Abstract
Aim of the study
Assessment of the concentrations of the soluble forms of the cell adhesion molecules sVCAM-1 and sICAM-1 in serum of female breast cancer patients. These concentrations were assessed in relation to factors such as: age, clinical stage of disease, histological grade of malignancy, the status of the local axillary lymph nodes, and the size of the primary tumour.
Material and methods
A total of 103 patients with primary breast cancer, aged 29 to 89 years, were investigated. The control group consisted of 40 healthy women. The concentration of sVCAM-1 and sICAM-1 was assessed using an enzyme-linked immunosorbent assay (ELISA).
Results
The results of the study suggest that the level of sVCAM-1 and sICAM-1 in the serum of women with breast cancer was significantly higher than that seen in the serum of healthy women. A relationship between the level of adhesion molecules and the stage of clinical disease advancement was discovered. There was a correlation between the increasing concentrations of sVCAM-1 and sICAM-1 and with the aggressiveness of the disease. Significant differences were also found in the group of women with metastases to the axillary lymph nodes and women with no metastasis. Similar correlations were found between sVCAM-1 and sICAM-1 levels and the size of primary tumour.
Conclusions
The results obtained suggest that the assessment of the soluble forms of sVCAM-1 and sICAM-1 may be useful indicators in the assessment of the clinical advancement of breast cancer.
Keywords: cell adhesion molecules, immunoglobin superfamily, breast cancer, sVCAM-1 and sICAM-1
Introduction
Angiogenesis plays a significant role in the growth of malignant tumours and the formation of remote metastases [1]. Vascular endothelial cells are of particular importance in the early stages of this process, owing to their adhesive properties, which maintains close contact between cells. This cell-to-cell adhesion, and adhesion to macromolecules of the extra cellular matrix (ECM), is possible because of the presence of so-called cell adhesion molecules (CAMs), expressed on the external surface of the endothelium [2]. Under normal, physiological circumstances, adhesion molecules take part in the processes of embryogenesis, the growth of organs and the protection of the endothelium against the migration of cells from the surrounding stroma through strong interactions between cells as well as between cells and elements of the ECM. It is worth noting that the loss of such interactions allows cancer cells to become detached from the mass of the primary tumour, to pass through the walls of the blood vessels, and to form new remote metastases [2, 3]. On the basis of differing biochemical structure and function, CAMs may be divided into five families of adhesion proteins: cadherins, integrins, selectins, immunoglobulin molecules, and CD 44 molecules [2].
Previously, it was shown that the cell adhesion molecules immunoglobulin superfamily (ICAM-1, ICAM-2 and ICAM-3), the vascular cell adhesion molecule (VCAM-1), and the platelet endothelial cell adhesion molecule (PECAM) have significant roles in tumour progression [4]. The adhesion protein ICAM-1 is built from five immunoglobulin-like domains, for which the ligand is integrin β2. To date, CAMs have been demonstrated on the surface of the endothelium, macrophages, lymphocytes, monocytes, and cells of the immune system. Similarly, VCAM-1 is a glycoprotein, characterised by the presence of seven immunoglobin domains, linked by disulphide bridges. The main ligands for VCAM-1 are integrins α4β1 and α4β7. The presence of these adhesive molecules has been demonstrated on the cells of the endothelium, macrophages, dendritic cells, and on the surface of cancer cells. Under the influence of pro-inflammatory cytokines, endothelial cells increase the expression of the proteins ICAM-1 and VCAM-1, thereby increasing the adhesion between the vessel walls and leucocytes. Besides being found as membrane-bound molecules, both ICAM-1 and VCAM-1 also have soluble forms, known as sICAM-1 and sVCAM-1 [5]. Increased concentrations of such molecules in the bodily fluids may be a prognostic marker in the course of many neoplastic diseases. During the last decade, scientific publications have described increased expression of adhesion molecules in patients suffering from cancers of the stomach, urinary bladder, urinary tract, and pancreas, as well as in cases of melanoma [6–9]. In these studies it was observed that changes to the expression or function of adhesion molecules sICAM-1 and sVCAM-1 leads to a loss of mutual binding between vascular endothelial cells, a necessary condition for the migration of cancer cells through damaged vascular walls and the formation of remote metastases [5].
The aim of the study was to assess the concentrations of the soluble forms of inter-cellular adhesion molecule-1 (sICAM-1) and vascular cell adhesion molecule-1 (sVCAM-1) in serum from women suffering from breast cancer. Other prognostic factors taken into account included the following: age, clinical stage of disease advancement, histological grade of malignancy, status of local axillary lymph nodes, and the size of the primary tumour.
Material and methods
The adhesion molecules sICAM-1 and sVCAM-1 were demonstrated in the serum of 103 women with breast cancer, prior to surgery. Patients with primary breast cancer, before first surgery, were included into the study. Patients who used neoadjuvant chemotherapy were excluded from the study group. The women were aged between 29 and 89 years (average age 56 years). The patients were treated in the Department of Oncological Surgery within the Oncology Division of Poznan University of Medical Sciences. Control material consisted of serum drawn from 40 healthy women aged between 24 and 75 years (average age 47 years). The Bioethics Committee of Poznan University of Medical Sciences gave consent prior to undertaking the study. Patient characteristics are shown in Table 1.
Table 1.
Clinical characteristics of the breast cancer patients
| Parameter | Number of patients | Percentage of tested patients (%) |
|---|---|---|
| Age Pre-menopausal Post-menopausal |
31 72 |
30.1 69.9 |
| Stage of clinical disease | ||
| advancement according to TNM I II III |
47 38 18 |
45.6 36.9 17.5 |
| Status of local axillary lymph | ||
| nodes pN0 pN1 |
51 52 |
49.5 50.5 |
| Size of the primary tumour pT < 20 mm pT ≥ 20 < 50 mm pT ≥ 50 mm |
54 40 9 |
52.4 38.9 8.7 |
| Histological grade of malignancy G1 G2 G3 |
11 50 42 |
10.7 48.5 40.8 |
The concentrations of sICAM-1 and sVCAM-1 were determined using an enzyme-linked immunosorbent assay (ELISA) (Quantikine tests, R&D Systems).
Statistical analysis
The concentration levels obtained for the analysed molecules were investigated statistically using the software Statistica 8.0. Prior to the statistical analysis the parameters were checked to ensure a normal distribution – this was done using a Shapiro-Wilk W test. For the purpose of testing for differences between the tested groups where normal distribution was not determined, a non-parametric U Mann-Whitney test was applied. Results were considered statistically significant where p < 0.05.
Results
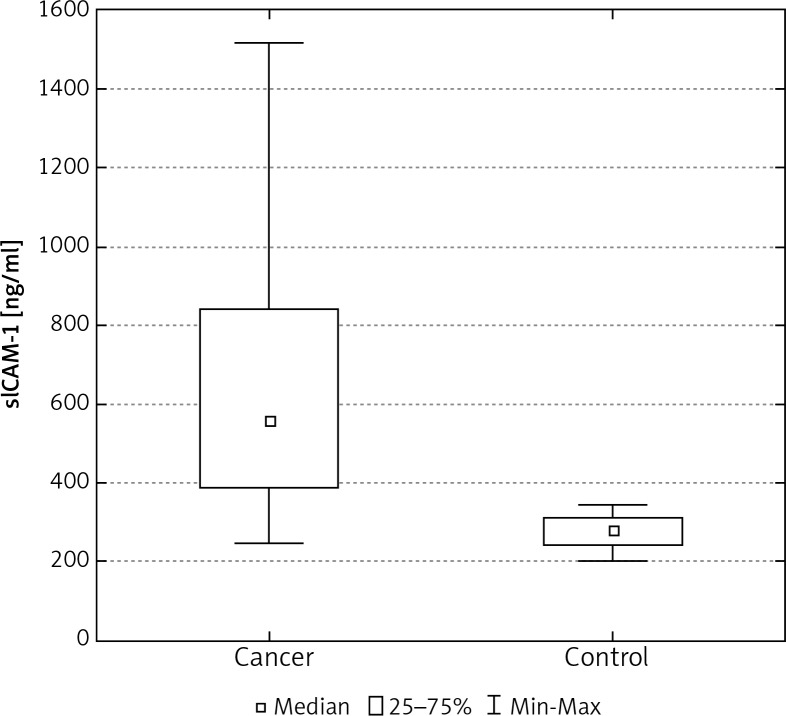
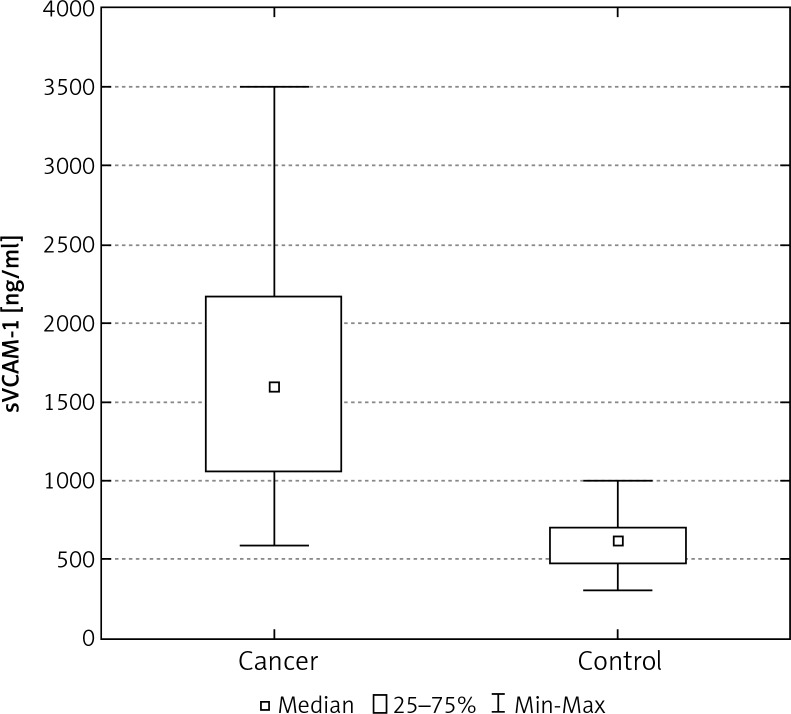
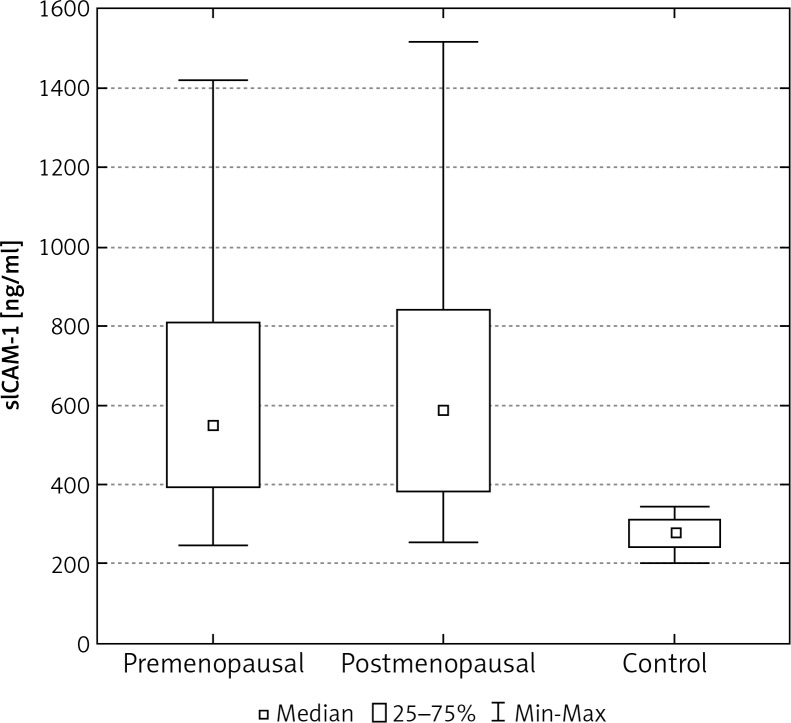
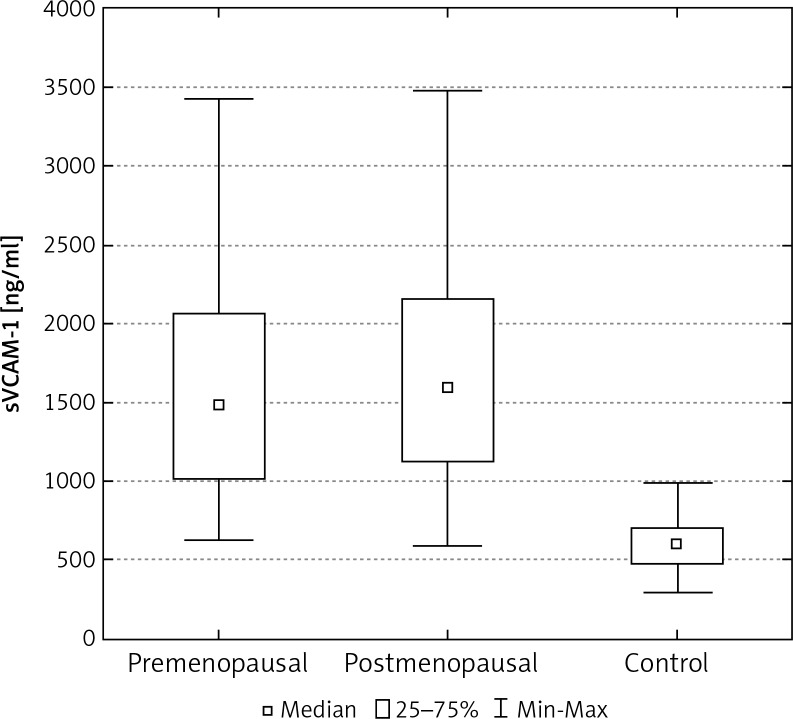
In the control group of 40 women, the average concentration of sICAM-1 and sVCAM-1 in the serum was significantly lower than in the group of 103 women with breast cancer (Tables 2, 3). The median concentration of sICAM-1 amongst women with malignant breast tumours was almost three times greater, and for sVCAM-1 more than twice as high as it was in the healthy women. These differences were statistically significant (Figs. 1, 5). The mean and median concentrations of sICAM-1 and sVCAM-1 in post-menopausal women was elevated in comparison to the concentrations of the same molecules in women who had not yet reached menopause. These tendencies were not statistically significant, however (Tables 2, 3, Figs. 2, 6). The median concentrations of sICAM-1 and sVCAM-1 rose in a statistically significant manner, in line with increasing grade of histological malignancy in cases of breast cancer. A significant relationship was found between the value for sICAM-1 and grades G1, G2, and G3 of histological malignancy (Table 2). A similar relationship was found during the analysis of sVCAM-1 results (Table 3).
Table 2.
Concentration of sICAM-1 in the serum of women with breast cancer in relation to age, clinical stage of disease, lymph node status, tumour size, and histological malignancy grade
| Patient characteristics | mean sICAM-1 concentration [ng/ml] | sICAM-1 median sICAM-1 concentration [ng/ml] | sICAM-1 concentration range [ng/ml] |
|---|---|---|---|
| Control group n = 40 | 591.8 ±170.5 | 611.9 | 305.8–998.4 |
| Breast cancer patients n = 103 | 1674.9* ±765.8 | 1602.1* | 595.9–3492.1 |
| Age Pre-menopausal Post-menopausal |
1610.9* ±779.6 1702.0* ±763.7 |
1554.5* 1602.2* |
632.0–3450.0 595.9–3492.1 |
| Stage of clinical disease advancement according to the TNM | |||
|
I (n = 47) II (n = 38) III (n = 18) |
1133.8* ±457.4 1904.2*& ±481 2603.7*&# ±780 |
1030.0* 1884.7*& 2758.2*&# |
595.9–2417.0 939.0–3147.7 1054.4–3492.1 |
| Axillary lymph nodes status pN0 (n = 51) pN1 (n = 52) |
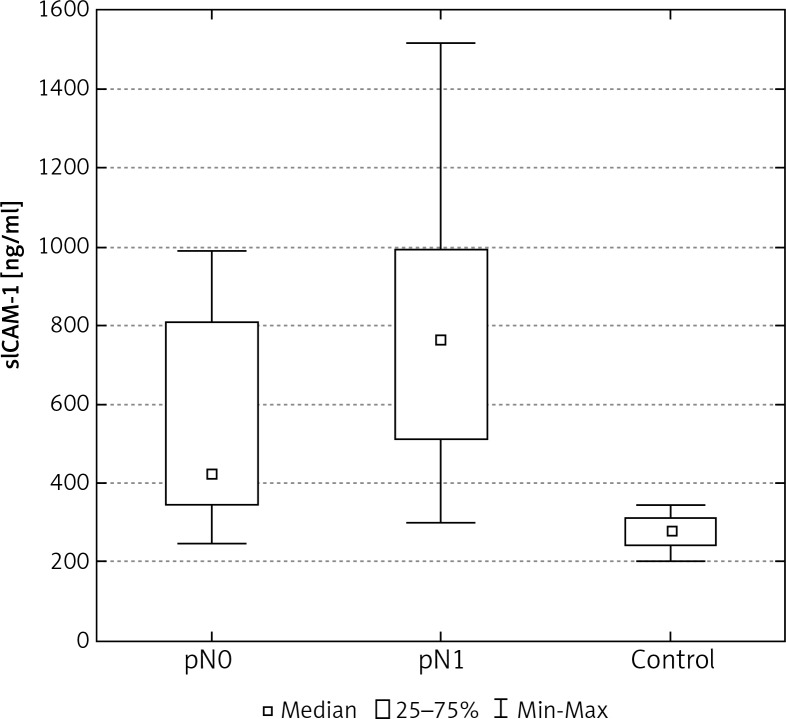
1237.7* ±558.0 2103.8*▼ ±714 |
1129.4* 2046.5*▼ |
595.9–2758.2 710.3–3492.1 |
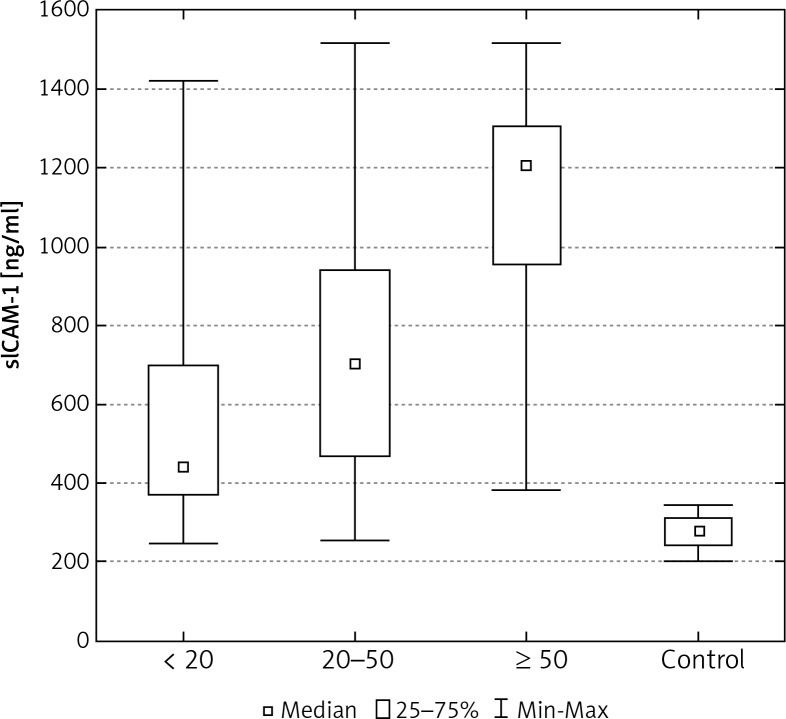
| Tumour size pT < 20 mm (n = 54) pT ≥ 20 < 50 mm (n = 40) pT ≥ 50 mm (n = 9) |
274.5* ±241.2 712.4*◆ ±286.1 1132.4*◆■±3404 |
439.9* 704.6*◆ 1207.5*◆■ |
245.0–1420.3 254.2–1516.3 383.4–1516.3 |
| Histological grade of malignancy G1 (n = 11) G2 (n = 50) G3 (n = 42) |
1055.4* ±338.2 1506.1*△ ±682.2 2038.2*△❑ ±775 |
997.2* 1417.0*△ 2042.0*△❑ |
699.1–1826.3 632.0–3450.0 595.9–3492.1 |
n – number of tested individuals
Statistically significant difference for sICAM-1 in comparison to the control group p < 0.05
Statistically significant difference for sICAM-1 in comparison to the women in stage I of clinical disease advancement p < 0.05
Statistically significant difference for sICAM-1 in comparison to the women in stage II of clinical disease advancement p < 0.05
Statistically significant difference for sICAM-1 in comparison to the group of women without metastases to the axillary lymph nodes p < 0.05
Statistically significant difference for sICAM-1 in comparison to the group of women with tumours measuring < 20 mm, p < 0.05
Statistically significant difference for sICAM-1 in comparison to women with tumours measuring ≥ 20 < 50 mm, p < 0.05
Statistically significant difference for sICAM-1 in comparison to women with a G1 histological grade of malignancy, p < 0.05
Statistically significant difference for sICAM-1 in comparison to women with a G2 histological grade of malignancy G2, p < 0.05
Table 3.
Concentration of sVCAM-1 in the serum of women with breast cancer in relation to age, clinical stage of disease, lymph node status, tumour size, and histological malignancy grade
| Patient characteristic | mean sVCAM-1 concentration [ng/ml] | sVCAM-1 median sVCAM-1 concentration [ng/ml] | sVCAM-1 concentration range [ng/ml] |
|---|---|---|---|
| Control group n = 40 | 274.5 ±41.2 | 280.8 | 201.3–344.4 |
| Breast cancer patients n = 103 | 648.0* ±312.6 | 559.9* | 240.0–1516.3 |
| Age Pre-menopausal Post-menopausal |
635.4* ±303.0 653.5* ±318.6 |
548.0* 585.2* |
245.0–1420.1 254.2–1516.3 |
| Stage of clinical disease advancement according to TNM I (n = 47) II (n = 38) III (n = 18) |
439.6* ±176.7 730.0*& ±236.3 782.6*&# ±335 |
387.8* 704.9*& 754.5*&# |
245.0–1047.0 318.4–1420.3 383.4–1516.3 |
| Axillary lymph node status pN0 (n = 51) pN1 (n = 52) |
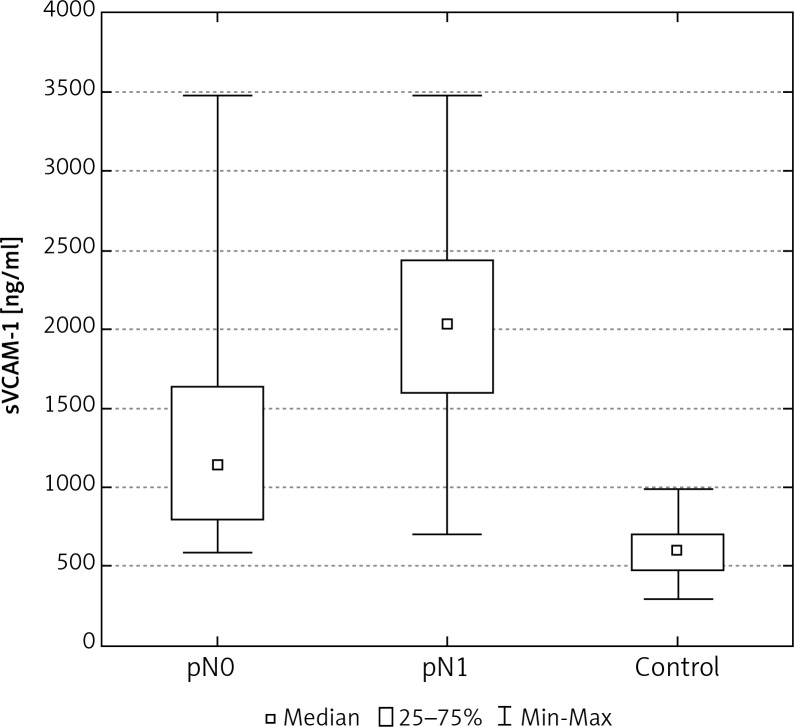
510.9* ±216.2 782.6*▼ ±335.1 |
421.3* 763.6*▼ |
245.0–992.1 297.3–1516.3 |
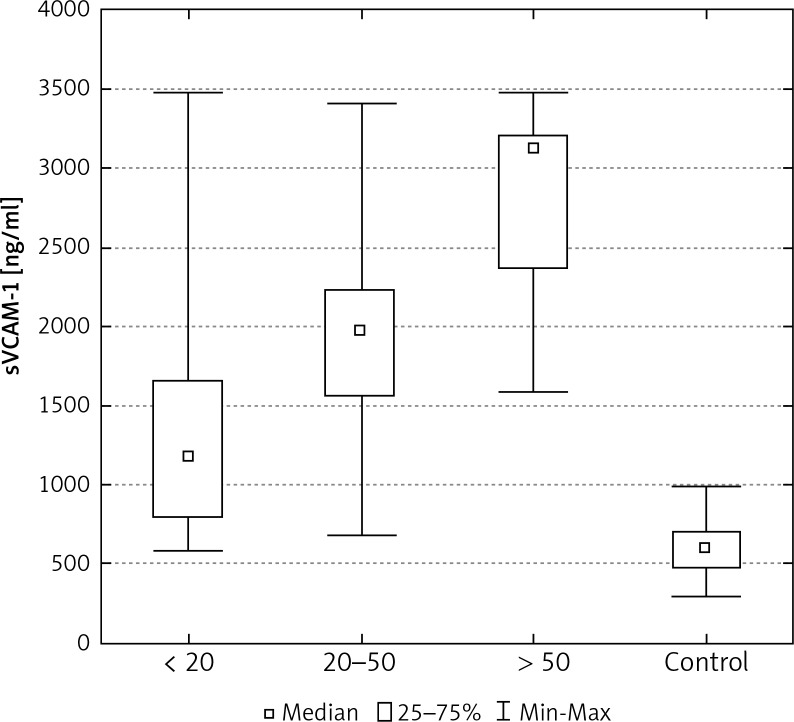
| Tumour size pT < 20 mm (n = 54) pT ≥ 20 < 50 mm (n = 40) pT ≥ 50 mm (n = 9) |
1257.3* ±545.3 1975.1*▼ ±631.8 2846.6*■ ±624 |
1154.0* 1988.0*◆ 3147.7*◆▼ |
595.9–2944.0 688.0–3425.9 1595.2–3492.1 |
| Histological grade of malignancy G1 (n = 11) G2 (n = 50) G3 (n = 42) |
451.1* ±218.6 595.1*△ ±273.0 762.7*△❑ ±339.2 |
332.4* 506.1*△ 739.6*△❑ |
286.7–954.5 245.0–1420.1 292.7–1516.3 |
n – number of tested individuals
Statistically significant difference for sVCAM-1 in comparison to the control group p < 0.05
Statistically significant difference for sVCAM-1 in comparison to the women in stage I of clinical disease advancement p < 0.05
Statistically significant difference for sVCAM-1 in comparison to the women in stage II of clinical disease advancement p < 0.05
Statistically significant difference for sVCAM-1 in comparison to the group of women without metastases to the axillary lymph nodes p < 0.05
r statistically significant difference for sVCAM-1 in comparison to the group of women with tumours measuring < 20 mm, p < 0.05
Statistically significant difference for sVCAM-1 in comparison to the group of women with tumours measuring ≥ 20 < 50 mm, p < 0.05
Statistically significant difference for sVCAM-1 in comparison to the women with a G1 histological grade of malignancy, p < 0.05
Statistically significant difference for sVCAM-1 in comparison to the women with a G2 histological grade of malignancy, p < 0.05
Fig. 1.
The concentration of sICAM-1 in the serum of women with breast cancer
Fig. 5.
The concentration of sVCAM-1 in the serum of women with breast cancer
Fig. 2.
The concentration of sICAM-1 in the serum of women with breast cancer in relation to age
Fig. 6.
The concentration of sVCAM-1 in the serum of women with breast cancer in relation to age
The median concentrations of sICAM-1 and sVCAM-1 increased together with advancing stage of the disease process (Tables 2, 3). The highest median concentrations for sICAM-1 and sVCAM-1 in serum were seen in women with stage III of the neoplastic disease processes (Tables 2, 3). A significant relationship was also found between the values of the tested molecules and stages I and II of the clinical disease process.
A statistically significant difference was also found between the determined levels of adhesion molecules in the serum of women with metastases to the axillary lymph nodes, in comparison to cases without metastases (Tables 2, 3, Figs. 3, 7).
Fig. 3.
The concentration of sICAM-1 in the serum of women with breast cancer in relation to lymph node status
Fig. 7.
The concentration of sVCAM-1 in the serum of women with breast cancer in relation to lymph node status
Increasing size of tumour was also related to higher concentrations of sICAM-1 and sVCAM-1 in patients with breast cancer (Tables 2, 3). Statistically significant differences were found between values of these proteins in patients where the tumour measured less than 20 mm in diameter, in patients where the tumour measured from 20 to 50 mm, and in patients where the diameter of the tumour was greater than 50 mm. Significant differences were also identified between the concentrations of sICAM-1 and sVCAM-1 in the control group and the levels of these CAMs, and amongst patients grouped according to the size of the tumour (Figs. 4, 8).
Fig. 4.
The concentration of sICAM-1 in the serum of women with breast cancer in relation to tumour size
Fig. 8.
The concentration of sVCAM-1 in the serum of women with breast cancer in relation to tumour size
Discussion
The occurrence and development of a tumour is a multi-stage and long-lasting process, involving disturbances both intracellular and extracellular, which, in turn, allow cancer cells to spread through the tissues and organs [10]. Despite the enormous progress that has been made in the diagnosis and treatment of cancer, mortality from tumours remains high and the tendency does not appear to be falling [10]. It is evident that a significant factor in this situation is the formation of remote metastases. For that reason, scientific studies are carried out with the aim of discovering the mechanisms responsible for the development of the primary tumour and the formation of secondary metastatic tumours. Reports exist showing that CAMs present on the endothelial surface play an important role in the process of pathogenesis [11]. These proteins are responsible for intercellular adhesive conditions and for the attachment of leucocytes to activated endothelial cells in the blood vessels, both of which are important in extravasation and in the process of inflammation [12]. Assuming that inflammation is a state that generally precedes the formation of a tumour, disruptions to the expression of CAMs may lead to the loss of intercellular links and links between cells and the ECM. They may also promote the attachment of cancer cells to endothelial cells, thus further enabling the formation of metastases in remote organs [12].
Up to now, it has been shown that particles belonging to the immunoglobulin-like adhesins, apart from the transmembrane proteins (mCAM) associated with the cell membranes of the endothelium, can also occur in soluble forms (sCAM) [12]. The mechanism by which these molecules arise in the circulation remains partly unexplained. It is possible that sICAM-1 and sVCAM-1 may be secreted into the bloodstream from the surface of activated endothelium. Circulating, soluble CAMs are smaller than those bound to cell surfaces as they are, in fact, only the extracellular domains of cell adhesion molecules. The concentration of these molecules in body fluids may reflect the degree of activation of lymphocytes, fibroblasts, and endothelial cells [13].
Currently, a number of research centres are committed to defining the role of adhesion molecules in angiogenesis and metastasis. It is believed that the concentration of the proteins sICAM-1 and sVCAM in peripheral blood and in cerebrospinal fluid may be useful in the early diagnosis of cancer, in the staging of the disease, and in monitoring treatment [14].
Of particular interest is the participation of the soluble forms of the adhesion molecules sICAM-1 and sVCAM-1 in the pathogenesis of metastases in women with breast cancer. The number of reports in the literature for this problem remains small. Fox et al., in their experiments, demonstrated the presence of the protein sVCAM-1 only in the vascular endothelial cells of the tumour, but did not detect the presence of this molecule in normal vascular endothelium [15]. The presence of the adhesin VCAM-1 in the cells of primary tumours of breast tissue was observed by Guerrero-Esteo et al. [16]. The authors suggested that the expression of VCAM-1 is dependent on the presence of the ligand VLA4 and affects not only the activation of endothelial cells, but also contributes to cancer cell shedding from the surface of the tumour, thus enabling infiltration to the surrounding tissues [16].
In this paper we evaluated the concentration of the soluble forms of vascular cell adhesion molecule-1 (sVCAM-1) and intercellular adhesion molecule-1 (sICAM-1) in women with breast cancer, taking account of prognostic factors. The studies demonstrated a significant increase in the concentration of the studied adhesion molecules in patients suffering from this cancer in comparison to the control group. Similar observations were made by Sheen-Chen et al., who observed a statistically significant increase in the concentration of sICAM-1 in the serum of patients with invasive breast cancer [17]. Similarly, Madej and O'Hanlon, in their study, showed elevated levels of adhesion molecules in the serum of patients with breast cancer [18, 19]. An increase in the concentration of the protein was also obtained in the case of other types of cancer. The Alexiou research group showed increased levels of ICAM-1 in the serum of patients with gastric cancer [6]. Sun et al. described high concentrations of sICAM-1 particles in the serum of patients with hepatocellular carcinoma and also observed a high expression of adhesin in cancerous tissue, compared to benign tumours and a control group [20]. De Vita et al. showed statistically significant increases in levels of the adhesion molecule ICAM-1 in the serum of patients with lung cancer, in comparison to a control group [21].
In the present study, we also observed a high level of the soluble form of vascular cell adhesion molecule-1 (sVCAM-1) in patients with breast cancer, and these levels were significantly higher than in the control group. However, Byrne et al. demonstrated, in patients with breast cancer, not only a significant increase in the level of the protein in comparison to healthy controls, but they also hypothesised that sVCAM-1 production in the epithelial cells may promote production of vascular endothelial growth factor-VEGF [14]. Elevated concentrations of sVCAM-1 have also been demonstrated in the course of other types of cancer. The researchers Ke and Ding et al. studied patients with gastric cancer and showed not only a high content of VCAM-1 in the tumour tissue, but also obtained a positive correlation between adhesin and the density of blood vessels, in comparison to healthy tissue [22, 23]. The authors suggest that the molecule VCAM-1 may be an important proangiogenic factor responsible for the process of tumour vascularisation. Assuming that the soluble forms of adhesion molecules are activators of angiogenesis, they suggest that the increased production of the adhesion molecules ICAM-1 and sVCAM-1 by endothelial cells influences not only the process of tumour growth, but also the migration of cancer cells to distant tissues and organs.
One of the most important prognostic factors in breast cancer patients is age. Epidemiological studies carried out in recent years show that women under 35 years of age with this cancer have a significantly worse prognosis when compared with older women. In our study we found that postmenopausal women had median concentrations of sICAM-1 and sVCAM-1, which were slightly higher than the values for the same parameters in premenopausal women, but without evidence of statistical significance. In his experience, Madej identified no differences in the concentrations of sICAM-1, relative to menopausal status, in the serum of patients with breast cancer [18]. The lack of data in the literature regarding the concentration of sVCAM-1 in pre- and post-menopausal patients with breast cancer makes it impossible to compare their results with those obtained in other research centres. Czekierdowski et al. evaluated the concentrations of sVCAM-1, but in the serum of women with ovarian cancer before and after the menopause. They found no significant age dependent differences in protein concentration [24].
It is important, in determining the prognosis and in selecting the right treatment, to correctly assess the cancer staging. Our study showed that the higher the clinical stage of the disease according to the TNM classification, the higher the concentration levels of the adhesion molecules sICAM-1 and sVCAM-1, in the serum of women with breast cancer. Statistical analysis showed significant differences between values for sICAM-1 and sVCAM-1 in stages I, II, and III of the disease. These results suggest that the assessment of soluble forms of the adhesion molecules sICAM-1 and sVCAM-1 complement the clinical staging of the disease. Sheen-Chen et al. analysed the concentration of sICAM-1 in the serum of patients with invasive breast cancer and showed a significant correlation between serum sICAM-1 levels and the severity of the disease [17]. Nothing is to be found in the literature, however, regarding the concentration of the soluble molecule sVCAM-1 in women with breast cancer, depending on the stage of cancer.
Another important prognostic factor in patients with breast cancer is the status of the axillary lymph nodes. The presence or absence of lymphatic metastases is considered to be one of the most important prognostic parameters in the course of the disease. The greater the number of positive axillary lymph nodes, the more unfavourable the prognosis. In the absence of axillary lymph node involvement, cancer recurrence is likely only in about 20–30% of patients [25]. In our study, patients with metastases to the axillary lymph nodes showed higher values than were found in women without metastases, and these differences were statistically significant. These results confirm the experimental findings of other authors. Madej also observed a significant increase in the concentration of sICAM-1 in the serum of patients with breast cancer with distant metastases, when compared to the levels of adhesins in patients without metastatic disease [17]. Sheen-Chen et al. showed a similar relationship [17]. Byrne described a statistically significant increase in the concentration of sVCAM-1 levels in breast cancer patients who developed metastases in comparison to those in whom metastases were not present [14]. The statistically significant findings of Hyun et al. showed high levels of sVCAM-1 in patients with operable breast cancer and metastasis [26]. Similar findings, regarding sICAM-1 and sVCAM-1, were found in the cases of patients with stomach cancer in the study by Yoo et al. [27]. They found a positive correlation between the concentration of the studied molecules in the serum of patients, the stage of disease advancement, and the occurrence of distant metastases.
In analysing the results of the assessment of adhesion molecules, the size of the primary tumour was also taken into account. It is generally accepted that the likelihood of metastases occurring in women with tumours measuring greater than 55 mm is around 60%, whereas when the tumour measures less than 11 mm in diameter, that likelihood falls to 15%, and in patients whose tumours measure less than about 5 mm, the risk amounts to only 5% [25]. Our findings show that the greater the size of the primary tumour, the higher the mean and median concentrations were for the soluble forms of the adhesion molecules sICAM-1 and sVCAM-1. A lack of published reports concerning the levels of soluble forms of adhesion molecules, in relation to primary tumour size, makes it impossible for us to compare our results with those of other authors.
In conclusion, we found that the assessment of the soluble forms of the adhesion molecules sICAM-1 and sVCAM-1 in the serum of women with breast cancer extends the panel of laboratory tests available to monitor the progress of the disease. In our work, we found that quantitative changes in the levels of the soluble forms of adhesion molecules may be related to impaired functioning of vascular endothelium and may be an important factor in the activation of endothelial cells. In turn, increased activation of the vascular endothelium can trigger inflammation processes, which may cause damage within the endothelium. Consequences of such damage include local growth of the tumour and the formation of remote metastases. Further studies to ascertain how components of the vascular endothelium participate and interact during the process of metastasis will be of interest to our department.
In conclusions:
Concentrations of the soluble forms of the adhesion molecules sICAM-1 and sVCAM-1 in the serum of women with breast cancer were significantly higher than in the serum from the control group, which may be indicative of increased activity in vascular endothelial cells.
Increased concentrations of the molecules sICAM-1 and sVCAM-1 in the serum of women with breast cancer may point to reduced interaction between endothelial cells and elements of the extracellular matrix. This phenomenon promotes the invasion of cancer cells into neighbouring organs and tissues.
There is a positive relationship between the concentrations of sICAM-1 and sVCAM-1 in women with breast cancer and the stage of the disease, including the presence of metastases to local axillary lymph nodes, indicating that assessment of these molecules may be used to monitor the clinical state of the patient.
Acknowledgement
The authors would like to thank Professor Jan Bręborowicz of the Department of Tumour Pathology, Poznan University of Medical Sciences, for histopathological analyses.
The authors declare no conflict of interest.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 3.Kamezaki S, Kurozawa Y, Iwai N, Hosoda T, Okamoto M, Nose T. Serum levels of soluble ICAM-1 and VCAM-1 predict pre-clinical cancer. Eur J Cancer. 2005;41:2355–9. doi: 10.1016/j.ejca.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Skubitz AP. Adhesion molecules. Cancer Treat Res. 2002;107:305–29. doi: 10.1007/978-1-4757-3587-1_15. [DOI] [PubMed] [Google Scholar]
- 5.Pasieka Z. The role of cell adhesion molecules in progression and metastasis process of solid tumors and their usefulness as a neoplasmatic marker in clinical practice. Onkol Pol. 2003;6:39–44. [Google Scholar]
- 6.Alexiou D, Karayiannakis AJ, Syrigos KN, Zbar A, Sekara E, Michail P, Rosenberg T, Diamantis T. Clinical significance of serum levels of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in gastric cancer patients. Am J Gastroenterol. 2003;98:478–85. doi: 10.1111/j.1572-0241.2003.07259.x. [DOI] [PubMed] [Google Scholar]
- 7.Perabo F, Sharma S, Gierer R, Wirger A, Fimmers R, Steiner G, Adam M, Schultze-Seemann W. Circulating intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin in urological malignancies. Indian J Cancer. 2001;38:1–7. [PubMed] [Google Scholar]
- 8.Torer N, Kayaselcuk F, Nursal TZ, Yildirim S, Tarim A, Nòyan T, Karakayali H. Adhesion molecules as prognostic markers in pancreatic adenocarcinoma. J Surg Oncol. 2007;96:419–23. doi: 10.1002/jso.20654. [DOI] [PubMed] [Google Scholar]
- 9.Yasasever V, Tas F, Duranyildiz D, Camlica H, Kurul S, Dalay N. Serum levels of the soluble adhesion molecules in patients with malignant melanoma. Pathol Oncol Res. 2000;6:42–5. doi: 10.1007/BF03032657. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad A, Hart IR. Mechanism of metastasis. Crit Rev Oncol Hematol. 1997;26:1021–8. doi: 10.1016/s1040-8428(97)10002-6. [DOI] [PubMed] [Google Scholar]
- 11.Ilyas M. Adhesion molecule expression in breast cancer: the phoenix in tumour metastasis. J Pathol. 2000;190:3–5. doi: 10.1002/(SICI)1096-9896(200001)190:1<3::AID-PATH490>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Gho YS, Kim PN, Li HC, Elkin M, Kleinman HK. Stimulation of tumour growth by human soluble intercellular adhesion molecule-1. Cancer Res. 2001;61:4253–7. [PubMed] [Google Scholar]
- 13.Pietruczuk M, Pietruczuk A, Pancewicz S, Hermanowska-Szpakowicz T. ICAM-1: structure, biological role and clinical significance. Pol Merk Lek. 2004;17:507–11. [PubMed] [Google Scholar]
- 14.Byrne GJ, Ghellal A, Iddon J, Blann AD, Venizelos V, Kumar S, Howell A, Bundred NJ. Serum soluble vascular cell adhesion molecule-1: role as a surrogate marker of angiogenesis. J Natl Cancer Inst. 2000;92:1329–36. doi: 10.1093/jnci/92.16.1329. [DOI] [PubMed] [Google Scholar]
- 15.Fox SB, Turner GD, Gatter KC, Harris AL. The increased expression of adhesion molecules ICAM-3, E- and P- selectins on breast cancer endothelium. J Pathol. 1995;177:369–76. doi: 10.1002/path.1711770407. [DOI] [PubMed] [Google Scholar]
- 16.Guerrero-Esteo M, Ruiz-Velasco N, Muñoz M, Teixidó J. Role of two conserved glycine residues in the beta-propeller domain of the integrin alpha4 subunit in VLA-4 conformation and function. FEBS Lett. 1998;5:123–8. doi: 10.1016/s0014-5793(98)00576-6. [DOI] [PubMed] [Google Scholar]
- 17.Sheen-Chen SM, Eng HL, Sheen CW, Cheng YF, Chou FF, Chen WJ. Serum levels of circulating intercellular adhesion molecule-1 in patients with breast cancer. Anticancer Res. 1997;17:2823–6. [PubMed] [Google Scholar]
- 18.Madej B. Analysis of selected neoplastic factors in breast cancer in reference to formation of distant metastases. Poland: Lublin University of Medical Sciences; 2005. [Google Scholar]
- 19.O'Hanlon DM, Fitzsimons H, Lynch J, Tormey S, Malone C, Given HF. Soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 2002;38:2252–7. doi: 10.1016/s0959-8049(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 20.Sun JJ, Zhou XD, Liu YK, Tang ZY, Feng JX, Zhou G, Xue Q, Chen J. Invasion and metastasis of liver cancer: expression of intercellular adhesion molecule 1. J Cancer Res Clin Oncol. 1999;125:28–34. doi: 10.1007/s004320050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vita F, Infusino S, Auriemma A, Orditura M, Catalano G. Circulating levels of soluble intercellular adhesion molecule-1 in non-small cell lung cancer patients. Oncol Rep. 1998;5:393–6. doi: 10.3892/or.5.2.393. [DOI] [PubMed] [Google Scholar]
- 22.Ke JJ, Shao QS, Ling ZQ. Expression of E-selectin, integrin beta 1 and immunoglobulin superfamily member in human gastric carcinoma cells and its clinicopathologic significance. World J Gastroenterol. 2006;12:3609–11. doi: 10.3748/wjg.v12.i22.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2003;9:1409–14. doi: 10.3748/wjg.v9.i7.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czekierdowski A, Czekierdowska S. The assessment of angiogenic factors VEGF, VEGFR-2 and VCAM-1 in the serum of women with malignant ovarian tumours bevore and after the menopause. Prz Menopauz. 2007;3:128–33. [Google Scholar]
- 25.Olszewski WT. Pathological prognostic factors in breast cancer. New Med. 2002;119:2–5. [Google Scholar]
- 26.Hyun KK, Kyoung RL, Eun SL. Circulating vascular cell adhesion molecule-1 in patients with operable breast cancer. J Thromb Haemostasis. 2003;1:12–8. [Google Scholar]
- 27.Yoo NC, Chung HC, Chung HC, et al. Synchronous elevation of soluble intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) correlates with gastric cancer progression. Yonsei Med J. 1998;39:27–36. doi: 10.3349/ymj.1998.39.1.27. [DOI] [PubMed] [Google Scholar]