Abstract
Early studies described CD69 as a leukocyte activation marker, and suggested its involvement in the activation of different leukocyte subsets as well as in the pathogenesis of chronic inflammation. However, recent investigations have showed that CD69 knockout mice exhibit an enhanced susceptibility to different inflammatory diseases, mainly those mediated by Th17 lymphocytes. The recent discovery of a ligand for CD69 expressed on Dendritic cells, Galectin-1, has confirmed the immunoregulatory role of CD69 mainly by the inhibition of Th17 differentiation and function in mice and humans. In this regard, the expression of CD69, both in Th17 lymphocytes and by a subset of regulatory T cells, has an important role in the control of the immune response and the inflammatory phenomenon. Therefore, different evidences indicate that CD69 exerts a complex immuno-regulatory role in humans, and that it could be considered as target molecule for the therapy of immune-mediated diseases.
Keywords: CD69; Inflammatory diseases; Treg lymphocytes; Th17 lymphocytes, Immuno-regulation
CD69 IS AN EARLY ACTIVATION ANTIGEN OF IMMUNE CELLS
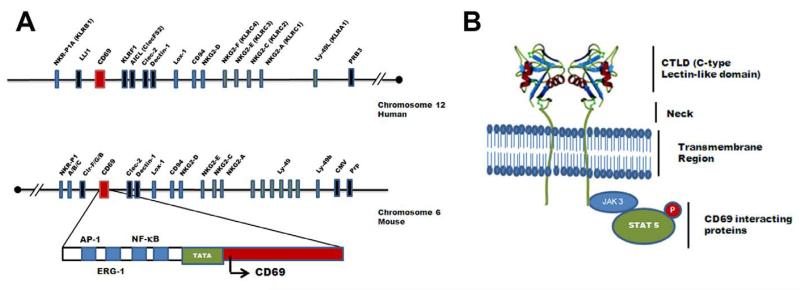
CD69 is a type II C-lectin membrane receptor with a scarce expression in resting lymphocytes that is rapidly induced upon cell activation. CD69 gene maps at human chromosome 12, and behaves as an early activation gene that contains responsive elements for the transcription factors NF-κB, ERG-1 and AP-1 (Fig. 1A). Early in vitro data as well as the prominent expression of CD69 in chronic inflammatory cell infiltrates suggested that this receptor has an important role in the activation of leukocytes, exerting a pro-inflammatory effect. Recent studies in CD69-deficient mice indicate that this molecule exerts a complex and interesting role in the modulation of the immune response and the inflammatory phenomenon [1]. However, these studies have been controversial for a while since two different models of CD69 deficient mice have been described with opposite effects in the development of T-cell independent arthritis and Th2-dependent asthma. Murata et al. 2003 [2] described a CD69 knockout mice that were protected towards arthritis induced by anti-type II collagen antibodies whereas Lamana et al. 2006 [3] revisited the same disease model with an independent CD69-deficient mice and found that the lack of CD69 did not inhibits joint inflammation. Lamana et al. used monoclonal antibodies against CD69 in wild type mice, that downregulates the expression of the molecule in the membrane, to bypass putative different genetic alterations in the process of generation of these two different animal models. These investigations confirmed that the lack of CD69 do not protects the mice towards the T-independent model of neutrophil-mediated arthritis. Regarding the role of CD69 in asthma, Miki-Hosokawa et al. 2009 described that the reduced migration of Th2 cells accounts for the inhibition of lung inflammation in a model of CD69 null mice [4], whereas Martin et al. 2010 using an independent mouse model of CD69 deficiency, found that Th2 and Th17 responses were enhanced in the lungs with higher expression of S1P1 receptors which therefore, induces an increase in the migration of these cells [5]. The use of the neutralizing anti-CD69 antibodies in vivo in wild type animals, to avoid differences by genetic alterations, mimicked the phenotype of CD69 deficient mice in the asthma model and in a model of skin contact hypersensitivity. Therefore, apart form the different animal models, the studies in vivo with monoclonal antibodies against CD69 demonstrated that the blocking of this molecule enhances Th17-dependent inflammatory responses and that this process is independent of the genetic alteration between the two animal models. Besides asthma, different models of T-cell independent lung inflammation have been studied in CD69-deficient mice. Fibrosis and inflammation were attenuated in CD69-deficient mice after intratracheal bleomycin and cigarette smoking, although both models are mediated mostly by macrophages and neutrophils [6, 7].
Fig. 1. Main characteristics of CD69.
(A) Gene encoding for CD69 is localized in the natural killer (NK) gene complex. CD69 belongs to a family of receptors that modulate the immune response, the NK gene complex. NK cluster encodes several proteins as CD94 receptors or NKG2 receptor family of proteins. CD69 gene is localized at human chromosome 12 and mouse chromosome 6, and the sequence contains a potential TATA element with a number of putative binding sites for inducible transcription factors (NF-κB, ERG-1, AP-1) involved in the control of CD69 gene expression. (B) CD69 protein structure and interacting proteins. CD69 belongs to the C-type lectin receptors family (CTLD), and its structure is shared by other members as CD94. Three-dimensional analysis of human CD69 structure has revealed that it is expressed as a homodimer, formed by two identical polypeptide chains with different degrees of glycosylation (and molecular weights of 33 and 27 kDa), linked by a disulphide bridge. The cytoplasmic tail of mouse and human CD69 associates with Jak3/Stat5 signaling proteins, which regulates the transcription of RORγt/RORC2 and therefore the differentiation of Th17 lymphocytes.
The recent discovery of the CD69 ligand Galectin-1, expressed on Dendritic cells (DCs), that has remained a question for decades, clarifies the controversy of the previous data indicating that CD69 could be an activator or an regulator of immune responses. The selective binding of CD69 to Galectin-1 on DCs modulates the differentiation of Th17 helper cells, therefore confirming that this pair is involved in the negative control of pro-inflammatory responses [8].
Until the counter-receptor for CD69, Galectin-1, has been characterized, most functional studies on CD69 have been performed with agonistic monoclonal antibodies (mAbs). Thus, it has been described that the engagement of CD69 in T lymphocytes in the presence of phorbol esters triggers Ca2+ influx, ERK1/2 kinases activation, induction of synthesis of IL-2 and interferon-γ, and cell proliferation [9, 10]. However, recent experiments have shown that the cytoplasmic tail of CD69 is able to interact with the transcription factor STAT5 through Jak3 activation. The activation of this signaling pathway inhibits the differentiation of Th17 lymphocytes by blocking the synthesis of IL-17 and inactivating RORγt transcription factor. The lack of CD69 expression results in enhanced Th17 pro-inflammatory responses in vivo in CD69-deficient mice [11].
CD69 exerts its regulatory functions in various specialized “activated” T cell subsets. The role of CD69 in the control of T helper memory cells [12] has been described and also the differential role of CD69 targeting by antibodies on bystander and antigen specific T cell proliferation [13]. Moreover, a subset of recently activated CD69+ FoxP3+ regulatory T lymphocytes showed site-specific accumulation after transfer to lymphoid compartments, indicating that this molecule could be a potential marker to develop cellular-based therapies to treat autoimmune diseases [14]. Furthermore, it has been showed that CD69 negatively regulates the migration of effector T lymphocytes and bone marrow-derived dendritic cells induced by the chemotactic factor sphingosine-1-phosphate (S1P) [15, 16]. All these data indicate that CD69 exerts a complex regulatory effect on the immune system, affecting the differentiation of cells, the synthesis of cytokines, and modulating the inflammatory response.
CD69 AS AN IMMUNO-REGULATORY RECEPTOR
The important role of CD69 as a brake for the inflammatory responses is supported by evidences in different experimental animal models (experimental diseases are summarized in Table 1). Thus, in the murine model of collagen-induced arthritis, the absence of CD69 exacerbates the inflammatory and destructive phenomenon in the affected joints, which is accompanied by a significant diminution in the synthesis of TGF-β [17]. In addition, these animals show an increased generation of Th1 lymphocytes and an enhanced synthesis of soluble inflammatory mediators, including IL-1β, and the chemokines RANTES, MIP-1α, and MIP-1β. Accordingly, in this experimental model the in vivo blockade of TGF-β with mAbs induces a similar effect than the absence of CD69 [17]. On the other hand, it has also been shown that CD69-deficient mice develop an exacerbated form of ovalbumin-induced allergic airway inflammation, with an enhanced recruitment of eosinophils, and an increased synthesis of Th2 and Th17 cytokines in lung tissue [5]. Likewise, in an experimental model of contact dermatitis, the absence of CD69 or its blockade enhances the tissue damage and the recruitment of Th1 and Th17 lymphocytes [5]. The relevant immuno-regulatory role of CD69 has been also observed in experimental autoimmune myocarditis. In this model of cardiomyopathy, CD69 deficiency enhances the inflammatory phenomenon and aggravates myocardial tissue damage mediated by Th17 lymphocytes, favoring the progression to heart failure [18]. In addition, it has been reported that the transfer of CD4+ T cells from CD69 knockout mice into RAG(−/−) immuno-deficient animals induces an accelerated form of colitis, with an enhanced production of IFN-γ, TNF-α, and IL-17 [19]. However, the atherosclerosis induced by high-fat-diet in apoE−/− mice is not enhanced by the absence of CD69, and when atherosclerosis is induced in these mice by immunization with conalbumin, the CD69+ lymphocytes seem to participate in the pathogenesis of vascular lesions, as effector cells [20, 21].
Table 1. CD69 role in animal models of disease.
| Disease | Animal model | Cell Type | Tissue | References |
|---|---|---|---|---|
| Collagen-induced arthritis | C57BL/6J CD69−/− | T and B cells | Synovial leukocytes | [17] |
| Anti-type-II collagen induced arthritis | C57BL/6J CD69−/− | Neutrophils | Joints | [2, 3] |
| Ovalbumin-induced allergic airway inflammation | Balb/c CD69−/− | Th2/Th17 cells | Lugs and airways | [4, 5] |
| Lung inflammation after Bleomycin | C57BL/6J CD69−/− | Macrophages, Neutrophils and lymphocytes | Lungs | [6] |
| Cigarette smoke Induced inflammation | C57BL/6J CD69−/− | Macrophages and Neutrophils | Lungs | [7] |
| Contact hypersensitivity to oxazolone | C57BL/6J CD69−/− | Th1/Th17 | Skin | [5] |
| Experimental autoimmune myocarditis | Balb/c CD69−/− | Th17 cells | Myocardium. | [18] |
| Systemic lupus erythematosus | NZB×NZW mice | CD4+CD69+ T cells | Peripheral lymphoid tissues, kidney, and lung | [67] |
| Listeria monocytogenes infection | C57BL/6J CD69−/− and Balb/c CD69−/− | Th1 | Liver and spleen | [66] |
| Hepatocarcinoma | C57BL/6J and BALB/c mice | New subset of regulatory CD4+ T cells | Spleen and mesenteric lymph nodes | [23] |
| CD4 T cell transfer colitis | C57BL/6J CD69−/− | Th17 cells | Bowel | [19] |
THE COMPLEX ROLE OF CD69 IN AUTOIMMUNE DISEASES
In humans, the in vivo expression of CD69 has been mainly detected in leukocyte inflammatory infiltrates (Table 2). As stated above, although this pattern of expression of CD69 initially suggested that this molecule exerts a pro-inflammatory pathogenic effect, several recent reports indicate that this receptor is an important element of the immuno-regulatory machinery, aimed to limit the inflammatory phenomenon and tissue damage. However, since CD69 is expressed by different subsets of activated leukocytes, it is feasible that in humans this receptor may have effects other than the down-regulation of immune response. In this regard, the prominent expression of CD69 in the synovial fluid and/or tissue from patients with rheumatoid arthritis (RA) or chronic viral hepatitis suggested its involvement in the pathogenesis of the inflammatory phenomenon seen in these conditions [22, 23]. In this regard, it is of interest that most T cells from the rheumatoid synovial fluid show the unconventional phenotype CD69+ HLA-DR+ CD25−, and that CD69 is involved in the induction of synthesis of TNF-α by macrophages, with the participation of IL-15 [24, 25]. A similar phenomenon seems to occur in the case of CD69+ NK lymphocytes from the synovial fluid, which are also able to induce the release of TNF-α by monocytes, an effect that is abolished when CD69 is blocked [26]. Finally, although osteoarthritis has been largely considered as a non-inflammatory condition, it is of interest that a significant proportion of CD69+ lymphocytes are detected in both the synovial fluid and synovial membrane from patients with this disease [27].
Table 2. CD69 in human diseases.
| Disease | Cell Type | Tissue | References |
|---|---|---|---|
| Rheumatic diseases | |||
| Rheumatoid arthritis | T and NK cells | Synovial fluid | [22, 24, 27] |
| Osteoarthritis | T cells | Synovial membrane and fluid | [27] |
| Systemic sclerosis | Th17 and Treg cells | Peripheral blood | [29, 71] |
| Systemic lupus erythematosus | T, Treg and NK cells | Peripheral blood | [31, 63, 67] |
| Wegener’s granulomatosis | Th17 cells | Peripheral blood | [43] |
| Autoimmune diseases | |||
| Multiple sclerosis | Th17 cells | Cerebrospinal fluid | [35, 36] |
| Neuromyelitis optica | T cells | Peripheral blood | [37] |
| Autoimmune thyroiditis | T and Treg cells | Peripheral blood, thyroid gland | [40, 42] |
| Inflammatory diseases | |||
| Sarcoidosis | T cells | Peripheral blood, lung | [44] |
| Pneumonitis | CD4+ Tcells | Lung | [66] |
| Chronic bronchitis | CD4+ T cells | Peripheral blood, lung | [47] |
| Atherosclerosis | T cells | Blood vessels | [48] |
| Other diseases | |||
| Asthma | T cells, eosinophils | Peripheral blood, lung | [55, 56, 58] |
| Atopic dermatitis | Eosinophils | Peripheral blood | [57] |
| Hepatocellular carcinoma | Treg cell subset | Peripheral blood, liver | [64] |
| Kidney transplant | Treg cells | Peripheral blood, kidney | [52, 54] |
Patients with systemic sclerosis (SSc) show an increased differentiation of Th17 lymphocytes, and an enhanced synthesis of TGF-β. Interestingly, during the inflammatory phase of skin damage of SSc, a significant infiltration by CD69+ leukocytes is observed, mainly in those patients with the limited form of this condition [28]. In addition, the peripheral blood from these patients show enhanced levels of CD69+ T cells, which synthesize IL-17 [29]. In this regard, it is worth remembering that CD69 engagement induces the release of TGF-β, a cytokine with a relevant role in the fibrotic phenomenon that occurs in patients with SSc [30]. However, it is also true that CD69 seems to be an important negative modulator for Th17 cells [11].
In other rheumatic diseases, as systemic lupus erythematous (SLE), it has been also described that NK cells from peripheral blood show an enhanced expression of CD69 as well as an increased synthesis of interferon-γ, which correlates with increased levels of interferon-α in serum, a key cytokine in the pathogenesis of this condition [31]. Furthermore, these patients show enhanced levels of CD8+CD69+ cytotoxic lymphocytes, which are able to induce the apoptosis of different cells [32], a phenomenon that also has a key role in the pathogenesis of SLE. It is of interest that a significant fraction of patients with SLE and RA synthesize auto-antibodies directed against CD69, which could modify the function of this receptor, contributing thus to de dysregulation of the immune system observed in these conditions [33]. A similar finding has been described in patients with chronic viral hepatitis [34]..
CD69 seems to be also involved in the pathogenesis of other autoimmune diseases. Thus, patients with multiple sclerosis (MS), mainly those with active brain lesions, show an enhanced proportion of CD69+ T cells in cerebrospinal fluid and peripheral blood [35]. In addition, these patients show an increased frequency of CD4+CD69+ lymphocytes that are activated by myelin, which release interferon-γ and/or TNF-α [36]. Accordingly, patients with neuromyelitis optica (a demyelinating condition related to MS, which is associated with autoimmunity against the cell membrane protein aquaporin 4) also show enhanced levels of peripheral blood CD69+ T cells, and an enhanced proportion of lymphocytes that are activated by aquaporin 4 derived peptides [37]. Interestingly, in a murine model of MS, the oral administration of the myelin basic protein (MBP) induces the preferential localization of these CD69+ cells to the Peyer’s patches, where they are deleted by apoptosis, a phenomenon that confers protection against the disease [38]. As in other autoimmune diseases, Th17 cells have an important pathogenic role in MS [39]. Thus, an enhanced proportion of Th17 cells has been detected in the cerebrospinal fluid of these patients, and CD69+ Th17 lymphocytes show a high pathogenic potential [39]. In addition, patients with autoimmune thyroid disease (AITD) also show a high proportion of CD69+ leukocytes in peripheral blood and thyroid tissue as well as an enhanced differentiation of Th17 cells, which infiltrate the inflamed gland [40, 41]. However, it is of interest that at least a proportion of these CD69+ cells seems to correspond to lymphocytes with a regulatory function [42]. On the other hand, it has been described that when lymphocytes from patients with Wegener’s granulomatosis are stimulated with the proteinase 3 auto-antigen, an enhanced proportion of CD69+ lymphocytes with a Th17 phenotype is generated [43]. Furthermore, the bronchoalveolar lavage fluids (BALF) from patients with sarcoidosis and hypersensitivity pneumonitis contain a high proportion of CD69+ T cells, which shows a significant positive correlation with tissue damage [44, 45]. Similar findings have been reported in patients with chronic pulmonary obstructive disease [46, 47]. Since in all these conditions the tissue damage is, at least in part, mediated by Th17 cells, it is tempting to speculate that the increased proportion of CD69+ is reflecting a failed attempt to suppress the inflammatory phenomenon by regulatory lymphocytes. However, it is also feasible that at least a fraction of the CD69+ cells observed in these inflammatory diseases correspond to pathogenic activated effector cells.
In the case of the inflammatory phenomenon that is involved in the pathogenesis of atherosclerosis, it has been described that in patients with acute coronary syndromes the percentage of CD69+ lymphocytes in atheroma plaques correlates with disease severity and patient outcome [48]. In the case of chronic viral hepatitis, most intrahepatic CD8+ lymphocytes express CD69, but not CD25 [23], resembling those described in the synovial fluid of patients with RA [24, 25], and likely related to the regulatory CD69+CD25− lymphocytes described in mice [49]. On the other hand, patients with dementia associated to AIDS show increased levels of peripheral blood CD69+ monocytes, which exert in vitro a cytotoxic effect on neural cells [50]. Finally, in the case of organ transplantation, CD69 expression has been initially associated to graft rejection, and CD69+ leukocytes seem to exert a pathogenic role in this phenomenon [51, 52]. However, it has also been reported that CD69+ T regulatory (Treg) cells are potent inhibitors of skin allograft damage [53]. Accordingly, long-term surviving patients with kidney transplants show increased levels of peripheral blood CD4+CD25+CD69+ Treg lymphocytes [54].
CD69 AND ALLERGIC DISEASES
In patients with allergic asthma there is an increased number of CD69+ eosinophils in BALF, mainly after antigenic challenge [55, 56]. Furthermore, in patients with atopic dermatitis, the presence of CD69+ eosinophils in peripheral blood is associated with very high levels of total IgE [57]. Thus, the presence of CD69+ eosinophils in the peripheral blood or tissues from patients with allergic conditions is strongly associated with an active inflammatory phenomenon, which requires appropriate therapy [57]. On the other hand, the sputum of patients with asthma contains high levels of CD69+ lymphocytes, and as in the case of eosinophils, the number of those cells is enhanced by antigenic challenge [58]. In addition, it has been reported that the exposure of the peripheral blood mononuclear cells from allergic individuals to allergens results in the appearance of a large proportion of CD69+ NK cells [59]. These data indicate that in patients with allergic conditions, CD69 behaves as an activation marker of the leukocytes involved in the tissue damage, which seem to be refractory to the suppressive effect of Treg cells [60].
CD69 AND REGULATORY T CELLS
As stated above, it has been described in mice a CD4+CD25−CD69+Foxp3− T cell subset that exerts a suppressive function through TGF-β [49] (regulatory properties of the different subsets of Tregs are summarized in Box 1). In this regard, we have previously detected the presence of CD69+TGF-β+ and CD69+IL-10+ lymphocytes in the peripheral blood and thyroid gland from patients with AITD [42]. The existence of CD4+CD69+ regulatory cells has been confirmed in humans by Gandhi R, et al. [61], who characterized a CD4+CD69+TGF-β+ small cell subset (0.1 to 1.5% of T lymphocytes in peripheral blood). These cells show a variable expression of CD25, their differentiation is independent of Foxp3, and are able to exert a strong immunosuppressive function in vitro, which is mediated by TGF-β and IL-10 [61]. Interestingly, patients with SLE show a diminished number of peripheral blood CD4+CD69+ lymphocytes, which is associated with resistance of effector T lymphocytes to suppression by conventional Treg cells [62, 63]. In addition, it has been reported that the levels of CD4+CD69+CD25− cells are increased in the peripheral blood and liver from patients with hepatocellular carcinoma, and that these levels correlate with tumor size or the presence of metastases [64]. In contrast, children with intestinal parasite infection [65] and patients with kidney transplants [54] show enhance levels of peripheral blood CD4+CD69+ Treg cells. Finally, it has been reported that CD69-deficient mice show increased tissue inflammation during the early stage of Listeria monocytogenes infection, with an increased synthesis of Th1 cytokines [66].
Box 1. Regulatory T cells.
Most than four decades ago it was described that an immune cell subset is able to suppress the immune response and autoimmune phenomena [73]. Since the so called “T suppressor cells” were never properly characterized, their existence was subsequently denied [74]. However, in 1995 Sakaguchi S. et al. reported that a CD4+ T cell subset with a high constitutive expression of the alpha chain of IL-2 receptor (CD25high) was responsible for the immune tolerance to self antigens [75]. It was subsequently described that the differentiation of these natural T regulatory (nTreg) cells in thymus is dependent on the expression of the transcription factor Foxp3, and that they have a relevant role in the homeostasis of the immune system and the pathogenesis of autoimmune disease [76].
Additional lymphocyte subsets with immuno-regulatory activity have been described [76, 77]. Induced T regulatory (iTreg) cells (CD4+CD25+Foxp3+) are generated in the periphery, under the effect of different cytokines, mainly TGF-β. CD8+ lymphocytes expressing Foxp3 have been also described.
There are also Foxp3− regulatory lymphocytes, including the Tr1, Th3, and Tr35 cell subsets. Tr1 lymphocytes (CD4+CD25−Foxp3−) are generated in the periphery, and exert their suppressive effect mainly through IL-10 synthesis, acting on monocytes and macrophages. Th3 lymphocytes (CD4+CD25−Foxp3−TGFβ+) are mainly involved in oral induced tolerance, having an important effect on Foxp3+ Treg cells. Tr35 lymphocytes (CD4+Foxp3−) are induced by IL-10 and IL-35 and are characterized by the synthesis of the latter cytokine, which exerts a potent immunosuppressive effect [78].
As stated in the text, it has been characterized a lymphocyte subset (CD4+Foxp3−TGFβ+) that show a constitutive expression of CD69, and variable levels of CD25 [49, 61]. These cells exert a remarkable regulatory effect, apparently mediated by TGF-β and IL-10. On the other hand, it seems evident that CD69 may be expressed, under certain circumstances, by other regulatory cells, including nTreg and iTreg lymphocytes. Finally, the precise relationship among the different CD4+ T regulatory cell subsets remains as an important point to be fully elucidated.
There are additional data on the involvement of CD69 in T cells with immunosuppressive function. Thus, an early report showed that in the NZBxNZW mice, a T-cell dependent model of SLE, there is an increased number of CD4+CD69+ T cells, which exert in vitro a suppressive effect, inhibiting the synthesis of IL-2 by CD4+CD69− lymphocytes [67]. In contrast, and as stated above, patients with SLE show a decreased number of CD69+ Treg cells with a defective suppressive function, which is paradoxically associated with an enhanced synthesis of TGF-β [63]. These patients also show increased levels of effector CD69+ lymphocytes in their peripheral blood. In this regard, it is worth noting that patients with SLE are characterized by a defective production of IL-2 and that this cytokine has a critical role in the differentiation of conventional Foxp3+ Treg lymphocytes.
A role of CD69 in the generation of Foxp3+ Treg cells has been recently proposed. In this regard, the differentiation of Treg cells in human thymus is linked to CD69 expression, and dendritic cells are able to induce the differentiation of CD4+CD8+CD69high thymocytes into CD4+CD25highFoxp3+ Treg cells [68]. In addition, when Treg cells are activated in vitro with allogenic dendritic cells or mesenchymal stem cells, they become CD69+ and exert a potent suppressive function [69]. Accordingly, in patients with Chagas’ disease, the in vitro exposure of lymphocytes to T. cruzi antigens also results in the expression of CD69 by CD4+CD25high Treg cells [70]. In this regard, in SSc patients the diminished expression of CD69 by CD4+CD25high Treg lymphocytes is associated with a poor suppressive function and a diminished synthesis of TGF-β [71]. Finally, it has been demonstrated that oral tolerance is impaired in CD69-deficient mice, and that their CD4+ T lymphocytes show a reduced potential to differentiate into Foxp3+ Treg cells [19]. All these data strongly suggest that CD69 is involved in the function and generation of different types of Treg cells, mainly in the CD4+CD25highFoxp3+ natural regulatory lymphocytes, and the recently described CD4+Foxp3−TGF-β+ lymphocyte subset.
CONCLUDING REMARKS
Early studies on CD69 indicated that both in mice and humans this molecule behaves as a leukocyte activation marker, and strongly suggested that it exerts a relevant role on the activation of different leukocyte subsets as well as in the pathogenesis of the tissue damage seen in different inflammatory conditions [1, 9, 10]. However, the generation of CD69 knockout mice demonstrated that, although these animals exhibit few immune abnormalities in steady state conditions, they clearly show an enhanced susceptibility to several inflammatory diseases, mainly those mediated by Th17 lymphocytes [5, 17-19]. Accordingly, a T lymphocyte subset expressing CD69 and exerting in vitro a potent immunosuppressive function through TGF-β and IL-10 was subsequently described in mice [61][53]. Obviously, these data indicated that the main biological function of CD69 in vivo is the negative regulation of the immune response and the inflammatory phenomenon. However, the current challenge is to confirm that all these phenomena also occur in humans, i.e., to corroborate that the main function of CD69 in humans is the negative regulation of the immune reactivity, and that its deficiency results in an enhanced risk for autoimmune and chronic inflammatory diseases. In this regard, it is worth noting that most reports on CD69 in humans suggest that its expression in vivo is reflecting an ongoing immune response, associated to tissue damage. In addition, no individuals with deficiency of CD69 have been described yet, and in the single report on the possible association between allelic variants (SNP’s) of CD69 gene and chronic inflammatory diseases, no significant skewing in the distribution of the five SNP’s studied in patients with RA was observed [72]. Therefore, we consider that it is very important, on the basis of the interesting data generated in the CD69 knockout mice, to investigate in humans, through future studies, the following points: 1) Identification and study of individuals with congenital deficiency of CD69; 2) Characterization of the natural ligand(s) for CD69; 3) To establish the precise relationship between CD4+CD69+CD25−Foxp3− regulatory T cells and other subsets of lymphocytes with regulatory function; 4) To perform detailed and prospective studies on the number and function of CD4+CD69+CD25−Foxp3− regulatory T cells in different autoimmune and chronic inflammatory conditions in humans, and; 5) To define the functional role of CD69 in activated Foxp3+ Treg cells. The information generated through these studies would allow us to establish the biological role of CD69 in humans in health and disease, making feasible the targeting of this molecule for the therapy of autoimmune and chronic inflammatory diseases.
Outstanding Question Box.
What is the functional role of CD69 in activated effector T lymphocytes?
As occurs in mice, the main physiological role of CD69 in humans is the suppression of the immune response and the down-regulation of the inflammatory phenomenon?
Which are the endogenous ligands of CD69?
Is CD69 a plausible target for the therapy of autoimmune inflammatory diseases?
It would be a useful maneuver the blockade of CD69 to enhance the immune reactivity during immunization procedures?
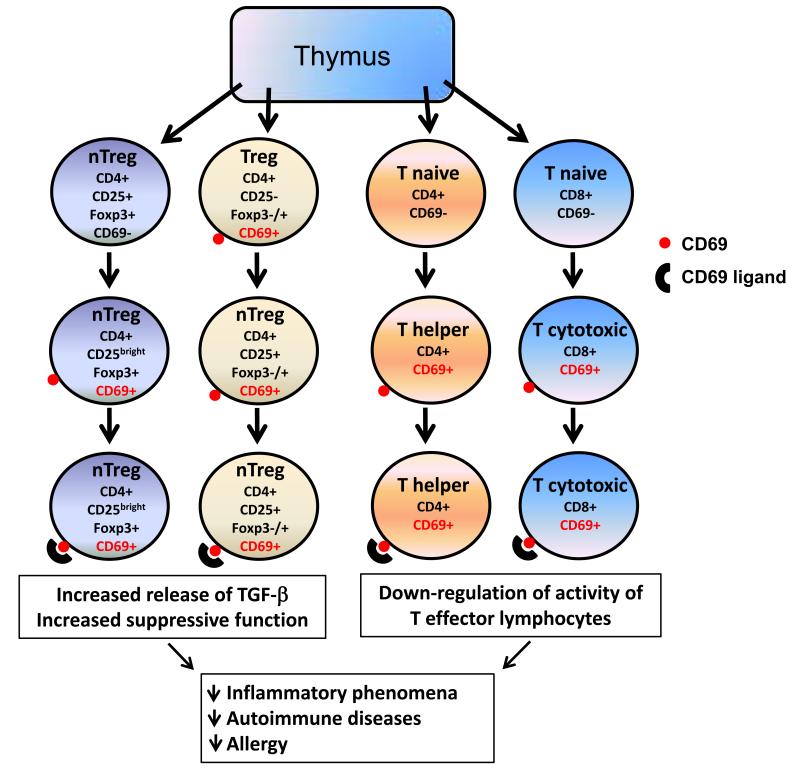
Fig. 2. Immuno-regulatory role of CD69.
Natural T regulatory (nTreg, left) lymphocytes, which do not constitutively express CD69, are able to synthesize this molecule, when are activated in the periphery. Upon its engagement with their putative endogenous ligands (expressed on the surface of immune cells, including antigen presenting cells), the intracellular signals generated through CD69 would enhance the immunosuppressive activity of nTreg cells and would increase the synthesis of TGF-β. Another subset of Treg lymphocytes (CD4+ CD25−, with a variable expression of Foxp3, middle left) is characterized by the constitutive expression of CD69. Upon activation, these cells become CD25+ and, as in the case of nTreg lymphocytes they would increase their immuno-regulatory activity after the engagement of their CD69 molecules. In the case of naïve non-regulatory CD4+ and CD8+ T cells (middle right, and right, respectively), CD69 behaves as an activation molecule, appearing on the cell surface when these cells are activated by antigen presenting cells or other stimuli. When these cells are differentiated into effector (helper and cytotoxic) lymphocytes, they remain as CD69+, and the engagement of this molecule would diminish the pro-inflammatory activity of these cells. However, and as stated in the text, it is also feasible that the expression of CD69 by CD4+ or CD8+ effector lymphocytes is associated, under certain circumstances, with an enhanced pathogenic capability of these cells Thus, CD69 seems to exert a complex but relevant immuno-regulatory role at different cell levels, affecting the severity of immune-mediated diseases, mainly those characterized by chronic inflammation.
ACKNOWLEDGMENTS
This work was funded by grants SAF2011-27330 and SAF2011-25834 from the Spanish Ministry of Economy and Competitiveness to P.M. and F.S-M.; grant INDISNET 01592006 from Comunidad de Madrid to P.M and F.S-M.; and ERC-2011-AdG294340-GENTRIS to FSM; The CNIC is supported by the Ministry of Science and Innovation and the Pro CNIC Foundation.
Meter referencia RIC!!
Glossary
- Th17 lymphocytes
are a CD4+ T cell lineage highly relevant for the defense against fungi and bacteria and the development of autoimmune diseases. This subset of helper T cells differs from Th1 and Th2 lymphocytes mainly in the release of distinctive cytokines such as IL-17A, IL-17F, IL-22 or IL-21. Th17 lymphocytes are specialized in initiating inflammatory responses in epithelial and mucosal surfaces by the activation of neutrophils.
- RORγt
retinoic acid receptor-related orphan nuclear receptor gamma, is a key transcription factor for the development of Th17 cells both in human and mouse.
- STAT5
signal transducer and activator of transcription protein STAT5 is and essential mediator of IL-2 signaling in T cells. FoxP3 is activated by STAT5 enhancing the function of regulatory T cells, whereas Th17 cell differentiation is inhibited by this transcription factor through the inhibition of STAT3 phosphorylation and translocation to the nucleus.
- RAG(−/−) mice
are animals with a mutation in the germline of the V(D)J recombination activation gene RAG-1. These mice have immunodeficient responses, defective B and T lymphocyte maturation and small lymphoid organs.
- NZBxN2W mice
animal model for the study of systemic lupus erythematosus (SLE). These animals develop the disease spontaneously by generating auto-antibodies with specificity to nucleic acid antigens, and die by lethal immune-complex glomerulonephritis.
- S1P1
a G-protein coupled receptor of the Egd gene family of Sphingosine-1-phosphate (S1P) specific receptors. This receptor is critical for proper lymphocyte development and egress to lymphoid organs. During the migratory process of mature T cells from the thymus to the periphery, the gene encoding S1P1 (Egd1) is positively regulated from double-positive to single-positive thymocytes transition, which gives rise to an increase in the expression of the receptor favouring the egress to periphery.
Footnotes
DISCLOSURE The authors declare that they have no competing interest as defined by Trends in Molecular Medicine, or other interest that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Sancho D, et al. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Murata K, et al. CD69-null mice protected from arthritis induced with anti-type II collagen antibodies. Int Immunol. 2003;15:987–992. doi: 10.1093/intimm/dxg102. [DOI] [PubMed] [Google Scholar]
- 3.Lamana A, et al. The role of CD69 in acute neutrophil-mediated inflammation. Eur J Immunol. 2006;36:2632–2638. doi: 10.1002/eji.200636355. [DOI] [PubMed] [Google Scholar]
- 4.Miki-Hosokawa T, et al. CD69 controls the pathogenesis of allergic airway inflammation. J Immunol. 2009;183:8203–8215. doi: 10.4049/jimmunol.0900646. [DOI] [PubMed] [Google Scholar]
- 5.Martin P, et al. The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity. J Allergy Clin Immunol. 2010;126:355–365. 365, e351–353. doi: 10.1016/j.jaci.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi K, et al. Attenuation of lung inflammation and fibrosis in CD69-deficient mice after intratracheal bleomycin. Respir Res. 2011;12:131. doi: 10.1186/1465-9921-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuyusaki J, et al. Cigarette smoke-induced pulmonary inflammation is attenuated in CD69-deficient mice. Journal of receptor and signal transduction research. 2011;31:434–439. doi: 10.3109/10799893.2011.631929. [DOI] [PubMed] [Google Scholar]
- 8.de la Fuente HC-A,A, Martinez del Hoyo G, Bonay P, Perez-Hernandez D, Vazquez J, Navarro P, Gutierrez-Gallego R, Ramirez-Huesca M, Martin P, Sanchez-Madrid F. The leukocyte activation receptor CD69 controls T cell differentiation through its interaction with Galectin-1 on dendritic cells. Blood. 2013 doi: 10.1128/MCB.00348-14. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cebrian M, et al. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621–1637. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testi R, et al. T cell activation via Leu-23 (CD69) J Immunol. 1989;143:1123–1128. [PubMed] [Google Scholar]
- 11.Martin P, et al. CD69 association with Jak3/Stat5 proteins regulates Th17 cell differentiation. Mol Cell Biol. 2010;30:4877–4889. doi: 10.1128/MCB.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinoda K, et al. Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc Natl Acad Sci U S A. 2012;109:7409–7414. doi: 10.1073/pnas.1118539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alari-Pahissa E, et al. CD69 does not affect the extent of T cell priming. PLoS One. 2012;7:e48593. doi: 10.1371/journal.pone.0048593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieberman SM, et al. Site-specific accumulation of recently activated CD4+ Foxp3+ regulatory T cells following adoptive transfer. Eur J Immunol. 2012;42:1429–1435. doi: 10.1002/eji.201142286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 16.Lamana A, et al. CD69 modulates sphingosine-1-phosphate-induced migration of skin dendritic cells. J Invest Dermatol. 2011;131:1503–1512. doi: 10.1038/jid.2011.54. [DOI] [PubMed] [Google Scholar]
- 17.Sancho D, et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J Clin Invest. 2003;112:872–882. doi: 10.1172/JCI19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Adalia A, et al. CD69 limits the severity of cardiomyopathy after autoimmune myocarditis. Circulation. 2010;122:1396–1404. doi: 10.1161/CIRCULATIONAHA.110.952820. [DOI] [PubMed] [Google Scholar]
- 19.Radulovic K, et al. CD69 Regulates Type I IFN-Induced Tolerogenic Signals to Mucosal CD4 T Cells That Attenuate Their Colitogenic Potential. J Immunol. 2012 doi: 10.4049/jimmunol.1100765. [DOI] [PubMed] [Google Scholar]
- 20.Gomez M, et al. Atherosclerosis development in apolipoprotein E-null mice deficient for CD69. Cardiovasc Res. 2009;81:197–205. doi: 10.1093/cvr/cvn227. [DOI] [PubMed] [Google Scholar]
- 21.Khallou-Laschet J, et al. The proatherogenic role of T cells requires cell division and is dependent on the stage of the disease. Arterioscler Thromb Vasc Biol. 2006;26:353–358. doi: 10.1161/01.ATV.0000198401.05221.13. [DOI] [PubMed] [Google Scholar]
- 22.Laffon A, et al. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991;88:546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Monzon C, et al. Expression of a novel activation antigen on intrahepatic CD8+ T lymphocytes in viral chronic active hepatitis. Gastroenterology. 1990;98:1029–1035. doi: 10.1016/0016-5085(90)90030-5. [DOI] [PubMed] [Google Scholar]
- 24.Afeltra A, et al. Expression of CD69 antigen on synovial fluid T cells in patients with rheumatoid arthritis and other chronic synovitis. Ann Rheum Dis. 1993;52:457–460. doi: 10.1136/ard.52.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afeltra A, et al. Coexpression of CD69 and HLADR activation markers on synovial fluid T lymphocytes of patients affected by rheumatoid arthritis: a three-colour cytometric analysis. Int J Exp Pathol. 1997;78:331–336. doi: 10.1046/j.1365-2613.1997.290360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Alvaro I, et al. Interleukin-15 and interferon-gamma participate in the cross-talk between natural killer and monocytic cells required for tumour necrosis factor production. Arthritis Res Ther. 2006;8:R88. doi: 10.1186/ar1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rollin R, et al. Early lymphocyte activation in the synovial microenvironment in patients with osteoarthritis: comparison with rheumatoid arthritis patients and healthy controls. Rheumatol Int. 2008;28:757–764. doi: 10.1007/s00296-008-0518-7. [DOI] [PubMed] [Google Scholar]
- 28.Kalogerou A, et al. Early T cell activation in the skin from patients with systemic sclerosis. Ann Rheum Dis. 2005;64:1233–1235. doi: 10.1136/ard.2004.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radstake TR, et al. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFbeta and IFNgamma distinguishes SSc phenotypes. PLoS One. 2009;4:e5903. doi: 10.1371/journal.pone.0005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga J, Whitfield ML. Transforming growth factor-beta in systemic sclerosis (scleroderma) Front Biosci (Schol Ed) 2009;1:226–235. doi: 10.2741/s22. [DOI] [PubMed] [Google Scholar]
- 31.Hervier B, et al. Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-gamma production in patients with active disease. Arthritis Rheum. 2011;63:1698–1706. doi: 10.1002/art.30313. [DOI] [PubMed] [Google Scholar]
- 32.Rus V, et al. Increased expression and release of functional tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by T cells from lupus patients with active disease. Clin Immunol. 2005;117:48–56. doi: 10.1016/j.clim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, et al. Anti-CD69 autoantibodies cross-react with low density lipoprotein receptor-related protein 2 in systemic autoimmune diseases. J Immunol. 2001;166:1360–1369. doi: 10.4049/jimmunol.166.2.1360. [DOI] [PubMed] [Google Scholar]
- 34.Ooka S, et al. Autoantibodies to low-density-lipoprotein-receptor-related protein 2 (LRP2) in systemic autoimmune diseases. Arthritis Res Ther. 2003;5:R174–180. doi: 10.1186/ar754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrella O, et al. Markers of activated T lymphocytes and T cell receptor gamma/delta+ in patients with multiple sclerosis. Eur Neurol. 1993;33:152–155. doi: 10.1159/000116923. [DOI] [PubMed] [Google Scholar]
- 36.Lunemann JD, et al. Cross-sectional and longitudinal analysis of myelin-reactive T cells in patients with multiple sclerosis. J Neurol. 2004;251:1111–1120. doi: 10.1007/s00415-004-0493-1. [DOI] [PubMed] [Google Scholar]
- 37.Matsuya N, et al. Increased T-cell immunity against aquaporin-4 and proteolipid protein in neuromyelitis optica. Int Immunol. 2011;23:565–573. doi: 10.1093/intimm/dxr056. [DOI] [PubMed] [Google Scholar]
- 38.Song F, et al. The Peyer’s patch is a critical immunoregulatory site for mucosal tolerance in experimental autoimmune encephalomylelitis (EAE) J Autoimmun. 2008;30:230–237. doi: 10.1016/j.jaut.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brucklacher-Waldert V, et al. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132:3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 40.Gessl A, Waldhausl W. Elevated CD69 expression on naive peripheral blood T-cells in hyperthyroid Graves’ disease and autoimmune thyroiditis: discordant effect of methimazole on HLA-DR and CD69. Clin Immunol Immunopathol. 1998;87:168–175. doi: 10.1006/clin.1998.4524. [DOI] [PubMed] [Google Scholar]
- 41.Marazuela M, et al. Adhesion molecules from the LFA-1/ICAM-1,3 and VLA-4/VCAM-1 pathways on T lymphocytes and vascular endothelium in Graves’ and Hashimoto’s thyroid glands. Eur J Immunol. 1994;24:2483–2490. doi: 10.1002/eji.1830241034. [DOI] [PubMed] [Google Scholar]
- 42.Marazuela M, et al. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91:3639–3646. doi: 10.1210/jc.2005-2337. [DOI] [PubMed] [Google Scholar]
- 43.Abdulahad WH, et al. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2008;58:2196–2205. doi: 10.1002/art.23557. [DOI] [PubMed] [Google Scholar]
- 44.Wahlstrom J, et al. Phenotypic analysis of lymphocytes and monocytes/macrophages in peripheral blood and bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Thorax. 1999;54:339–346. doi: 10.1136/thx.54.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heron M, et al. T-cell activation profiles in different granulomatous interstitial lung diseases--a role for CD8+CD28(null) cells? Clin Exp Immunol. 2010;160:256–265. doi: 10.1111/j.1365-2249.2009.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glader P, et al. Systemic CD4+ T-cell activation is correlated with FEV1 in smokers. Respiratory medicine. 2006;100:1088–1093. doi: 10.1016/j.rmed.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Ekberg-Jansson A, et al. The expression of lymphocyte surface antigens in bronchial biopsies, bronchoalveolar lavage cells and blood cells in healthy smoking and never-smoking men, 60 years old. Respir Med. 2000;94:264–272. doi: 10.1053/rmed.1999.0735. [DOI] [PubMed] [Google Scholar]
- 48.Hosono M, et al. Increased expression of T cell activation markers (CD25, CD26, CD40L and CD69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis. 2003;168:73–80. doi: 10.1016/s0021-9150(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 49.Han Y, et al. CD69+ CD4+ CD25− T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- 50.Kusdra L, et al. Changes in monocyte/macrophage neurotoxicity in the era of HAART: implications for HIV-associated dementia. AIDS. 2002;16:31–38. doi: 10.1097/00002030-200201040-00005. [DOI] [PubMed] [Google Scholar]
- 51.Creemers P, et al. Evaluation of peripheral blood CD4 and CD8 lymphocyte subsets, CD69 expression and histologic rejection grade as diagnostic markers for the presence of cardiac allograft rejection. Transpl Immunol. 2002;10:285–292. doi: 10.1016/s0966-3274(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 52.Posselt AM, et al. CD69 expression on peripheral CD8 T cells correlates with acute rejection in renal transplant recipients. Transplantation. 2003;76:190–195. doi: 10.1097/01.TP.0000073614.29680.A8. [DOI] [PubMed] [Google Scholar]
- 53.Sagoo P, et al. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez CM, et al. Kidney transplant patients with long-term graft survival have altered expression of molecules associated with T-cell activation. Transplantation. 2004;78:1541–1547. doi: 10.1097/01.tp.0000140968.17770.c1. [DOI] [PubMed] [Google Scholar]
- 55.Hartnell A, et al. CD69 is expressed by human eosinophils activated in vivo in asthma and in vitro by cytokines. Immunology. 1993;80:281–286. [PMC free article] [PubMed] [Google Scholar]
- 56.Julius P, et al. CD69 surface expression on human lung eosinophils after segmental allergen provocation. Eur Respir J. 1999;13:1253–1259. doi: 10.1183/09031936.99.13612609. [DOI] [PubMed] [Google Scholar]
- 57.Toma T, et al. Expansion of activated eosinophils in infants with severe atopic dermatitis. Pediatr Int. 2005;47:32–38. doi: 10.1111/j.1442-200x.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- 58.Lourenco O, et al. T cells in sputum of asthmatic patients are activated independently of disease severity or control. Allergol Immunopathol (Madr) 2009;37:285–292. doi: 10.1016/j.aller.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Werfel T, et al. Rapid expression of the CD69 antigen on T cells and natural killer cells upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy. 1997;52:465–469. doi: 10.1111/j.1398-9995.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 60.Thunberg S, et al. Allergen provocation increases TH2-cytokines and FOXP3 expression in the asthmatic lung. Allergy. 2010;65:311–318. doi: 10.1111/j.1398-9995.2009.02218.x. [DOI] [PubMed] [Google Scholar]
- 61.Gandhi R, et al. Cutting edge: human latency-associated peptide+ T cells: a novel regulatory T cell subset. J Immunol. 2010;184:4620–4624. doi: 10.4049/jimmunol.0903329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonelli M, et al. Quantitative and qualitative deficiencies of regulatory T cells in patients with systemic lupus erythematosus (SLE) Int Immunol. 2008;20:861–868. doi: 10.1093/intimm/dxn044. [DOI] [PubMed] [Google Scholar]
- 63.Alvarado-Sanchez B, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–118. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Zhu J, et al. Increased CD4(+) CD69(+) CD25(-) T cells in patients with hepatocellular carcinoma are associated with tumor progression. J Gastroenterol Hepatol. 2011;26:1519–1526. doi: 10.1111/j.1440-1746.2011.06765.x. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Hernandez MH, et al. Regulatory T Cells in children with intestinal parasite infection. Parasite Immunol. 2009;31:597–603. doi: 10.1111/j.1365-3024.2009.01149.x. [DOI] [PubMed] [Google Scholar]
- 66.Vega-Ramos J, et al. CD69 limits early inflammatory diseases associated with immune response to Listeria monocytogenes infection. Immunol Cell Biol. 2010;88:707–715. doi: 10.1038/icb.2010.62. [DOI] [PubMed] [Google Scholar]
- 67.Ishikawa S, et al. A subset of CD4+ T cells expressing early activation antigen CD69 in murine lupus: possible abnormal regulatory role for cytokine imbalance. J Immunol. 1998;161:1267–1273. [PubMed] [Google Scholar]
- 68.Martin-Gayo E, et al. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366–5375. doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 69.Saldanha-Araujo F, et al. Mesenchymal stem cells promote the sustained expression of CD69 on activated T lymphocytes: roles of canonical and non-canonical NF-kappaB signalling. Journal of cellular and molecular medicine. 2012;16:1232–1244. doi: 10.1111/j.1582-4934.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Araujo FF, et al. Regulatory T cells phenotype in different clinical forms of Chagas’ disease. PLoS Negl Trop Dis. 2011;5:e992. doi: 10.1371/journal.pntd.0000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radstake TR, et al. Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expression. PLoS One. 2009;4:e5981. doi: 10.1371/journal.pone.0005981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rueda B, et al. Investigation of CD69 as a new candidate gene for rheumatoid arthritis. Tissue Antigens. 2008;72:206–210. doi: 10.1111/j.1399-0039.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 73.Gershon RK, et al. Suppressor T cells. J Immunol. 1972;108:586–590. [PubMed] [Google Scholar]
- 74.Green DR, Webb DR. Saying the ‘s’ word in public. Immunology today. 1993;14:523–525. doi: 10.1016/0167-5699(93)90180-S. [DOI] [PubMed] [Google Scholar]
- 75.Sakaguchi S, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 76.Miyara M, Sakaguchi S. Human FoxP3(+)CD4(+) regulatory T cells: their knowns and unknowns. Immunol Cell Biol. 2011;89:346–351. doi: 10.1038/icb.2010.137. [DOI] [PubMed] [Google Scholar]
- 77.Weiner HL, et al. Oral tolerance. Immunological reviews. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collison LW, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]