Abstract
Background
Prenatal alcohol exposure has been linked to impairment in cerebellar structure and function, including eyeblink conditioning. The deep cerebellar nuclei, which play a critical role in cerebellar-mediated learning, receive extensive inputs from brain stem and cerebellar cortex and provide the point of origin for most of the output fibers to other regions of the brain. We used in vivo 1H magnetic resonance spectroscopy (MRS) to examine effects of prenatal alcohol exposure on neurochemistry in this important cerebellar region.
Methods
MRS data from the deep cerebellar nuclei were acquired from 37 children with heavy prenatal alcohol exposure and 17 non- or minimally exposed controls from the Cape Coloured (mixed ancestry) community in Cape Town, South Africa.
Results
Increased maternal alcohol consumption around time of conception was associated with lower N-acetylaspartate (NAA) levels in the deep nuclei (r=−0.33, p<0.05). Higher levels of alcohol consumption during pregnancy were related to lower levels of the choline-containing metabolites (r=−0.37, p<0.01), glycerophosphocholine plus phosphocholine (Cho). Alcohol consumption levels both at conception (r=0.35, p<0.01) and during pregnancy (r=0.38, p<0.01) were related to higher levels of glutamate plus glutamine (Glx). All these effects continued to be significant after controlling for potential confounders.
Conclusions
The lower NAA levels seen in relation to prenatal alcohol exposure may reflect impaired neuronal integrity in the deep cerebellar nuclei. Our finding of lower Cho points to disrupted Cho metabolism of membrane phospholipids, reflecting altered neuropil development with potentially reduced content of dendrites and synapses. The alcohol-related alterations in Glx may suggest a disruption of the glutamate–glutamine cycling involved in glutamatergic excitatory neurotransmission.
Keywords: fetal alcohol spectrum disorders (FASD), prenatal alcohol exposure, magnetic resonance spectroscopy (MRS), N-acetylaspartate (NAA), choline (Cho), glutamate (Glu)
Introduction
Fetal alcohol spectrum disorders (FASD) include a range of physical growth and neurobehavioral deficits in children whose mothers drank alcohol during pregnancy. We used magnetic resonance spectroscopy (MRS) to study effects of prenatal alcohol exposure on potential neurochemical indicators in the deep cerebellar nuclei of children. The incidence of fetal alcohol syndrome (FAS) is estimated to be 18 to 141 times greater in the Cape Coloured (mixed ancestry) population in the Western Cape Province of South Africa (May et al., 2007) than in the United States. This is among the highest reported incidences in the world. FAS, the most severe FASD, is characterized by distinctive craniofacial dysmorphology, small head circumference, and pre- and/or postnatal growth retardation (Hoyme et al., 2005). A diagnosis of partial FAS (PFAS) requires the same facial dysmorphology as well as small head circumference, retarded growth, or neurobehavioral impairment. A large proportion of exposed children lack the characteristic FAS facial features but exhibit a broad range of cognitive and/or behavioral deficits.
The earliest autopsy studies in humans reporting damaging effects of prenatal alcohol exposure identified errors in cell migration, agenesis or thinning of the corpus callosum, and anomalies in the cerebellum and brain stem (Jones and Smith, 1973; Clarren, 1977). Cerebellar structural anomalies (Jones and Smith, 1973; Clarren, 1977) have been consistently reported in FAS, and studies of effects on brain volume have reported disproportionate size reductions in the cerebellum (Archibald et al., 2001; Chen et al., 2012). In addition, many of the behavioural deficits seen in individuals with FASD, including spatial recognition, motor learning, and fine motor control, are mediated, in part, by the cerebellum (Guerri, 1998). In the 5-year follow-up of our Cape Town Longitudinal Cohort, a remarkably striking deficit was observed in delay eyeblink conditioning. In this cerebellar-mediated nonverbal classical conditioning paradigm, the subject learns to associate a conditioned stimulus, typically a pure tone, with a brief air puff to the eye (unconditioned stimulus) that elicits a reflexive blink. None of the children in the Cape Town sample with full FAS met criteria for conditioning, compared to 75% of the healthy controls; only 33.3% of the children with PFAS and 37.9% of the heavily exposed nonsyndromal children met criteria for conditioning (Jacobson et al., 2008). These findings were subsequently confirmed in a school-aged cohort (Jacobson et al., 2011).
The deep cerebellar nuclei, which play a critical role in cerebellar-mediated learning (Christian and Thompson, 2003), receive extensive inputs from brain stem and cerebellar cortex, and most output fibers from the cerebellum to other brain regions originate from this region. Neural plasticity in the deep nuclei is known to be essential to create the new neural pathways that mediate the conditioned response (Freeman and Nicholson, 2000; Green et al., 2002). Neurodegeneration via apoptosis of cerebellar cells has been demonstrated in the deep cerebellar nuclei of alcohol-exposed rats (Dikranian et al., 2005), leading to decreased numbers of neurons in this region (Green et al., 2002).
In the present study, we performed single-voxel in vivo 1H MRS in the deep cerebellar nuclei of children with FASD and non- or minimally-exposed control children to assess biochemical alterations in this region. In contrast to previous studies that have examined the relation of prenatal alcohol exposure to MRS endpoints (Cortese et al., 2006; Fagerlund et al., 2006; Astley et al., 2009), we performed both real-time motion and first-order shim correction to ensure accurate anatomical localization throughout acquisition and improved magnetic field homogeneity (Hess et al., 2011a). We report absolute metabolite concentrations using water scaling, calculated after adjustment for tissue fractions of gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) in the voxel. The metabolites measured reliably using this procedure were N-acetylaspartate (NAA), glycerophosphocholine plus phosphocholine (Cho), phosphocreatine plus creatine (tCr), myo-inositol (Ins), glutamate (Glu), and glutamate plus glutamine (Glx). Given that NAA is considered a marker of functioning in neuroaxonal tissue and that decreased numbers of neurons have been reported in the deep nuclei of alcohol-exposed rats (Green et al., 2002), we hypothesized that prenatal alcohol exposure would be associated with lower levels of NAA in this region. The relation of prenatal alcohol exposure to the levels of the other metabolites was also examined. In addition, given that the children with full FAS were particularly vulnerable to impairment in eyeblink conditioning (Jacobson et al., 2008, 2011), we hypothesized that this group would show the greatest reduction in NAA compared to controls.
Methods
Participants
Pregnant women from the Cape Coloured community in Cape Town, South Africa, were recruited between 1999 and 2002 at their first antenatal clinic visit (Jacobson et al., 2008). The Cape Coloured, composed mainly of descendants of white European settlers, Malaysian slaves, indigenous Khoi-San aboriginals, and black Africans, have historically comprised the large majority of workers on grape and fruit farms in the wine-producing region of the Western Cape. The Western Cape supports a large wine-producing industry, in which farm workers were traditionally paid, in part, with wine. Socioeconomic deprivation combined with easy access to alcohol has led to excessive maternal drinking, even during pregnancy, which has persisted in both the urban and rural Cape Coloured communities, leading to a high prevalence of FASD (Jacobson et al., 2008).
Cape Coloured pregnant women who reported consuming at least 14 standard drinks/week or binge drinking (≥5 drinks/occasion) were recruited into the study, as was a control group of pregnant women who abstained or drank minimally during pregnancy (<7 drinks/week and no binge drinking). Excluded were women <18 years of age and those with diabetes, epilepsy, cardiac problems requiring treatment, and religiously observant Moslem women, whose religious practices prohibit alcohol consumption. Infant exclusionary criteria included major chromosomal anomalies, neural tube defects, multiple births, and seizures.
Maternal alcohol consumption was assessed using a timeline follow-back approach (Jacobson et al., 2002; 2008). At recruitment the mother was interviewed regarding incidence and amount of drinking on a day-by-day basis during a typical 2-week period at time of conception. She was also asked whether her drinking had changed since conception; if so, when the change occurred and how much she drank on a day-by-day basis during the preceding 2 weeks. This procedure was repeated in mid-pregnancy, and again at 1 month postpartum to provide information about drinking during the latter part of pregnancy. Volume was recorded for each type of beverage consumed each day, converted to ounces absolute alcohol (AA) using multipliers proposed by Bowman and colleagues (1975), and averaged to provide three summary measures of alcohol consumption at conception and during pregnancy: average ounces of AA consumed/day, AA/drinking day (dose/occasion) and frequency of drinking (days/week). Number of cigarettes smoked on a daily basis and frequency of marijuana and other drug use (days/week) were also obtained.
Each child was examined for growth and FAS dysmorphology by two U.S.-based expert dysmorphologists (HEH and LKR) following the revised Institute of Medicine criteria (Hoyme et al., 2005) during a 6-day clinic in 2005 (Jacobson et al., 2008). Five children who did not attend the clinic were assessed by a Cape Town-based dysmorphologist (NK) with expertise in FAS diagnosis. There was substantial agreement among the dysmorphologists on assessment of all dysmorphic features, including the three principal fetal alcohol-related characteristics—palpebral fissure length, and philtrum and vermilion measured using the Lip-Philtrum Guide (Astley and Clarren, 2001) (median r=0.78). The dysmorphologists (HEH and LKR), SWJ, JLJ, and CDM, subsequently conducted a case conference to reach consensus regarding which children met criteria for the FAS and PFAS diagnoses (Jacobson et al., 2008).
IQ was assessed on the Wechsler Intelligence Scale for Children-IV (WISC-IV) at 9 years (see detailed description in Jacobson et al., 2011 and Diwadkar et al., 2013). The WISC-IV was administered in English or Afrikaans, depending on the language used in the child’s elementary school classroom. The WISC-IV was translated into Afrikaans by a clinical psychologist whose first language is Afrikaans and back-translated by another native Afrikaans speaker. At the 5-year follow-up of this cohort, we had administered the Junior South African Intelligence Scale [JSAIS; Madge et al., 1981], which is available in Afrikaans and English and has been normed for South African children. Seventy-one of the children from that follow-up were administered the WISC-IV at 9 years. IQ scores obtained using the JSAIS at 5 years were strongly correlated with the 9-year WISC scores, r=0.79, p<0.001.
In vivo 1H MRS Acquisition
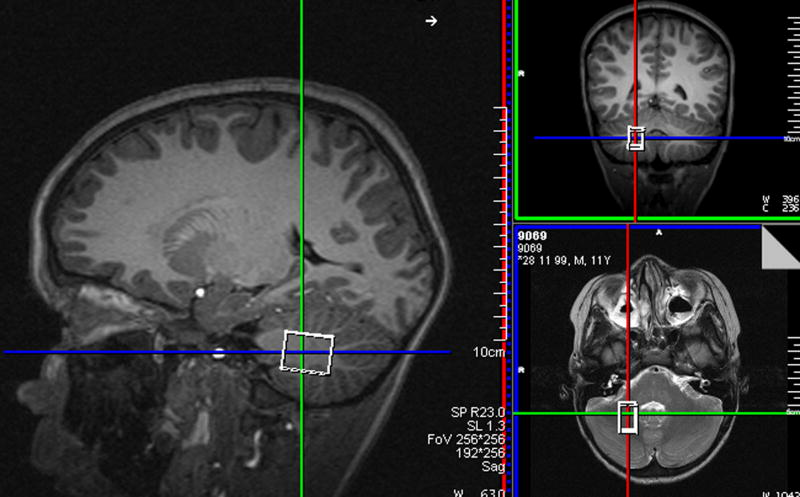
Magnetic resonance spectroscopy data were acquired from 72 right-handed children (age 8.8 to 12.0 years). Those with braces, metal implanted in their body, or claustrophobia were not scanned. All scans were performed on a 3T Allegra (Siemens Medical Systems, Erlangen, Germany) scanner at the Cape Universities Brain Imaging Centre. The volume of interest (VOI) was localized in the deep cerebellar nuclei (Fig. 1) using a T2-weighted acquisition immediately preceding the spectroscopy. Signal loss due to iron deposits in this region (Dimitrova et al., 2006) facilitates accurate localization on T2-weighted images and reproducible positioning of the VOI. Iron deposits in the deep nuclei are low in children but increase with age (Aoki et al., 1989) and the deep nuclei were visible in 67% of the children scanned in this study.
Fig. 1.
Voxel placement for MRS data acquisition
1H MRS data for the 3.8 cm3 voxel were obtained using an EPI volumetric navigated point-resolved spectroscopy (PRESS) sequence (Hess et al., 2011a) with real-time first-order shim and motion correction (TR=2000 ms, TE=30 ms, 128 measurements, vector size 1024, spectral bandwidth 1000 kHz). Water unsuppressed 1H MRS measurements were acquired for 7 different TE’s (TE=30 ms, 50 ms, 75 ms, 100 ms, 144 ms, 500 ms and 1000 ms, TR=4000 ms; 2 averages) to estimate tissue fraction composition (Ernst et al., 1993).
All procedures were performed according to protocols approved by ethics committees at Wayne State University and University of Cape Town. All parents/guardians provided informed written consent, and the children provided oral assent.
1H MRS Pre-processing and Quantification
Individual measurements were recorded separately and frequency- and phase-corrected offline before averaging (Hess et al., 2011b). Briefly, this procedure involved convolving the spectrum from each measurement with a simulated spectrum to robustly detect the frequency shift of the metabolites, followed by singular value decomposition (SVD). The primary component of the SVD provides a set of complex weights that were used to recombine the spectra in a weighted and phase-coherent manner. A spectral range of −448 Hz to 128 Hz was used for both the convolution and SVD. This technique ensured narrower linewidths and higher signal-to-noise ratios (SNRs).
Metabolite levels of the resulting corrected water-suppressed spectra were quantified using LCModel (Provencher, 2008). The water signal of the water-unsuppressed spectra were also quantified using LCModel and modelled as a function of TE using a tri-exponential function (Sigma Plot; version 11; Systat Software Inc, San Jose, California, USA) to estimate the tissue fraction of GM, WM and CSF in the voxel (Ernst et al 1993). GM, WM, and CSF voxel content values, along with the other appropriate relaxation correction factors were then utilized to obtain absolute quantification values (Kreis et al., 1993; Provencher, 2008) from the frequency- and phase-corrected data (Gasparovic et al., 2006).
Exclusionary criteria
Several exclusionary criteria were applied to ensure extraction of only the most reliable results. Spectra from nine children were excluded due to LCModel SNRs<8; seven due to full-width at half maximum (FWHM) linewidths of NAA>9.6 Hz. SNR in LCModel is defined as the ratio of the maximum in the spectrum minus baseline over the analysis window to twice the root mean square residuals. Statistical analyses were performed only on the metabolite peaks with an overall mean Cramer-Rao Lower Bound value <20%; namely, NAA, Cho, tCr, Ins, Glu and Glx. Two additional subjects were excluded as extreme outliers (>2 standard deviations above or below the mean), one on the continuous alcohol exposure measures, the other on the metabolite concentrations.
Statistical Analyses
Statistical analyses were performed using SPSS (version 20; IBM, New York, USA). Nine control variables were considered as potential confounders: child’s sex and age at assessment and maternal education (years), marital status (married/unmarried), verbal intellectual competence (Peabody Picture Vocabulary Test-Revised), nonverbal intellectual competence (Raven Progressive Matrices), smoking (cigarettes/day) and marijuana use (days/month) during pregnancy, and maternal age at delivery.
Each metabolite level was examined in relation to prenatal alcohol exposure at conception and during pregnancy in separate hierarchical multiple regression analyses. Prenatal alcohol exposure was entered in the first step of each analysis; all control variables related to the metabolite (at p<0.20) were entered in the second step to determine if the effect of prenatal alcohol on the metabolite continued to be significant after statistical adjustment for potential confounders. Each metabolite was also examined in relation to FASD diagnosis using analysis of variance (ANOVA). In addition, given that the FAS group was particularly vulnerable to impairment in eyeblink conditioning and that Green et al. (2002) found fewer neurons in the deep nuclei of the most heavily ethanol-exposed rats, we ran a planned contrast of NAA levels in controls vs. the FAS group, which is comprised of the most severely exposed and affected children.
Results
Sample Characteristics
After applying exclusionary criteria, we report data for 54 (34 male, 20 female) right-handed children. Table 1 summarizes demographic information for the children included in the study. The low IQ scores reflect the highly disadvantaged backgrounds of the children in this population (Jacobson et al., 2008). Due to the small number of children with FAS, the FAS and PFAS groups were combined in the data analysis. There were no significant between-group differences in spectral SNR (F(2,53)=0.02, p=0.98) or FWHM (F(2,53)=0.22, p=0.80). Prenatal alcohol exposure was very high, averaging 7.6 standard drinks/occasion for the FAS/PFAS group and 6.6 for the nonsyndromal heavily exposed (HE) group. All but 2 of the 17 control mothers abstained from drinking during pregnancy; one drank 2 drinks on 3 occasions; the other, 1 drink on 6 occasions.
Table 1.
Sample Characteristics (N = 54)
| Child Sex (male) | 34 (63.0%) |
| Child’s age at assessment | 10.7 ± 0.6 |
| WISC-IV IQ | 71.7 ± 12.3 |
| Maternal education (yr) | 8.7 ± 2.9 |
| Maternal marital status (married) | 29 (53.7%) |
| Maternal Peabody Picture Vocabulary Test (PPVT) IQa | 61.6 ± 18.5 |
| Maternal Raven scoreb | 29.7 ± 10.9 |
| Absolute alcohol consumed per day at conception (oz) (n = 35)c | 1.2 ± 0.9 |
| Absolute alcohol consumed per day across pregnancy (oz) (n = 39)c | 0.8 ± 0.7 |
| Absolute alcohol consumed per occasion across pregnancy (oz) (n = 39)c | 3.5 ± 2.0 |
| Drinking days per week across pregnancy (n = 39)c | 1.5 ± 1.1 |
| FASD Diagnosis | |
| Fetal alcohol syndrome | 5 (9.3%) |
| Partial FAS | 15 (27.8%) |
| Heavily exposed nonsyndromal | 17 (31.5%) |
| Controls | 17 (31.5%) |
| Cigarettes smoked per day during pregnancy (n = 42)c | 8.5 ± 6.9 |
| Marijuana use during pregnancy (occasions/month) (n = 6)c | 1.3 ± 1.3 |
Values are mean ± standard deviation or number (%).
Missing for 5 participants.
Missing for 3 participants.
Consumers only.
1H MRS Findings
Figure 2 shows the improvement to our prospectively motion-corrected data after pre-processing (Hess et al., 2011a). For the example shown in Figure 2a, moderate motion during the scan resulted in relatively poor SNR (Fig. 2b) despite prospective motion and shim correction, which was substantially improved after pre-processing (Fig. 2c). Absolute metabolite concentrations are summarized in Table 2. Choline levels in the deep nuclei were similar to those seen in neocortical white matter and hippocampus in the Astley et al. (2009) study, where the median exposure group average was 1.4 mM; NAA and creatine levels in the deep nuclei were somewhat lower (7.7 mM and 5.5 mM, respectively). Virtually none of the correlations between the nine control variables and the six metabolites were significant at p<0.20; the sole exceptions were a positive correlation between maternal smoking during pregnancy and Glx (r=0.24, p<0.10) and lower levels of Ins in the boys (r=−0.24, p<0.10)
Fig. 2.
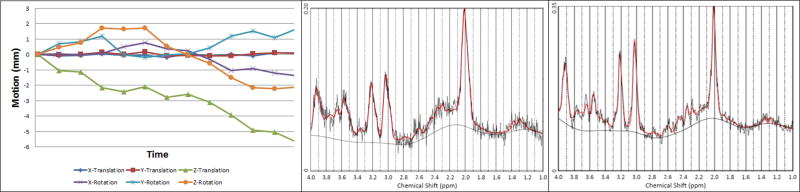
Spectrum for a single subject: (a) Translational and rotational motion (mm) during the scan as determined by the volumetric navigator. (b) LCModel output without offline frequency and phase correction prior to averaging (Hess et al., 2011b) [full-width at half maximum (FWHM) = 10.2 Hz, signal-to-noise ratio (SNR) = 9]. (c) LCModel output with offline frequency and phase correction prior to averaging (FWHM = 7.7 Hz, SNR = 13).
Table 2.
Absolute Metabolite Levels
| Metabolite | Levels (mM) |
|---|---|
| N-Acetylaspartate (NAA) | 5.2 ± 0.5 |
| Glycerophosphocholine + Phosphocholine (Cho) | 1.2 ± 0.2 |
| Creatine + Phosphocreatine (tCr) | 4.7 ± 0.6 |
| Glutamate (Glu) | 5.0 ± 1.2 |
| Glutamate + Glutamine (Glx) | 6.0 ± 1.4 |
| Myo-Inositol (Ins) | 3.7 ± 0.8 |
Values are means ± standard deviation
Table 3 shows the results of the multiple regression analyses, both before and after controlling for potential confounders. As hypothesized, higher levels of maternal alcohol consumption at conception were associated with lower NAA levels (Fig. 3). Following the Hochberg (1988) procedure to correct for multiple comparisons, the threshold for statistical significance for the three other associations with p-values <0.05 was adjusted by dividing 0.05 by 3, generating a critical p-value of 0.017. Using this cut-off, higher levels of alcohol consumption during pregnancy were significantly related to lower Cho levels (Fig. 4), and both measures of alcohol consumption were related to higher levels of Glx (Fig. 5). By contrast to the continuous measures of prenatal alcohol exposure, diagnostic group was not related to the levels of any of the metabolites (all p’s>0.20). However, as predicted, NAA was significantly lower in the FAS group than in the controls, (t (20)= −2.30, p<0.03).
Table 3.
Relation of Prenatal Alcohol Exposure to Absolute Metabolite Concentrations in the Deep Cerebellar Nuclei
| Metabolite | N | AA/day at time of conception |
AA/day across pregnancy |
||
|---|---|---|---|---|---|
| r | β | r | β | ||
| N-Acetylaspartate (NAA) | 49 | −0.33* | −0.33* | −0.26† | −0.26† |
| Glycerophosphocholine plus + Phosphocholine (Cho) | 54 | −0.23† | −0.23† | −0.37** | −0.37** |
| Creatine + Phosphocreatine (tCr) | 51 | 0.01 | 0.01 | −0.10 | −0.10 |
| Glutamate (Glu) | 54 | 0.21† | 0.21† | 0.23† | 0.23† |
| Glutamate + Glutamine (Glx)a | 54 | 0.35** | 0.32* | 0.38** | 0.35** |
| Myo-Inositol (Ins)b | 51 | −0.16 | −0.13 | −0.14 | −0.13 |
AA = absolute alcohol; r represents the simple correlation between alcohol exposure and the metabolite; β is the standardized regression coefficient, after adjustment for the potential confounding variables listed below. Ns vary in the regression analyses due to missing cases for certain of the control variables.
Control variables: smoking during pregnancy
Control variables: child gender
p < 0.10
p < 0.05
p < 0.01
Fig. 3.
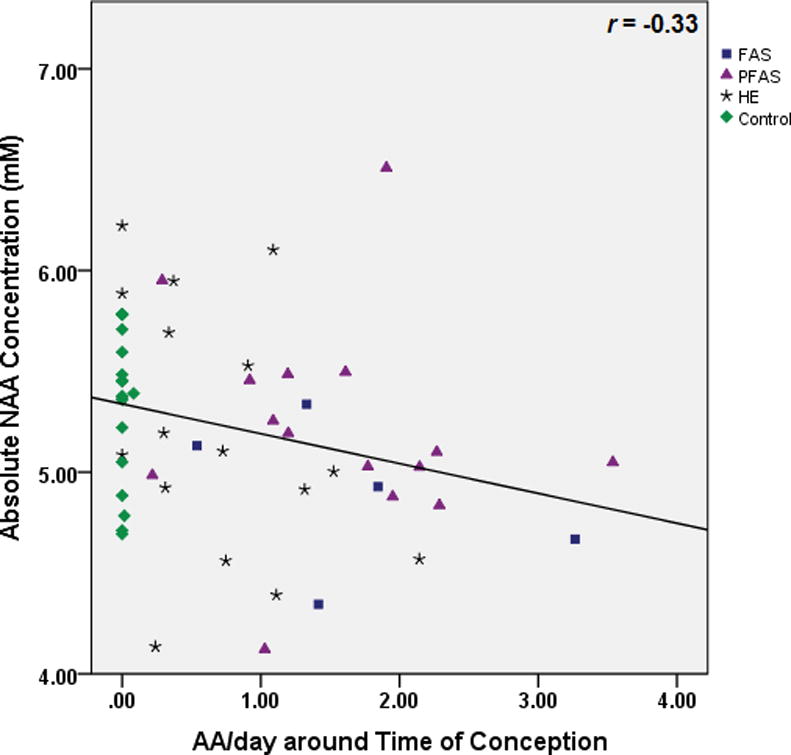
Relation of prenatal alcohol exposure at conception to N-Acetylaspartate (NAA) levels in the deep cerebellar nuclei.
Fig. 4.
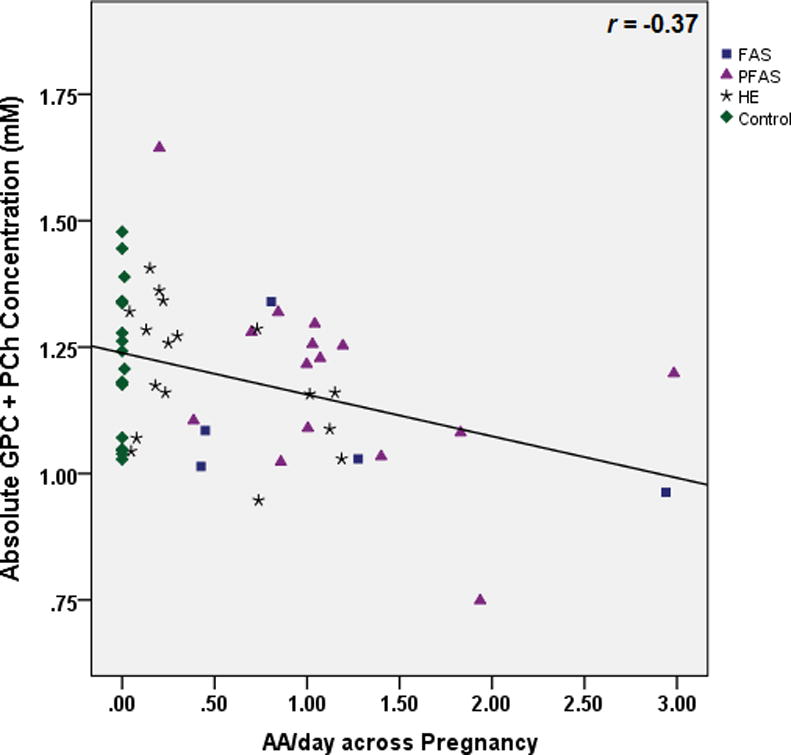
Relation of prenatal alcohol exposure during pregnancy to glycerophosphocholine plus phosphocholine (Cho) levels in the deep cerebellar nuclei
Fig. 5.
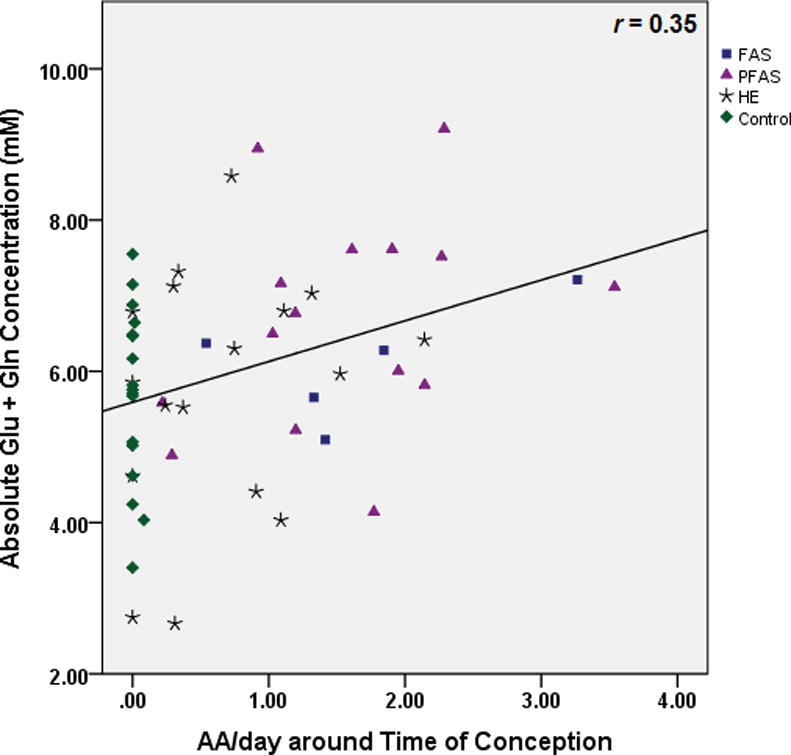
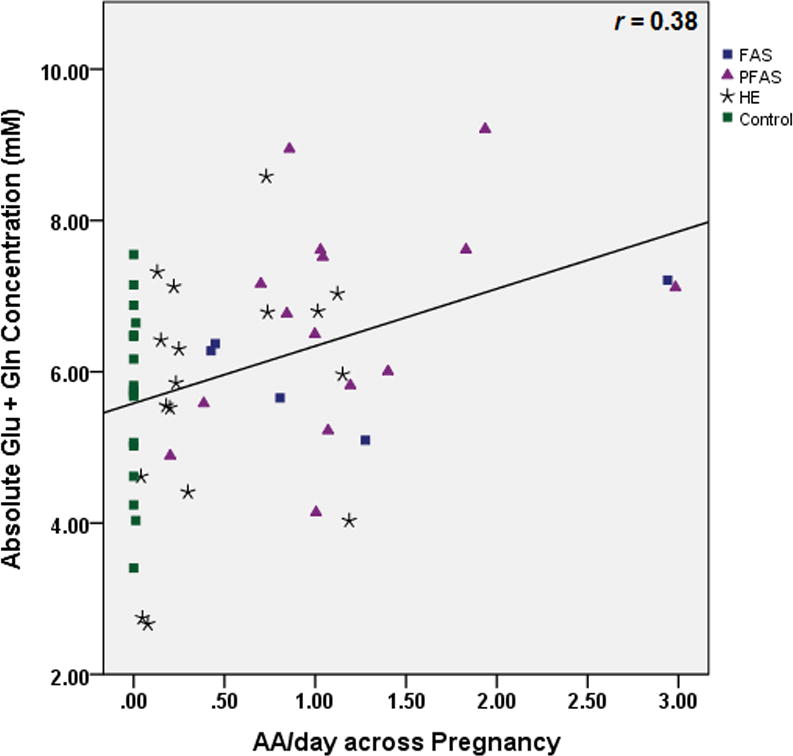
Relation of (a) prenatal alcohol exposure at conception and (b) prenatal alcohol exposure during pregnancy to glutamate plus glutamine (Glx) levels in the deep cerebellar nuclei
Partial correlation analysis was used to determine the degree to which the effects of maternal drinking during pregnancy on Cho and Glx might be attributable to poorer neuronal integrity, as measured by NAA. After controlling for NAA, the relation of AA/day during pregnancy to Cho remained significant (partial r=−0.35, p<0.01), as did the relation of AA/day at conception and during pregnancy to Glx (partial r’s=0.34 and 0.38, respectively, both p’s <0.01), indicating that the effects of prenatal alcohol exposure on the levels of these metabolites are mediated by independent mechanisms. The effect of alcohol consumption during pregnancy on Glx was also not attributable to its effect on Cho (partial r=0.35, p=0.01).
With regard to the relation of these metabolites with child outcome, none of the metabolites were significantly related to IQ scores or eyeblink conditioning performance for the sample as a whole (all p’s>0.10). In the control group, however, higher levels of NAA were related to delay eyeblink conditioning performance at both 5 years (r=0.72 p<0.01) and 9 years (r=0.54, p<0.05). By contrast, NAA was unrelated to eyeblink conditioning in the exposed children at either age (r’s=−0.16 and −0.02 at 5 and 9 years, respectively, both p’s>0.20).
Discussion
This study is the first to use in vivo 1H MRS to examine effects of prenatal alcohol exposure on the neurochemistry of the deep cerebellar nuclei. Prenatal alcohol was associated with lower levels of NAA, which is indicative of poorer neuronal integrity and consistent with findings of decreased numbers of neurons found in this region in heavily exposed laboratory rats (Green et al., 2002). As predicted, NAA levels were also lower in the FAS group when compared with the controls. NAA concentrations increase rapidly during early brain development, particularly in the cerebellum, thalamus and gray matter (Van der Knaap et al., 1992), areas where formation of dendritic arborizations and synaptic connections occur at a high rate during childhood (Pouwels et al., 1999). The lower levels of NAA seen in the children with prenatal alcohol exposure may, therefore, reflect impairment in the early developmental formation of dendritic arborizations and synaptic connections (Stanley et al., 2007; Pouwels et al., 1999). NAA is also an important osmolyte in the brain, participates in oligodendrocyte myelin formation, and plays a role in neuroimmune reactions and intercellular signalling (Baslow, 2003). Decreased NAA levels are seen in neurodegenerative disorders, such as Alzheimer’s disease (Schott et al., 2010) and disorders characterized by a lack of progressive cortical development, such as early onset schizophrenia (Stanley et al., 2007).
One strength of this study is the detailed drinking histories obtained prospectively during pregnancy using timeline follow-back interviews (Jacobson et al., 2002), which enabled us to assess metabolite levels as a function of continuous measures of prenatal alcohol exposure. As in several previous studies (Woods et al., 2013; Meintjes et al., 2013), the continuous measures were more sensitive than fetal alcohol diagnosis. Our finding that lower NAA levels in the deep nuclei were related to poorer eyebink conditioning but not to overall IQ in the control children is consistent with the critical role of this region in cerebellar-mediated learning. The lack of association of NAA with eyeblink performance in the exposed children suggests that the effect of fetal alcohol exposure on this elemental form of learning may not be mediated by poorer neuronal integrity but rather by impairment in other aspects of the eyeblink conditioning cerebellar-brain stem circuit (Spottiswoode et al., 2011).
In this study, prenatal alcohol exposure was assessed both at time of conception and averaged across pregnancy. The effect on NAA was significant for exposure at conception but fell short of significance for exposure across pregnancy; conversely, the effects on Cho were significant only for the across pregnancy measure. Because these two exposure measures were highly correlated (r=0.89) and the effects of both measures were similar in magnitude, we do not believe that these findings reflect differences in timing of exposure but are instead likely the product of fluctuations associated with small sample size.
Only a few studies have used in vivo 1H MRS to examine neurochemical effects of prenatal alcohol exposure, and only one of these (Astley et al., 2009) adjusted for tissue fractions in the voxel. One primate study (Astley et al., 1995) reported an increased ratio of Cho to tCr with increasing prenatal alcohol exposure in a VOI that included the thalamus, basal ganglia, and adjacent white matter. However, failure to adjust for tissue fractions was problematic in this primate study due to the large voxel size, which included both white and grey matter. Another study (Cortese et al., 2006) that found increased NAA levels in the caudate nuclei in children with FASD compared with non-exposed controls, both when reported as a ratio relative to tCr and absolute levels, was limited by small sample size (n=13). Fagerlund et al. (2006) performed multi-voxel in vivo 1H MRS in a large portion of the cerebrum and the cerebellum and found decreased NAA/Cho and NAA/tCr ratios in voxels from the cerebral cortex, white matter, thalamus and cerebellum. Although the authors viewed the absolute signals with caution, as no adjustment was made for tissue fractions, the absolute metabolite levels suggested that the findings of lower NAA/Cho and NAA/tCr ratios were driven by higher levels of Cho and tCr. The voxels sampled in the cerebellum were not isolated to specific structures but included segments from different cerebellar regions. Also, the sample size for this study was small, consisting of 10 subjects each in the exposure and control groups.
In the only prior study that adjusted for tissue fractions in the voxel, in vivo 1H MRS was used to assess the metabolites in a fronto-parietal white matter and a hippocampal/basal nuclear region (Astley et al., 2009). Adjustment for tissue fractions was performed by segmentation of the MR image, and absolute metabolite concentrations were calculated by means of water scaling using LCModel. This study found significantly lower levels of Cho in fronto-parietal white matter of children prenatally exposed to alcohol. Lower Cho was also seen in the hippocampal/basal nuclear region of children with FASD compared to healthy controls, but this difference fell short of statistical significance. Thus, the principal consistent finding that emerges from the two larger studies that adjusted for tissue fractions of GM, WM, and CSF is an inverse relation between prenatal alcohol exposure and Cho.
Given that Cho comprises phosphocholine (PCh) and glycerophosphocholine (GPC), which are precursors and breakdown products, respectively, of membrane phospholipids, the observed inverse relation with prenatal alcohol exposure suggests decreased membrane phospholipid content in the deep cerebellar nuclei. It will be of interest to see whether this effect of fetal alcohol exposure, which was seen in two very different brain regions—fronto-parietal white matter previously (Astley et al., 2009) and deep cerebellar nuclei in this study—will be evident in other brain regions in future studies. The finding that the correlations of prenatal alcohol exposure with Cho remain significant even after controlling for NAA indicates that the effect of prenatal alcohol exposure on Cho is independent of its effect on neuronal integrity indicated by the reduction in NAA.
Glu, the primary excitatory neurotransmitter in the brain, plays a critical role in learning. During excitatory action Glu is released at the pre-synaptic terminal, taken up by the surrounding glia, converted to glutamine (Gln), transported back to the pre-synaptic terminal and converted to Glu, completing the Glu-Gln cycling process. The finding that prenatal alcohol exposure was significantly related to increased Glx but not to Glu per se suggests a disruption in the Glu-Gln cycling during excitatory neurotransmission in the deep cerebellar nuclei. This effect was independent of the effects of prenatal alcohol on both NAA and Cho. Although Glx was not measured in the previous in vivo 1H MRS studies of FASD, glutamatergic transmission is known to be affected in adult alcoholism, as alcohol has an inhibitory effect on N-methyl-D-aspartate (NMDA) receptors, which play a critical role in glutamatergic neurotransmission (Tsai et al., 1998). The glutamatergic system attempts to compensate for this inhibition by up-regulation of NMDA receptors. Once chronic alcohol exposure ends, as would be experienced by a newborn prenatally exposed to alcohol, increased glutamatergic excitability and, ultimately, glutamatergic excitotoxicity (Tsai et al., 1998) remains.
Conclusion
In conclusion, the hypothesis that prenatal alcohol exposure would be associated with a reduction in NAA levels in the deep cerebellar nuclei was confirmed in this study, consistent with evidence from animal studies of alcohol exposure-related impairment in neuronal integrity in this region. We also found evidence of an alcohol-related disruption in choline metabolism that could influence learning by adversely affecting synaptogenesis and/or expansion and maintenance of dendritic arborization. The observed alcohol-related alterations in Glx suggest disruption of the excitatory neurotransmission of glutamatergic neurons. It is possible that prenatal alcohol exposure will be found to be associated with similar alterations of these metabolites in other regions, thereby providing information regarding aspects of cellular function at the neurochemical level that may mediate prenatal alcohol effects on a range of neurobehavioral endpoints.
Acknowledgments
This research was funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA): R01 AA016781 and an administrative supplement to R01 AA09524 (S. Jacobson, PI); U01 AA014790 (S. Jacobson, PI); R21AA017410 (E. Meintjes and A. van der Kouwe, PIs); and U24 AA014815 (K. Jones, PI) in conjunction with the NIAAA Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD). This research was also supported by a grant from the NIH Office of Research on Minority Health, the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa, Focus Area Grant FA2005040800024 from the South African National Research Foundation (E. Meintjes, PI), and seed money grants from the University of Cape Town, the President of Wayne State University, and the Joseph Young, Sr., Fund from the State of Michigan. We thank Bruce Spottiswoode, Ph.D., the CUBIC radiographers Marie-Louise de Villiers and Nailah Maroof, and our UCT and WSU research staff Maggie September, Mariska Pienaar, Nicolette Hamman, and Neil Dodge. We also thank the dysmorphologists, H. Eugene Hoyme, Luther K. Robinson, and Nathanial Khaole, who conducted the dysmorphology examinations of the children for the FASD diagnosis. We greatly appreciate the participation of the mothers and children in the longitudinal study.
Footnotes
The authors declare no competing financial interests.
References
- Aoki S, Okada Y, Nishimura K, Barkovich AJ, Kios BO, Brasch RC, Norman D. Normal deposition of brain iron in childhood and adolescence: MR imaging at 1.5 T. Radiol. 1989;172:381–385. doi: 10.1148/radiology.172.2.2748819. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Astley SF, Weinberger E, Shaw DWW, Richards TL, Clarren SK. Magnetic resonance imaging in ethanol exposed Macaca nemestrina. Neurotoxicol Teratol. 1995;17:523–530. doi: 10.1016/0892-0362(95)00012-g. [DOI] [PubMed] [Google Scholar]
- Astley SF, Clarren SK. Measuring the facial phenotype of individuals with prenatal alcohol exposure: Correlations with brain dysfunction. Alcohol Alcohol. 2001;36:147–159. doi: 10.1093/alcalc/36.2.147. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Richards T, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K. Magnetic resonance spectroscopy outcomes from a comprehensive magnetic resonance study of children with fetal alcohol syndrome. Magn Reson Imaging. 2009;27:760–778. doi: 10.1016/j.mri.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslow MH. N-Acetyl aspartate in the vertebrate brain: Metabolism and function. Neurochem Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Bowman RS, Stein LI, Newton JR. Measurement and interpretation of drinking behavior: I. On measuring patterns of alcohol consumption: II. Relationships between drinking behavior and social adjustment in a sample of problem drinkers. J Stud Alcohol. 1975;36:1154–1172. doi: 10.15288/jsa.1975.36.1154. [DOI] [PubMed] [Google Scholar]
- Chen X, Coles CD, Lynch ME, Hu X. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Hum Brain Mapp. 2012;33:1663–1676. doi: 10.1002/hbm.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clarren SK. Central nervous system malformations in two offspring of alcoholic women. Birth Defects-Orig. 1977;13:151–153. [PubMed] [Google Scholar]
- Cortese BM, Moore GJ, Bayley BA, Jacobson SW, Delaney-Black V, Hannigan JH. Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: preliminary findings in the caudate nucleus. Neurotoxicol Teratol. 2006;28:597–606. doi: 10.1016/j.ntt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Dikranian K, Qin YQ, Labruyere J, Nemmers B, Olney JW. Ethanol induced neuroapoptosis in the developing rodent cerebellum and related brain structures. Brain Res Dev Brain Res. 2005;155:1–13. doi: 10.1016/j.devbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, Zeljko D, Schwarze F, Maschke M, Gerwig M, Frings M, Beck A, Aurich V, Forsting M, Timmann D. Probabilistic 3D MRI atlas of the human cerebellar dentate/interposed nuclei. Neuroimage. 2006;30:12–25. doi: 10.1016/j.neuroimage.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Diwadkar V, Meintjes EM, Goradia D, Dodge NC, Molteno CD, Jacobson SW, Jacobson JL. Differences in cortico-striatal-cerebellar activations during working memory in syndromal and non-syndromal children with prenatal alcohol exposure. Hum Brain Mapp. 2013;34:1931–1945. doi: 10.1002/hbm.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson Ser B. 1993;102:1–8. [Google Scholar]
- Fagerlund A, Heikkinen S, Autti-Rämö I, Korkman M, TImonen M, Kuusi T, Riley EP, Lundbom N. Brain metabolic alterations in adolescents and young adults with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30:2097–2104. doi: 10.1111/j.1530-0277.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Nicholson DA. Developmental changes in eye-blink conditioniing and neuronal activity in the cerebellar interpositus nucleus. The Journal of Neuroscience. 2000;20:813–819. doi: 10.1523/JNEUROSCI.20-02-00813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnet Reson Med. 2006;55:1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Green JT, Tran T, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Hess AT, Tisdall MD, Andronesi OC, Meintjes EM, Van der Kouwe AJW. Real-time motion and B0 corrected singel voxel spectroscopy using volumetric navigators. Magn Reson Med. 2011a;66:314–323. doi: 10.1002/mrm.22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AT, Van der Kouwe AJ, Meintjes EM. Water-independent frequency-and phase-corrected spectroscopic averaging using cross-correlation and singular value decomposition. Proceedings of the International Society for Magnetic Resonance in Medicine, ISMRM 19th Scientific Meeting; Montréal, Canada. May 2011; 2011b. p. 148. ISSN# 1545-4428. [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatr. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Jacobson JL, Sokol RJ. Validity of maternal report of alcohol, cocaine, and smoking during pregnancy in relation to infant neurobehavioral outcome. Pediatr. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2011;35:250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Madge EM, Van den Berg AR, Robinson M, Landman J. Junior South African Individual Scales. Pretoria: Human Sciences Research Council; 1981. [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depen. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes EM, Narr KL, van der Kouwe AJW, Molteno CD, Gutman B, Woods RP, Thompson PM, Jacobson JL, Jacobson SW. Tensor-based morphometry reveals brain midline tissue reduction in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2013;37:105A. [Google Scholar]
- Pouwels PJ, Brockmann K, Kruse B, Wilken B, Wick M, Hanefeld F, Frahm J. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res. 1999;46:474–485. doi: 10.1203/00006450-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Provencher SW. LCModel & LCMgui User’s Manual. 2008 Available at: http://www.s-provencher.com/pages/lcm-manual.shtml.
- Schott JM, Frost C, MacManus DG, Ibrahim F, Waldman AD, Fox NC. Short echo time proton magnetic resonance spectroscopy in Alzheimer’s disease: a longitudinal multiple time point study. Brain. 2010;133:3315–3322. doi: 10.1093/brain/awq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, Gore JC, Peterson BS, Jacobson JL, Jacobson SW. Diffusion tensor imaging of the cerebellum and eyeblink conditioning in fetal alcohol spectrum disorder. Alc Clin Exp Res. 2011;35(12):2174–2183. doi: 10.1111/j.1530-0277.2011.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JA, Vemulapalli M, Nutche J, Montrose DM, Sweeney JA, Pettegrew JW, MacMaster FP, Keshavan MS. Reduced N-acetyl-aspartate levels in schizophrenia patients with a younger onset age: A single-voxel 1H spectroscopy study. Schizophr Res. 2007;93:23–32. doi: 10.1016/j.schres.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE, Ragan P, Chang R, Chen S, Linnoila VMI, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatr. 1998;155:726–732. doi: 10.1176/ajp.155.6.726. [DOI] [PubMed] [Google Scholar]
- Van der Knaap MS, Van der Grond J, Luyten PR, Den Hollander JA, Nauta JJ, Valk J. 1H and 31P magnetic resonance spectroscopy of the brain in degenerative cerebral disorders. Ann Neurol. 1992;31:202–211. doi: 10.1002/ana.410310211. [DOI] [PubMed] [Google Scholar]
- Woods K, Jacobson JL, Molteno CD, Gore J, Jacobson SW, Meintjes EM. Poorer recruitment of intraparietal sulcus in number processing in fetal alcohol spectrum disorders. Proceedings of the 19th Annual Meeting of the Organization for Human Brain Mapping (OHBM 2013); Seattle, WA, USA. June 16–20, 2013; 2013. #2667. [Google Scholar]