Abstract
The aim of this study was to investigate the efficacy of topical application of colchicine in reducing intraarticular adhesion in rabbits. Thirty-six rabbits were randomly and equally divided into three groups. An approximately 10 × 10 mm2 area of cortical bone was removed from both sides of the left femoral condyle, and the cancellous bone underneath was exposed. Cotton pads soaked with different concentrations of colchicine or saline were applied to the decorticated areas for 10 minutes. The surgical limb was fixed in a flexed position for 4 weeks postoperatively. To evaluate knee intraarticular adhesion, we performed macroscopic evaluation, histological and collagen density analyses, hydroxyproline content determination, fibroblast counting and densitometric analyses. The results showed that loose collagen tissues with little or no adhesion were present around the decorticated areas in the group treated with 0.5 mg/ml colchicine. The intraarticular adhesion score, hydroxyproline content, number of fibroblasts and densitometric value in this group were also significantly lower than those in the other groups. There was moderate intraarticular adhesion in the group treated with 0.1 mg/ml colchicine. However, dense scar tissue with dense adhesions was found in the control group. In conclusion, topical application of 0.5 mg/ml colchicine may reduce knee intraarticular adhesion.
Intraarticular adhesion following knee surgery is a familiar complication and remains a challenging problem during recovery1,2. The physiological mechanism underlying adhesion formation is unclear, and there are multiple origins of adhesion. Following activation by cytokines, fibroblasts migrate to the sites of injury, where they proliferate. They then produce collagen and release extracellular matrix, which ultimately results in the formation of abundant scar tissue and adhesion. Intraarticular adhesion may cause joint stiffness, pain, cartilage degeneration and other severe functional impairments3,4,5.
For many years, intraarticular adhesion has been one of the primary concerns in joint surgery, and many strategies have been sought to reduce intraarticular adhesion. Minimally invasive techniques and meticulous hemostasis are generally applied to reduce tissue damage and avoid adverse complications. Arthroscopic lysis of adhesions is also used to relieve arthrofibrotic symptoms6,7. Moreover, in clinical and experimental studies, numerous pharmacological agents, biological materials and synthetic barriers have been used to reduce intraarticular adhesions2,8,9. However, the results have either been conflicting or involve adverse events, and in many cases, symptomatic intraarticular adhesions have recurred. Recently, some anticancer drugs, such as mitomycin C, hydroxycamptothecin and fluorouracil, have been topically applied to reduce tissue adhesions and have shown satisfactory results in clinical and experimental studies10,11,12.
Colchicine, an ancient drug extracted from the flowers of Colchicum autumnale, is generally used to treat acute gout attacks and familial Mediterranean fever. It can inhibit microtubule polymerization, disrupt the cytoskeleton and inhibit intracellular motility13,14. Recently, colchicine has been proven to be effective in idiopathic pulmonary fibrosis, actinic keratoses and cystic fibrosis owing to its strong anti-inflammatory and anti-fibrotic effects15,16. In addition to these diseases, colchicine has shown satisfactory results when used locally to prevent epidural fibrosis17.
The aim of this study was to investigate the efficacy of topical use of colchicine in reducing intraarticular adhesion following knee surgery in rabbits. We hypothesized that topically applied colchicine may be a preventive therapy for reducing intraarticular adhesion. The results of this study may be useful for future human trials of topical colchicine for clinical use.
Results
The recovery of all rabbits was uneventful following the operation, and there was no cutaneous necrosis, wound infection or mortality in the rabbits during the follow-up period.
Macroscopic evaluation
Macroscopic evaluation revealed no or partial weak fibrous adhesions between the decorticated areas of the femoral condyle and the joint capsule in the group treated with 0.5 mg/ml colchicine. In the group treated with 0.1 mg/ml colchicine, the decorticated areas were covered with moderate scar adhesions that could be eliminated by manual traction. However, dense fibrous adhesions were observed around the decorticated areas of the femoral condyle in the control group. The intraarticular adhesion scores were assessed based on the visual scoring system described in Table 1.
Table 1. Knee intraarticular adhesion grade based on the visual scoring system. Six rabbits were randomly selected from each group for analysis.
| Grade | ||||
|---|---|---|---|---|
| Group | 0 | 1 | 2 | 3 |
| Colchicine (0.5 mg/ml) | 2 | 4 | 0 | 0 |
| Colchicine (0.1 mg/ml) | 0 | 3 | 3 | 0 |
| Control (saline) | 0 | 0 | 0 | 6 |
Hydroxyproline content determination
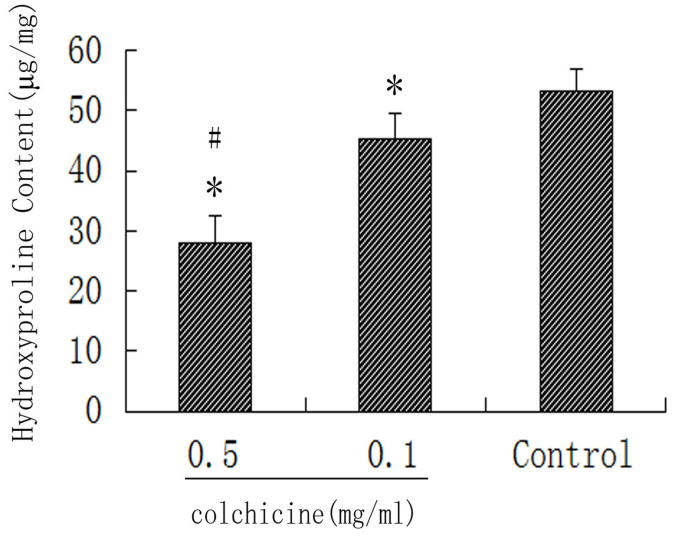
The hydroxyproline content in the colchicine-treated group was significantly less than that in the control group (P < 0.01). The hydroxyproline content in the 0.5 mg/ml colchicine-treated group was also less than that in the 0.1 mg/ml colchicine-treated group (P < 0.05). The hydroxyproline content in the intraarticular scar tissues for each group is shown in Figure 1.
Figure 1. The hydroxyproline content in each group.
*P < 0.01 compared with the hydroxyproline content in the control group. #P < 0.05 compared with the hydroxyproline content in the 0.1 mg/ml colchicine group.
Histological analysis
All the decorticated areas of rabbits in the control group showed markedly dense scar tissues with dense adhesions to the joint capsule and surrounding tissues. Extensive collagen-tissue hyperplasia was observed, and a large number of fibroblasts were observed in the decorticated areas. In the 0.1 mg/ml colchicine-treated group, the decorticated areas were primarily covered with moderate scar tissue. Collagen-tissue hyperplasia was decreased, and the number of fibroblasts was also reduced compared with that in the control group. In the 0.5 mg/ml colchicine-treated group, loose scar tissue with little or no adhesion was observed around the decorticated areas of the femoral condyle. Collagen-tissue hyperplasia was markedly decreased, and the number of fibroblasts was also significantly reduced compared with that in the control group (Fig. 2 and Fig. 3).
Figure 2. Representative histological images of intraarticular adhesion in rabbits treated with 0.5 mg/ml colchicine, 0.1 mg/ml colchicine or saline.
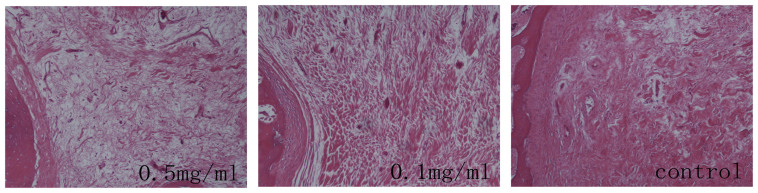
The decorticated area in the control group showed marked scar tissue with dense adhesions. The decorticated area in the 0.1 mg/ml colchicine-treated group was covered primarily with moderate scar tissue. The decorticated area in the 0.5 mg/ml colchicine-treated group showed loose scar tissue with few adhesions. The sections were stained with hematoxylin-eosin, and the magnification is 200×, the size of the scale bar is 100 μm.
Figure 3. Collagen density in intraarticular scar tissue in rabbits treated with 0.5 mg/ml colchicine, 0.1 mg/ml colchicine or saline.
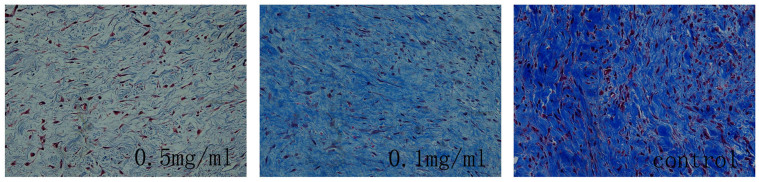
The collagen tissue is shown in blue because the sections were stained with Masson's trichrome. The density of collagen in the sections from the 0.5 mg/ml colchicine-treated group was significantly less than that in tissue from the 0.1 mg/ml colchicine-treated group and the saline group. The magnification is 200×, the size of the scale bar is 100 μm.
Fibroblast density
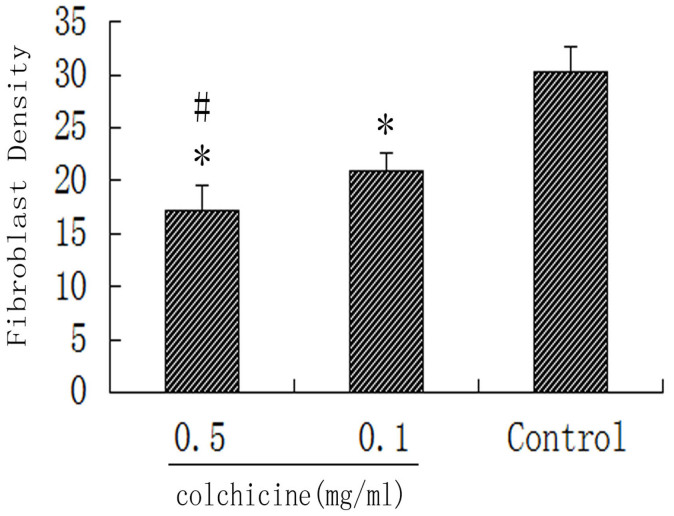
Fibroblast density in the intraarticular scar tissue in the 0.5 mg/ml colchicine-treated group was significantly reduced compared with the 0.1 mg/ml colchicine-treated group and the control group (P < 0.01). Moreover, fibroblast density in the 0.1 mg/ml colchicine-treated group was also less than in the control group (P < 0.05). The fibroblast densities in the intraarticular scar tissue of each treatment group are shown in Figure 4.
Figure 4. Fibroblast counts of tissue from each group.
*P < 0.05 compared with the fibroblast count of the control group. #P < 0.01 compared with the fibroblast count of the 0.1 mg/ml colchicine-treated group.
Densitometric analysis
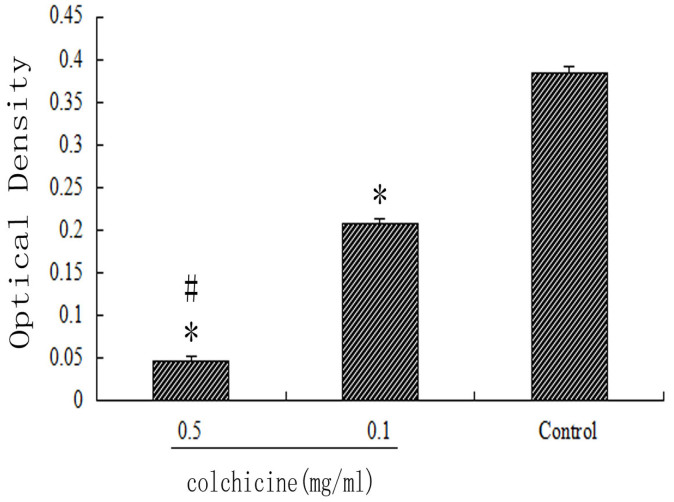
The optical density value of collagen tissue in the 0.5 mg/ml colchicine-treated group was significantly less than that in the 0.1 mg/ml colchicine-treated group and the control group (P < 0.01). Moreover, the optical density value of collagen tissue in the 0.1 mg/ml colchicine-treated group was less than that in the control group (P < 0.01). The optical density values of collagen tissue in the intraarticular scar tissue of each treatment group are shown in Figure 5.
Figure 5. Collagen optical density in each group.
*P < 0.01 compared with the optical density value of the control group. #P < 0.01 compared with the optical density value of the 0.1 mg/ml colchicine-treated group.
Discussion
This study showed that topically applied colchicine reduced intraarticular scar adhesion in rabbits following knee surgery by inhibiting fibroblast infiltration and collagen synthesis. Moreover, 0.5 mg/ml colchicine showed better effects than 0.1 mg/ml colchicine.
In this study, multiple methods, including macroscopic evaluation and histological analysis, were used to evaluate the efficacy of colchicine in reducing intraarticular adhesion. The formation of fibrotic adhesions in response to injury is not well understood, and adhesion can arise as a result of multiple factors. However, it is known that fibroblasts produce a large amount of collagen and extracellular matrix components in decorticated areas. This excess or sustained production may lead to the formation of scar tissue and result in intraarticular adhesion. Thus, collagen density measurements, hydroxyproline content determination, fibroblast counts and densitometric analysis of collagen may be used to evaluate the efficacy of colchicine in reducing intraarticular adhesion. In this study, macroscopic evaluation and histological observation detected weak or moderate fibrous adhesions around the decorticated areas in the colchicine-treated groups. Collagen density, hydroxyproline content, fibroblasts and collagen density were also all significantly lower in the colchicine-treated groups than in the control group. Moreover, these parameters were reduced in the 0.5 mg/ml colchicine-treated group compared with the 0.1 mg/ml colchicine-treated group, which indicates that 0.5 mg/ml colchicine is better able to reduce intraarticular adhesion compared with 0.1 mg/ml colchicine.
The efficacy of colchicine in reducing scar formation has been shown in the treatment of idiopathic pulmonary fibrosis, cystic fibrosis and actinic keratoses16,18. Colchicine has shown potential in prolonging myringotomy patency when applied as a solution to the external ear19. Another study showed that topically applied colchicine reduced spinal epidural fibrosis following total laminectomy in rat17.
In the past, colchicine was widely used in the treatment of arthritic conditions. The mechanism of action of colchicine is based primarily on its inhibition of microtubule polymerization14,18,19,20. It can bind to microtubular proteins, form high-affinity complexes and disrupt the cytoskeleton, which may result in the inhibition of cell division and secretion of cytokines. Colchicine exerts this effect primarily on fibroblasts and leucocytes. This inhibition, which is prominent in fibroblasts, decreases scar formation and cytokine production14,15,17. Thus, the anti-adhesion effect of colchicine is confirmed. Our study shows that colchicine inhibits fibroblast infiltration and reduces intraarticular adhesion, based on macroscopic evaluation and histological analysis following knee surgery.
Many studies have reported that colchicine has an inhibitory effect on the transport and secretion of collagen based on microtubule assembly inhibition and that it may also increase collagenase activity. These factors may underlie its anti-adhesion effects21.
Collagen is an important component of scar tissue that is primarily synthesized and secreted by fibroblasts. Moreover, hydroxyproline accounts for 12.5% of the amino acid content of collagen fibers; thus, hydroxyproline content may reflect the formation of collagen in scar tissue22. Using sections stained with Masson's trichrome, we measured collagen optical density, which is positively correlated with collagen levels. Densitometric analysis was then used to examine collagen content in the scar tissue. The consistency of scar adhesion was assessed on the basis of collagen density, hydroxyproline content and densitometric analyses. We observed that collagen density and hydroxyproline content were significantly decreased following colchicine treatment; these results confirm the findings of previous reports as well as our hypothesis. These results should be of interest to clinicians because they indicate that colchicine could be used as an anti-fibrotic drug.
Colchicine is an ancient drug with a narrow therapeutic-toxicity window, and there is marked interindividual variability in responses to this drug15. Intravenous injections of 0.015 mg/kg colchicine are effective; however, the drug is toxic in doses greater than 0.1 mg/kg and lethal at 0.8 mg/kg. Deaths have occurred following administration of intravenous colchicine to a cumulative dose of 4 mg during a course of therapy23. Recently, topical application of colchicine was used in a clinical and experimental study18. In another study, Tetik reported that arthroscopic washout fluid combined with 0.5625 mg/ml colchicine had effects on the biological properties of joint cartilage in a rat model, without any systemic side effects24. Haim observed that topical application of 0.01% colchicine to the middle-ear cavity reduced granulation formation and prolonged myringotomy patency; however, colchicine at concentrations of 0.1% and greater showed ototoxic effects19. It has also been reported that 0.005 mg/ml colchicine prevented epidural fibrosis; this concentration was based on its toxicity to the spinal cord and nerve roots17.
In the current study, the maximum concentration of topically applied colchicine used was 0.5 mg/ml, based on a previous study24. Our findings confirmed that colchicine reduced intraarticular adhesion effectively in rabbits. No systemic complications, such as cutaneous necrosis, wound infection or mortality, were noted following topical application. However, the toxicity of colchicine via topical absorption is unknown, and higher concentrations or application to a larger surface area may cause substantial absorption. Therefore, the surface area of application should be limited, and the safety margins determined.
In conclusion, topical application of suitable concentrations of colchicine reduced knee intraarticular adhesion without significant side effects, and this method may be an easy and low-cost technique for the prevention of intraarticular adhesion. However, the margin of safety, the format used and the long-term effects require further study and should be clarified prior to clinical application.
Methods
Ethics statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal care and experiments were performed in accordance with the guidelines and were approved by the Ethics Committee of Clinical Medical College of Yangzhou University.
Animal population
The colchicine solution was diluted to 0.5 mg/ml or 0.1 mg/ml using sterile physiological saline. Thirty-six male New Zealand white rabbits, weighing 3.5 to 4.0 kg, were housed in a controlled environment. The animals were randomly and equally divided into 3 groups: the 0.5 mg/ml colchicine group, the 0.1 mg/ml colchicine group and the control group. The rabbits were acclimated to the environment for 1 week prior to the experiment.
Surgical procedure
The rabbit knee intraarticular adhesion model was performed according to a previous study1,25. After induction of anesthesia using intravenous administration of 20% urethane, the fur around the left knee was shaved and the exposed skin was sterilized using iodophor. The knee was opened using a medial parapatellar approach, and the medial and lateral sides of the femoral condyle were exposed. An approximately 10 × 10 mm2 area of cortical bone was removed from both sides of the femoral condyle using a dental burr until the cancellous bone underneath was exposed. The articular cartilage was left intact. Following hemostasis, cotton pads soaked with 0.1 and 0.5 mg/ml colchicine or physiological saline were applied to the decorticated areas for 10 minutes. The surrounding tissues were covered with wet gauze to prevent contact with the agent. After the cotton pads were removed, the articular capsule and skin were closed with silk sutures. The surgical limbs were subjected to extra-articular knee-joint immobilization in the fully flexed position, using Kirschner wires, for 4 weeks. The animals were housed individually in cages and had free access to standard chow and water.
Macroscopic evaluation
After four weeks, six rabbits were randomly selected from each group for macroscopic evaluation following induction of anesthesia by intravenous administration of 20% urethane. The presence and severity of intraarticular adhesion were assessed by three professional pathologists according to the following visual scoring system25,26: 0, no adhesions; 1, weak, mild, filmy adhesions that were eliminated by minimal manual traction; 2, moderate adhesions that were eliminated by manual traction; and 3, dense and firm adhesions that had to be removed surgically.
Hydroxyproline content determination
The six rabbits were euthanized after the macroscopic evaluation. Scar tissues were obtained from the center of the decorticated areas, and the hydroxyproline content was determined using a previously described method1,22. The knee was opened, and approximately 20 mg (wet weight) of scar tissue was obtained from the decorticated areas. The samples were lyophilized, ground separately and hydrolyzed with 6 mol/l HCl at 130°C for 12 h. The samples were then neutralized with 2.5-N NaOH using methyl red as the indicator. One milliliter of chloramine T was added to the hydrolyzed samples and hydroxyproline standards (four known concentrations). Following incubation for 20 min at room temperature, 1 ml of p-dimethylaminobenzaldehyde solution was added to the samples and the standards. The absorbance of the solution was determined at 558 nm using a spectrophotometer, and the hydroxyproline content per milligram of scar tissue was calculated based on a standard curve constructed using serially diluted concentrations of commercial hydroxyproline.
Histological analysis
Six rabbits were selected from each group at four weeks postoperatively and were euthanized using an overdose of urethane. The entire knee joint, including all connective tissue and fibrotic scar tissue, was removed. The specimens were fixed in 10% buffered formalin and subsequently dehydrated and embedded in paraffin following decalcification in 6% nitric acid. Twelve successive 4-μm transverse sections perpendicular to the femoral axis were obtained.
Six odd-numbered sections were stained with hematoxylin-eosin, and the intraarticular scar adhesions were evaluated using a light microscope at a magnification of 200×. Three counting areas were selected in the middle and at the margins of the decorticated sites within each section, and each counting area was approximately 100 × 100 μm. Fibroblast density was calculated at a magnification of 400×. The number of fibroblasts in each section was defined as the mean number from three fields, and the number for each rat was defined as the mean number from the six sections.
Six even-numbered sections were stained with Masson's trichrome, and the optical density of collagen was observed using a light microscope at a magnification of 200×. Densitometric analysis of collagen tissue was also performed. The sections stained with Masson's trichrome were photographed using a light microscope (Olympus BX50, Japan) connected to a CCD camera (Olympus DP70, Japan). The optical density value of positively stained collagen was determined using Image Pro Plus 6.0 image analysis software.
Statistical analysis
Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) software (version 15.0). The data are shown as the mean ± standard deviation. Tukey's test was used to calculate significant differences in hydroxyproline content, fibroblast number and optical density. For all analyses, P < 0.05 was considered statistically significant.
Author Contributions
This study was conceived and designed by Y.L.Q., L.X.L., S.Y. and L.Y.; S.Y., L.Y., H.J.L., W.J.C. and W.D.X. performed the experiments. All authors analyzed the data and discussed the results. S.Y. and L.Y. wrote the paper, and the other authors commented on the manuscript.
Acknowledgments
Funding was provided by the National Natural Science Foundation of China (grants 81301550, 81371971 and 81271994). The authors express their sincere appreciation to all workers in the pathology laboratory of Yangzhou University.
References
- Fukui N., Tashiro T., Hiraoka H., Oda H. & Nakamura K. Adhesion formation can be reduced by the suppression of transforming growth factor-beta1 activity. J Orthop Res 18, 212–219 (2000). [DOI] [PubMed] [Google Scholar]
- Hayashi M., Sekiya H., Takatoku K., Kariya Y. & Hoshino Y. Experimental model of knee contracture in extension: its prevention using a sheet made from hyaluronic acid and carboxymethylcellulose. Knee Surg Sports Traumatol Arthrosc 12, 545–551 (2004). [DOI] [PubMed] [Google Scholar]
- Monument M. J. et al. The mast cell stabilizer ketotifen reduces joint capsule fibrosis in a rabbit model of post-traumatic joint contractures. Inflamm Res 61, 285–292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand K. A., Zhang M., Salo P. T. & Hart D. A. Joint capsule mast cells and neuropeptides are increased within four weeks of injury and remain elevated in chronic stages of posttraumatic contractures. J Orthop Res 26, 1313–1319 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand K. A., Zhang M., Germscheid N. M., Wang C. & Hart D. A. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta Orthop 79, 116–125 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy A. M. & Elsoufy M. A. Arthroscopic arthrolysis for arthrofibrosis of the knee after total knee replacement. HSS J 7, 130–133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerosch J. & Aldawoudy A. M. Arthroscopic treatment of patients with moderate arthrofibrosis after total knee replacement. Knee Surg Sports Traumatol Arthrosc 15, 71–77 (2007). [DOI] [PubMed] [Google Scholar]
- Brunelli G., Longinotti C., Bertazzo C., Pavesio A. & Pressato D. Adhesion reduction after knee surgery in a rabbit model by Hyaloglide, a hyaluronan derivative gel. J Orthop Res 23, 1377–1382 (2005). [DOI] [PubMed] [Google Scholar]
- Fukui N., Nakajima K., Tashiro T., Oda H. & Nakamura K. Neutralization of fibroblast growth factor-2 reduces intraarticular adhesions. Clin Orthop Relat Res 250–258 (2001). [DOI] [PubMed] [Google Scholar]
- Kocaoglu B., Akgun U., Nalbantoglu U., Poyanli O. & Karahan M. Adhesion reduction after knee surgery in a rat model by mitomycin C. Knee Surg Sports Traumatol Arthrosc 19, 94–98 (2011). [DOI] [PubMed] [Google Scholar]
- Li X. et al. Comparison of the effects of mitomycin C and 10-hydroxycamptothecin on an experimental intraarticular adhesion model in rabbits. Eur J Pharmacol 703, 42–45 (2013). [DOI] [PubMed] [Google Scholar]
- Sun Y. et al. A comparison of the effectiveness of mitomycin C and 5-fluorouracil in the prevention of peridural adhesion after laminectomy. J Neurosurg Spine 7, 423–428 (2007). [DOI] [PubMed] [Google Scholar]
- Rigante D. et al. The pharmacologic basis of treatment with colchicine in children with familial Mediterranean fever. Eur Rev Med Pharmacol Sci 10, 173–178 (2006). [PubMed] [Google Scholar]
- Andreu J. M. & Timasheff S. N. Interaction of tubulin with single ring analogues of colchicine. Biochemistry 21, 534–543 (1982). [DOI] [PubMed] [Google Scholar]
- Niel E. & Scherrmann J. M. Colchicine today. Joint Bone Spine 73, 672–678 (2006). [DOI] [PubMed] [Google Scholar]
- Entzian P. et al. Antiinflammatory and antifibrotic properties of colchicine: implications for idiopathic pulmonary fibrosis. Lung 175, 41–51 (1997). [DOI] [PubMed] [Google Scholar]
- Ozdemir O. et al. Topical use of colchicine to prevent spinal epidural fibrosis in rats. Neurol Res 32, 1117–1120 (2010). [DOI] [PubMed] [Google Scholar]
- Akar A. et al. Efficacy and safety assessment of 0.5% and 1% colchicine cream in the treatment of actinic keratoses. J Dermatolog Treat 12, 199–203 (2001). [DOI] [PubMed] [Google Scholar]
- Haim G., Ephraim E., Ronen P. & Haim S. Colchicine prolongs patency of myringotomy in an animal model. Int J Pediatr Otorhinolaryngol 75, 554–557 (2011). [DOI] [PubMed] [Google Scholar]
- Maduri S. & Atla V. R. Formulation of colchicine ointment for the treatment of acute gout. Singapore Med J 53, 750–754 (2012). [PubMed] [Google Scholar]
- Harris E. D. Jr & Krane S. M. Effects of colchicine on collagenase in cultures of rheumatoid synovium. Arthritis Rheum 14, 669–684 (1971). [DOI] [PubMed] [Google Scholar]
- Woessner J. F. Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93, 440–447 (1961). [DOI] [PubMed] [Google Scholar]
- Bonnel R. A., Villalba M. L., Karwoski C. B. & Beitz J. Deaths associated with inappropriate intravenous colchicine administration. J Emerg Med 22, 385–387 (2002). [DOI] [PubMed] [Google Scholar]
- Tetik O., Doral M. N., Atay A. O. & Leblebicioglu G. Influence of irrigation solutions combined with colchicine and diclofenac sodium on articular cartilage in a rat model. Knee Surg Sports Traumatol Arthrosc 12, 503–509 (2004). [DOI] [PubMed] [Google Scholar]
- Yan L. et al. The effect of mitomycin C in reducing intraarticular adhesion after knee surgery in rabbits. Eur J Pharmacol 643, 1–5 (2010). [DOI] [PubMed] [Google Scholar]
- Rothkopf D. M., Webb S., Szabo R. M., Gelberman R. H. & May J. W. Jr An experimental model for the study of canine flexor tendon adhesions. J Hand Surg Am 16, 694–700 (1991). [DOI] [PubMed] [Google Scholar]