Abstract
Background
One of the main aims of routine antenatal care is to identify the ‘at risk’ fetus in order to apply clinical interventions which could result in reduced perinatal morbidity and mortality. Doppler ultrasound study of umbilical artery waveforms helps to identify the compromised fetus in ‘high-risk’ pregnancies and, therefore, deserves assessment as a screening test in ‘low-risk’ pregnancies.
Objectives
To assess the effects on obstetric practice and pregnancy outcome of routine fetal and umbilical Doppler ultrasound in unselected and low-risk pregnancies.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group Trials Register (May 2010).
Selection criteria
Randomised and quasi-randomised controlled trials of Doppler ultrasound for the investigation of umbilical and fetal vessels waveforms in unselected pregnancies compared to no Doppler ultrasound. Studies where uterine vessels have been assessed together with fetal and umbilical vessels have been included.
Data collection and analysis
Two authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction.
Main results
We included five trials involving 14,185 women. The methodological quality of the trials was generally unclear because of insufficient data included in the reports.
Routine fetal and umbilical Doppler ultrasound examination in low-risk or unselected populations did not result in increased antenatal, obstetric and neonatal interventions, and no overall differences were detected for substantive short term clinical outcomes such as perinatal mortality. There is no available evidence to assess the effect on substantive long term outcomes such as childhood neurodevelopment and no data to assess maternal outcomes, particularly psychological effects.
Authors’ conclusions
Existing evidence does not provide conclusive evidence that the use of routine umbilical artery Doppler ultrasound, or combination of umbilical and uterine artery Doppler ultrasound in low-risk or unselected populations benefits either mother or baby. Future studies should be designed to address small changes in perinatal outcome, and should focus on potentially preventable deaths.
Medical Subject Headings (MeSH): *Ultrasonography, Doppler; Perinatal Mortality; Pregnancy Outcome; Randomized Controlled Trials as Topic; Ultrasonography, Prenatal [*methods]; Umbilical Arteries [*ultrasonography]; Uterine Artery [ultrasonography]
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Description of the condition
One of the main aims of routine antenatal care is to identify the ‘at risk’ fetus in order to apply clinical interventions which could result in reduced perinatal morbidity and mortality (RCOG 1997). The routine use of a screening test should be based on proven clinical effectiveness, to avoid subjecting a large group of normal women to anxiety and inappropriate intervention with subsequent risk of iatrogenic morbidity and mortality.
In majority of cases the fetal death can be attributed to a ‘known’ cause such as maternal disorder (hypertension, diabetes and others), fetal pathology (congenital abnormalities, intrauterine growth restrictions (IUGR)), placental pathologies or intrapartum complications. The rate of unexplained fetal deaths decreased from 3.5 per 1000 total births in the 1960s to 1.1 to 1.9 per 1000 in the 1990s (Chibber 2005; Fretts 1992; Huang 2000). Unrecognised IUGR remains the main cause of unexplained stillbirths in otherwise uncomplicated pregnancy. Two recent studies identified the fetal growth restriction in 43% (Gardosi 2005) and 52% (Froen 2004) of unexplained stillbirth respectively, concluding that the IUGR was the strongest risk factor for an unexplained intrauterine death.
It is important to highlight that the fetal growth restriction is often confused with the concept of being small-for-gestational age. Some fetuses are constitutionally small and they do not have increased perinatal mortality or morbidity. Inability to distinguish easily between small but healthy fetuses and those who are failing to reach their growth potential has hampered attempts to find appropriate treatment for growth restriction (Soothill 1993). Growth restricted fetuses are at increased risk of mortality and morbidity (Bernstein 2000; Fisk 2001). The serious morbidity includes intraventricular haemorrhage, bronchopulmonary dysplasia, necrotising enterocolitis, infection, pulmonary haemorrhage, hypothermia and hypoglycaemia (Fisk 2001). Early antenatal detection, treatment where appropriate, and timely delivery could minimise the risks significantly.
Description of the intervention
Doppler ultrasound technology is based on the Doppler shift, a physical principle of the change of ultrasound frequency when aimed at the moving object (e.g. red cell) (Campbell 1983; Fitzgerald 1977; Nelson 1988; Owen 2001). Different Doppler methods are used in obstetrics: continuous-wave, pulsed-wave, colour and power Doppler flow (Eik-Nes 1980; Mires 2000).
Doppler ultrasound examination can be performed as a part of a more detailed ultrasound assessment that includes fetal biometry and anatomical survey or as a separate ultrasound examination. Flow of the umbilical and fetal arteries is most often quantified either by pulsatility index or resistant index (Burns 1993; Nelson 1988). These indices reflect the down stream vascular resistance by quantifying the differences between the peak systolic and the enddiastolic velocity within blood vessels of interest in each cardiac cycle. A high ratio in umbilical artery indicates a high vascular impedance and possible feto-placental compromise. In extreme circumstance the blood flow at the end of diastole may be absent or even reversed (Figure 2 - we plan to insert a scan picture in a final version).
Initial Doppler studies have been restricted to the umbilical artery, but other fetal vessels have recently become a focus of interest including middle cerebral artery and ductus venosus.
How the intervention might work
Although stillbirths and fetal complications related to placental problems are rare in uncomplicated pregnancy, the impact is devastating. Current methods for the assessment of fetal well-being and detection of compromised fetus in the routine antenatal care include: symphysis fundal height measurement from the 24th week (Neilson 1998a; NICE 2008), fetal movements charts (Mangesi 2007) and antenatal cardiotocography (Pattison 1999). None of them, however, have proven ability to make an impact on perinatal mortality and morbidity.
Observational and longitudinal studies of Doppler ultrasound in unselected or low-risk pregnancies have raised doubts about its efficacy and authors have cautioned against its introduction into obstetric practice without supportive evidence from randomised trials (Beattie 1989; Goffinet 1997; Sijoms 1989). The relatively low incidence of preventable adverse perinatal outcomes in low-risk and unselected populations present a challenge in evaluating the clinical effectiveness of routine Doppler ultrasound, as large numbers are required to provide definitive evidence.
Why it is important to do this review
Any screening test has not only potential for benefit, but also for harm (Barnett 1995). Subjecting a large group of low-risk patients to a screening test with relatively high false positive rate is likely to cause anxiety and lead to inappropriate intervention and subsequent risk of iatrogenic morbidity and mortality.
Although the epidemiological studies and Cochrane review have found no correlation between the use of fetal Doppler ultrasound and adverse neurological outcome in childhood development, childhood malignancies and birth weight (Neilson 1998b; Salvesen 2007), some concern about the association between the left-handedness in males and exposure to Doppler ultrasound has been expressed (Kieler 2001; Kieler 2002; Salvesen 1999).
Considering that no recent studies have been done regarding the fetal exposure to Doppler ultrasound and the fact that the acoustic output of a modern equipment has increased (Barnett 2001; Duck 1991; Henderson 1997) indicates that Doppler ultrasound in obstetrics should be used only if of proven value (in terms of improved outcome, good specificity and sensitivity).
The continuous assessment of the evidence to provide the balanced view of effectiveness, safety and cost effectiveness is, therefore, essential. In this review, we will focus on fetal and umbilical Doppler ultrasound in low-risk and unselected pregnancies. There are other reviews on ‘Fetal and umbilical Doppler ultrasound in high-risk pregnancies’ (Alfirevic 2009) and on ‘Utero-placental Doppler ultrasound for improving pregnancy outcome’ (a Cochrane review in progress).
OBJECTIVES
To assess the effects of routine fetal and umbilical Doppler ultrasound, or a combination of uterine Doppler ultrasound and umbilical Doppler ultrasound, in unselected and low-risk pregnancies on obstetric practice and pregnancy.
A low-risk population is defined as a population where those considered at risk have been excluded. Criteria of ‘at risk’ are defined variably and this is taken into consideration.
In the context of this review ‘unselected’ pregnant population refers to a mixture of pregnant women with no identified risk factors and those who may have some risk factors but the trialists have not reported them separately.
METHODS
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of routine fetal and umbilical Doppler ultrasound, or a combination of uterine Doppler ultrasound and umbilical Doppler ultrasound, in unselected or low-risk pregnancies. We included quasi-randomised trials, but planned to undertake sensitivity analysis by trial quality. Had we identified studies that were published as conference abstracts only, we would have tried to contact the authors for further details. We would have included them but undertaken sensitivity analyses of trial quality (see Sensitivity analysis).
Types of participants
Pregnant women in both unselected and low-risk populations.
Types of interventions
Routine Doppler ultrasound of the fetal and umbilical artery circulation in pregnancy in unselected or low-risk populations. We included studies that considered the combination of utero-placental Doppler and fetal or umbilical Doppler in normal pregnancies in this review.
If appropriate, we performed stratified analyses of all outcome measures in the following comparisons:
all routine Doppler versus no Doppler/concealed Doppler examinations (i.e. caregivers not aware of results);
single Doppler measurement versus no Doppler/concealed Doppler examinations;
multiple Doppler measurement versus no Doppler/concealed Doppler examinations.
Types of outcome measures
We have selected outcome measures with the help of a proposed core data set of outcome measures (Devane 2007).
Primary outcomes
Any perinatal death after randomisation.
Serious neonatal morbidity - composite outcome including hypoxic ischaemic encephalopathy, intraventricular haemorrhage, bronchopulmonary dysplasia, necrotising enterocolitis.
Secondary outcomes
Stillbirth.
Neonatal death.
Any potentially preventable perinatal death after randomisation (excluding congenital malformations, chromosomal abnormalities, termination of pregnancy).
Fetal acidosis.
Apgar score less than seven at five minutes.
Caesarean section (both elective and emergency).
Elective caesarean section.
Emergency caesarean section.
Spontaneous vaginal birth.
Operative vaginal birth.
Induction of labour.
Neonatal resuscitation required.
Infant requiring intubation/ventilation.
Neonatal fitting/seizures.
Preterm birth (before 37 completed weeks of pregnancy).
Infant respiratory distress syndrome.
Meconium aspiration.
Neonatal admission to special care or intensive care unit, or both.
Infant birthweight.
Gestational age at birth.
Length of infant hospital stay.
Long-term infant/child neurodevelopmental outcome.
Women’s views of care/satisfaction.
We have reported non-prespecified outcomes if we consider them to be important.
Search methods for identification of studies
Electronic searches
We have contacted the Trials Search Co-ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (May 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We have looked for additional studies in the reference lists of the studies identified.
We have not applied any language restrictions.
Data collection and analysis
The methodology for data collection and analysis was based on the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2009a).
Selection of studies
Two review authors have independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We have resolved any disagreement through discussion; there was no need to consult the third author.
Data extraction and management
We have designed a form to extract data. Two review authors have extracted the data using the agreed form. We have resolved discrepancies through discussion. We have entered data into Review Manager software (RevMan 2008), and checked for accuracy. We did not contact authors of the included studies for additional information.
Assessment of risk of bias in included studies
Two review authors have independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009a). We have resolved any disagreement through discussion.
1) Sequence generation (checking for possible selection bias)
We have described the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We have assessed the methods as:
adequate (any truly random process, e.g. random number table; computer random-number generator);
inadequate (any non-random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
2) Allocation concealment (checking for possible selection bias)
We have described the methods used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during, recruitment.
We have assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; alternation; date of birth);
unclear.
3) Blinding (checking for possible performance bias)
We have described all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We have also provided any information relating to whether the intended blinding was effective. Where blinding was not possible, we have assessed whether the lack of blinding was likely to have introduced bias. We have assessed blinding separately for different outcomes or classes of outcomes.
We have assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate inadequate or unclear for outcome assessors.
4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers (compared with the total randomised participants), reasons for attrition/exclusion where reported, and any re-inclusions in analyses which we undertook.
Had there been loss of data greater than 20% we would have considered whether this missing data might impact on outcomes acknowledging that with long-term follow up, complete data are difficult to attain.
5) Selective reporting bias
We have described how the possibility of selective outcome reporting bias was examined by us and what we found.
We have assessed the methods as:
adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre-specified outcomes have been reported; one or more reported primary outcomes were not pre-specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
6) Other sources of bias
We have described any important concerns we have about other possible sources of bias.
We have assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2009a). With reference to (1) to (6) above, we have assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We would have explored the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis) but there were insufficient high-quality studies.
Measures of treatment effect
Where there were multiple pregnancies, we have used the number of women who are randomised as the denominator for maternal outcomes, and the number of babies of women who are randomised as the denominator for neonatal outcomes.
We have also used as the denominator the number of babies of women who were randomised even though some babies could not have attained the outcome; for example, if there was a stillbirth then this baby would not have been able to attain the outcome of ‘Admission to special care baby unit’.
None of the studies reported data on twins, with three studies specifically excluding multiple pregnancies (Davies 1992; Mason 1993; Whittle 1994). We would have contacted a statistician concerning how to deal with the non-independence of this data for multiple pregnancies had it been necessary.
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes were measured in the same way between trials. We have used the standardised mean difference to combine trials that measure the same outcome, but used different methods.
Unit of analysis issues
If there had been several time points for assessment of an outcome, we would have performed separate analyses. In studies including multiple pregnancies because of non-independence, we would have used cluster trial methods in these situations and consulted a statistician to help with the analyses.
Dealing with missing data
For included studies, we have noted levels of attrition. We have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we have carried out analyses, as far as possible, on an intention-to-treat basis: i.e. we have attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised, minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We have assessed statistical heterogeneity in each meta-analysis using the T2 (tau-squared), I2 and Chi2 statistics. We have regarded heterogeneity as substantial if T2 was greater than zero and either I2 was greater than 30% or there was a low P-value (less than 0.10) in the Chi2 test for heterogeneity. Where we found heterogeneity and used random-effects, we have reported the average risk ratio, or average mean difference or average standard mean difference.
Assessment of reporting biases
If there had been 10 or more studies in a meta-analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually, and would have used formal tests for funnel plot asymmetry. For continuous outcomes, we would have used the test proposed by Egger 1997, and for dichotomous outcomes we would have used the tests proposed by Peters 2006. If we had detected asymmetry by any of these tests or by a visual assessment, we would have performed exploratory analyses to investigate it.
Had there been sufficient studies of high quality we would, where we have suspected reporting bias (see ‘Selective reporting bias’ above), have attempted to contact study authors asking them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we have explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We have carried out statistical analysis using the Review Manager software (RevMan 2008). We have used fixed-effect meta-analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we have used random-effects analysis to produce an overall summary, if this was considered clinically meaningful. If an average treatment effect across trials was not clinically meaningful, we have not combined heterogeneous trials. If we used random-effects analyses, the results have been presented as the average treatment effect and its 95% confidence interval, the 95% prediction interval for the underlying treatment effect, and the estimates of T2 and I2 (Higgins 2009b).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following a priori subgroup analyses on all outcomes:
umbilical Doppler ultrasound only or umbilical and uteroplacental Doppler ultrasound.
For fixed-effect meta-analyses, we would have conducted the planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001. For random-effects meta-analyses, we would have assessed differences between subgroups by inspection of the subgroups’ confidence intervals; non-overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
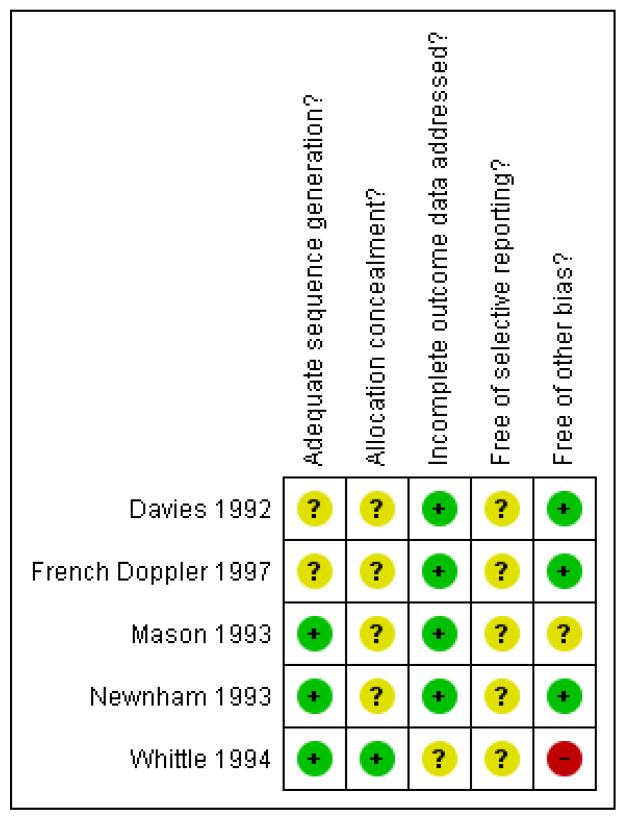
We were to carry out sensitivity analysis to explore the effect of trial quality for the primary outcomes in the review; however, there was only one study (Whittle 1994) of sufficient quality in terms of low risk of bias for sequence generation and concealment allocation although it did suffer from other potential bias (Figure 1). Where there was risk of bias associated with a particular aspect of study quality (e.g. inadequate allocation concealment), we were to explore this by sensitivity analysis.
Figure 1 .
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Publication bias
An assessment of publication bias was desired but considered inappropriate here as only five studies were identified, and it is generally recommended that 10 studies are required for publication bias assessments.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search identified 20 publications, of which we have included five studies involving 14,185 women and 26 meta-analyses. We have excluded eight studies. For further details of trial characteristics, please refer to the tables of Characteristics of included studies and Characteristics of excluded studies.
Included studies
The five included studies were all undertaken in the 1990s. Three studies used fetal/umbilical vessels only (French Doppler 1997; Mason 1993; Whittle 1994). Two studies used both uterine vessels and umbilical vessels (Davies 1992; Newnham 1993). One study looked at a single assessment at 28 to 34 weeks (French Doppler 1997), three studies looked more than one assessment (Davies 1992; Mason 1993; Newnham 1993) and one study had a mixture of some women receiving a single assessment and others more than one assessment (Whittle 1994).
Excluded studies
We excluded five studies because they studied uterine Doppler ultrasound only and not fetal and umbilical Doppler or a combination of uterine plus fetal and umbilical Doppler (Ellwood 1997; Goffinet 2001; Snaith 2006; Subtil 2000; Subtil 2003). These studies will be assessed in a separate review on ‘Utero-placental Doppler ultrasound for improving pregnancy outcome’. We excluded two studies because the previous review authors had tried to contact these authors for information needed for studies to be included and had received no response (Gonsoulin 1991; Schneider 1992). We excluded one study because it had high risk of bias; we needed further information before being able to include it and it only reported one outcome relevant to the review (induction of labour) (Scholler 1993).
Risk of bias in included studies
We assessed risk of bias of each included study according to the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2009a) and summarised in Figure 1.
Allocation
Only one study had adequate sequence generation and allocation concealment (Whittle 1994), although the imbalance between the number of women allocated to the two groups (1642 to the intervention group and 1344 to the control group) would suggest a problem with the randomisation process. Two studies had adequate sequence generation but unclear allocation concealment (Mason 1993; Newnham 1993) and two studies had unclear sequence generation and allocation concealment (Davies 1992; French Doppler 1997).
Blinding
None of the studies could be blinded for clinicians and only ones that had concealed versus revealed groups could be blinded for the women.
Incomplete outcome data
Four studies showed minimal loss of data either by withdrawal after randomisation or by loss to follow up less than 6% (Davies 1992; French Doppler 1997; Mason 1993; Newnham 1993). The fifth study reported no loss of outcome data (Whittle 1994).
Selective reporting
Since we did not assess the trial protocols of the included studies, we cannot comment on whether all the pre-specified outcomes are reported on.
Other potential sources of bias
Three studies appeared to be free from other potential biases (Davies 1992; French Doppler 1997; Newnham 1993) and for one study this seemed unclear (Mason 1993). The fifth study was assessed as having high risk of bias in that there was a considerable difference in the numbers of women allocated to the two groups (1642 and 1344) which probably indicates a problem with the randomisation (Whittle 1994). This was discussed and explained by the authors as “…due to secretarial error in preparation of the envelopes…previously used random numbers had been ‘recycled’ through the study.” This was considered to be a possible high risk of bias in this study.
Effects of interventions
1) All routine Doppler ultrasound versus no Doppler ultrasound (five studies, 14,185 women)
Five studies addressed this comparison (Davies 1992; French Doppler 1997; Mason 1993; Newnham 1993; Whittle 1994).
Primary outcomes
1) Any perinatal death after randomisation
Due to the large heterogeneity for this outcome (Tau2 = 0.18, Chi2 P = 0.10, I2 = 51%; ), we used a random-effects meta-analysis. The average risk ratio (RR) across studies was 0.85 (95% confidence interval (CI) 0.47 to 1.54; four studies, 11,190 women, Analysis 1.1), indicating that on average there is no statistically significant reduction identified in the risk of perinatal death when Doppler ultrasound is used. A prediction interval for the underlying relative risk in any future study is also very wide (95% prediction interval = 0.09 to 8.01), reflecting the large heterogeneity identified and the small number of studies.
Based on a single study, there was no significant difference identified in serious neonatal morbidity (RR 0.99, 95% CI 0.06 to 15.75; one study, 2016 woman, Analysis 1.2).
Secondary outcomes
We found no significant differences in either the average intervention effect estimate across studies where a random-effects meta-analysis was used, nor in the pooled estimate of the intervention effect from a fixed-effect meta-analysis for the whole range of the secondary outcomes (Analyses 1.3 to 1.21). These included Apgar scores less than seven at five minutes, caesarean section, operative vaginal births, spontaneous vaginal births, induction of labour, neonatal resuscitation and preterm birth.
When looking further into outcomes with substantive heterogeneity, we found the following.
Stillbirths: there was no evidence of between-study heterogeneity (Tau2 = 0). Overall, no effect was discernable (Analysis 1.3).
Neonatal death: there was only one study assessing fetal vessels only, showing no statistically significant difference (French Doppler 1997) and two studies using a combination of fetal and utero-placental Doppler (Davies 1992; Newnham 1993) showing large heterogeneity (Analysis 1.4).
Potentially preventable neonatal deaths: there was extremely large heterogeneity for this outcome and the average intervention effect across studies from a random-effects meta-analysis was not significant (average RR 0.82, 95% CI 0.15 to 4.67; three studies, 9395 babies, random effects (Tau2 = 1.87, Chi2 P < 0.01, I2 = 80%), Analysis 1.5). This was despite one subgroup appearing to make a difference.
In subgroup analysis with two studies that assessed Doppler ultrasound used only in fetal/umbilical vessels, the heterogeneity disappeared and, in a fixed-effect meta-analysis, there was a statistically significant reduction potentially preventable perinatal deaths (pooled RR 0.35, 95% CI 0.12 to 0.99; two studies, 6884 women, Analysis 1.5.1).
By contrast, in the one study that used a combination of fetal/umbilical vessels and uterine vessels Doppler assessment there was a statistically significant increase potentially preventable perinatal mortality (RR 3.95, 95% CI 1.32 to 11.77; one study, 2475 women, Analysis 1.5.2). Again, caution is strongly advised here as these are subgroup analyses of secondary outcomes containing small number of studies.
Preterm birth: the subgroup analysis failed to provide an explanation of significant heterogeneity for this outcome. (Analysis 1.17).
2) Single Doppler ultrasound assessment versus no Doppler ultrasound (one study, 3898 women)
One study addressed this comparison (French Doppler 1997).
Primary outcomes
Based on a single study, there was no statistically significant difference identified in any perinatal death after randomisation (RR 0.33, 95% CI 0.09 to 1.23; one study, 3898 women, Analysis 2.1). Serious neonatal morbidity was not assessed.
Secondary outcomes
There were no statistically significant differences identified in any of the secondary outcomes which were assessed (Graphs 2.3 to 2.21).
3) Multiple Doppler ultrasound assessments versus no Doppler ultrasound (three studies, 7301 women)
Three studies addressed this comparison (Davies 1992; Mason 1993; Newnham 1993). One study combined the data from women having one assessment and some having more than one assessment (Whittle 1994); these data are only included in Comparison 1 - ‘All routine Doppler ultrasound versus no Doppler routine ultrasound’.
Primary outcomes
Perinatal deaths showed large heterogeneity (Tau2 = 0.13, Chi2 P = 0.14, I2 = 49%). A random-effects meta-analysis showed that the average intervention effect across studies was not statistically significant (average RR 1.00, 95% CI: 0.55 to 1.80; three studies, 7292 women, Analysis 3.1); the prediction interval for the intervention effect in any future study was an even wider 95% CI. From a single study, for the serious neonatal morbidity outcome the intervention effect was again clearly not significant (RR 0.99, 95% CI 0.06 to 15.75; one study, 2016 women, Analysis 3.2) (Mason 1993).
Secondary outcomes
There were no statistically significant differences identified in any of the secondary outcomes which were assessed (Analyses 3.3 to 3.21).
DISCUSSION
This review includes data from 14,185 women from five studies (Davies 1992; French Doppler 1997; Mason 1993; Newnham 1993; Whittle 1994). No differences in perinatal mortality were demonstrated, although there was considerable heterogeneity and the number of participants remains too small to detect small but potentially significant changes in perinatal outcome (Chalmers 1989).
The pooled data from two studies using umbilical artery Doppler showed a significant reduction in potentially preventable perinatal mortality (French Doppler 1997; Whittle 1994). However, the results from Davies suggested that routine Doppler ultrasound in unselected pregnancies assessing both umbilical and uterine artery Doppler may do more harm than good, but authors acknowledged that increase in perinatal deaths was an unexpected finding which may have occurred by chance (Davies 1992). Furthermore, they state that the study was not designed to test the ability of routine Doppler ultrasound examinations to reduce perinatal mortality, as a much larger number of women would need to be included in a such a trial to test this hypothesis.
In the Perth study (Newnham 1993), there was an unexpected finding of a greater risk of intrauterine growth restriction in the serial ultrasound and Doppler examination group (i.e. the intensive monitoring group). The authors report “A written diagnosis of intrauterine growth restriction was observed more frequently in the medical records of women in the intensive group than in the regular group (relative risk 2.07; 95% CI 1.34 to 3.21)” but they do not provide data in a format in which we can include in our review (we have written to the authors and Lancet to try to obtain this data). The authors state that multiple logistic regression analyses indicated that this was probably not a chance effect, and it is possible that frequent exposure to ultrasound may have influenced fetal growth. This finding was not associated with increased perinatal morbidity and mortality, and follow up of these children at one year of age found that the difference in growth was no longer discernible (Newnham 1996). This is, however, a further finding which suggests more harm than good, and the authors stress the need for further investigation of the effects of frequent ultrasound exposure on fetal growth.
AUTHORS’ CONCLUSIONS
Implications for practice
Existing data do not provide conclusive evidence that the use of routine umbilical artery Doppler ultrasound, or combination of umbilical and uterine artery Doppler ultrasound in low-risk or unselected populations benefits either mother or baby. At present, Doppler ultrasound examination should be reserved for use in high-risk pregnancies (Alfirevic 2009).
Implications for research
If there is to be future research into fetal and umbilical Doppler ultrasound examination in low-risk or unselected populations, a large trial with adequate power to test hypotheses related to perinatal outcome is required. Trials should focus on potentially preventable deaths and inclusion criteria should reflect that. It would be also important to include assessment of neurodevelopment assessment of maternal outcomes and psychological effects on mother.
PLAIN LANGUAGE SUMMARY.
Doppler ultrasound of fetal blood vessels in normal pregnancies
One of the main aims of routine antenatal care is to identify babies who are not thriving in the womb. It is possible that medical interventions might improve outcomes for these babies, if they can be identified. Doppler ultrasound uses sound waves to detect the movement of blood in vessels. It is used in pregnancy to study blood circulation in the baby, uterus and placenta. Using it in high-risk pregnancies, where there is concern about baby’s condition, shows benefits. However, its value as a screening tool in all pregnancies needs to be assessed as there is a possibility of unnecessary interventions and adverse effects. The review of trials of routine Doppler ultrasound of the baby’s vessels in pregnancy identified five studies involving more than 14,000 women and babies. The studies were not of high quality and were all undertaken in the 1990s. There were no improvements identified for either the baby or the mother, though more data would be needed to prove whether it is effective or not for improving outcomes.
ACKNOWLEDGEMENTS
We are grateful to Professor AM Weindling (Professor of Perinatal Medicine, University of Liverpool) for advice on neonatal outcomes to the authors of the original version of this review (Bricker 2007). Also to Dr JA Davies, and to Professor J Newnham and Dr Sharon Evans for also providing further information on their trials (Davies 1992; Newnham 1993) to the authors of the original version of this review (Bricker 2007).
Our thanks to Leanne Bricker and James P Neilson for their work on the previous version of this review.
Richard Riley who provided help with the statistical analysis, in particular with the random-effects analyses and prediction intervals.
SOURCES OF SUPPORT
Internal sources
The University of Liverpool, UK.
External sources
National Institute for Health Research, UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews: CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. Individual women. | |
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental intervention: Doppler ultrasound of umbilical-artery and uterine-artery
Control/Comparison intervention: no Doppler ultrasound
Multiple estimations were at 20 and 32 weeks’ gestation. |
|
| Outcomes | Number of days of antenatal admission; number of CTG recordings and US scans; gestational age at birth; mode of birth; birthweight; Apgar scores; need for resuscitation (intermittent positive pressure ventilation either via a mask or endotracheal tube); admission to NICU; fetal and neonatal outcomes The study was not designed to test the ability of Doppler ultrasound to reduce PNM, so the fact that there were more preventable deaths in the Doppler group is likely to be due to chance. However, the authors do theorise that it is possible that a woman’s knowledge of a normal result may have resulted in her taking less notice of symptoms that might otherwise have resulted in a review of fetal well-being |
|
| Notes | London (UK) 1992 study in previous version of the review (Bricker 2007). | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No information provided. |
| Allocation concealment? | Unclear | “…cards in sealed opaque envelopes….” no mention of numbers sequence. The handbook says: “…sequentially numbered opaque sealed envelopes…” and that to be really sure “Envelopes were sequentially numbered and opened sequentially only after participants details were written on the envelope” |
| Incomplete outcome data addressed? All outcomes |
Yes | Describe any loss of participants to follow up at each data collection point. Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not has the data been able to be re-included?
|
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | If the study was stopped early, explain the reasons:
Describe any baseline in balance:
Describe any differential diagnosis:
|
| Methods | Multicentre randomised controlled trial. Individual women. Randomisation in blocks of 4 | |
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental intervention: umbilical Doppler ultrasound
Control/comparison intervention: no Doppler ultrasound
Single estimation between 28-34 weeks. |
|
| Outcomes |
|
|
| Notes | France 1997 study in previous version of the review (Bricker 2007). Authors did report umbilical cord pH < 7.20 but only on a subsample of women and the groups were not randomised groups. Findings were: Doppler 188 / 757 (24.8%) and no Doppler 181/761 (23.8%) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | “…randomly divided…” |
| Allocation concealment? | Unclear |
|
| Incomplete outcome data addressed? All outcomes |
Yes | Describe any loss of participants to follow up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not has the data been able to be re-included?
|
| Free of selective reporting? | Unclear | We have not assessed the trial protocol. |
| Free of other bias? | Yes | There appear to be no other biases. |
| Methods | Randomised controlled trial, individual women. | |
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental intervention: umbilical artery Doppler ultrasound
Control/comparison intervention: routine care, no Doppler ultrasound
Multiple estimations were at 28 and 34 weeks’ gestation. |
|
| Outcomes | Main outcome: obstetric intervention rate, short-term neonatal morbidity | |
| Notes | Leeds (UK) 1993 study in previous version of the review (Bricker 2007). | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | “Tables of random numbers were used to generate each random permuted block.” |
| Allocation concealment? | Unclear | “…opaque numbered envelopes which were opened on the fetal assessment unit by a radiographer who had no personal knowledge of the women or her history.” No mention if enveloped were sealed. |
| Incomplete outcome data addressed? All outcomes |
Yes | Describe any loss of participants to follow up at each data collection point:
Describe any exclusion of participants after randomisation:
|
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Unclear | If the study was stopped early, explain the reasons:
Describe any baseline in balance:
Describe any differential diagnosis:
|
| Methods | Randomised controlled trial. Individual women. | |
| Participants | Inclusion criteria
|
|
| Interventions | Experimental intervention: umbilical and uterine Doppler US - intense monitoring group
Control/Comparison intervention: no Doppler US - regular group
Multiple estimations were at 18, 24, 28, 34 and 38 weeks’ gestation |
|
| Outcomes | Induction of labour; caesarean section; ultrasound information | |
| Notes | Perth (Aus) 1993 study in previous version of the review (Bricker 2007). Authors report an increase in IUGR with the Doppler group (RR 2.07, 95% CI 1.34 to 3.21) but do not provide the data for us to enter into RevMan. They report “Multiple logistic regression analyses showed that the increased proportion of growth-restricted fetuses in the intensive arm was not due to a chance effect from differential clustering within the two groups…” though they go on to say that while this may have been a chance finding, it is possible that frequent exposure to ultrasound may have influenced fetal growth. This finding was not associated with increased perinatal morbidity and mortality, and follow up of these children at 1 year of age found that the difference in growth was no longer discernible. We are trying to contact the authors and are writing to the journal to seek further data |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | “…computer generated random numbers…” |
| Allocation concealment? | Unclear |
|
| Incomplete outcome data addressed? All outcomes |
Yes | Describe any loss of participants to follow up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not has the data been able to be re-included?
|
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | Yes | If the study was stopped early, explain the reasons:
Describe any baseline in balance:
Describe any differential diagnosis:
|
| Methods | Randomised controlled trial. Individual women. | |
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental intervention: umbilical Doppler US revealed
Control/Comparison intervention: no Doppler
Multiple estimations were at 26-30 weeks and 34-36 weeks. Of the 2986 women in the study, 1386 underwent examination at both gestational windows, 1056 at only the first and 544 at only the second |
|
| Outcomes | Antenatal complications; antenatal admissions; day care visits; elective delivery; elective CS; CS in labour; CS for FD; birth < 32 weeks; Apgar scores; small for dates; admission to SCBU; ventilations; stillbirth | |
| Notes | Glasgow (UK) 1994 study in previous version of the review (Bricker 2007). | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | “the order was generated by random-number tables.” |
| Allocation concealment? | Yes | “sealed opaque envelopes” “numbered.” |
| Incomplete outcome data addressed? All outcomes |
Unclear | Describe any loss of participants to follow up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not has the data been able to be re-included? |
| Free of selective reporting? | Unclear | We did not assess the trial protocol. |
| Free of other bias? | No | If the study was stopped early, explain the reasons:
Describe any baseline in balance:
Describe any differential diagnosis:
Also:
|
AN: antenatal
BP: blood pressure
CTG: cardiotocography
FD:
ITT: intention to treat
IUGR: intrauterine growth restriction
NICU: neonatal intensive care unit
PIH: pregnancy-induced hypertension
PNM: perinatal mortality
SCBU: special care baby unit
SGA: small-for-gestational age
US: ultrasound
VS: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ellwood 1997 | Trial studied uterine Doppler ultrasound and not fetal and umbilical |
| Goffinet 2001 | Trial studied uterine Doppler ultrasound and not fetal and umbilical |
| Gonsoulin 1991 | Conference abstract - not clear whether high-risk/low-risk/unselected pregnancies, and no data suitable for inclusion. Further details were sought from the authors by the authors of the previous version of this review (L Bricker and JP Neilson), without success. |
| Schneider 1992 | Conference abstract in English language identified - unexplained difference in numbers (250 vs 329) in Doppler vs control groups suggesting allocation bias. The definitive publication after translation from German did not explain this difference and failed to outline the trial methodology |
| Scholler 1993 | This study was translated from German for us. It was a quasi RCT of 211 women undergoing Doppler ultrasound vs no Doppler ultrasound. It was excluded for a combination of the following reasons: the only outcome relevant to our review was induction of labour; the study had high risk of bias being a quasi RCT; further information was needed from the authors before these data could be included. Data reported for induction of labour: Doppler group 37/108 and no Doppler group 41/103 |
| Snaith 2006 | Trial studied uterine Doppler ultrasound and not fetal and umbilical |
| Subtil 2000 | Trial studied uterine Doppler ultrasound and not fetal and umbilical |
| Subtil 2003 | Trial studied uterine Doppler ultrasound and not fetal and umbilical |
RCT: randomised controlled trial
vs: versus
DATA AND ANALYSES
Comparison 1.
All routine Doppler ultrasound versus no Doppler ultrasound
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 4 | 11190 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.47, 1.54] |
| 1.1 Fetal/umbilical vessels only | 2 | 5914 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.20, 1.29] |
| 1.2 Fetal/umbilical vessels + uterine artery | 2 | 5276 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.47, 2.38] |
| 2 Serious neonatal morbidity | 1 | 2016 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.06, 15.75] |
| 2.1 Fetal/umbilical vessels only | 1 | 2016 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.06, 15.75] |
| 2.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Stillbirth | 4 | 12160 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.32, 1.97] |
| 3.1 Fetal/umbilical vessels only | 2 | 6884 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.12, 0.95] |
| 3.2 Fetal/umbilical vessels + uterine artery | 2 | 5276 | Risk Ratio (M-H, Random, 95% CI) | 1.41 [0.44, 4.46] |
| 4 Neonatal death | 3 | 9174 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.09, 5.23] |
| 4.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Random, 95% CI) | 0.25 [0.03, 2.23] |
| 4.2 Fetal/umbilical vessels + uterine artery | 2 | 5276 | Risk Ratio (M-H, Random, 95% CI) | 1.44 [0.04, 54.93] |
| 5 Potentially preventable perinatal death | 3 | 9359 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.15, 4.67] |
| 5.1 Fetal/umbilical vessels only | 2 | 6884 | Risk Ratio (M-H, Random, 95% CI) | 0.35 [0.12, 0.99] |
| 5.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Random, 95% CI) | 3.95 [1.32, 11.77] |
| 6 Fetal acidosis | 1 | 1518 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.87, 1.25] |
| 6.1 Fetal/umbilical vessels only | 1 | 1518 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.87, 1.25] |
| 6.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Apgar score < 7 at 5 minutes | 4 | 11375 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.56, 1.39] |
| 7.1 Fetal/umbilical vessels only | 3 | 8900 | Risk Ratio (M-H, Fixed, 95% CI) | 0.78 [0.47, 1.29] |
| 7.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.48 [0.53, 4.14] |
| 8 Caesarean section (elective and emergency) | 2 | 6373 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.85, 1.13] |
| 8.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 8.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.74, 1.29] |
| 9 Elective caesarean section | 4 | 11375 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.87, 1.18] |
| 9.1 Fetal/umbilical vessels only | 3 | 8900 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.87, 1.23] |
| 9.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.70, 1.28] |
| 10 Emergency caesarean section | 2 | 6373 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.74, 1.18] |
| 10.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.91 [0.71, 1.17] |
| 10.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.52, 2.59] |
| 11 Spontaneous vaginal birth | 2 | 6373 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.96, 1.02] |
| 11.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.94, 1.02] |
| 11.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.95, 1.06] |
| 12 Operative vaginal birth | 2 | 6884 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.96, 1.12] |
| 12.1 Fetal/umbilical vessels only | 2 | 6884 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.96, 1.12] |
| 12.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13 Induction of labour | 4 | 11190 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.97, 1.12] |
| 13.1 Fetal/umbilical vessels only | 2 | 5914 | Risk Ratio (M-H, Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 13.2 Fetal/umbilical vessels + uterine artery | 2 | 5276 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.93, 1.10] |
| 14 Neonatal resuscitation | 2 | 6373 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.84, 1.24] |
| 14.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 14.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
| 15 Infant intubation/ventilation | 1 | 2986 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.54, 1.81] |
| 15.1 Fetal/umbilical vessels only | 1 | 2986 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.54, 1.81] |
| 15.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16 Neonatal seizures/fits | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Fetal/umbilical vessels only | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Preterm birth (before 37 weeks) | 4 | 12162 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.87, 1.18] |
| 17.1 Fetal/umbilical vessels only | 2 | 6884 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.81, 1.29] |
| 17.2 Fetal/umbilical vessels + uterine artery | 2 | 5278 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.83, 1.24] |
| 18 Infant respiratory distress syndrome | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Meconium aspiration | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Neonatal admission to SCBU/NICU | 3 | 7477 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.84, 1.17] |
| 20.1 Fetal/umbilical vessels only | 2 | 5002 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.82, 1.18] |
| 20.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.67, 1.53] |
| 21 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Birthweight | 2 | 5914 | Mean Difference (IV, Fixed, 95% CI) | −17.55 [−42.23, 7.13] |
| 22.1 Fetal/umbilical vessels only | 2 | 5914 | Mean Difference (IV, Fixed, 95% CI) | −17.55 [−42.23, 7.13] |
| 22.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23 Gestational age at birth | 2 | 5914 | Mean Difference (IV, Fixed, 95% CI) | −0.08 [−0.16, −0.00] |
| 23.1 Fetal/umbilical vessels only | 2 | 5914 | Mean Difference (IV, Fixed, 95% CI) | −0.08 [−0.16, −0.00] |
| 23.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 2.
Single Doppler ultrasound assessment versus no Doppler ultrasound
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.09, 1.23] |
| 1.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.33 [0.09, 1.23] |
| 1.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2 Serious neonatal morbidity | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 2.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Stillbirth | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 3.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 3.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 4 Neonatal death | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.03, 2.23] |
| 4.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.25 [0.03, 2.23] |
| 4.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5 Potentially preventable perinatal death | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.29 [0.06, 1.37] |
| 5.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.29 [0.06, 1.37] |
| 5.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6 Fetal acidosis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Apgar score < 7 at 5 minutes | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.38, 2.66] |
| 7.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.38, 2.66] |
| 7.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8 Caesarean section (elective and emergency) | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 8.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 8.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 9 Elective caesarean section | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 9.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 9.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10 Emergency caesarean section | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.91 [0.71, 1.17] |
| 10.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.91 [0.71, 1.17] |
| 10.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11 Spontaneous vaginal birth | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.94, 1.02] |
| 11.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.94, 1.02] |
| 11.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12 Operative vaginal birth | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.10 [0.95, 1.26] |
| 12.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.10 [0.95, 1.26] |
| 12.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13 Induction of labour | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.14 [0.99, 1.33] |
| 13.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.14 [0.99, 1.33] |
| 13.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14 Neonatal resuscitation | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 14.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 14.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15 Infant intubation/ventilation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16 Neonatal seizures/fits | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Fetal/umbilical vessels only | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Preterm birth (before 37 weeks) | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.20 [0.86, 1.69] |
| 17.1 Fetal/umbilical vessels only | 1 | 3898 | Risk Ratio (M-H, Fixed, 95% CI) | 1.20 [0.86, 1.69] |
| 17.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18 Infant respiratory distress syndrome | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Meconium aspiration | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Neonatal admission to SCBU/NICU | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Birthweight | 1 | 3898 | Mean Difference (IV, Fixed, 95% CI) | −14.0 [−42.94, 14.94] |
| 22.1 Fetal/umbilical vessels only | 1 | 3898 | Mean Difference (IV, Fixed, 95% CI) | −14.0 [−42.94, 14.94] |
| 22.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23 Gestational age at birth | 1 | 3898 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−0.19, −0.01] |
| 23.1 Fetal/umbilical vessels only | 1 | 3898 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−0.19, −0.01] |
| 23.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Comparison 3.
Multiple Doppler ultrasound assessments versus no Doppler ultrasound
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 3 | 7292 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.55, 1.80] |
| 1.1 Fetal/umbilical vessels only | 1 | 2016 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.21, 2.93] |
| 1.2 Fetal/umbilical vessels + uterine artery | 2 | 5276 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.47, 2.38] |
| 2 Serious neonatal morbidity | 1 | 2016 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.06, 15.75] |
| 2.1 Fetal/umbilical vessels only | 1 | 2016 | Risk Ratio (M-H, Fixed, 95% CI) | 0.99 [0.06, 15.75] |
| 2.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 3 Stillbirth | 2 | 5276 | Risk Ratio (M-H, Random, 95% CI) | 1.41 [0.44, 4.46] |
| 3.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 3.2 Fetal/umbilical vessels + uterine artery | 2 | 5276 | Risk Ratio (M-H, Random, 95% CI) | 1.41 [0.44, 4.46] |
| 4 Neonatal death | 2 | 5276 | Risk Ratio (M-H, Random, 95% CI) | 1.44 [0.04, 54.93] |
| 4.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 4.2 Fetal/umbilical vessels + uterine artery | 2 | 5276 | Risk Ratio (M-H, Random, 95% CI) | 1.44 [0.04, 54.93] |
| 5 Potentially preventable perinatal death | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 3.95 [1.32, 11.77] |
| 5.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 5.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 3.95 [1.32, 11.77] |
| 6 Fetal acidosis | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 6.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 7 Apgar score < 7 at 5 minutes | 2 | 4491 | Risk Ratio (M-H, Fixed, 95% CI) | 0.93 [0.48, 1.80] |
| 7.1 Fetal/umbilical vessels only | 1 | 2016 | Risk Ratio (M-H, Fixed, 95% CI) | 0.66 [0.27, 1.60] |
| 7.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.48 [0.53, 4.14] |
| 8 Caesarean section (elective and emergency) | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.74, 1.29] |
| 8.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 8.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.74, 1.29] |
| 9 Elective caesarean section | 2 | 4491 | Risk Ratio (M-H, Fixed, 95% CI) | 0.90 [0.70, 1.16] |
| 9.1 Fetal/umbilical vessels only | 1 | 2016 | Risk Ratio (M-H, Fixed, 95% CI) | 0.79 [0.49, 1.29] |
| 9.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.70, 1.28] |
| 10 Emergency caesarean section | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.52, 2.59] |
| 10.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 10.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 [0.52, 2.59] |
| 11 Spontaneous vaginal birth | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.95, 1.06] |
| 11.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 11.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.95, 1.06] |
| 12 Operative vaginal birth | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 12.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 13 Induction of labour | 3 | 7292 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 13.1 Fetal/umbilical vessels only | 1 | 2016 | Risk Ratio (M-H, Fixed, 95% CI) | 1.00 [0.83, 1.21] |
| 13.2 Fetal/umbilical vessels + uterine artery | 2 | 5276 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.93, 1.10] |
| 14 Neonatal resuscitation | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
| 14.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 14.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
| 15 Infant intubation/ventilation | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 15.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16 Neonatal seizures/fits | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.1 Fetal/umbilical vessels only | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 16.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 17 Preterm birth (before 37 weeks) | 3 | 8264 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.82, 1.15] |
| 17.1 Fetal/umbilical vessels only | 1 | 2986 | Risk Ratio (M-H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 17.2 Fetal/umbilical vessels + uterine artery | 2 | 5278 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.83, 1.24] |
| 18 Infant respiratory distress syndrome | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 18.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19 Meconium aspiration | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 19.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 20 Neonatal admission to SCBU/NICU | 2 | 4491 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.71, 1.34] |
| 20.1 Fetal/umbilical vessels only | 1 | 2016 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.56, 1.52] |
| 20.2 Fetal/umbilical vessels + uterine artery | 1 | 2475 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.67, 1.53] |
| 21 Women’s views/satisfaction | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.1 Fetal/umbilical vessels only | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 21.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Risk Ratio (M-H, Fixed, 95% CI) | Not estimable |
| 22 Birthweight | 1 | 2016 | Mean Difference (IV, Fixed, 95% CI) | −27.0 [−74.23, 20.23] |
| 22.1 Fetal/umbilical vessels only | 1 | 2016 | Mean Difference (IV, Fixed, 95% CI) | −27.0 [−74.23, 20.23] |
| 22.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 23 Gestational age at birth | 1 | 2016 | Mean Difference (IV, Fixed, 95% CI) | −0.02 [−0.19, 0.15] |
| 23.1 Fetal/umbilical vessels only | 1 | 2016 | Mean Difference (IV, Fixed, 95% CI) | −0.02 [−0.19, 0.15] |
| 23.2 Fetal/umbilical vessels + uterine artery | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
HISTORY
Protocol first published: Issue 2, 1999
Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 6 November 2008 | Amended | Converted to new review format. |
| 5 February 2007 | Amended | Review withdrawn from publication. |
| 14 January 2000 | New citation required and conclusions have changed | Substantive amendment |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
We updated the Background and Methods sections and changed the title from ‘Routine Doppler ultrasound in normal pregnancy’ to ‘Fetal and umbilical Doppler ultrasound in normal pregnancy’.
We changed the outcome of ‘Preterm labour (onset of labour before 37 weeks)’ to ‘Preterm birth (birth less than 37 weeks)’ because this was the outcome reported in the studies.
We have modified the wording in the methods sections for Assessment of heterogeneity, Assessment of reporting biases and Data synthesis to update them with the new methods being used by the group, developed in conjunction with the group’s statisticians, Simon Gates and Richard Riley. We have used these new methods in the review.
WHAT’S NEW
Last assessed as up-to-date: 30 May 2010.
| Date | Event | Description |
|---|---|---|
| 28 January 2010 | New citation required but conclusions have not changed | New review team substantially updated the review. |
| 20 May 2009 | New search has been performed | Search updated. Six new trials excluded (Ellwood 1997; Goffinet 2001; Scholler 1993; Snaith 2006; Subtil 2000; Subtil 2003). |
Footnotes
DECLARATIONS OF INTEREST None known.
References to studies included in this review
- Davies 1992 {published and unpublished data} .Breart G, Uzan S, Uzan M. Doppler ultrasound screening during pregnancy [Letter; comment] Lancet. 1993;341(8843):501–2. doi: 10.1016/0140-6736(93)90263-g. [DOI] [PubMed] [Google Scholar]; Davies J, Spencer J, Gallivan S. Randomised trial of Doppler screening in a general obstetric population. Proceedings of the 26th British Congress of Obstetrics and Gynaecology; Manchester, UK. July 7-10.1992. 1992. [Google Scholar]; Davies JA, Gallivan S, Spencer JAD. Randomised controlled trial of doppler ultrasound screening of placental perfusion during pregnancy. Lancet. 1992;340:1299–303. [PubMed] [Google Scholar]; Spencer JAD, Davies JA, Gallivan S. Randomised trial of routine Doppler screening during pregnancy. Journal of Maternal Fetal Investigation. 1992;1:126. [Google Scholar]
- French Doppler 1997 {published data only} .Doppler French Study Group A randomised controlled trial of Doppler ultrasound velocimetry of the umbilical artery in low risk pregnancies. British Journal of Obstetrics and Gynaecology. 1997;104:419–22. doi: 10.1111/j.1471-0528.1997.tb11492.x. [DOI] [PubMed] [Google Scholar]
- Mason 1993 {published data only} .Mason GC, Lilford RJ, Porter J, Nelson E, Tyrell S. Randomised comparison of routine versus highly selective use of Doppler ultrasound in low risk pregnancies. British Journal of Obstetrics and Gynaecology. 1993;100:130–3. doi: 10.1111/j.1471-0528.1993.tb15207.x. [DOI] [PubMed] [Google Scholar]
- Newnham 1993 {published and unpublished data} .Evans S, Newnham J, MacDonald W, Hall C. Characterisation of the possible effect on birthweight following frequent prenatal ultrasound examinations. Early Human Development. 1996;45(3):203–14. doi: 10.1016/0378-3782(96)01728-8. [DOI] [PubMed] [Google Scholar]; Newnham J, MacDonald W, Gurrin L, Evans S, Landau L, Stanley F. The effect of frequent prenatal ultrasound on birthweight: follow up at one year of age. Proceedings of the 14th Australian Perinatal Society in conjunction with the New Zealand Perinatal Society; Adelaide, Australia. 1996 March 24-27.1996. [Google Scholar]; Newnham JP, Doherty DA, Kendall GE, Zubrick SR, Landau LL, Stanley FJ. Effects of repeated prenatal ultrasound examinations on childhood outcome up to 8 years of age: follow-up of a randomised controlled trial. Lancet. 2004;364:2038–44. doi: 10.1016/S0140-6736(04)17516-8. [DOI] [PubMed] [Google Scholar]; Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet. 1993;342:887–91. doi: 10.1016/0140-6736(93)91944-h. [DOI] [PubMed] [Google Scholar]
- Whittle 1994 {published data only} .Hanretty KP. Randomized study of doppler waveforms in umbilical and uterine arteries as a screening method to identify the compromised fetus. Personal communication. 1988 [Google Scholar]; Whittle MJ, Hanretty KP, Primrose MH, Neilson JP. Screening for the compromised fetus: A randomised trial of umbilical artery velocimetry in unselected pregnancies. American Journal of Obstetrics and Gynecology. 1994;170(2):555–9. doi: 10.1016/s0002-9378(94)70226-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ellwood 1997 {unpublished data only} .Ellwood D, Peek M, Curren J. Predicting adverse pregnancy outcomes with ultrasound. A randomised controlled trial. Personal communication. 1997 [Google Scholar]
- Goffinet 2001 {published data only} .Goffinet F, Aboulker D, Paris-Llado J, Bucourt M, Uzan M, Papiernik E, et al. Screening with a uterine doppler in low risk pregnant women followed by low dose aspirin in women with abnormal results: a multicenter randomised controlled trial. British Journal of Obstetrics and Gynaecology. 2001;108:510–8. doi: 10.1111/j.1471-0528.2001.00116.x. [DOI] [PubMed] [Google Scholar]
- Gonsoulin 1991 {published data only} .Gonsoulin MD. Umbilical artery Doppler waveform analysis: a randomized study on effect on outcome. American Journal of Obstetrics and Gynecology. 1991;164:370. [Google Scholar]
- Schneider 1992 {published data only} .Schneider KT, Amberg-Wendland D, Renz S, Furstenau U. Prospective randomized study of the clinical value of Doppler sonography as a screening procedure. Gynakologische Rundschau. 1991;31(Suppl 1):139–40. [PubMed] [Google Scholar]; Schneider KTM, Renz S, Furstenau U, Amberg-Wendland D, Prochaska D, Graeff H. Doppler flow measurements as a screening method during pregnancy: Is it worth the effort? Journal of Maternal Fetal Investigation. 1992;1:125. [Google Scholar]
- Scholler 1993 {published data only} .Scholler J, Putz M, Sainz HG, Altrichter R, Philipp K. Value of Doppler sonography in management of non-risk pregnancies at term [Der Stellenwert der Dopplersonographie bei der Betreuung von Nicht–Risikoschwangerschaften am Geburtstermin] Gynakologische Rundschau. 1993;33(1 Suppl):118–9. doi: 10.1159/000272187. [DOI] [PubMed] [Google Scholar]
- Snaith 2006 {published data only} .Snaith V. [accessed 21 March 2006];Support and reassurance in antenatal care. Current Controlled Trials. http://controlled-trials.com/mrct
- Subtil 2000 {published data only} .Subtil D, Truffert P, Goeusse P, Dufour P, Uzan S, Breart G, et al. Value of systematic doppler +/− low dose aspirin to prevent vascular complications in primigravidae. Hypertension in Pregnancy. 2000;19(Suppl 1):9. [Google Scholar]
- Subtil 2003 {published data only} .Subtil D, Goeusse P, Houfflin-Debarge V, Puech F, Lequien P, Breart G, et al. Randomised comparison of uterine artery doppler and aspirin (100 mg) with placebo in nulliparous women: the essai regional aspirine mere-enfant study (part 2) BJOG: an international journal of obstetrics and gynaecology. 2003;110(5):485–91. doi: 10.1046/j.1471-0528.2003.t01-1-02097.x. [DOI] [PubMed] [Google Scholar]
Additional references
- Alfirevic 2009 .Alfirevic Z, Stampalija T, Gyte GML, Neilson JP. Fetal and umbilical Doppler ultrasound in high risk pregnancies. Cochrane Database of Systematic Reviews. 2009;(Issue 1) [DOI: 10.1002/14651858.CD007529] [Google Scholar]
- Barnett 1995 .Barnett SB. Ultrasound safety in obstetrics: What are the concerns? Ultrasound Quarterly. 1995;13(4):228–39. [Google Scholar]
- Barnett 2001 .Barnett SB, Maulik D. Guidelines and recommendations for safe use of Doppler ultrasound in perinatal applications. Journal of Maternal-Fetal Medicine. 2001;10:75–84. doi: 10.1080/714904312. [DOI] [PubMed] [Google Scholar]
- Beattie 1989 .Beattie RB, Dornan JC. Antenatal screening for intrauterine growth retardation with umbilical artery Doppler ultrasonography. BMJ. 1989;298(6674):631–5. doi: 10.1136/bmj.298.6674.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein 2000 .Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. American Journal of Obstetrics and Gynecology. 2000;182:198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- Burns 1993 .Burns PN. Principles of Doppler and color flow. Radiology in Medicine. 1993;85:3–16. [PubMed] [Google Scholar]
- Campbell 1983 .Campbell S, Diaz-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Wilson K, et al. New Doppler technique for assessing utero-placental blood flow. Lancet. 1983;i:675–77. doi: 10.1016/s0140-6736(83)91970-0. [DOI] [PubMed] [Google Scholar]
- Chalmers 1989 .Chalmers I. Evaluating the effects of care during pregnancy and childbirth. In: Chalmers I, Enkin M, Keirse MJNC, editors. Effective care in pregnancy and childbirth. Vol. 1. Oxford University Press; Oxford: 1989. pp. 3–38. [Google Scholar]
- Chibber 2005 .Chibber R. Unexplained antenatal fetal deaths: what are the determinants? Archives of Gynecology and Obstetrics. 2005;271:286–91. doi: 10.1007/s00404-004-0606-1. [DOI] [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Eggar M, Davey Smith G, Altman DJ, editors. Systematic reviews in health care: meta-analysis in context. BMJ; London: 2001. [Google Scholar]
- Devane 2007 .Devane D, Begley CM, Clarke M, Horey D, OBoyle C. Evaluating maternity care: a core set of outcome measures. Birth. 2007;34(2):164–72. doi: 10.1111/j.1523-536X.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- Duck 1991 .Duck FA, Martin K. Trends in diagnostic ultrasound exposure. Physics in Medicine and Biology. 1991;38:1423–32. doi: 10.1088/0031-9155/36/11/002. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eik-Nes 1980 .Eik-Nes SH, Brubaak AO, Ulstein MK. Measurement of human fetal blood flow. BMJ. 1980;280:283–4. [Google Scholar]
- Fisk 2001 .Fisk NM, Smith RP. Fetal growth restriction; small for gestational age. In: Chamberlain G, Steer P, editors. Turnbull’s Obstetrics. 3rd Edition Churchill Livingstone; Edinburgh: 2001. pp. 197–209. [Google Scholar]
- Fitzgerald 1977 .Fitzgerald DE, Drumm JE. Non-invasive measurement of the human circulation using ultrasound: a new method. BMJ. 1977;2:1450–1. doi: 10.1136/bmj.2.6100.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretts 1992 .Fretts RC, Boyd ME, Usher RH, Usher HA. The changing pattern of fetal death, 1961-1988. Obstetrics & Gynecology. 1992;79:35–9. [PubMed] [Google Scholar]
- Froen 2004 .Froen JF, Gardosi JO, Thurmann A, Francis A, Stray-Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstetricia et Gynecologica Scandinavica. 2004;83(9):801–7. doi: 10.1111/j.0001-6349.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- Gardosi 2005 .Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ. 2005;331:1113–7. doi: 10.1136/bmj.38629.587639.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffinet 1997 .Goffinet F, Paris J, Heim N, Nisand I, Breart G. Predictive value of Doppler umbilical artery velocimetry in a low risk population with normal fetal biometry. A prospective study of 2016 women. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1997;71(1):11–9. doi: 10.1016/s0301-2115(96)02606-1. [DOI] [PubMed] [Google Scholar]
- Henderson 1997 .Henderson J, Whittingham TA, Dunn T. A review of the acoustic output of modern diagnostic ultrasound equipment. BMUS Bulletin. 1997 Nov;:10–4. Vol. [Google Scholar]
- Higgins 2009a .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2. The Cochrane Collaboration; [updated September 2009]. 2009. www.cochrane-handbook.org Available from. [Google Scholar]
- Higgins 2009b .Higgins JP, Thompson SG, Spiegelhalter DJ. A reevaluation of random-effects meta-analysis. Journal of the Royal Statistical Society, Series A, (Statistics in Society) 2009;172(1):137–59. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang 2000 .Huang DY, Usher RH, Kramer MS, Yang H, Morin L, Fretts R. Determinants of unexplained fetal deaths. Obstetrics & Gynecology. 2000;95:215–21. doi: 10.1016/s0029-7844(99)00536-0. [DOI] [PubMed] [Google Scholar]
- Kieler 2001 .Kieler H, Cnattingius S, Haglund B, Palmgren J, Axelsson O. Sinistrality - a side-effect of prenatal sonography: a comparative study of young men. Epidemiology. 2001;12:618–23. doi: 10.1097/00001648-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Kieler 2002 .Kieler H, Cnattingius S, Palmgren J, Haglund B, Axelsson O. First trimester ultrasound scans and left-handedness. Epidemiology. 2002;13(3):370. doi: 10.1097/00001648-200205000-00022. [DOI] [PubMed] [Google Scholar]
- Mangesi 2007 .Mangesi L, Hofmeyr GJ. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD004909.pub2. [DOI: 10.1002/14651858.CD004909.pub2] [DOI] [PubMed] [Google Scholar]
- Mires 2000 .Mires GJ, Patel NB, Dempster J. The value of fetal umbilical artery flow velocity waveforms in the prediction of adverse fetal outcome in high risk pregnancies. Journal of Obstetrics and Gynaecology. 2000;10:261–70. [Google Scholar]
- Neilson 1998a .Neilson JP. Symphysis-fundal height measurement in pregnancy. Cochrane Database of Systematic Reviews. 1998;(Issue 1) doi: 10.1002/14651858.CD000944. [DOI: 10.1002/14651858.CD000944] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson 1998b .Neilson JP. Ultrasound for fetal assessment in early pregnancy. Cochrane Database of Systematic Reviews. 1998;(Issue 4) doi: 10.1002/14651858.CD000182. [DOI: 10.1002/14651858.CD000182] [DOI] [PubMed] [Google Scholar]
- Nelson 1988 .Nelson TR, Pretorius DH. The Doppler signal: where does it come from and what does it mean? American Journal of Radiology. 1988;151:439–47. doi: 10.2214/ajr.151.3.439. [DOI] [PubMed] [Google Scholar]
- Newnham 1996 .Newnham J, MacDonald W, Gurrin L, Evans S, Landau L, Stanley F. The effect of frequent prenatal ultrasound on birthweight: follow-up at one year of age. Proceedings of the 14th Annual Congress of the Australian Perinatal Society in conjunction with the New Zealand Perinatal Society; Adelaide, Australia. 1996 March 24-27.1996. p. A26. [Google Scholar]
- NICE 2008 .National Institute for Health and Clinical Excellence . NICE clinical guideline 62 (March 2008) RCOG Press; London: 2008. Antenatal Care: routine care for the healthy pregnant women. [Google Scholar]
- Owen 2001 .Owen P. Fetal assessment in the third trimester: fetal growth and biophysical methods. In: Chamberlain G, Steer P, editors. Turnbull’s obstetrics. 3rd Edition Churchill Livingstone; Edinburgh: 2001. [Google Scholar]
- Pattison 1999 .Pattison N, McCowan L. Cardiotocography for antepartum fetal assessment. Cochrane Database of Systematic Reviews. 1999;(Issue 1) doi: 10.1002/14651858.CD001068. [DOI: 10.1002/14651858.CD001068] [DOI] [PubMed] [Google Scholar]
- Peters 2006 .Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- RCOG 1997 .Royal College of Obstetricians and Gynaecologists . Ultrasound screening for fetal abnormalities: report of the RCOG Working Party. RCOG; London: 1997. [Google Scholar]
- RevMan 2008 .RevMan . Review Manager (RevMan) Version 5.0 for Windows. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Salvesen 1999 .Salvesen KA, Eik-Nes SH. Ultrasound during pregnancy and birthweight, childhood malignancies and neurological development. Ultrasound in Medicine and Biology. 1999;25:1025–31. doi: 10.1016/s0301-5629(99)00051-4. [DOI] [PubMed] [Google Scholar]
- Salvesen 2007 .Salvesen KA. Epidemiological prenatal ultrasound studies. Progress in Biophysics and Molecular Biology. 2007;93(7):295–300. doi: 10.1016/j.pbiomolbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Sijoms 1989 .Sijoms EA, Reuwer PJHM, Van Beek E, Bruinse HW. The validity of screening for small-for-gestational-age and low-weight-for-length infants by Doppler ultrasound. British Journal of Obstetrics and Gynaecology. 1989;96(5):557–61. doi: 10.1111/j.1471-0528.1989.tb03255.x. [DOI] [PubMed] [Google Scholar]
- Soothill 1993 .Soothill PW, Ajayi RA, Campbell S, Nicolaides KH. Prediction of morbidity in small and normally grown fetuses by fetal heart rate variability, biophysical profile score and umbilical artery Doppler studies. British Journal of Obstetrics and Gynaecology. 1993;100:742–5. doi: 10.1111/j.1471-0528.1993.tb14265.x. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Bricker 2007 .Bricker L, Neilson JP. Routine Doppler ultrasound in pregnancy. Cochrane Database of Systematic Reviews. 2007;(Issue 2) doi: 10.1002/14651858.CD001450. [DOI: 10.1002/14651858.CD001450.pub2] [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study