Abstract
Epithelial organ remodeling is a major contributing factor to worldwide death and disease, costing healthcare systems billions of dollars every year. Despite this, most fundamental epithelial organ research fails to produce new therapies and mortality rates for epithelial organ diseases remain unacceptably high. In large part, this failure in translating basic epithelial research into clinical therapy is due to a lack of relevance in existing preclinical models. To correct this, new models are required that improve preclinical target identification, pharmacological lead validation, and compound optimization. In this review, we discuss the relevance of human stem cell-derived, three-dimensional organoid models for addressing each of these challenges. We highlight the advantages of stem cell-derived organoid models over existing culture systems, discuss recent advances in epithelial tissue-specific organoids, and present a paradigm for using organoid models in human translational medicine.
Introduction
Irreversible epithelial organ remodelling is a major contributing factor to worldwide death and disease, costing healthcare systems billions of dollars every year. Diseases of epithelial remodelling include lung and gastrointestinal cancers as well as chronic diseases such as chronic obstructive pulmonary disease (COPD), liver cirrhosis and inflammatory bowel disease. Sadly, most epithelial organ research fails to produce new therapies for these diseases and mortality rates remain unacceptably high 1. This is because significant barriers exist at all levels for translating fundamental biomedical research into clinically relevant therapies 2.
Translating biomedical research into therapy typically involves preclinical target and assay identification, high-throughput in vitro compound screening, animal-based safety and pharmacodynamic testing, and eventual human clinical trials. Unfortunately, reliance on this pathway fails to capture many aspects necessary for taking discovery science into clinical practice 3. This is exemplified by the fact that 90% of compounds identified during high-throughput screening fail to progress beyond Phase 1 clinical trials. Of those compounds that do progress to clinical trials a further 90% will fail to become new drugs 4. The cost implications of these failures are potentially enormous, with the development of each new drug recently estimated to cost as much as $1.8 billion 4. The health implications of compounds failing in human clinical trials are also significant. An illustration of this point was the severe reaction caused by the agonist monoclonal antibody TGN1412 in a Phase I clinical trial5. Here, patients experienced a systemic cytokine storm and multi-organ failure despite the compound having exhibited a good safety profile in earlier animal models 6.
These exceptional costs and the relative paucity of new drug approvals highlight the fact that new approaches are needed for epithelial translational medicine. Key challenges include identifying which fundamental discoveries are most relevant as potential therapies (identifying appropriate targets), addressing the observation that in vitro and animal models often do not reflect human physiology (adequately validating lead compounds), and accounting for human population variability during preclinical testing (improving lead optimization). In this review, we discuss the relevance of human stem cell-derived epithelial organoids as a new tool to address these points. We highlight advantages of stem cell-derived epithelial organoid models relative to other culture systems, discuss recent advances in tissue-specific organoids, and present a paradigm for using human stem cell-derived organoid models to deliver a high-throughput and high content epithelial translational medicine platform.
Advantages of stem cell-derived organoid models
In the simplest terms, organoid models include three dimensional (3D) cell culture systems that closely resemble the in vivo organ or tissue from which they were derived. These 3D systems reproduce the complex spatial morphology of a differentiated epithelium to allow biologically relevant cell-cell and cell-matrix interactions. Ideally, the physical, cellular, and molecular characteristics of 3D organoid models mean that they share similar physiological responses with in vivo differentiated epithelia. This is in contrast to traditional two dimensional (2D) cell culture models that often bear little physical, molecular, or physiological similarity to their tissue of origin.
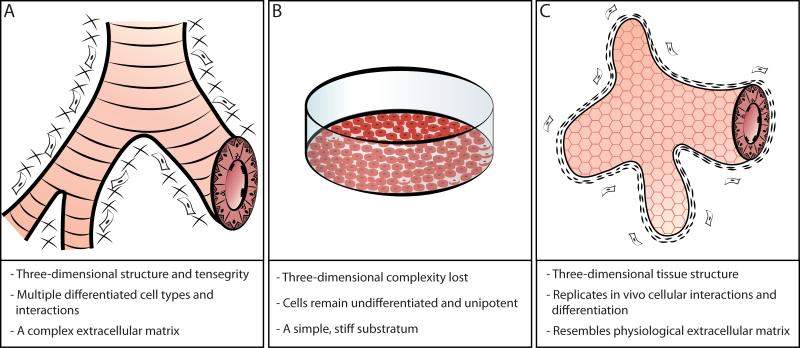
Although the earliest 3D epithelial organoid models were first described over 40 years ago their utility in translational medicine has until recently remained limited 7. This was because early organoid models required large numbers of starting cells, were not amenable to high-throughput screening, and often exhibited limited in vitro viability 8. These drawbacks have now been largely eliminated as advances in multipotent stem and progenitor cell isolation have allowed researchers to develop highly reproducible, long-lived organoids from single cells 9-11. Stem and progenitor cells maintain robust regenerative capacity, multipotent differentiation potential, and exhibit long-term residence within their tissue of origin. Properties of 3D epithelial organoids that distinguish them from 2D culture models include the presence of multipotent cellular differentiation, biologically relevant cellular signaling, and a complex intercellular communication and organization network (Figure 1).
Figure 1. The benefits of stem cell-derived organoid models for epithelial translational medicine.
(A) In vivo, epithelial cells inhabit complex organ microenvironments that are composed of epithelial cells, stromal cells, and the surrounding extracellular matrix. (B) Two dimensional culture models do not resemble normal in vivo tissues. (C) Stem cell-derived organoid models recapitulate in vivo cellular interactions, multipotent differentiation, and in vivo tissue architecture.
Multipotent cellular differentiation
By definition, epithelial organoids comprise multiple differentiated cell types which are found in the relevant organ in vivo. For example, all cell types of the intestinal epithelium are represented in the Matrigel-based model described by Sato and colleagues 9. This is in stark contrast to protocols which are reliant upon 2D primary cultures or cell lines which are often composed entirely of undifferentiated cell types and as such bear little resemblance to the intended parent organ. Sato and colleagues also found that the inclusion of differentiated epithelial Paneth cells increased organoid forming efficiency from approximately 7% to over 75% of Lgr5(+) stem cells 12. This supports the hypothesis that multipotent cellular differentiation is itself required for normal organoid formation.
Separately, epithelial organoid models have the capacity to integrate additional cells that improve their growth and differentiation 13. Airway epithelial studies have shown that the inclusion of niche-specific supporting cell populations enhances organoid growth and differentiation10. Separate work has also shown that including specific mesenchymal cell populations can further improve airway epithelial organoid formation10, 14. Impressively, organoid branching reminiscent of developmental airway morphogenesis has been reported following co-culture of lung epithelial organoids with human endothelial cells 15. These results suggest that both epithelial and non-epithelial cells provide specific factors and matrix molecules that are relevant for organoid growth and differentiation.
Biologically relevant signaling
A recurring observation in organoid models is that the signaling pathways governing organoid formation are identical to those utilized during in vivo organ development and homeostasis. Specifically, canonical Wnt/β-Catenin and Notch pathway signaling are known to be of central importance both in vitro and in vivo. This is perhaps unsurprising given that these pathways are widely involved in many important stages of endodermal organ development 16. For example, Notch signaling in liver development regulates fate decisions made by biliary epithelial cells while Wnt signaling initiates hepatic specification 16. In adult tissues, canonical Wnt and Notch signaling maintains proliferation of many epithelial cell types and their perturbation is strongly associated with the onset of epithelial disease 17. In vivo activation of the Wnt-β-catenin pathway in human lung and intestinal epithelia causes cellular hyperproliferation 18, 19, suppresses ciliated cell differentiation 19, and can promote early lung and intestinal neoplasias 17, 20. Separately, signaling via the Notch ligands Dll1 and Dll4 increases intestinal crypt-associated stem cell abundance 21 and determines in vivo ciliated versus muco-secretory cell differentiation 22.
Intercellular communication and organization networks
The complex intercellular communication and organization networks present in most tissues are difficult to study in 2D monolayer cultures. This is because in vivo, cells exist within a complex network that provides important signaling and biomechanical components. It is now known that cellular communication through force generation is important for normal cellular growth and differentiation. Specifically, work by Bissell and colleagues demonstrated that the phenotype of mammary epithelial cells varies in response to the overall stiffness and composition of the extracellular matrix (ECM). Cells cultured in elastic substrata comparable to the in vivo mammary tissue environment maintain an in vivo-like morphology whereas stiffer substrates induce uncontrolled cell spreading and proliferation 23. Further, Gjorevski and Nelson found that epithelial tissues cultured in three dimensions experience patterns of mechanical stress more comparable to those of in vivo tissues 24. Overall, 3D cultures recapitulate in vivo cell-cell and cell-matrix interactions more successfully than 2D plastic substrate cultures. Thus, 3D culture models allow for the emergence of more physiologically relevant cell phenotypes.
Tissue specific organoid models for translational medicine
Stem and multipotent progenitor cell-derived organoids exist for many tissues, with an ever-increasing number derived from both human and animal tissues. Of these, liver, lung, and gastrointestinal epithelial organoid models are of particular interest for translational medicine. This is because failures to predict toxicity and/or efficacy within these organs are a prominent cause of late-stage drug failures 25-28.
Liver organoid models
The demand for physiologically relevant models of human liver is high given that 20% of drugs fail during Phase III clinical trials due to unforeseen hepatotoxic side effects 29. Unfortunately, traditional 2D hepatocyte cultures respond abnormally to a wide variety of stimuli and are therefore often unsuitable for accurately determining drug safety profiles 30.
There are currently two main approaches for generating physiologically relevant liver organoids: sandwich cultures and self-assembling spheroids 28, 31. In sandwich culture models, patient-derived hepatocyte progenitor cells are seeded between layers of collagen or Matrigel in order to replicate in vivo liver morphology and maintain epithelial hepatocyte polarity 28. In contrast, self-assembling spheroids are formed when hepatocyte progenitor cells are cultured alongside stromal cells in a collagen sponge 31, 32. Spheroids such as these more closely resemble in vivo hepatocyte physiology with cells forming better tight junctions, cell polarity, protein expression and metabolic activity. These characteristics increase hepatic organoid sensitivity to pharmacotoxic compounds relative to conventional 2D hepatocyte cultures 33. In addition to toxicity assays, preclinical targets and techniques that could be investigated in a high throughput manner using hepatic organoids include phase 1 and phase 2 metabolic function, ex vivo bile production, and hepatic coagulation cascade protein expression.
Unfortunately, both hepatocyte sandwich culture and self-assembling spheroid model systems currently exhibit only limited in vitro growth and longevity. This is most likely because identified populations of human hepatocyte progenitor cells are not sufficient for long-term organoid culture as was initially found during attempts to generate intestinal organoids 34. As such it may be necessary to identify and isolate different cell populations from human liver that are capable of sustaining long-term organoid viability. Currently, the identity of long-term human liver stem and progenitor cells remains controversial 35.
Lung organoid models
Respiratory diseases account for nearly 1 in 5 deaths worldwide and lung cancer survival rates remain poor despite numerous therapeutic advances during the past 30 years 36. These figures highlight the need for new, physiologically relevant models for translational lung research. In recent years, Matrigel-based organoid models have been established that enable proximal airway stem or progenitor cells to differentiate in a physiologically relevant manner 13, 37-39. In humans organoids are basal cell-derived and demonstrate intrinsic self-renewal 38, multipotent secretory, basal and ciliated cell differentiation 38, and in rare cases airway-like branching 15. Although it remains unclear whether all basal cells represent stem cells or instead multipotent committed progenitor cells40, mcroarray analysis nonetheless shows that differentiated human basal epithelial cell cultures share a similar transcriptional profile to in vivo airways 41. Further work is now required to improve the efficiency of airway organoid formation and better characterize the cell types responsible for 3D organoid differentiation.
Although airway organoid assays remain in their infancy, it is becoming realistic to imagine the generation of a range of organoid models derived from multiple healthy and diseased patient biopsies. These could be selected to represent a range of respiratory diseases and to account for interindividual population variability. These properties suggest that airway organoid models will be useful in a wide range of translational research including toxicity, drug efficacy and mucociliary clearance studies. Indeed Balharry and colleagues report that airway organoid cultures and in vivo airways exhibit similar stress responses following toxin exposure 42.Preclinical high throughput screening of airway organoids will likely include assays for airway mucous production and secretion, ciliated cell differentiation and function, and airway pathogen resistance.
Gastrointestinal organoid models
The need for clinically relevant models of the gastrointestinal (GI) system is high, particularly given the worldwide prevalence of human intestinal disease. Historically, preclinical GI translational medical research has relied entirely on animal models and cancer cell cultures that are of limited relevance to human physiology 34, 43. However, in 2009 the first long-term organoid models of the small intestine were established using 3D Matrigel 9 systems. GI organoid models have since been adapted to support derivation of normal stomach 44, intestine and colonic organoids 34. The multipotent differentiation capacity of these models suggests that they will provide a valuable resource for translational medicine. They are also likely to benefit investigations of the role commensal bacteria populations play in maintaining intestinal health 43.
In addition to deriving organoid models from healthy patient tissues, the Clevers group has also established epithelial organoid models from human colorectal cancers and metaplastic Barrett’s oesophagus epithelia 34. These disease-specific organoid models now provide the potential for testing therapeutic target efficacy as well as compound toxicity in a preclinical, high-throughput setting. Specific preclinical targets and high throughput assays could include screening for cellular proliferation and monitoring intestinal epithelial cell differentiation. Overall, recent progress suggests that human stem cell-derived organoid models will be an invaluable tool for improving the efficiency of GI translational medicine.
Other epithelial organoid models
Clinically, the pancreas is an important translational medicine target given that type I and type II diabetes now affects 350 million people worldwide. Separately, the development of kidney organoids is an additional high-priority target as the prevalence of renal disease has increased significantly in recent years. Although the identity of human kidney stem cells amenable to organoid derivation remains elusive 45, the recent description of a multipotent stem cell population in adult human pancreas raises the possibility of developing biologically relevant pancreatic organoid models 46.
Pluripotent cell-derived epithelial organoids
In recent years the capacity to generate organoids from pluripotent embryonic stem (ES) and induced pluripotent stem (iPS) cells has been reported, generally using approaches that mimic normal developmental processes through the step-wise application of various signaling molecules 47. Using these techniques, intestinal organoids have been derived from both ES and iPS cells 48. More recently, protocols to direct human pluripotent cells towards airway differentiation have also emerged 49, 50. Despite these advances, it is not yet known how well ES and iPS-derived epithelial organoids will replicate true in vivo differentiation. This is because the long-term influence of epigenetic changes introduced during iPS and ES cell derivation and differentiation remains incompletely understood 51. It therefore remains to be determined whether ES and iPS-derived organoids will be useful for preclinical translational medicine.
A paradigm for organoid models in translational medicine
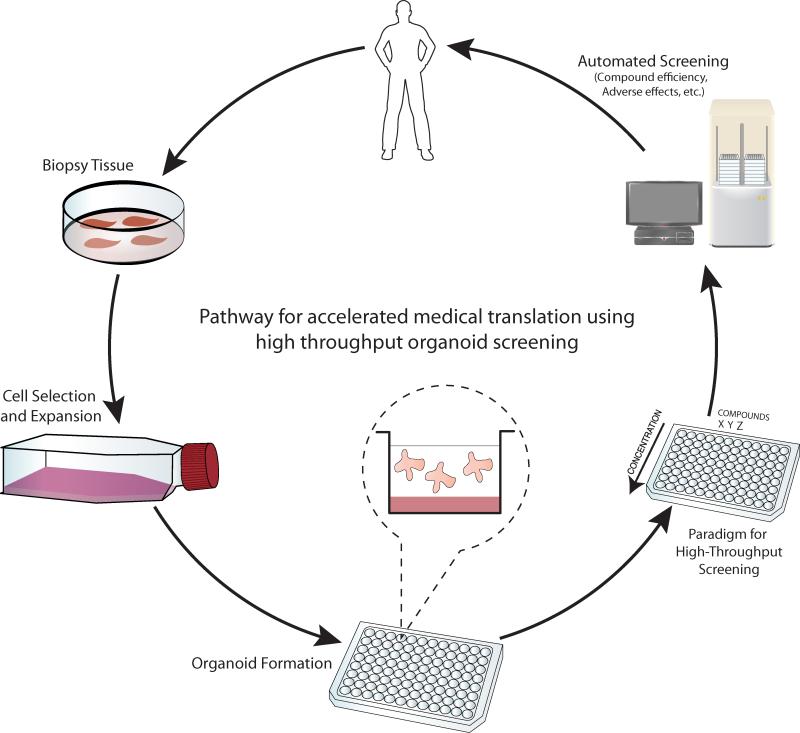
Improving translational medicine from fundamental biomedical discovery to pharmaceutical product requires models capable of producing high-quality, high content and high-throughput data. Here, we propose a paradigm in which human stem cell-derived organoid models may be used in a high-throughput and high content epithelial translational medicine pipeline (Figure 2). We propose that tissue samples could be obtained using minimally invasive biopsy procedures from healthy and diseased patients. Stem or long term progenitor cells present in patient biopsies could then be isolated based on unique cell surface proteins, rapidly expanded in number following a brief 2D culture, and seeded in organoid forming assays as described above. The validity of this approach was recently demonstrated using human intestinal SCs derived from patient biopsies 34. Multiwell plate-based organoid assays would then be channeled into compound toxicity and efficacy screening systems such as gene expression microarray, protein mass-spectrometry, and multiplex ELISA platforms. High throughput and high content analysis would be achieved using automated cell manipulation and readout systems.
Figure 2. A paradigm for high content, high throughput epithelial organoid screening in translational medicine.
Stem cells or multipotent progenitor cells derived from human biopsies will be expanded in vitro and used to derived patient-specific organoids. These will be amenable to high throughput, high content automated compound screening given their resemblance to normal in vivo tissues.
Summary and future directions
In order for high-throughput, high content organoid assays to become a reality several outstanding questions remain to be addressed. Existing organ-specific 3D cell culture models must be optimized to allow consistent, efficient and reproducible organoid formation. This will involve reducing heterogeneity within the organoid-initiating cell population through better characterization of initiating cell types and improving tissue-specific organoid growth conditions. It will also be necessary to optimize organoid forming assays to better replicate in vivo organ physiology at a functional, cellular, and molecular level. Finally, it is of critical importance that biologically relevant preclinical targets are identified to facilitate organoid-based pharmacological lead validation.
Overall, we feel that our paradigm for stem cell-derived organoid models in epithelial translational medicine has the potential to improve preclinical testing and pharmacological compound validation. We believe that 3D organoid modeling will allow one to assess both compound efficacy and safety in a high throughput and high content manner while additionally addressing interindividual human population variability. Thus, our paradigm should provide improved target identification, lead validation, and lead optimization in a single cost-effective, high content and high-throughput assay. In future studies, we envisage expansion of this paradigm beyond simple epithelial organoids and towards development of novel ectodermal and mesodermal-derived organoid models relevant for human translational medicine.
Acknowledgements
The authors gratefully acknowledge the support of members of the UCL Lungs for Living Research Centre for critical reading of this manuscript. REH is funded by a BBSRC-CASE studentship with industrial support from Unilever; AG is funded by a European Research Council Starting Investigator Grant.
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Albani S, Colomb J, Prakken B. Translational medicine 2.0: from clinical diagnosis-based to molecular-targeted therapies in the era of globalization. Clin Pharmacol Ther. 2010;87:642–645. doi: 10.1038/clpt.2010.60. [DOI] [PubMed] [Google Scholar]
- 3.Albani S, Prakken B. The advancement of translational medicine-from regional challenges to global solutions. Nat Med. 2009;15:1006–1009. doi: 10.1038/nm0909-1006. [DOI] [PubMed] [Google Scholar]
- 4.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 5.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 6.Stebbings R, Findlay L, Edwards C, et al. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol. 2007;179:3325–3331. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- 7.Hackney JD, Bils RF, Takahashi Y, et al. Organotypic culture of mammalian lung--studies on morphology, ultrastructure, and surfactant. Aspen Emphysema Conf. 1968;9:13–34. [PubMed] [Google Scholar]
- 8.Stoker AW, Streuli CH, Martins-Green M, et al. Designer microenvironments for the analysis of cell and tissue function. Curr Opin Cell Biol. 1990;2:864–874. doi: 10.1016/0955-0674(90)90085-s. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 10.McQualter JL, Yuen K, Williams B, et al. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertoncello I, McQualter J. Isolation and clonal assay of adult lung epithelial stem/progenitor cells. Curr Protoc Stem Cell Biol. 2011:1. doi: 10.1002/9780470151808.sc02g01s16. Chapter 2: Unit 2G. [DOI] [PubMed] [Google Scholar]
- 14.Teisanu RM, Chen H, Matsumoto K, et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2011;44:794–803. doi: 10.1165/rcmb.2010-0098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franzdottir SR, Axelsson IT, Arason AJ, et al. Airway branching morphogenesis in three dimensional culture. Respir Res. 2010;11:162. doi: 10.1186/1465-9921-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schepers A, Clevers H. Wnt signaling, stem cells, and cancer of the gastrointestinal tract. Cold Spring Harb Perspect Biol. 2012;4:a007989. doi: 10.1101/cshperspect.a007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KA, Kakitani M, Zhao J, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds SD, Zemke AC, Giangreco A, et al. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konigshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol. 2010;42:21–31. doi: 10.1165/rcmb.2008-0485TR. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrinet L, Rodilla V, Liu Z, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240. doi: 10.1053/j.gastro.2011.01.005. e1231-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rock JR, Gao X, Xue Y, et al. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alcaraz J, Xu R, Mori H, et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr Biol. 2010;2:424–434. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell JC, Wells JM. Generating intestinal tissue from stem cells: potential for research and therapy. Regen Med. 2011;6:743–755. doi: 10.2217/rme.11.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McQualter JL, Bertoncello I. Concise review: Deconstructing the lung to reveal its regenerative potential. Stem Cells. 2012;30:811–816. doi: 10.1002/stem.1055. [DOI] [PubMed] [Google Scholar]
- 27.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia L, Arooz T, Zhang S, et al. Hepatocyte function within a stacked double sandwich culture plate cylindrical bioreactor for bioartificial liver system. Biomaterials. 2012;33:7925–7932. doi: 10.1016/j.biomaterials.2012.06.078. [DOI] [PubMed] [Google Scholar]
- 29.Meng Q. Three-dimensional culture of hepatocytes for prediction of drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2010;6:733–746. doi: 10.1517/17425251003674356. [DOI] [PubMed] [Google Scholar]
- 30.Bokhari M, Carnachan RJ, Cameron NR, et al. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J Anat. 2007;211:567–576. doi: 10.1111/j.1469-7580.2007.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugimoto S, Harada K, Shiotani T, et al. Hepatic organoid formation in collagen sponge of cells isolated from human liver tissues. Tissue Eng. 2005;11:626–633. doi: 10.1089/ten.2005.11.626. [DOI] [PubMed] [Google Scholar]
- 32.Lazaro CA, Croager EJ, Mitchell C, et al. Establishment, characterization, and long-term maintenance of cultures of human fetal hepatocytes. Hepatology. 2003;38:1095–1106. doi: 10.1053/jhep.2003.50448. [DOI] [PubMed] [Google Scholar]
- 33.Du Y, Han R, Ng S, et al. Identification and characterization of a novel prespheroid 3-dimensional hepatocyte monolayer on galactosylated substratum. Tissue Eng. 2007;13:1455–1468. doi: 10.1089/ten.2006.0381. [DOI] [PubMed] [Google Scholar]
- 34.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 35.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med. 2007;175:547–553. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- 37.LeSimple P, van Seuningen I, Buisine MP, et al. Trefoil factor family 3 peptide promotes human airway epithelial ciliated cell differentiation. Am J Respir Cell Mol Biol. 2007;36:296–303. doi: 10.1165/rcmb.2006-0270OC. [DOI] [PubMed] [Google Scholar]
- 38.Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Peters-Hall JR, Bose S, et al. Human bronchial epithelial cells differentiate to 3D glandular acini on basement membrane matrix. American journal of respiratory cell and molecular biology. 2011;44:914–921. doi: 10.1165/rcmb.2009-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler C, Birchall M, Giangreco A. Interventional and intrinsic airway homeostasis and repair. Physiology. 2012;27:140–147. doi: 10.1152/physiol.00001.2012. [DOI] [PubMed] [Google Scholar]
- 41.Pezzulo AA, Starner TD, Scheetz TE, et al. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2011;300:L25–31. doi: 10.1152/ajplung.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balharry D, Sexton K, BeruBe KA. An in vitro approach to assess the toxicity of inhaled tobacco smoke components: nicotine, cadmium, formaldehyde and urethane. Toxicology. 2008;244:66–76. doi: 10.1016/j.tox.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Wildenberg ME, van den Brink GR. A major advance in ex vivo intestinal organ culture. Gut. 2012;61:961–962. doi: 10.1136/gutjnl-2012-302021. [DOI] [PubMed] [Google Scholar]
- 44.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Diep CQ, Ma D, Deo RC, et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smukler SR, Arntfield ME, Razavi R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8:281–293. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longmire TA, Ikonomou L, Hawkins F, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mou H, Zhao R, Sherwood R, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobin SC, Kim K. Generating pluripotent stem cells: Differential epigenetic changes during cellular reprogramming. FEBS Lett. 2012;586:2874–2881. doi: 10.1016/j.febslet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]