Abstract
Background:
While the trajectory of blood pressure (BP) with aging is well known, there is a lack of data on how cardiorespiratory fitness (fitness) impacts age-associated BP changes.
Objectives
The objective of the study was to investigate whether fitness alters the aging-BP trajectory.
Methods:
A cohort from the Aerobics Center Longitudinal Study totaling 13,953 men aged 20 to 90 years, free of hypertension, cardiovascular disease, and cancer, completed from 3 to 28 (mean, 3.8) follow-up medical examinations between 1970 and 2006. Fitness was measured by a maximal treadmill exercise test. Longitudinal data were analyzed with a linear mixed model .
Results:
Diastolic BP (DBP) tended to increase until nearly age 60, when a decrease was observed. Systolic BP (SBP) tended to increase over all age periods. In multivariable analysis, average SBP increased by 0.31 mm Hg (95% CI, 0.29 to 0.32) with 1 year age increments, after adjusting for body fat %, fitness, resting heart rate, glucose, triglycerides, cholesterol, current smoking, heavy alcohol drinking, and parental history of hypertension. DBP also increased with age, with a yearly increase of 0.15 mm Hg (95% CI, 0.14 to 0.16). Overall, abnormal SBP (>120 mm Hg) began to occur at about age 50 and abnormal DBP (>80 mm Hg) began at to occur at age 60. Men with higher fitness levels experienced abnormal SBP later than those with low fitness levels.
Conclusions:
Our findings underscore the potential modifying effect of fitness on BP trajectory with aging over the male adult life span. Improving fitness levels might extend the normal SBP and DBP ranges, delaying the development of hypertension.
Keywords: aging, blood pressure, cardiorespiratory fitness, cohort study
Introduction
Approximately one-third of U.S. adults (~68 million) have high blood pressure (BP), which significantly increases the risk for cardiovascular diseases (CVD), the leading cause of death in the United States (1) and worldwide (2,3). High BP is also a risk factor for other chronic diseases, such as diabetes (4) and renal disease (5). There is substantial evidence that diet and physical activity are the major modifiable life-style factors for preventing BP increases (6, 7). Over the last century, progressively elevated BP was thought to be part of the human aging process (8, 9) in the industrialized world, even though little or no age-related BP increase is observed in some populations, including American Indian populations such as the Yanomamo and Xingu (10, 11). The Framingham Heart Study (12,13) showed that the aging patterns of elevated BP for systolic BP (SBP) and diastolic BP (DBP) differed; SBP increased continuously from age 30 years while DBP increased until the 50s, then decreased (13,14).
Previous studies have demonstrated that increased cardiorespiratory fitness (fitness) levels result in lowered BP in an inverse relationship that varied across age strata (15-17). However, little knowledge exists regarding the impact of fitness on age-associated BP changes, and the mechanism is currently unclear. Identifying the age-related BP trajectory and exploring factors that modify the trajectory are important public health goals with significant clinical implications. In this study, we evaluated the effects of aging and fitness on BP (SBP, DBP, and pulse pressure [PP]) using data from the Aerobics Center Longitudinal Study. The purposes of the study were to: 1) define the longitudinal, aging BP trajectory; and 2) determine whether fitness is an independent modifier of the age-associated BP trajectory.
Methods
Study population
The Aerobics Center Longitudinal Study is a large cohort study of healthy adults. A majority (95%) of the study population is white, graduated from college, is employed in professional or executive occupations, and comes from middle to high socioeconomic strata. The current analytical sample consisted of 13,953 men aged 20-90 years old who had at least three preventive medical evaluations at the Cooper Clinic in Dallas, TX, between 1970 and 2006. These men completed from 3 to 28 evaluations (mean, 3.8) for a total of 56,140 observations. All participants in this sample had complete data on SBP and DBP, normal resting and exercising electrocardiograms, and a body mass index (BMI) ≥18.5, and were able to reach 85% of age-predicted maximal heart rate during their treadmill tests. No subjects had a history of myocardial infarction, stroke, cancer, or hypertension at baseline. Participants with a history of self-reported hypertension during any visit were excluded to remove treatment effects on BP levels. The Cooper Institute Institutional Review Board approved the study protocol annually, and all participants gave their informed consent for the baseline examination and follow-up study.
Clinical Examinations
The clinical examination included a physical examination, anthropometry measurements, fasting blood chemistry analysis, resting BP, symptom-limited maximal exercise test for the measurement of fitness, and a standardized questionnaire on personal and family medical history. All measures were obtained after subjects had fasted overnight for at least 12 h.
Resting SBP and DBP were measured in the seated position after at least a 5 min rest, by trained technicians who used mercury manometers and followed a standard procedure (18). More than 2 readings were taken and the average was used in the analysis. PP was defined as the difference between SBP and DBP. Hypertension was defined as SBP of 140 mm Hg or higher and/or DBP of 90 mm Hg or higher. Fasting glucose and lipid profiles were analyzed by automated laboratory techniques in the Cooper Clinic, which meets the quality control standards of the Centers for Disease Control and Prevention Lipid Standardization Program (18). Hypercholesterolemia was defined as a total cholesterol concentration of 240 mg/dL or higher or physician diagnosis. Diabetes was defined as a fasting glucose concentration of 126 mg/dL or higher, insulin use, or physician diagnosis.
Age, smoking status, and alcohol drinking status were obtained by self-administered questionnaire. BMI was calculated from measured weight and height in accordance with standard procedures. Waist circumference was measured at the level of the umbilicus with a plastic tape measure. Physical inactivity was assessed by self-report and defined as reporting no physical activity during leisure time in the 3 months before the examination.
Body fat % was assessed either by hydrostatic weighing, skin fold-thickness measurements, or both. When both methods were available, hydrostatic weighing was preferred. Cardiorespiratory fitness was assessed by a Balke (19) maximal treadmill exercise test. Total treadmill time, which is highly correlated with maximal oxygen uptake, was used as an index of aerobic power. All participants were classified into low (<33rd percentile), moderate (33rd ~66th percentile) or high fit categories (>66th percentile) according to the distribution of age-specific treadmill time. Information about measuring body fat % and fitness are presented in detail elsewhere (20).
Statistical analysis
Means and standard deviations were calculated for continuous variables and percentages for categorical variables. The random intercept model (RIM), a simple version of the linear mixed model (LMM) (21,22), was used to analyze the longitudinal data. The rationale behind LMM is that it can accommodate an unbalanced study design with unequally spaced repeated measures over time. We used age as the measure of time for growth within each subject. The random intercept model takes into account within-subject measure dependency and also variability in subject-specific intercepts not explained by the independent variables included in the model. Conditional on the unobserved random intercept, the observations within each subject are assumed to be independent. The correlations among repeated measurements of the same subject can be obtained through a specified structure. Considering that only a few measurements are unequally spaced over time, the correlation structure is modeled by an autoregressive (AR) process of order 1. The dependent variables for each RIM analysis were BP (SBP, DBP, and PP). All the models were fitted after adjusting for the baseline exam time, which took cohort effects into account. We first examined the longitudinal change in BP associated with aging. Both linear and quadratic models were examined. We then evaluated the influence of body fat % on the age- BP association, because it has been significantly linked to BP (23). Next, we investigated the independent influence of fitness and body fat % on age-related BP changes. Finally, we accounted for time-varying covariates, including waist circumference, smoking status, fasting glucose, and cholesterol. A log likelihood ratio test was used to determine if the model improved the fit (24,25). In addition, the Bayesian information criterion (BIC) (24) and Akaike information criterion (AIC) (25) were used to assess the balance between achieving a good fit of the model to the data and obtaining precise parameter estimates in order to compare models. Each regression coefficient was tested with a z-statistic to determine if it was significantly different from 0. Statistical tests were 2-sided: P < 0.05 was accepted to indicate statistical significance. We fitted all the models using the function lme in the package nlme in R (version 3.0.1). This R function implements 2 likelihood-based methods: maximum likelihood (ML) and restricted maximum likelihood (REML). The REML estimation method is preferred in most cases, while the likelihood ratio test comparing the full and reduced models is only valid under ML estimation.
Results
The baseline characteristics of the current study sample according to the 3 baseline fitness levels are described in Table 1. The overall sample size and each specific sample size are provided on the basis of fitness levels. Overall, participants in the high fitness category had more favorable baseline characteristics, which included lower BMI, waist circumference, body fat %, resting heart rate, triglycerides, and cholesterol compared to participants in the lower fitness levels. Those levels included higher percentages of current smokers and participants who were physically inactive, had diabetes, and had hypercholesterolemia. However, the percentages of heavy alcohol drinking, and parental history of hypertension displayed opposite trends across the fitness levels.
Table 1.
Baseline Characteristics for Male Participants According to Fitness Froups: Aerobics Center Longitudinal Study, 1970-2006.
| Level of fitness | |||||
|---|---|---|---|---|---|
| Overall (n = 13,953), mean (SD) or % |
Low (n = 1,532), mean (SD) or % |
Moderate (n = 5,416), mean (SD) or % |
High (n = 7,005), mean (SD) or % |
P for linear trend |
|
| Age, yrs | 42.98 (8.84) | 41.44 (7.72) | 42.76 (8.49) | 43.49 (9.29) | <0.001 |
| Height, cm | 179.26 (6.48) | 178.33 (6.56) | 179.23 (6.49) | 179.48 (6.43) | <0.001 |
| Weight, kg | 83.17 (11.86) | 90.83 (16.44) | 84.89 (11.64) | 80.16 (9.60) | <0.001 |
| BMI, kg/m2 | 25.85 (3.21) | 28.50 (4.56) | 26.4 (3.1) | 24.85 (2.41) | <0.001 |
| Waist circumference, cm | 92.41 (9.59) | 102.83 (12.49) | 95.36 (9.03) | 89.06 (7.67) | <0.001 |
| Body fat % | 20.39 (6.28) | 25.40 (6.60) | 22.13 (5.7) | 18.01 (5.53) | <0.001 |
| Systolic blood pressure (mm Hg) | 119.33 (11.94) | 120.94 (12.75) | 119.44 (11.56) | 118.89 (12.01) | <0.001 |
| Diastolic blood pressure (mm Hg) | 79.68 (8.73) | 81.55 (9.08) | 80.37 (8.80) | 78.74 (8.47) | <0.001 |
| Treadmill time duration, min | 18.91 (4.61) | 12.09 (2.12) | 16.44 2.19 | 22.32 (3.35) | <0.001 |
| Maximal METS | 12.12 (2.29) | 8.93 (0.98) | 10.91 (1.02) | 13.75 (1.85) | <0.001 |
| Resting heart rate | 59.50 (10.34) | 66.76 (10.09) | 62.43 9.69 | 55.66 (9.23) | <0.001 |
| Fasting plasma glucose, mg/dl | 99.07 (13.59) | 102.55 18.09 | 99.7 14.49 | 97.81 (11.37) | <0.001 |
| Triglycerides, mg/dl | 127.52 (94.34) | 177.26 128.54 | 141.26 100.04 | 105.92 (72.17) | <0.001 |
| Total cholesterol, mg/dl | 207.98 (39.13) | 218.42 (42.16) | 212.33 39.26 | 202.31 (37.40) | <0.001 |
| Current smoker, % | 14.9 | 27.3 | 18.3 | 9.6 | <0.001 |
| Heavy drinker, % | 6.3 | 4.6 | 5.6 | 7.2 | <0.001 |
| Physical inactivity, % | 30.3 | 68.4 | 41.3 | 13.5 | <0.001 |
| Diabetes, % | 3.4 | 7.1 | 3.7 | 2.4 | <0.001 |
| Hypercholesterolemia, % | 25.2 | 32.8 | 27.9 | 21.4 | <0.001 |
| Parental history of hypertension | 13.2 | 9.9 | 12.2 | 14.8 | <0.001 |
BMI = body mass index; MET = metabolic equivalent.
Heavy drinker is defined as more than 14 alcoholic drinks per week for men.
Diabetes is defined as a fasting glucose level of at least 126 mg/dL, physician-diagnosed diabetes, or insulin use.
Hypercholesterolemia is defined as a total cholesterol level of at least 240 mg/dL or physician-diagnosed hypercholesterolemia.
On the basis of the estimated correlation matrix, we found high correlations between fitness and body fat %, waist circumference, and resting heart rate, which could lead to the issue of multicolinearity and affect the relationship of fitness with BP trajectories with aging. To reduce multicolinearity, 3 RIM models were fitted with fitness as a predictor, and body fat %, waist circumference, and resting heart rate as a response, respectively. The residuals of those 3 variables were the variations unexplained by fitness; they were then added into the multivariate model with other covariates. However, although it has been shown that fitness has a nonlinear relationship with aging (26), we discovered that fitness levels varied substantially across all age groups. Therefore, we did not standardize fitness by age.
The RIM results for SBP, DBP and PP trajectories with aging, after adjusting for the other covariates, are presented in Table 2, Table 3, and Table 4. We found that the SBP trajectory with aging is linear, while DBP and PP are nonlinear. SBP increased by 0.30 mm Hg (95% confidence interval [95% CI], 0.29 to 0.31) with each year of age (Table 2). Body fat % was added into model II and the β coefficient for age dropped significantly to 0.25, indicating that body fat % is a significant confounder and independently correlates with SBP. After controlling for body fat %, SBP increased by 0.25 mm Hg (95% CI, 0.24-0.26) with each 1 year increase in age. When fitness and age*fitness interaction were added to model III, model fit was improved on the basis of AIC and BIC values. Model III showed that fitness was negatively associated with SBP and that the age*fitness interaction was significant. Finally, all covariates, including waist circumference, resting heart rate, fasting glucose, triglycerides, cholesterol, smoking status, alcohol drinking status, and parental history of hypertension were included in model IV. The results showed that age, body fat%, waist circumference, resting heart rate, fasting glucose, triglycerides, cholesterol, alcohol drinking status and parental history of hypertension were all positively associated with SBP. In contrast, smoking status was inversely associated with SBP. The interaction term of age*fitness was still significant in model IV.
Table 2.
Partial Regression Coefficients (β) and Standard Errors (SE) for the Systolic Blood Pressure Predictor Variables in the RIM analyses: Aerobics Center Longitudinal Study, 1970–2006.
| Variable | Model I | Model II | Model III | Model IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Fixed Effects | ||||||||||||
| Intercept | 118.78 | 0.17 | <0.001 | 114.67 | 0.27 | <0.001 | 118.48 | 0.18 | <0.001 | 111.05 | 0.62 | <0.001 |
| Age | 0.30 | 0.007 | <0.001 | 0.25 | 0.007 | <0.001 | 0.23 | 0.008 | <0.001 | 0.31 | 0.008 | <0.001 |
| Baseline exam date | 0.00016 | 0.00003 | <0.001 | 0.00013 | 0.00003 | <0.001 | 0.00014 | 0.00003 | <0.001 | 0.00022 | 0.00003 | <0.001 |
| Body fat % | 0.20 | 0.01 | <0.001 | 0.15 | 0.01 | <0.001 | 0.05 | 0.02 | 0.0051 | |||
| Fitness | −0.60 | 0.03 | <0.001 | −0.35 | 0.03 | <0.001 | ||||||
| Age × fitness | −0.02 | 0.003 | <0.001 | −0.03 | 0.003 | <0.001 | ||||||
| Waist circumference | 0.14 | 0.0099 | <0.001 | |||||||||
| Resting heart rate | 0.13 | 0.007 | <0.001 | |||||||||
| Fasting plasma glucose | 0.04 | 0.005 | <0.001 | |||||||||
| Triglycerides | 0.006 | 0.0007 | <0.001 | |||||||||
| Total cholesterol | 0.009 | 0.002 | <0.001 | |||||||||
| Current smoker | −1.56 | 0.19 | <0.001 | |||||||||
| Heavy drinker | 1.76 | 0.22 | <0.001 | |||||||||
| Parental history of hypertension |
1.86 | 0.16 | <0.001 | |||||||||
| Random Effects | ||||||||||||
| SD intercept (95% CI) | 7.78 (7.63, 7.94) | 7.71 (7.55, 7.88) | 7.80 (7.64, 7.97) | 7.62(7.45,7.79) | ||||||||
| SD error (95% CI) | 9.26 (9.18, 9.34) | 9.15 (9.07, 9.24) | 9.10(9.01, 9.18) | 8.74 (8.65, 8.83) | ||||||||
SD intercept = SD of the individual variance of the random part of the model; SD error = standard error of linear mixed model.
CI = confidence interval.
Body fat %, waist circumference, and resting heart rate were normalized by fitness.
Table 3.
Partial Regression Coefficients (β) and Standard errors (SE) for the Diastolic Blood Pressure Predictor Variables in the RIM analyses: Aerobics Center Longitudinal Study, 1970–2006.
| Variable | Model I | Model II | Model III | Model IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Fixed Effects | ||||||||||||
| Intercept | 78.53 | 0.12 | <0.001 | 73.46 | 0.19 | <0.001 | 78.50 | 0.12 | <0.001 | 74.16 | 0.45 | <0.001 |
| Age | 0.16 | 0.005 | <0.001 | 0.12 | 0.005 | <0.001 | 0.11 | 0.005 | <0.001 | 0.14 | 0.006 | <0.001 |
| Age2 | −0.003 | 0.0003 | <0.001 | −0.002 | 0.0003 | <0.001 | −0.003 | 0.0003 | <0.001 | −0.002 | 0.0004 | <0.001 |
| Baseline exam date | 0.0003 | 0.00002 | <0.001 | 0.0002 | 0.00002 | <0.001 | 0.0003 | 0.00002 | <0.001 | 0.0002 | 0.00002 | <0.001 |
| Body fat % | 0.25 | 0.008 | <0.001 | 0.20 | 0.009 | <0.001 | 0.08 | 0.01 | <0.001 | |||
| Fitness | −0.61 | 0.02 | <0.001 | −0.49 | 0.02 | <0.001 | ||||||
| Waist circumference | 0.12 | 0.007 | <0.001 | |||||||||
| Resting heart rate | 0.13 | 0.005 | <0.001 | |||||||||
| Fasting plasma glucose | 0.007 | 0.003 | 0.0338 | |||||||||
| Triglycerides | 0.005 | 0.0006 | <0.001 | |||||||||
| Total cholesterol | 0.02 | 0.001 | <0.001 | |||||||||
| Current smoker | −1.02 | 0.14 | <0.001 | |||||||||
| Heavy drinker | 1.37 | 0.17 | <0.001 | |||||||||
| Parental history of hypertension |
1.39 | 0.12 | <0.001 | |||||||||
| Random Effects | ||||||||||||
| SD intercept (95% CI) | 5.11 (4.99, 5.23) | 4.99 (4.87, 5.12) | 5.02 (4.91, 5.13) | 4.84 (4.72, 4.96) | ||||||||
| SD error 95% CI | 6.90 (6.84, 6.96) | 6.79 (6.73, 6.86) | 6.77 (6.71, 6.83) | 6.59 (6.53, 6.66) | ||||||||
SD intercept = SD of the individual variance of the random part of the model; SD error = standard error of linear mixed model.
CI = confidence interval.
Table 4.
Partial Regression Coefficients (β) and Standard Errors (SE) for the Pulse Pressure Predictor Variables in the RIM Analyses: Aerobics Center Longitudinal Study, 1970–2006.
| Variable | Model I | Model II | Model III | Model IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Fixed Effects | ||||||||||||
| Intercept | 38.93 | 0.128 | <0.0001 | 39.63 | 0.22 | <0.0001 | 38.81 | 0.13 | <0.0001 | 33.81 | 0.54 | <0.0001 |
| Age | 0.12 | 0.005 | <0.0001 | 0.12 | 0.006 | <0.0001 | 0.13 | 0.006 | <0.0001 | 0.16 | 0.007 | <0.0001 |
| Age2 | 0.014 | 0.0004 | <0.0001 | 0.013 | 0.0004 | <0.0001 | 0.014 | 0.0004 | <0.0001 | 0.015 | 0.0004 | <0.0001 |
| Baseline exam date | −0.00009 | 0.00002 | <0.0001 | −0.00007 | 0.00002 | <0.0001 | −0.00008 | 0.00002 | <0.0001 | 0.00005 | 0.00002 | 0.0185 |
| Body fat % | −0.04 | 0.009 | <0.0001 | −0.02 | 0.01 | <0.0001 | −0.03 | 0.02 | 0.0949 | |||
| Fitness | 0.17 | 0.02 | <0.0001 | 0.33 | 0.03 | <0.0001 | ||||||
| Waist circumference | 0.05 | 0.009 | <0.0001 | |||||||||
| Resting heart rate | 0.02 | 0.006 | 0.0026 | |||||||||
| Fasting plasma glucose | 0.04 | 0.004 | <0.0001 | |||||||||
| Triglycerides | 0.001 | 0.0007 | 0.1565 | |||||||||
| Total cholesterol | −0.002 | 0.002 | 0.2814 | |||||||||
| Current smoker | −0.55 | 0.17 | 0.001 | |||||||||
| Heavy drinker | 0.58 | 0.20 | 0.0036 | |||||||||
| Parental history of hypertension |
0.81 | 0.15 | <0.0001 | |||||||||
| Random Effects | ||||||||||||
| SD intercept (95% CI) | 4.92 (4.79, 5.04) | 4.92 (4.79, 5.06) | 4.90 (4.77, 5.03) | 4.86 (4.71, 5.02) | ||||||||
| SD error (95% CI) | 8,47 (8.41, 8.53) | 8.41 (8.34, 8.48) | 8.41 (8.35, 8.48) | 8.34 (8.27, 8.42) | ||||||||
SD intercept = SD of the individual variance of the random part of the model; SD error = standard error of linear mixed model.
CI = confidence interval.
Table 3 shows that DBP was positively associated with age, body fat %, waist circumference, resting heart rate, fasting glucose, triglycerides, cholesterol, alcohol drinking status and parental history of hypertension. Inverse associations were observed between DBP and current smoking and age-squared. Additionally, DBP was inversely related to age-squared, indicating a quadratic age-DBP pattern. After adjusting all covariates, DBP still maintained a positive association with age (0.14 mm Hg [95% CI, 0.13-0.15]). However, no significant interaction between age and fitness was found for DBP changes.
The pattern of PP is opposite that of DBP, with a much steeper rate of increase after approximately age 50 years. The interaction term of age*fitness for PP was negative, but not statistically significant, and thus was not included in our models. Table 4 summarizes the PP data, showing the curvilinear association of PP with aging. After the multivariate adjustment, PP increased by 0.16 mm Hg (95% CI, 0.15 to 0.17).
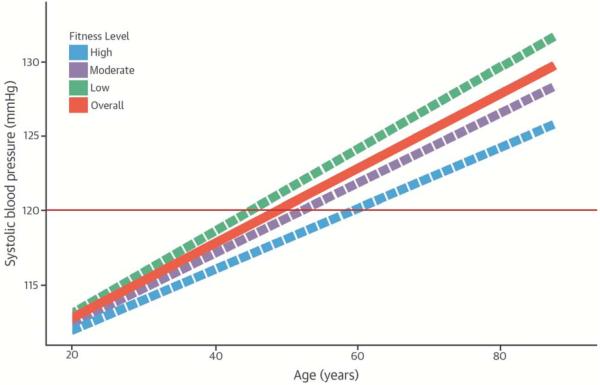
To better visualize the effect of fitness on this trajectory, we provided the estimated SBP trajectory with aging and corresponding 95% confidence bands for people with 3 different fitness levels, assuming a constant body fat % residual (−0.06, mean of the residual) and the same baseline examination time under model III. The three levels were chosen on the basis of medians from low (<33rd percentile), moderate (33rd ~66th percentile) or high (>66th percentile) fitness. The results for SBP are reported in the Central Illustration. We also provided the overall BP trajectory with aging under model II, assuming the same baseline examination time and a constant body fat % (20.31). Constant body fat % was calculated on the basis of the normalization equation with body fat % residual = −0.06 and fitness = mean of the fitness. The Central Illustration shows that age and SBP interact around age 20 years, indicating that the relationship between SBP and aging changes across three fitness levels and that after the age 20 years turning point, fitness is a protective factor for SBP increment with aging.
Discussion
There were several key findings in this prospective, population-based cohort study. First, BP is inversely associated with fitness levels. People in higher fitness categories had lower BP than those in lower fitness categories. Second, the interaction between age and fitness was significant for the SBP trajectory but not for DBP, which partially confirmed our hypothesis that fitness is an effect modifier for the SBP aging trajectory. This finding also indicated that fitness is likely to modify SBP, but not DBP. Third, body fat % had a strong confounding effect. However, after adjusting for body fat %, the SBP and DBP aging trajectories were not changed significantly, supporting our hypothesis that the age-BP trajectory was independent of body fat %. Finally, the SBP trajectory increased linearly with age, while the DBP trajectory had a nonlinear relationship with aging. These overall trend findings were consistent with previous cohort studies on BP trajectory over the life course, but the amounts of change were different, perhaps due to population differences (10, 12, 27).
Arterial and arteriolar stiffness are the main factors contributing to the increase in BP with aging. Previous studies showed that aortic stiffening with age is mainly caused by elastin fiber fracture, with stresses transferred to collagenous elements in an aorta which becomes progressively dilated (28, 29), and also by vascular aging manifestations, displayed at the levels of large elastic arteries by arteriosclerosis originating from a gradual mechanical senescence of fibrosis and calcification of elastic fibers (30, 31). However, there is significant heterogeneity in this process among different arteries because not all arterial trees exhibit the same age-dependent stiffening (32). Our results showed that in a large cohort of healthy men, for each increase in age of 1 year, SBP linearly increased by 0.30 mm Hg, with a standard error of 0.0007 mm Hg, and DBP increased quadratically by 0.14 mm Hg, with a standard error of 0.006 mm Hg, consistent with a prior study (12). The current findings are in agreement with the previous studies (12, 33), which showed that DBP was quadratically associated with aging and that after 60 to 65 years of age, DBP decreased with aging. Although the potential mechanism remains unclear, several possible explanations were proposed, including a theory of “burned out” diastolic hypertension and “selective survival.” However, Framingham Heart Study investigators found that after “burned out” diastolic hypertension and “selective survival” were removed, DBP still had a quadratic shape (33), leading them to suggest increasing large artery stiffness as the most likely explanation. As PP might act as a surrogate measure of arterial stiffness, we also examined the longitudinal aging trajectory of PP (Table 4). We found that after approximately 50 years of age, PP increased steeply, suggesting that arterial stiffness becomes severe with aging. In addition, the quadratic nature of DBP among all categorized BP groups, including the lowest BP group from the Framingham Heart Study, might cancel out the age-related physical fitness relationship. However, this potential mechanism needs further investigation.
Epidemiological studies have consistently demonstrated that fitness is inversely and independently associated with metabolic syndrome, a cluster of CVD risk factors (34,35). Additional evidence shows that physical activity has a dose-dependent relationship with fitness (36). Therefore, fitness plays an important role in the age-BP trajectory. Our results showed that SBP becomes >120 mm Hg around 46 years of age for those in the low fitness level and at 54 years of age for those in the high fitness level. In contrast, at approximately age 42 years, DBP becomes >80 mm Hg for those at the low fitness level, which does not occur until advanced ages (beyond 90 years) for those at a high fitness level. This suggests that highly fit men are likely to reach abnormal SBP values about a decade later than men in the low fitness category, implying that improving fitness levels may reduce the duration of elevated SBP (37). Although much studied, the physiological mechanism for how BP is lowered by fitness remains unclear. Previous studies proposed that improved endothelial function, with reduction in early wave reflection due to physical activity, could result in lower SBP at a higher level of fitness (38-40). These data have important clinical implications, particularly considering that elevated regular physical activity and aerobic exercise yield important SBP and DBP reductions in adults. Mean reductions in resting BP ranged from 2 to 5 mm Hg for resting SBP and 2 to 3 mm Hg for resting DBP (41,42). Although these BP reductions may appear small, they are clinically significant because it has been estimated that as little as a 2 mm Hg reduction in population average resting SBP would reduce coronary heart disease mortality by 4%, while a reduction of 5 mm Hg would reduce coronary heart disease mortality by 9% (43).
The key strength of this study is its design, which included longitudinal measures of SBP, DBP and other time-varying covariates so that BP trajectories with aging could be assessed. Another advantage is the relatively large sample size, as it provided stable estimates with small standard errors. The third strength is that instead of only using BMI, which may not reflect the increased risk of body fatness (44), we used body fat % to determine body composition; nevertheless, we did not separate visceral from subcutaneous adiposity, which may have different impacts on CVD risk. Finally, we used the RIM method to quantify SBP and DBP trajectories with age by incorporating the unequal-spaced repeated measurements. This RIM method also included random intercepts to account for within-subject dependency and variability in subject-specific intercepts not explained by the independent variables included in the model.
Several limitations should be acknowledged. The most important concern in longitudinal study design is losses to follow-up. However, this was studied in the ACLS and it is not a serious problem. Another limitation is that there is no information on antihypertensive medication status. We thus excluded those who reported a history of hypertension from baseline and each follow-up visit. Among these initially hypertension-free men, 1146 developed hypertension during follow-up, with incidence rates across low, moderate, and high fitness groups of 11.2%, 9.3%, and 6.7%, respectively. This again shows the association of higher fitness with a lower rate of developing hypertension, as we have published previously (45).
Due to concerns about the effect of medication on BP trajectory, men who developed hypertension during follow-up were excluded from the analysis. To determine the implications of these exclusions on the results, we repeated the analysis, including the observations from those who developed hypertension during follow-up (Tables 2a-4a and Figure 1a are available as an online appendix). Although no significant difference between Figure 1 and Figure 1a was observed, the overall line in Figure 1a was shifted slightly back, which might indicate that men who developed hypertension received antihypertensive therapy to lower their BP, resulting in a delay in reaching the SBP level of 120 mm Hg. Thus, the aging-BP trajectory was no longer natural. This was also a concern in the Framingham study (33), where the authors stated that because 1948 was the start point of this study, the efficacy of treating hypertension was not well established, which offered the unique opportunity to study the effect of the untreated BP-aging trajectory. Since the current study started in the 1970s, when anti-hypertension therapy was widely administered, we excluded these men from the main analysis due to this concern. Heart rate was included in the multivariate models to further control the possible effect of beta-adrenergic blocking agents on BP, since these agents have been used since early in this study. In addition, since we were limited to available clinical measurements obtained at irregular clinical intervals, we cannot eliminate the possibility of confounding by unmeasured or unknown factors. The current study includes relatively healthy men who were primarily white and of middle-to-upper socioeconomic status. We should, therefore, be cautious about generalizing these results to other populations, including those with preexisting conditions.
Table 2a.
Partial regression coefficients (β) and standard errors (SE) for the systolic blood pressure predictor variables in the RIM analyses among men with incident hypertension during follow-up: Aerobics Center Longitudinal Study, 1970–2006.
| Variable | Model I | Model II | Model III | Model IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Fixed Effects | ||||||||||||
| Intercept | 118.95 | 0.17 | <0.001 | 114.74 | 0.27 | <0.001 | 118.67 | 0.18 | <0.001 | 111.14 | 0.61 | <0.001 |
| Age | 0.30 | 0.007 | <0.001 | 0.26 | 0.007 | <0.001 | 0.24 | 0.008 | <0.001 | 0.32 | 0.008 | <0.001 |
| Baseline exam date | 0.00015 | 0.00003 | <0.001 | 0.00011 | 0.00003 | <0.001 | 0.00014 | 0.00003 | <0.001 | 0.0002 | 0.00003 | <0.001 |
| Body fat % | 0.20 | 0.01 | <0.001 | 0.16 | 0.01 | <0.001 | 0.05 | 0.02 | 0.0051 | |||
| Fitness | −0.61 | 0.03 | <0.001 | −0.36 | 0.03 | <0.001 | ||||||
| Age × Fitness | −0.02 | 0.003 | <0.001 | −0.03 | 0.003 | <0.001 | ||||||
| Waist circumference | 0.14 | 0.0099 | <0.001 | |||||||||
| Resting heart rate | 0.14 | 0.007 | <0.001 | |||||||||
| Fasting plasma glucose | 0.04 | 0.005 | <0.001 | |||||||||
| Triglycerides | 0.006 | 0.0007 | <0.001 | |||||||||
| Total cholesterol | 0.01 | 0.002 | <0.001 | |||||||||
| Current smoker | −1.58 | 0.19 | <0.001 | |||||||||
| Heavy drinker | 1.77 | 0.22 | <0.001 | |||||||||
| Parental history of hypertension |
1.94 | 0.16 | <0.001 | |||||||||
| Random Effects | ||||||||||||
| SD intercept (95% CI) | 7.79 (7.64, 7.95) | 7.73 (7.57, 7.89) | 7.81 (7.65, 7.98) | 7.62 (7.45,7.79) | ||||||||
| SD error (95% CI) | 9.35 (9.27, 9.43) | 9.25 (9.16, 9.34) | 9.19 (9.11, 9.28) | 8.83 (8.74, 8.93) | ||||||||
SD intercept: SD of the individual variance of the random part of the model; SD error: standard error of linear mixed model; CI = confidence interval.
Body fat%, waist circumference, and resting heart rate were normalized by fitness.
Table 4a.
Partial regression coefficients (β) and standard errors (SE) for the pulse pressure predictor variables in the RIM analyses among men with incident hypertension during follow-up: Aerobics Center Longitudinal Study, 1970–2006.
| Variable | Model I | Model II | Model III | Model IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Fixed Effects | ||||||||||||
| Intercept | 39.02 | 0.128 | <0.0001 | 39.69 | 0.22 | <0.0001 | 38.92 | 0.13 | <0.0001 | 33.96 | 0.54 | <0.0001 |
| Age | 0.12 | 0.005 | <0.0001 | 0.13 | 0.006 | <0.0001 | 0.13 | 0.006 | <0.0001 | 0.16 | 0.007 | <0.0001 |
| Age2 | 0.014 | 0.0004 | <0.0001 | 0.013 | 0.0004 | <0.0001 | 0.014 | 0.0004 | <0.0001 | 0.015 | 0.0004 | <0.0001 |
| Baseline exam date | −0.0001 | 0.00002 | <0.0001 | −0.00008 | 0.00002 | <0.0001 | −0.00009 | 0.00002 | <0.0001 | 0.00004 | 0.00002 | 0.0185 |
| Body fat % | −0.04 | 0.009 | <0.0001 | −0.01 | 0.01 | <0.0001 | −0.02 | 0.02 | 0.0949 | |||
| Fitness | 0.17 | 0.02 | <0.0001 | 0.33 | 0.03 | <0.0001 | ||||||
| Waist circumference | 0.05 | 0.009 | <0.0001 | |||||||||
| Resting heart rate | 0.02 | 0.006 | 0.0026 | |||||||||
| Fasting plasma glucose | 0.04 | 0.004 | <0.0001 | |||||||||
| Triglycerides | 0.001 | 0.0007 | 0.1565 | |||||||||
| Total cholesterol | −0.002 | 0.002 | 0.2814 | |||||||||
| Current smoker | −0.58 | 0.17 | 0.001 | |||||||||
| Heavy drinker | 0.59 | 0.20 | 0.0036 | |||||||||
| Parental history of hypertension |
0.83 | 0.15 | <0.0001 | |||||||||
| Random Effects | ||||||||||||
| SD intercept (95% CI) | 4.93 (4.79, 5.07) | 4.94 (4.81, 5.07) | 4.91 (4.78, 5.05) | 4.89 (4.74, 5.04) | ||||||||
| SD error (95% CI) | 8,52 (8.46, 8.59) | 8.46 (8.39, 8.52) | 8.46 (8.39, 8.53) | 8.39 (8.31, 8.46) | ||||||||
SD intercept: SD of the individual variance of the random part of the model; SD error: standard error of linear mixed model; CI = confidence interval.
Body fat%, waist circumference, and resting heart rate were normalized by fitness.
Figure 1a. Systolic blood pressure trajectories with aging among men with incident hypertension during follow-up by fitness levels.
Provided are the trajectories of the overall sample (purple), low (blue), moderate (green) and high fit level (pink), assuming a constant percent body fat of 20.31%. The solid red line represents the cutoff point for an abnormal systolic blood pressure level of 120 mmHg.
Figure 1. Central Illustration. Systolic Blood Pressure Trajectories With Aging in Healthy Men by Fitness Levels.
Trajectories of the overall sample (orange), low (green), moderate (purple), and high fit level (blue) are provided, assuming a constant 20.31% body fat. Solid red line represents the cutoff point of 120 mmHg for a prehypertension level of systolic blood pressure.
Conclusions
Our findings underscore the potential effects of fitness on SBP trajectories with aging over the adult life course. Promoting fitness to extend the duration of normal SBP levels might reduce the potential risk for developing hypertension, CVD, and other BP-related chronic diseases, as well as reduce medical costs, major morbidity, and mortality. Since regular physical activity is the primary and most modifiable determinant of fitness level, our results underscore the potential importance of increasing regular physical activity to prevent the aging-related progressive rise in BP.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Human aging is associated with progressively increasing blood pressure. Better cardiorespiratory fitness can lower systolic and diastolic blood pressure, and the inverse relationship between fitness and blood pressure varies with age.
TRANSLATIONAL OUTLOOK: The mechanisms by which fitness modifies the relationship between aging and blood pressure deserves further investigation, particularly in individuals with hypertension or other forms of cardiovascular disease.
Table 3a.
Partial regression coefficients (β) and standard errors (SE) for the diastolic blood pressure predictor variables in the RIM analyses among men with incident hypertension during follow-up: Aerobics Center Longitudinal Study, 1970–2006.
| Variable | Model I | Model II | Model III | Model IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Fixed Effects | ||||||||||||
| Intercept | 78.63 | 0.12 | <0.001 | 73.53 | 0.19 | <0.001 | 78.62 | 0.12 | <0.001 | 74.15 | 0.45 | <0.001 |
| Age | 0.16 | 0.005 | <0.001 | 0.12 | 0.005 | <0.001 | 0.11 | 0.005 | <0.001 | 0.14 | 0.006 | <0.001 |
| Age2 | −0.003 | 0.0003 | <0.001 | −0.002 | 0.0003 | <0.001 | −0.003 | 0.0003 | <0.001 | −0.002 | 0.0004 | <0.001 |
| Baseline exam date | 0.0003 | 0.00002 | <0.001 | 0.0002 | 0.00002 | <0.001 | 0.0003 | 0.00002 | <0.001 | 0.0002 | 0.00002 | <0.001 |
| Body fat % | 0.25 | 0.008 | <0.001 | 0.20 | 0.009 | <0.001 | 0.08 | 0.01 | <0.001 | |||
| Fitness | −0.62 | 0.02 | <0.001 | −0.49 | 0.02 | <0.001 | ||||||
| Waist circumference | 0.12 | 0.007 | <0.001 | |||||||||
| Resting heart rate | 0.13 | 0.005 | <0.001 | |||||||||
| Fasting plasma glucose | 0.007 | 0.003 | 0.0338 | |||||||||
| Triglycerides | 0.005 | 0.0006 | <0.001 | |||||||||
| Total cholesterol | 0.02 | 0.001 | <0.001 | |||||||||
| Current smoker | −1.01 | 0.14 | <0.001 | |||||||||
| Heavy drinker | 1.36 | 0.16 | <0.001 | |||||||||
| Parental history of hypertension |
1.45 | 0.12 | <0.001 | |||||||||
| Random Effects | ||||||||||||
| SD intercept (95% CI) | 5.13 (5.02, 5.24) | 5.01 (4.90, 5.12) | 5.04 (4.93, 5.15) | 4.85 (4.73, 4.96) | ||||||||
| SD error 95% CI | 6.94 (6.88, 6.99) | 6.83 (6.77, 6.89) | 6.81 (6.75, 6.87) | 6.63 (6.57, 6.70) | ||||||||
SD intercept: SD of the individual variance of the random part of the model; SD error: standard error of linear mixed model; CI = confidence interval. Body fat%, waist circumference, and resting heart rate were normalized by fitness.
Acknowledgements
This work was supported by National Institutes of Health grants AG06945, HL62508, and R21DK088195. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the Cooper Clinic physicians and technicians for collecting the baseline data and Cooper Institute staff for data entry and management.
ABBREVIATIONS LIST
- ACLS
Aerobics Center Longitudinal Study.
- BP
Blood pressure
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- PP
Pulse pressure
- MET
metabolic equivalent.
- CVD
cardiovascular disease.
- RIM
Random intercept model
- REML
Restricted maximum likelihood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have reported that they have no relevant relationships to the contents of this paper to disclose.
Data sharing: As part of his separation agreement from the Cooper Institute in 2006, Dr. Steven Blair obtained the Aerobics Center Longitudinal Study (ACLS) database used in this project. This agreement prevents us from sharing the data with other investigators. However, we pledge to collaborate with interested investigators on research using the database.
References
- 1.Vital signs: Prevalence, treatment, and control of hypertension--United States, 1999-2002 and 2005-2008. MMWR Morb Mortal Wkly Rep. 2011;60:103–8. [PubMed] [Google Scholar]
- 2.World Health Organization A global brief on hypertension: silent killer, global public health crisis. 2013 Available at: http://apps.who.int/iris/bitstream/10665/79059/1/WHO/DCO/WHD/2013.2/eng.pdf. Accessed July 15, 2014.
- 3.Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–9. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 5.Cozzolino M, Gentile G, Mazzaferro S, et al. Blood pressure, proteinuria, and phosphate as risk factors for progressive kidney disease: a hypothesis. Am J Kidney Dis. 2013;62:984–92. doi: 10.1053/j.ajkd.2013.02.379. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Stampfer MJ, Hu FB, Manson JE, et al. Primary prevention of coronary heart disease in women through diet and lifestyle. New Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 8.Gurven M, Blackwell AD, Rodriguez DE, et al. Does blood pressure inevitably rise with age?: Longitudinal evidence among forager-horticulturalists. Hypertension. 2012;60:25–33. doi: 10.1161/HYPERTENSIONAHA.111.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–8. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez BL, Labarthe DR, Huang B, et al. Rise of blood pressure with age. New evidence of population differences. Hypertension. 1994;24:779–85. doi: 10.1161/01.hyp.24.6.779. [DOI] [PubMed] [Google Scholar]
- 11.Mancilha-Carvalho Jde J, Souza e Silva NA. The Yanomami indians in the INTERSALT Study. Arq Bras Cardiol. 2003;80:289–300. doi: 10.1590/s0066-782x2003000300005. [DOI] [PubMed] [Google Scholar]
- 12.Cheng S, Xanthakis V, Sullivan LM, et al. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension. 2012;60:1393–9. doi: 10.1161/HYPERTENSIONAHA.112.201780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin SS. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J Hypertens Suppl. 1999;17:S29–36. [PubMed] [Google Scholar]
- 14.McaLeavy KMA, Fiumara MC. Eating disorders: Are they addictions? A dialogue. J Soc Work Pract Addict. 2001;1:107–13. [Google Scholar]
- 15.Barlow CE, LaMonte MJ, Fitzgerald SJ, et al. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006;163:142–50. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 16.Rheaume C, Arsenault BJ, Belanger S, et al. Low cardiorespiratory fitness levels and elevated blood pressure: what is the contribution of visceral adiposity? Hypertension. 2009;54:91–7. doi: 10.1161/HYPERTENSIONAHA.109.131656. [DOI] [PubMed] [Google Scholar]
- 17.Shook RP, Lee DC, Sui X, et al. Cardiorespiratory fitness reduces the risk of incident hypertension associated with a parental history of hypertension. Hypertension. 2012;59:1220–4. doi: 10.1161/HYPERTENSIONAHA.112.191676. [DOI] [PubMed] [Google Scholar]
- 18.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–80. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 19.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–88. [PubMed] [Google Scholar]
- 20.Sui X, Jackson AS, Church TS, et al. Effects of cardiorespiratory fitness on aging: glucose trajectory in a cohort of healthy men. Ann Epidemiol. 2012;22:617–22. doi: 10.1016/j.annepidem.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twisk JWR. Applied longitudinal data analysis for epidemiology. Cambridge University Press; New York, NY: 2013. [Google Scholar]
- 22.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer; New York, NY: 2009. [Google Scholar]
- 23.Ruiz JR, Ortega FB, Loit HM, et al. Body fat is associated with blood pressure in school-aged girls with low cardiorespiratory fitness: The European Youth Heart Study. J Hypertens. 2007;25:2027–34. doi: 10.1097/HJH.0b013e328277597f. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–4. [Google Scholar]
- 25.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Proceeding of the Second International Symposium on Information Theory. Akademiai Kiado; Budapest, Hungary: 1973. pp. 267–81. [Google Scholar]
- 26.Jackson AS, Sui X, Hebert JR, et al. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781–87. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills AK, Lawlor DA, Muniz-Terrera G, et al. Population heterogeneity in trajectories of midlife blood pressure. Epidemiology. 2012;23:203–11. doi: 10.1097/EDE.0b013e3182456567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Rourke MF. Arterial aging: Pathophysiological principles. Vasc Med. 2007;12:329–41. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 28.Steppan J, Barodka V, Berkowitz DE, et al. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;2011:263585. doi: 10.4061/2011/263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dao HH, Essalihi R, Bouvet C, et al. Evolution and modulation of age-related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307–17. doi: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson J. Age-related medial elastocalcinosis in arteries: mechanisms, animal models, and physiological consequences. J Appl Physiol. 2008;105:1643–51. doi: 10.1152/japplphysiol.90476.2008. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 33.Franklin SS, Gustin W, 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart study. Circulation. 1997;96:308–15. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 34.Brage S, Wedderkopp N, Ekelund U, et al. European Youth Heart Study (EYHS). Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: The European Youth Heart Study (EYHS) Diabetes Care. 2004;27:2141–8. doi: 10.2337/diacare.27.9.2141. [DOI] [PubMed] [Google Scholar]
- 35.LaMonte MJ, Barlow CE, Jurca R, et al. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: A prospective study of men and women. Circulation. 2005;112:505–12. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 36.Lee IM. Dose-response relation between physical activity and fitness: even a little is good; more is better. JAMA. 2007;297:2137–9. doi: 10.1001/jama.297.19.2137. [DOI] [PubMed] [Google Scholar]
- 37.Stamler J, Rose G, Stamler R, et al. INTERSALT study findings. Public health and medical care implications. Hypertension. 1989 Nov;14(5):570–7. doi: 10.1161/01.hyp.14.5.570. [DOI] [PubMed] [Google Scholar]
- 38.Pahkala K, Heinonen OJ, Simell O, et al. Association of physical activity with vascular endothelial function and intima-media thickness. Circulation. 2011;124:1956–63. doi: 10.1161/CIRCULATIONAHA.111.043851. [DOI] [PubMed] [Google Scholar]
- 39.Di Francescomarino S, Sciartilli A, Di Valerio V, et al. The effect of physical exercise on endothelial function. Sports Med. 2009;39:797–812. doi: 10.2165/11317750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Walther C, Gielen S, Hambrecht R. The effect of exercise training on endothelial function in cardiovascular disease in humans. Exercise and sport sciences reviews. 2004;32:129–34. doi: 10.1097/00003677-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 41.U.S.Department of Health and Human Services Physical Activity Guidelines Advisory Committee Report 2008. doi: 10.1111/j.1753-4887.2008.00136.x. Available at: http://www.health.gov/paguidelines/report/pdf/CommitteeReport.pdf). 2008. Accessed July 15, 2014. [DOI] [PubMed]
- 42.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stamler J, Rose G, Stamler R, et al. INTERSALT study findings. Public health and medical care implications. Hypertension. 1989;14:570–7. doi: 10.1161/01.hyp.14.5.570. [DOI] [PubMed] [Google Scholar]
- 44.Etchison WC, Bloodgood EA, Minton CP, et al. Body mass index and percentage of body fat as indicators for obesity in an adolescent athletic population. Sports Health. 2011;3:249–52. doi: 10.1177/1941738111404655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chase NL, Sui X, Lee DC, et al. The association of cardiorespiratory fitness and physical activity with incidence of hypertension in men. Am J Hypertens. 2009;22:417–24. doi: 10.1038/ajh.2009.6. [DOI] [PubMed] [Google Scholar]