needed. Volume of autologous blood collected/retransfused: autologous group donated 2 units of blood. Iron supplementation: All patients received iron supplementation consisting of 100 mg Fe2+ given orally 2 times a day after the first donation until the day of the operation. Use of transfusion threshold: Not reported. Length of surgery: Average of 107 and 97 minutes in PAD and control groups respectively. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood (n), allogeneic blood transfused (units), blood loss (mL), haemostatic parameters (bleeding times). | |
| Notes | Period of study: Not reported. Length of study: Not reported. A priori sample size: Not reported. Baseline comparability: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | Patients were randomly allocated by the use of sealed envelopes. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | High risk | Data were not analysed on an intention‐to‐treat basis. |
Heiss 1993.
| Methods | Design: Randomised controlled trial. Setting: Germany. | |
| Participants | N = 120 patients undergoing colorectal surgery.
Inclusion criteria: intention of curative tumour resection and eligibility for enrolment in an autologous blood donation programme (Hb concentration ≥12.5 g/dL). Exclusion criteria: Acute infections, aged 75 years and over, history of seizures, unstable coronary disease, severe morbidity, or a likelihood that the tumour could not be resected. |
|
| Interventions |
Timing of autologous blood collection/retransfusion: patients were scheduled to deposit two units of blood 7 and 10 days before surgery. Each unit was separated and stored as RBC concentrate preserved in citrate‐phosphate‐dextrose‐adenine (CPDA‐1) and fresh frozen plasma. The RBC concentrates were buffy coat poor, but not leukocyte‐depleted. Before every donation, a complete blood cell count was performed, and if the Hb value was less than 11.0g/dL, a second unit was not obtained. The predeposited units were matched and made ready. Additionally, 2 units of standard allogeneic leukocyte‐poor RBC concentrates were routinely kept in reserve in the transfusion centre. If more blood was needed, allogeneic blood was used. Iron supplementation: Iron supplementation was given to the intervention group as ferrous sulphate 100 mg orally twice daily. Transfusion thresholds: Transfusions were recommended at a haemoglobin concentration below 10.0 g/dL, based on measured blood loss. Other active intervention given to both arms: Decisions about the use of colloid and crystalloid fluids and blood transfusion were made by each patient's attending anaesthetist or surgeon. |
|
| Outcomes | Outcomes reported: Number of subjects exposed to allogeneic blood (n), allogeneic blood transfused (units), blood loss (mL), haemoglobin levels, postoperative infections (n), DTH responses. | |
| Notes | Period of study: November 1987 and March 1991. Length of study: Participants were followed up for 3 months. A priori sample size: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | Low risk | Data were analysed on an intention‐to‐treat basis. |
Heiss 1997.
| Methods | Design: Randomised controlled trial. Setting: Germany. | |
| Participants | N = 56 patients undergoing colorectal surgery with a primary diagnosis of colorectal cancer that was potentially curable (resectable).
NB: No demographic data provided Inclusion criteria: Diagnosis of colorectal cancer that was potentially curatively resectable and were eligible for autologous blood donation. |
|
| Interventions |
Timing of autologous blood collection/retransfusion: PAD group donated blood on the seventh and tenth day before surgery. Patients received their autologous units (packed red cells, buffy coat poor) and additional allogeneic blood units if necessary. Volume of autologous blood collected/retransfused: 2 units per donation. Iron supplementation: No supplementation reported. Use of transfusion threshold: Blood transfusions were given when the Hb value declined to less than 9.0 g/dL. Length of surgery: Not reported. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood (n), modulation of immune responses (IL‐2 receptor serum levels, TNF‐alpha serum levels, TNF‐alpha and IL10 serum levels, Tetanus‐immunoglobulin titre, Serum cytokine responses) | |
| Notes | Period of study: From 1992 to 1996. Length of study: Not reported. A priori sample size: Not reported. Baseline comparability: No statistically significant difference between the study groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | Low risk | Data were analysed on an intention‐to‐treat basis. |
Hoynck 1992.
| Methods | Design: Randomised controlled trial. Setting: The Netherlands. | |
| Participants | N = 282 male and female patients undergoing colorectal surgery (analysis based on 268 patients).
Inclusion criteria: Histologically proven colorectal carcinoma or a radiologically suspected lesion for malignancy. Exclusion criteria: Previous malignancy, history of severe cardiovascular or respiratory disease, convulsions after infancy, colitis ulcerosa or polyposis coli, pre‐operative irradiation, emergency operation, blood transfusion during pre‐operative period, pre‐operative Hb < 120 g/L and/or Hct < 35%, evidence of metastatic disease. |
|
| Interventions |
Timing of autologous blood collection/retransfusion: donated blood between 14 and 7 days before operation. The interval between the two donations was at least 3 days. In all but one hospital, patients donated their blood in the regional blood banks where autologous and allogeneic blood was processed to erythrocyte concentrates (without buffy coat) and fresh frozen plasma. All unused autologous units were discarded. Volume of autologous blood collected/retransfused: 2 units Iron supplementation: Before the first donation, autologous patients started with iron supplementation (ferrous fumarate 3 times daily 200 mg). Use of transfusion threshold: Patients received a transfusion when blood loss was greater than 500 mL and the Hb level < 10.5 g/dL. When blood loss was less than 500 mL, blood transfusions were only given when Hb concentration was repeatedly below 10.5 g/dL. A blood loss greater than 500 mL had to be followed by an administration of 2 units of blood (autologous or allogeneic), and depending on Hb concentration (Hb < 10.5 g/dL) more allogeneic units were allowed to be given. Autologous units of fresh frozen plasma were available for the patients. Length of surgery: Not reported. |
|
| Outcomes | Outcomes reported : Number of patients exposed to allogeneic blood (n), allogeneic blood transfused (units), blood loss (mL). | |
| Notes | Period of study: September 1986 to January 1988. Length of study: Not reported. A priori sample size: Not reported. Baseline comparability: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | High risk | Data were not analysed on an intention‐to‐treat basis. |
Kajikawa 1994.
| Methods | Design: Randomised controlled trial. Setting: Japan. | |
| Participants | N = 42 patients (32 male, 10 female) undergoing hepatectomy for hepatocellular carcinoma.
NB: No demographic data provided. Exclusion criteria: Hematocrit level < 33.0%. |
|
| Interventions |
Timing of autologous blood collection/retransfusion: Donation beginning 2 to 3 weeks before the operation. Volume of autologous blood collected/retransfused: 400 mL (2 units) autologous blood was collected once or twice weekly (total = 400 to 1200 mL) from each patient. Iron supplementation: PAD patients received 80 mg iron sulphate and 500 mL of Ringer's lactate solution intravenously on the day of blood collection, followed by 100 mg iron sulphate given orally once daily until the operation. Use of transfusion threshold: Not reported. Length of surgery: Mean ± sem = 318 ± 23 and 290 ± 20 minutes in the PAD and control groups respectively. |
|
| Outcomes | Outcomes reported: number of patients exposed to allogeneic blood (n), allogeneic blood transfusion (units), blood loss (mL), mortality (n), complications (n). | |
| Notes | Period of study: August 1991 and November 1992. Length of study: Not reported. A priori sample size: Not reported. Baseline comparability: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | High risk | Not blinded. |
| Intention‐to‐treat analysis? | Low risk | Data were analysed on an intention‐to‐treat basis. |
Kostopanagiotou 2007.
| Methods | Design: Randomised controlled trial. Setting: Greece | |
| Participants | N = 35, liver resection patients.
Inclusion criteria: Pre‐operative haematocrit within normal levels. Exclusion criteria: Patients with co‐morbid diseases, cirrhosis or receiving immunosuppressive medication were excluded. |
|
| Interventions |
Timing of autologous blood collection/retransfusion: no details presented. Volume of autologous blood collected/retransfused: 2 units. Patients in PAD group who needed more than the 2 units and those (in either group) who did not require transfusion were excluded from the analysis. Iron supplementation: Not mentioned. Use of transfusion threshold: Serum haemoglobin levels were maintained at > 9.0 g/dL. Length of surgery: Mean ± SD = 175 ± 45 and 190 ± 50 minutes in the PAD and control groups respectively. |
|
| Outcomes |
Outcomes reported: Number exposed to autologous and allogeneic blood*, length of hospital stay, mortality. [*These outcome data were not presented in the trial report, obtained from author.] |
|
| Notes | Period of study: Not reported. Length of study: Patients followed up for 1 year after surgery. A priori sample size: Not reported. Baseline comparability: Reported that demographics and type of surgery were similar in both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | Low risk | Data were analysed on an intention‐to‐treat basis. |
Lorentz 1991.
| Methods | Design: Randomised controlled trial. Setting: Germany. | |
| Participants | N = 64 patients scheduled for total hip arthroplasty (included 63 patients in analysis).
[* not included in review's analyses] |
|
| Interventions |
Timing of autologous blood collection/retransfusion: Preoperative autologous donations were stored in CPDA‐1 buffer. A predonation Hb concentration of 11 g/dL was required. Surgery was carried out in the 5th week after the first donation. Volume of autologous blood collected/retransfused: Three units of 450 mL. Iron supplementation: Not mentioned. Use of transfusion threshold: If the Hb concentration fell below 9.0 g/dL in the operating room and intensive care unit or below 10.0 g/dL in the general ward, autologous blood or allogeneic packed red cells were transfused. |
|
| Outcomes | Outcomes reported: Number of patients exposed to allogeneic blood (n), allogeneic blood transfused (units), blood loss (mL). | |
| Notes | Period of study: Not reported. Length of study: Not reported. A priori sample size: Not reported. Baseline comparability: Reported that the general data for the patients were comparable for both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information. |
| Allocation concealment? | Unclear risk | No information. |
| Blinding? All outcomes | Unclear risk | No information. |
| Intention‐to‐treat analysis? | High risk | Data were not analysed on an intention‐to‐treat basis. |
CS = Cell salvage; EPO = Erythropoietin; EVF = Erythrocyte volume fraction; Hb = Haemoglobin; PAD = Pre‐operative autologous donation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avall 1997 | Fifty‐six patients were enrolled in the study, but more than half were excluded after randomisation. Patients not receiving transfused blood, or receiving too much blood (> 3 units of allogeneic blood or autologous + allogeneic blood) were excluded. |
| Goodnough 1999 | Randomised trial of acute normovolemic haemodilution compared to preoperative autologous donation in total knee arthroplasty. No appropriate control/comparator group. |
| Goodnough 2000 | Randomised trial of acute normovolemic haemodilution compared to preoperative autologous donation in total hip arthroplasty. No appropriate control/comparator group. |
| Kiyama 1999 | Randomised controlled trial of patients undergoing elective cardiac surgery who were randomised to either perioperative recombinant human erythropoietin (Epoetin alpha) and preoperative autologous donation or to iron supplementation. No appropriate control/comparator group. |
| Rubens 2000 | Randomised trial of acute normovolemic haemodilution compared to preoperative autologous donation in adult cardiac surgery. No appropriate control/comparator group. |
| Stowell 1999 | A multicentre, randomised, open‐label, parallel‐group study comparing the safety and efficacy of perioperative recombinant human erythropoietin (Epoetin alpha) with the safety and efficacy of preoperative autologous donation in total joint arthroplasty. This trial was comparing one active treatment versus another active treatment. No appropriate control/comparator group. |
Characteristics of studies awaiting assessment [ordered by study ID]
Gomez‐Barrera 2006.
| Methods | Controlled trial. Spain. |
| Participants | N = 388, patients undergoing orthopedic surgery. |
| Interventions |
|
| Outcomes | Number of patients receiving allogeneic blood, amount of allogeneic blood units, number of patients receiving autologous blood, amount of autologous blood units. |
| Notes | Abstract only. Unclear if allocation was random. |
Naumenko 2003.
| Methods | Randomised controlled trial. |
| Participants | N = 143 patients. |
| Interventions | |
| Outcomes | |
| Notes | Article in Russian. |
Contributions of authors
Contributors (names are listed alphabetically):
Paul Carless (University of Newcastle, Australia) obtained relevant papers, applied inclusion/exclusion criteria to retrieved papers, quality assessed trials, extracted data from the trials, entered data into MetaView 4.1, entered all study details into Review Manager 4.1, and co‐wrote the review;
Dean Fergusson (*ISPOT International Research Coordinator/University of Ottawa Centre for Transfusion Research, Canada) coordinated the conduct of the original review as part of the International Study of Peri‐Operative Transfusion;
David Henry (Institute of Clinical Evaluative Sciences, Toronto, Canada) obtained funding for the study, was involved in study design, screened abstracts and titles for relevant articles, and co‐wrote the review;
Katharine Ker (London School of Hygiene & Tropical Medicine, UK) undertook the following tasks for the 2009 update ‐ screened search output, obtained articles, applied inclusion/exclusion criteria to retrieved papers, assessed risk of bias, extracted data, performed data analysis and revised the text of the review;
Annette Moxey (University of Newcastle) obtained relevant papers, applied inclusion/exclusion criteria to retrieved papers, quality assessed trials, extracted data from the trials and entered data into MetaView 4.1;
Dianne O'Connell (Cancer Council, Sydney, Australia) provided statistical consultancy for the review, checked data for consistency, analysed and interpreted the results, provided methodological content, and co‐wrote the review.
* ISPOT ‐ International Study of Peri‐Operative Transfusion
Sources of support
Internal sources
Special purpose grant, Hunter Area Pathology Services, NSW, Australia.
External sources
Australian Health Ministers' Advisory Committee. National Health and Medical Research Council of Australia, Australia.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bezwada 2003 {published data only}
- Bezwada HP, Nazarian DG, Henry DH, Booth RE. Preoperative use of recombinant human erythropoietin before total joint arthroplasty. Journal of Bone and Joint Surgery. American volume 2003;85‐A:1795‐800. [DOI] [PubMed] [Google Scholar]
Billote 2002 {published data only}
- Billote DB, Glisson SN, Green D, Wixson RL. A prospective, randomized study of preoperative autologous donation for hip replacement surgery. Journal of Bone and Joint Surgery. American volume 2002;84‐A:1299‐304. [DOI] [PubMed] [Google Scholar]
Bouchard 2008 {published data only}
- Bouchard D, Marcheix B, Al‐Shamary S, Vanden Eynden F, Demers P, Robitaille D, et al. Preoperative autologous blood donation reduces the need for allogeneic blood products: a prospective randomized study. Canadian Journal of Surgery 2008;51(6):422‐7. [PMC free article] [PubMed] [Google Scholar]
Busch 1993 {published data only}
- Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. New England Journal of Medicine 1993;328(19):1372‐6. [DOI] [PubMed] [Google Scholar]
- Busch OR, Hop WC, Marquet RL, Jeekel J. The effect of blood transfusions on survival after surgery for colorectal cancer. European Journal of Cancer 1995;31A(7‐8):1226‐8. [DOI] [PubMed] [Google Scholar]
Christopoulou 2001 {published data only}
- Christopoulou M, Derartinian H, Hatzidimitriou G, Iatrou L. Autologous blood transfusion in oral and maxillofacial surgery patients with the use of erythropoietin. Journal of Craniomaxillofacial Surgery 2001;29:118‐25. [PubMed] [Google Scholar]
Ekback 1995 {published data only}
- Ekback G, Schott U, Axelsson K, Carberg M. Perioperative autotransfusion and functional coagulation analysis in total hip replacement. Acta Anaesthesiologica Scandinavica 1995;39:390‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elawad 1991 {published data only}
- Elawad AA, Jonsson S, Laurell M, Fredin H. Predonation autologous blood in hip arthroplasty. Acta Orthopaedica Scandinavica 1991;62(3):218‐22. [DOI] [PubMed] [Google Scholar]
- Elewad AA, Fredin HO, Laurell M, Jonsson S. Elderly patients' responses to preoperative autologous blood collection. Medical Journal of Australia 1991;155(3):147‐50. [PubMed] [Google Scholar]
Hedstrom 1996 {published data only}
- Hedstrom M, Flordal PA, Ahl T, Svensson J, Dalen N. Autologous blood transfusion in hip replacement. No effect on blood loss but less increase of plasminogen activator inhibitor in a randomized series of 80 patients. Acta Orthopaedica Scandinavica 1996;67(4):317‐20. [DOI] [PubMed] [Google Scholar]
Heiss 1993 {published data only}
- Heiss MM, Mempel W, Delanoff C, Jauch KW, Gabka C, Mempel M, et al. Blood transfusion‐modulated tumor recurrence: first results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. Journal of Clinical Oncology 1994;12(9):1859‐67. [DOI] [PubMed] [Google Scholar]
- Heiss MM, Mempel W, Jauch KW, Delanoff C, Mayer G, Mempel M, et al. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery [published erratum appears in Lancet 1994 Jan 1;343(8888):64]. Lancet 1993;342(8883):1328‐33. [DOI] [PubMed] [Google Scholar]
Heiss 1997 {published data only}
- Heiss MM, Fasol‐Merten K, Allgayer H, Strohlein MA, Tarabichi A, Wallner S, et al. Influence of autologous blood transfusion on natural killer and lymphokine‐activated killer cell activities in cancer surgery. Vox Sanguinis 1997;73(4):237‐45. [DOI] [PubMed] [Google Scholar]
- Heiss MM, Fraunberger P, Delanoff C, Stets R, Allgayer H, Strohlein MA, et al. Modulation of immune response by blood transfusion: evidence for differential effect of allogeneic and autologous blood in colorectal cancer surgery. Shock 1997;8(6):402‐8. [DOI] [PubMed] [Google Scholar]
Hoynck 1992 {published data only}
- Hoynck van Papendrecht MA, Hop W, Langenhorst BL, Kothe FC, Marquet RL, Jeekel J. Feasibility of a predeposit autologous blood donation program in colorectal cancer patients: results from a randomized clinical study. Vox Sanguinis 1992;62(2):102‐7. [DOI] [PubMed] [Google Scholar]
Kajikawa 1994 {published data only}
- Kajikawa M, Nonami T, Kurokawa T, Hashimoto S, Harada A, Nakao A, et al. Autologous blood transfusion for hepatectomy in patients with cirrhosis and hepatocellular carcinoma: use of recombinant human erythropoietin. Surgery 1994;115:727‐34. [PubMed] [Google Scholar]
Kostopanagiotou 2007 {published data only}
- Kostopanagiotou G, Pandazi A, Matsota P, Arkadopoulos N, Dalamanga N, et al. Effect of packed red blood cells transfusion on plasma fibronectin during liver resection. Transfusion Medicine 2007;17:115‐8. [DOI] [PubMed] [Google Scholar]
Lorentz 1991 {published data only}
- Lorentz A, Osswald PM, Schilling M, Jani L. A comparison of autologous transfusion procedures in hip surgery. Anaesthesist 1991;40(4):205‐13. [PubMed] [Google Scholar]
References to studies excluded from this review
Avall 1997 {published data only}
- Avall A, Hyliner M, Bengtson J, Carlsson L, Bengtsson A. Postoperative inflammatory response after autologous and allogeneic blood transfusion. Anesthesiology 1997;87:511‐6. [DOI] [PubMed] [Google Scholar]
Goodnough 1999 {published data only}
- Goodnough LT, Monk TG, Despotis GJ, Merkel K. A randomized trial of acute normovolemic hemodilution compared to preoperative autologous blood donation in total knee arthroplasty. Vox Sanguinis 1999;77(1):11‐6. [DOI] [PubMed] [Google Scholar]
Goodnough 2000 {published data only}
- Goodnough LT, Despotis GJ, Merkel K, Monk TG. A randomized trial comparing acute normovolemic hemodilution and preoperative autologous blood donation in total hip arthroplasty. Transfusion. 2000;40(9):1054‐7. [DOI] [PubMed] [Google Scholar]
Kiyama 1999 {published data only}
- Kiyama H, Ohshima N, Imazeki T, Yamada T. Autologous blood donation with recombinant human erythropoietin in anemic patients. Annals of Thoracic Surgery. 1999;68(5):1652‐6. [DOI] [PubMed] [Google Scholar]
Rubens 2000 {published data only}
- Rubens FD, Dupuis JY, Robblee J, Rock G, Bryson GL, Wells PS. Feasibility of blinding in a randomized controlled trial comparing preoperative autologous blood donation and acute normovolemic hemodilution in adult cardiac surgery. Transfusion 2000;40(9):1058‐62. [DOI] [PubMed] [Google Scholar]
Stowell 1999 {published data only}
- Stowell CP, Chandler H, Jove M, Guilfoyle M, Wacholtz MC. An open‐label, randomized study to compare the safety and efficacy of perioperative epoetin alfa with preoperative autologous blood donation in total joint arthroplasty. Orthopedics 1999;22:s105‐12. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gomez‐Barrera 2006 {published data only}
- Gomez‐Barrera M, Izuel‐Rami M, Garcia‐Erce JA, Recasens V, Mendaza M, Rabanaque MJ. Study about the use of Epoetin Alfa, with or without autologous blood donation, in patients undergoing hip or knee orthopedic major surgery ‐ poster 50. Transfusion Alternatives in Transfusion Medicine 2006;8(Suppl. 1):79. [Google Scholar]
Naumenko 2003 {published data only}
- Naumenko SE, Pokrovskii MG, Belavin AS, Kim SF. Blood‐saving effectiveness of preoperative reservation of autoblood for surgical treatment of ischemic heart disease. Vestnik Khirurgii Imeni i ‐ i ‐ Grekova 2003;162(2):59‐64. [PubMed] [Google Scholar]
Additional references
Blumberg 1997
- Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Seminars in Hematology 1997;34(3 Suppl 2):34‐40. [PubMed] [Google Scholar]
Bryson 1998
- Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta‐analysis. The International Study of Perioperative Transfusion. Anesthesia & Analgesia 1998;86(1):9‐15. [DOI] [PubMed] [Google Scholar]
Carless 2004
- Carless P, Moxey AJ, O'Connell D, Henry D. Autologous transfusion techniques: a systematic review of their efficacy. Transfusion Medicine 2004;14:123‐44. [DOI] [PubMed] [Google Scholar]
Carson 1998
- Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al. A pilot randomized trial comparing symptomatic vs hemoglobin‐level‐driven red blood cell transfusion following hip fracture. Transfusion 1998;38(6):522‐9. [DOI] [PubMed] [Google Scholar]
Coyle 1999
- Coyle D, Lee KM, Fergusson DA, Laupacis A. Economic analysis of erythropoietin use in orthopaedic surgery. Transfusion Medicine 1999;9(1):21‐30. [DOI] [PubMed] [Google Scholar]
Faught 1998
- Faught C, Wells P, Fergusson D, Laupacis A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfusion Medicine Reviews 1998;12(3):206‐25. [DOI] [PubMed] [Google Scholar]
Fergusson 1999a
- Fergusson D, Walraven C, Coyle D, Laupacis A. Economic evaluations of technologies to minimize perioperative transfusion: a systematic review of published studies. International Study of Peri‐operative Transfusion (ISPOT) investigators. Transfusion Medicine Reviews 1999;13(2):106‐17. [DOI] [PubMed] [Google Scholar]
Fergusson 1999b
- Fergusson D, Blair A, Henry D, Hisashige A, Huet C, Koopman‐van G, et al. Technologies to minimize blood transfusion in cardiac and orthopedic surgery. Results of a practice variation survey in nine countries. International Study of Peri‐operative Transfusion (ISPOT) Investigators. International Journal of Technology Assessment in Health Care 1999;15(4):717‐28. [PubMed] [Google Scholar]
Forgie 1998
- Forgie MA, Wells PS, Laupacis A, Fergusson D. Preoperative autologous donation decreases allogeneic transfusion but increases exposure to all red blood cell transfusion: results of a meta‐analysis. International Study of Perioperative Transfusion (ISPOT) Investigators. Archives of Internal Medicin 1998;158(6):610‐6. [DOI] [PubMed] [Google Scholar]
Goldman 1997
- Goldman M, Remy‐Prince S, Trepanier A, Decary F. Autologous donation error rates in Canada. Transfusion 1997;37(5):523‐7. [DOI] [PubMed] [Google Scholar]
Hebert 1995
- Hebert PC, Wells G, Marshall J, Martin C, Tweeddale M, Pagliarello G, et al. Transfusion requirements in critical care. A pilot study. Canadian Critical Care Trials Group [published erratum appears in JAMA 1995 Sep 27;274(12):944]. JAMA 1995;273(18):1439‐4. [DOI] [PubMed] [Google Scholar]
Hebert 1999
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. New England Journal of Medicine 1999;340(6):409‐17. [DOI] [PubMed] [Google Scholar]
Henry 2001
- Henry DA, Moxey AJ, Carless PA, O'Connell D, Mcclelland B, Henderson KM, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001886.pub2] [DOI] [PubMed] [Google Scholar]
Huet 1999
- Huet C, Salmi LR, Fergusson D, Koopman‐van Gemert AW, Rubens F, Laupacis A. A meta‐analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesthesia & Analgesia 1999;89(4):861‐9. [DOI] [PubMed] [Google Scholar]
Kimball 1995
- Kimball AM, Berkley S, Ngugi E, Gayle H. International aspects of the AIDS/HIV epidemic. Annual Review of Public Health 1995;16:253‐82. [DOI] [PubMed] [Google Scholar]
Laupacis 1997
- Laupacis A, Fergusson D. Drugs to minimize perioperative blood loss in cardiac surgery: meta‐analyses using perioperative blood transfusion as the outcome. The International Study of Peri‐operative Transfusion (ISPOT) Investigators. Anesthesia & Analgesia 1997;85(6):1258‐67. [DOI] [PubMed] [Google Scholar]
Laupacis 1998
- Laupacis A, Fergusson D. Erythropoietin to minimize perioperative blood transfusion: a systematic review of randomized trials. The International Study of Peri‐operative Transfusion (ISPOT) Investigators. Transfusion Medicine 1998;8(4):309‐17. [DOI] [PubMed] [Google Scholar]
McFarland 1997
- McFarland W, Mvere D, Shandera W, Reingold A. Epidemiology and prevention of transfusion‐associated human immunodeficiency virus transmission in sub‐Saharan Africa. Vox Sanguinis 1997;72(2):85‐92. [DOI] [PubMed] [Google Scholar]
McVay 1990
- McVay PA, Andrews A, Hoag MS, Polan D, Skettino S, Stehling LC, et al. Moderate and severe reactions during autologous blood donations are no more frequent than during homologous blood donations. Vox Sanguinis 1990;59(2):70‐2. [DOI] [PubMed] [Google Scholar]
Whyte 1997
- Whyte GS, Savoia HF. The risk of transmitting HCV, HBV or HIV by blood transfusion in Victoria. Medical Journal of Australia 1997;166(11):584‐6. [DOI] [PubMed] [Google Scholar]


