Abstract
Autoantibodies to the islet-specific Zn transporter ZnT8 (Slc30a8), as well as CD4 T cells, have been identified in patients with type 1 diabetes. Here we examined for CD4 T-cell reactivity to ZnT8 epitopes in the NOD mouse. Immunization with a cytoplasmic domain of the protein or with peptides predicted to bind to I-Ag7 resulted in a CD4 T-cell response, indicating a lack of deletional tolerance. However, presentation by intraislet antigen-presenting cells (APC) to the T cells was not detectable in prediabetic mice. Presentation by islet APC was found only in islets of mice with active diabetes. In accordance, a culture assay indicated the weak transfer of ZnT8 reactivity from insulinomas or primary β-cells to APC for presentation to T cells. A T cell directed to one peptide (345–359) resulted in the transfer of diabetes, but only in conditions in which the recipient NOD mice or NOD.Rag1−/− mice were subjected to light irradiation. In late diabetic NOD mice, CD4 T cells were found as well as a weak antibody response. We conclude that in NOD mice, ZnT8 is a minor diabetogenic antigen that can participate in diabetes in conditions in which the islet is first made receptive to immunological insults.
Introduction
Several autoantigens have been identified in the autoimmune diabetes of the NOD mouse (1,2). The degree to which they are causative and responsible for the initiation and persistence of the diabetic process is an important issue to determine. Among the important autoantigens is insulin, which by a number of experimental findings appears to be a major driver of the process (3–10). Not only have CD4 T cells been identified as causing diabetes, but also high expression in antigen-presenting cells (APC) or therapeutic manipulations involving immunization with insulin chains have resulted in an effect on diabetes penetrance (11,12). Aside from insulin, other β-cell proteins have been identified as being autoantigens (2,13–16), but whether they have as important a role as insulin or arise primarily from the broad autoreactive diabetogenesis as a result of an initial insult, an epitope-spreading effect, needs to be determined.
In an effort to anlyze components from β-cells that may be involved in the T-cell autoreactivity, we evaluate here the immunogenicity of ZnT8, all in the NOD mouse. ZnT8 (Slc30a8) is an islet-specific Zn membrane transporter required for the transport of Zn ions necessary for the assembly of insulin hexamers in secretory granules (17–20). That ZnT8 is an immunogen in autoimmune diabetes was first made evident by the findings of autoantibodies to it in sera from patients with type 1 diabetes (T1D) (21). Indeed, antibodies to ZnT8, together with antibodies to insulin, IA2, and GAD have been used to make predictions on the development of T1D (21–26). Moreover, T cells to ZnT8 have been identified in patients (27–31).
Here we show that there is no immunological tolerance to ZnT8 protein and that T cells can be induced to various segments of the molecule. Yet, presentation of ZnT8 epitopes by islet APC was weak. T cells directed to one peptide, 345–359, located on the carboxy-cytosolic (C-Cyt) segment, caused diabetes in cell-transfer protocols but required an inflamed islet as a result of sublethal irradiation. ZnT8 is a minor diabetogenic antigen in the NOD mouse.
Research Design and Methods
Mice, Immunizations, and ELISA Spot Assays
NOD (NOD/ShiLtJ), NOD.Rag1−/− (NOD.129S7(B6)- Rag1tm1Mom/J), C57/B6, and B10.BR mice were initially obtained from The Jackson Laboratory (Bar Harbor, ME); the T-cell receptor (TCR) transgenic mice were BDC2.5 (32) and NOD.8F10 (10). All mouse strains were bred and maintained at Washington University School of Medicine, St. Louis, MO, by approved protocols.
Mice were immunized with peptide or protein antigens (10 nmol) emulsified in complete Freund’s adjuvant (Difco, Detroit, MI) subcutaneously in the footpads of hind legs. The popliteal lymph nodes were collected 7 days later, dispersed into single-cell suspension, and analyzed by interleukin-2 (IL-2) and interferon-γ (IFN-γ) ELISA spot (ELISPOT) assays (BD Biosciences, San Jose, CA) in a 96-well format with 1 × 106 cells/well, according to the manufacturer’s protocol. Blocking of class II MHC molecules was performed by addition of anti I-Ag7 (AG2.42.7) monoclonal antibody to lymph node cells 30 min before addition of antigen. The plates were evaluated with a CTL Immunospot Analyzer (Cellular Technology, Shaker Heights, OH) and plotted with GraphPad Prism 6.0 software.
Cloning and Production of Recombinant ZnT8 C-Cyt Protein
The ZnT8 constructs were cloned from a cDNA pool generated from total RNA isolated from NOD islet cells. The recombinant protein was designed to contain the C-Cyt segment. The cDNA was amplified using a pair of oligo nucleotides, Forward: 5′-CATCTTACATATGGAAGGTGTTCCAAAGGGCCT-3′ and Reverse: 5′-GTGTGACCCTCGAGGTCCTGAGGGTCTTCGCA-3′, and directionally cloned into a pET29b+ (EMD Millipore, Billerica, MA) expression vector at NdeI and XhoI restriction sites. The cloned ZnT8-pET29b plasmid encodes the C-Cyt ZnT8 protein (AA 274–367) that is tagged with a COOH-terminal polyhistidine epitope. Chemically competent BL21 star Escherichia coli cells (Life Technologies, Carlsbad, CA) were transformed with ZnT8-pET29b plasmid and grown in lysogeny broth-kanamycin at 30°C until optical density at 600 nm of ∼0.6 was reached. Production of protein was induced by addition of 1 mmol/L isopropyl β-d-1-thiogalactopyranoside (Sigma-Aldrich, St. Louis, MO) for 6 h, after which bacterial cells were harvested by centrifugation and washed and lysed to release the cytoplasmic proteins. The histidine-tagged ZnT8 protein (∼11 kDa) was purified by a Ni-nitrilotriacetic acid agarose affinity column (Qiagen, Valencia, CA), analyzed by Western blotting with anti-His antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and verified by mass spectrometry.
Generation of ZnT8-Overexpressing Cell Lines
The ZnT8 open reading frame, including signal sequence, was cloned into a retroviral delivery vector that incorporates a COOH-terminal AcGFP1 fluorescent tag into ZnT8. Briefly, the cDNA was amplified by primer pairs, Forward: 5′-TGTCCCAGCTAGCATGGAGTTTCTTGAGAGA-3′ and Reverse: 5′-GTGTGAGGATCCAAGTCCTGAGGGTCTTCGCA-3′, and cloned in-frame into the pRetroQ-AcGFP1-N1 vector (Clontech, Mountain View, CA) at NheI and BamHI restriction sites. The ZnT8-pRetroQ plasmid was amplified in Stellar-competent cells (Clontech), and pantropic retroviral particles were made by cotransfecting the Phoenix-GP (National Gene Vector Biorepository, Indianapolis, IN) packaging line with ZnT8-pRetroQ along with VSV-G–containing plasmids in Lipofectamine 2000, a lipid-based transfection (Life Technologies).
M12.C3.g7 B-cell lymphoma and Nit-1 insulinoma (American Type Culture Collection, Manassas, VA) cells were transduced with the ZnT8-AcGFP1 retrovirus in the presence of 10 mg/mL polybrene (Sigma-Aldrich) and enriched for green fluorescence by cell sorting. The Nit-1 and Nit-ZnT8 insulinoma lines were cultured in F-12K medium (Sigma-Aldrich) supplemented with dialyzed FBS at 10% and mildly trypsinized between subcultures while the M12.C3.g7 lines were grown in DMEM (Life Technologies) supplemented with 10% FBS.
Binding Assay
A computational program developed by Chang et al. (33) was used to predict the nine amino acid segments that preferentially bind to I-Ag7. Synthetic peptides were made and then tested for their binding to purified I-Ag7 proteins. Binding was performed, as detailed before, by competition of the peptide to soluble I-Ag7 by standard 125I-labeled peptide (GKKVATTVHAGYG). The labeled peptide was used at a concentration that bound ∼25% of input counts per minute. Binding is expressed as the concentration that inhibits 50% of the response (34).
Development of Primary Cell Lines and Adoptive Transfer
The ZnT8 primary cell lines were generated by immunization with peptides in complete Freund’s adjuvant. The lines were maintained by weekly stimulations with 1 μmol/L peptide, 50 units/mL IL-2, and 1.25 × 106/mL irradiated splenocytes and tested by proliferation before adoptive transfers. Blocking antibodies for I-Ag7 (AG2.42.7) and I-Ak (40F) were used at 10 μg/mL. For adoptive cell transfer, activated T cells on 3-day postactivation were intravenously injected into recipients through the tail vein. Irradiated mice were exposed to ionizing radiation from a 137Cs source (6 Gy) immediately before the cell transfer. Mice were monitored for ∼20 weeks, and mice with blood glucose levels ≥250 mg/dL for two consecutive readings were considered diabetic. The pancreata were collected and formalin fixed for histopathologic assessments.
T-Cell Hybridoma and Antigen Presentation by Islets of Langerhans Cells
T-cell hybridomas were generated by fusion of activated lymph node cells elicited by peptide immunization with BW5147 fusion partner following standard protocols. The T-hybrid clones were screened for reactivity with their respective peptides and ZnT8 protein on the M12.C3.g7 APC line and with the ZnT8-overexpressing M12.C3.g7 line. Typically in a T-cell assay, 5 × 104 T-hybrids were added to 5 × 104 APC in a 96-well plate for 18 h, and IL-2 release was measured by proliferation of an IL-2–dependent cell line (CTLL-2) in a [3H] thymidine incorporation assay.
Islets of Langerhans were isolated from pancreata perfused with collagenase solution and were hand picked (35). Single-cell suspension of islets cells was prepared with nonenzymatic cell dispersion solution (Sigma-Aldrich) and used at 5 × 104 cells/well in T-cell assay with ZnT8 T-hybridomas. IL-2 production was measured by CTLL-2 cells as described (35).
Antigen Transfer Protocols
The transfer of ZnT8 epitopes to APC was evaluated in three different sets of β-cells: Nit-1 insulinoma, a variant of Nit-1 overexpressing ZnT8-AcGFP1 (Nit-ZnT8), and primary β-cells (dispersed islet cells from B10.BR mice, an allogeneic source, H-2k). A modified T-cell assay was used to analyze the transfer of granule antigens to APC. We used Flt3L-elicited splenic CD11c dendric cells (DCs) and macrophage colony–stimulating factor–induced bone marrow macrophages as APC. Mice were given three daily intraperitoneal injections with Flt3L (10 μg/mouse), and on the seventh day after the last injection, spleens were harvested and DCs enriched by CD11c+ magnetic beads (Miltenyi Biotec, Auburn, CA). For generation of macrophages, bone marrow cells were cultured in the presence of 10 units/mL macrophage colony–stimulating factor (from L-929 culture supernatant).
Insulinoma cells (2–2.5 × 105) or primary β-cells (5 × 104) were cocultured with DCs or macrophages (5 × 104) for 24 h in 96-well plates, followed by addition of T-hybridomas (5 × 104) for an additional 18 h. Reactivities to three candidate T cells (viz. insulin, chromogranin A, and ZnT8) were analyzed to study passage and processing to secretory granule antigens. All of the T cells belonged to the conventional or “type A” group and retained reactivities to their respective protein antigens and overexpressing C3.g7 lines (this report, and data not shown) (9, 34). The contact dependency of antigen transfer was studied by introduction of polycarbonate transwell membranes (Corning, Corning, NY) with 0.4, 3.0, and 8.0 μmol/L pore sizes. Insulinoma cells were cultured in the upper compartment, and the APC and T cells were added to the lower compartment. For paraformaldehyde fixation, insulinoma cells were treated with 2% paraformaldehyde for 10 min. To induce apoptosis, cells were treated with 50 mmol/L streptozocin (Sigma-Aldrich) for 20 h, which induced ∼80% cell death measured by JC-1 staining to analyze depolarized mitochondria.
Antibody and T-Cell Evaluations
Analysis of antibodies to the ZnT8 construct and to the various peptides was done by ELISA by techniques used to detect various autoantibodies in NOD sera: 96-well MaxiSorp plates (Nunc, Roskilde, Denmark) were coated overnight with 0.5 mg/mL ZnT8 protein. Sample preparation involved various dilutions of mouse serum with or without ZnT8 protein as a competitor. Plates were incubated for 1 h at room temperature and at 4°C for overnight incubation. The next day, the plate was washed, and an anti-mouse IgG-horseradish peroxidase conjugate (Jackson ImmunoResearch Laboratories, West Grove, PA) was added for 2 h. Antibody binding was visualized using the OptEIA TMB reagent (BD Biosciences, San Diego, CA) for 10 min at ambient temperature, and the plate was read at 450 nm.
CD4 T cells to the ZnT8 peptides were tested by ELISPOT assay. Cells were isolated from the popliteal lymph nodes and islets and pooled; cells were also harvested from inguinal nodes cells. CD4 T cells were purified using anti-CD4 magnetic beads (Miltenyi Biotec, San Diego, CA). Spleen DCs were isolated from flt3-ligand-3L–treated mice. NOD mice were given intraperitoneal injections of 10 μg for 3 consecutive days, and 10 days later the spleen cells were isolated. The single-cell suspension was then irradiated with 3,000 RAD. CD4 cells (at 3 × 106) and FLT-3L DCs (at 5 × 107) were then cultured for 4 days in a T-25 flask in a total volume of 20 mL Dulbecco’s minimal essential media plus 10% FCS, with or without 5 mmol/L concentration of the ZnT8 peptides (ZnT8 314–328, 330–343, and 345–359). Cells were collected and plated to an ELISPOT plate (Millipore) coated with anti–IL-2 or anti–IFN-γ at 105 cultured CD4 cells/well. These cells were plated with or without added ZnT8-pooled peptides at 10 mmol/L. ELISPOT plates were developed and spots quantitated using the CTL Immunospot Analyzer (Cellular Technology) and plotted with GraphPad Prism 6.0 software.
Results
Immunogenicity of ZnT8 Peptides
An algorithm-based program was used that predicts the binding strength of peptides to I-Ag7 to form peptide-MHC complexes. The Chang et al. (33) program, based on the analysis of several hundred natural peptides isolated from an I-Ag7 peptidome (36,37), identifies the favorable amino acids on the five MHC-binding residues of the peptide (i.e., P1, P4, P6, P7, and P9). Nine amino acid segments were selected, and synthetic peptides were made by adding amino and carboxy terminal flanks as required for optimal binding (Table 1). A total of 13 peptides were selected located on the various segments of the ZnT8 protein, including cytosolic and transmembrane segments (Table 1). Binding affinities of each peptide toward I-Ag7 were measured in a standard inhibition assay. Notably, two peptides, one from the transmembrane segment (212–225) and the other from the cytoplasmic segment (345–359), exhibited higher logarithm of odds scores, indicating better binding to I-Ag7, which was confirmed. Other peptides showed intermediate-to-poor binding scores. Those peptides with high logarithm of odds scores yielded a lower but variable half-maximal inhibitory concentration in the low micromoles per liter range.
Table 1.
Prediction, distribution, and binding analyses of ZnT8 epitopes with NOD MHC-II allele (I-Ag7)
| Epitope | Location | Peptide sequence | Logarithm of odds scores | IC50 (μmol/L)* |
|---|---|---|---|---|
| 57–70 | N-CYT | KATGNRSSKQAHAK | NB | |
| 91–104 | TM-1/GC/TM-2 | GHVAGSLAILTDAA | 0.990 | 0.6 |
| 98–111 | TM-2 | AILTDAAHLLIDLT | −0.211 | NB |
| 128–141 | CYT | SKRLTFGWYRAEIL | NB | |
| 166–179 | GC/TM-4 | LYPDYQIQAGIMIT | −0.272 | NB |
| 201–214 | CYT | GYNHKDVQANASVR | 0.931 | 88.2 |
| 212–225 | TM-5 | SVRAAFVHALGDVF | 1.938 | 0.6 |
| 257–270 | TM-6 | LVLASTVMILKDFS | 0.936 | 0.5 |
| 284–297 | C-CYT | NSVKEIILAVDGVI | 0.940 | 3.3 |
| 292–305 | C-CYT | AVDGVISVHSLHIW | 0.431 | 5.1 |
| 313–326 | C-CYT | ILSVHVATAASQDS | 0.616 | 3.1 |
| 330–344 | C-CYT | RTGIAQALSSFDLHS | −0.011 | 5.7 |
| 345–359 | C-CYT | LTIQIESAADQDPSC | 1.365 | 1.1 |
IC50, half-maximal inhibitory concentration.
The peptide segments were identified in a binding prediction algorithm (33). The program identified the preferred nine amino acid segments of the ZnT8 protein. These are underlined for each peptide. Synthetic peptides were made, adding residues from the natural sequence at each end. Flanking residues are known to be important in the binding of the nine amino acid core to class II MHC molecules (36,37).
*NB: nonbinder (IC50 >500 mmol/L).
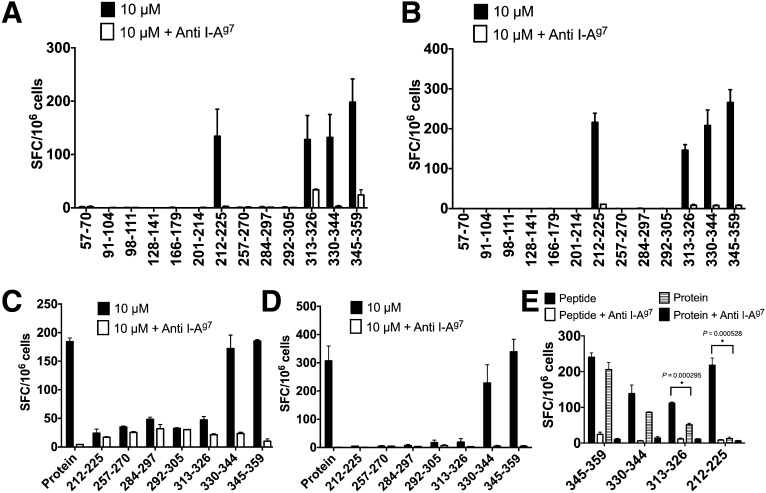
A schematic representation of ZnT8 and a brief review of ZnT8-targeted autoreactivity is presented in Fig. 1A–C. In the current study, immunogenicity was evaluated after immunization and restimulation with the immunizing peptides in ELISPOT assays. Of 13 peptides, 4 generated T-cell responses, as evidenced by IL-2– and IFN-γ–producing cells (212–225, 313–326, 330–344, and 345–359; Fig. 2A and B). All of the responses were blocked by anti I-Ag7 antibody. There was limited correlation between the half-maximal inhibitory concentration and the extent of the response. For example, the response was about equal in the four immunogenic peptides, despite binding differences. Peptides that demonstrated a relatively high affinity binding were not necessarily immunogenic (i.e., 91–104 and 257–270). These issues are not unique to the ZnT8 peptides and have been found in the analysis of model proteins. Table 2 is a summary of all findings testing the four epitopes.
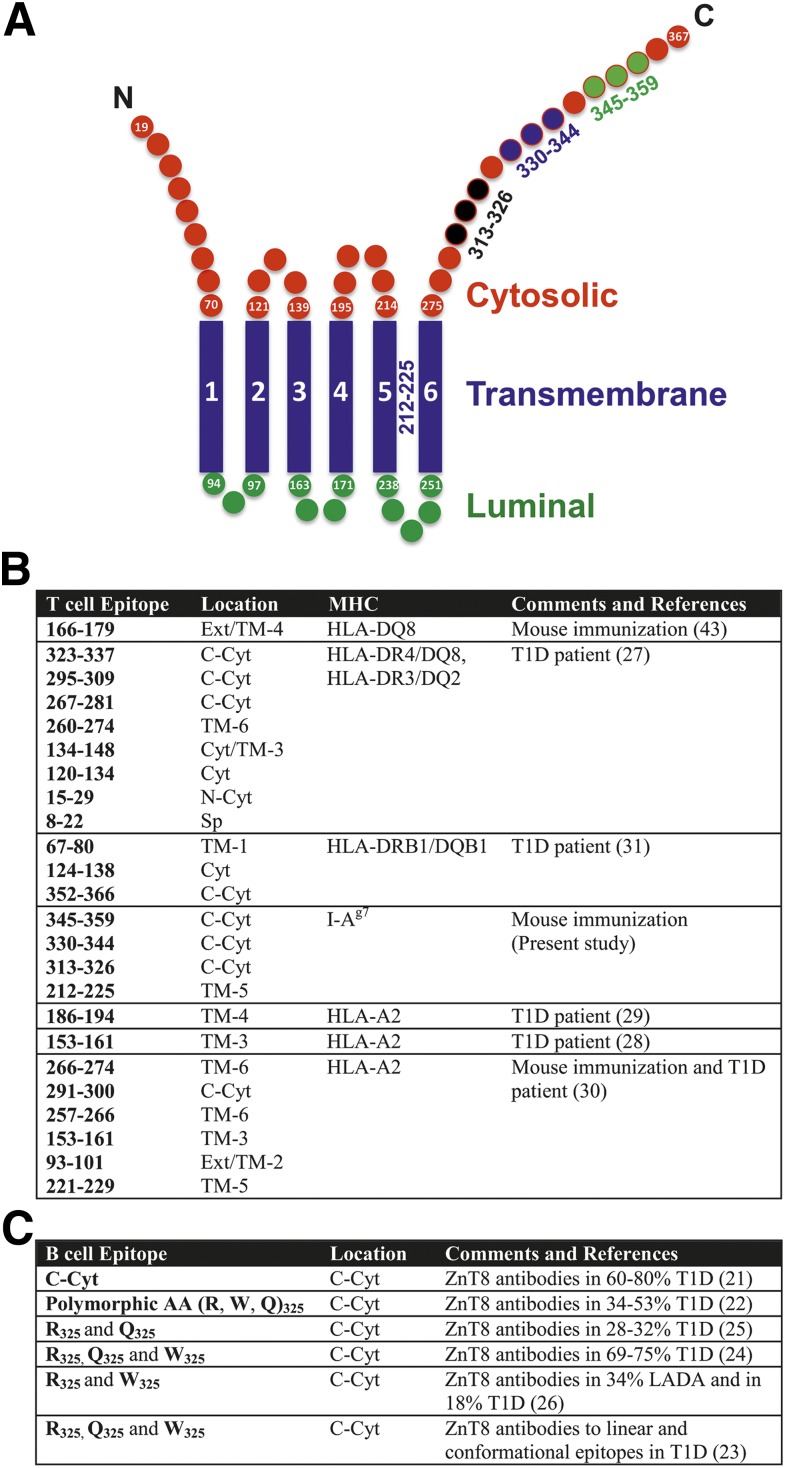
Figure 1.
A: Representation of ZnT8 with predicted positions of amino acid residues for transmembrane, cytosolic, and luminal/extracellular segments (TMHMM v2.0). Four immunogenic epitopes are marked (not drawn to scale). Synopsis of T-cell epitopes (B) and B-cell epitopes (C) on ZnT8. Ext, extracellular; LADA, latent autoimmune diabetes of adult; Sp, signal peptide; TM, transmembrane.
Figure 2.
Immunogenicity of ZnT8 protein and peptides. A and B: ELISPOT assay after immunization and restimulation with the indicated peptides. Shown are the number of IL-2–positive (A) and IFN-γ–positive (B) spot forming cells (SFC) per 106 plated lymph node cells. C and D: ELISPOT after immunization with ZnT8 C-Cyt protein (274–367) and restimulation with the immunizing protein or the peptides indicated. C and D represent number of IL-2–positive and IFN-γ–positive cells, respectively. Blocking of MHC-II was achieved by addition of 5–6 μg/mL anti I-Ag7 antibody. E: IL-2 ELISPOT assay on lymph node cells from NOD mice immunized with the indicated peptide antigen. The cells were restimulated with the immunizing peptide (10 μmol/L) or ZnT8 C-Cyt protein (10 μmol/L), with and without MHC II–blocking antibody (anti I-Ag7). *P values are provided from paired t test. Data are representative of six (A and B), three (C and D), and two (E) experiments. Baseline spots never exceeded 20, were not subtracted, and are indicated in baselines. Bars indicate mean and standard deviation.
Table 2.
Summary of ZnT8 epitopes, T cells, and their reactivities
| Epitope | |||||
|---|---|---|---|---|---|
| 345–359 | 330–359 | 313–326 | 212–225 | ||
| Binding (μmol/L) | I-Ag7 | 1.1 | 5.7 | 3.1 | 0.6 |
| Immunization* | Peptide | + + + | + + + | + + | + + + |
| Protein | + + + | + + + | +/− | — | |
| T-cell response† | Peptide | + + + | + + + | + + + | + + + |
| Protein | + + + | + + + | — | — | |
| ZnT8-C3.g7 | + + + | + + + | — | — | |
| T-cell transfer‡ | NOD-Irr | Positive | ND | Negative | Negative |
| NOD.Rag1−/− | Negative | Negative | ND | ND | |
| NOD.Rag1−/−-Irr | Positive | Negative | ND | ND | |
Irr, sublethally irradiated; ND, not determined.
*ELISPOT response to immunizations.
†Responsiveness of T-cell hybridoma to the indicated antigens.
‡Diabetogenesis after adoptive cell transfer into indicated recipients.
Importantly, immunization with ZnT8 protein elicited a response. The recombinant protein segment encompassed the C-Cyt from residues 274 to 367 with molecular mass ∼11 kDa (from Fig. 1A). The T-cell response was recalled by the immunizing protein and by two peptides, 330–344 and 345–359, and all were I-Ag7 dependent (i.e., vs. class II MHC epitopes; Fig. 2C and D). The 313–326 peptide, which elicited a response by peptide immunization, was very weakly reactive, if at all, after protein immunization. Peptides 292–305 and 284–297 also did not elicit a response. There was no T-cell reactivity to the transmembrane-5 (212–225) or to the transmembrane-6 (257–270) segments not represented in the immunizing protein.
A recall response to the peptide as well as to the protein was elicited after immunization with the peptides 330–334 and 345–359, confirming that these peptide segments were presented after protein immunization (Fig. 2E). The recall response to protein after immunization with peptide 313–326 was weak in accordance with its poor immunogenicity after protein immunization. There was no recall to peptide 212–225 by the protein.
T-Cell Hybridomas and Their Reactivities
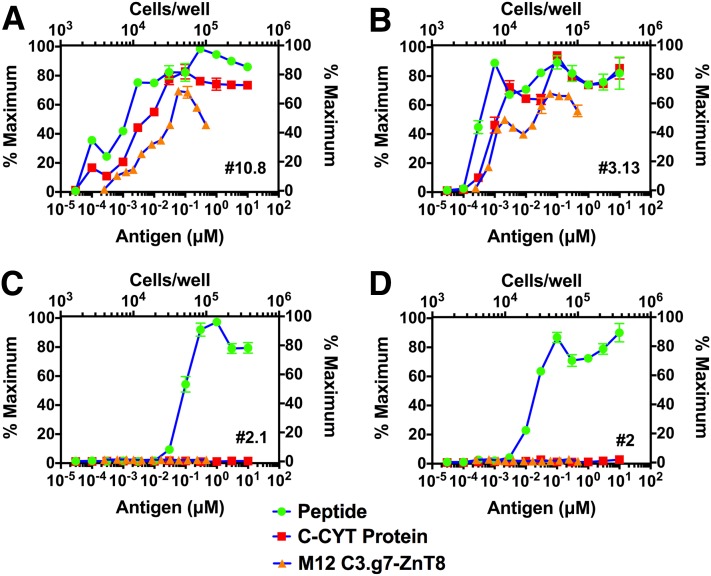
T-cell hybridomas were made after immunization with the four immunogenic peptides. Notably, two sets of hybridomas were found: 1) those that recognized peptide and protein antigens from exogenous source and that also reacted with endogenously derived epitopes in a C3.g7 line expressing the ZnT8 protein, the ZnT8-C3.g7 line (Fig. 3A and B); and 2) those that recognized only peptides but failed to react with protein and the ZnT8-C3.g7 line (Fig. 3C and D). A number of 330–344 (5 of 5) and 345–359 (6 of 8) clones were reactive to the three forms of antigen, whereas clones reactive to 313–326 (6 of 7) and 212–225 (6 of 6) responded only to exogenous peptides.
Figure 3.
Reactivity of ZnT8-specific T cells. T-hybridomas were derived after peptide immunization, and the antigen response was measured to exogenous peptides or ZnT8 C-Cyt protein presented on the M12 C3.g7 APC line and to a ZnT8 expressing the M12 C3.g7 line. IL-2 production was measured by [3H] thymidine incorporation assay with an IL-2–dependent cell line (CTLL-2). A: 345–359 specific (clone #10.8). B: 330–344 specific (clone #3.13). C: 313–326 specific (clone #2.1). D: 212–225 specific (clone #2). Values are presented as percent maximum of the counts per minute (CPM) obtained for the respective clone. Maximum CPM obtained for clones #10.8, #3.13, #2.1, and #2 were 51,875, 50,647, 49,594, and 51,634, respectively. Data are representative of 10 experiments. The lower abscissa in the panel represents the amounts of antigen in the reaction, the left ordinate represents the degree of IL-2 stimulation, the top abscissa represents the number of M12 C3.g7 APC line used in the assay, and the right ordinate shows the results. Bars indicate mean and standard deviation.
Our laboratory previously identified two sets of autoreactive T cells to insulin. One is a conventional T cell, which we termed type A, that recognizes protein- and peptide-derived antigens, and a second nonconventional set that recognizes only exogenous insulin peptide and denatured insulin, termed type B (9,34,38). These latter T cells were important because they bypassed thymic control (9,10). The findings with the ZnT8 suggest a similar breakdown of the clones into these two sets. In contrast to the insulin-reactive type A, to which there is limited immunological reactivity because insulin is presented in the thymus, the T cells directed to 345–359 and 330–344 were elicited by immunization with the cytosolic protein segment and were reactive to the C3.g7-ZnT8 line (see Discussion).
Adoptive T-Cell Transfer and Diabetogenesis
We developed primary T-cell lines to evaluate their diabetogenic potential. First, lines were developed against pooled peptides 212–225 and 313–326 (group I) and against 330–344 and 345–359 (group II). Cells were transferred into sublethally irradiated (6 Gy) NOD recipients (Table 3). The lines responded to individual peptides in proliferative assays before transfer (not shown). Upon transfer, the group II T-cell line induced diabetes in irradiated NOD mice, whereas all four recipient mice of the group I line remained free of diabetes (monitored for 22 weeks).
Table 3.
Summary of diabetogenesis by adoptive transfer of ZnT8 primary T-cell lines
| T-cell | Recipient | Diabetes incidence [n/N (%)] | Kinetics* |
|---|---|---|---|
| 212–225 + 313–326 group I | NOD-Irr | 0/4 (0) | |
| 330–344 + 345–359 group II | NOD-Irr | 3/4 (75) | 13 (3) |
| 345–359 | NOD.Rag1−/− | 0/10 (0) | |
| NOD.Rag1−/−-Irr | 8/10 (80) | 23 (4), 24 (1), 31 (2), 52 (1) | |
| NOD-Irr | 8/8 (100) | 11 (4), 23 (4) | |
| 330–344 | NOD.Rag1−/− | 0/4 (0) | |
| NOD.Rag1−/−-Irr | 0/5 (0) | ||
| BDC2.5 | NOD.Rag1−/− | 3/3 (100) | 6 (3) |
Irr, sublethally irradiated.
*First observation of blood glucose ≥250 mg/dL in day post T-cell transfer, and the number of mice tested positive is indicated in the parentheses. Mice were usually tested at an interval of 5–7 days.
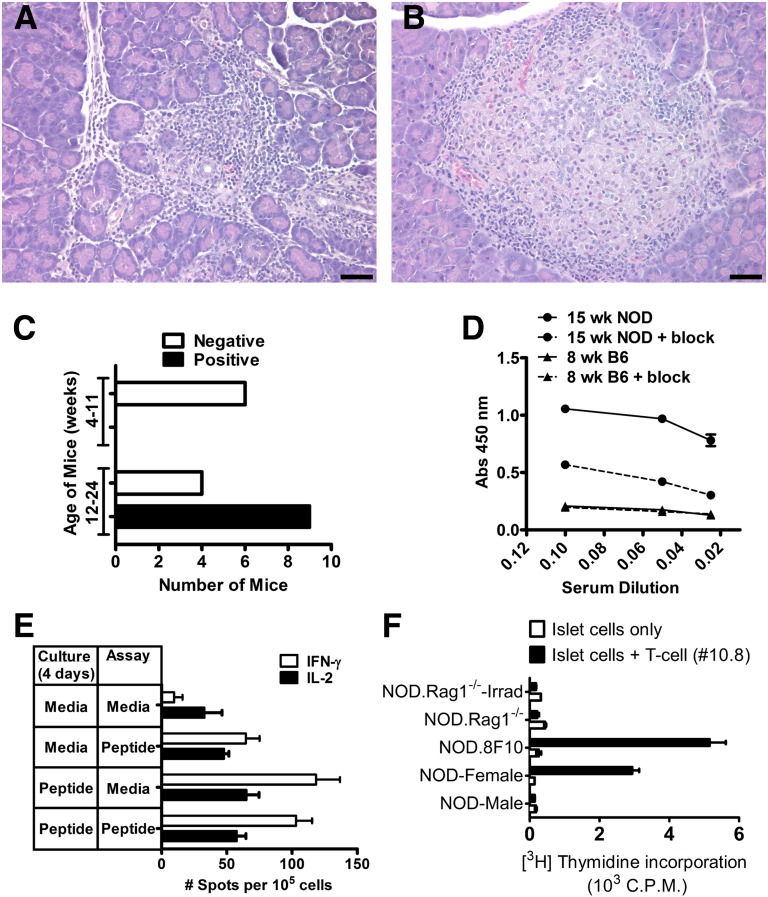
We examined independently the 345–359 and 330–344 reactive T-cell lines (Table 3). The 345–359 T cells were diabetogenic when transferred into irradiated mice (NOD or NOD.Rag1−/−). The nonirradiated NOD.Rag1−/− recipients at the same time remained free of diabetes. In general, diabetes developed faster when transferred into irradiated NOD mice than when transferred to NOD.Rag1−/− mice. Irradiated NOD that did not receive cells did not develop diabetes. Pathological assessment of pancreata from irradiated (NOD and NOD.Rag1−/−) mice, all of which developed diabetes, revealed a pronounced inflammation of islets with lymphocytes, macrophages, and apoptotic β-cells (Fig. 4A). By contrast, the same cells transferred into the nonirradiated NOD.Rag1−/− mice showed a variable degree of pathology. Most islets showed nondestructive insulitis with a typical peri-insulitic lesion and normal-appearing β-cells (Fig. 4B).
Figure 4.
Pathogenicity of ZnT8 T cells. Histopathology of pancreatic lesion after transfer of 2 × 107 of activated ZnT8 345–359 T cells into sublethally irradiated (6 Gy) NOD.Rag1−/− (A) and NOD.Rag1−/− mice (B). Bars, 50 µm. C: ZnT8 antibodies in NOD female mice at 4–11 and 12–24 weeks of age. A positive reaction is one giving an optical density (OD) of more than 0.5 at a 1:10 dilution of sera. Positive reactions were only found after 12 weeks. Experiment compiles data from 19 mice screened. D: A representative assay from sera from 15-week-old NOD and B6 females positive or negative for ZnT8 antibodies, respectively, tested for binding to ZnT8 with or without ZnT8-blocking peptide at different dilutions. E: Sorted CD4 T cells from pancreatic lymph nodes and islets from 16-week-old NOD females were cultured for 4 days with or without the ZnT8 peptide (indicated in panel A under Culture); the ELISPOT assay was done with or without the addition of the ZnT8 peptide to detect IFN-γ and IL-2 production by ELISPOT (indicated under Assay). The results indicate a positive IFN-γ response. Experiment is a representative of three independent experiments. Four experiments examining T cells at 8–12 weeks did not show positive reactivity. F: Antigen presentation by dispersed islet cells to ZnT8 345–359 (#10.8) T-cell hybridoma. NOD male (10–12 weeks), NOD female (16 weeks), NOD.8F10 female (11 weeks), NOD.Rag1−/− male (12 weeks), and irradiated (Irrad) NOD.Rag1−/− male (12 weeks) mice (three to five per group) were used. Values are representative from four experiments using three to five mice per group. Not shown in the graph are results of four other different experiments using islets from 4- to 8-week-old mice in which no response was obtained. Bars indicate mean and standard deviation.
The 330–344 reactive T cells were nondiabetogenic in irradiated and nonirradiated NOD.Rag1−/− recipients. Activated T cells from a diabetogenic TCR transgenic mouse, BDC2.5 (32) were included as a positive reference that transferred diabetes in NOD.Rag1−/−. T cells to 330–344 did not lead to islet pathology in irradiated and nonirradiated groups. The two ZnT8 primary lines (i.e., 345–359 and 330–344) used in transfer experiments retained proliferative responses to their cognate peptides and, as expected, were inhibited by anti–MHC-II blocking (data not shown).
Parallel to these studies, the antibody response to ZnT8 was examined in NOD mice at various ages. The C-Cyt ZnT8 protein included the segment from residues 274–367 and was tagged with a COOH-terminal polyhistidine epitope. This is the same protein segment to which antibodies have been targeted in human patients (Fig. 1). There was no reactivity in sera from C57/B6 mice tested in 10 samples at various ages. Antibody reactivity to the C-Cyt segment was detected only in sera of mice late in diabetogenesis (9 of 13 mice, after 12 weeks; Fig. 4C and D). NOD sera were considered autoantibody positive when showing a 0.5 optical density reading over the background level: the sera showed a level of reactivity that was not inhibited by an excess of the free protein. (The explanation for the background is not apparent; it may represent autoantibodies directed to segments of the protein that are denatured upon binding to the assay plate as occurs with some antiprotein sera.) Positivity was found at high dilution of sera, suggesting a low level of antibodies. Further studies are needed to characterize the fine antibodies to ZnT8 in NOD sera.
CD4 T cells isolated from pancreatic lymph nodes and islets were reactive to ZnT8 peptides but only at 16 weeks of age and not before at 8 to 12 weeks (Fig. 4E).
Transfer of Secretory Granule Antigens to APC
In an effort to explain the results of the T cell transfers, the islet presentation of the functionally strong ZnT8 345–359 peptide was examined in vivo and in culture. β-Cells do not express class II MHC molecules. The diabetogenic antigens are transferred to APC. Islets contain a network of APC that are heavily charged with peptides from secretory granules (9,35,39,40).
Dispersed islet cells were placed in culture with the ZnT8 345–359 specific T-cell hybridoma (clone 10.8). In four different experiments, there was no presentation examining islets from 4- to 8-week-old NOD females or males or NOD.Rag.1−/− mice. However, there was presentation, albeit weakly, from islets of prediabetic NOD female and NOD.8F10 islets (Fig. 4F). The latter are islets from a TCR transgenic mouse of an insulin-reactive T cell (10). These islets from NOD females and NOD.8F10 exhibited a degree of insulitis with myeloid and lymphoid infiltrations. Thus the findings indicate that ZnT8 epitopes are presented when an active diabetogenic process is evident and not before. Lastly, the islets from irradiated NOD.Rag1−/− mice minimally presented antigens.
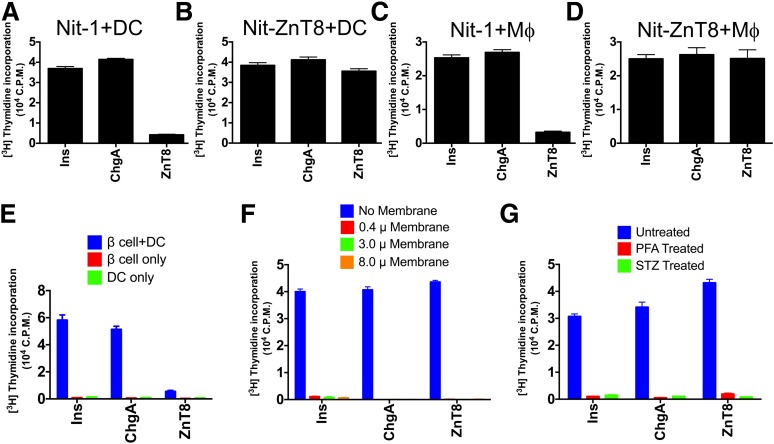
To examine further the transfer of the ZnT8 epitope from β-cells to the islet APC, a culture assay testing the Nit-1 insulinomas or β-cells was developed. These were incubated first with APC, after which presentation was examined using the strong T-cell hybridoma (clone 10.8) directed to ZnT8 345–359. By comparison, the reactivity was also examined to peptide 13–21 of the B chain of insulin (34) or to peptide 111–125 of chromogranin A.
Coculturing Nit-1 or allogeneic β-cells with APC transferred insulin and chromogranin A reactivity, as evidenced by activation of T cells (Fig. 5A, C, and E). However in comparison, the presentation of ZnT8 was present but at a very low level. These findings ex vivo paralleled the results of presentation by NOD islets referred to above and indicate a limited amount of ZnT8 epitopes being handled by the APC. In contrast, strong presentation was observed in Nit-1 overexpressing the ZnT8 transgene (Nit-ZnT8; Fig. 5B and D).
Figure 5.
Transfer of β-cell antigens in coculture. The indicated cells Nit-1 (A and C), Nit-ZnT8 (B and D), and islet cells (E) isolated from allogeneic mice (B10.BR, H-2k) were cocultured with the indicated T-cell clones as described in the text: #IIT3 reacting with Ins B 13–21, #35 reacting with peptide ChgA 111–125, and #10.8 reacting with ZnT8 345–359. The APC were CD11c-enriched DCs from Flt3L-elicited spleen or from macrophage colony–stimulating factor–induced bone marrow macrophages from NOD mice. F: Shows results of coculture experiments in which Nit-ZnT8 cells were cultured in the upper chamber of transwell dishes (pore sizes 0.4–8.0 mm) to separate them from Flt3L DCs added to the bottom chamber. The same T-cell hybridomas were used in the assay and were added to the chamber with DC. The bar in blue indicates the results of insulinoma and DCs cocultured together without membrane separation; and next is in the transwell having different pore sizes. G: Coculture experiments in which Nit-ZnT8 cells were treated with paraformaldehyde (PFA; 2%, 10 min) or streptozocin (STZ; 50 mmol/L, 20 h) and washed before addition of Flt3L DCs in antigen-presentation assay with T-cell hybridomas. Bars in blue indicates coculture with untreated Nit-ZnT8. Data are representative of seven (A), five (B), one (C and D), two (E), three (E), and two (G) independent experiments.
We analyzed whether the transfer involved a contact reaction or the release of soluble immunogens by using transwell membrane barriers between Nit-ZnT8 and APC (Fig. 5F). Separating the insulinoma cells from APC in transwells abolished the transfer of all three antigens. There was no improvement in presentation despite the use of the membrane with largest pore size (8.0 μm). The transwell membranes were permeable to peptides, insulin protein, and isolated granules (data not shown). Therefore, transfer of antigens in coculture requires cell-to-cell contact between donor (insulinoma) and acceptor cells (APC). Lastly, the transfer of immunogen in primary β-cells or in Nit-ZnT8 required live cells. Figure 5G shows the results with Nit-ZnT8: the transfer did not take place after paraformaldehyde fixation of cells or after treatment with streptozocin.
Discussion
We decided to examine the immunogenicity of ZnT8 based on the clinical findings pointing to an antibody response to it in patients with T1D. Our findings indicate that ZnT8 is not a major autoantigen in the NOD autoreactive process. We found autoreactive T cells late in diabetogenesis and also showed that these are weakly pathogenic.
Immunization with ZnT8 proteins resulted in a relatively strong CD4 T-cell response as strong as that obtained in our laboratory to a foreign protein or peptide. These findings indicate that immunological tolerance to this antigen is weak or nonexistent. Analysis of transcriptional profiles reported in the Immunological Genome Project (www.immgen.org) indicate a low expression, if at all, of ZnT8 in medullary thymic epithelial cells in contrast to other β-cells autoantigens such as insulin, for one example (Supplementary Fig. 1). This limited thymic expression may well explain the lack of a deletional tolerance.
Our results documented the weak passage of ZnT8 epitopes from β-cells to APC in vivo, when examining islet APC, or ex vivo. The proteome analyses of β-cells indicated a very low expression of ZnT8 (41,42). Islet APC contain a very high density of peptide-MHC complexes derived from β-cells as a result of their capture of secretory granules and present particularly well to insulin, including to C-peptide, both abundant in the secretory granule (39,40). These quantitative issues are likely to contribute to the strong diabetogenicity of insulin and the weak autoreactivity to ZnT8. However, another important consideration not only in the framework of ZnT8 but also to all β-cells autoantigens is the structure of the autoantigen and its localization in the secretory granule, which appears to be the target of CD4 and B cell epitopes. (For CD8 epitopes, however, the mechanisms and dynamics of presentation become more complex because it involves also epitopes such as that from the islet-specific glucose-6-catalytic subunit-related protein, a protein residing in the endoplasmic reticulum [43].) In contrast to insulin, which is in the core of the granule, ZnT8 is a membrane protein, and conceivably, its processing may be less efficient in donating immunogenic epitopes. From the analysis of the autoantibodies and T cells in patients with T1D, B-cell epitopes are localized to the COOH-terminal cytosolic domain (Fig. 1C; 21–26), whereas there is a spread of CD4 and CD8 T-cell epitopes to cytosolic and transmembrane segments (Fig. 1B; 27–31,43). The intriguing question is how these segments then become accessible for immune recognition by B cells and APC. Our coculture experiments of insulinomas with APC indicated a contact-dependent transfer of granule core and membrane-bound antigens. Clearly, the insulinomas and the β-cells both had to be live for transfer to take place: neither fixed cells nor dying cells after streptozocin treatment resulted in transfer. We are now examining this transfer system in depth in an effort to explain the recognition of protein segments that at face value appear inaccessible. We expect that as more epitopes are examined (44,45) a consensus should be reached on the factors playing a role in presentation by APC and in those that contribute to the various stages in diabetes (2,13–16).
The 345–359 specific T cells were diabetogenic upon adoptive transfer, but only in situations where mice were lightly irradiated. This requirement was rather distinct compared with that other T cells like BDC2.5 or NOD.8F10, which readily transfer into nonirradiated Rag.1−/− mice. The 345–359 T cells did induce a mild insulitis in standard NOD.Rag1−/− mice, whereas in striking contrast, a rather aggressive form was transferred into the irradiated NOD and NOD.Rag1−/−. In accordance with the transfer findings, the presentation of ZnT8 peptide-MHC complexes in immunologically quiescent islets such as young NOD male or NOD.Rag1−/− was undetectable. However, there was a clear increase in presentation in older NOD and NOD.8F10, where a degree of islet inflammation was evident. This finding indicates that minor epitopes become more evident as diabetes progresses and gives credence to the epitope-spreading concept. The presentation during diabetogenesis may result from epitopes derived from β-cells in apoptosis or during a stress reaction. Concerning the effects of radiation, exposure to an ionizing radiation is known to induce multiple radioadaptive changes. Most prominent of those include upregulation of cell adhesion molecules such as intracellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin in vascular endothelial cells (46–48). Upregulation of intracellular adhesion molecule, intracellular adhesion molecule-1, and chemokines took place in islets after arrival of specific T cells (49,50). Moreover, the increase in adhesion molecules facilitated the entry of T cells (49). Current experiments are examining the cellular and molecular responses of the islets to various inflammatory stimuli, including irradiation, and their influence on cell localization and diabetes incidence. Our initial findings are pointing to a rise in the expression of adhesion molecules as predicted from the findings cited above.
Article Information
Acknowledgments. The authors thank Shirley Petzold (Washington University School of Medicine) for assistance in peptide-binding experiments, Katherine Frederick (Washington University School of Medicine) for help in mouse handling, and Lindsay Moore (Washington University School of Medicine) in isolating islets.
Funding. This work was supported by National Institutes of Health grants AI-024742, DK-058177, and DK-20579.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.K.N., B.C., A.N.V., and E.R.U. designed experiments and evaluated the data. D.K.N. and A.N.V. conducted most of the experiments. B.C. and E.R.U. evaluated islet cytology and histology findings. D.K.N., B.C., and E.R.U. wrote the manuscript. E.R.U. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1882/-/DC1.
References
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005;23:447–485 [DOI] [PubMed] [Google Scholar]
- 2.Arvan P, Pietropaolo M, Ostrov D, Rhodes CJ. Islet autoantigens: structure, function, localization, and regulation. Cold Spring Harb Perspect Med 2012;2 [DOI] [PMC free article] [PubMed]
- 3.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol 1994;24:1853–1857 [DOI] [PubMed] [Google Scholar]
- 4.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol 1995;25:1056–1062 [DOI] [PubMed] [Google Scholar]
- 5.Halbout P, Briand J-P, Bécourt C, Muller S, Boitard C. T cell response to preproinsulin I and II in the nonobese diabetic mouse. J Immunol 2002;169:2436–2443 [DOI] [PubMed] [Google Scholar]
- 6.French MB, Allison J, Cram DS, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes 1997;46:34–39 [DOI] [PubMed] [Google Scholar]
- 7.Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol 2004;5:1028–1035 [DOI] [PubMed] [Google Scholar]
- 8.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005;435:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol 2010;11:350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4⁺ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J Exp Med 2013;210:2403–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muir A, Peck A, Clare-Salzler M, et al. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferon-gamma transcription. J Clin Invest 1995;95:628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med 2011;208:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman SM, DiLorenzo TP. A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens 2003;62:359–377 [DOI] [PubMed] [Google Scholar]
- 14.Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med 2012;2:a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol 2005;87:123–162 [DOI] [PubMed] [Google Scholar]
- 16.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol 2014;32:579–608 [DOI] [PubMed] [Google Scholar]
- 17.Foster MC, Leapman RD, Li MX, Atwater I. Elemental composition of secretory granules in pancreatic islets of Langerhans. Biophys J 1993;64:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes 2004;53:2330–2337 [DOI] [PubMed] [Google Scholar]
- 19.Lemaire K, Ravier MA, Schraenen A, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci U S A 2009;106:14872–14877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolson TJ, Bellomo EA, Wijesekara N, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 2009;58:2070–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenzlau JM, Liu Y, Yu L, et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 2008;57:2693–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skärstrand H, Lernmark A, Vaziri-Sani F. Antigenicity and epitope specificity of ZnT8 autoantibodies in type 1 diabetes. Scand J Immunol 2013;77:21–29 [DOI] [PubMed] [Google Scholar]
- 24.Ingemansson S, Vaziri-Sani F, Lindblad U, Gudbjornsdottir S, Törn C, Diss-Study Group Long-term sustained autoimmune response to beta cell specific zinc transporter (ZnT8, W, R, Q) in young adult patients with preserved beta cell function at diagnosis of diabetes. Autoimmunity 2013;46:50–61 [DOI] [PubMed] [Google Scholar]
- 25.Tiberti C, Yu L, Lucantoni F, et al. Detection of four diabetes specific autoantibodies in a single radioimmunoassay: an innovative high-throughput approach for autoimmune diabetes screening. Clin Exp Immunol 2011;166:317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen MK, Härkönen T, Forsblom C, Groop PH, Knip M, Tuomi T. Zinc transporter type 8 autoantibodies (ZnT8A): prevalence and phenotypic associations in latent autoimmune diabetes patients and patients with adult onset type 1 diabetes. Autoimmunity 2013;46:251–258 [DOI] [PubMed] [Google Scholar]
- 27.Dang M, Rockell J, Wagner R, et al. Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol 2011;186:6056–6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Xu X, Gu R, et al. Prediction of HLA class I-restricted T-cell epitopes of islet autoantigen combined with binding and dissociation assays. Autoimmunity 2012;45:176–185 [DOI] [PubMed] [Google Scholar]
- 29.Scotto M, Afonso G, Larger E, et al. Zinc transporter (ZnT)8(186-194) is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia 2012;55:2026–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Énée É, Kratzer R, Arnoux JB, et al. ZnT8 is a major CD8+ T cell-recognized autoantigen in pediatric type 1 diabetes. Diabetes 2012;61:1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chujo D, Foucat E, Nguyen TS, Chaussabel D, Banchereau J, Ueno H. ZnT8-Specific CD4+ T cells display distinct cytokine expression profiles between type 1 diabetes patients and healthy adults. PLoS ONE 2013;8:e55595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell 1993;74:1089–1100 [DOI] [PubMed] [Google Scholar]
- 33.Chang KY, Suri A, Unanue ER. Predicting peptides bound to I-Ag7 class II histocompatibility molecules using a novel expectation-maximization alignment algorithm. Proteomics 2007;7:367–377 [DOI] [PubMed] [Google Scholar]
- 34.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an insulin peptide-MHC complex allows diabetogenic T cells to escape thymic deletion. J Exp Med 2011;208:2375–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci U S A 2008;105:6121–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest 2005;115:2268–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suri A, Vidavsky I, van der Drift K, Kanagawa O, Gross ML, Unanue ER. In APCs, the autologous peptides selected by the diabetogenic I-Ag7 molecule are unique and determined by the amino acid changes in the P9 pocket. J Immunol 2002;168:1235–1243 [DOI] [PubMed] [Google Scholar]
- 38.Mohan JF, Unanue ER. Unconventional recognition of peptides by T cells and the implications for autoimmunity. Nat Rev Immunol 2012;12:721–728 [DOI] [PubMed] [Google Scholar]
- 39.Levisetti MG, Lewis DM, Suri A, Unanue ER. Weak proinsulin peptide-major histocompatibility complexes are targeted in autoimmune diabetes in mice. Diabetes 2008;57:1852–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calderon B, Carrero JA, Unanue ER. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Curr Opin Immunol 2014;26:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petyuk VA, Qian WJ, Hinault C, et al. Characterization of the mouse pancreatic islet proteome and comparative analysis with other mouse tissues. J Proteome Res 2008;7:3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waanders LF, Chwalek K, Monetti M, Kumar C, Lammert E, Mann M. Quantitative proteomic analysis of single pancreatic islets. Proc Natl Acad Sci U S A 2009;106:18902–18907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieberman SM, Evans AM, Han B, et al. Identification of the β cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A 2003;100:8384–8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurr W, Yavari R, Wen L, et al. A Reg family protein is overexpressed in islets from a patient with new-onset type 1 diabetes and acts as T-cell autoantigen in NOD mice. Diabetes 2002;51:339–346 [DOI] [PubMed] [Google Scholar]
- 45.Baker RL, Delong T, Barbour G, Bradley B, Nakayama M, Haskins K. Cutting edge: CD4 T cells reactive to an islet amyloid polypeptide peptide accumulate in the pancreas and contribute to disease pathogenesis in nonobese diabetic mice. J Immunol 2013;191:3990–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci U S A 1997;94:6432–6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallahan DE, Virudachalam S. Ionizing radiation mediates expression of cell adhesion molecules in distinct histological patterns within the lung. Cancer Res 1997;57:2096–2099 [PubMed] [Google Scholar]
- 48.Heckmann M, Douwes K, Peter R, Degitz K. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp Cell Res 1998;238:148–154 [DOI] [PubMed] [Google Scholar]
- 49.Calderon B, Carrero JA, Miller MJ, Unanue ER. Entry of diabetogenic T cells into islets induces changes that lead to amplification of the cellular response. Proc Natl Acad Sci U S A 2011;108:1567–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS ONE 2013;8:e59701. [DOI] [PMC free article] [PubMed] [Google Scholar]